Abstract
Purpose:
To identify treatment, intrapersonal, and provider factors that influence childhood cancer survivors' adherence to recommended mammography screening.
Design:
Secondary analysis of data derived from 3 consecutive surveys within the Childhood Cancer Survivors' Study.
Sample:
Female childhood cancer survivors: N = 335, mean age = 30.92, mean years after diagnosis = 21.79.
Methods:
T-tests and structural equation modeling.
Main Research Variables:
Mammogram recency, health concerns, affect, motivation, survivor-provider interaction.
Findings:
Forty-three percent of the variance was explained in mammogram recency. Survivors most likely to follow the recommended mammogram schedule were directly influenced by cancer treatment exposure to mantle radiation (p = 0.01), less intrinsic motivation (p = 0.01), positive affect (p = 0.05), recent visits to oncology clinic (p = 0.01), discussion of subsequent cancer risks with physician (p = 0.001), perceptions of more severe late effects (p = 0.05), age ≥ 40 years (p ≤ 0.001), and having received a print media intervention detailing breast cancer risks and follow-up strategies.
Conclusions:
Perceived symptoms, motivation, affect, provider influences, readiness for medical follow-up, and knowledge of treatment exposures are potential modifiable targets for intervention to support mammography screening in childhood cancer survivors at risk.
Implications for Nursing:
(1) Provide written summaries of treatment exposures and recommended schedule of mammography screening at end of cancer treatment and throughout follow-up; (2) identify and address survivor symptoms and concerns that may potentially negate screening; (3) enhance motivation for screening through tailoring personal risk information to health concerns, affect and readiness for follow-up.
INTRODUCTION AND BACKGROUND
There is now a sizable cohort of women between the ages of 20 and 40 who are at an elevated risk for breast cancer because their developing breast tissue was exposed to radiation during childhood cancer treatment (Hewitt, Weiner, & Simone, 2003; Ries et al., 2007). Survivors of Hodgkin's disease comprise the largest proportion of childhood cancer survivors in the group at risk for secondary breast cancer. However, chest radiation is also routinely used in treatment protocols for metastatic Wilms' tumor and soft tissue sarcomas, as well as for other refractory or recurrent pediatric malignancies. Previous investigations indicate that by 45 years of age, 12% to 20% of young women treated with radiation therapy will be diagnosed with breast cancer (Bhatia et al., 2003; Kenney et al., 2004; Taylor, Winter, Stiller, Murphy, & Hawkins, 2006). Thus the risk of breast cancer after chest radiation for a pediatric malignancy rivals that of women with a BRCA mutation, who have an estimated cumulative incidence of breast cancer at age 40 that ranges from 10% to 19% (Bhatia et al., 2003; Bishop, 1999; Ford et al., 1998; Struewing et al., 1997).
Information about secondary breast cancer following radiation for pediatric malignancies is largely derived from studies of survivors of Hodgkin's disease. The risk of breast cancer in this group begins to increase about 8 years after chest radiation (Bhatia et al., 2003; Kenney et al., 2004; Metayer et al., 2000) and the interval from Hodgkin's disease to breast cancer for both pediatric and adult groups is about 15 to 20 years (Bhatia et al., 2003; Cutuli et al., 2001; Kenney et al., 2004; Metayer et al., 2000; Taylor et al., 2006; Wolden et al., 2000). The median age of breast cancer diagnosis is 32 to 35 years old, (Bhatia et al., 2003; Kenney et al., 2004; Taylor et al., 2006), which is well below the average age of breast cancer onset (e.g., > 50 years) (Ries et al., 2007) in the general population and below the age at which most women routinely undergo mammography (e.g., 40 years)(American Cancer Society, 2007).
Consistent with the general population (Berry et al., 2005; Vlastos & Verkooijen, 2007), early detection of breast cancer in the high-risk population of childhood cancer survivors may lead to increased diagnoses of breast cancers at early stages, thereby requiring less invasive treatments, incurring improved outcomes and enhanced quality of life. Annual screening mammography with adjunct breast MRI is recommended for childhood cancer survivors, starting at age 25 or eight years after completion of radiation therapy, whichever occurs last (Children's Oncology Group, 2006). Among Hodgkin's disease survivors who develop secondary breast cancer, 27% to 100% of the cancers were detected by mammography (Dershaw, Yahalom, & Petrek, 1992; Diller et al., 2002; Wolden et al., 2000); however, many survivors do not adhere to the treatment exposure–based guidelines for screening. For example, only 169 (41%) of 414 survivors at increased risk of breast cancer underwent mammography (Nathan et al., 2007), and fewer long-term survivors of childhood cancer (21%, N = 4414) report ever having had a mammogram (Yeazel et al., 2004) compared to survivors of adult cancers (75%–92%, N = 4785) (Bellizzi, Rowland, Jeffery, & McNeel, 2005). There is a critical need to educate and promote mammography screening in this high risk population in order to potentially reduce breast cancer morbidity and mortality.
Quite similar to the general population (Cui et al., 2007; Cummings, Whetstone, Shende, & Weismiller, 2000; Goodwin, Visintainer, Facelle, & Falvo, 2006; Williams, Lindquist, Sudore, Covinsky, & Walter, 2008) factors that predict mammography utilization in survivors of breast cancer and Hodgkin disease include visits to the oncologist (Field et al., 2008), gynecologist (Doubeni et al., 2006), or primary care physician (Doubeni et al., 2006), having health insurance (Bober, Park, Schmookler, Medeiros Nancarrow, & Diller, 2007), physician support (Bober et al., 2007), worry about breast cancer (Bloom, Stewart, & Hancock, 2006), older age (Bloom et al., 2006), and higher education and income (Breen, Yarbroff, & Meissner, 2007). Childhood cancer survivors who are least likely to report receiving routine mammography are younger and express a lack of concern for future health issues (Yeazel et al., 2004).
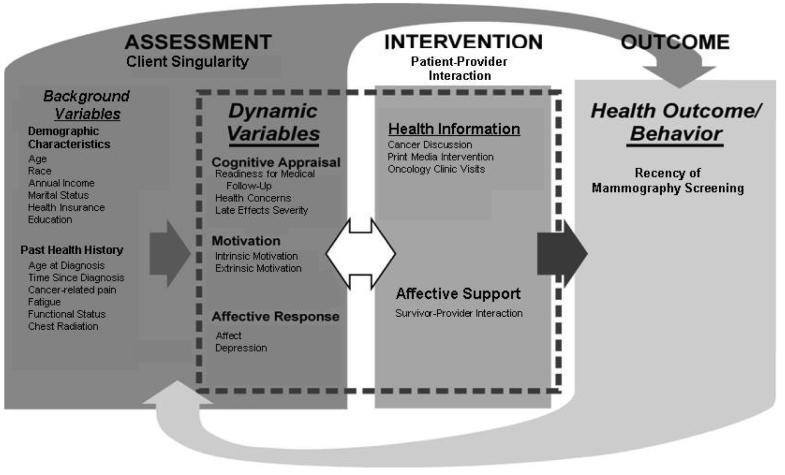
In addition to disease and treatment factors, personal and contextual factors influence health behavior choices (Breslow, Lloyd & Shumaker, 1994; Cox, McLaughlin, Rai, Steen, & Hudson, 2005; Cox, McLaughlin, Steen, & Hudson, 2006; Kraemer, Wilson, Fairburn, & Agras, 2002; Prochaska, 2005; Rejeski, Brawley, McAuley, & Rapp, 2000). To describe the multiple influences on survivors' adherence to mammography screening guidelines, we selected the Interaction Model of Client Health Behavior (IMCHB) (Cox, 1982; Cox, 2003; Cox et al., 2006; Cox et al., 2008a, 2008b, 2008c), which incorporates both intrapersonal and contextual variables and has been adapted to the study of childhood cancer survivors (Fig. 1). The IMCHB incorporates physical, social, cognitive, motivational, affective, provider, and environmental antecedents to health behavior. The original empirical support for the model concepts and their relationships is reported in detail elsewhere (Cox, 1982, 1984). Briefly, the model comprises three elements: client singularity (the unique intrapersonal and contextual configuration of the individual), client-professional interaction (the therapeutic content and process that occurs between a provider and patient), and health outcomes (the behavior or behaviorally related outcome subsequent to a patient-professional interaction). The model's working hypothesis is that the potential for positive health outcomes increases as the provider intervention is tailored to the unique manifestation of each patient relative to a constellation of their background variables, cognitive appraisal, affect, and motivation.
Fig. 1.
Correspondence of the Interaction Model of Client Health Behavior (IMCHB) with Study Variables
Structural equation modeling (SEM), which combines factor and path analyses into a comprehensive methodology (Kaplan, 2000), allowed us to test the hypotheses generated by the conceptual model. SEM tests all hypothesized relationships simultaneously rather than sequentially. Our goal was to identify disease, treatment, survivor, provider, and contextual factors that could be targeted with behavioral interventions to support recommended mammography screening.
METHODS
Data Source
The CCSS is a multi-institutional retrospective cohort study that was started in 1994 to examine the late effects of pediatric cancer treatment. Survivors complete a baseline questionnaire at study entry and respond to follow-up questionnaires sent at regular intervals. Participants consented to release their medical records from their participating treatment centers. Questionnaires and sampling methods are detailed in Robison, et al. (2002) and available for review at http://www.stjude.org/ccss.
The Follow-up 2 and Health Care Needs Surveys provided the data used for this study. The Follow-up 2 questionnaire contains questions on: demographics, medical care received during the most recent two-year period, medical conditions recently diagnosed, surgical procedures, cancer recurrence or new malignancies, marital status, pregnancy history, offspring, health habits, education, employment, insurance, income, and family history. The majority of the questions used in the Follow-up 2 questionnaire came from the National Health Interview Survey and were validated in a population of childhood cancer survivors (Louie et al., 2000). The Health Care Needs Survey addressed, socio-demographic factors, survivor-related psychological factors, knowledge of late effects, access to health care, and multidimensional health locus of control.
Sample
Originally 20,346 survivors were contacted to participate in CCSS. Eligible participants were those who had survived 5 or more years after being treated for a malignant disease diagnosed (before the age of 21 years) between 1970 and 1986. The Health Care Needs Survey (HCNS; initiated by KO), randomly sampled 1600 of the survivors. Of the 978 (61%) participants who completed and returned the survey, 838 (86%) returned the Follow-up 2 survey of the CCSS within the same data collection period. Nonrespondents to the HCNS were typically male (59%), minorities (37%), or had less than a high school education (56%). Survivors who completed the HCNS but not the Follow-up 2 survey were younger at diagnosis (P = 0.019) and diagnosed more recently (P ≤ 0.001). Data were self-reported (Table 1). The sample for this analysis includes females who had responded to the HCNS and Follow-up 2 surveys (N = 453); for descriptive comparative purposes (Tables 1 and 2), we selected a subset of young women at highest risk for secondary breast neoplasm (exposure to mantle radiation during cancer therapy) (N = 82) and compared them to the total female sample (N = 453).
Table 1.
Descriptive Summary for Female Survivors at Lower (N = 453) and Highest Risk (N=82) for Breast Neoplasma
| Variables |
Lower-Risk Group [N(%)] |
Higher-Risk Group [N(%)] |
P-Value (X2) |
||||
|---|---|---|---|---|---|---|---|
| Race | 0.133 | ||||||
| White | 275 (74.7) | 61 (74.4) | |||||
| Black | 36 (9.8) | 4 (4.9) | |||||
| Hispanic | 40 (10.9) | 15 (18.3) | |||||
| Other | 17(4.60) | 2(2.4) | |||||
| Personal Annual Income ($U.S.) | 0.283 | ||||||
| None | 63 (17.6) | 13 (16.5) | |||||
| <19,999 – 39,999 | 235 (65.7) | 46 (58.3) | |||||
| 40,000-59,999 | 40 (11.2) | 15 (19.0) | |||||
| ≥60,000 | 20 (5.6) | 5 (6.3) | |||||
| Marital Status | ≤0.001 | ||||||
| Ever Married | 136 (36.8) | 57 (69.5) | |||||
| Never Married | 234 (63.2) | 25 (30.5) | |||||
| Health Insurance | 0.164 | ||||||
| Yes | 320 (87.2) | 76 (92.7) | |||||
| No | 47 (12.8) | 6 (7.3) | |||||
| Education | 0.570 | ||||||
| 1-12 years | 7 (1.9) | 1 (1.2) | |||||
| Completed High School/GED | 52 (14.2) | 7 (8.6) | |||||
| Post High School Training/Some College | 132 (36.0) | 31 (38.3) | |||||
| College Graduate/Post-Graduate Work | 176 (48.0) | 42 (51.9) | |||||
| Seen at Oncology Clinic Within Past 2 Years | 0.004 | ||||||
| Yes | 30(8.6) | 18 (22.5) | |||||
| No | 318(91.4) | 62(77.5) | |||||
| Mean | SD | Range | Mean | SD | Range |
P-Value (T-test) |
|
| Age (years) | 29.78 | 6.94 | 17.20-50.40 | 36.06 | 7.63 | 19.10-51.50 | ≤0.001 |
| Age at Diagnosis (years) | 8.18 | 5.59 | 0-20.90 | 13.44 | 5.63 | 0.4-20.90 | ≤0.001 |
| Time Since Diagnosis (years) | 21.61 | 4.43 | 14.30-31.90 | 22.62 | 5.02 | 14.5-31.70 | 0.069 |
N varies because of missing data.
Table 2.
Descriptive Summary of Study Measures Comparing Survivors at Lower and Highest Secondary Breast Neoplasms
| Screening Behavior | Lower-Risk Group [N (%)] |
Highest Risk Group [N (%)] |
P-Value Between Lower and Highest Risk Groups (X2) |
||
|---|---|---|---|---|---|
| Recency of Mammogram | ≤0.001 | ||||
| Never | 265 (58.6) | 22 (26.8) | |||
| 5 or more years ago | 86 (19.0) | 30 (36.6) | |||
| More than 2 years but less than 5 years | 39 (8.6) | 15 (18.3) | |||
| 1-2 years ago | 28 (6.2) | 9 (11.0) | |||
| Less than a year ago | 26 (5.8) | 6 (7.3) | |||
| Don't know | 8 (1.8) | 0 (0) | |||
| INDEPENDENT VARIABLES | P-Value Between Lower and Highest Risk Groups (X2) | ||||
| Physician Discussed Risk of Developing Cancer | 0.001 | ||||
| Yes | 125 (29.2) | 41 (51.3) | |||
| No | 303 (70.8) | 39 (48.8) | |||
| Perceived Severity of Late Effects | 0.065 | ||||
| Moderate, Severe, Life-threatening | 101(22.5) | 30 (36.6) | |||
| Mild or No Chronic Problems | 348(77.5) | 52 (63.4) | |||
| Received Print Media Intervention | 0.023 | ||||
| Yes | 126 (27.8) | 33 (40.2) | |||
| No | 327 (72.2) | 49 (59.8) | |||
| Age 40 Years or More | <0.001 | ||||
| Yes | 65 (14.3) | 29 (35.4) | |||
| No | 388 (85.7) | 53 (64.6) | |||
| Likelihood of Cancer-Related Follow-up | 0.0009 | ||||
| Precontemplation | 220 (50.3) | 22 (27.8) | |||
| Contemplation | 127 (29.1) | 36 (45.6) | |||
| Action | 90 (20.6) | 21 (26.6) | |||
| Mean | SD | Mean | SD |
P-Value Between Lower and Highest Risk Groups (t test) |
|
| Intrinsic Motivation | 17.91 | 3.78 | 17.93 | 3.65 | 0.966 |
| Extrinsic Motivation | 7.77 | 3.37 | 7.32 | 2.75 | 0.260 |
| Affect | 18.15 | 3.91 | 18.88 | 3.84 | 0.601 |
| Health Concerns | 3.85 | 0.90 | 3.56 | 0.78 | 0.007 |
| Patient-Physician Relationship | 13.63 | 3.92 | 13.31 | 3.27 | 0.498 |
| No. of Cancer-related Physician Visits | 1.78 | 1.37 | 2.49 | 1.72 | 0.001 |
| Exercise Frequency at Baseline | 2.12 | 2.08 | 1.82 | 1.87 | 0.230 |
Outcome Measures
Single items addressed the recency of the last mammogram (1 = Never; 2 = 5 or more years ago; 3 = More than 2 years but less than 5 years; 4 = 1-2 years ago; 5 = Less than 1 year ago) (see Table 2). Survivors who answered “don't know” for any of the screening exams were excluded from the analysis.
Independent Measures
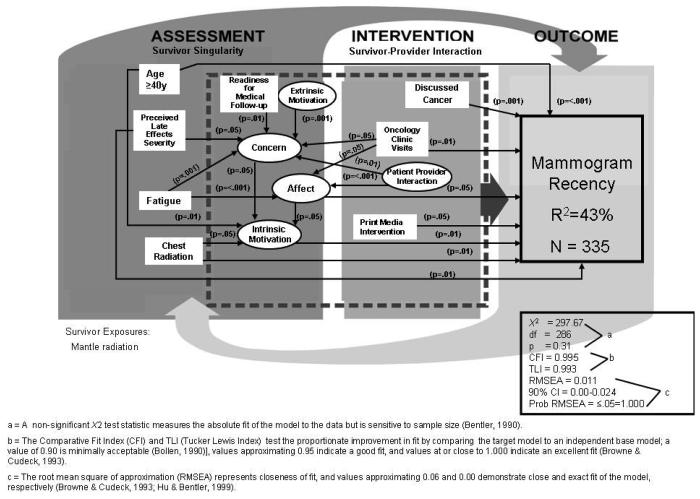
Two types of variables are modeled in SEM: observed and latent. In contrast to observed variables that can be directly measured (e.g., test scores, diagnostic criteria), latent variables (e.g., depression) are measured indirectly by a set of observed variables (Muthen & Muthen, 2007). Our final model has 8 directly observed measures (represented in Fig. 2 as rectangles) and 5 latent measures (represented in Fig. 2 as ovals) that contributed directly and/or indirectly to the explained variance in frequency of mammography.
Fig. 2.
Predictors of Mammography Recency
Directly observed independent variables
Although all variables corresponding to the conceptual model were examined as potential covariates, the following directly observed independent variables were statistically significant in the final models: (1) number of cancer-related visits in the last 2 years (1 = None; 7 = More than 20); (2) physician–survivor discussion of subsequent cancer (1 = Yes, 2 = No); (3) survivors' perceptions of their late effects (1 = moderate, severe, life-threatening; 2 = mild or no chronic problems); (4) follow-up care at an oncology clinic in the past 2 years (1 = Yes; 2 = No); (5) age ≥ 40 years (1 = Yes; 0 = No); (6) receipt of a print media intervention detailing exposure risks and recommended follow-up for breast sequelae (1 = Yes; 0 = No); (7) exposure to mantle radiation during cancer treatment (1=Yes; 0 = No), (8) fatigue (1 = All of the time; 6 = None of the time); and (9) stage or level of readiness for medical follow-up (1 = Pre-contemplation – no cancer-related check-up from a physician in the last 2 years and little likelihood of having a check-up within the next 2 years; 2 = Contemplation – no cancer-related check-up in the last 2 years, but likely or very likely to have a cancer-related check-up in the next 2 years; 3 = Action – had a cancer-related check-up in the last 2 years and likely or very likely to have a cancer-related check-up in the next 2 years).
Latent independent variables
The following latent measures were significant in the final models.
Health Concerns: Three observed variables comprise this variable – survivors' general concerns about their health, their concerns about the chances of getting sick, and their perceptions about the importance of a check-up (1 = Moderate, quite a bit, or extremely concerned; 2 = Not at all or a little concerned) (α = 0.79).
Affect: Four items from the SF-36 Mental Health subscale (Ware, Snow, & Kosinski, 2000) comprise this variable (1 = All of the time; 6 = None of the time) – peaceful, happy, downhearted and blue, or not cheerful. Reverse-scoring allowed higher scores to reflect a more positive affect (α = 0.78).
Intrinsic Motivation: Five observed items from the Multidimensional Health Locus of Control Scale (MHLC) (Wallston, Wallston, & DeVellis, 1978) comprise this variable (1 = Strongly disagree; 6 = Strongly agree) (α = 0.79).
Extrinsic Motivation: Five MHLC (Wallston et al., 1978) items comprise this variable (1 = Strongly disagree; 6 = Strongly agree) (α = 0.80).
Survivor–Physician Relationship: Four observed items rated (1 = Not at all; 5 = Extremely) comprise this variable – doctors took enough time to answer questions, could ask doctor questions about cancer, fears and concerns had been addressed by doctors and nurses, and PCP could handle cancer-related problems (α = 0.78).
Statistical Analyses
SEM has two components: (1) the measurement model evaluates whether observed measures (e.g., scales, self-reports) adequately represent the latent variables and (2) model hypotheses (Fig. 1) are then tested with respect to the interrelation of the latent variables and covariates (Raykov & Marcoulides, 2000). SEM was performed with Mplus 4.2 (Muthen & Muthen, 2007). The models are based on a complete data matrix. A sample size of more than 200 is considered large in SEM (Kline, 2005).
Multiple indicators assess how well the model fits the data (Bentler, 1990; Bollen, 1990; Browne & Cudeck, 1993; Hu & Bentler, 1999). Factor loading values for the latent variables were less than or equal to p = 0.01 and factor score determinacy values were more than 0.90, suggesting strong latent construct measures (Muthen & Muthen, 2007). The final model has significant parameter estimates corresponding to the hypothesized relationships (Table 3), meets the established SEM fit criteria (Fig. 2), and offers the highest percentage of explained variance for mammography screening recency.
Table 3.
Structural Equation Modeling (SEM) Results for Mammogram Recency (R2 = 43%)
| Estimate | SE | Estimate/SEa | Std YXb | |
|---|---|---|---|---|
| Mammogram Recency | ||||
| Intrinsic motivation | −0.257 | 0.089 | −2.880 | −0.151 |
| Affect | 0.168 | 0.085 | 1.968 | 0.095 |
| Discussed subsequent CA | −0.505 | 0.157 | −3.221 | −0.140 |
| Perceived severity of late effects | −0.514 | 0.172 | −2.995 | −0.131 |
| Age ≥ 40 years | 1.853 | 0.208 | 8.919 | 0.404 |
| Exposure to chest radiation | 0.566 | 0.196 | 2.880 | 0.132 |
| Print media intervention | 0.361 | 0.155 | 2.329 | 0.100 |
| Seen at oncology clinic in the past 2 years | −0.773 | 0.229 | −3.376 | −0.150 |
| Affect | ||||
| Survivor-provider interaction | 0.266 | 0.050 | 5.334 | 0.307 |
| Seen at oncology clinic in the past 2 years | −0.304 | 0.144 | −2.115 | −0.104 |
| Fatigue | 0.407 | 0.041 | 10.031 | 0.567 |
| Health Concerns | ||||
| Fatigue | 0.068 | 0.019 | 3.652 | 0.233 |
| Readiness for medical follow-up | −0.087 | 0.030 | −2.893 | −0.187 |
| Extrinsic motivation | −0.146 | 0.037 | −3.918 | −0.308 |
| Survivor-provider interaction | 0.094 | 0.025 | 3.696 | 0.268 |
| Seen at oncology clinic in the past two years | 0.159 | 0.075 | 2.119 | 0.135 |
| Intrinsic Motivation | ||||
| Affect | 0.188 | 0.075 | 2.520 | 0.182 |
| Health concerns | 0.479 | 0.209 | 2.294 | 0.187 |
| Age ≥ 40 years | −0.504 | 0.172 | −2.922 | −0.187 |
| Exposure to chest radiation | 0.399 | 0.161 | 2.483 | 0.159 |
z score = 1.96, significant at P=0.05; z score =2.58, significant at P=0.01
an approximation of the strength of the relative contribution of the background variable to the outcome (either the latent construct or the path outcome) obtained by using data that adjust for the differences in measurement scales
RESULTS
The typical respondent was a white, unmarried female college graduate with a personal income of $19,999–39,999; she had health insurance and had not been seen at an oncology clinic in the past 2 years. Tables 1 and 2 compare the total female sample (N = 453) with women at highest risk (exposure to mantle radiation) for secondary breast neoplasm (N = 82). Women in the high-risk group were currently older, older at diagnosis, had been married, and were more likely to have been seen at an oncology clinic more recently. No one in the high-risk group reported not knowing whether or not they had ever had a mammogram, and they were more likely than those in the lower-risk group to have had a mammogram more recently. Notably, 77.6% of those in the lower-risk group had never or not within the last 5 years had a mammogram compared to 63.4% of those at highest risk of secondary breast neoplasm. Compared to survivors at lower risk, those at highest risk for breast cancer were more likely to have discussed with their physician the risk of developing a subsequent cancer, had received a print media intervention detailing their risks and suggested follow-up, be ≥ 40 years, be more ready for medical follow-up, be more concerned about their health, and report more cancer-related physician visits.
Structural Equation Model of Mammogram Recency
The total sample (lower and highest risk groups combined) of females was used to test the SEM. The mantle radiation variable was used as an independent predictor of mammogram recency. The mammogram model fit the data very well (N = 335; X2 = 297.67, df = 286, P = 0.31; CFI = 0.995, TLI = 0.993; RMSEA = 0.011; 90% CI = 0.00–0.024; Probability RMSEA ≤ .05 = 1.000) and explained 43% of the variance in recency of mammogram screening. Eight variables predicted more frequent mammograms in keeping with the recommended schedule: (1) a more positive affect, (2) discussion of subsequent cancer with physician, (3) age ≥ 40 years, (4) less intrinsic motivation, (5) visits to the oncology clinic within the last 2 years, (6) exposure to mantle radiation, (7) receipt of a print media intervention detailing risks and recommended follow-up for those at increased risk for breast cancer, and (8) perceptions of moderate to severe late effects of therapy. A more positive perception of physician interaction, less frequent fatigue, and more recent visits to the oncology clinic predicted a more positive affect. Higher levels of health concern were predicted by higher levels of extrinsic motivation, negative perceptions of the provider, more frequent fatigue, more recent oncology clinic visits, and a higher stage of readiness for medical follow-up. Higher levels of intrinsic motivation were predicted by lower levels of health concern, a more positive affect, age ≥ 40 years, and not having been exposed to chest radiation. Being age ≥ 40 years directly predicted mammography recency and indirectly predicted screening recency through intrinsic motivation (p = 0.05).
DISCUSSION
Female adult survivors of childhood cancer often do not adhere to recommended mammography screening guidelines. Approximately 59% of those at lower risk and 27% of those at highest risk had never had a mammogram, despite their older current age and longer interval since diagnosis and treatment. In keeping with recommendations for the general population (U.S. Preventive Services Task Force, 2002), however, female survivors ≥ 40 years were more likely than those ≤ 40 years to adhere to mammography screening guidelines.
Nearly half of those at highest risk of secondary breast neoplasm reported having never discussed subsequent cancer risks with a physician. Fewer survivors in both groups reported being at the later action stage of readiness for medical follow-up than the earlier precontemplation and contemplation stages.
Fatigue and perceptions of severity of late effects were strong exogenous variables (unaffected by other variables) in the model. Perceptions of more severe late effects supported more recent mammography and contributed to greater health concerns; more frequent fatigue contributed to greater health concerns and to a more negative affect, which in turn was more likely to result in less frequent mammography. In recent reports of adult survivors of childhood cancer, 19% of 2645 survivors (Mulrooney et al., 2008), and 30% of 161 survivors reported fatigue (Meeske, Siegel, Globe, Mack, & Bernstein, 2005). Fatigue can negatively impact quality of life in survivors (Meeske et al., 2005) as well as deter health behaviors that can modify late effects (Cox et al, 2008b; Cox, Rai, Rosenthal, Phipps, & Hudson, 2008d).
More health concerns contributed to a poorer affect and lower levels of intrinsic motivation; these findings may reflect those patients who are already experiencing more late effects sequelae. More problems increase health concerns and may make survivors feel that their health issues are beyond their control (low intrinsic motivation). Moreover, lack of specific information on risk factors and misconceptions about risk can exacerbate concerns or make survivors deny that significant health problems exist (Hopwood, 2000; Mahdy, Fatohy, Mounir, & El-Deghedi, 1998; Pohls et al., 2004). Having discussed subsequent cancer risks with a health-care provider, having received a print media intervention detailing personalized risk and recommended follow-up for a secondary breast neoplasm, and having been followed more recently at an oncology clinic predicted more recent mammography screening. These findings are similar to the trend seen in the general population, in which specific health-care provider recommendation is associated with a higher rate of screening for cervical (Coughlin, Breslau, Thompson, & Bernard, 2005), breast (Garbers & Chiasson, 2006; Mayer et al., 2007), prostate (Mayer et al., 2007), colorectal (Katz et al., 2004; Ling, Klein, & Dang, 2006; Matthews, Nattinger, Venkatesan, & Shaker, 2007), and skin cancers (Manne & Lessin, 2006). The extent to which more recent oncology visits predicted more recent mammography screening may reflect an increase in sequelae of treatment, increase in confidence in the knowledge of the specialty provider, familiarity with the facility and its staff in case the treatment was more recent, or more targeted delivery of care than that available in a non-specialty facility.
Consistent with results found in a population of survivors of Hodgkin disease, a more positive perception of health-care provider interaction supported a more positive affect (Bober et al., 2007) and decreased health concerns; the model identified a more positive affect as a predictor of more recent mammography screening. Women in particular tend to view health-care provider interaction as supportive (Hall, Irish, Roter, Ehrlich, & Miller, 1994; Hall & Roter, 1995), rely on provider input for their health care decisions, and value their relationship with the health-care provider (Hall et al., 1994; Hall & Roter, 1995; Oeffinger et al., 2004; Shaw et al., 2006; Xu & Borders, 2003).
Motivation and stage or level of readiness for medical follow-up were key factors in the model. Extrinsic motivation and a higher stage of readiness for follow-up predicted a higher intensity of health concerns. Extrinsically motivated individuals are more worried and fearful about their health and perceive that they have less control over health matters (Cox, 2003; Cox et al., 2005; Deci & Ryan, 2002); similarly, individuals who are in the precontemplation or contemplation stage of readiness for health behavior action perceive themselves as less efficacious in exerting control over health matters than those in the action stage (Emmons et al., 2003; Hogenmiller et al., 2007; Tung, Nguyen, & Tran, 2008) and they are more likely to rely on health professionals for direction.
Survivors ≤40 years, not exposed to chest radiation during cancer therapy, less concerned about their health, and more positive in affect were more intrinsically motivated; greater intrinsic motivation, however, resulted in less frequent mammograms. Intrinsically motivated individuals are more self-reliant, self-directed, and generally more autonomous in their behavior choices (Deci & Ryan, 2002) than are extrinsically motivated individuals. When more intrinsically motivated individuals do not have accurate information about risk and risk modifications, they are likely to be at greater risk for lack of medical follow-up than are those who are more extrinsically motivated who rely more on provider input to direct their health care decisions.
STUDY LIMITATIONS
The study sample reflects a subset of the overall CCSS population – those who responded to the Health Care Needs and CCSS Follow-up 2 Surveys; therefore, survivors included in the current analysis may not be fully representative of the population from which they were derived. The information utilized to classify the mammography screening outcome, as well as the independent measures, was based upon self-reported data. Lastly, while the CCSS population represents a large and heterogeneous cohort of five year survivors, results may not be generalizable to all childhood cancer survivors. As a group, CCSS participants may be more informed regarding risks and health promotion because of newsletters received as part of participation in the study.
CLINICAL IMPLICATIONS FOR NURSING
Regardless of the time since a survivor's diagnosis and treatment (Hudson et al., 2003; Langeveld, Ubbink, & Smets, 2000; Meeske et al., 2005), nurses and advanced practice nurses are encouraged to specifically inquire about any treatment-related symptoms, particularly pain, fatigue, and anxiety. These symptoms may share common biological mechanisms (Cleeland et al., 2003; Lee et al., 2004; Miaskowski & Aouizerat, 2007) and, until addressed, may be a significant deterrent to recommended screening. Nurses/advanced practice nurses should elicit survivors' concerns and address any misconceptions that may contribute to survivors' lack of understanding about the significance of their risks for a secondary breast neoplasm. Personalized information on survivors' specific risks and recommended follow-up verbally and in print will emphasize the seriousness of the potential for this late effect and reinforce the need to adhere to the recommended mammography schedule. A focused responsive interaction between the nurse and survivor can explore survivors' fears, concerns, readiness for follow-up, and misconceptions and is important to reduce survivors' anxiety about screening, support their motivation to follow the recommended screening schedule, and contribute to a more positive affect.
CONCLUSIONS
Several factors can influence female childhood cancer survivors' adherence to mammography screening recommendations, including already established sequelae (e.g., pain, fatigue), the survivor–provider relationship, important intrapersonal factors (affect, motivation, health concerns, level of readiness to seek appropriate follow-up for their cancer), and in-print information that details specific risks for secondary breast neoplasm as well as the recommended follow-up for this risk. Tailored interventions (verbal and print format) (Cox et al., 2006; Cox et al., 2008a; Cox et al., 2008d; Wu & West, 2007) that consider patients' age, motivation, readiness for follow-up, risk perceptions, and affective response to their illness and treatment should be offered to patients and their families nearing treatment completion and in post-therapy follow-up. Supporting patients with written summaries of their treatment, late effects risks, and specific recommendations for follow-up as they transition to survivorship and non-specialty primary care providers may be useful in promoting continued awareness of the seriousness of the potential for this late effect of treatment and the importance of regular mammography.
Acknowledgements
The authors acknowledge the contributions of Sharon Naron (for editorial assistance) and Kelly Shempert (for illustrations).
This work was supported by grants RO3 NR009203 and U24 CA55727 from the U.S. Public Health Service, a grant from the Robert Wood Johnson Foundation, and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
KEY POINTS
Although female childhood cancer survivors are at increased risk of breast cancer as a result of treatment exposures, they do not adhere to the recommended schedule for mammography screening.
Intrapersonal (motivation, affect, concerns), contextual (symptoms, treatment exposures), and provider (survivor–provider relationship and discussions) factors are strong influences for adherence to mammography screening.
Nursing interventions to support mammography screening in survivors should: (1) consider the impact of motivation, affect, and level of readiness for follow-up on health behavior; (2) provide written summaries of treatment exposures and the recommended interval for mammography screening at treatment completion and throughout follow-up; (3) identify specific fears, anxieties, and misconceptions that may prevent adherence to mammography screening.
References
- American Cancer Society How is breast cancer found? 2007 Retrieved July 24, 2008, from http://www.cancer.org/docroot/CRI/content/CRI_2_2_3X_How_is_breast_cancer_found _5.asp?sitearea=
- Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. Journal of Clinical Oncology. 2005;23(34):8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletins. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. New England Journal of Medicine. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. Journal of Clinical Oncology. 2003;21(23):4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- Bishop DT. BRCA1 and BRCA2 and breast cancer incidence: a review. Annals of Oncology. 1999;10(Suppl 6):113–119. [PubMed] [Google Scholar]
- Bloom JR, Stewart SL, Hancock SL. Breast cancer screening in women surviving Hodgkin disease. American Journal of Clinical Oncology. 2006;29(3):258–266. doi: 10.1097/01.coc.0000209447.63640.5a. [DOI] [PubMed] [Google Scholar]
- Bober SL, Park ER, Schmookler T, Medeiros Nancarrow C, Diller L. Perceptions of breast cancer risk and cancer screening: a qualitative study of young, female Hodgkin's disease survivors. Journal of Cancer Education. 2007;22(1):42–46. doi: 10.1007/BF03174374. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Overall fit in covariance structure models: two types of sample size effects. Psychological Bulletins. 1990;107:256–259. [Google Scholar]
- Breen N, Yabroff KR, Meissner HI. What proportion of breast cancers are detected by mammography in the United States? Cancer Detection and Prevention. 2007;31(3):220–224. doi: 10.1016/j.cdp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Breslow L, Lloyd D, Shumaker SA. Disease Prevention Research at NIH: An agenda for all. Workshop B: Health behaviors-predictors, mediators, and endpoints. Preventive Medicine. 1994;23:552–553. doi: 10.1006/pmed.1994.1079. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JA, editors. Testing structural equation models. Sage; Newbury Park, CA: 1993. [Google Scholar]
- Children's Oncology Group The Children's Oncology Group Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. 2006 Retrieved October 22, 2007, from http://www.survivorshipguidelines.org.
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Breslau ES, Thompson T, Benard VB. Physician recommendation for papanicolaou testing among U.S. women, 2000. Cancer Epidemiology, Biomarkers, and Prevention. 2005;14(5):1143–1148. doi: 10.1158/1055-9965.EPI-04-0559. [DOI] [PubMed] [Google Scholar]
- Cox CL. An interaction model of client health behavior: theoretical prescription for nursing. ANS Advances in Nursing Science. 1982;5(1):41–56. doi: 10.1097/00012272-198210000-00007. [DOI] [PubMed] [Google Scholar]
- Cox CL. The individual as client. In: Sullivan J, editor. Directions for community health nursing. Blackwell Scientific; Boston: 1984. pp. 129–172. [Google Scholar]
- Cox CL. Online exclusive: a model of health behavior to guide studies of childhood cancer survivors. Oncology Nursing Forum. 2003;30(5):E92–99. doi: 10.1188/03.ONF.E92-E99. [DOI] [PubMed] [Google Scholar]
- Cox CL, Hudson MM, Mertens A, Oeffinger K, Whitton J, Montgomery M, et al. Determinants of medical screening participation in adult survivors of childhood cancer at highest risk of treatment-related late effects. Archives of Internal Medicine, In Review. 2008a [Google Scholar]
- Cox CL, McLaughlin RA, Rai SN, Steen BD, Hudson MM. Adolescent survivors: a secondary analysis of a clinical trial targeting behavior change. Pediatric Blood and Cancer. 2005;45(2):144–154. doi: 10.1002/pbc.20389. [DOI] [PubMed] [Google Scholar]
- Cox CL, McLaughlin RA, Steen BD, Hudson MM. Predicting and modifying substance use in childhood cancer survivors: application of a conceptual model. Oncology Nursing Forum. 2006;33(1):51–60. doi: 10.1188/06.ONF.51-60. [DOI] [PubMed] [Google Scholar]
- Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: Targets for intervention. Cancer, In Review. 2008b doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Montgomery M, Rai SN, McLaughlin R, Steen BD, Hudson MM. Supporting breast self-examination in female childhood cancer survivors: a secondary analysis of a behavioral intervention. Oncology Nursing Forum. 2008c;35(3):423–430. doi: 10.1188/08.ONF.423-430. [DOI] [PubMed] [Google Scholar]
- Cox CL, Rai SN, Rosenthal D, Phipps S, Hudson MM. Subclinical late cardiac toxicity in childhood cancer survivors: impact on self-reported health. Cancer. 2008d;112(8):1835–1844. doi: 10.1002/cncr.23378. [DOI] [PubMed] [Google Scholar]
- Cui Y, Peterson NB, Hargreaves M, Wen W, Patel K, Drake J, et al. Mammography use in the Southern Community Cohort Study (United States) Journal of Health Care for the Poor and Underserved. 2007;18(4 Suppl):102–117. doi: 10.1353/hpu.2007.0115. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Whetstone L, Shende A, Weismiller D. Predictors of screening mammography: implications for office practice. Archives of Family Medicine. 2000;9(9):870–875. doi: 10.1001/archfami.9.9.870. [DOI] [PubMed] [Google Scholar]
- Cutuli B, Borel C, Dhermain F, Magrini SM, Wasserman TH, Bogart JA, et al. Breast cancer occurred after treatment for Hodgkin's disease: analysis of 133 cases. Radiotherapy and Oncology. 2001;59(3):247–255. doi: 10.1016/s0167-8140(01)00337-1. [DOI] [PubMed] [Google Scholar]
- Deci E, Ryan R. Intrinsic motivation and self-determination in human behavior. Plenum Press; New York: 1985. [Google Scholar]
- Dershaw DD, Yahalom J, Petrek JA. Breast carcinoma in women previously treated for Hodgkin disease: mammographic evaluation. Radiology. 1992;184(2):421–423. doi: 10.1148/radiology.184.2.1320281. [DOI] [PubMed] [Google Scholar]
- Diller L, Medeiros Nancarrow C, Shaffer K, Matulonis U, Mauch P, Neuberg D, et al. Breast cancer screening in women previously treated for Hodgkin's disease: a prospective cohort study. Journal of Clinical Oncology. 2002;20(8):2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Field TS, Ulcickas Yood M, Rolnick SJ, Quessenberry CP, Fouayzi H, et al. Patterns and predictors of mammography utilization among breast cancer survivors. Cancer. 2006;106(11):2482–2488. doi: 10.1002/cncr.21893. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Butterfield RM, Puleo E, Park ER, Mertens A, Gritz ER, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health Study. Journal of Clinical Oncology. 2003;21(2):189–196. doi: 10.1200/JCO.2003.06.130. [DOI] [PubMed] [Google Scholar]
- Field TS, Doubeni C, Fox MP, Buist DS, Wei F, Geiger AM, et al. Under utilization of surveillance mammography among older breast cancer survivors. Journal of General Internal Medicine. 2008;23(2):158–163. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. American Journal of Human Genetics. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers S, Chiasson MA. Breast cancer screening and health behaviors among African American and Caribbean Women in New York City. Journal of Health Care for the Poor and Underserved. 2006;17(1):37–46. doi: 10.1353/hpu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Goodwin SS, Visintainer PF, Facelle J, Falvo CE. Breast cancer screening in Rockland County, New York: a survey of attitudes and behaviors. Ethnicity and Disease. 2006;16(2):428–434. [PubMed] [Google Scholar]
- Hall JA, Irish JT, Roter DL, Ehrlich CM, Miller LH. Satisfaction, gender, and communication in medical visits. Medical Care. 1994;32(12):1216–1231. doi: 10.1097/00005650-199412000-00005. [DOI] [PubMed] [Google Scholar]
- Hall JA, Roter DL. Patient gender and communication with physicians: results of a community-based study. Womens Health. 1995;1(1):77–95. [PubMed] [Google Scholar]
- Hewitt M, Weiner S, Simone J. Childhood Survivorship: Improving Care and Quality of Life. The National Academy of Sciences; 2003. [PubMed] [Google Scholar]
- Hogenmiller JR, Atwood JR, Lindsey AM, Johnson DR, Hertzog M, Scott JC., Jr. Self-efficacy scale for Pap smear screening participation in sheltered women. Nursing Research. 2007;56(6):369–377. doi: 10.1097/01.NNR.0000299848.21935.8d. [DOI] [PubMed] [Google Scholar]
- Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Research. 2000;2(6):387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Journal of the American Medical Association. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- Kaplan D. Structural Equation Modeling: Foundations and Extensions. Sage Publications; Newbury Park, CA: 2000. [Google Scholar]
- Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, et al. Colorectal cancer screening among African American church members: a qualitative and quantitative study of patient-provider communication. BMC Public Health. 2004;4:62. doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney LB, Yasui Y, Inskip PD, Hammond S, Neglia JP, Mertens AC, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Annals of Internal Medicine. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling (Methodology in the Social Sciences) 2nd ed. The Guilford Press; New York, NY: 2005. [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Langeveld NE, Ubbink M, Smets E. ‘I don't have any energy’: The experience of fatigue in young adult survivors of childhood cancer. European Journal of Oncology Nursing. 2000;4:20–28. doi: 10.1054/ejon.1999.0063. [DOI] [PubMed] [Google Scholar]
- Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: results from HINTS. Journal of Health Communication. 2006;11(Suppl 1):181–190. doi: 10.1080/10810730600639190. [DOI] [PubMed] [Google Scholar]
- Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplantion. 2000;25(11):1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- Mahdy NH, Fatohy IM, Mounir GM, El-Deghedi BM. Assessment of students' knowledge, attitude and practice concerning cancer and its prevention. Part I. Journal of the Egyptian Public Health Association. 1998;73(34):399–431. [PubMed] [Google Scholar]
- Manne S, Lessin S. Prevalence and correlates of sun protection and skin self-examination practices among cutaneous malignant melanoma survivors. Journal of Behavioral Medicine. 2006;29(5):419–434. doi: 10.1007/s10865-006-9064-5. [DOI] [PubMed] [Google Scholar]
- Matthews BA, Nattinger AB, Venkatesan T, Shaker R. Colorectal cancer screening among midwestern community-based residents: indicators of success. Journal of Community Health. 2007;32(2):103–120. doi: 10.1007/s10900-006-9038-0. [DOI] [PubMed] [Google Scholar]
- Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, et al. Screening practices in cancer survivors. Journal of Cancer Survivorship. 2007;1(1):17–26. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. Journal of Clinical Oncology. 2005;23(24):5501–5510. doi: 10.1200/JCO.2005.03.210. [DOI] [PubMed] [Google Scholar]
- Metayer C, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. Journal of Clinical Oncology. 2000;18(12):2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Seminars in Oncology Nursing. 2007;23(2):99–105. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Ness KK, Neglia JP, Whitton JA, Green DM, Zeltzer LK, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS) Sleep. 2008;31(2):271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen K, Muthen BO. Mplus User's Guide. 4th ed. Muthen & Muthen; Los Angeles, CA: 2007. [Google Scholar]
- Nathan PC, Greenberg ML, Ness KK, Mahoney MC, Gurney JG, Hudson MM, et al. 2007 ASCO Annual Meeting Proceedings Part 1. Journal of Clinical Oncology. 2007;25:6502. [Google Scholar]
- Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Annals of Family Medicine. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohls UG, Renner SP, Fasching PA, Lux MP, Kreis H, Ackermann S, et al. Awareness of breast cancer incidence and risk factors among healthy women. European Journal of Cancer Prevention. 2004;13(4):249–256. doi: 10.1097/01.cej.0000136718.03089.a5. [DOI] [PubMed] [Google Scholar]
- Prochaska JO. Health behavior change research: a consortium approach to collaborative science. Annals of Behavioral Medicine. 2005;29(Suppl):4–6. doi: 10.1207/s15324796abm2902s_2. [DOI] [PubMed] [Google Scholar]
- Raykov T, Marcoulides GA. A first course in structural equation modeling. 1st ed. Lawrence Erlbaum Associates; Mahway, NJ: 2000. [Google Scholar]
- Rejeski WJ, Brawley LR, McAuley E, Rapp S. An examination of theory and behavior change in randomized clinical trials. Controlled Clinical Trials. 2000;21(5 Suppl):164S–170S. doi: 10.1016/s0197-2456(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975-2004. 2007 Retrieved March 1, 2008, from http://seer.cancer.gov/csr/1975_2004.
- Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Medical and Pediatric Oncology. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- Shaw AK, Pogany L, Speechley KN, Maunsell E, Barrera M, Mery LS. Use of health care services by survivors of childhood and adolescent cancer in Canada. Cancer. 2006;106(8):1829–1837. doi: 10.1002/cncr.21798. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. New England Journal of Medicine. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Winter DL, Stiller CA, Murphy M, Hawkins MM. Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: a population-based study. International Journal of Cancer. 2007;120(2):384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- Tung WC, Nguyen DH, Tran DN. Applying the transtheoretical model to cervical cancer screening in Vietnamese-American women. International Nursing Review. 2008;55(1):73–80. doi: 10.1111/j.1466-7657.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force Screening for Breast Cancer: Recommendations and Rationale. 2002 2008, from http://www.ahrq.gov/clinic/3rduspstf/breastcancer/brcanrr.htm.
- Vlastos G, Verkooijen HM. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. Oncologist. 2007;12(1):1–10. doi: 10.1634/theoncologist.12-1-1. [DOI] [PubMed] [Google Scholar]
- Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Education Monographs. 1978;6(2):160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. QualityMetric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- Williams BA, Lindquist K, Sudore RL, Covinsky KE, Walter LC. Screening mammography in older women. Effect of wealth and prognosis. Archives of Internal Medicine. 2008;168(5):514–520. doi: 10.1001/archinternmed.2007.103. [DOI] [PubMed] [Google Scholar]
- Wolden SL, Hancock SL, Carlson RW, Goffinet DR, Jeffrey SS, Hoppe RT. Management of breast cancer after Hodgkin's disease. Journal of Clinical Oncology. 2000;18(4):765–772. doi: 10.1200/JCO.2000.18.4.765. [DOI] [PubMed] [Google Scholar]
- Wu TY, West BT. Mammography stage of adoption and decision balance among Asian Indian and Filipino American women. Cancer Nursing. 2007;30(5):390–398. doi: 10.1097/01.NCC.0000290812.14571.2c. [DOI] [PubMed] [Google Scholar]
- Xu KT, Borders TF. Gender, health, and physician visits among adults in the United States. American Journal of Public Health. 2003;93(7):1076–1079. doi: 10.2105/ajph.93.7.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeazel MW, Oeffinger KC, Gurney JG, Mertens AC, Hudson MM, Emmons KM, et al. The cancer screening practices of adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2004;100(3):631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]