Abstract
Twenty years after the discovery of sarcolemmal ATP-sensitive K+ channels and 12 years after the discovery of mitochondrial KATP (mitoKATP) channels, progress has been remarkable, but many questions remain. In the case of the former, detailed structural information is available, and it is well accepted that the channel couples bioenergetics to cellular electrical excitability; however, in the heart, a clear physiological or pathophysiological role has yet to be defined. For mitoKATP, structural information is lacking, but there is abundant evidence linking the opening of the channel to protection against ischemia-reperfusion injury or apoptosis. This review updates recent progress in understanding the physiological role of mitoKATP and highlights outstanding questions and controversies, with the intent of stimulating additional investigation on this topic.
Keywords: ischemia, reperfusion, mitochondria, ATP-sensitive potassium channels, calcium-activated potassium channels
Since their discovery in cardiac myocytes using single-channel patch-clamp techniques in the early 1980s,1 surface membrane ATP-sensitive K+ channels have been characterized extensively at the molecular level. Progress has been rapid, facilitated by the cloning of the major components of the heteromeric channel—the sulfonylurea receptor (SUR12 and SUR23), an ABC transporter family member, and the pore-forming inward rectifier K+ channel protein (Kir6.14 and Kir6.25). ATP-sensitive K+ channels play an important role in translating the metabolic status of a cell into a physiological effect, with perhaps the best examples being the initiation of insulin secretion in pancreatic β cells and modulation of vascular tone. The opening of cardiac sarcolemmal ATP-sensitive K+ (sarcKATP) channels by metabolic inhibition dramatically affects cardiac myocyte electrical excitability6 and is responsible for ischemia-induced suppression of excitability,7 but it is still not clear why this adaptation is important to the cell, organ, or organism. This issue has been revisited in several recent studies of Kir8–10 or SUR11–13 knockout mice.
Another door was opened in 1991, with the discovery of mitochondrial KATP (mitoKATP) channels in the liver mitochondrial inner membrane.14 This finding was bolstered by evidence that a variety of K+ channel openers and inhibitors influenced mitochondrial function, 15–18 culminating in the establishment of a link between the mitoKATP channel and protection against ischemic injury in intact hearts18 and isolated myocytes.19 This hypothesis explained the paradoxical finding that the effects of K+ channel openers on the cardiac action potential and their ability to suppress ischemia-reperfusion injury were not correlated.20–22 Since then, mitoKATP has been implicated in cellular protection against metabolic stress in a variety of tissues, including liver, gut, brain, and kidney, and has been shown to be an essential component of the mechanism of ischemic preconditioning in the heart.21,23–25
Although progress has been made in delineating the mechanisms involved in mitoKATP-mediated protection, the absence of a clear molecular definition of the protein has hampered structure-function studies of this intracellular channel. Nevertheless, the recent development of novel, more specific, mitochondrial K+ channel openers and inhibitors lends support to the general paradigm that increased mitochondrial K+ influx contributes to the cell’s defense against ischemic injury.
Herein, a synthesis and critical review of available information on mitoKATP is presented, with the hope of crystallizing important questions that need to be addressed as we move toward a more complete understanding of the functional role of mitochondrial ion channels in health and disease.
Evidence Supporting Mitochondrial KATP Channels
Electrophysiological Recordings of Mitochondrial Channel Activity
Electrophysiological recordings, although technically challenging for intracellular ion channels, are perhaps the most concrete proof of the existence of mitoKATP channels. Indeed, although the various other methods lend compelling support for a specific mitochondrial K+ influx pathway, direct detection of single-channel activity in the mitochondrial inner membrane provided the foundation for subsequent interpretations. The electrophysiological evidence began with the original work of Inoue et al,14 who directly patch-clamped mitoplasts (mitochondria stripped of the outer membrane) prepared from fused liver mitochondria. Single K+ selective channels were identified and were inhibited by ATP (Ki≈0.8 mmol/L) applied to the mitochondrial matrix face of the channel. They were also blocked by the K+ channel inhibitor 4-aminopyridine. Channel properties resembled those of surface membrane KATP channels, although with a much lower unitary conductance (10 pS in 100 mmol/L cytosolic K+ and 33 mmol/L matrix K+). Importantly, the channels were inhibited by the sulfonylurea glibenclamide (5 µmol/L), additional evidence that a KATP-like channel was present.
There have been no additional publications demonstrating KATP channel activity directly on the intact mitochondrial inner membrane since the original discovery. However, several independent groups have observed channels sensitive to K+ channel openers and inhibitors in reconstituted membranes using highly purified mitochondrial inner membrane preparations. Paucek et al15 reported the purification and reconstitution of a 54-kDa mitochondrial protein that conferred channel activity to a lipid bilayer with a saturating conductance of 30 pS (1 mol/L KCl), consistent with earlier studies showing K+ channel activity attributed to a mitochondrial K+ uniporter in a similar fraction.26,27 It was subsequently found that nucleotide regulation of mitoKATP in bilayers was polarized28; ATP added to the trans but not the cis chamber inhibited the channels (the trans chamber conventionally represents the matrix face of the bilayer; however, the orientation of protein insertion was shown to be specific to medium conditions). Based on the argument that GTP and palmitoyl CoA (assumed to be impermeable to the mitochondrial inner membrane) modulated the activity of mitoKATP in intact mitochondria, these authors concluded that the regulatory site faced the cytosol (or, rather, the mitochondrial intermembrane space).
Zhang et al29 observed K+-selective channel activity in planar lipid bilayers reconstituted with purified bovine mitochondrial membranes, having a conductance of 56 pS in 150 mmol/L KCl. These channels were dose-dependently inhibited by MgATP and activated by GTP when applied only to the trans (matrix) side of the bilayer. They were blocked by 10 to 100 µmol/L 5-hydroxydecanoate (5-HD) or 10 to 100 µmol/L glibenclamide and competitively activated by 10 µmol/L diazoxide. The K+ channel blocker HMR1098, a subtype-selective KATP antagonist that preferentially blocks the sarcKATP channel subtype (see pharmacology below), did not inhibit the channels. Interestingly, mitoKATP channel open probability was enhanced in the presence of superoxide, exogenously generated by a xanthine/xanthine oxidase reaction, and this activation was abolished by pretreatment with 5-HD or a thiol-reducing agent. Redox regulation of mitoKATP channel activity in bilayers was earlier demonstrated by Marinov and Mironova and colleagues,26,30,31 who reported the opposite effect of the reducing agent dithiothreitol, ie, increased activity, but decreased selectivity.26 KATP channels sensitive to diazoxide and 5-HD were also recently observed by Nakae et al32 in reconstitution experiments with purified heart mitochondrial inner membranes. The K+ channels were activated by the anesthetic isoflurane, known to confer protection against infarction,33 and inhibited by application of ATP to the cis (cytosolic) face of the bilayer. Consistent with other studies, the channels were inhibited by 5-HD (100 µmol/L) but not by HMR1098.
Mitochondrial K+ Uptake
A second line of evidence supporting a specific ATP-sensitive K+ channel in the mitochondrial inner membrane is the measurement of K+ uptake into proteoliposomes reconstituted with purified mitochondrial protein fractions. This method has been used extensively by Garlid and colleagues15,18,28,34,35 to characterize the channel’s regulation by Mg2+, ATP and ADP, GTP and GDP, and acyl-CoA esters, as well as its sensitivity to pharmacological openers and inhibitors (see the online data supplement, available at http://circres.ahajournals.org, for more detail). In a key study, Garlid et al36 reported a striking distinction between the pharmacology of sarcKATP and mitoKATP; diazoxide had a potency for the mitoKATP channel (K1/2, 0.4 µmol/L) roughly 2000-fold higher than for the sarcolemmal channel (855 µmol/L). Cromakalim and two of its highly potent congeners, EMD60480 and EMD57970 (K1/2, <10 nmol/L), activated mitoKATP in the ATP-inhibited state, but with no particular selectivity for the mitochondrial isoform.
Mitochondrial Swelling
To avoid rupture of the mitochondrial outer membrane due to swelling, as well as to limit unnecessary energy wastage, mitochondrial cation flux is tightly regulated. The mitochondrial K+ cycle, counterbalancing mitochondrial uniporter activity with K+/H+ exchange, is an important component in mitochondrial volume homeostasis.37,38 With the opening of a mitochondrial K+ channel, K+ influx, accompanied by the movement of permeable anions and H2O, can transiently exceed the extrusion capacity of the K+/H+ exchanger. This results in an increase in steady-state matrix volume, assessed by the extent of light scattering at 520 nm. This assay has been extensively used to investigate the opening of mitoKATP in intact isolated mitochondria.16,39 The advantage of this method compared with reconstitution studies is that the mitochondrial ion channels and the bioenergetic apparatus are intact and regulatory agents can be applied directly to the mitochondria. Limitations of the method include the need to work under hypoosmotic conditions (eg, 115 mOsm) to maintain linearity of the response,39,40 the use of unphysiological ion gradients, nucleotide concentrations, or substrates, and the elimination of normal cytoskeletal architecture and the influence of neighboring organelles or membranes. Nevertheless, in addition to confirming the results of reconstitution experiments, this method has proven useful for characterizing the regulation of mitoKATP. For example, Jaburek et al39 demonstrated that pharmacological inhibition of mi-toKATP depends on how the channels are activated. Mitochondrial swelling activated by removal of ATP (but with Mg2+ present) was completely insensitive to 5-HD or gliben-clamide; however, swelling activated by diazoxide, cromakalim, or GTP in the presence of ATP and Mg2+ was inhibited by glibenclamide (low µmol/L) or by 5-HD (45 to 85 µmol/L). K+ channel openers induced swelling at concentrations roughly five times higher than those required to activate K+ uptake into proteoliposomes; the K1/2 for cromakalim, diazoxide, and EMD60480 was 6.3 µmol/L, 2.3 µmol/L, and 5.4 nmol/L, respectively, in 0.1 mmol/L ATP plus 1 mmol/L Mg2+.36
Flavoprotein Oxidation
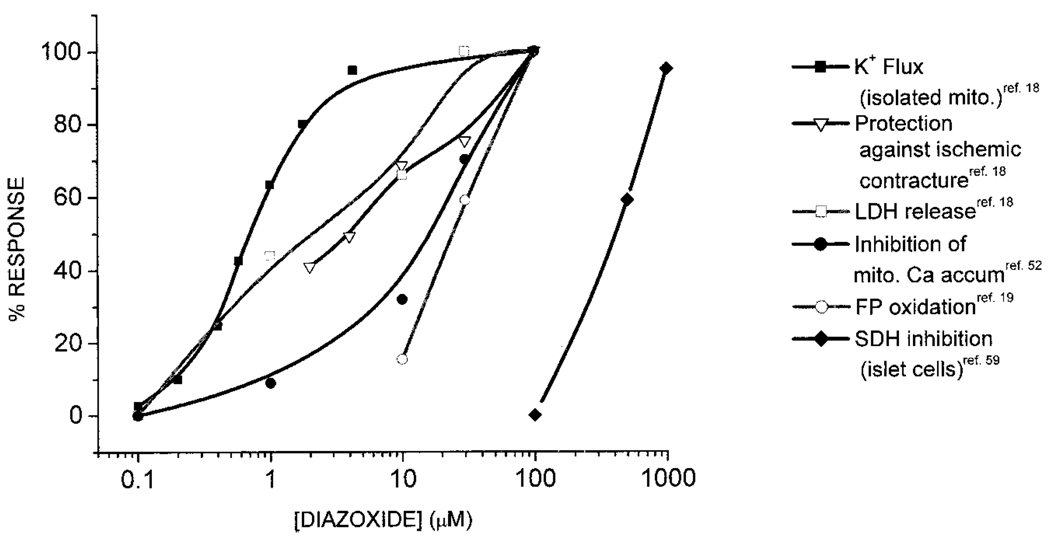
The autofluorescence of NADH and flavoproteins in the mitochondrial matrix provides a built-in sensor of mitochondrial redox state in intact cells and tissues.41,42 In isolated cardiac myocytes excited with 480 nm light, the major component of fluorescence (500- to 550-nm emission band) arises from FAD tightly associated with Krebs cycle dehydrogenases (eg, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase) in equilibrium with the NAD+/NADH redox couple. Under the right conditions, flavoprotein fluorescence can therefore be used to detect changes in the rate of mitochondrial oxidative phosphorylation and the minimum and maximum oxidation states of mitochondria can be calibrated in situ using an inhibitor of electron transport (cyanide) and an uncoupler (eg, 2,4-dinitrophenol), respectively. A study by Liu et al19 and subsequent studies43–47 used native flavoprotein fluorescence to assay the opening of mitoKATP in intact cardiac myocytes. The opening of a mitochondrial K+ channel dissipates energy that would normally be used by the F1,F0 ATPase to produce ATP; hence, mitoKATP opening results in mild uncoupling of mitochondrial energy production. This can be detected as a net change in the redox balance of the mitochondria and an increase in flavoprotein fluorescence if the stimulation of electron flow (NADH oxidation) exceeds the rate of production of NADH. The optimal conditions for measuring a flavoprotein response were found by culturing myocytes overnight and applying K+ channel openers in the absence of external substrates.19 Under these conditions, the K+ channel opener diazoxide induced a reproducible and reversible net oxidation of the flavoprotein pool to a maximal level of 40% to 50% of the fully uncoupled state without any effect on sarcKATP currents measured simultaneously. The K1/2 for this response was 27 µmol/L (Figure 1), ≈10-fold higher than that found for diazoxide-mediated swelling in isolated mitochondria but approximately equal to its potency for cardio-protection in whole hearts,18 intact cells,19 or whole-animal studies.48 The difference in diazoxide sensitivity in intact cells compared with isolated mitochondria can be explained by the 10- to 80-fold higher concentration (≈8 mmol/L) of ATP in the cytosol of intact myocytes (the potency of K+ channel openers strongly depends on the ATP concentration) and the additional sarcolemmal barrier to diazoxide diffusion in the myocyte.
Figure 1.
Concentration-dependent effects of diazoxide. Diazoxide potently activates mitoKATP in isolated mitochondria and protects against ischemic contracture and LDH release with saturation near 50 µmol/L Diazoxide inhibits mitochondrial Ca2+ accumulation during simulated ischemia and oxidizes mitochondrial flavoproteins in isolated myocytes with similar potency (K1/2 ≈25 µmol/L). All of the above effects are inhibited by KATP channel blockers. Depolarization of Δψm attributed to SDH inhibition under normoxic conditions in pancreatic islet cells occurs in the 0.1- to 1-mmol/L concentration range and is not inhibited by KATP channel blockers.
In subsequent studies, flavoprotein oxidation has been used to determine the pharmacology and regulation of mitoKATP in cardiac myocytes.43,44,46,47,49–51 Regarding the selectivity of K+ channel openers and inhibitors for the mitochondrial versus sarcolemmal isoform of the KATP channel, the results, with two exceptions (the opener P-1075 did not open mitoKATP and HMR1098 did not inhibit the diazoxide response in intact cells), are in good agreement with those obtained using isolated mitochondria.
Assaying the mitochondrial flavoprotein oxidation state has provided the only convenient measure of mitochondrial channel opening in intact cells and permits one to study the response of sarcKATP channels simultaneously; however, this method, as with the others mentioned above, has some limitations. The mitochondrial redox potential is a steady-state variable that is the product of the net balance between NADH production by the Krebs cycle and the rate of NADH oxidation. The redox balance therefore does not report the overall rate of oxidative phosphorylation and it could either remain constant or differ over a range of respiratory rates. For example, when cardiac workload increases, there is often no net change in NAD+/NADH due to the simultaneous stimulation of upstream and downstream flows. Furthermore, in most cases, mitochondrial membrane potential (ΔΨm) is well compensated by enhanced proton pumping, so that ΔΨm does not change dramatically despite partial oxidation of the redox pool. In fact, diazoxide-induced FP oxidation is accompanied by only a small depolarization of ΔΨm in intact myocytes under normoxic conditions.52 Measuring oxygen consumption as an index of electron flow helps to resolve the extent of mitochondrial uncoupling induced by mitoKATP openers, as discussed below.
Effects on Respiration and ΔΨm
The opening of a mitochondrial ion channel will partially dissipate the energy stored as the proton motive force (µH=ΔΨm–RT/FΔpH) by contributing inward current and, in the case of a K+ channel, by expending a portion of the proton gradient to eject K+ via the K+/H+ exchanger. This partial uncoupling (energy dissipation not coupled to ATP production) will result in a compensatory increase in proton pumping and oxygen consumption to maintain ΔΨm and oxidative phosphorylation. Because the relative change in K+ influx due to KATP channel opening has been estimated to be relatively small (≈30 nmol K+ min−1mg−1),53 the subtle influence of mitoKATP opening on respiration can often be masked by other nonspecific actions of K+ channel openers on respiration. For example, the most widely investigated mitoKATP opening compound, diazoxide, inhibits succinate-supported respiration in isolated mitochondria at a concentration range that overlaps with the high end of the cardio-protective dose range (discussed below). This decrease in respiration has been attributed to an effect of diazoxide on succinate dehydrogenase (SDH) and is not evident when NADH-linked substrates are used. The latter conditions can thus be used to detect the uncoupling effect of mitoKATP opening. K+ channel inhibitor-sensitive stimulation of mitochondrial oxygen consumption usually amounts to an increase of ≈20% in liver or heart mitochondria53 and up to 50% in brain mitochondria,34,54 presumably due to a higher channel density in the brain.
In intact C2C12 skeletal myoblast and human-derived Giradi cell lines, Minners et al55 reported that pharmacological (diazoxide or adenosine) or ischemic preconditioning results in mild uncoupling, evidenced by stimulation of cellular oxygen consumption, a small decrease in ΔΨm, and depression of cellular ATP levels. These effects, as well as the protection against ischemic damage, were attenuated by 5-HD, suggesting a link between the mechanism of protection and the uncoupling effects.
Similar effects of K+ channel openers on mitochondrial respiration, ΔΨm, and NADH were recently reported in a comprehensive study by Debska et al54 using isolated rat skeletal muscle mitochondria and intact L6 skeletal myoblast cells. In both preparations, diazoxide or nicorandil stimulated the rate of respiration in a saturable, concentration-dependent manner, with a K1/2 of ≈20 µmol/L. Consistent with other studies,52,53,56 the K+-specific depolarization of ΔΨm, determined by subtracting off a small nonspecific effect of the openers in K+-free medium, was diminutive; approaching 8 mV for 100 µmol/L diazoxide (4 mV for nicorandil). Glibenclamide completely inhibited the depolarization, whereas 5-HD partially inhibited the response. Like the effects on respiration and ΔΨm, a K+-specific and saturable oxidation of the NADH redox pool was observed for diazoxide and nicorandil, with K1/2 <50 µmol/L (similar to FP oxidation in cardiomyocytes). The swelling response of the mitochondria followed a similar concentration-response profile.
Although the effects of the prototype mitoKATP opener diazoxide on respiration and ΔΨm have been characterized by several investigators, the use of this drug is complicated by channel-independent actions on mitochondrial metabolism. Unfortunately, more potent openers (eg, BMS-191095)22 of mitoKATP have not been studied as intensively but could provide needed confirmation in the future. In the meantime, certain questions should be kept in mind when trying to determine if a compound’s effect is due to mitoKATP opening. Does the effect occur in the right concentration range (ie, saturating at < 100 µmol/L)? Is the effect inhibited by 5-HD, glibenclamide, or (preferably) both? Is the effect consistent with the pharmacology for structurally distinct mitoKATP openers and inhibitors that have been shown to confer or block protection against ischemia-reperfusion injury?
Inconsistencies in the Mitochondrial KATP Channel Story
Nonspecific Effects of K+ Channel Openers
The antihypertensive and diabetogenic properties of diazoxide predate the discovery of ATP-sensitive K+ channels. Thus, early studies of diazoxide’s effect on energy metabolism were designed to explain its role in insulin secretion and glucose homeostasis. In 1969, Schäfer et al57 reported that diazoxide inhibited succinate oxidation in isolated rat liver mitochondria or beef heart submitochondrial particles. The effect was highly specific for succinate, and the rate of NADH oxidation was unaffected or slightly stimulated (≈20%) by diazoxide (β-hydroxybutyrate substrate). Inhibition of succinate-supported respiration by diazoxide was not saturable up to 1 mmol/L and was attributed to reduced succinate uptake, with consequent suppression of SDH (complex II) activity. Subsequently,58 a mild uncoupling effect of diazoxide (400 µmol/L) on state 4 respiration, ranging from 37% to 52%, was noted for all substrates (glutamate, α-ketoglutarate, and β-hydroxybutyrate) except succinate (respiration decreased by 22%). Retrospective interpretation of these findings is complicated by the lack of comparison with K+-free medium and differing states of polarization of the mitochondrial membrane in different experiments. However, it is clear from these early studies that (1) high concentrations of diazoxide strongly inhibit succinate-supported mitochondrial respiration but not NADH-linked substrate oxidation,57–60 (2) the inhibitory effect on SDH can obscure the mild uncoupling effect of diazoxide,61 (3) the concentration-response curve for SDH inhibition overlaps with the high end of the diazoxide effect on mitoKATP channel opening but does not saturate until the millimolar range (Figure 1),58 and (4) glibenclamide does not inhibit SDH inhibition by diazoxide but does inhibit the uncoupling effect.61
Grimmsmann and Rustenbeck59 later explored the effects of high concentrations of diazoxide on metabolism in liver mitochondria and intact pancreatic B-cells. Diazoxide (0.5 mmol/L) slightly improved state 4 mitochondrial ATP production in succinate or tetramethylphenylenediamine (TMPD) plus ascorbate but inhibited energy production from α-ketoisocaproate. When respiration was stimulated with 400 µmol/L ADP, ATP production by succinate or α-ketoisocaproate, but not TMPD plus ascorbate, was inhibited by diazoxide (0.5 mmol/L), consistent with an inhibitory effect on SDH. The K+ channel openers pinacidil and levcromakalim had no effect on succinate-supported oxidative phosphorylation, whereas they inhibited or stimulated α-ketoisocaproate metabolism, respectively. In intact cells, diazoxide concentrations from 0.1 to 1 mmol/L depolarized ΔΨm, as did pinacidil (0.5 mmol/L), whereas levcromakalim (0.5 mmol/L) had no effect. In agreement with earlier studies,62 neither tolbutamide nor glibenclamide could reverse the inhibition of respiration by the K+ channel openers.
Recent studies have confirmed the finding that diazoxide60,63,64 inhibits succinate-supported mitochondrial respiration, and some have postulated that inhibition of complex II may be involved in the mechanism of ischemic preconditioning. Ovide-Bordeaux et al,64 using saponin-permeabilized left ventricular subendocardial fibers, found that 100 µmol/L diazoxide had no effect on mitochondrial respiration in the presence of glutamate plus malate but inhibited succinate-supported respiration by ≈22%. Glibenclamide did not block this effect. In addition, these investigators found no effect of diazoxide on the ADP sensitivity of respiration. Reproducing the results of the studies mentioned above, Hanley et al63 reported that diazoxide (10 to 100 µmol/L) inhibited succinate-, but not NADH-supported, respiration in beef heart submitochondrial particles in a nonsaturable manner. Pinacidil had no effect on succinate oxidation but partially inhibited NADH oxidation, probably as a result of inhibition of complex I.65 Glibenclamide and 5-HD were not tested in this study, but evidence was presented that 5-HD could be activated to 5-HD-CoA by acyl CoA synthetase, the first step toward entry into the β oxidation pathway.
In a study investigating the effects of ischemic or pharmacological preconditioning on mitochondrial parameters, Lim et al60 found parallel increases in mitochondrial matrix volume and succinate oxidation in mitochondria isolated from hearts subjected to 30 minutes of ischemia and 30 minutes of reperfusion. Diazoxide (50 µmol/L) similarly increased matrix volume, but inhibited succinate-supported respiration by ≈30%, while having no effect on palmitoyl carnitine or glutamate plus malate oxidation. Although activation of 5-HD to 5-HD-CoA was confirmed in this study, 5-HD was not metabolized by the fatty acid β-oxidation pathway in heart mitochondria, and it partially inhibited the oxidation of all substrates at concentrations from 300 µmol/L to 2 mmol/L. Importantly, 5-HD did not reverse the impairment of succinate oxidation by diazoxide. Contrary to these findings, a subsequent study from the same group reported that an increase in mitochondrial matrix volume by diazoxide could not be detected in isolated mitochondria, claiming this as evidence against the existence of mitoKATP channels.66
Taken together, these results demonstrate that it is unlikely that inhibition of SDH underlies the protection afforded by K+ channel openers for the following reasons: (1) the inhibition of respiration by diazoxide only applies to succinate-supported respiration and not physiologically relevant substrates, (2) inhibition of SDH by diazoxide does not saturate and does not parallel the dose-response for protection, (3) SDH inhibition is not reversed by glibenclamide or tolbutamide, and (4) many potent K+ channel openers that have strong protective effects do not inhibit SDH activity.
Nevertheless, the diazoxide interaction with SDH begs the question of whether this protein is in any way involved in the activation of mitoKATP. Studies in heart67 and brain68 have suggested that enhanced tolerance to ischemia conferred by the SDH inhibitor 3-nitropriopionic acid might involve mitoKATP opening, as evidenced by attenuation of the protection by 5-HD. Recent experiments suggest that 3-nitropriopionic acid activates mitoKATP channels reconstituted from a highly purified mitochondrial membrane preparation,69 and this effect was inhibited by 5-HD or glibenclamide, implying that SDH modulates mitoKATP as part of a macromolecular complex.
Lack of K+ Selectivity of Mitochondrial Response to Diazoxide
Suppression of pathological reactive oxygen species (ROS) production during ischemia/reperfusion is one mechanism by which K+ channel openers seem to be acting to protect against cell injury. A recent study by Ozcan et al70 found that on reoxygenation after 5 minutes of anoxia, the levels of ROS produced by isolated mitochondria were decreased by diazoxide or nicorandil. This reduction in ROS production was 5-HD sensitive and was associated with better preservation of oxidative phosphorylation and maintenance of the structural integrity of the mitochondria. Interestingly, the suppression of ROS production was evident even in K+-free medium (sucrose substitution), suggesting that K+-selective channels were not required for the beneficial effect. Furthermore, malonate, an inhibitor of SDH, also reduced ROS production. Again, a link was suggested between diazoxide-mediated SDH inhibition and mitochondrial preservation. Because this study used pyruvate plus malate to support respiration, which is not inhibited by diazoxide, it is unclear what role SDH inhibition could be playing in the response. Follow-up studies using mitochondrial K+ channel openers that do not inhibit SDH (eg, pinacidil or cromakalim) and sensitivity of the response to glibenclamide would be useful to resolve the discrepancies raised by these findings.
Pharmacology of mitoKATP Channels
The amphipathic nature of compounds that interact with intracellular targets introduces possible nonspecific actions on metabolism that complicate interpretation of results. In the case of mitoKATP, classification as a selective K+ channel resembling an isoform of the ATP-sensitive K+ channel has been inferred from the effects of a wide variety of K+ channel openers or blockers. Misinterpreting a nonspecific effect of any particular compound can only be avoided by the use of multiple agents having dissimilar structures but the singular common property of a demonstrated action on K+ channels.
In this context, the number and variety of K+ channel openers that activate mitoKATP provide some reassurance that the target is indeed an inner-membrane K+ channel. These are compiled in the Table. In terms of isoform selectivity, the findings in cardiomyocytes and isolated mitochondria can be summarized as follows: diazoxide, nicorandil, and the highly potent BMS-191095 open mitoKATP channels with minimal effects on the cardiac sarcKATP isoform. A variety of other K+ channel openers (eg, cromakalim or its stereoselective form levcromakalim, EMD60480, EMD57970, pinacidil, RP66471, minoxidil sulfate, and KRN2391), some with submicromolar potencies, also activate mitoKATP but do not discriminate between the two KATP channel isoforms. With respect to mitoKATP inhibition, glibenclamide blocks both isoforms, whereas HMR1098 is generally found to be selective for the sarcolemmal channel (however, see the studies by Birincioglu et al,71 Tsuchida et al,72 and Krenz et al73). 5-HD selectively inhibits the mitochondrial isoform with little effect on sarcKATP.51
Several new compounds have also been reported to interact with mitoKATP, including sildenafil,74 levosimedan,75 YM934,76 and MCC-134.47 MCC-134, an aprikahm analogue known to inhibit pancreatic KATP channels but open smooth muscle KATP channels, recently has been shown to inhibit mitoKATP while activating cardiac sarcKATP.47 Importantly, this compound prevented diazoxide-mediated protection against simulated ischemia but did not confer protection by itself, supporting the argument that mitoKATP rather than sarcKATP channels were responsible.
Confirming the general hypothesis that enhanced mitochondrial K+ influx induces cardioprotection, recent single-channel and K+ uptake studies demonstrate that mitochondrial Ca2+-activated K+ channels are also present on the cardiac mitochondrial inner membrane.77 NS-1619, a KCa opener, was protective against infarction, and this effect was inhibited by the KCa blocker paxilline. These findings support the development of a new class of protective K+ channel openers targeted to the mitochondria.
Caution should be used when extrapolating results obtained in isolated myocytes or mitochondria to the intact heart or animal. For example, although diazoxide is not very potent for activation of cardiomyocyte KATP channels, it is quite effective at activating smooth muscle or pancreatic KATP isoforms. Thus, the infarct-limiting effects of this drug theoretically could be due to nonmitochondrial targets; however, in most models, such extrinsic factors have not been found to mediate protection.
The drug selectivity of the target may also be altered under ischemic conditions, either as a result of altered high-energy phosphate content or changes in pH. D’Hahan et al78 have demonstrated that the sensitivity of sarcKATP channels to K+ channel openers may increase under simulated ischemic conditions (ie, high ADP), and the selective inhibitor 5-HD was originally characterized by its ability to block sarcKATP currents activated by high ADP (1 mmol/L), low pH of 6.6,79 or metabolic inhibition.80 Conclusions about selectivity need to reexamined under many different conditions. How and when mitoKATP channels open during ischemia is still undetermined, and limited information is available about the effects of ischemia on the efficacy of mitoKATP channel openers.
Links to Ischemic Preconditioning/Cardioprotection
mitoKATP: A Common Effector of Diverse Stimuli
In the years since it was proposed that the opening of mitoKATP channels may be involved in the mechanism of protection against ischemic injury, there has been an explosion of interest in this topic. MitoKATP has been implicated in the cardioprotective effects of a variety of stimuli (see the online data supplement) and is involved in the mechanism of both early and delayed preconditioning. In many of these studies, the conclusion that mitoKATP is involved is based solely on inhibition of protection by 5-HD. Given the caveats described above, a direct link between protection and the mitochondrial channel should be regarded as incomplete and could be strengthened by additional pharmacological evidence (eg, sensitivity to glibenclamide and HMR1098).
Trigger Versus Effector?
In the absence of a direct reporter of mitoKATP activation in the intact heart, it is unclear when or how mitoKATP channels open during the course of ischemia and reperfusion. It is assumed that physiological activation occurs as a result of impaired metabolism or in response to a potentiating stimulus such as NO,44 protein kinase C (PKC) activation,51 ROS,29 or intracellular signaling pathways. Pharmacological inhibition with 5-HD or glibenclamide, applied either during a preconditioning stimulus or during the long ischemia, has been the principal means of examining this question. It should be recognized that inhibition of mitoKATP does not seem to influence the extent of infarction in the absence of preconditioning. Thus, if mitoKATP opens during a long ischemia, it does not seem to be conferring any protection. However, it is now well accepted that blocking mitoKATP during the preconditioning ischemia inhibits protection against infarction. It is presently disputed whether 5-HD or glibenclamide, applied just before the long ischemia, abrogates protection.23,81 Although several studies have shown that 5-HD or gliben-clamide, applied after the preconditioning stimulus but before the index ischemia, failed to block protection,82 others have found these inhibitors effective within the same time window83–85 or when applied days after a delayed preconditioning stimulus.86–88 Thus, it appears likely that mitoKATP opening is both a trigger and an effector of the recruitable protection associated with preconditioning but does not provide a background level of protection for the first episode of ischemia.
Role of sarcKATP
With the wealth of recent evidence implicating mitoKATP in protection against ischemic injury, one is left wondering what role sarcKATP channels play in the process. These channels are the premier sensors of the energy state of the myocyte and, when activated, shorten or interrupt the action potential.6,89 In times of metabolic stress, decreasing cellular electrical excitability could protect cells against injury; however, the performance of the heart would suffer and the risk of arrhythmias may increase.90 Before the mitoKATP hypothesis, activation of sarcKATP channels and action potential shortening was viewed as the possible mechanism underlying the anti-ischemic effects of K+ channel openers.21,91,92 This idea was additionally supported by inhibition of protection by glibenclamide, but whereas both glibenclamide and 5-HD blocked the anti-ischemic effect of cromakalim, only glibenclamide inhibited action potential shortening.83,93 Several subsequent studies also showed a lack of correlation between the extent of action potential shortening and the reduction of infarct size.20,94–96 Doses of bimakalim,94 cromakalim,95 or BMS-18044820 that had little or no effect on the action potential could still significantly reduce infarct size, demonstrating that sarcKATP channel-mediated effects on the action potential were not obligatory for cardioprotection.
The role of sarcKATP in ischemia-reperfusion injury has been revisited using transgenic mice in which Kir6.2, the pore-forming subunit of cardiac KATP channels,97,98 has been knocked out. The principal effect of knocking out Kir6.2 in the mouse is that hearts go into contracture within 5 minutes of exposure to ischemia and do not recover function during reperfusion after 20 minutes of ischemia. This differs markedly from wild-type controls, which partially recover from this protocol, but resembles the effect of blocking sarcKATP channels with HMR1098. These results suggest that sarcKATP channels protect mouse hearts from the pathological effects of early ischemia. It should be noted that this conclusion does not seem to apply to larger animals, because HMR1098 has little or no effect on contractility during ischemia in dogs90 or human myocardium99 and does not alter infarct size in rats100 or rabbits.101 Thus, mice seem to be exquisitely sensitive to block of sarcKATP channels during ischemia, perhaps because they are normally operating at a high level of contractile performance with little cardiac reserve102 and depend on sarcKATP channels to modulate the action potential and cellular Ca2+ in times of stress.10 Unfortunately, this hypersensitivity to ischemia in the knockout mouse precludes firm conclusions regarding the role of mitoKATP in cardioprotection. It has been argued that a failure to observe a preconditioning response in Kir6.2 knockout mice suggests that sarcolemmal, rather than mitochondrial, KATP channels are responsible for protection8; however, it is hard to justify this conclusion given that the ischemic damage in the absence of preconditioning is much more severe than in wild-type mice (pressure-rate product in knockout mice after ischemia/reper-fusion was about half that in wild-type mice). On the other hand, these results do provide renewed motivation to define the relative contributions of sarcolemmal and mitochondrial KATP channels to early ischemic injury, as addressed pharmacologically in several studies.72,103–105
Mechanisms of Protection
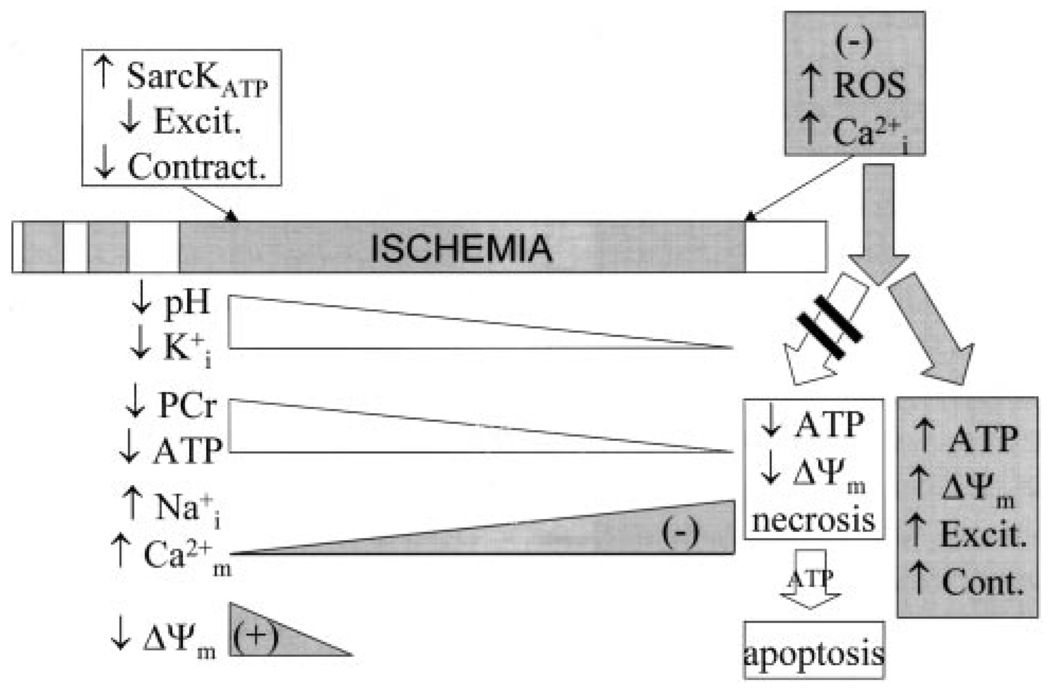
Investigation of the mechanism of protection by mitochondrial K+ channel opening has spawned several hypotheses, which, in general, are not mutually exclusive and probably all contribute to preservation of mitochondrial and contractile function. Much of the data in support of a particular mechanism have been gleaned from isolated cell or mitochondrial studies, making it difficult to determine whether the conclusions are relevant in the context of the whole heart. Nevertheless, progress is being made toward validation of the influence of mitoKATP opening on injury in the intact myocardium, and consensus is developing regarding several key hypotheses. There is good evidence that mitoKATP activation increases ROS production under normoxic conditions, decreases ROS production during reperfusion, blunts mitochondrial Ca2+ accumulation during ischemia, and improves mitochondrial energy production after ischemia (Figure 2). Precisely how these effects are brought to bear is a subject of active investigation.
Figure 2.
Mitigation of ischemic injury by preconditioning. Ischemic or pharmacological preconditioning diminishes accumulation of intracellular Na+ and Ca2+ ions during a long ischemia and blunts reperfusion-induced Ca2+ overload and ROS generation. These actions favor improved mitochondrial energy generation and suppress necrotic and apoptotic cell death.
Mitochondrial Swelling and Improved Oxidative Phosphorylation
As discussed above, swelling of the mitochondria occurs as a consequence of activation of the mitochondrial K+ cycle38 and has been extensively used to characterize the regulation and pharmacology of mitoKATP in isolated mitochondria. Expansion of the mitochondrial matrix improves fatty acid oxidation, respiration, and ATP production.106 Thus, this mechanism has become one of several leading hypotheses to explain protection.
It has been argued that physiological swelling of the mitochondria would be impossible to detect in intact myocardium,60 because a 25% increase in volume would result in a mere 3% increase in mitochondrial diameter, too small to detect within the error limits of electron microscopy (pathological swelling, on the other hand, can be detected and is inhibited by mitoKATP opening107). By isolating mitochondria from perfused hearts under normoxic, preconditioned, ischemic, or reperfused conditions with or without treatment with diazoxide or diazoxide plus 5-HD, Lim et al60 measured mitochondrial matrix volume using 3H2O and [14C]sucrose and found that two 5-minute cycles of preconditioning or diazoxide (50 µmol/L) increased matrix volume by 58% and 88%, respectively. Neither increase was inhibited by 5-HD, and it was found that 5-HD by itself significantly increased matrix volume. Mitochondrial function was also assessed in this study and depended on which substrates were used. In mitochondria isolated under preischemic conditions, IPC increased (by 30% to 40%) state 3 respiration of 2-oxoglutarate or succinate but not that of ascorbate plus TMPD. In preischemic mitochondria, diazoxide inhibited state 3 respiration of 2-oxoglutarate (by 22%) and slightly inhibited that of succinate but had no effect on ascorbate plus TMPD, glutamate plus malate, or palmitoyl carnitine plus malate oxidation. For mitochondria isolated at the end of 30-minute ischemia, state 3 respiration was inhibited in all groups relative to preischemic controls, regardless of substrate. Diazoxide significantly improved end-ischemic mitochondrial respiration (55% higher), but this effect was not inhibited by 5-HD, which paradoxically increased respiration by itself. Although the beneficial effects of IPC or diazoxide on cardiac hemodynamics were inhibited by 5-HD, none of the effects on isolated mitochondria were. Partial recovery of mitochondrial function was achieved by reperfusion; however, IPC or diazoxide treatment provided no additional benefit. Changes in matrix volume or respiration of these mitochondria, therefore, were uncorrelated with protection.
A similar approach was used by Fryer et al100 for mitochondria isolated from preconditioned or diazoxide-treated hearts. Mtochondria from hearts exposed to ischemia-reperfusion had markedly suppressed ATP synthesis compared with nonischemic controls, and the depression was reversed by IPC. 5-HD, but not HMR1098, eliminated this protective effect. In contrast, despite its infarct-sparing effect, diazoxide treatment did not reverse the decline in mitochondrial ATP synthesis.
In chronically hypoxic rabbit hearts, postischemic recovery of left ventricular function is enhanced compared with normoxic control animals, and this extra protection is attenuated by glibenclamide or 5-HD,108 which had no effect on recovery in controls. Mtochondria isolated from the chronically hypoxic hearts had improved basal ATP synthesis rates. Bimkalim treatment decreased ATP synthesis in control mitochondria in a 5-HD–sensitive or glibenclamide-sensitive manner, but in mitochondria from hypoxic hearts, this opener had no effect, although the inhibitors slowed basal ATP synthesis rates. These data suggest that mitoKATP channels are open in the chronically hypoxic animals, providing enhanced resistance to ischemia.
Several other studies have reported better preservation of ATP production by mitochondria treated with K+ channel openers,53,70,109 although, again, this effect depends on the choice of substrate and whether ΔΨm is maintained. Kowaltowski et al53 have argued that improved function is the result of expansion of the mitochondrial matrix space, which may preserve the mitochondrial inner and outer membrane contact sites in the optimal orientation for ADP import.110 In contrast, in skinned cardiac trabeculae, Ovide-Bordeaux et al64 reported no effects of diazoxide (100 µmol/L) on the Km for ADP stimulation of respiration by diazoxide.
It is apparent that although mitochondrial swelling is a likely consequence of mitoKATP channel opening and has been demonstrated for isolated mitochondria, linking this effect directly to mitochondrial preservation after ischemia-reperfusion is a challenging task.
Suppression of Ca2+ Overload
Several recent studies have confirmed the hypothesis, initially suggestion by Liu et al,19 that mitochondrial Ca2+ accumulation during ischemia and reperfusion may be attenuated by mitoKATP opening. Holmuhamedov et al111 reported that diazoxide and pinacidil decreased the rate and magnitude of Ca2+ uptake into isolated mitochondria and that, in intact cardiomyocytes, this effect was inhibited by 5-HD. They attributed the effect to partial depolarization (10 to 24 mV) of ΔΨm, which was shown to occur in response to pinacidil (100 µmol/L), cromakalim (25 µmol/L),or levcromakalim (20 µmol/L).56 This small depolarization was K+-dependent and inhibited by a K+ channel blocker. This group also reported a second effect of K+ channel openers, release of Ca2+ and intermembrane components such as cytochrome c from Ca2+-loaded mitochondria. The latter finding is at odds with the antiapoptotic effects of mitoKATP. Murata et al52 demonstrated that mitochondrial Ca2+ accumulation during simulated ischemia was attenuated by mitoKATP opening (Figure 1), and this mechanism reduced the magnitude of PTP-opening on reperfusion. Wang et al,112 using either ischemic preconditioning or diazoxide treatment, also showed that mitoKATP activation blunts mitochondrial Ca2+ accumulation during ischemia in intact hearts. Recently, Korge et al113 found that diazoxide protected isolated mitochondria from anoxic injury in a 5-HD–sensitive manner. This effect was mimicked by phor-bol myristic acid, implying that mitoKATP could also be activated by PKC. The protective effect was associated with strong depolarization of ΔΨm under anoxic conditions and a consequent decrease in mitochondrial Ca2+ loading, which prevented a mitochondrial permeability transition on reoxygenation. These data are consistent with other reports indicating that mitoKATP opening prevents apoptosis,88 presumably by inhibiting the activation of the PTP.107,114–120 In neurons, the antiapoptotic effect of mitoKATP opening and a reduction in cortical infarct size were attributed to a shift in the balance between apoptotic and antiapoptotic proteins; diazoxide treatment suppressed Bax translocation and cytochrome c release and enhanced Bcl2 levels.
Increased/Decreased ROS Production
ROS play an essential, but double-edged, role in mitoKATP-mediated protection. ROS generation is a trigger of the preconditioning response,121 and protection by K+ channel openers can be inhibited by ROS scavengers.122 Several reports have measured increased mitochondrial ROS generation in response to preconditioning or mitoKATP activation. 122–124 This mechanism activates protective, PKC-dependent, signaling pathways.121 Another physiologically important free radical, NO, produced from cytosolic and mitochondrial NO synthases, plays an important role as a trigger of both early and delayed preconditioning125–127 and is known to potentiate mitoKATP opening.44
In contrast, it is well-known that ROS produced on reperfusion after a long ischemia can cause irreversible cell injury.128 This postischemic burst of ROS is suppressed by pretreatment with mitoKATP openers.70,129 Hence, the consensus is that mitoKATP facilitates the production of protective ROS during preconditioning but decreases injurious postischemic ROS production. The mechanistic details of this effect remain to be determined.
Molecular Structure?
Determination of the structure of the pore-forming protein underlying mitoKATP conductance will resolve many of the controversial issues raised in this review. Unfortunately, several early leads have not produced definitive results. In general, K+ uniport activity has been observed when purified mitochondrial proteins in the molecular weight range of 50 to 60 kDa have been reconstituted into proteoliposomes,27,130 and a 54-kDa protein was tentatively identified as a component of mitoKATP.15 These data, together with the similar pharmacology of known plasma membrane isoforms of KATP (particularly the combination of SUR1/Kir6.1131), suggest that mitoKATP might be composed of an inward rectifier potassium channel subunit (Kir) in association with a sulfonylurea receptor (SUR). Binding of Kir6.1 antibodies to a 51-kDa protein in a mitochondrial membrane preparation and intact mitochondria132 fortified this hypothesis. In contrast, dominant-negative knockout of Kir6.1 or Kir6.2 using adenoviruses did not affect mitoKATP responses in intact myocytes.45 More recently, transgenic knockout of Kir6.1, a component of vascular KATP channels, resulted in a phenotype of sudden cardiac death associated with vasospasm.9 The response of these mice to ischemic injury has not yet been determined, but mitoKATP opening was apparently not disrupted by elimination of this KATP subunit.9
Regarding the possibility that SUR is present in mitochondrial membranes, low-affinity sulfonylurea binding sites were identified in purified mitochondrial preparations,133,134 and a putative 63-kD sulfonylurea binding protein was reported by Grover and Garlid21; however, the molecular structure of this binding site remains unresolved. A recent study by Munoz et al11 tested whether elimination of SUR1 influenced ischemic preconditioning in the brain. Double carotid occlusion for 20 minutes protected hippocampal neurons from damage induced by a subsequent 40 minutes of ischemia, as did treatment with diazoxide. The extent of protection by ischemic preconditioning or diazoxide did not differ between WT and knockout mice and was inhibited by 5-HD. Nevertheless, 5-HD exacerbated neurodegeneration in the absence of preconditioning, and the authors concluded that this result was consistent with the involvement of mitoKATP channels. SUR1 was apparently not a required component of mitoKATP.
Conclusions
The role of the mitochondria in ischemic preconditioning and cell survival during ischemia-reperfusion continues to be a fertile area of investigation. Several lines of evidence, using a variety of techniques, support the hypothesis that K+-selective channels are present and confer protection against ischemic and apoptotic injury. These include direct electrophysiological recordings, K+ flux assays, mitochondrial swelling, flavoprotein oxidation, effects on mitochondrial bioenergetics, and pharmacological responses. Pharmacological evidence is not simply based on a single compound or class of compounds but on a plethora of structurally dissimilar K+ channel openers and a lesser number of available inhibitors that have as their sole common factor a demonstrated effect on K+ channel activity. Any one piece of evidence cannot definitively prove (or disprove) the existence of mitoKATP, but taken together, the argument in favor is quite strong.
Overall, the links between mitoKATP and cardioprotection are robust; however, when individual studies are considered, using a single K+ channel opener or inhibitor to support the primary argument renders the interpretation vulnerable. This is exemplified by the widespread use of diazoxide alone as the activator of mitoKATP. There is ample evidence that this compound, at concentrations that overlap but do not reproduce the dose-response relationship for ischemic protection, inhibits SDH activity, but it is highly unlikely that SDH inhibition per se is responsible for protection. However, there is still the possibility that a diazoxide interaction with SDH may be important for inducing the opening of mitoKATP, a topic that will require additional investigation.
Consensus is building that mitoKATP opening evokes a response involving several different protective mechanisms, including matrix swelling, ROS modulation, and effects on mitochondrial Ca2+ homeostasis. A unifying hypothesis has yet to be elaborated because of the complexity of control of mitochondrial oxidative phosphorylation and the dangers of extrapolating in vitro data to the conditions mitochondria experience during ischemia and reperfusion in the intact heart.
Finding the pore-forming proteins underlying the protective effects of K+ channel openers will undoubtedly resolve many outstanding questions and convince skeptics, but, in the meantime, novel and selective pharmacological agents are already being developed, facilitating the translation of basic knowledge about mitoKATP into clinical treatments for ischemia-related and apoptotic diseases.
Selectivity of K+ Channel Openers and Inhibitors Toward Cardiac Mitochondrial KATP (mitoKATP), Sarcolemmal KATP (sarcKATP), or Mitochondrial KCa (mitoKCa
| mitoKATP | sarcKATP | mitoKCa | |
|---|---|---|---|
| K+ channel openers | |||
| Diazoxide | ✓ | a | |
| Nicorandil | ✓ | b | |
| BMS-180448 | ✓ | c | |
| BMS-191095 | ✓ | ||
| Cromakalim | ✓ | ✓ | |
| EMD60480, 57970 | ✓ | ✓ | |
| Pinacidil | ✓ | ✓ | |
| P-1060 | ✓ | ✓ | |
| P-1075 | d | ✓ | |
| Minoxidil sulfate | ✓ | ✓ | |
| KRN2391 | ✓ | ✓ | |
| Sildenafil | ✓ | ✓ | |
| Isoflurane | ✓ | ✓ | |
| Aprikalim | ✓ | ✓ | |
| MCC-134 | ✓ | ||
| Levosimendan | ✓ | ✓ | |
| NS-1619 | ✓ | ||
| K+ channel blockers | |||
| 5-Hydroxydecanoate | ✓ | e | |
| MCC-134 | ✓ | ||
| Glibenclamide, glipizide, glisoxepide | ✓ | ✓ | |
| Glimepiride | f | ✓ | |
| HMR1098 (1883) | g | ✓ | |
| ChTx, IbTx | ✓ | ||
| Paxilline | ✓ | ||
MitoKATP activation has been reported for all KATP channel openers listed except MCC-134. Mitochondrial selectivity reported for the openers diazoxide, BMS-191095, and BMS-180448 and for the inhibitors 5-hydroxydecanoate and MCC-134, whereas HMR1098 and glimepiride show selectivity for the sarcKATP channel. Selectivity depends on the cell type examined and the conditions used:
Diazoxide activates sardATP at high concentrations or when ADP is high78;
nicorandil activates sarcKATP at high concentrations50;
BMS-180448 is cardioprotective at concentrations that do not shorten the action potential20;
P-1075 was selective for sarcKATP in adult rabbit myocytes43 but activated mitoKATP in isolated rat mitochondria;
5-hydroxydecanoate inhibits sarcKATP at low pH135;
glimepiride blocks sarcKATP but does not inhibit cardioprotection48;
Acknowledgments
This work was supported by the NIH National Heart, Lung, and Blood Institute (R01-HL54598 and R01-HL61711).
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1107. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 6.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991;68:280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 7.Smallwood JK, Ertel PJ, Steinberg MI. Modification by glibenclamide of the electrophysiological consequences of myocardial ischaemia in dogs and rabbits. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:214–220. doi: 10.1007/BF00166967. [DOI] [PubMed] [Google Scholar]
- 8.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 9.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 10.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz A, Nakazaki M, Goodman JC, Barrios R, Onetti CG, Bryan J, Aguilar-Bryan L. Ischemic preconditioning in the hippocampus of a knockout mouse lacking SUR1-based KATP channels. Stroke. 2003;34:164–170. doi: 10.1161/01.str.0000048215.36747.d1. [DOI] [PubMed] [Google Scholar]
- 12.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 14.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 15.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- 16.Szewczyk A, Mikolajek B, Pikula S, Nalecz MJ. Potassium channel openers induce mitochondrial matrix volume changes via activation of ATP-sensitive K+ channel. Pol J Pharmacol. 1993;45:437–443. [PubMed] [Google Scholar]
- 17.Garlid KD. Cation transport in mitochondria: the potassium cycle. Biochim Biophys Acta. 1996;1275:123–126. doi: 10.1016/0005-2728(96)00061-8. [DOI] [PubMed] [Google Scholar]
- 18.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels: possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Sato T, O’Rourke B, Marbán E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 20.Grover GJ, D’Alonzo AJ, Hess T, Sleph PG, Darbenzio RB. Glyburide-reversible cardioprotective effect of BMS-180448 is independent of action potential shortening. Cardiovasc Res. 1995;30:731–738. [PubMed] [Google Scholar]
- 21.Grover GJ, Garlid KD. ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 22.Grover GJ, D’Alonzo AJ, Garlid KD, Bajgar R, Lodge NJ, Sleph PG, Darbenzio RB, Hess TA, Smith MA, Paucek P, Atwal KS. Pharmacologic characterization of BMS-191095, a mitochondrial KATP opener with no peripheral vasodilator or cardiac action potential shortening activity. J Pharmacol Exp Ther. 2001;297:1184–1192. [PubMed] [Google Scholar]
- 23.Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial KATP channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- 25.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 26.Mironova GD, Skarga YY, Grigoriev SM, Negoda AE, Kolomytkin OV, Marinov BS. Reconstitution of the mitochondrial ATP-dependent potassium channel into bilayer lipid membrane. J Bioenerg Biomembr. 1999;31:159–163. doi: 10.1023/a:1005408029549. [DOI] [PubMed] [Google Scholar]
- 27.Diwan JJ, Haley T, Sanadi DR. Reconstitution of transmembrane K+ transport with a 53 kilodalton mitochondrial protein. Biochem Biophys Res Commun. 1988;153:224–230. doi: 10.1016/s0006-291x(88)81212-9. [DOI] [PubMed] [Google Scholar]
- 28.Yarov-Yarovoy V, Paucek P, Jaburek M, Garlid KD. The nucleotide regulatory sites on the mitochondrial KATP channel face the cytosol. Biochim Biophys Acta. 1997;1321:128–136. doi: 10.1016/s0005-2728(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]
- 30.Grigoriev SM, Skarga YY, Mironova GD, Marinov BS. Regulation of mitochondrial KATP channel by redox agents. Biochim Biophys Acta. 1999;1410:91–96. doi: 10.1016/s0005-2728(98)00179-0. [DOI] [PubMed] [Google Scholar]
- 31.Marinov BS, Grigoriev SM, Skarga Y, Olovjanishnikova GD, Mironova GD. Effects of pelargonidine and a benzocaine analogue p-diethylaminoethyl benzoate on mitochondrial KATP channel. Membr Cell Biol. 2001;14:663–671. [PubMed] [Google Scholar]
- 32.Nakae Y, Kwok WM, Bosnjak ZJ, Jiang MT. Isoflurane activates rat mitochondrial ATP-sensitive K+ channels reconstituted in lipid bilayers. Am J Physiol Heart Circ Physiol. 2003;284:H1865–H1871. doi: 10.1152/ajpheart.01031.2002. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- 35.Paucek P, Yarov-Yarovoy V, Sun X, Garlid KD. Inhibition of the mitochondrial KATP channel by long-chain acyl-CoA esters and activation by guanine nucleotides. J Biol Chem. 1996;271:32084–32088. doi: 10.1074/jbc.271.50.32084. [DOI] [PubMed] [Google Scholar]
- 36.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 37.Brierley GP, Jurkowitz M, Chavez E, Jung DW. Energy-dependent contraction of swollen heart mitochondria. J Biol Chem. 1977;252:7932–7939. [PubMed] [Google Scholar]
- 38.Garlid KD, Paucek P. The mitochondrial potassium cycle. IUBMB Life. 2001;52:153–158. doi: 10.1080/15216540152845948. [DOI] [PubMed] [Google Scholar]
- 39.Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- 40.Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta. 1986;853:187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 41.Hassinen I, Chance B. Oxidation-reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun. 1968;31:895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- 42.Chance B, Salkovitz IA, Kovach AG. Kinetics of mitochondrial flavoprotein and pyridine nucleotide in perfused heart. Am J Physiol. 1972;223:207–218. doi: 10.1152/ajplegacy.1972.223.1.207. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Sasaki N, Seharaseyon J, O’Rourke B, Marbán E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal KATP channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki N, Sato T, Ohler A, O’Rourke B, Marbán E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 45.Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, O’Rourke B, Marbán E. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J Mol Cell Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Sasaki N, O’Rourke B, Marbán E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki N, Murata M, Guo Y, Jo SH, Ohler A, Akao M, O’Rourke B, Xiao RP, Bolli R, Marbán E. MCC-134, a single pharmacophore, opens surface ATP-sensitive potassium channels, blocks mitochondrial ATP-sensitive potassium channels, and suppresses preconditioning. Circulation. 2003;107:1183–1188. doi: 10.1161/01.cir.0000051457.64240.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mocanu MM, Maddock HL, Baxter GF, Lawrence CL, Standen NB, Yellon DM. Glimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxide. Circulation. 2001;103:3111–3116. doi: 10.1161/01.cir.103.25.3111. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Sato T, Seharaseyon J, Szewczyk A, O’Rourke B, Marbán E. Mitochondrial ATP-dependent potassium channels: viable candidate effectors of ischemic preconditioning. Ann N Y Acad Sci. 1999;874:27–37. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Sasaki N, O’Rourke B, Marbán E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol. 2000;35:514–518. doi: 10.1016/s0735-1097(99)00552-5. [DOI] [PubMed] [Google Scholar]
- 51.Sato T, O’Rourke B, Marbán E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- 52.Murata M, Akao M, O’Rourke B, Marbán E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 53.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 54.Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97–105. doi: 10.1016/s0005-2728(02)00340-7. [DOI] [PubMed] [Google Scholar]
- 55.Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 56.Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998;275:H1567–H1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- 57.Schafer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol. 1969;18:2678–2681. [PubMed] [Google Scholar]
- 58.Schafer G, Portenhauser R, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide, I: action on succinate dehydrogenase and TCA-cycle oxidations. Biochem Pharmacol. 1971;20:1271–1280. doi: 10.1016/0006-2952(71)90358-3. [DOI] [PubMed] [Google Scholar]
- 59.Grimmsmann T, Rustenbeck I. Direct effects of diazoxide on mitochondria in pancreatic B-cells and on isolated liver mitochondria. Br J Pharmacol. 1998;123:781–788. doi: 10.1038/sj.bjp.0701663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Portenhauser R, Schafer G, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide, II: interaction with energy conservation and ion transport. Biochem Pharmacol. 1971;20:2623–2632. doi: 10.1016/0006-2952(71)90171-7. [DOI] [PubMed] [Google Scholar]
- 62.Lenzen S, Panten U. Characterization of succinate dehydrogenase and a-glycerophosphate dehydrogenase in pancreatic islets. Biochem Med. 1983;30:349–356. doi: 10.1016/0006-2944(83)90027-3. [DOI] [PubMed] [Google Scholar]
- 63.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial KATP channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 65.Lembert N, Idahl LA, Ammon HP. K-ATP channel independent effects of pinacidil on ATP production in isolated cardiomyocyte or pancreatic β-cell mitochondria. Biochem Pharmacol. 2003;65:1835–1841. doi: 10.1016/s0006-2952(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 66.Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ockaili RA, Bhargava P, Kukreja RC. Chemical preconditioning with 3-nitropropionic acid in hearts: role of mitochondrial KATP channel. Am J Physiol Heart Circ Physiol. 2001;280:H2406–H2411. doi: 10.1152/ajpheart.2001.280.5.H2406. [DOI] [PubMed] [Google Scholar]
- 68.Horiguchi T, Kis B, Rajapakse N, Shimizu K, Busija DW. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke. 2003;34:1015–1020. doi: 10.1161/01.STR.0000063404.27912.5B. [DOI] [PubMed] [Google Scholar]
- 69.Ardehali H. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Circulation. 2003;108(suppl IV) doi: 10.1073/pnas.0401703101. IV–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 71.Birincioglu M, Yang XM, Critz SD, Cohen MV, Downey JM. S-T segment voltage during sequential coronary occlusions is an unreliable marker of preconditioning. Am J Physiol. 1999;277:H2435–H2441. doi: 10.1152/ajpheart.1999.277.6.H2435. [DOI] [PubMed] [Google Scholar]
- 72.Tsuchida A, Miura T, Tanno M, Sakamoto J, Miki T, Kuno A, Matsumoto T, Ohnuma Y, Ichikawa Y, Shimamoto K. Infarct size limitation by nicorandil: roles of mitochondrial KATP channels, sarcolemmal KATP channels, and protein kinase C. J Am Coll Cardiol. 2002;40:1523–1530. doi: 10.1016/s0735-1097(02)02268-4. [DOI] [PubMed] [Google Scholar]
- 73.Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002;97:365–373. doi: 10.1007/s003950200045. [DOI] [PubMed] [Google Scholar]
- 74.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 75.Kopustinskiene DM, Pollesello P, Saris NE. Levosimendan is a mitochondrial KATP channel opener. Eur J Pharmacol. 2001;428:311–314. doi: 10.1016/s0014-2999(01)01350-4. [DOI] [PubMed] [Google Scholar]
- 76.Tanonaka K, Taguchi T, Koshimizu M, Ando T, Morinaka T, Yogo T, Konishi F, Takeo S. Role of an ATP-sensitive potassium channel opener, YM934, in mitochondrial energy production in ischemic/reperfused heart. J Pharmacol Exp Ther. 1999;291:710–716. [PubMed] [Google Scholar]
- 77.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 78.D’Hahan N, Moreau C, Prost AL, Jacquet H, Alekseev AE, Terzic A, Vivaudou M. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc Natl Acad Sci USA. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Notsu T, Ohhashi K, Tanaka I, Ishikawa H, Niho T, Fukutake K, Mizota M. 5-Hydroxydecanoate inhibits ATP-sensitive K+ channel currents in guinea-pig single ventricular myocytes. Eur J Pharmacol. 1992;220:35–41. doi: 10.1016/0014-2999(92)90008-r. [DOI] [PubMed] [Google Scholar]
- 80.Notsu T, Tanaka I, Takano M, Noma A. Blockade of the ATP-sensitive K+ channel by 5-hydroxydecanoate in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1992;260:702–708. [PubMed] [Google Scholar]
- 81.Gross GJ, Fryer RM. Mitochondrial KATP channels: triggers or distal effectors of ischemic or pharmacological preconditioning? Circ Res. 2000;87:431–433. doi: 10.1161/01.res.87.6.431. [DOI] [PubMed] [Google Scholar]
- 82.Schulz R, Gres P, Heusch G. Activation of ATP-dependent potassium channels is a trigger but not a mediator of ischaemic preconditioning in pigs. Br J Pharmacol. 2003;139:65–72. doi: 10.1038/sj.bjp.0705225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- 84.Fryer RM, Hsu AK, Gross GJ. Mitochondrial KATP channel opening is important during index ischemia and following myocardial reperfusion in ischemic preconditioned rat hearts. J Mol Cell Cardiol. 2001;33:831–834. doi: 10.1006/jmcc.2001.1350. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Cone J, Liu Y. Dual roles of mitochondrial KATP channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol. 2001;280:H246–H255. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Kudo M, Xu M, Ayub A, Ashraf M. Mitochondrial KATP channel as an end effector of cardioprotection during late preconditioning: triggering role of nitric oxide. J Mol Cell Cardiol. 2001;33:2037–2046. doi: 10.1006/jmcc.2001.1468. [DOI] [PubMed] [Google Scholar]
- 87.Carroll R, Yellon DM. Delayed cardioprotection in a human cardiomyocyte-derived cell line: the role of adenosine, p38MAP kinase and mitochondrial KATP. Basic Res Cardiol. 2000;95:243–249. doi: 10.1007/s003950050187. [DOI] [PubMed] [Google Scholar]
- 88.Takashi E, Wang Y, Ashraf M. Activation of mitochondrial KATP channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 89.O’Rourke B, Ramza BM, Marbán E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 90.Billman GE, Englert HC, Scholkens BA. HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel, part II: effects on susceptibility to ventricular fibrillation induced by myocardial ischemia in conscious dogs. J Pharmacol Exp Ther. 1998;286:1465–1473. [PubMed] [Google Scholar]
- 91.Grover GJ, Sleph PG, Dzwonczyk S. Pharmacologic profile of cromakalim in the treatment of myocardial ischemia in isolated rat hearts and anesthetized dogs. J Cardiovasc Pharmacol. 1990;16:853–864. doi: 10.1097/00005344-199012000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Grover GJ, Dzwonczyk S, Sleph PG. Reduction of ischemic damage in isolated rat hearts by the potassium channel opener, RP 52891. Eur J Pharmacol. 1990;191:11–18. doi: 10.1016/0014-2999(90)94091-b. [DOI] [PubMed] [Google Scholar]
- 93.McCullough JR, Normandin DE, Conder ML, Sleph PG, Dzwonczyk S, Grover GJ. Specific block of the anti-ischemic actions of cromakalim by sodium 5-hydroxydecanoate. Circ Res. 1991;69:949–958. doi: 10.1161/01.res.69.4.949. [DOI] [PubMed] [Google Scholar]
- 94.Yao Z, Gross GJ. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation. 1994;89:1769–1775. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]
- 95.Grover GJ, D’Alonzo AJ, Parham CS, Darbenzio RB. Cardioprotection with the KATP opener cromakalim is not correlated with ischemic myocardial action potential duration. J Cardiovasc Pharmacol. 1995;26:145–152. doi: 10.1097/00005344-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 96.Grover GJ, D’Alonzo AJ, Dzwonczyk S, Parham CS, Darbenzio RB. Preconditioning is not abolished by the delayed rectifier K+ blocker dofetilide. Am J Physiol. 1996;271:H1207–H1214. doi: 10.1152/ajpheart.1996.271.3.H1207. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marbán E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 99.Ghosh S, Standen NB, Galinanes M. Evidence for mitochondrial KATP channels as effectors of human myocardial preconditioning. Cardiovasc Res. 2000;45:934–940. doi: 10.1016/s0008-6363(99)00407-1. [DOI] [PubMed] [Google Scholar]
- 100.Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial KATP channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- 101.Jung O, Englert HC, Jung W, Gogelein H, Scholkens BA, Busch AE, Linz W. The KATP channel blocker HMR 1883 does not abolish the benefit of ischemic preconditioning on myocardial infarct mass in anesthetized rabbits. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:445–451. doi: 10.1007/s002109900212. [DOI] [PubMed] [Google Scholar]
- 102.Kass DA, Hare JM, Georgakopoulos D. Murine cardiac function: a cautionary tail. Circ Res. 1998;82:519–522. doi: 10.1161/01.res.82.4.519. [DOI] [PubMed] [Google Scholar]
- 103.Sanada S, Kitakaze M, Asanuma H, Harada K, Ogita H, Node K, Takashima S, Sakata Y, Asakura M, Shinozaki Y, Mori H, Kuzuya T, Hori M. Role of mitochondrial and sarcolemmal KATP channels in ischemic preconditioning of the canine heart. Am J Physiol Heart Circ Physiol. 2001;280:H256–H263. doi: 10.1152/ajpheart.2001.280.1.H256. [DOI] [PubMed] [Google Scholar]
- 104.Tanno M, Miura T, Tsuchida A, Miki T, Nishino Y, Ohnuma Y, Shimamoto K. Contribution of both the sarcolemmal KATP and mitochondrial KATP channels to infarct size limitation by KATP channel openers: differences from preconditioning in the role of sarcolemmal KATP channels. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:226–232. doi: 10.1007/s002100100448. [DOI] [PubMed] [Google Scholar]
- 105.Light PE, Kanji HD, Fox JE, French RJ. Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J. 2001;15:2586–2594. doi: 10.1096/fj.01-0188com. [DOI] [PubMed] [Google Scholar]
- 106.Halestrap AP. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989;973:355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- 107.Akao M, O’Rourke B, Kusuoka H, Teshima Y, Jones SP, Marbán E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- 108.Eells JT, Henry MM, Gross GJ, Baker JE. Increased mitochondrial KATP channel activity during chronic myocardial hypoxia: is cardioprotection mediated by improved bioenergetics? Circ Res. 2000;87:915–921. doi: 10.1161/01.res.87.10.915. [DOI] [PubMed] [Google Scholar]
- 109.Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 110.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP-sensitive K+ channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 111.Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol (Lond) 1999;519(pt 2):347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho SA, Figueredo V, Schaefer S. Preconditioning limits mitochondrial Ca2+ during ischemia in rat hearts: role of KATP channels. Am J Physiol Heart Circ Physiol. 2001;280:H2321–H2328. doi: 10.1152/ajpheart.2001.280.5.H2321. [DOI] [PubMed] [Google Scholar]
- 113.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc Natl Acad Sci USA. 2002;99:3312–3317. doi: 10.1073/pnas.052713199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akao M, Ohler A, O’Rourke B, Marbán E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- 115.Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial KATP channel activation reduces anoxic injury by restoring mitochondrial membrane potential. Am J Physiol Heart Circ Physiol. 2001;281:H1295–H1303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- 116.Liu H, Zhang HY, McPherson BC, Baman T, Roth S, Shao Z, Zhu X, Yao Z. Role of opioid δ1 receptors, mitochondrial KATP channels, and protein kinase C during cardiocyte apoptosis. JMol Cell Cardiol. 2001;33:2007–2014. doi: 10.1006/jmcc.2001.1464. [DOI] [PubMed] [Google Scholar]
- 117.Akao M, Teshima Y, Marbán E. Antiapoptotic effect of nicorandil mediated by mitochondrial ATP-sensitive potassium channels in cultured cardiac myocytes. J Am Coll Cardiol. 2002;40:803–810. doi: 10.1016/s0735-1097(02)02007-7. [DOI] [PubMed] [Google Scholar]
- 118.Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 120.Akao M, O’Rourke B, Teshima Y, Seharaseyon J, Marbán E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- 121.Oldenburg O, Cohen MV, Downey JM. Mitochondrial KATP channels in preconditioning. J Mol Cell Cardiol. 2003;35:569–575. doi: 10.1016/s0022-2828(03)00115-9. [DOI] [PubMed] [Google Scholar]
- 122.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 123.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 124.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 125.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, Jadoon AK. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 126.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 128.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 130.Paliwal R, Costa G, Diwan JJ. Purification and patch clamp analysis of a 40-pS channel from rat liver mitochondria. Biochemistry. 1992;31:2223–2229. doi: 10.1021/bi00123a003. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, Ren G, O’Rourke B, Marbán E, Seharaseyon J. Pharmacological comparison of native mitochondrial KATP channels with molecularly defined surface KATP channels. Mol Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- 132.Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Commun. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- 133.Szewczyk A, Pikula S, Wojcik G, Nalecz MJ. Glibenclamide inhibits mitochondrial K+ and Na+ uniports induced by magnesium depletion. Int J Biochem Cell Biol. 1996;28:863–871. doi: 10.1016/1357-2725(96)00040-4. [DOI] [PubMed] [Google Scholar]
- 134.Szewczyk A, Wojcik G, Lobanov NA, Nalecz MJ. The mitochondrial sulfonylurea receptor: identification and characterization. Biochem Biophys Res Commun. 1997;230:611–615. doi: 10.1006/bbrc.1996.6023. [DOI] [PubMed] [Google Scholar]
- 135.Notsu T, Tanaka I, Mizota M, Yanagibashi K, Fukutake K. A cAMP-dependent protein kinase inhibitor modulates the blocking action of ATP and 5-hydroxydecanoate on the ATP-sensitive K+ channel. Life Sci. 1992;51:1851–1856. doi: 10.1016/0024-3205(92)90036-o. [DOI] [PubMed] [Google Scholar]