Abstract
Previous studies show that intravascular injection of human bone marrow stromal cells (hBMSCs) significantly improves neurological functional recovery in a rat model of intracerebral hemorrhage (ICH). In the present study, we tested the hypothesis that mannitol improves the efficiency of intra-arterial MSC delivery (i.e., fewer injected cells required for therapeutic efficacy) after ICH. There were four post-ICH groups (N=9): group 1, negative control with only intra-arterial injection of 1 million human fibroblasts in phosphate-buffered saline (PBS); group 2, intravenous injection of mannitol alone in PBS (1.5 g/kg); group 3, intra-arterial injection of 1 million hBMSCs alone in PBS; and group 4, intravenous injection of mannitol (1.5 g/kg) in PBS followed by intra-arterial injection of 1 million hBMSCs in PBS. Group 4 exhibited significantly improved neurological functional outcome as assessed by neurological severity score (NSS) and corner test scores. Immunohistochemical staining of group 4 suggested increased synaptogenesis, proliferating immature neurons, and neuronal migration. The number of hBMSCs recruited to the injured region increased strikingly in group 4. Tissue loss was notably reduced in group 4. In summary, the beneficial effects of intra-arterial infusion of MSCs are amplified with intravenous injection of mannitol. Preadministration of mannitol significantly increases the number of hBMSCs located in the ICH region, improves histochemical parameters of neural regeneration, and reduces the anatomical and pathological consequences of ICH.
Keywords: bone marrow stromal cell, neural regeneration, intracerebral hemorrhage, stroke
1. Introduction
Much attention has turned to neurorestorative treatments to improve recovery after various forms of brain injury. One major experimental approach is to explore undifferentiated pluripotent stem cells in improving neurological outcomes following experimental neurological injury, including ischemic stroke, head injury, and spinal cord injury (Eglitis et al., 1999, Chopp et al., 2000, Mahmood et al., 2003). Bone marrow stem cells (MSCs) can serve as precursors of nonhematopoietic tissue and have a capacity for self-renewal and differentiation in a number of nonhematologic tissues, thereby serving as a potential medium for cell therapy (Prockop, 1997). MSCs have been shown to pass through the blood-brain interface to target sites of brain lesions under experimental conditions (Li et al., 2001, Zhang et al., 2002, Mahmood et al., 2003). MSCs have shown the capacity to differentiate into neurons and astrocytes and have the ability to preferentially migrate to damaged cortex (Kopen et al., 1999). Of particular significance is the ability of MSCs to secrete or stimulate secretion of growth factors which create a local environment conducive for neuroregeneration (Villars et al., 2000).
In numerous reports, animals treated with human MSCs have shown significant improvement after ischemic stroke and traumatic brain injury (TBI) (Li et al., 2000, Li et al., 2001, Li et al., 2002, Mahmood et al., 2003, Lu et al., 2004). We recently demonstrated the beneficial effects of human bone marrow stromal cell (hBMSC) infusion in rats subjected to intracerebral hemorrhage (ICH), as evidenced by reduced tissue loss, mitotic activity, immature neuron formation, synaptogenesis, and neuronal migration (Seyfried et al., 2006). Obtaining the maximum therapeutic delivery of the hBMSCs using the lowest injection concentration is a clinically relevant concern. We hypothesize that high-dose mannitol may beneficially influence hBMSC administration and the migration of cells. In this study we measure the effects of intraarterial administration of hBMSCs coupled with prior intravenous injection of high-dose mannitol on neurological outcome and cellular recovery following experimental ICH.
2. Results
All animals survived the 2-week experimental period. The left panel of Figure 1 demonstrates that at day 1 after ICH, no apparent difference was implied among all four groups using NSS and corner turn tests. At the end of the first week, neurological functional outcome measured by NSS started to show statistically significant improvement for the combination group. At the end of the second week after the ictus, the combination group continuously exhibited significant neurological functional improvement as assessed by both NSS and the corner turn test (Figure 1). Neither the mannitol group nor the hBMSC group showed any significant neurological improvement when compared to the control group.
Fig. 1.
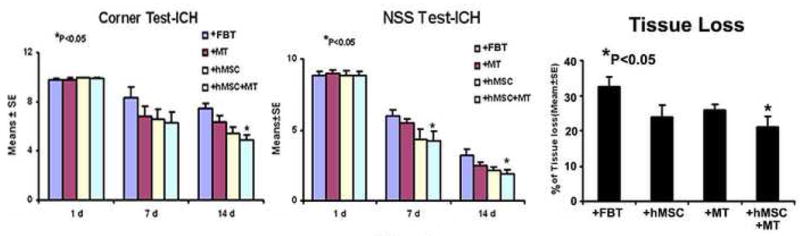
Neurological functional tests and striatal tissue loss percentage of the injured side. Quantitative bar graph results of NSS (left panel) and corner turn test (middle panel) of four groups (control, FB; mannitol, MT; hBMSC; combination treatment, hBMSC+MT) are presented in the left and middle panels. Bar graphs of quantitative striatal tissue loss percentages in the ICH region relative to the contralateral normal region of four groups are shown in right panel. Statistical significance level is: *P<0.05.
The percentage of striatal tissue loss on the side of hemorrhage is presented in the right panel of Figure 1. The area of tissue loss as a percentage of the normal hemisphere was calculated as follows: control, 32.4 ± 2.8%; mannitol, 25.9 ± 1.75% (P>0.05); 1 million hBMSCs, 24 ± 3.4% (P>0.05); and combination treatment, 21 ± 3.2% (P<0.01). Therefore, the percentage of striatal tissue loss was significantly reduced in the combination group when compared to the control group. A trend of improvement was also observed when either reagent was used alone, but was not statistically significant. Figure 3 also shows that the combination group reduces the tissue loss. Average body weights at the beginning of the experiment were similar (control, 296.4 ± 34.1 g); mannitol, 291.2 ± 35.2 g, P>0.1; 1 million hBMSCs, 297.7 ± 36.3 g, P>0.1; combination treatment, 296.9 ± 37.1, P>0.1), and those after 2 weeks were not significantly different (319.2 ± 42.3 g, 316.6 ± 45.7 g, P>0.1; 321.9 ± 52.7 g, P>0.1; 325.1 ± 47.4 g, P>0.1), respectively.
Fig. 3.
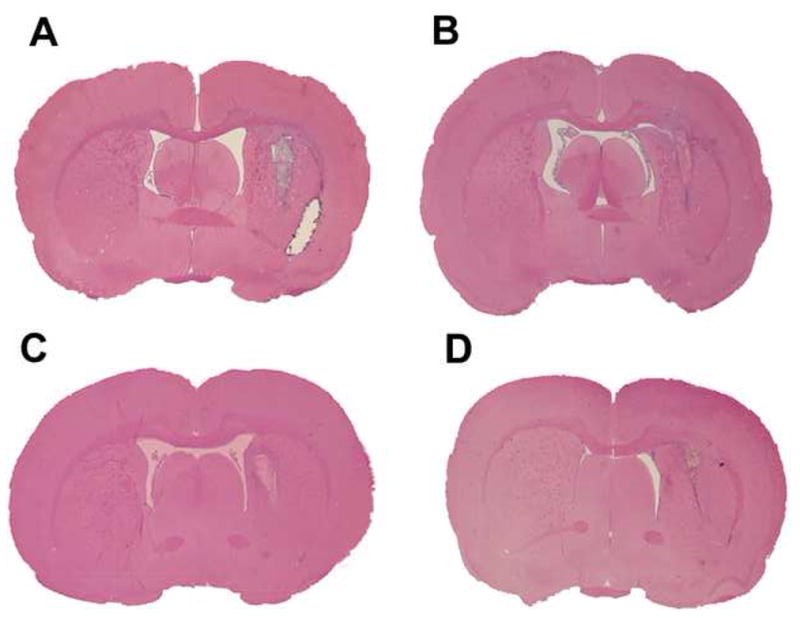
H&E staining showing representative sections of fibroblast control group (A), mannitol group (B), hBMSC group (C) and the combination group (D).
As shown on the upper panel of Figure 4, immunohistochemistry using mAb1281 revealed substantially more positive-staining cells (216±16, P<0.05) in the injured region of the combination group than in the injured region of the hBMSC group (157±21), suggesting that mannitol effectively potentiates hBMSCs to migrate to the injured site. Further immunostaining against BrdU illustrated that there are significantly more BrdU-positive staining cells in the boundary zone (P<0.05) around the injured site of the combination group than the control group, suggesting that mannitol and hBMSCs may act synergistically to promote cells to proliferate and migrate to the injured region. One intriguing observation is that mannitol alone significantly increases the BrdU labeling significantly in the vicinity of the injury (P<0.05), suggesting that mannitol may contain mitogenic activity to initiate DNA replication.
Fig. 4.
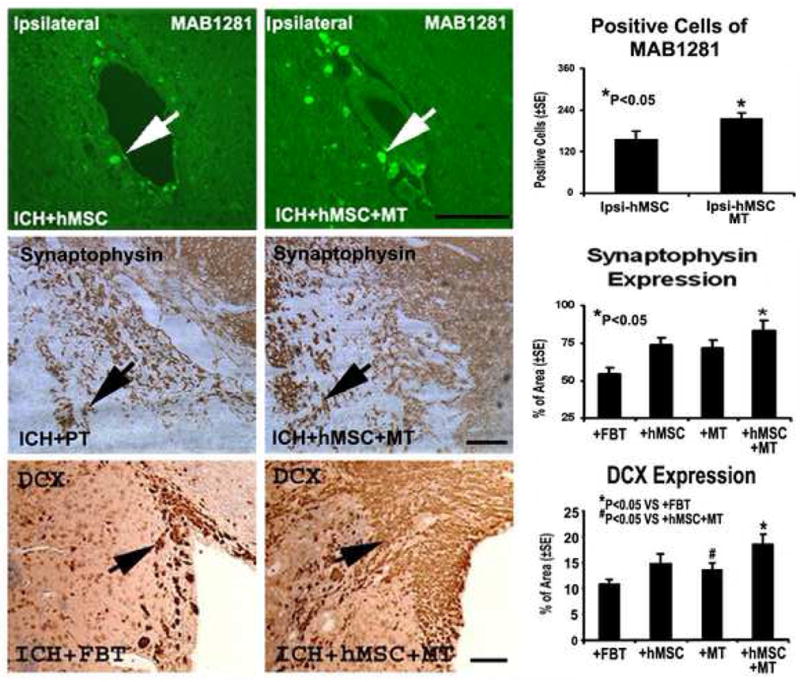
Immunostaining of the control and combination treatment sections showing the quantitative immunoreactivities of mAb 1281, synaptophysin, and DCX. The other two groups are not shown. Note that a higher number of cells per area are stained to mAb 1281, synaptophysin and DCX. Quantitative immunoreactivities for all four groups are presented as bar graphs on the right side of each panel. Scale bar = 50 um.
As demonstrated in Figures 4 and 5, immunohistochemical staining also showed significantly increased expression of synaptophysin, TUJ1 and DCX (P<0.05) in the combination group compared to the control group. Co-immunostaining with both BrdU and TUJ1 was also performed to detect any newly formed immature neurons. Double staining for BrdU and TUJ1 revealed a subpopulation of cells that express a neuronal marker while still dividing, suggesting that the cells positive for immature neuron marker are newly formed during the recovery stage in the combination group.
Fig. 5.
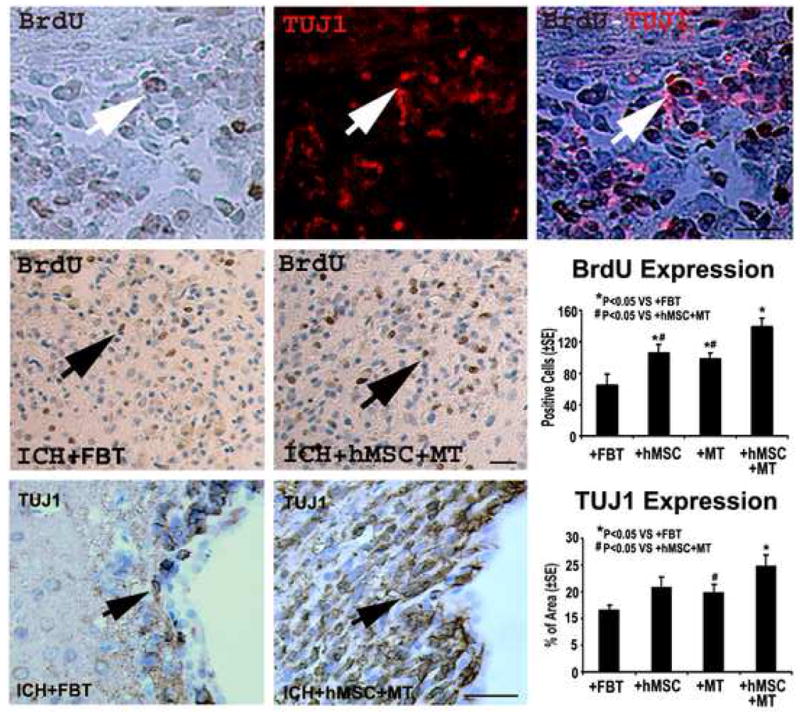
Immunostaining of the control and combination treatment sections showing the quantitative immunoreactivities of BrdU and TUJ1. Quantitative immunoreactivities for all four groups are presented as bar graphs on the right side of each panel. Colocalization of BrdU and TUJ1 in a subpopulation of cells near the injured region of the combination group is shown on the bottom panel. Arrows indicate cells positively stained for both BrdU and TUJ1. Scale bar = 50 um.
3. Discussion
The potential use of MSCs for brain repair and regeneration has been reported using different animal models of injury (Kopen et al., 1999, Chopp et al., 2000, Li et al., 2000, Li et al., 2001, Li et al., 2002, Mahmood et al., 2003). Cell therapy induces neurorestorative changes in the brain reflective of several mechanisms of action; however, the ability of MSCs to localize to a region of brain injury and increase local concentrations of growth factors such as BDNF and VEGF may be paramount (Villars et al., 2000, Li et al., 2002, Lu et al., 2004). Various cell concentrations and routes of administration have been applied to demonstrate the therapeutic efficacy of MSC treatment of neural injury (Li et al., 2002). Investigation using the MCA occlusion model demonstrated that 1 million hBMSCs administered intravenously failed to show significant benefit in animal recovery, whereas 2 million cells injected intraarterially improved neurological function (Chen et al., 2001a). Previous work in experimental TBI using intraarterial cell therapy, provided a direct route of administration, but resulted in increased cerebral ischemia, likely due to small vessel thrombosis by the cells (Lu et al., 2001). The application of MSCs to treat experimental ICH has been studied less extensively than its use in treating ischemic stroke and TBI. Four doses of 2 million mesenchymal stem cells delivered through the carotid artery to treat collagenase-induced ICH, improved motor function in rats (Zhang et al., 2006). In this study we demonstrate that intravenous mannitol increases the effectiveness of low-dose intraarterial hBMSCs as evident by improved neurological functional recovery in an injection model of ICH. Tissue loss was also significantly reduced in the mannitol/hBMSC group compared to hBMSCs alone. Accordingly, immunostaining for BrdU and neuronal markers illustrated significantly more newly generated cells expressing immature neuronal marker and enhanced neuronal migration in the perilesional region of the combination treatment group.
As an experimental model, the direct injection of autologous blood into the striatum in the rat has proven reliable for studying the pathophysiological changes occurring after spontaneous ICH (Seyfried et al., 2004). In the ICH model of injury we have demonstrated that MSCs localize around the ICH and that features of active neuroregeneration are present after intravenous administration of 3–8 million cells (Seyfried et al., 2006). Maximizing the effectiveness of cell therapy is of concern clinically, particularly if autologous donation is considered (Kopen et al., 1999, Chopp et al., 2000). From a theoretical perspective the intraarterial route for hBMSC delivery is appealing, because the cells could be delivered directly to the vascular territory of the affected tissue. From the clinical standpoint the intraarterial route is not completely excluded since it is used extensively with other therapies, including chemotherapy, embolization of tumors and arteriovenous malformations, and endovascular treatment of intracranial arterial stenosis or acute thrombotic occlusion (Loew and Papavero, 1988, Ueda et al., 1998). When considering intraarterial delivery of MSCs after ICH, two major issues need to be addressed: 1) possible arterial occlusion in the already compromised microvasculature of the perihematomal region caused by the cell mixture, and 2) aggravating brain edema caused by the space-occupying hematoma. To address these issues we explored the potential advantage of adding mannitol to the treatment protocol, and to our knowledge, this is the first description of its use with hBMSCs after experimental ICH.
Mannitol is a sugar alcohol and an osmolyte and has been used to prevent or treat medical conditions that are caused by an increase in body fluids/water (Winkler and Munoz-Ruiz, 1995). As an adjunctive treatment, mannitol has been frequently used to decrease edema or intracranial pressure with massive brain lesions (McGraw and Howard, 1983, Schwarz et al., 1998). Mannitol has also been used to open the blood-brain barrier (BBB) by temporarily shrinking the tightly coupled endothelial cells that make up the barrier, thus allowing for drugs delivered directly to the brain (Kroll and Neuwelt, 1998). Another suggested mechanism of mannitol’s effect on cerebral vasculature is increased endothelial permeability and small vessel dilation (Machi et al., 1996). Rheology of cerebral blood flow can be improved by mannitol, as it lowers the viscosity and allows better capillary flow (Burke et al., 1981). Although it is still somewhat controversial, high doses of mannitol have been reported to treat acute TBI and reduce elevated intracranial pressure (Cruz et al., 2001, Wakai et al., 2005). Our previous study indicates that the number of hBMSCs that were recruited to the injured site reached a plateau after intravenous infusion of 3 to 8 million hBMSCs, suggesting that the BBB may be one of the rate-limiting steps in hBMSCs reaching the injured site (Seyfried et al., 2006). hBMSCs delivered by intraarterial infusion, although anatomically following a direct route, still need to pass through the BBB before they can successfully target and migrate to the injured region. Mannitol, by its known effects on the BBB, could allow easier passage of the MSCs to the damaged region. The current study suggests that mannitol facilitates the migration of hBMSCs into the perihematomal region as evidenced by the increased immunostaining pattern of mAb1281 after mannitol and hBMSCs compared to hBMSCs alone.
In addition to its effects on BBB permeability, mannitol may improve the results of MSC treatment of ICH by other mechanisms. High doses of mannitol have been reported to improve clinical outcome for acute TBI, and this effect would not be expected to be secondary to increased BBB permeability (Schierhout and Roberts, 2000). The basis behind this phenomenon is most likely related to mannitol’s osmotic and rheologic properties and its potential mitotic activity, which was an unexpected observation in this study (Paczynski et al., 1997). In our study, mannitol alone increased new cell formation around the ICH. Mannitol can prevent the swelling of cells and alleviate the subsequent cell damage at the vicinity of the injured site (Tranum-Jensen et al., 1981). It is therefore a possibility that mannitol may enhance tissue tolerance to acute stress after injury and that mannitol may salvage more viable cells at the vicinity of the injury site (Lizasoain et al., 2006). In this way, mannitol may attenuate the stroke shock and therefore prolong the survival time window of injured tissues (Mulhern et al., 2007).
Other osmolytes are being pursued in a number of disease situations including cerebral ischemia. One particular instance is the endoplasmic reticulum stress-related chaperones, such as 4-phenyl butyric acid and tauroursodeoxycholic acid (Mulhern et al., 2007). These small chemicals apparently attenuate the endoplasmic reticulum stress, one of the universal inducers of cell death. Recent pre-clinical studies support the possibility of using these small chemicals to treat or delay cell injury (Rodrigues et al., 2002). However, to our knowledge, mannitol, which is similar to tauroursodeoxycholic acid, has not been shown to be a classic chaperone (Scumpia et al., 2007). Additional investigation is necessary to see if mannitol can delay acute neuronal necrosis and subsequent neuronal apoptosis during brain ischemia or ICH.
In the combination therapy group, mannitol followed by intraarterial hBMSC proved to be safe and effective in the ICH model in the rat: there was no premature mortality and there was significant functional benefit only when hBMSCs were preceded by mannitol. The number of hBMSCs reaching the area of injury was significantly increased by mannitol infusion, suggesting that there was improved delivery through the microvasculature and/or better BBB penetration. The amount of encephalomalacia, or tissue loss, was reduced only when the animals received mannitol and hBMSCs, and not from the intraarterial hBMSCs alone. While intraarterial hBMSCs have been effective in models of cerebral ischemia, it is likely that the benefit of mannitol in the ICH model becomes more apparent because of the differing nature of the inherent pathophysiology of a blood clot. A parenchymal hematoma creates an acute mass effect, compresses surrounding small vessels, and secretes vasogenic blood breakdown products; mannitol may counteract these effects while simultaneously improving cell delivery (Boulard et al., 2003). That mannitol is potentially an effective aid to the process of neurorestoration after ICH, is supported by the increased presence of BrdU-positive cells in the region of the ICH compared to controls. Even though no improvement of neurological outcome was noted in groups 2 and 3, either mannitol or a low dose of hBMSCs alone can significantly elicit cells to proliferate at the injured site. This suggests that there is true synergy in the combination treatment of hBMSCs with mannitol, which results in significant neurological recovery in this ICH model.
In summary, low-dose intraarterial hBMSCs are a safe and effective treatment for ICH in the experimental model when they are preceded by an intravenous mannitol bolus. This study suggests that the combination therapy of mannitol and hBMSCs is more effective than either therapy alone when given intraarterially to treat ICH.
4. Experimental Procedures
4.1 Animals and Reagents
Adult male Wistar rats were purchased from the Jackson Laboratory. All animal studies have been carried out under the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System. Human primary fibroblasts were from American Type Culture Collection (Manassas, VA). Mannitol and 5′-bromo-2′ deoxyuridine (BrdU) were obtained from Sigma (St. Louis, MO). The following primary antibodies were used: monoclonal antibody against BrdU (1:100 Dako, Carpenteria, CA); synaptophysin (1:1,000 mAb, Clone SY 38; Chemicon, Temecula, CA); doublecortin (DCX) (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), tubulin isotype III (TUJ1) (1:5,000 mAb; Covance, Berkeley, CA) and anti-Nuclei (1:500; specific for all human cell types; Chemicon, Temecula, CA).
4.2 Isolation and culture expansion of hBMSCs
hBMSCs were provided by Cognate BioServices, Inc. (Baltimore, MD). HBMSCs at P4 that are CD34 and CD45 negative and greater than 90% CD90, CD105 and CD13 positive were used in the experiments. Human bone marrow was diluted with PBS and mixed with Hespan. The nucleated cells in the supernatant layer were collected, washed and counted. Nucleated cells were cultured in polysterene vessels in DME low glucose, with 10% FBS. Adherent, spindle-shaped cells were passaged using trypsin. Cells were re-plated and kept in culture for one week, with one media change for every subsequent passage. Cells were harvested and cryopreserved in appropriate dose-related aliquots in Plasma-Lyte containing human serum albumin and dimethyl sulfoxide. Cells were tested for purity at the end of each passage by flow cytometry.
4.3 Animal surgical procedures of intravenous hemorrhage, intravenous infusion of mannitol and intraarterial infusion of hBMSCs
Thirty-six male Wistar rats weighing 270 to 320 g were used for ICH studies. ICH was induced by injecting 100 μl of autologous blood as previously described (Seyfried et al., 2006). Briefly, the rat under general anesthesia was placed in a stereotactic frame (David Kopf Instruments, Tujunga, CA). A 1-mm craniectomy was performed and a stereotactically guided needle was placed into the right striatum (3.5 mm lateral to midline, 0.5 mm anterior to bregma, depth 5.5 mm below the surface to midline). The autologous blood was taken from the femoral artery at a steady infusion rate of 10 μl per minute with an infusion pump (600–910/920, Harvard Apparatus, Holliston, MA) (Seyfried et al., 2004). The weight of each rat was recorded daily for 2 weeks.
Twenty-four hours after ICH, the animals were divided into four experimental groups. Group 1 received only intraarterial (via internal carotid artery) injection of 1 million human primary fibroblasts in phosphate-buffered saline (PBS) as a control. Group 2 received intravenous injection of mannitol at a dose of 1.5 g/kg in PBS via the tail vein. Group 3 received intraarterial (i.e., internal carotid artery) injection of 1 million hBMSCs in PBS. Group 4 received intravenous injection of mannitol at a dose of 1.5 g/kg followed 10 minutes later by intraarterial injection of 1 million hBMSCs in PBS. In a previous study, treatment of stroke in rat with fibroblasts provided no functional benefit and immunostaining compared with PBS treated rat (Li et al., 2002). Therefore, the present study uses fibroblast as the control treatment. All treatments were administered 24 h after induction of ICH. All rats also received a daily injection of 100 mg/kg BrdU intraperitoneally starting at day 1 after ICH for 14 days.
hBMSCs and human primary fibroblasts obtained from the companies were used directly without subculture. The rats were anesthetized and a modified polyethelene catheter (PE-50) was advanced through a small puncture in the external carotid artery into the lumen of internal carotid artery for a distance of ~15mm. The vial containing the transplanted cells was thawed quickly in a 37 °C water bath. After washing and resuspending in PBS, the cell number was counted. Only the vial with more than 85% viability will be used. Approximately 1 × 106 cells in 100 ul PBS was slowly injected over a 5-min period into each rat through the internal carotid artery.
4.4 Neurological functional tests
Functional outcomes were measured by neurological severity score (NSS)(Chen et al., 2001b) and corner turn testing score (Zhang et al., 2002), as described previously (Seyfried et al., 2006). The mNSS is a composite of motor (i.e., muscle status and abnormal movement), sensory (i.e., visual, tactile, and proprioceptive), beam balance, and reflex tests. The total score has 14 points. The higher the score is, the more severe the injury. The corner turn test was developed for measuring long-term functional recovery in the rat. An uninjured rat randomly turns left or right, whereas an injured rat preferentially turns toward the unimpaired ipsilateral (left) side. We recorded the number of right turns from 10 trials for each test and used the results for statistical analysis.
4.5 Histology and immunohistochemistry
At 14 days postoperatively, the animals were anesthetized and perfused transcardially with PBS followed by 4% paraformaldehyde. Brain tissues were excised, fixed in formalin and sliced into 2-mm thick blocks. Each block was embedded in paraffin. Every 40th coronal section was cut at a thickness of 6 μm between +0.1–0.86 mm of the bregma from each rat for a total of six blocks. These sections were used for H & E and immunochemical staining. Percentage of the striatal tissue loss compared to the contralateral striatum was calculated using an image analysis system (Data Translation, Marlboro, MA).
The brain tissue residing between +0.1–0.86 mm of the bregma on the third block was the most severely injured and therefore the third block was specifically selected for immunostaining. Every 40th coronal section from +0.1–0.86 mm of the bregma was used for immunochemical staining with the same antibody. Sections were blocked in a Tris-buffered saline containing 5% normal goat serum, 1% BSA and 0.05% Tween-20. Sections were then incubated with the primary antibodies for localization of BrdU (1:100; a marker for proliferation cells), TUJ1 (1:5,000; a marker for immature neurons), DCX (1:50; a marker for migrating neuroblasts) (Feng and Walsh, 2001), Synaptophysin (1:1000; a marker for presynapic plasticity and synaptogenesis) and mAb 1281 (1:500; a marker specific for human nuclei) (Mahmood et al., 2003). After sequential incubation with biotinylated IgG, the sections were treated with an avidin-biotin peroxidase system (ABC Kit, Vector Laboratories, CA). DAB was then used as a sensitive chromogen for light microscopy. The slides stained for anti-Nuclei were visualized with FITC-conjugated secondary antibody. Double staining was performed to determine the BrdU-reactive cells expressing TUJ1. The sections were stained by DAB with anti-BrdU mouse monoclonal antibody, which was then followed by an hour of blocking. After a brief washing, the sections were incubated with polyclonal rabbit anti-TUJ1 overnight at 4 °C and subsequently with anti-rabbit Cy5 for 30 min. All immunostainings were performed at the same time with two negative controls (i.e., the omission of primary antibody and the use of pre-immune serum) for quality control of the immunoassay procedure.
Figure 2 describes a representative ICH coronal section illustrating three regions (i.e., damaged striatum core, boundary zone and subventricular zone [SVZ]). For semiquantitative measurements of synaptophysin, TUJ1 and DCX, a series of six slides at various levels from the same block were used. Synaptophysin was measured in the striatal region. TUJ1 and DCX were measured at the SVZ. Synaptophysin, TUJ1 and DCX were digitized under a 20X objective lens (Olympus BX40; Olympus Optical Co, Tokyo, Japan) by using a 3D-CCD color video camera (model DXC-970MD; Sony Corp, Tokyo, Japan) interfaced with the MCID image analysis system (Imaging Research, Inc., St. Catharine’s, ON, Canada). For synaptophysin, TUJ1 and DCX, data are presented as a percentage of the immunopositive areas in each field divided by the total areas in the field (628 × 480 μm2) (Chen et al., 2003). The number of BrdU-positive cells was counted in the boundary around the lesion. mAb 1281 quantitative data were presented as the total number of immunoreactive cells within the ipsilateral areas of each slide.
Fig. 2.
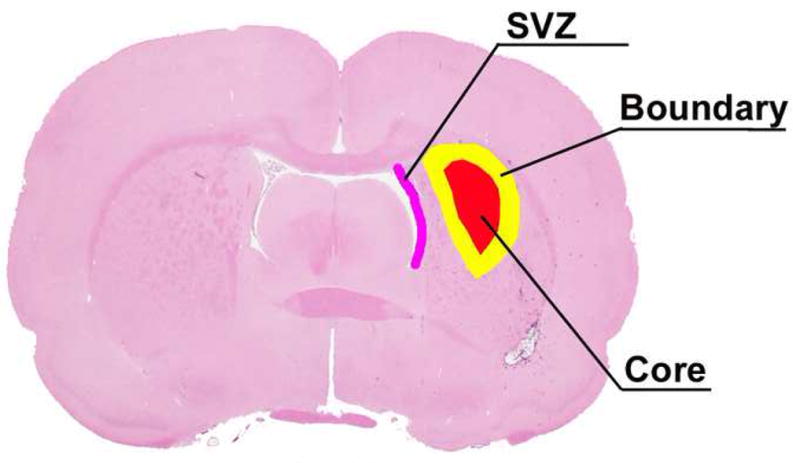
A representative H&E stained coronal section at the level of the anterior commissure of rat brain. The detected areas of the injured hemisphere recognized under the microscope are the hemorrhage core area (red), the boundary zone (yellow), and the subventricular zone (SVZ, pink).
4.6 Statistical Analysis
Statistical analysis of functional scores, area of ICH-related tissue damage, and histochemical results were all performed using the independent Student t-test. Data were presented as the mean ± standard error and P values <0.05 were considered significant.
Acknowledgments
Special thanks to Susan MacPhee-Gray for editorial assistance.
This work was supported by Internal (Departmental) Funds and National Institutes of Health grant NSO58581.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boulard G, Marguinaud E, Sesay M. Osmotic cerebral oedema: the role of plasma osmolarity and blood brain barrier. Ann Fr Anesth Reanim. 2003;22:215–219. doi: 10.1016/s0750-7658(03)00009-1. [DOI] [PubMed] [Google Scholar]
- Burke AM, Quest DO, Chien S, Cerri C. The effects of mannitol on blood viscosity. J Neurosurg. 1981;55:550–553. doi: 10.3171/jns.1981.55.4.0550. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- Cruz J, Minoja G, Okuchi K. Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial. Neurosurgery. 2001;49:864–871. doi: 10.1097/00006123-200110000-00016. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999;10:1289–1292. doi: 10.1097/00001756-199904260-00025. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci. 2001;2:408–416. doi: 10.1038/35077559. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099; discussion 1099–1100. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Lizasoain I, Cardenas A, Hurtado O, Romera C, Mallolas J, Lorenzo P, Castillo J, Moro MA. Targets of cytoprotection in acute ischemic stroke: present and future. Cerebrovasc Dis. 2006;21(Suppl 2):1–8. doi: 10.1159/000091698. [DOI] [PubMed] [Google Scholar]
- Loew F, Papavero L. The intraarterial route of drug delivery in the chemotherapy of malignant brain tumours. Adv Tech Stand Neurosurg. 1988;16:51–79. doi: 10.1007/978-3-7091-6954-4_2. [DOI] [PubMed] [Google Scholar]
- Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- Machi T, Kassell NF, Scheld MW, Lehmann GA. Effect of mannitol on the permeability of cultured endothelial cells. Fukuoka Igaku Zasshi. 1996;87:178–183. [PubMed] [Google Scholar]
- Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702; discussion 702–693. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- McGraw CP, Howard G. Effect of mannitol on increased intracranial pressure. Neurosurgery. 1983;13:269–271. doi: 10.1227/00006123-198309000-00009. [DOI] [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Kador PF, Randazzo J, Shinohara T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol Vis. 2007;13:1397–1405. [PubMed] [Google Scholar]
- Paczynski RP, He YY, Diringer MN, Hsu CY. Multiple-dose mannitol reduces brain water content in a rat model of cortical infarction. Stroke. 1997;28:1437–1443; discussion 1444. doi: 10.1161/01.str.28.7.1437. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Spellman SR, Sola S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ. Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab. 2002;22:463–471. doi: 10.1097/00004647-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2000:CD001049. doi: 10.1002/14651858.CD001049. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Schwab S, Bertram M, Aschoff A, Hacke W. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke. 1998;29:1550–1555. doi: 10.1161/01.str.29.8.1550. [DOI] [PubMed] [Google Scholar]
- Scumpia AJ, Kafel J, Hallas BH, Horowitz JM, Torres G. Endothelial heat shock response in cerebral ischemia. Histol Histopathol. 2007;22:815–823. doi: 10.14670/HH-22.815. [DOI] [PubMed] [Google Scholar]
- Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104:313–318. doi: 10.3171/jns.2006.104.2.313. [DOI] [PubMed] [Google Scholar]
- Seyfried D, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg. 2004;101:104–107. doi: 10.3171/jns.2004.101.1.0104. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J, Janse MJ, Fiolet WT, Krieger WJ, D’Alnoncourt CN, Durrer D. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ Res. 1981;49:364–381. doi: 10.1161/01.res.49.2.364. [DOI] [PubMed] [Google Scholar]
- Ueda T, Sakaki S, Nochide I, Kumon Y, Kohno K, Ohta S. Angioplasty after intra-arterial thrombolysis for acute occlusion of intracranial arteries. Stroke. 1998;29:2568–2574. doi: 10.1161/01.str.29.12.2568. [DOI] [PubMed] [Google Scholar]
- Villars F, Bordenave L, Bareille R, Amedee J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem. 2000;79:672–685. doi: 10.1002/1097-4644(20001215)79:4<672::aid-jcb150>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2005:CD001049. doi: 10.1002/14651858.CD001049.pub2. [DOI] [PubMed] [Google Scholar]
- Winkler SR, Munoz-Ruiz L. Mechanism of action of mannitol. Surg Neurol. 1995;43:59. doi: 10.1016/0090-3019(95)80039-j. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006;28:104–112. doi: 10.1179/016164106X91960. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]


