Abstract
AIM: To evaluate the accuracy of endoscopic ultrasound (EUS), EUS-fine needle aspiration (FNA) in evaluating mediastinal lymphadenopathy.
METHODS: Only EUS and EUS-FNA studies confirmed by surgery or with appropriate follow-up were selected. Articles were searched in Medline, Pubmed, and Cochrane control trial registry. Only studies from which a 2 × 2 table could be constructed for true positive, false negative, false positive and true negative values were included. Two reviewers independently searched and extracted data. The differences were resolved by mutual agreement. Meta-analysis for the accuracy of EUS was analyzed by calculating pooled estimates of sensitivity, specificity, likelihood ratios, and diagnostic odds ratios. Pooling was conducted by both Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). The heterogeneity of studies was tested using Cochran’s Q test based upon inverse variance weights.
RESULTS: Data was extracted from 76 studies (n = 9310) which met the inclusion criteria. Of these, 44 studies used EUS alone and 32 studies used EUS-FNA. FNA improved the sensitivity of EUS from 84.7% (95% CI: 82.9-86.4) to 88.0% (95% CI: 85.8-90.0). With FNA, the specificity of EUS improved from 84.6% (95% CI: 83.2-85.9) to 96.4% (95% CI: 95.3-97.4). The P for chi-squared heterogeneity for all the pooled accuracy estimates was > 0.10.
CONCLUSION: EUS is highly sensitive and specific for the evaluation of mediastinal lymphadenopathy and FNA substantially improves this. EUS with FNA should be the diagnostic test of choice for evaluating mediastinal lymphadenopathy.
Keywords: Endoscopic ultrasound, EUS-fine needle aspiration, Mediastinal lymphadenopathy
INTRODUCTION
Management of patients with mediastinal lymphadeno-pathy depends on the etiology of lymphadenopathy. Differentiating inflammatory from neoplastic processes in the mediastinal lymph nodes is not only important from the treatment standpoint, but also vital in predicting survival. Multiple diagnostic modalities are available to evaluate mediastinal lymphadenopathy. Computer tomography (CT) of the chest does not clearly image the aortopulmonary, subcarinal, and paraesophageal areas due to the lowering of image resolution because of the movement and partial volume effect of pulmonary vessels, aortic arch, and left atrium[1]. Also, for lesions smaller than 1 cm, the sensitivity of CT is low[2–5], and the size-based criteria to diagnose metastatic involvement of the lymph nodes have lower accuracy[6]. Therefore, other methods were introduced, including transbronchial biopsy, CT-guided transthoracic fine-needle aspiration (FNA), mediastinoscopy, or thoracoscopic biopsy.
In the transbronchial technique, the FNA needle is advanced blindly, reducing the yield of diagnosing subcarinal and paraesophageal nodes to approximately 50%[7,8]. Due to the potential danger of inadvertent vascular puncture, transthoracic biopsy is avoided when the mass is close to major vessels. This procedure is also associated with significant complications, including bleeding and pneumothorax in up to 25%-35% of cases[9,10]. Extended cervical mediastinoscopy or anterior mediastinoscopy can be used to access level 5 (aortopulmonary window) mediastinal nodes, which is not inspected by the standard methods[11–13]. Extended cervical mediastinoscopy has a sensitivity of 83% in examining the paraaortic and subaortic lymph node chains, but the subcarinal group is inaccessible[11]. Thoracoscopy can visualize the inferior mediastinum effectively, but it is limited only to accessing the level of major bronchi, leaving the superior mediastinum non-visualized[14]. Both procedures are invasive, require hospitalization and general anesthesia, and both have limitations.
With the introduction of endoscopic ultrasonography (EUS), it is now possible to visualize not only the gastrointestinal tract but also surrounding structures. However, EUS is limited in its ability to distinguish an inflammatory/reactive process from a malignancy, particularly within lymph nodes[15,16]. The accuracy of EUS in diagnosing mediastinal lymphadenopathy has been varied[17–21]. FNA during EUS may be performed safely in a short outpatient procedure setting without general anesthesia. It is not clear to what extent, if any, FNA adds in improving the accuracy of EUS to diagnose mediastinal lymphadenopathy[22–25].
The goal of this meta-analysis was to evaluate the accuracy of EUS alone and EUS with FNA in correctly diagnosing mediastinal lymphadenopathy. Due to multiple studies scattered in the literature and no published meta-analysis in this area, this meta-analysis was performed in an attempt to answer this essential clinical question. This meta-analysis and systematic review was written in accordance with the proposal for reporting by the QUOROM (Quality of Reporting of Meta-analyses) statement[26]. Since this manuscript looks at diagnostic accuracy of a test, the study design for this meta-analysis and systematic review conformed to the guidelines of Standards for Reporting of Diagnostic Accuracy (STARD) initiative[27].
MATERIALS AND METHODS
Study selection criteria
Only EUS-FNA studies confirmed by surgery or appropriate follow-up were selected. From this pool, only studies from which a 2 × 2 table could be constructed for true positive, false negative, false positive and true negative values were included.
Data collection and extraction
Articles were searched in Medline, Pubmed, Ovid journals, Cumulative Index for Nursing & Allied Health Literature, ACP journal club, DARE, International Pharmaceutical Abstracts, old Medline, Medline non-indexed citations, OVID Healthstar, and Cochrane Control Trial Registry. The search terms used were endoscopic ultrasound, EUS, ultrasound, mediastinal lymphadenopathy, nodal invasion, fine needle aspiration, FNA, staging, surgery, sensitivity, specificity, positive predictive value, and negative predictive value. 2 × 2 tables were constructed with the data extracted from each study. To give validity to the data, two authors (SP and JR) independently searched and extracted the data into an abstraction form. Any differences were resolved by mutual agreement.
Quality of studies
Clinical trial with a control arm can be assessed for the quality of the study. A number of criteria have been used to assess this quality of a study (e.g. randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome)[28,29]. There is no consensus on how to assess studies without a control arm. Hence, these criteria do no apply to studies without a control arm[29]. Therefore, for this meta-analysis and systematic review, studies were selected based on completeness of data and inclusion criteria.
Statistical analysis
Meta-analysis for the accuracy of EUS in diagnosing the etiology of mediastinal lymphadenopathy was performed by calculating pooled estimates of sensitivity, specificity, likelihood ratios, and diagnostic odds ratios. EUS studies were grouped into time periods to standardize the change in EUS technology and EUS criteria for lymph node involvement[30]. These time periods were 1988 to 1994, 1995 to 1999, and 2000 to 2006. Pooling was conducted using both Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). The confidence intervals were calculated using the F distribution method[31]. The width of the point estimates in the Forrest plots indicates the assigned weight to that study. For 0 value cells, a 0.5 was added as described by Cox[32]. The heterogeneity of the sensitivities and specificities was tested by applying the likelihood ratio test[33]. The heterogeneity of likelihood ratios and diagnostic odds ratios were tested using Cochran’s Q test based upon inverse variance weights[34]. Heterogeneity among studies was also tested by using summary receiver operating characteristic (SROC) curves. SROC curves were used to calculate the area under the curve (AUC). The effect of publication and selection bias on the summary estimates was tested by Harbord-Egger bias indicator[35] and Begg-Mazumdar indicator[36]. Also, funnel plots were constructed to evaluate potential publication bias using the standard error and diagnostic odds ratio[37,38].
RESULTS
The initial search using the search terms identified 4310 reference articles. Among these, 460 relevant articles were selected and reviewed by two authors independently. Data was extracted from 76 studies (n = 9310) which met the inclusion criteria. Of these, 44 studies used EUS alone[17,18,39–80] and 32 studies used EUS-FNA[19–25,81–107]. Figure 1 shows the search results. Table 1 shows the characteristics for EUS studies without FNA and Table 2 depicts characteristics of EUS studies with FNA. All the 76 selected studies were published as full-text articles in peer review journals. The pooled estimates given are estimates calculated by the fixed effect model.
Figure 1.
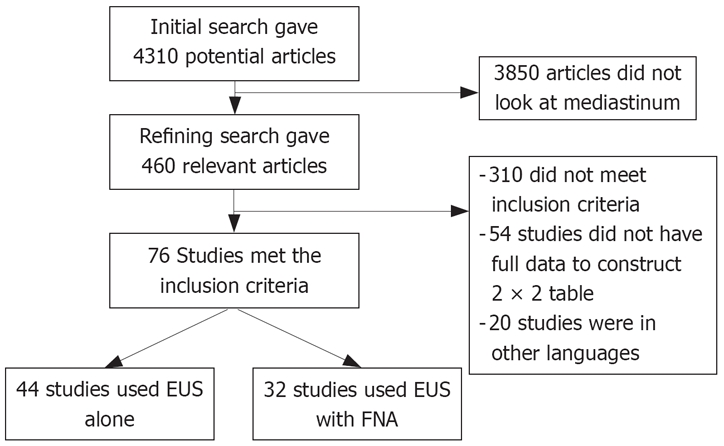
The search results.
Table 1.
Characteristics of studies included in this meta-analysis for EUS without FNA
| Author | Year of publication | No. of patients | Type of recruitment | Confirmatory procedure |
| Tio et al[71] | 1986 | 26 | Prospective | Surgery |
| Murata et al[57] | 1988 | 173 | Consecutive | Surgery |
| Tio et al[69] | 1989 | 75 | Prospective | Surgery |
| Vilgrain et al[75] | 1990 | 51 | Consecutive | Surgery |
| Tio et al[68] | 1990 | 102 | Consecutive | Surgery |
| Rice et al[63] | 1991 | 22 | Consecutive | Surgery |
| Heintz et al[52] | 1991 | 40 | Consecutive | Surgery |
| Botet et al[40] | 1991 | 50 | Consecutive | Surgery |
| Tio et al[70] | 1989 | 74 | Prospective | Surgery |
| Ziegler et al[80] | 1991 | 52 | Consecutive | Surgery |
| Rosch et al[64] | 1992 | 44 | Consecutive | Surgery |
| Fok et al[46] | 1992 | 54 | Consecutive | Surgery |
| Yoshikane et al[79] | 1993 | 28 | Consecutive | Surgery |
| Grimm et al[49] | 1993 | 63 | Prospective | Surgery |
| Dittler et al[45] | 1993 | 167 | Consecutive | Surgery |
| Peters et al[61] | 1994 | 42 | Consecutive | Surgery |
| Catalano et al[43] | 1994 | 100 | Consecutive | Surgery |
| McLoughlin et al[18] | 1995 | 15 | Consecutive | Surgery |
| Binmoeller et al[39] | 1995 | 87 | Prospective | Surgery |
| HunerBein et al[53] | 1996 | 19 | Consecutive | Surgery |
| Hasegawa et al[50] | 1996 | 22 | Consecutive | Surgery |
| Francois et al[47] | 1996 | 29 | Consecutive | Surgery |
| Natsugoe et al[58] | 1996 | 37 | Consecutive | Surgery |
| Milena et al[54] | 1997 | 40 | Prospective | Surgery |
| Vikers et al[73] | 1997 | 50 | Consecutive | Surgery |
| Shimizu et al[67] | 1997 | 431 | Consecutive | Surgery |
| Pham et al[62] | 1998 | 28 | Consecutive | Surgery |
| Vikers et al[74] | 1998 | 50 | Prospective | Surgery |
| Salminen et al[65] | 1999 | 32 | Consecutive | Surgery |
| Krasna et al[56] | 1999 | 88 | Consecutive | Surgery |
| Browrey et al[41] | 1999 | 98 | Prospective | Surgery |
| Catalano et al[42] | 1999 | 149 | Prospective | Surgery |
| Giovannini et al[48] | 1999 | 198 | Prospective | Surgery |
| Nishimaki et al[60] | 1999 | 224 | Consecutive | Surgery |
| Heidemann et al[51] | 2000 | 68 | Consecutive | Surgery |
| Nesje et al[59] | 2000 | 68 | Prospective | Surgery |
| Vazquez-Sequeiros et al[105] | 2001 | 37 | Consecutive | Surgery |
| Wiersema et al[77] | 2001 | 82 | Prospective | Surgery |
| Wakelin et al[76] | 2002 | 36 | Consecutive | Surgery |
| Kienle et al[55] | 2002 | 117 | Prospective | Surgery |
| Schwartz et al[66] | 2002 | 188 | Consecutive | Surgery |
| Wu et al[78] | 2003 | 31 | Prospective | Surgery |
| Arima et al[17] | 2003 | 58 | Consecutive | Surgery |
| DeWitt et al[44] | 2005 | 102 | Prospective | Surgery |
Table 2.
Characteristics of studies included in this meta-analysis for EUS with FNA
| Author | Year of publication | No. of patients | Type of recruitment | Confirmatory procedure |
| Kondo et al[6] | 1990 | 503 | Consecutive | Surgery |
| Schuder et al[25] | 1991 | 32 | Consecutive | Surgery |
| Silvestri et al[83] | 1995 | 27 | Prospective | Surgery |
| Giovannini et al[82] | 1995 | 141 | Prospective | Surgery or appropriate follow-up |
| Pedersen et al[21] | 1996 | 9 | Consecutive | FNA and appropriate follow-up |
| HunerBein et al[90] | 1996 | 19 | Consecutive | Surgery |
| Gress et al[19] | 1997 | 52 | Prospective | Surgery |
| Wiersema et al[104] | 1997 | 60 | Consecutive | FNA and appropriate follow-up |
| HunerBein et al[91] | 1998 | 15 | Consecutive | Surgery |
| HunerBein et al[98] | 1998 | 16 | Consecutive | Surgery |
| Fritscher-Ravens et al[101] | 1999 | 16 | Consecutive | FNA and appropriate follow-up |
| Mishra et al[102] | 1999 | 111 | Consecutive | FNA and appropriate follow-up |
| Giovannini et al[81] | 1999 | 198 | Prospective | Surgery or appropriate follow-up |
| Williams et al[89] | 1999 | 333 | Prospective | Surgery or appropriate follow-up |
| Fritscher-Ravens et al[84] | 2000 | 35 | Prospective | Surgery |
| Fritscher-Ravens et al[98] | 2000 | 35 | Consecutive | FNA and appropriate follow-up |
| Savides et al[100] | 2000 | 54 | Consecutive | FNA and appropriate follow-up |
| Fritscher-Ravens et al[103] | 2000 | 153 | Consecutive | FNA and appropriate follow-up |
| Vazquez-Sequeiros et al[105] | 2001 | 37 | Consecutive | Surgery |
| Wallace et al[91] | 2001 | 43 | Consecutive | FNA and appropriate follow-up |
| Wiersema et al[85] | 2001 | 82 | Prospective | Surgery |
| Chhieng et al[96] | 2001 | 103 | Consecutive | Surgery |
| Devereaux et al[22] | 2002 | 49 | Consecutive | Surgery |
| Catalano et al[92] | 2002 | 62 | Consecutive | Surgery |
| Schwartz et al[66] | 2002 | 188 | Consecutive | Surgery |
| Arima et al[95] | 2003 | 58 | Consecutive | Surgery |
| Pellise et al[23] | 2004 | 11 | Consecutive | Surgery |
| Kramer et al[86] | 2004 | 81 | Prospective | Surgery |
| Walsh et al[97] | 2005 | 27 | Consecutive | Surgery or appropriate follow-up |
| Tournoy et al[88] | 2005 | 67 | Prospective | Surgery |
| Khoo et al[93] | 2006 | 20 | Prospective | Surgery |
| Beek et al[87] | 2006 | 43 | Prospective | Surgery |
Accuracy of EUS with and without FNA
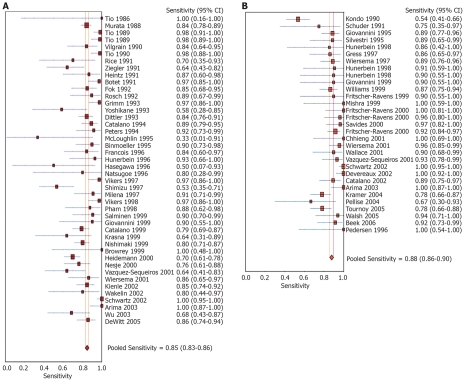
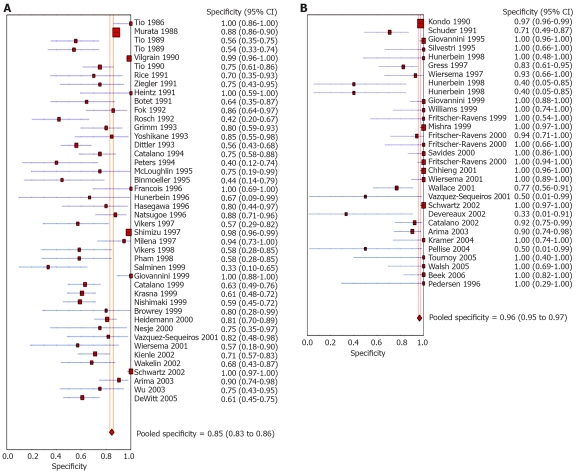
Pooled sensitivity to diagnose the cause for mediastinal lymphadenopathy was 84.7% (95% CI: 82.9-86.4) for EUS alone versus 88.0% (95% CI: 85.8-90.0) for EUS with FNA. The Forrest plot showing the sensitivity of EUS with and without FNA in various studies is shown in Figure 2A and B, respectively. EUS without FNA had a pooled specificity of 84.6% (95% CI: 83.2-85.9) and with FNA was 96.4% (95% CI: 95.3-97.4). Forrest plots showing specificity from various studies with and without FNA is depicted in Figure 3A and B, respectively.
Figure 2.
Forrest plots. A: Sensitivity of EUS alone in diagnosing mediastinal lymphadenopathy; B: Sensitivity of EUS-FNA in diagnosing mediastinal lymphadenopathy.
Figure 3.
Forrest plots. A: Specificity of EUS alone in diagnosing mediastinal lymphadenopathy. B: Specificity of EUS-FNA alone in diagnosing mediastinal lymphadenopathy.
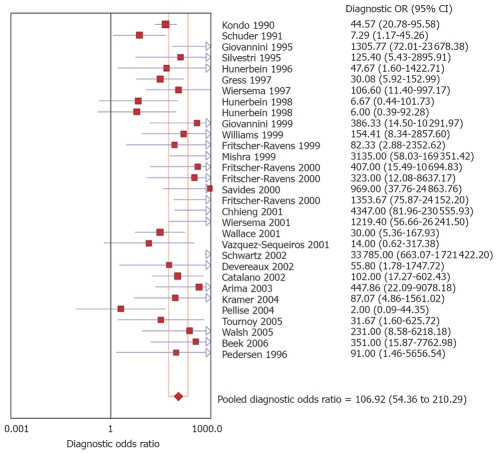
The pooled positive likelihood ratio of EUS without FNA was 3.3 (95% CI: 2.6-4.3) and with FNA was 11.2 (95% CI: 5.9-21.2). The pooled negative likelihood ratio was 0.24 (95% CI: 0.1-0.3) for EUS without FNA and 0.13 (95% CI: 0.1-0.2) for EUS with FNA. The diagnostic odds ratio, the odds of having nodal metastasis in positive as compared to negative EUS studies, was 19.1 (95% CI: 12.7-28.5) for EUS without FNA and 106.9 (95% CI: 54.4-210.3) for EUS with FNA. Figure 4 shows a Forrest plot of various studies with FNA and their DOR. All the pooled estimates calculated by random effect models were similar to the estimates of fixed effect model.
Figure 4.
Forrest plot showing diagnostic odds ratio of EUS-FNA in identifying mediastinal lymphadenopathy.
SROC curves for EUS without FNA showed an area under the curve (AUC) of 0.91. EUS with FNA showed an AUC of 0.97. Figure 5 shows the SROC curve. The P for Chi-squared heterogeneity for all the pooled accuracy estimates was > 0.10. Table 3 shows the accuracy estimates of EUS alone and EUS-FNA.
Figure 5.
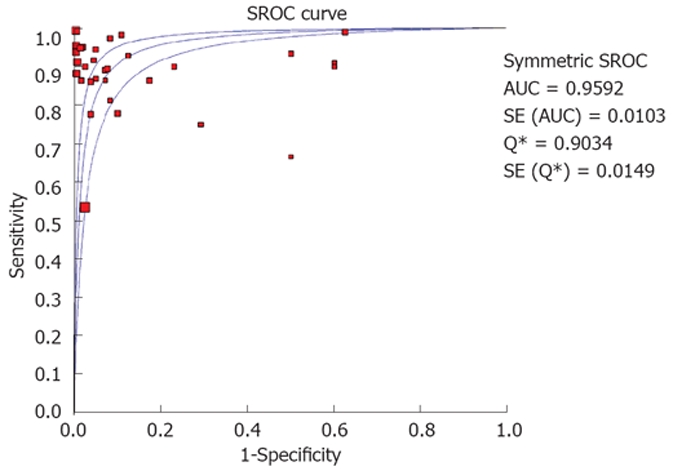
SROC for EUS to diagnose mediastinal lymphadenopathy.
Table 3.
Pooled diagnostic accuracy estimates of EUS alone and EUS-FNA
| EUS | EUS-FNA | |
| Studies | 44 | 32 |
| Pooled sensitivity | 84.7% (82.9-86.4) | 88.0% (85.8-90.0) |
| Pooled specificity | 84.6% (83.2-85.9) | 96.4% (95.3-97.4) |
| Positive likelihood ratio | 3.3 (2.6-4.3) | 11.2 (5.9-21.2) |
| Negative likelihood ratio | 0.24 (0.1-0.3) | 0.13 (0.1-0.2) |
| Diagnostic odds ratio | 19.1 (12.7-28.5) | 106.9 (54.4-210.3) |
| Area under the curve | 0.91 | 0.97 |
Effect of technology over time
To standardize the criteria for lymph node involvement and change in technology, the studies were grouped into three time periods[30]. These time periods were 1988 to 1994, 1995 to 1999, and 2000 to 2006. During these time periods, the number of studies that met the inclusion criteria for EUS alone were 17, 17, and 10, respectively. Studies that met inclusion criteria for EUS-FNA were 4, 10, and 18, respectively. For the most recent time period, EUS alone had a sensitivity of 81.6% (95% CI: 77.8-85.1) and specificity of 82.4% (95% CI: 78.2-86.1). During the same time period, EUS-FNA had a sensitivity of 91.7% (95% CI: 89.3-93.7) and specificity of 96.8% (95% CI: 94.9-98.2). All pooled estimates during the three time periods are given in Table 4. The P for chi-squared heterogeneity for all the pooled accuracy estimates was > 0.1.
Table 4.
Pooled diagnostic accuracy estimates of EUS alone and EUS-FNA for different time periods with 95% CI
| Time period | No. of studies | Pooled sensitivity | Pooled specificity | Pooled LR+1 | Pooled LR-2 | Pooled DOR3 |
| EUS without FNA | ||||||
| 1988 to 1994 | 17 | 88.0% (85.4-90.2) | 85.2% (83.4-86.9) | 3.6 (2.4-5.4) | 0.2 (0.1-0.3) | 27.5 (14.5-52.4) |
| 1995 to 1999 | 17 | 82.6% (78.8-85.9) | 84.4% (81.6-86.9) | 3.0 (2.0-4.5) | 0.3 (0.2-0.4) | 14.8 (7.5-29.3) |
| 2000 to 2005 | 10 | 81.6% (77.8-85.1) | 82.4% (78.2-86.1) | 3.4 (2.2-5.3) | 0.3 (0.2-0.4) | 14.9 (6.7-33.1) |
| EUS-FNA | ||||||
| 1988 to 1994 | 4 | 71.8% (63.9-78.9) | 96.8% (94.9-98.1) | 15.5 (2.4-101.2) | 0.3 (0.1-0.6) | 61.8 (10.5-63.8) |
| 1995 to 1999 | 10 | 88.9% (83.5-93.0) | 94.7% (90.7-97.3) | 8.1 (2.8-23.3) | 0.1 (0.1-0.2) | 57.0 (20.7-57.1) |
| 2000 to 2005 | 18 | 91.7% (89.3-93.7) | 96.8% (94.9-98.2) | 12.5 (5.2-29.8) | 0.1 (0.1-0.2) | 17.7 (5.0-62.8) |
1LR+: Positive likelihood ratio; 2LR-: Negative likelihood ratio; 3DOR: Diagnostic odds ratio.
Bias estimates
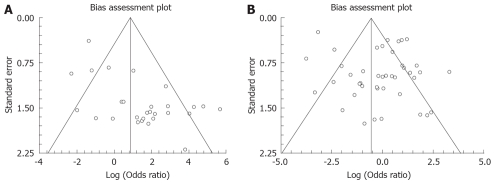
The bias calculations using Harbord-Egger bias indicator gave a value of 1.08 (95% CI: -0.79-2.95, P = 0.29) for EUS studies without FNA and 2.02 (95% CI: 0.29-3.74, P = 0.04) for studies with FNA. The Begg-Mazumdar indicator for bias gave a Kendall’s tau b value of 0.13 (P = 0.36) for studies without FNA and -0.19 (P = 0.07) for studies with FNA. The funnel plots for the studies without and with FNA are shown in Figure 6A and B.
Figure 6.
Funnel plots. A: Bias assessment for EUS studies without FNA in examining mediastinal lymphadenopathy; B: Bias assessment for EUS-FNA studies in examining mediastinal lymphadenopathy.
DISCUSSION
Diagnosing the correct etiology for mediastinal lymph-adenopathy helps direct precise therapy and prognosis. Thoracoscopic procedures for tissue biopsy carry a risk of complications in 25%-35% of cases[9,10]. The advantage of EUS is the ability to perform FNA during the procedure for tissue diagnosis. The procedure is, in comparison with other alternative options, safe, less invasive, and does not require general anesthesia or hospitalization[107]. The complication rate is extremely low (0.5%-2.3%) with several studies reporting no complications[48,77,83,107]. Modalities using FNA, such as transbronchial, computed tomography, or thoracoscopic procedure, cannot be used for the entire mediastinum[2–13]. EUS has the ability to image the aortopulmonary window, the subcarinal nodes, inferior mediastinum, and entire posterior part of the mediastinum.
This meta-analysis and systematic review was written in accordance with the proposal for reporting by the QUOROM (Quality of Reporting of Meta-analyses) statement[7]. This meta-analysis and systematic review shows that the pooled sensitivity of EUS for mediastinal lymphadenopathy is high and use of FNA during the procedure, further increases such sensitivity. The pooled specificity for diagnosing mediastinal lymphadenopathy is also high with substantial improvement if FNA is performed during the procedure (from 84.6% to 96.4%). Diagnostic odds ratio is defined as the odds of having a positive test in patients with true anatomic disease when compared to patients who do not have the disease. EUS has a very high diagnostic odds ratio for mediastinal lymphadenopathy. For example, if EUS indicates mediastinal lymphadenopathy and if FNA is performed on the enlarged nodes, the patient has odds of 106 times to have the correct etiology for lymph node enlargement. If EUS shows mediastinal lymphadenopathy, then the nodes should be biopsied by FNA to improve the diagnostic accuracy.
The positive likelihood ratio measures how well a test identifies a disease state. The higher the positive likelihood ratio, the better the test performs in identifying the correct disease state. The negative likelihood ratio of the same test measures how well the test performs in excluding a disease state. The lower the negative likelihood ratio, the better the test performs in excluding a disease. For mediastinal lymphadenopathy, EUS has a high positive likelihood ratio and low negative likelihood ratio. This indicates that EUS performs better in diagnosing and excluding mediastinal lymphadenopathy. For mediastinal lymphadenopathy, all the pooled accuracy estimates of EUS are higher if FNA is performed during the procedure. Also, these pooled estimates give a baseline for future study comparisons.
The EUS studies with FNA were grouped into time periods and analyzed to standardize the criteria and the technology of EUS over the past two decades. Over the last two decades, the sensitivity and specificity of EUS with FNA has substantially improved.
Due to the possibility of different studies using slightly different criteria for diagnosis, heterogeneity among the studies was tested by drawing SROC curves and finding the AUC. An AUC of 1 for any test indicates that the test is excellent. SROC curves for EUS showed that the value for AUC was very close to 1, indicating that EUS is an excellent test to diagnose mediastinal lymphadenopathy. Publication bias and selection bias may affect the summary estimates. Studies with statistically significant results tend to be published and cited. Smaller studies may show larger treatment effects due to fewer case-mix differences (e.g. patients with only early or late disease) than larger trials. This bias can be estimated by bias indicators and construction of funnel plots. Bias among studies can affect the shape of the funnel plot. In this meta-analysis and systematic review, bias calculations using Harbord-Egger indicator[36] and Begg-Mazumdar indicator[37] showed no statistically significant bias for EUS studies without FNA. Furthermore, funnel plot analyses showed no significant bias for EUS without FNA and EUS-FNA studies (Figure 6B).
In conclusion, EUS has high sensitivity and specificity to evaluate mediastinal lymphadenopathy. This meta-analysis demonstrates that FNA substantially improves the specificity of EUS in evaluating mediastinal lymphadenopathy. EUS with FNA should be the diagnostic test of choice for evaluating mediastinal lymphadenopathy.
Peer reviewer: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Ma L L- Editor Alpini GD E- Editor Liu Y
References
- 1.Genereux GP, Howie JL. Normal mediastinal lymph node size and number: CT and anatomic study. AJR Am J Roentgenol. 1984;142:1095–1100. doi: 10.2214/ajr.142.6.1095. [DOI] [PubMed] [Google Scholar]
- 2.Arita T, Kuramitsu T, Kawamura M, Matsumoto T, Matsunaga N, Sugi K, Esato K. Bronchogenic carcinoma: incidence of metastases to normal sized lymph nodes. Thorax. 1995;50:1267–1269. doi: 10.1136/thx.50.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izbicki JR, Thetter O, Karg O, Kreusser T, Passlick B, Trupka A, Haussinger K, Woeckel W, Kenn RW, Wilker DK. Accuracy of computed tomographic scan and surgical assessment for staging of bronchial carcinoma. A prospective study. J Thorac Cardiovasc Surg. 1992;104:413–420. [PubMed] [Google Scholar]
- 4.McLoud TC, Bourgouin PM, Greenberg RW, Kosiuk JP, Templeton PA, Shepard JA, Moore EH, Wain JC, Mathisen DJ, Grillo HC. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–323. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 5.McKenna RJ Jr, Libshitz HI, Mountain CE, McMurtrey MJ. Roentgenographic evaluation of mediastinal nodes for preoperative assessment in lung cancer. Chest. 1985;88:206–210. doi: 10.1378/chest.88.2.206. [DOI] [PubMed] [Google Scholar]
- 6.Kondo D, Imaizumi M, Abe T, Naruke T, Suemasu K. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–593. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 7.Harrow EM, Oldenburg FA Jr, Lingenfelter MS, Smith AM Jr. Transbronchial needle aspiration in clinical practice. A five-year experience. Chest. 1989;96:1268–1272. doi: 10.1378/chest.96.6.1268. [DOI] [PubMed] [Google Scholar]
- 8.Harrow EM, Wang KP. The staging of lung cancer by bronchoscopic transbronchial needle aspiration. Chest Surg Clin N Am. 1996;6:223–235. [PubMed] [Google Scholar]
- 9.Salazar AM, Westcott JL. The role of transthoracic needle biopsy for the diagnosis and staging of lung cancer. Clin Chest Med. 1993;14:99–110. [PubMed] [Google Scholar]
- 10.Gardner D, vanSonnenberg E, D'Agostino HB, Casola G, Taggart S, May S. CT-guided transthoracic needle biopsy. Cardiovasc Intervent Radiol. 1991;14:17–23. doi: 10.1007/BF02635526. [DOI] [PubMed] [Google Scholar]
- 11.Lopez L, Varela A, Freixinet J, Quevedo S, Lopez Pujol J, Rodriguez de Castro F, Salvatierra A. Extended cervical mediastinoscopy: prospective study of fifty cases. Ann Thorac Surg. 1994;57:555–557; discussion 557-558. doi: 10.1016/0003-4975(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 12.Barendregt WB, Deleu HW, Joosten HJ, Berg W, Janssen JP. The value of parasternal mediastinoscopy in staging bronchial carcinoma. Eur J Cardiothorac Surg. 1995;9:655–658. doi: 10.1016/s1010-7940(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 13.Merav AD. The role of mediastinoscopy and anterior mediastinotomy in determining operability of lung cancer: a review of published questions and answers. Cancer Invest. 1991;9:439–442. doi: 10.3109/07357909109084642. [DOI] [PubMed] [Google Scholar]
- 14.Landreneau RJ, Hazelrigg SR, Mack MJ, Fitzgibbon LD, Dowling RD, Acuff TE, Keenan RJ, Ferson PF. Thoracoscopic mediastinal lymph node sampling: useful for mediastinal lymph node stations inaccessible by cervical mediastinoscopy. J Thorac Cardiovasc Surg. 1993;106:554–558. [PubMed] [Google Scholar]
- 15.Heintz A, Mildenberger P, Georg M, Braunstein S, Junginger T. Endoscopic ultrasonography in the diagnosis of regional lymph nodes in esophageal and gastric cancer--results of studies in vitro. Endoscopy. 1993;25:231–235. doi: 10.1055/s-2007-1010298. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman AR, Sivak MV Jr. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. Gastrointest Endosc. 1989;35:214–219. doi: 10.1016/s0016-5107(89)72761-9. [DOI] [PubMed] [Google Scholar]
- 17.Arima M, Tada M. Endoscopic ultrasound-guided fine needle aspiration biopsy in esophageal and mediastinal diseases: Clinical indications and results. Dig Endosc. 2003;15:93–99. [Google Scholar]
- 18.McLoughlin RF, Cooperberg PL, Mathieson JR, Stordy SN, Halparin LS. High resolution endoluminal ultrasonography in the staging of esophageal carcinoma. J Ultrasound Med. 1995;14:725–730. doi: 10.7863/jum.1995.14.10.725. [DOI] [PubMed] [Google Scholar]
- 19.Gress FG, Savides TJ, Sandler A, Kesler K, Conces D, Cummings O, Mathur P, Ikenberry S, Bilderback S, Hawes R. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: a comparison study. Ann Intern Med. 1997;127:604–612. doi: 10.7326/0003-4819-127-8_part_1-199710150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868–872. doi: 10.1067/mge.2002.129610. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BH, Vilmann P, Folke K, Jacobsen GK, Krasnik M, Milman N, Hancke S. Endoscopic ultrasonography and real-time guided fine-needle aspiration biopsy of solid lesions of the mediastinum suspected of malignancy. Chest. 1996;110:539–544. doi: 10.1378/chest.110.2.539. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux BM, Leblanc JK, Yousif E, Kesler K, Brooks J, Mathur P, Sandler A, Chappo J, Lehman GA, Sherman S, et al. Clinical utility of EUS-guided fine-needle aspiration of mediastinal masses in the absence of known pulmonary malignancy. Gastrointest Endosc. 2002;56:397–401. doi: 10.1016/s0016-5107(02)70045-x. [DOI] [PubMed] [Google Scholar]
- 23.Pellise M, Castells A, Gines A, Agrelo R, Sole M, Castellvi-Bel S, Fernandez-Esparrach G, Llach J, Esteller M, Bordas JM, et al. Detection of lymph node micrometastases by gene promoter hypermethylation in samples obtained by endosonography- guided fine-needle aspiration biopsy. Clin Cancer Res. 2004;10:4444–4449. doi: 10.1158/1078-0432.CCR-03-0600. [DOI] [PubMed] [Google Scholar]
- 24.Kondo D, Imaizumi M, Abe T, Naruke T, Suemasu K. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–593. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 25.Schuder G, Isringhaus H, Kubale B, Seitz G, Sybrecht GW. Endoscopic ultrasonography of the mediastinum in the diagnosis of bronchial carcinoma. Thorac Cardiovasc Surg. 1991;39:299–303. doi: 10.1055/s-2007-1019991. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 27.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44:635–638. [PubMed] [Google Scholar]
- 28.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788–797. doi: 10.1016/j.gie.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Leemis LM, Trivedi KS. A Comparison of Approximate Interval Estimators for the Bernoulli Parameter. Am Stat. 1996;50:63–68. [Google Scholar]
- 32.Cox DR. The analysis of binary data. Vol. 50. London: Methuen; 1970. [Google Scholar]
- 33.Agresti A. Analysis of ordinal categorical data. Vol. 50. New York: John Wileys & Sons; 1984. [Google Scholar]
- 34.Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in context. Vol. 50. London: BMJ Books; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 37.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 39.Binmoeller KF, Seifert H, Seitz U, Izbicki JR, Kida M, Soehendra N. Ultrasonic esophagoprobe for TNM staging of highly stenosing esophageal carcinoma. Gastrointest Endosc. 1995;41:547–552. doi: 10.1016/s0016-5107(95)70188-5. [DOI] [PubMed] [Google Scholar]
- 40.Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:419–425. doi: 10.1148/radiology.181.2.1924783. [DOI] [PubMed] [Google Scholar]
- 41.Bowrey DJ, Clark GW, Roberts SA, Maughan TS, Hawthorne AB, Williams GT, Carey PD. Endosonographic staging of 100 consecutive patients with esophageal carcinoma: introduction of the 8-mm esophagoprobe. Dis Esophagus. 1999;12:258–263. doi: 10.1046/j.1442-2050.1999.00071.x. [DOI] [PubMed] [Google Scholar]
- 42.Catalano MF, Alcocer E, Chak A, Nguyen CC, Raijman I, Geenen JE, Lahoti S, Sivak MV Jr. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc. 1999;50:352–356. doi: 10.1053/ge.1999.v50.98154. [DOI] [PubMed] [Google Scholar]
- 43.Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–446. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 44.DeWitt J, Kesler K, Brooks JA, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Dis Esophagus. 2005;18:21–27. doi: 10.1111/j.1442-2050.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 45.Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in esophageal carcinoma. Endoscopy. 1993;25:156–161. doi: 10.1055/s-2007-1010275. [DOI] [PubMed] [Google Scholar]
- 46.Fok M, Cheng SW, Wong J. Endosonography in patient selection for surgical treatment of esophageal carcinoma. World J Surg. 1992;16:1098–1103; discussion 1103. doi: 10.1007/BF02067067. [DOI] [PubMed] [Google Scholar]
- 47.Francois E, Peroux J, Mouroux J, Chazalle M, Hastier P, Ferrero J, Simon J, Bourry J. Preoperative endosonographic staging of cancer of the cardia. Abdom Imaging. 1996;21:483–487. doi: 10.1007/s002619900109. [DOI] [PubMed] [Google Scholar]
- 48.Giovannini M, Monges G, Seitz JF, Moutardier V, Bernardini D, Thomas P, Houvenaeghel G, Delpero JR, Giudicelli R, Fuentes P. Distant lymph node metastases in esophageal cancer: impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536–540. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 49.Grimm H, Binmoeller KF, Hamper K, Koch J, Henne-Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy. 1993;25:224–230. doi: 10.1055/s-2007-1010297. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa N, Niwa Y, Arisawa T, Hase S, Goto H, Hayakawa T. Preoperative staging of superficial esophageal carcinoma: comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc. 1996;44:388–393. doi: 10.1016/s0016-5107(96)70086-x. [DOI] [PubMed] [Google Scholar]
- 51.Heidemann J, Schilling MK, Schmassmann A, Maurer CA, Buchler MW. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg. 2000;17:219–224. doi: 10.1159/000018838. [DOI] [PubMed] [Google Scholar]
- 52.Heintz A, Hohne U, Schweden F, Junginger T. Preoperative detection of intrathoracic tumor spread of esophageal cancer: endosonography versus computed tomography. Surg Endosc. 1991;5:75–78. doi: 10.1007/BF00316841. [DOI] [PubMed] [Google Scholar]
- 53.Hunerbein M, Dohmoto M, Rau B, Schlag PM. Endosonography and endosonography-guided biopsy of upper-GI-tract tumors using a curved-array echoendoscope. Surg Endosc. 1996;10:1205–1209. doi: 10.1007/s004649900280. [DOI] [PubMed] [Google Scholar]
- 54.Kallimanis GE, Gupta PK, al-Kawas FH, Tio LT, Benjamin SB, Bertagnolli ME, Nguyen CC, Gomes MN, Fleischer DE. Endoscopic ultrasound for staging esophageal cancer, with or without dilation, is clinically important and safe. Gastrointest Endosc. 1995;41:540–546. doi: 10.1016/s0016-5107(95)70187-7. [DOI] [PubMed] [Google Scholar]
- 55.Kienle P, Buhl K, Kuntz C, Dux M, Hartmann C, Axel B, Herfarth C, Lehnert T. Prospective comparison of endoscopy, endosonography and computed tomography for staging of tumours of the oesophagus and gastric cardia. Digestion. 2002;66:230–236. doi: 10.1159/000068360. [DOI] [PubMed] [Google Scholar]
- 56.Krasna MJ, Mao YS, Sonett J, Gamliel Z. The role of thoracoscopic staging of esophageal cancer patients. Eur J Cardiothorac Surg. 1999;16 Suppl 1:S31–S33. doi: 10.1016/s1010-7940(99)00180-3. [DOI] [PubMed] [Google Scholar]
- 57.Murata Y, Suzuki S, Hashimoto H. Endoscopic ultrasonography of the upper gastrointestinal tract. Surg Endosc. 1988;2:180–183. doi: 10.1007/BF02498796. [DOI] [PubMed] [Google Scholar]
- 58.Natsugoe S, Yoshinaka H, Morinaga T, Shimada M, Baba M, Fukumoto T, Stein HJ, Aikou T. Ultrasonographic detection of lymph-node metastases in superficial carcinoma of the esophagus. Endoscopy. 1996;28:674–679. doi: 10.1055/s-2007-1005575. [DOI] [PubMed] [Google Scholar]
- 59.Nesje LB, Svanes K, Viste A, Laerum OD, Odegaard S. Comparison of a linear miniature ultrasound probe and a radial-scanning echoendoscope in TN staging of esophageal cancer. Scand J Gastroenterol. 2000;35:997–1002. doi: 10.1080/003655200750023101. [DOI] [PubMed] [Google Scholar]
- 60.Nishimaki T, Tanaka O, Ando N, Ide H, Watanabe H, Shinoda M, Takiyama W, Yamana H, Ishida K, Isono K, et al. Evaluation of the accuracy of preoperative staging in thoracic esophageal cancer. Ann Thorac Surg. 1999;68:2059–2064. doi: 10.1016/s0003-4975(99)01171-6. [DOI] [PubMed] [Google Scholar]
- 61.Peters JH, Hoeft SF, Heimbucher J, Bremner RM, DeMeester TR, Bremner CG, Clark GW, Kiyabu M, Parisky Y. Selection of patients for curative or palliative resection of esophageal cancer based on preoperative endoscopic ultrasonography. Arch Surg. 1994;129:534–539. doi: 10.1001/archsurg.1994.01420290080012. [DOI] [PubMed] [Google Scholar]
- 62.Pham T, Roach E, Falk GL, Chu J, Ngu MC, Jones DB. Staging of oesophageal carcinoma by endoscopic ultrasound: preliminary experience. Aust N Z J Surg. 1998;68:209–212. doi: 10.1111/j.1445-2197.1998.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 63.Rice TW, Boyce GA, Sivak MV. Esophageal ultrasound and the preoperative staging of carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1991;101:536–543; discussion 543-544. [PubMed] [Google Scholar]
- 64.Rosch T, Lorenz R, Zenker K, von Wichert A, Dancygier H, Hofler H, Siewert JR, Classen M. Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum by endoscopic ultrasonography. Gastrointest Endosc. 1992;38:460–467. doi: 10.1016/s0016-5107(92)70477-5. [DOI] [PubMed] [Google Scholar]
- 65.Salminen JT, Farkkila MA, Ramo OJ, Toikkanen V, Simpanen J, Nuutinen H, Salo JA. Endoscopic ultrasonography in the preoperative staging of adenocarcinoma of the distal oesophagus and oesophagogastric junction. Scand J Gastroenterol. 1999;34:1178–1182. doi: 10.1080/003655299750024670. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868–872. doi: 10.1067/mge.2002.129610. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu Y, Mera K, Tsukagoshi H, Takamasa M, Kawarazaki M, Watanabe Y, Nakasato T, Oohara M, Hosokawa M, Fujita M, et al. Endoscopic Ultrasonography for the Detection of Lymph Node Metastasis in Superficial Esophageal Carcinoma. Dig Endosc. 1997;9:178–182. [Google Scholar]
- 68.Tio TL, Coene PP, den Hartog Jager FC, Tytgat GN. Pre-operative TNM classification of esophageal carcinoma by endosonography. Hepatogastroenterology. 1990;37:376–381. [PubMed] [Google Scholar]
- 69.Tio TL, Coene PP, Schouwink MH, Tytgat GN. Esophagogastric carcinoma: preoperative TNM classification with endosono-graphy. Radiology. 1989;173:411–417. doi: 10.1148/radiology.173.2.2678255. [DOI] [PubMed] [Google Scholar]
- 70.Tio TL, Cohen P, Coene PP, Udding J, den Hartog Jager FC, Tytgat GN. Endosonography and computed tomography of esophageal carcinoma. Preoperative classification compared to the new (1987) TNM system. Gastroenterology. 1989;96:1478–1486. doi: 10.1016/0016-5085(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 71.Tio TL, den Hartog Jager FC, Tytgat GN. The role of endoscopic ultrasonography in assessing local resectability of oesophagogastric malignancies. Accuracy, pitfalls, and predictability. Scand J Gastroenterol Suppl. 1986;123:78–86. doi: 10.3109/00365528609091867. [DOI] [PubMed] [Google Scholar]
- 72.Vazquez-Sequeiros E, Norton ID, Clain JE, Wang KK, Affi A, Allen M, Deschamps C, Miller D, Salomao D, Wiersema MJ. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751–757. doi: 10.1067/mge.2001.112741. [DOI] [PubMed] [Google Scholar]
- 73.Vickers J, Alderson D. Influence of luminal obstruction on oesophageal cancer staging using endoscopic ultrasonography. Br J Surg. 1998;85:999–1001. doi: 10.1046/j.1365-2168.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 74.Vickers J. Role of endoscopic ultrasound in the preoperative assessment of patients with oesophageal cancer. Ann R Coll Surg Engl. 1998;80:233–239. [PMC free article] [PubMed] [Google Scholar]
- 75.Vilgrain V, Mompoint D, Palazzo L, Menu Y, Gayet B, Ollier P, Nahum H, Fekete F. Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol. 1990;155:277–281. doi: 10.2214/ajr.155.2.2115251. [DOI] [PubMed] [Google Scholar]
- 76.Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol. 2002;41:161–167. doi: 10.1016/s0720-048x(01)00418-1. [DOI] [PubMed] [Google Scholar]
- 77.Wiersema MJ, Vazquez-Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US-guided fine-needle aspiration biopsy. Radiology. 2001;219:252–257. doi: 10.1148/radiology.219.1.r01ap44252. [DOI] [PubMed] [Google Scholar]
- 78.Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219–224. doi: 10.3748/wjg.v9.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Shimodaira M, Maruta S, Miyata A, Yoshida M. Superficial esophageal carcinoma: evaluation by endoscopic ultrasonography. Am J Gastroenterol. 1994;89:702–707. [PubMed] [Google Scholar]
- 80.Ziegler K, Sanft C, Zeitz M, Friedrich M, Stein H, Haring R, Riecken EO. Evaluation of endosonography in TN staging of oesophageal cancer. Gut. 1991;32:16–20. doi: 10.1136/gut.32.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giovannini M, Monges G, Seitz JF, Moutardier V, Bernardini D, Thomas P, Houvenaeghel G, Delpero JR, Giudicelli R, Fuentes P. Distant lymph node metastases in esophageal cancer: impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536–540. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 82.Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171–177. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 83.Silvestri GA, Hoffman BJ, Bhutani MS, Hawes RH, Coppage L, Sanders-Cliette A, Reed CE. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441–1445; discussion 1445-1446. doi: 10.1016/0003-4975(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 84.Fritscher-Ravens A, Soehendra N, Schirrow L, Sriram PV, Meyer A, Hauber HP, Pforte A. Role of transesophageal endosonography-guided fine-needle aspiration in the diagnosis of lung cancer. Chest. 2000;117:339–345. doi: 10.1378/chest.117.2.339. [DOI] [PubMed] [Google Scholar]
- 85.Wiersema MJ, Vazquez-Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US-guided fine-needle aspiration biopsy. Radiology. 2001;219:252–257. doi: 10.1148/radiology.219.1.r01ap44252. [DOI] [PubMed] [Google Scholar]
- 86.Kramer H, van Putten JW, Post WJ, van Dullemen HM, Bongaerts AH, Pruim J, Suurmeijer AJ, Klinkenberg TJ, Groen H, Groen HJ. Oesophageal endoscopic ultrasound with fine needle aspiration improves and simplifies the staging of lung cancer. Thorax. 2004;59:596–601. doi: 10.1136/thx.2003.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Beek FT, Maas KW, Timmer R, Seldenrijk CA, de Bruin PC, Schramel FM. Oesophageal endoscopic ultrasound with fine-needle aspiration biopsy in the staging of non-small-cell lung carcinoma; results from 43 patients. Ned Tijdschr Geneeskd. 2006;150:144–150. [PubMed] [Google Scholar]
- 88.Tournoy KG, Praet MM, Van Maele G, Van Meerbeeck JP. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: high accuracy for the diagnosis of mediastinal lymphadenopathy. Chest. 2005;128:3004–3009. doi: 10.1378/chest.128.4.3004. [DOI] [PubMed] [Google Scholar]
- 89.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hunerbein M, Dohmoto M, Rau B, Schlag PM. Endosonography and endosonography-guided biopsy of upper-GI-tract tumors using a curved-array echoendoscope. Surg Endosc. 1996;10:1205–1209. doi: 10.1007/s004649900280. [DOI] [PubMed] [Google Scholar]
- 91.Hunerbein M, Ghadimi BM, Haensch W, Schlag PM. Transesophageal biopsy of mediastinal and pulmonary tumors by means of endoscopic ultrasound guidance. J Thorac Cardiovasc Surg. 1998;116:554–559. doi: 10.1016/S0022-5223(98)70160-6. [DOI] [PubMed] [Google Scholar]
- 92.Catalano MF, Nayar R, Gress F, Scheiman J, Wassef W, Rosenblatt ML, Kochman M. EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc. 2002;55:863–869. doi: 10.1067/mge.2002.124637. [DOI] [PubMed] [Google Scholar]
- 93.Khoo KL, Ho KY, Nilsson B, Lim TK. EUS-guided FNA immediately after unrevealing transbronchial needle aspiration in the evaluation of mediastinal lymphadenopathy: a prospective study. Gastrointest Endosc. 2006;63:215–220. doi: 10.1016/j.gie.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 94.Hunerbein M, Dohmoto M, Haensch W, Schlag PM. Endosonography-guided biopsy of mediastinal and pancreatic tumors. Endoscopy. 1998;30:32–36. doi: 10.1055/s-2007-993725. [DOI] [PubMed] [Google Scholar]
- 95.Arima M, Tada M. Endoscopic ultrasound-guided fine needle aspiration biopsy in esophageal and mediastinal diseases: Clinical indications and results. Dig Endosc. 2003;15:93–99. [Google Scholar]
- 96.Chhieng DC, Jhala D, Jhala N, Eltoum I, Chen VK, Vickers S, Heslin MJ, Wilcox CM, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a study of 103 cases. Cancer. 2002;96:232–239. doi: 10.1002/cncr.10714. [DOI] [PubMed] [Google Scholar]
- 97.Walsh PR, Williams DB. Mediastinal adenopathy: finding the answer with endoscopic ultrasound-guided fine-needle aspiration biopsy. Intern Med J. 2005;35:392–398. doi: 10.1111/j.1445-5994.2005.00857.x. [DOI] [PubMed] [Google Scholar]
- 98.Fritscher-Ravens A, Sriram PV, Topalidis T, Hauber HP, Meyer A, Soehendra N, Pforte A. Diagnosing sarcoidosis using endosonography-guided fine-needle aspiration. Chest. 2000;118:928–935. doi: 10.1378/chest.118.4.928. [DOI] [PubMed] [Google Scholar]
- 99.Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey W, Hawes RH, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–447. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 100.Savides TJ, Binmoeller K and Sarlin R. Effectiveness of EUS/FNA for diagnosing lung cancer in a managed care setting. Gastrointest Endosc. 2005;51:AB143. [Google Scholar]
- 101.Fritscher-Ravens A, Petrasch S, Reinacher-Schick A, Graeven U, Konig M, Schmiegel W. Diagnostic value of endoscopic ultrasonography-guided fine-needle aspiration cytology of mediastinal masses in patients with intrapulmonary lesions and nondiagnostic bronchoscopy. Respiration. 1999;66:150–155. doi: 10.1159/000029357. [DOI] [PubMed] [Google Scholar]
- 102.Mishra G, Sahai AV, Penman ID, Williams DB, Judson MA, Lewin DN, Hawes RH, Hoffman BJ. Endoscopic ultrasonography with fine-needle aspiration: an accurate and simple diagnostic modality for sarcoidosis. Endoscopy. 1999;31:377–382. doi: 10.1055/s-1999-32. [DOI] [PubMed] [Google Scholar]
- 103.Fritscher-Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C, Jaeckle S, Thonke F, Soehendra N. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278–2284. doi: 10.1111/j.1572-0241.2000.02243.x. [DOI] [PubMed] [Google Scholar]
- 104.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 105.Vazquez-Sequeiros E, Norton ID, Clain JE, Wang KK, Affi A, Allen M, Deschamps C, Miller D, Salomao D, Wiersema MJ. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751–757. doi: 10.1067/mge.2001.112741. [DOI] [PubMed] [Google Scholar]
- 106.Shimizu Y, Mera K, Tsukagoshi H, Takamasa M, Kawarazaki M, Watanabe Y, Tomohiko Nakasato, Oohara M, Hosokawa M, Fujita M, et al. Endoscopic ultrasonography for the detection of lymph node metastasis in superficial esophageal carcinoma. Dig Endosc. 1997;9:178–182. [Google Scholar]
- 107.Vilmann P. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lymph nodes. Gastrointest Endosc. 1996;43:S24–S29. doi: 10.1016/s0016-5107(96)81510-0. [DOI] [PubMed] [Google Scholar]