Abstract
Objectives
To evaluate long-term mortality effects of a home-based intervention previously shown to reduce functional difficulties, and whether survivorship benefits differed by risk level.
Design
Two-group randomized trial with survivorship followed up to 4 years from study entry.
Setting
Homes of urban community-living elders.
Participants
319 adults 70+ years with difficulties performing daily activities.
Intervention
Occupational and physical therapy sessions to instruct participants in compensatory strategies, home modifications, safety, fall recovery techniques, and balance and muscle strength exercises.
Measures
Survival time was number of days between baseline interview and date of death as determined by data from the National Death Index or December 31, 2005. Participants were stratified by baseline mortality risk (low, moderate, high) using a prognostic indicator.
Results
At two years, intervention participants (n=160) had a 5.6% mortality rate (n=9 deaths) and controls (n=159) a 13.2% rate (n=21 deaths; p=.020). Mortality rates remained relatively lower for intervention participants up to 3.5 years from study entry. At two years, intervention participants with moderate mortality risk had a 16.7% mortality rate (n=16 deaths/96) compared to 28.2% for equivalent control group participants (n=24 deaths/85; p=.021). By three years, mortality rates were not statistically significantly different between experimental and control groups.
Conclusions
The intervention extended survivorship up to 3.5 years, and maintained statistically significant differences for two years. Those at moderate mortality risk derived the most intervention benefit. Findings suggest that the intervention could be a low-cost clinical tool to delay both functional decline and mortality.
Keywords: Home care, frailty, survivorship, occupational therapy, physical therapy
INTRODUCTION
Functional disability due to age-related chronic or debilitating conditions is common among older adults and its prevalence is expected to increase as the population ages, particularly among the oldest old.1 As a marker of frailty, functional difficulties have significant negative consequences including social disengagement, increased fall risk, depression, reliance on personal assistance, relocation, and high service utilization and healthcare costs.2,3 Moreover, functional disability is predictive of mortality.4
Numerous interventions to address disability have been evaluated, with most studies showing small to no treatment effects.5,6 Studies examining mortality effects of interventions designed to reduce disability have been conducted mostly in countries other than the United States, focus on specific diseases or clinical populations, or report inconsistent results.7,8,9 Thus, the best treatment for minimizing disability, and whether this reduces mortality, is unclear.
We previously reported that a home-based occupational (OT) and physical therapy (PT) intervention (ABLE program), tested in a randomized controlled trial, reduced functional difficulties, fear of falling, and home hazards; and enhanced self-efficacy and use of control-oriented strategies (e.g., assistive devices, home modifications, energy conservation techniques).10,11 We also found that by 12 months, ABLE participants had a 1% mortality rate compared to a 10% mortality rate among control group participants with those hospitalized prior to study enrollment having a greater survivorship advantage (0% mortality rate among ABLE participants compared to 21% mortality rate for control group participants).12 These statistically significant differences in mortality rates at 12 months suggested the possibility of a survivorship effect beyond this initial study period and the importance of evaluating how long the intervention benefit lasted.
This follow-up study examined National Death Index (NDI) records to determine length of time a survivorship benefit was sustained by ABLE participants, and whether survivorship benefits differed by level of risk for mortality at time of study enrollment.13 As an exploratory study, there was no prediction concerning length of time of survivorship. However, based on our initial findings concerning the benefits conferred for participants hospitalized prior to study enrollment, we expected that ABLE would have the strongest impact on those entering the study with a moderate mortality risk level. Alternately, we expected less intervention benefit for those at high risk who, we reasoned, would require medical intervention beyond the scope of ABLE. We also anticipated that those at low mortality risk would enter the study with a survivorship advantage such that ABLE would have minimal impact on survivorship.
METHODS
Study Sample and Procedures
All study procedures including CONSORT flow chart, recruitment, and randomization are reported elsewhere.10 Briefly, participants were recruited from the community, ambulatory, 70 years or older, English-speaking, cognitively intact (Mini Mental State Examination [MMSE] score >24),14 and reported one or more functional difficulties. Participants were randomized to treatment (ABLE) or to a usual care control group. Baseline interviews were conducted from September 14, 2000 to July 14, 2003. NDI records were obtained for deaths up to December 31, 2005. Thus, follow-up ranged from 2½ to 5¼ years from study entry.
ABLE Intervention
As described elsewhere,10,11,12 ABLE compensated for declining abilities by introducing modifications to home environments and task performance. The active phase consisted of five OT contacts (four 90-minute visits and one brief telephone contact) and one 90-minute PT visit during the first six months. OTs worked with participants to identify and prioritize problem areas. For each identified targeted area, OTs observed and evaluated performance for safety, efficiency, difficulty level, environmental barriers and supports. OTs problem-solved with participants to refine personal goals, and identify behavioral and environmental contributors to performance difficulties. OTs then instructed elders in strategies tailored to needs, preferences, and environmental contingencies. Strategies included cognitive (e.g., reframing), behavioral (e.g., pace self), and environmental (e.g., grab bars) modifications. PTs provided balance and muscle strengthening exercises to support participation in targeted areas, fall recovery techniques to reduce fear of falling, and referrals for additional therapy if necessary.
The maintenance phase (from six to 12-months), consisted of three brief OT telephone calls to reinforce strategy use and help generalize use to newly identified problems. In a final home visit, OTs reviewed and reinforced strategies and obtained closure.
Control group participants did not receive intervention contact and at study completion (12-months), they received a home safety education booklet.
Measures
Background characteristics included age, gender, education (<high school, high school, >high school), living arrangement (alone, with others), race (White, African American, Other), and financial difficulty (0–4, higher scores representing greater difficulty).
Risk Groups
To categorize participants by mortality risk level, we used 11 of Lee et al.’s 12-item validated prognostic indicator and followed their scoring approach.15 One prognostic item, body mass (point=1 for high body mass), derived from weight and height information, was not included since these data were not collected in the trial. The 11-items and assigned risk points included age (70–74=3 points; 75–79=4 points; 80–84=5 points; ≥85=5); gender (male sex=2); comorbidities and health behavior (Diabetes mellitus=1; Cancer=2; Lung disease=2; Heart failure=2; Current smoker=2); and functional difficulties (Bathing=2; Managing finances=2; Walking several blocks=2; Pushing/pulling heavy objects=1). Higher scores indicated greater mortality risk.
A risk score (range =1–15) was calculated for each participant by adding points for each risk factor and then categorizing overall risk scores into three groups: low risk (I) scores (1–5), moderate risk (II) scores (6–9), and high risk (III) scores (≥10) based on Lee et al. Whereas Lee et al. had a fourth risk group (>14), only two individuals had these scores and thus we included them in risk group III. Average mortality risk scores were 4.1=low risk group, 7.8=moderate risk group; 11.3=high risk group; and 8.1 for the entire sample. Similar to the original 12-item index, we found that the 11-item version differentiated between risk groups. While our mortality risk scores were slightly higher for each risk group than Lee et al., this was expected given that our sample was older.
The three risk groups were compared on each risk factor and demographic characteristics using χ2 procedures. As in Lee et al., statistically significant differences emerged between risk groups in the expected direction, for each health and functional risk item, except for smoking. The high risk group had greater functional difficulties in all areas and a higher proportion of health conditions compared to moderate and low risk groups (p values=.001 to .003 for each item). Risk groups also differed by education (χ2=16.1, p=.003) and income (χ2=10.2, p=.037), with greater risk for those with <high school education and incomes <$20,000 per year.
As expected and similar to Lee et al., risk groups differed by mortality rate. At two years, the mortality rate among low risk participants was 5.9% (n = 3/51 deaths) compared to 8.3% (n=15/181 deaths) among moderate risk participants, and 13.8% (n=12/87 deaths) among those at highest mortality risk.
Data Analysis
The dependent variable, survival time, was defined as number of days between baseline interview (randomization date) and date of death or censoring. The only censoring was administrative; all participants’ follow-up was administratively censored at December 31, 2005, corresponding to the last date for which we have NDI information. The main effects of treatment and risk level were analyzed using standard statistical methods for censored data, with graphs of survival plotted using the Kaplan-Meier method. Statistical significance of treatment and risk level was determined by log rank tests. We estimated survival rates for treatment condition at 2, 3 and 4 years from date of study entry and risk level at 2 years from study entry. There was no censoring prior to two years of follow-up. Rates reported for time periods longer than two years were based on the Kaplan-Meier analyses. SPSS version 15.0 was used with significance level set at .05.
RESULTS
A detailed description of the study sample has been presented elsewhere.10,11 Briefly, at baseline, participants (N=319) were on average 79 years old, primarily female (81.8%), living alone (61.8%), and reporting low income. Close to one third of participants had < high school education, 53% were White and 46% were African American. At baseline, participants reported “some” to “a lot of” difficulty ambulating (Mean=2.5, ±SD=.8), carrying out self-care (Mean=1.8, ±SD=.6), and instrumental activities (Mean=2.1, ±SD=.6). Also at baseline, participants reported a mean of seven health conditions, most common being arthritis (84%), hypertension (71%), cataracts or macular degeneration (43%), cardiovascular problems (39%), and diabetes (23%). Furthermore, at baseline, 69.6% of participants rated their health as fair to poor and 51% indicated their health was not as good as one year ago. As previously reported, there were no large or statistically significant differences between treatment (n=160) and control group (n=159) participants on these background characteristics, health status indices, or primary outcome measures.10
Long-term Mortality Effects
Overall, as of the administrative cutoff (December 31, 2005), 76 (24%) of 319 participants died, of whom 42 (55%) were control group participants and 34 (45%) treatment participants. By four years from study entry, 74/319 individuals had died (40 control; 34 treatment). Two additional deaths occurred in the control group after four years but prior to the administrative cut off.
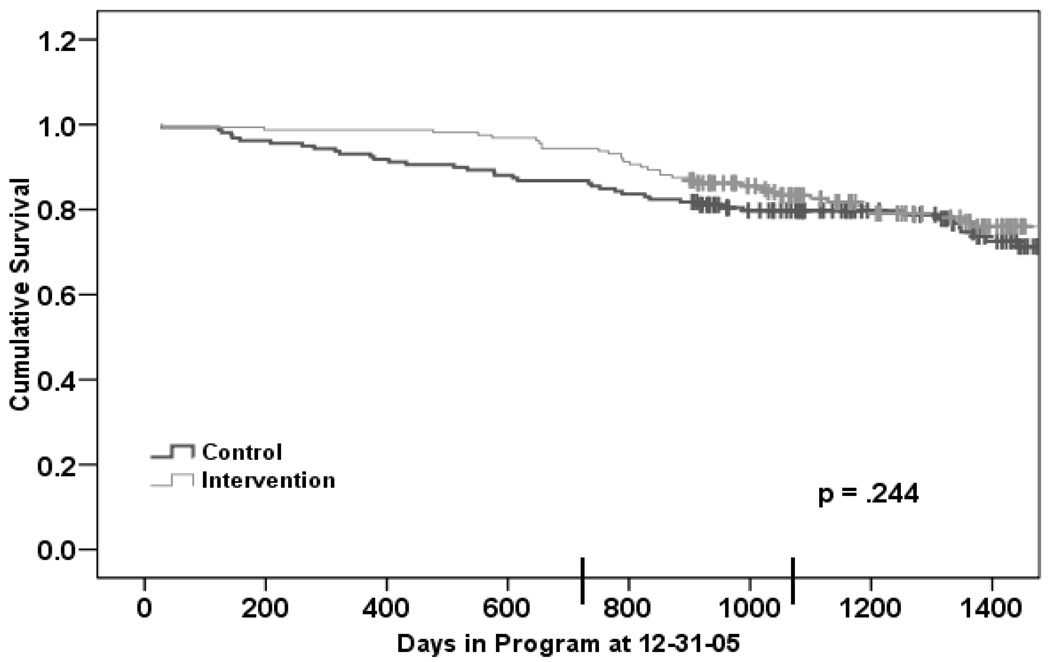
Figure 1 presents an estimate of a life table to four years from baseline interview for participants by treatment assignment without stratification by level of risk. As shown, the survival curves separate and then come together with an initial survival benefit for the treatment group remaining up to 3.5 years. However, the statistical significance of the comparison depends on the point at which the data are examined. At two years, the treatment condition had a substantial and statistically significant effect (by log-rank test) on survival (χ2=5.8, p=.016) such that only 5.6% (n=9/160) of intervention participants had died compared to 13.2% (n=21/159) of control participants. At three years, intervention participants exhibited a 16.6% Kaplan-Meier mortality rate and controls a 20.3% (p=.248); at four years, intervention participants exhibited a 24.0 % Kaplan-Meier rate and controls, a 28.7 % rate (p=.244).
Figure 1.
Estimates of survival by treatment group.
Note: Vertical lines on the survival curves show dates of censoring. The two vertical lines on the x-axis mark two and three years of follow-up.
The curves presented in Figure 1 suggest a postponement of some mortality in the intervention group, with a later catch-up period. To examine this further, we determined conditional six-month mortality rates for up to two years. These are rates of dying in the next six months among those alive at the start of that six-month period. The six-month mortality rate in the control group remained about 4% throughout the first two years of follow-up. In the intervention group, the mortality rate was near zero for one year and then began increasing. Prior to18 months, the six-month mortality rate was lower in the intervention group. Beginning at 18 months, the six-month mortality was higher in the intervention group (4% in intervention, versus 3% in control), corresponding to the point in Figure 1 where the curves begin to come together.
Treatment Benefits by Risk Groups
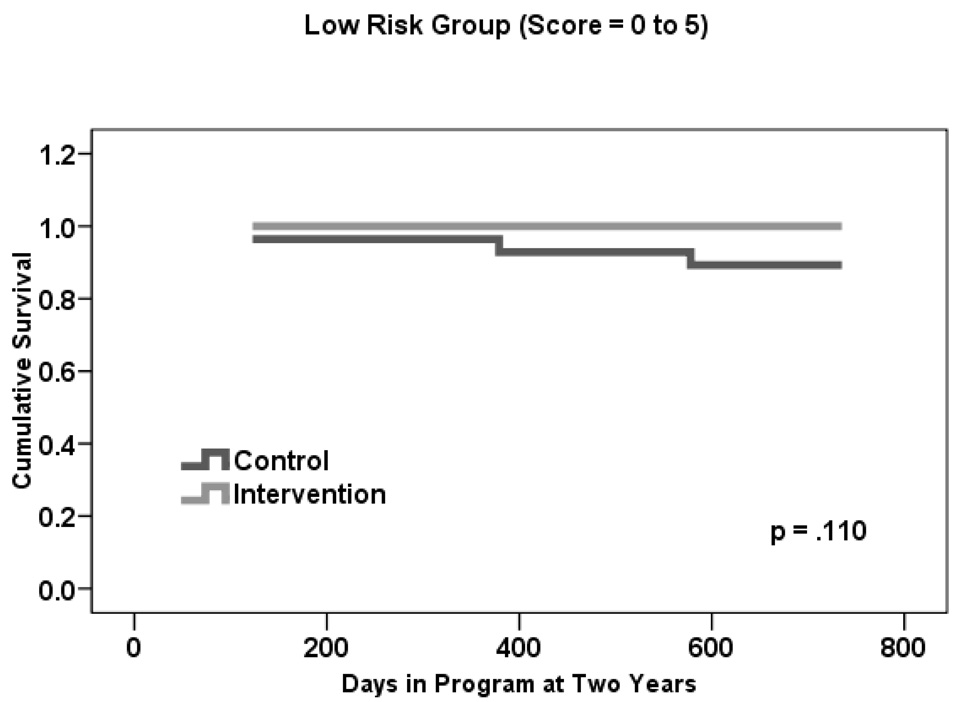
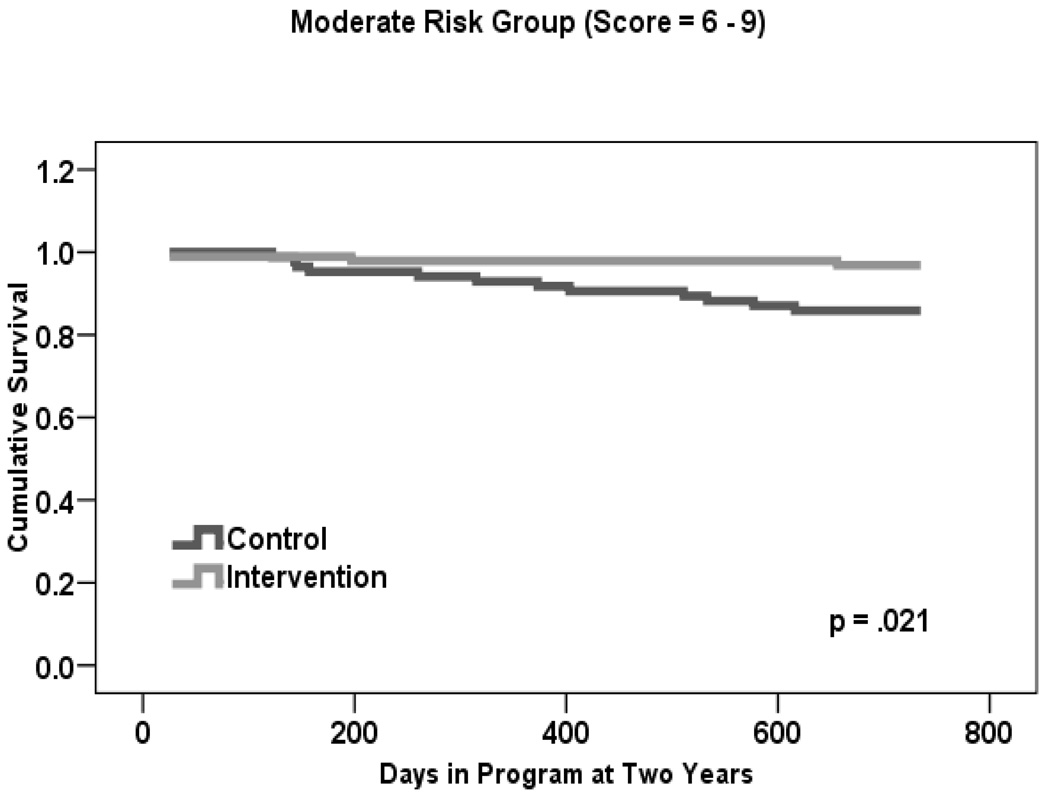
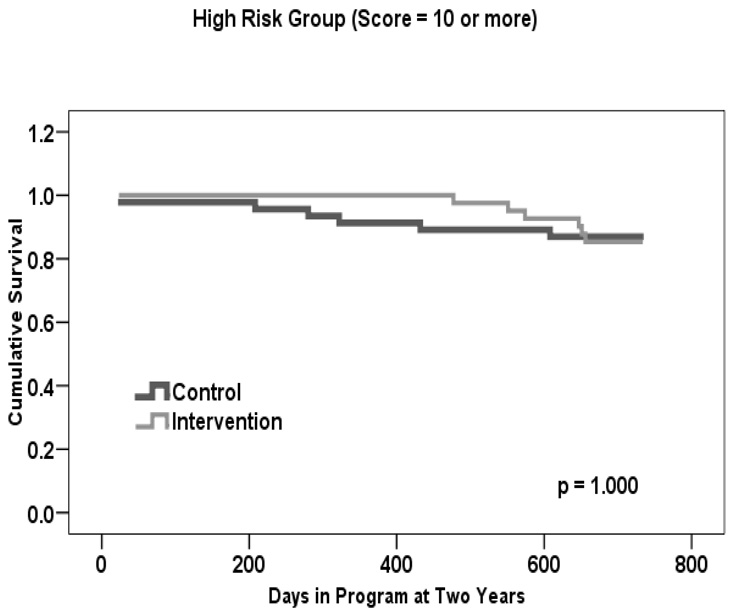
Given that a statistically significant difference in mortality rates was found at two versus three or four years, we used this time frame to examine whether there was a treatment effect within each risk group. Table 1 shows that the estimated two-year mortality rate for treatment participants was 0.0% in Group I, 3.0% in Group II, and 15.0% in Group III. This is in comparison to control participants, for whom rates were 11.0% in Group I, 14.0% in Group II, and 13.0% in Group III.
Table 1.
Two Year Mortality Rates by Risk Group (N = 319).
| Risk Group |
Risk Index Scorea |
Usual Care Groupb | Intervention Groupc | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Deaths | % | N | Deaths | % | N | Deaths | % | ||
| I | 0 to 5 | 28 | 3 | 11 | 23 | 0 | 0 | 51 | 3 | 5.9 |
| II | 6 to 9 | 85 | 12 | 14 | 96 | 3 | 3 | 181 | 15 | 8.3 |
| III | 10 or more | 46 | 6 | 13 | 41 | 6 | 15 | 87 | 12 | 13.8 |
Risk score is derived from Lee et al., 2006.
N = 159.
N = 160.
Figures 2a–c present two-year life table survival estimates for participants at each risk level stratified by treatment condition. The general trend for all three risk groups was similar to that of Figure 1, with survival curves separating for each level of risk and favoring the intervention group. By log rank test, treatment assignment had a statistically significant effect on survival for those participants who were at moderate risk of mortality, χ2 = 5.3, p=.021. However, no statistically significant differences were observed between groups at the lowest and highest levels of risk, though the magnitude of the difference in rates was similar in Group I to that of Group II.
Figure 2.
Estimates of survival by level of risk.
DISCUSSION
This study builds upon our previous findings that ABLE reduced functional difficulties and conferred a one year survivorship benefit. In this study, we show that mortality rates were statistically significantly lower for the intervention group compared to the control group for up to two years, with mortality rates remaining lower for ABLE participants for up to 3.5 years from study entry. The risk of dying within 2 years from study entry was close to 8 times higher in control group participants than ABLE participants.
The mortality benefit to two years was similar in low and moderate mortality risk groups, although only attaining statistical significance in the moderate group (the largest of the three groups in our sample). This may be partially due to the small sample size in the low risk group. For this moderate risk group, risk of dying within 2 years from study entry was close to 11 times higher in control group participants than in ABLE participants. The pattern was slightly different in the high risk group, in which there was an early benefit for ABLE participants. However, that benefit did not last to the two year point. Although ABLE participants at each level of mortality risk (low, moderate, high) derived some intervention benefit, intervention participants at moderate risk sustained a statistically significant two year survival advantage compared to their control-group counterparts.
These findings suggest that a relatively brief, nonpharmacologic intervention that helps older people use cognitive, behavioral and environmental strategies to reach self-identified functional goals has survivorship benefits that persist. The survivorship advantage extended well beyond ABLE’s six-month active phase of hands-on intensive skills-training.
Reasons for prolonged survivorship are unclear. One explanation may be ABLE’s preventive home safety and referral functions. Previous research has shown that health professional visits reduced mortality.7,9,16 Nevertheless, medical referrals were not typical in ABLE. Another explanation may be social attention, although it seems unlikely that approximately 10 professional contact hours explains survivorship extending 3.5 years from study entry.
A more plausible explanation is that ABLE offered strategies that helped participants achieve personal functional goals. As reported previously, ABLE participants reported greater use of control-oriented strategies than control group participants at 6 and 12-month follow-ups.10 The Life Span Theory of Control suggests that as impairments encroach on performance abilities, individuals experience heightened vulnerability to environmental complexities and threats to personal control.17 Threats to or actual loss of control have significant negative health consequences; alternately, enhancing control contributes to well-being and survivorship.18,19,20
How does enhancing control support survivorship? Control-oriented strategies enable continued engagement in everyday activities, which may have some physiological as well as psychosocial benefits. Although pathophysiologic mechanisms are unclear,21 our findings are consistent with recent evidence on the dynamic interaction between frailty and living environments.22
There are other competing explanations than the benefits conferred by ABLE. Findings may be a result of attrition bias or selective mortality in the intervention group such that the highest risk patients die early, leaving a remaining pool of lower risk patients. Mortality rates are higher in the higher risk group, so the baseline risk distribution of those remaining alive slowly moves towards the lower risk groups. Since there is little mortality in the intervention group early on, however, we do not see this effect in the intervention group in the early period and so this does not explain the increase in the 6-month rates in the intervention group. Also, as a randomized study, any attrition effect should be the same in both groups in the absence of an intervention effect. It appears that the intervention delays mortality in a subset but eventually medical issues take over.
A potential limitation is that the database does not allow for multivariate risk adjustments or control of clinical variables (e.g., comorbidities, health service utilization, hospitalizations). Also, survival analyses were unplanned and post-hoc. Nevertheless, these are minor limitations given that the original study was a randomized trial with no large or statistically significant differences between treatment and control groups on study outcomes or health variables.
Another issue is representativeness. Similar to population-based studies of older adults with similar ages and functional problems, we found a 10% mortality rate at two years for our study sample overall.4 This suggests that our sample may be representative of the larger population of elders living at home with performance difficulties. ABLE participants had a mortality rate of 5.6%, below the rate for the control group (13.2%) and those in representative studies (10%). Although not our main focus, in our sample, individuals of low income and education were at the highest risk of mortality. This association is consistent with population-based health disparities research, again suggesting the representativeness of our sample.23,24
Several clinical implications can be derived. Interventions addressing disability are not typically part of medical management. Evidence shows that those most in need, frail, post-hospitalized, vulnerable elders, receive inadequate follow-up care.25,26,27 ABLE may be most beneficial for risk groups.
Yet, older adults with no functional difficulties also report using accommodations to perform activities. Use of accommodations by this independent group has been shown to reflect a subclinical stage predictive of frailty.28 Thus, older adults with incipient functional difficulties may also benefit from ABLE as suggested by the slight intervention advantage afforded to the low risk mortality group.
Essential to ABLE is its client-centered focus, use of problem-solving, active engagement of older adults in problem-identification and strategy-generating processes, and tailoring of strategies to fit needs, cultural preferences and environments. These elements resonate with an emerging vision for geriatric care,29,30 which emphasizes that patient-centered care and symptom management be integrated with medical management and become standard practice.
Overall, ABLE demonstrated that teaching elders new approaches to performing valued activities resulted in additional years of life. Further research is necessary to substantiate mortality findings using a pre-planned, hypothesis-driven randomized trial. Equally important is determining why ABLE lost its benefit, whether different dose, intensity or boosters prolong benefit, and how to integrate ABLE into other proven interventions (e.g., physical activity) to extend positive effects. Future research should clarify physiological mechanisms by which survivorship benefits are conferred, effects of ABLE on health utilization, and cost and cost-effectiveness of this promising program.
ACKNOWLEDGMENTS
The research reported in this paper was supported by funds from the National Institute on Aging Grant #R01 AG13687. We gratefully thank the interventionist and interviewing staff and study participants for their time and responses.
Footnotes
Clinical Trial Registration: NCT00249925
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Federal Interagency Forum on Aging-Related Statistics. [Accessed April 15, 2008];Older Americans 2008: Key indicators of well-being. Available at: http://www.agingstats.gov/chartbook2000/OlderAmericans2000.pdf.
- 2.Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003;58(1):M70–M75. doi: 10.1093/gerona/58.1.m70. [DOI] [PubMed] [Google Scholar]
- 3.Spillman BC. Changes in elderly disability rates and the implications for health care utilization and cost. Milbank Q. 2004;82:157–194. doi: 10.1111/j.0887-378X.2004.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey EC, Covinsky KE, Lui LY, et al. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008;56:68–75. doi: 10.1111/j.1532-5415.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- 5.van Haastregt JC, Diederiks JP, van Rossum E, et al. Effects of preventive home visits to elderly people living in the community: Systematic review. BMJ. 2000;320:754–758. doi: 10.1136/bmj.320.7237.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: Systematic review and meta-regression analysis. JAMA. 2002;287:1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 7.Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: Systematic review and meta-analysis. BMJ. 2001;323:719–724. doi: 10.1136/bmj.323.7315.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, Vandenbroek AJ, Pearson S, et al. Prolonged beneficial effects of a home-based intervention on unplanned readmissions mortality among patients with congestive heart failure. Arch Intern Med. 1999;159:257–261. doi: 10.1001/archinte.159.3.257. [DOI] [PubMed] [Google Scholar]
- 9.Sahlen KG, Dahlgren L, Hellner BM, et al. Preventive home visits postpone mortality – a controlled trial with time-limited results. BMC Public Health. 2006;6:220. doi: 10.1186/1471-2458-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitlin LN, Winter L, Dennis MP, et al. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc. 2006;54:809–816. doi: 10.1111/j.1532-5415.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin LN, Winter L, Dennis MP, et al. Variation in response to a home intervention to support daily function by age, race, sex, and education. J Gerontol A Biol Sci Med Sci. 2008;63A:745–750. doi: 10.1093/gerona/63.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gitlin LN, Hauck WW, Winter L, et al. Effect of an in-home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: Preliminary findings. J Am Geriatr Soc. 2006;54:950–955. doi: 10.1111/j.1532-5415.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Department of Health and Human Services, Health Statistics. 2007 [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 16.Petersson I, Lilja M, Hammel J, et al. Impact of home modification services on ability in everyday life for people ageing with disabilities. J Rehabil Med. 2008;40:253–260. doi: 10.2340/16501977-0160. [DOI] [PubMed] [Google Scholar]
- 17.Heckhausen J, Schulz R. A life-span theory of control. Psychol Rev. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- 18.Yates LB, Djoussé L, Kurth T, et al. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168:284–290. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 19.Wrosch C, Schulz R, Heckhausen J. Health stresses and depressive symptomatology in the elderly: The importance of health engagement control strategies. Health Psychol. 2002;21:340–348. doi: 10.1037//0278-6133.21.4.340. [DOI] [PubMed] [Google Scholar]
- 20.Gitlin LN, Hauck WW, Dennis M, et al. Depressive symptoms in African American and White older adults with functional difficulty: The role of control strategies. J Am Geriatr Soc. 2007;55:1023–1030. doi: 10.1111/j.1532-5415.2007.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 22.Armi F, Guilley E, Lalive d'Epinay CJ. Health support provided and received in advanced old age: A five-year follow-up. Z Gerontol Geriatr. 2008;41:56–62. doi: 10.1007/s00391-007-0457-z. [DOI] [PubMed] [Google Scholar]
- 23.Bosma H, Dike van de Mheen H, Borsboom G, et al. Neighborhood socioeconomic status and all-cause mortality. Am J Epidemiol. 2001;153:363–371. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 24.Jagger C, Matthews R, Melzer D, et al. Educational differences in the dynamics of disability incidence, recovery and mortality: Findings from the MRC Cognitive Function and Ageing Study. Int J Epidemiol. 2007;36:358–365. doi: 10.1093/ije/dyl307. [DOI] [PubMed] [Google Scholar]
- 25.Hortobagyi T, Mizelle C, Beam S, et al. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 26.Chodosh J, Solomon DH, Roth CP, et al. The Quality of Medical Care Provided to Vulnerable Older Patients with Chronic Pain. J Am Geriatr Soc. 2004;52:756–761. doi: 10.1111/j.1532-5415.2004.52214.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Rantz M. Correlates of post-hospital physical function at 1 year in skilled nursing facility residents. [Accessed April 16, 2008];J Adv Nurs. 2008 doi: 10.1111/j.1365-2648.2008.04612.x. Available at: http://www.blackwell-synergy.com/action/showPdf?submitPDF=Full+Text+PDF+%2876+KB%29&doi=10.1111%2Fj.1365-2648.2008.04612.x. [DOI] [PubMed]
- 28.Wolinsky FD, Miller DK, Andresen EM, et al. Effect of subclinical status in functional limitation and disability on adverse health outcomes 3 years later. J Gerontol A Biol Sci Med Sci. 2007;62:101–106. doi: 10.1093/gerona/62.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Reuben DB. Better care for older people with chronic diseases: An emerging vision. JAMA. 2007;298:2673–2674. doi: 10.1001/jama.298.22.2673. [DOI] [PubMed] [Google Scholar]
- 30.Wagner EH, Austin BT, Davis C, et al. Improving chronic illness care: Translating evidence into action. Health Aff. 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]