Abstract
Vaccinia Virus (VACV) elicits a robust CD8 T cell response that plays an important role in host resistance. To date, there is little information on the molecules that are essential to generate large pools of VACV-specific effector CD8 T cells. Here, we show that the adaptor molecule MyD88 is critical for the magnitude of primary CD8 T cell responses to both dominant and subdominant VACV epitopes. MyD88−/− mice exhibit profound reduction in CD8 T cell expansion and anti-viral cytokine production. Surprisingly, the defect was not due to impaired antigen-presenting cell function, as MyD88−/− DC matured normally and were able to promote strong CD8 T cell priming following VACV infection. Rather, adoptive transfer experiments demonstrated that intrinsic MyD88-dependent pathways in CD8 T cells were critical. MyD88-deficient CD8 T cells failed to accumulate in wild-type hosts, and poor expansion of MyD88-deficient VACV-specific CD8 T cells resulted after virus infection. In contrast, no defect was evident in the absence of TRIF, TLR2, TLR4, TLR9, and IL-1R. Together, our results highlight an important role for MyD88 in initial anti-viral CD8 T cell responses and suggest that targeting this pathway may be useful in promoting and sustaining anti-VACV immunity.
Keywords: MyD88, Vaccinia Virus, Poxviruses, TLR, CD8 T cells
Introduction
Vaccinia virus (VACV) is a large DNA virus and is a member of the genus Orthopoxvirus, which includes variola, monkeypox, buffalopox, and cowpox. Variola, the etiological agent of smallpox, long considered to be the most deadly and persistent human pathogenic disease, was eradicated by 1977 through worldwide outbreak search and vaccination with live VACV (1). In humans and mice, immunization with VACV elicits a robust CD8 T cell response that plays an important role in the final clearance of primary infection and in subsequent protection from reinfection (2–5). The potency and longevity of CD8 T cell responses induced in response to VACV has led to the development of recombinant VACV as a vaccine vehicle for a number of other infectious diseases including influenza, HIV, malaria, and for cancer immunotherapy. However, despite their great efficacy in inducing strong CD8 T cell responses, we know little about the immune mechanisms behind vaccines made with VACV as a vector. Defining those that govern the efficient generation of antigen-specific CD8 T cells is of importance for the development of safer and more effective vaccines.
It is thought that anti-viral CD8 T cell responses are primarily regulated by innate signals induced by the invading virus. Profesional APC, such as dendritic cells (DC), play a central role in activation of naïve CD8 T cells. DC can sense viruses directly through various pattern recognition receptors (PPR) such as members of the Toll-like receptor (TLR) superfamily (6–9). The recognition of viruses by DC through TLRs leads primarily to their maturation, accompanied by changes in antigen presentation, costimulatory molecule expression, proinflammatory cytokine production, and migratory behavior and secondarily to enhanced adaptive immune responses (10). Myeloid differentiation primary-response gene 88 (MyD88) is an adaptor protein that is required for signal transduction by most TLRs and the IL-1R/IL-18R family (11, 12). The importance of this pathway to host response to pathogens has been demonstrated by enhanced suceptibility of MyD88-deficient (MyD88−/−) mice to a variety of eukaryotic, bacterial, and viral pathogens (13–17). In the context of viral infections, MyD88 has been shown to be important in host defence to a number of viruses including, LCMV (14, 18), CMV (19), and HSV (15, 20). Although the protective role of MyD88 has largely been attributed to its importance in cells of the innate immune system, recent studies have shown that naive and antigen primed CD8 T cells can express MyD88 and TLRs (21–23). This has added an additional layer of complexity to their potential role in anti-viral immunity and led to the notion that MyD88 dependent pathways could be directly involved in promoting virus-specific T cell responses.
VACV has evolved elaborate strategies that counteract many of the innate and adaptive immune responses of the host. Of potential significance are the VACV proteins encoded by the A46R, A52R, and N1L genes. Early studies using in vitro systems and cell lines showed that the A46R protein can block IL-1R and TLR signaling by interacting with MyD88, TRIF (Toll-IL-1R domain-containing adaptor-inducing IFN-beta), and TRAM (TRIF-related adapter molecule), and consequently interfere with downstream activation of MAPK and NF-κB (24, 25). The A52 protein has been implicated in blocking IL-1R-TLR-induced NF-κB activation and proinflammatory cytokine production by targeting TRAF6 and IRAK2 (24, 26, 27), while N1L protein suppresses signaling to NF-κB by TLR-IL-1R by associating with, and inhibiting, the IKK complex (28). Importantly, VACV deletion mutants lacking either, A46R (25), A52R (27), and N1L (29) genes are highly attenuated in vivo, providing direct evidence for an important role for TLR-IL-1R and MyD88-dependent pathways in anti-VACV immune responses. However, the precise in vivo role of MyD88 in innate and adaptive immunity to VACV is not clear.
Here, we demonstrate that T cell expression of MyD88 is necessary for the generation of large anti-VACV CD8 T cell populations. MyD88−/− mice have a severe defect in mounting normal CD8 T cell responses against VACV. This is not due to a defect in the DC compartment as MyD88−/− DC can mature and present viral antigen to CD8 T cells similar to wild-type DC. In contrast, adoptive T cell transfer studies show that CD8 T cells require intrinsic expression of MyD88 since wild-type CD8 cells can expand and differentiate into effector cells in a MyD88−/− host, while MyD88−/− CD8 T cells failed to expand in a MyD88-sufficient environment upon viral challenge. Our observations indicate a previously unappreciated requirement for MyD88 in the generation and accumulation of VACV-specific CD8 T cells.
Materials and Methods
Mice
The studies reported here conform to the animal Welfare Act and the NIH guidelines for the care and use of animals in biomedical research. All experiments were done in compliance with the regulations of the La Jolla Institute Animal care committee in accordance with the guidelines by the Association for assessment and Accreditation of laboratory Animal Care. 8–12 wk-old female C57BL/6, TLR2−/−, TLR4−/−, IL-1R−/−, RAG−/− mice were all purchased from the Jackson Laboratory (Bar Harbor, ME). OT-I TCR-transgenic mice were used as a source of Vβ5/Vα2 CD8+ T cells responsive to OVA-derived SIINFEKL peptide. TLR9CpG1−/− mice and TrifLps2/Lps2−/− mice generated on the C57BL/6 background by ENU mutagenesis were obtained from Dr. Bruce Beutler (30). MyD88-deficient mice were obtained from Dr. Sujan Shresta (constructed by Dr. S. Akira and Dr. B. Beutler).
Peptides and Tetramers
Vaccinia virus peptide epitopes used in this study were predicted and synthesized as described previously (31, 32). B8R (20–27; TSYKFESV), A3L (270-227; KSYNYMLL), A8R (189–196; ITYRFYLI), B2R (54–62; YSQVNKRYI), A23R (297–305; IGMFNLTFI). MHC/peptide tetramers for the VACV-WR epitope B8R (20–27; TSYKFESV)/H-2Kb, which were conjugated to allophycocyanin, were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Viruses
The VACV Western Reserve (VACVWR) strain was purchased from the American Type Culture Collection (Manassas, VA), grown in HeLa cells, and titered on VeroE6 cells.
Virus infections
For most experiments, mice were infected intraperitonealy (i.p.) with 2 × 105 PFU of VACV. Effector responses were analyzed between days 4 and 7 post-infection, after restimulating in vitro with VACV peptides as described before (33).
DC and CD8 T cell isolation from lymph nodes and spleen
DC were isolated essentially as described before (34, 35) with minor modifications. Briefly, popliteal lymph nodes (LNs) or spleen fragments were digested for 20 min at room temperature with collagenase/DNase (1 mg/ml collagenase D and 1 µg/ml grade II bovine pancreatic DNase I (Boehringer-Mannheim, Mannheim, Germany)) and then treated for 5 min with EDTA to disrupt T cell-DC complexes. CD11c+ cells were enriched by positive selection using the Miltenyi microbeads as per the manufacturer’s instructions ((Miltenyi Biotec; Auburn, CA) and subsequently sorted into different subsets based on CD4 and CD8 expression by FACS.
For adoptive transfer experiments, 1 × 105 or 1 × 104 naive wt OT-I CD8 T cells were purified from spleens and lymph nodes of indicated naïve donor mice with MACS technology (Miltenyi Biotec; Auburn, CA) and transferred into wt non-transgenic B6 or MyD88−/− mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA protein (VACV-OVA; 2 × 106 PFU/mouse) or PBS as indicated. OT-I expansion and effector formation were detected by FACS staining of transgenic TCR α and β chains after gating on CD8 T cells and in some cases after restimulating in vitro with OVA (SINFEKL) peptide.
CFSE-labeling of transgenic CD8 T cells
LN (inguinal, brachial, axillary, superficial cervical, and mesenteric) were obtained from CD8+ OTI TCR transgenic mice and purified using positive selection with MACS beads (Miltenyi Biotec). Enriched cells contained 90–95% specific CD8+ TCR transgenic T cells. These were labeled with CFSE (Molecular Probes, Eugene, OR) by incubating 107 purified cells per milliliter with 5 µM CFSE for 10 min at 37°C. Cells were then washed three times in HBSS containing 2.5% FCS.
Analysis of in vitro activation of naive T cells by DC
A total of 5 × 104 CD8+-purified CFSE-labeled TCR transgenic cells were added to 1.25 × 104 fluorescence activated cell sorted DC in 200 µl RPMI 1640 containing 10% FCS, 50 µM 2-ME, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (complete medium) in 96-well V-bottom plates (Costar, Corning, Corning, NY). Each culture was performed in duplicate. Cultures were analyzed for proliferation after 60 h. Cells were stained with anti-CD8α-Percp (53-6.7; BD PharMingen, San Diego, CA), anti-Vα2-PE (B20.1; BD PharMingen), and anti-Vβ5-APC cells from the entire well were analyzed for proliferation by flow cytometry.
Flow cytometry
Cytokine production in T cells was performed as previously described (36), with some modifications. Briefly, after lysing red blood cells (RBC), splenocytes from infected mice were resuspended in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 mg/ml streptomycin, 100 U/ml penicillin and 50 mM 2-mercaptoethanol (Sigma). 1–2 × 106 cells were plated in round-bottomed 96-well microtiter plates in 200 µl with medium or the indicated VACV peptides at 1 µg/ml for 1 hr at 37°C. GolgiPlug (BD Biosciences) was then added to the cultures according to the manufacture’s instructions and the incubation continued for 7 hrs. Cells were stained with anti-CD8 (PerCP; 53–6.7) and CD62L (PE; MEL-14), followed by fixation with cytofix-cytosperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular cytokine staining in BD Perm/Wash buffer for 30 min at 4°C. Anti-TNF (FITC; MP6-XT22) and IFN-γ (APC; XMG1.2) were obtained from e-Biosience and used at a 1:100 dilution. Samples were analyzed for their proportion of cytoplasmic cytokines after gating on CD8+CD62Llow T cells by FACSCaliburTM flow cytometer using CellQuest (BD Biosciences) and FlowJo software (Tree Star, san Carlos, CA).
Statistics
Statistical significance was analyzed by Student’s t test. Unless otherwise indicated, data represent the mean ± SEM, with p < 0.05 considered statistically significant.
Results
MyD88 is required for optimal CD8 T cell response against VACV-WR
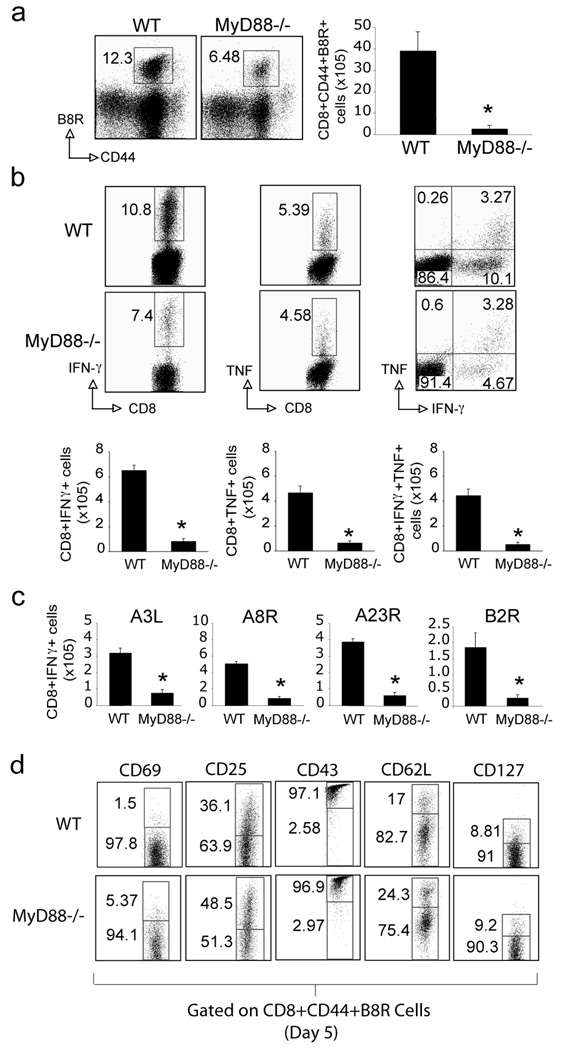
Over 7 days of primary i.p. infection with VACV-WR, we quantified the number of virus-specific CD8 T cells in spleens by using a tetramer of B8R, the immunodominant class I epitope of VACV. MyD88−/− mice generated greatly reduced B8R-reactive CD8 T cell responses compared with WT mice, based on analyses of percentages and absolute numbers of tetramer positive cells (Fig. 1a). Reduced percentages of CD8 T cells capable of producing IFN-γ in response to B8R were also observed, although TNF production was not strongly affected. However, because of the reduced numbers of CD8 T cells generated, this translated into significant deficiencies in the accumulation of both IFN-γ and TNF producing effector T cells (Fig. 1b). MyD88−/− mice also showed a similar defect in generating CD8 T cell populations responsive to a range of subdominant VACV epitopes, which included A3L, A8R, A23R and B2R (Fig. 1c). To assess whether the differentiation state of VACV-specific CD8 T cells was impacted by MyD88 the expression of CD25, CD43, CD62L, and CD127 were analyzed on CD8+B8R-tetramer+ cells in the spleen five days postinfection with VACV-WR. Virus-specific CD8 T cells from MyD88−/− mice expressed slightly higher levels of CD25, but other activation indicators including CD69, CD43, CD62L and CD127 were comparable with wt cells suggesting early activation of T cells upon virus stimulation is normal in MyD88−/− mice (Fig. 1d).
Figure 1. MyD88−/− mice mount reduced CD8 T cell responses against VACV-WR.
(a–c) WT or MyD88−/− mice were infected i.p. with VACV-WR (2×105 PFU/mouse) and analyzed after 7 days. (a) Splenocytes were stained with anti-CD8, -CD44, and B8R-Tetramer. Left: Representative dot plots of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers in each dot plot indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Splenocytes were stimulated with B8R peptide in vitro for intracellular IFN-γ and TNF staining. Top: Representative plots of IFN-γ (left), TNF (middle), and IFN-γ/TNF (right), gating on CD8+CD62L- cells. Percentages that stained for each cytokine are indicated. Bottom: Total numbers of CD8+IFN-γ+ cells (left), or CD8+TNF+ (middle), and CD8+IFN-γ+TNF+ cells (right) per spleen. (c) Splenocytes were stimulated with A3L, A8R, A23R or B2R peptide for intracellular IFN-γ staining. (d) WT or MyD88−/− mice were infected i.p. with VACV-WR and analyzed after 5 days. Splenocytes were stained with anti-CD8, -CD44, B8R-Tetramer and -CD69, -CD25, -CD43, -CD62L, or -CD127 as indicated. Representative plots of gated CD8+CD44+B8R-tetramer positive cells are shown. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout). Similar results were obtained in 3 separate experiments.
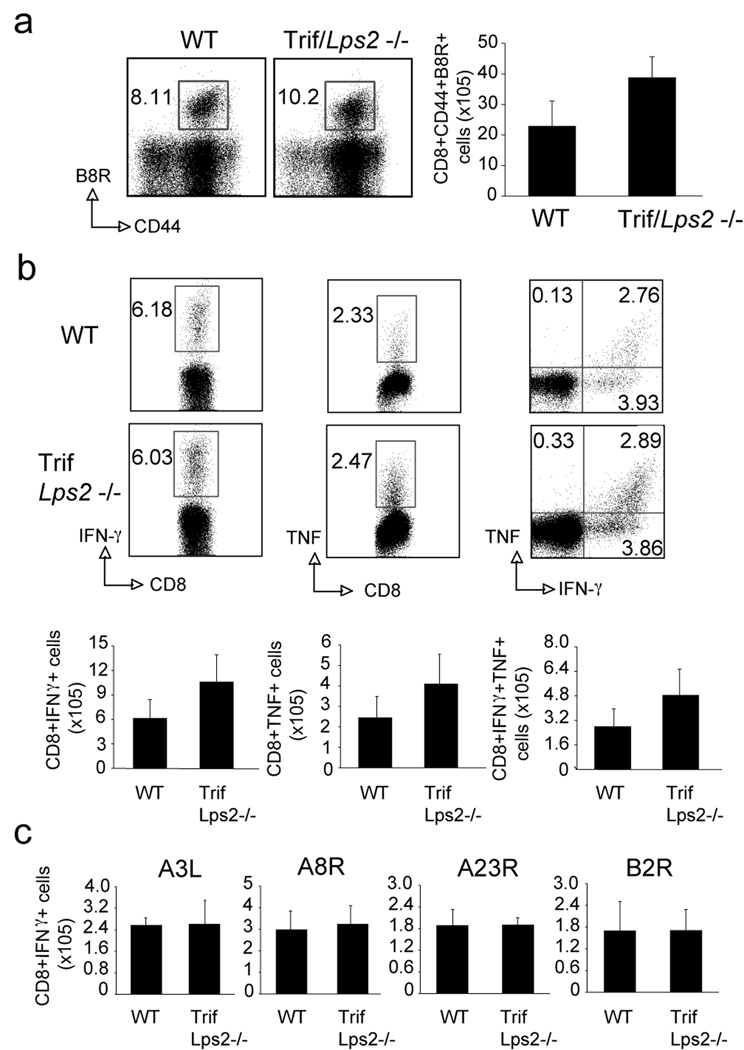
Interestingly, the defect found in MyD88−/− mice was not found in TrifLps2−/− mice. Normal B8R-reactive CD8 T cell responses were generated in TrifLps2−/− mice (Fig. 2a). Production of IFN-γ and TNF by CD8 T cells in response to a range of dominant and subdominant VACV epitopes was also not dependent on Trif expression (Fig. 2b and 2c) indicating some specificity in the importance of TLR-IL-1R type adaptors.
Figure 2. TrifLps2−/− mice mount normal CD8 T cell responses against VACV-WR.
(a–c) WT or TrifLps2−/− mice were infected i.p. with VACV-WR (2×105 PFU/mouse) and analyzed after 7 days. (a) Splenocytes were stained with anti-CD8, -CD44, and B8R-Tetramer. Left: Representative dot plots of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers in each dot plot indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Splenocytes were stimulated with B8R peptide in vitro for intracellular IFN-γ and TNF staining. Top: Representative plots of IFN-γ (left), TNF (middle), and IFN-γ/TNF (right), gating on CD8+CD62L- cells. Percentages that stained for each cytokine are indicated. Bottom: Total numbers of CD8+IFN-γ+ cells (left), or CD8+TNF+ (middle), and CD8+IFN-γ+TNF+ cells (right) per spleen. (c) Splenocytes were stimulated with A3L, A8R, A23R or B2R peptide for intracellular IFN-γ staining. Results are mean number ± SEM (n=4 mice/group) from one experiment. Similar results were obtained in 2 separate experiments.
VACV induces MyD88 independent DC maturation
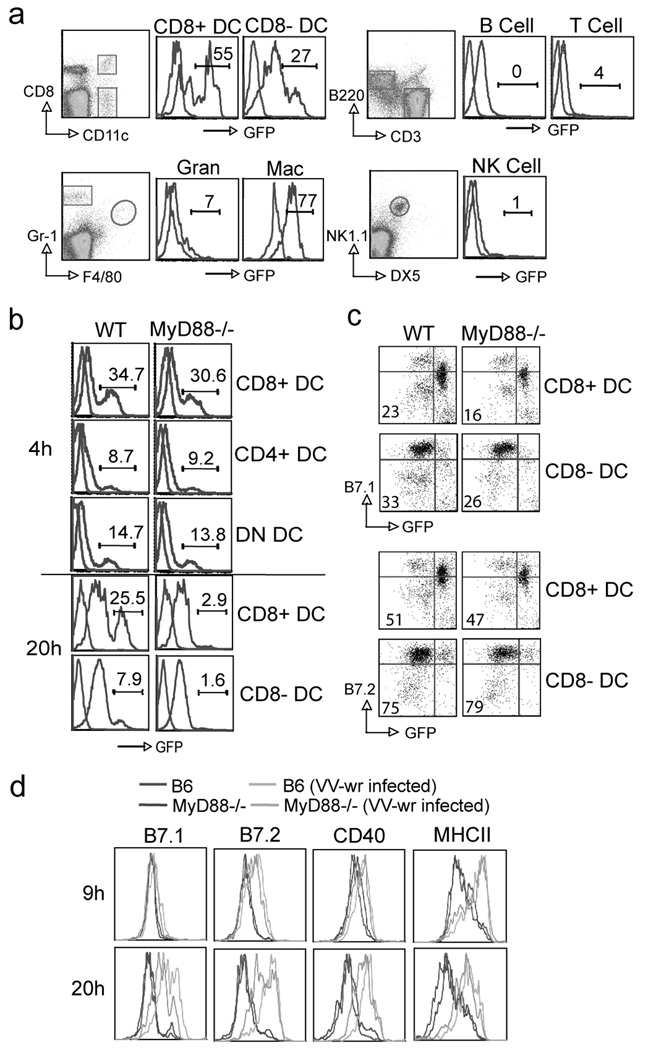
To test whether the impaired priming of CD8 T cells might have been related to a defective capacity of antigen-presenting cells to mature during the initial stages of infection, a recombinant virus expressing GFP (VACV-GFP) was used to infect different cell populations in vitro. VACV infected both CD8+ and CD8− DC and macrophages, with low/neglible amounts found in T cells, B cells, granulocytes and NK cells (Fig. 3a). We focused on DC as they have previously been shown to be responsible for priming naive CD8 T cells to VACV (35). Four hours after in vitro infection, there was no difference observed in the capacity of any subset of MyD88−/− DC to be infected by VACV-GFP (Fig. 3b). Although some death of MyD88−/− DC occurred after 20 hr of viral infection (Fig. 3b), infected DC upregulated comparable levels of B7.2 (Fig. 3c) as well as displaying normal levels of CD40 and class II MHC (not shown). B7.1 expression was slightly lower in infected MyD88−/− DC compared with wt controls (Fig. 3c). Although we could not use the recombinant VACV-GFP in vivo to specifically focus on virus-infected cells, due to rapid loss of expression of GFP, a similar conclusion was evident from analyzing total CD11c+ DC from VACV-WR infected mice. B7.1, B7.2, CD40, and class II were equivalently expressed in the absence of MyD88 over 20 hr, although some evidence of delayed kinetics was apparent (Fig 3d and not shown). These data strongly suggested that MyD88−/− DC can be infected and mature comparable to WT DC after VACV infection.
Figure 3. VACV induces MyD88 independent DC maturation.
(a) Splenocytes from WT mice were infected with recombinant VACV-GFP (MOI=10) in vitro. Nine hours later, cells were stained with anti-CD8/CD11c, B220/CD3, Gr-1/F4/80, or NK1.1/DX5 to identify populations of CD8+ DC, CD8- DC, B cells, T cells, granulocytes, macrophages and NK cells. Representative histograms: GFP expression after infection (blue line), non-infected cells as staining control (red line). Percentages of GFP expression are indicated. (b-c) Purified DC populations or splenocytes from WT or MyD88−/− mice were cultured with VACV-GFP in vitro. (b) Four or 20 hours later, DC were stained with anti-CD11c, CD4 and CD8. GFP expression in different purified DC populations was examined. Blue line: Infected cells. Red line: Non infected cell control. Percentages of GFP expression are indicated. (c) 18 hours later, splenocytes were stained with anti-CD11c, CD8, B7.1 or B7.2. Representative plots of gated CD11c+CD8+ or CD11c+CD8- DC staining for B7.1 (top) and B7.2 (bottom). The numbers indicate the percentages of GFP+B7.1+, or GFP+B7.2+ cells. (d) WT or MyD88−/− mice were infected with VACV-WR. Nine or 20 h later, splenocytes were harvested and stained with anti-CD11c, CD8, B7.1 or B7.2 or CD40 or MHCII. Representative plots of gated CD11c+CD8+ cells staining for indicated costimulatory molecules. Results are mean number ± SEM (n=4 mice/group) from one experiment. Similar results were obtained in 3 separate experiments.
WT CD8 T cells can expand and develop effector function in response to VACA-OVA in MyD88−/− mice
To further examine where MyD88 was functional, we transferred high or low numbers of OT-I CD8 T cells into MyD88−/− or WT mice, and one day later infected with VACV-OVA. Significantly, strong expansion of OVA-specific OT-I CD8 T cells was observed by tracking the transgenic TCR, and many OVA-reactive IFN-γ-producing CD8 T cells were generated, regardless of whether they were transferred into MyD88−/− mice (Fig. 4a and 4b). Notably, similar results were found when low number of OT-I cells (1 × 104) were transferred (Fig. 4a and 4b) even though it was reported that a high number of antigen-specific CD8 cells might influence DC maturation. These data suggested that the absence of MyD88 in the host environment does not limit the expansion and differentiation of naïve CD8 T cells driven by VACV infection and that any impaired activity was not due to defective antigen-presentation. In line with this, WT OT-I CD8 T cells co-cultured with MyD88−/− DC, purified from a VACV-OVA infected host, proliferated to OVA at a rate comparable to those cultured with WT DC in vitro (Fig. 4c).
Figure 4. WT CD8 T cells can expand in response to VACV infection in MyD88−/− mice.
(a) 1 × 105 or 1 × 104 WT OT-I CD8 T cells were adoptively transferred into WT or MyD88−/− mice. One day later, mice were infected i.p. with VACV-OVA or PBS. After 8 days, OT-I CD8 T cells were analyzed. Top: experimental scheme. Middle: Representative dot plots of co-staining for Vα2 and Vβ5 after gating on CD8+ cells. Bottom: Contour plots of IFN-γ staining after ex vivo OVA peptide restimulation, gating on CD8+Vα2+Vβ5+ cells. Numbers indicate the percentage of CD8+Vα2+Vβ5+IFN-γ positive cells. (b) Total numbers of CD8+ Vα2+Vβ5+INF-γ+ cells per spleen after transfer of 1 × 105 (Left) or 1 × 104 (right) WT OT-I cells into recipient. (c) WT or MyD88−/− mice were infected i.v. with VACV-OVA. Twenty four hours later, splenocytes were harvested and sorted for CD11c+CD8+ and CD11c+CD8- DC, and then cultured with naïve CFSE labeled OT-I CD8 T cells. Two and half days later, division of OT-I cells was examined. Left: experimental scheme. Middle: Representative dot plots of CFSE dilution after gating on CD8+Vα2+Vβ5+ cells. Percentages of proliferating OT-I cells are indicated. Right: Representative histogram of CFSE dilution gating on CD8+Vα2+Vβ5+ cells. Results are mean number ± SEM (n=4 mice/group) from one experiment. Similar results were obtained in 3 separate experiments.
MyD88−/− CD8 T cells have an intrinsic defect in survival and expansion in response to VACV
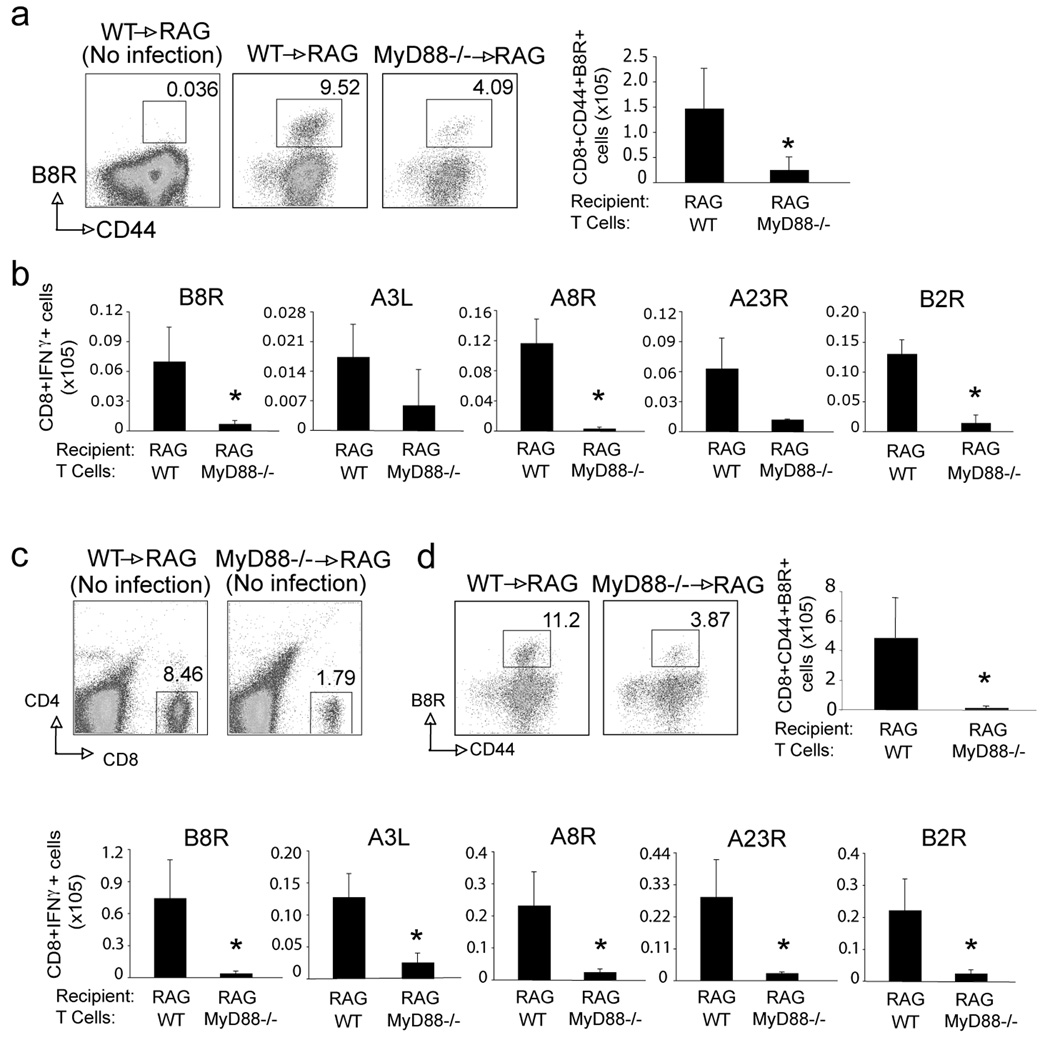
To test if the impaired CD8 T cell response in MyD88−/− mice reflected an intrinsic role for MyD88, we transferred WT or MyD88−/− CD8 T cells into WT RAG−/− mice and infected the recipient mice with VACV-WR. RAG−/− mice were used as recipients to prevent any endogenous T cell response. A large number of T cells were transferred as prior studies have shown that this limits any homeostatic proliferation, allowing us to effectively examine the VACV antigen-induced response. Seven days post infection, there were greatly reduced numbers of B8R-tetramer reactive CD8 T cells generated from the MyD88−/− donor cells (Fig. 5a). The overall number of IFN-γ-producing VACV-specific effector CD8 T cells generated from MyD88−/− donor populations was also strongly reduced, as shown by ex vivo restimulation with dominant and subdominant peptides (Fig. 5b). This data shows that MyD88 specifically regulates CD8 T cell expansion after encounter with viral peptides. However, it was also possible that MyD88 was required for survival/maintenance of CD8 T cells in the absence of antigen. To address this question, we transferred MyD88−/− CD8 cells into RAG−/− mice without virus infection. One week post transfer, there were approximately 4-fold fewer donor MyD88−/− CD8 T cells that survived compared to WT T cells (Fig. 5c), showing an intrinsic survival defect in the absence of MyD88. However, when these mice were subsequently infected and generation of VACV-reactive CD8 T cells was examined, a similar strong reduction in all VACV-specific CD8 T cell populations was observed (Fig. 5d). These data show that MyD88 is directly required within CD8 T cells for survival as well as expansion to VACV-derived antigen.
Figure 5. MyD88−/− CD8 T cells have an intrinsic defect in survival and expansion in response to VACV.
(a-b) Ten million WT or MyD88−/− CD8 T cells were adoptively transferred into RAG−/− mice. One day later, mice were infected i.p. with VACV-WR (2×105 PFU/mouse). After 8 days, splenocytes were harvested and stained with anti-CD8, -CD44, and B8R-Tetramer, or stimulated with the indicated peptides for intracellular IFN-γ staining. (a) Left: Representative dot plots of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Total number of IFN-γ positive cells gating on CD8+CD62L- cells per spleen after stimulation with the indicated VACV peptides. (c-d) Ten million WT or MyD88−/− CD8 T cells were adoptively transferred into RAG−/− mice without infection. (c) Seven days later, blood was collected and analyzed for CD4 and CD8 cells. Representative plots of gated T cells staining for CD4 and CD8. (d) Seven days later recipient mice were infected i.p. with VACV-WR (2×105 PFU/mouse). A further 7 days after infection, recipient splenocytes were stained with B8R-Tetramer, or stimulated with the indicated peptides for intracellular staining for IFN-γ. Results are mean number ± SEM (n=4 mice/group) from one experiment. *,p < 0.05 (wt mice vs knockout). Similar results were obtained in 2 separate experiments.
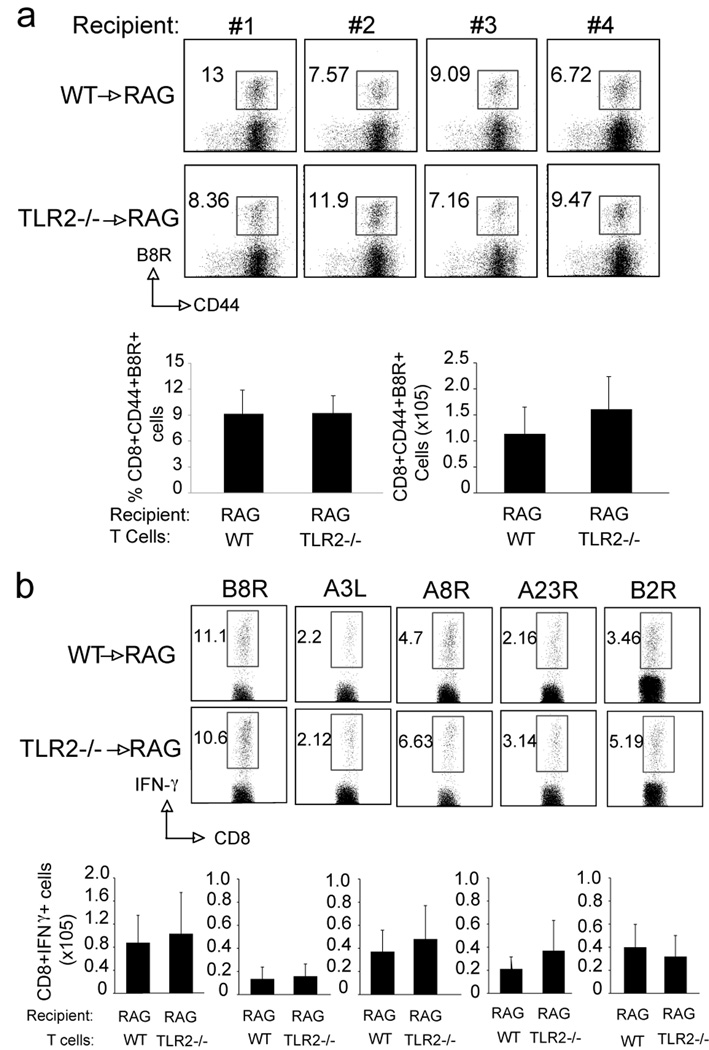
The T cell requirement for MyD88 is not explained by the use of TLR2
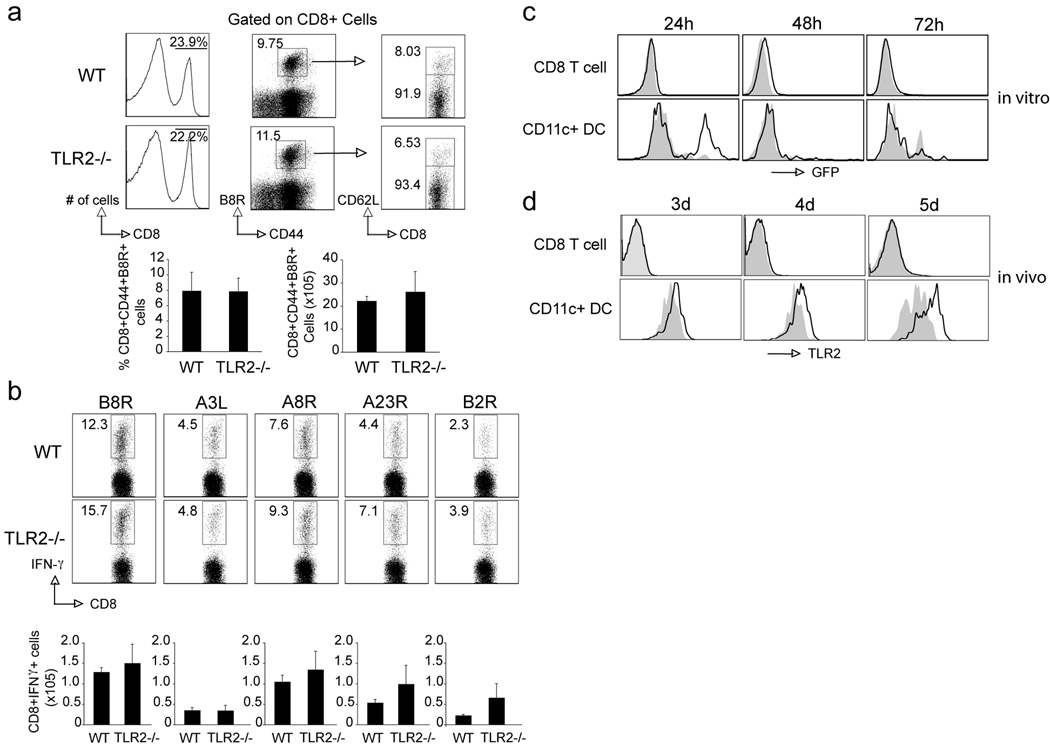
It has been reported that TLR2 agonists can directly enhance survival and proliferation of activated murine T cells in vitro and in vivo (21, 22, 37). We therefore first investigated whether a direct effect of this pathway on CD8 T cells could account for the MyD88 requirement. CD8 T cells from TLR2−/− mice were transferred into RAG−/− mice, followed by infection with VACV. Seven days post-infection, CD8 T cells from TLR2−/− mice mounted a normal response to VACV compared with wt cells. These cells expanded normally upon viral stimulation and also produced comparable levels of virus-specific effector cytokines (Fig. 6a and 6b). Consistent with this, VACV infection induced robust CD8 T cell responses to both dominant and subdominant VACV epitopes in TLR2−/− mice that was comparable to that seen in wt mice (Fig. 7a and 7b). To further confirm the redundant role of TLR2 on T cells during VACV infection, we infected splenocytes with VACV-GFP in vitro and found that, unlike DC, CD8 T cells could not be infected in vitro (Fig. 7c). Moreover, cell surface expression of TLR2 was significantly upregulated on DC following VACV infection in vivo, but TLR2 was undetectable on CD8 T cells (Fig. 7d). Consistent with previous reports, the TLR2 agonist Pam3Cys directly promoted CD8 T cell proliferation in vitro when combined with anti-CD3, but a highly purified preparation of VACV did not enhance anti-CD3-induced proliferation (data not shown). Taken together, our data suggested that in our experiment model, TLR2 is not the upstream receptor of MyD88-dependent signaling pathways.
Figure 6. TLR2-deficient CD8 T cells respond normally to VACV.
(a–b) Ten million WT or TLR2−/− CD8 T cells were adoptively transferred into RAG−/− mice. One day later, mice were infected i.p. with VACV-WR (2×105 PFU/mouse). After 8 days, splenocytes were harvested and stained with anti-CD8, -CD44, and B8R-Tetramer, or stimulated with the indicated peptides for intracellular IFN-γ staining. (a) Top: Individual dot plot of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Bottom, left: Percentage of CD44+B8R-tetramer positive cells gating on CD8+ cells per spleen. Bottom, right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Splenocytes were stimulated with B8R, A3L, A8R, A23R or B2R peptide for intracellular IFN-γ staining. Top: Representative plots of IFN-γ, gating on CD8+CD62L- cells. Percentages that stained for INF-γ are indicated. Bottom: Total numbers of CD8+IFN-γ+ cells per spleen after stimulation of individual peptide indicated previously. Results are mean number ± SEM (n=4 mice/group) from one experiment. Similar results were obtained in 2 separate experiments.
Figure 7. TLR2-deficient mice respond normally to VACV.
(a–b) WT or TLR2−/− mice were infected i.p. with VACV-WR (2×105 PFU/mouse) and analyzed after 7 days. (a) Splenocytes were stained with anti-CD8, -CD44, -CD62L and B8R-Tetramer. Left: Representative histogram of total splenocytes staining for CD8. The numbers in each histogram indicate the percentage of CD8 positive cells. Middle: Representative dot plot of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Representative dot plot of gated CD8+CD44+B8R tetramer positive cells staining for CD62L. Bottom left: Percentage of CD8+CD44+B8R-tetramer positive cells per spleen. Bottom right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Splenocytes were stimulated with B8R, A3L, A8R, A23R or B2R peptide for intracellular IFN-γ staining. Top: Representative plots of IFN-γ, gating on CD8+CD62L- cells. Percentages that stained for INF-γ are indicated. Bottom: Total numbers of CD8+IFN-γ+ cells per spleen after stimulation of individual peptide indicated previously. Results are mean number ± SEM (n=4 mice/group) from one experiment. Similar results were obtained in 2 separate experiments. (c–d) VACV does not infect T cells or induces TLR2 expression. (c) Splenocytes from WT mice were cultured with recombinant VACV-GFP in vitro. Twenty-four, 48 and 72 hours later, cells were stained with anti-CD8, -CD11c to identify CD8 T cells and CD11c+ DC population. Representative histograms: GFP expression after infection (black line), non-infected cells as staining control (grey filled). (d) WT mice were infected i.p. with VACV-WR (2×105 PFU/mouse) and analyzed after 3, 4 and 5 days. Splenocytes were stained with anti-CD8, -CD11c and -TLR2. Representative histograms: TLR2 expression after infection (black line), naive cells as staining control (grey filled).
The T cell requirement for MyD88 is not explained by the use of TLR4, TLR9 or IL-1R neither
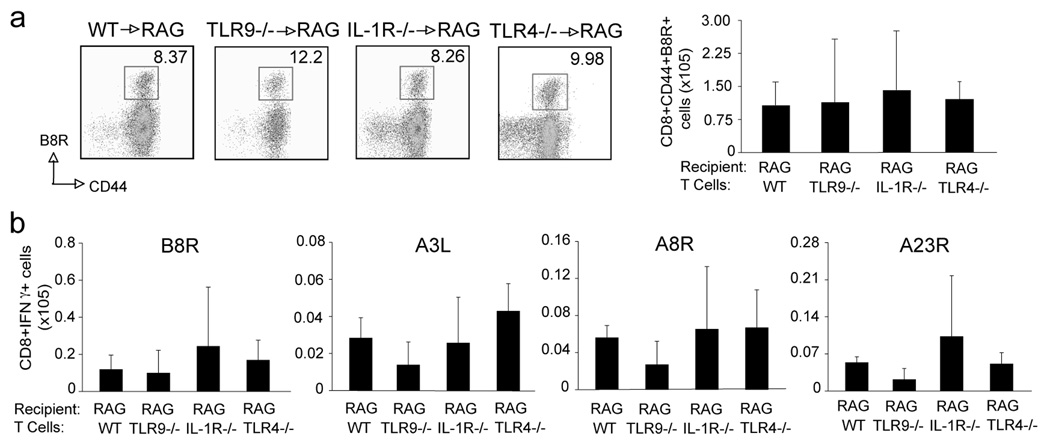
Besides TLR2, previous studies have also shown the involvement of TLR9 in direct regulation of CD4 T cell survival (38). Moreover, TLR4 mediates a protective innate immune response against VACV infection (39). Thus, we also investigated whether a direct effect of pathways linked by these two receptors on CD8 T cells could account for the MyD88 requirement. We transferred TLR9−/− or TLR4−/− CD8 T cells into RAG−/− mice and challenged the recipient mice with VACV one day later. Similar to TLR2−/− cells, CD8 T cells from these mice responded to the virus normally and generated comparable VACV-reactive effector CD8 T cell populations (Fig. 8). Since MyD88 also plays a role in signaling downstream of the IL-1R family, and the cytokines using these receptors, IL-1 and IL-18, have been shown to influence T cell survival and effector function (40), we furthermore transferred IL-1R−/− CD8 T cells into RAG−/− mice. Similarly, there was also no difference in responsiveness to VACV (Fig. 8). Consistent with this, VACV infection induced robust CD8 T cell responses to both dominant and subdominant VACV epitopes in TLR9−/−, and IL-1R−/− mice that was comparable to that seen in wt mice (data not shown). Therefore, while MyD88 plays a critical role in T cells during VACV infection, the upstream receptors of MyD88-dependent signaling pathways remain unclear.
Figure 8. TLR4, TLR9, or IL-1R-deficient CD8 T cells respond normally to VACV.
Ten million WT, TLR4−/−, TLR9−/−, and IL-1R−/− CD8 T cells were adoptively transferred into RAG−/− mice. One day later, mice were infected i.p. with VACV-WR (2×105 PFU/mouse). After 8 days, splenocytes were harvested. (a) Left: Representative plots of gated CD8+ cells staining for CD44 and B8R tetramer using TLR9−/−, TLR4−/−, IL-1R−/− donor cells. The numbers indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Total number of CD8+CD44+B8R-tetramer positive cells per spleen. (b) Total number of CD8+IFN-γ+ cells per spleen after stimulation with the indicated VACV peptides. Results are mean number ± SEM (n=4 mice/group) from one experiment.
Discussion
Here we show that T cell expression of MyD88 is critical for the magnitude of primary CD8 T cell responses to both dominant and subdominant VACV epitopes, with MyD88−/− T cells exhibiting profoundly reduced expansion and anti-viral cytokine production. These experiments help define the precise regulatory mechanisms that govern the efficient generation of adaptive immuity. The adaptor molecule MyD88 participates in host defence to a number of viruses including RNA viruses such as LCMV, and DNA viruses including herpesvirus.
To date, most studies of MyD88 in anti-viral immunity have focused on cells of the innate immune system, such as DC and macrophages. Depending on the experimental system, MyD88−/− DC show profound defects in maturation, costimulatory molecule expression, pro-inflammatory cytokine production, and/or antigen presenting capacity. In response to LCMV infection, pDC and cDC from MyD88−/− mice produce less IFN-α (18), and other inflammatory cytokines such as MCP-1, IL-1 and IL-6 (14), which can play an important role in regulating CD8 T cell expansion and survival (41, 42). Moreover, DC are dependent on the presence of MyD88 to efficiently cross-present viral antigens from infected cells (43). In the context of VACV infection, a recent study demonstrated that, similar to LCMV, production of IL-1 and IL-6 by DC was mediated through TLR-2 in a MyD88-dependent but TRIF-independent manner (44). Notably, 4 to 6-fold higher VACV titers were detected in TLR-2−/− or MyD88−/− mice than in wt mice as early as 3 days post infection, implying that production of innate cytokines by DC through a TLR-2/MyD88 pathway may in part contribute to innate immune control of VACV (44). In the present study we investigated the effects of VACV infection on DC function by focusing on their capacity to mature and activate CD8 T cells. We show that VACV can infect multiple DC subsets and induce their maturation as measured by cell surface upregulation of CD80, CD86, CD40, and MHC Class II. Splenic CD8α+ DC were the major subset infected by VACV and this correlated with their superior capacity to activate naïve CD8 T cells. Using several complementary approaches we found that the defective CD8 T cell responses observed in MyD88−/− mice could not be explained by a defect in DC maturation and antigen presentation. First, MyD88−/− DC infected with VACV were able to upregulate several costimulatory molecules comparable to wt DC. Second, strong expansion and cytokine production of wt CD8 T cells responding to antigen in the context of VACV infection was observed after transfer into MyD88−/− mice. Third, both VACV-infected wt and MyD88−/− DC efficiently induced antigen-specific CD8 T cell responses ex vivo. Our studies then extend previous reports by showing that in contrast to production of IL-1 and IL-6, MyD88 is not required for DC to generate normal CD8 T cell responses directed against VACV epitopes. The capacity of MyD88−/− DC to upregulate costimulatory molecules and efficiently stimulate VACV-specific CD8 T cells might be related to production of inflammatory cytokines that can mimic and hence replace the need for the MyD88 signal. Potentially, consistent with this idea are recent reports showing that two cytokines implicated in DC maturation and CD8 T cell priming, namely IFN-β (44) and IL-12 (45), can be induced in DC in response to VACV in a MyD88-independent manner. Together these results demonstrate that DC can respond to VACV infection via distinct mechanisms that involve MyD88-dependent and independent pathways.
An important observation in our study is that VACV-specific CD8 T cells need to express MyD88 for optimal expansion and cytokine production. Although much evidence implicates MyD88 as a key signaling component of innate responses, this molecule is also expressed in CD8 T cells (21, 22). We show that wild-type CD8 cells can expand and differentiate into effector cells in a MyD88−/− host, while MyD88−/− CD8 T cells failed to expand in a MyD88-sufficient environment upon VACV challenge, fully supporting the conclusion that T cells are the direct recipient of MyD88 signals. Interestingly, a recent study demonstrated that while MyD88 was dispensable for early T cell division and effector differentiation, it played an important role in supporting the survival and accumulation of LCMV-specific CD8 T cells during clonal expansion (46). Thus, MyD88-dependence by anti-viral CD8 T cells may be a general phenomenon that applies to a variety of viruses.
Recently several groups have shown that both human and murine CD4 and CD8 T cells can express functional TLR. Engagement of TLR-1/2, TLR5, TLR7/8, and TLR9 directly on CD4 T cells augments their proliferation in vitro in part, by increasing IL-2 production and maintaining IL-2Rα chain expression (38). Moreover, TLR3 and TLR9 signaling in CD4 T cells has been shown to promote survival by increasing the expression of anti-apoptotic molecules such as Bcl-xL through activation of PI-3K and NF-kB pathways (37, 47). The effects of TLR engagement on CD8 T cells are less well characterized. Naïve CD8 T cells can express TLR1, TLR2, TLR6, TLR7 and TLR9 mRNA, whereas TLR3, TLR4, TLR5, and TLR8 mRNA expression is very low or undetectable (21, 22). To date, only TLR-1/2 engagement with lipopeptide Pam3CysSk4, a synthetic analog of bacterial and mycoplasmal lipoprotein, on CD8 T cells has been shown to enhance proliferation, survival and cytokine production after TCR engagement (21, 22).
In the present study we investigated whether a direct effect of TLR2 pathway on CD8 T cells could account for the MyD88 requirement. Using an adoptive transfer system where we transferred purified naïve polyclonal CD8 T cells from wt and TLR2−/− mice into RAG−/− mice that were then infected with VACV, we show that both percentages and absolute numbers of CD8 T cells reactive with the immunodominant (B8R) and four subdominant epitopes (A3L, A8R, A23R, B2R) of VACV were comparable between the two groups. The responses we chose account for up to 70–80% of the total VACV CD8 response (32). In all cases we did not observe any requirement for TLR2 in either expansion or cytokine production by VACV-specific CD8 T cells. Similarly, infection of TLR2−/− mice with VACV resulted in the generation of robust CD8 T cell responses directed against both dominant and subdominant VACV epitopes. Consistent with a lack of a role for TLR2 on CD8 T cells, we were also unable to detect any TLR2 expression on the surface of CD8 T cells at various times postinfection with VACV either in vivo or in vitro. Moreover, using recombinant VACV expressing GFP, we show that while VACV readily infects CD11+ DCs it cannot infect CD8 T cells in culture. Moreover, we ruled out several other upstream receptors such as IL-1R, TLR4, and TLR9. Together, our results suggest that it is unlikely that MyD88 is downstream of TLRs sensing a VACV PAMP directly on CD8 T cells. Similar to our results with VACV, mice deficient in TLR2, TLR4, TLR7, TLR8, TLR9, IL-1R, IL-18R, and caspase-1-deficient mice infected with LCMV do not reproduce the phenotype of MyD88−/− mice since they mount normal LCMV-specific CD8 T cell responses (18, 46). Thus, while MyD88 plays a critical role in CD8 T cells during viral infections, the identity of its upstream receptor/s remain unclear. As has been proposed before (46), and also supported by our studies, one possibility is that MyD88-dependent viral recognition may occur through novel, as yet unidentified TLRs or other cell surface receptors. Another nonmutually exclusive possibility is that viruses such as LCMV and VACV can activate MyD88-dependent pathways in CD8 T cells indirectly, for example via cytokines (48).
While this manuscript was in the review process Quigley et al. (49) also reported that VACV-specific CD8 T cells need to express MyD88 for optimal expansion/survival. Interestingly however, while in our study we did not find any role for TLR2 in either expansion or cytokine production by VACV-specific CD8 T cells, the published paper by Quigley et al. (49) concluded that TLR2 is the receptor that links to MyD88 and TLR2 is required by these CD8 T cells. The discrepancies between our data with regards to TLR2 usage and Quigley’s finding are not clear at present, but they are likely due to the different experimental systems. For example, C57BL/6 mice were used throughout in our study, while B10.D2 mice were used in Quigley’s study. Consistent with our data, Rahman et al. (46) have recently shown that mice deficient in TLR2 on the C57LB/6 background infected with LCMV also do not reproduce the phenotype of MyD88−/− mice. Therefore, the backgrounds of the mice might simply explain the differences. Further studies are therefore required to examine the precise mechanisms by which the genetic background of mice can influence the differential usage of TLR2 by virus-specific CD8 T cells.
In conclusion, we demonstrate that while T cell expression of MyD88 is necessary for the generation of large anti-VACV CD8 T cell populations, MyD88 is not required for DC maturation and antigen presentation. Our finding that VACV-specific CD8 T cells directly require MyD88 for their expansion and survival together with other studies showing that VACV can modulate this pathway raises an intriguing idea that this virus may have evolved strategies to subvert anti-viral immune responses by directly targeting CD8 T cells.
Acknowledgments
This work was supported by NIH grants AI77079 to S.S.-A., AI67341 to M.C., AI33068, AI048073, and AI057840 to CFW. This is publication #1064 from the La Jolla Institute for Allergy and Immunology.
Reference
- 1.Smith GL, McFadden G. Smallpox: anything to declare? Nat Rev Immunol. 2002;2:521–527. doi: 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- 2.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 4.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 6.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 8.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 10.Kaisho T, Akira S. Regulation of dendritic cell function through toll-like receptors. Curr Mol Med. 2003;3:759–771. doi: 10.2174/1566524033479366. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 13.Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 14.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 15.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 17.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- 20.Tengvall S, Harandi AM. Importance of myeloid differentiation factor 88 in innate and acquired immune protection against genital herpes infection in mice. J Reprod Immunol. 2008;78:49–57. doi: 10.1016/j.jri.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008;22:3628–3637. doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 23.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK, O'Neill LA. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci U S A. 2000;97:10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–1018. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maloney G, Schroder M, Bowie AG. Vaccinia virus protein A52R activates p38 mitogen-activated protein kinase and potentiates lipopolysaccharide-induced interleukin-10. J Biol Chem. 2005;280:30838–30844. doi: 10.1074/jbc.M501917200. [DOI] [PubMed] [Google Scholar]
- 27.Harte MT, Haga IR, Maloney G, Gray P, Reading PC, Bartlett NW, Smith GL, Bowie A, O'Neill LA. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003;197:343–351. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiPerna G, Stack J, Bowie AG, Boyd A, Kotwal G, Zhang Z, Arvikar S, Latz E, Fitzgerald KA, Marshall WL. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. J Biol Chem. 2004;279:36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- 29.Mathew A, O'Bryan J, Marshall W, Kotwal GJ, Terajima M, Green S, Rothman AL, Ennis FA. Robust intrapulmonary CD8 T cell responses and protection with an attenuated N1L deleted vaccinia virus. PLoS ONE. 2008;3:e3323. doi: 10.1371/journal.pone.0003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 31.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 33.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 36.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 37.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Hutchens MA, Luker KE, Sonstein J, Nunez G, Curtis JL, Luker GD. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4:e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 41.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 43.Chen M, Barnfield C, Naslund TI, Fleeton MN, Liljestrom P. MyD88 expression is required for efficient cross-presentation of viral antigens from infected cells. J Virol. 2005;79:2964–2972. doi: 10.1128/JVI.79.5.2964-2972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yammani RD, Pejawar-Gaddy S, Gurley TC, Weimer ET, Hiltbold EM, Alexander-Miller MA. Regulation of maturation and activating potential in CD8+ versus CD8- dendritic cells following in vivo infection with vaccinia virus. Virology. 2008;378:142–150. doi: 10.1016/j.virol.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun D, Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol. 2006;7:375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- 49.Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2008 doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]