Abstract
The pathogenesis of high‐grade serous carcinoma of the ovary has come into sharper focus as closer attention has been paid to the earlier phases of this disease. The study of patients with BRCA mutation has been of particular value, in as much as the examination of prophylactic salpingo‐oophorectomies will reveal an early cancer in approximately 5% of individuals. Recently studies have shown that about 80% of these early carcinomas originate in the distal fallopian tube. This review summarizes the recent data supporting the distal fallopian tube as an important site for serous carcinogenesis, stressing both the presence of a novel precursor (the p53 signature) and the application of this model to all women irrespective of BRCA status. The challenges and unmet needs unmasked by this paradigm shift in ovarian cancer research are discussed.
Keywords: Serous carcinoma, Ovarian carcinoma, Intraepithelial carcinoma, p53, BRCA
1. Introduction
Most epithelial cancers are preceded by a non‐malignant condition (precursor) that in a variable percentage of cases, progressively evolves into a malignancy. In the case of cervical cancer, the precursor is both a clinically or microscopically visible abnormality and treatable by conservative ablation. These two properties have resulted in a dramatic reduction in cervical cancer deaths in the past 50 years, notwithstanding the promise of the recently introduced preventive vaccine. It underscores further that fact that cervical cancer can be prevented by blocking the succession of biologic events leading to cancer by simply preventing HPV infection from occurring.
Ovarian cancer accounts for 3% of malignancies in women and strikes approximately 24,000 women each year and eventually kills over 60% of those afflicted because about two in three are discovered after the disease has spread beyond the reproductive organs (American Cancer Society, 2007). In terms of control, it represents a striking contrast to its counterpart in the cervix. The disease is not caused by a virus that can be controlled with a vaccine, it originates in the upper female genital tract, a site with no direct access for cellular sampling and screening, and has until recently not been linked to a precursor that could be characterized biologically and temporally.
The lifetime risk of developing an ovarian cancer is much higher for women with hereditary mutations in BRCA1 or BRCA2 (Lancaster et al., 2007). Because serous carcinoma is usually discovered at a late clinical stage after spreading to the ovarian surface and peritoneum, it has a poor outcome.
Recent molecular studies have suggested that epithelial ovarian malignancies can be divided into two groups based on shared genetic mutations and observed progression from precursor lesions (Shih and Kurman, 2004; Singer et al., 2005). The type I tumors show mutations in a variety of pathways including mismatch repair genes, BRAF, KRAS, Beta‐catenin, and PTEN. This family includes clear cell, endometrioid, mucinous, and low‐grade serous carcinomas as well as the borderline (serous, mucinous, endometrioid) tumors. Type I tumors seem to evolve in a stepwise fashion from cortical inclusion cysts and endometriosis to borderline tumors (except clear cell) to invasive malignancies. Low‐grade serous carcinomas, for instance, are more likely to have mutations in BRAF and KRAS similar to their borderline counterparts. High‐grade serous carcinomas (type II tumors) commonly show mutations in p53 and are usually not found in association with adjacent borderline serous tumors. High‐grade serous malignancies are usually discovered at an advanced stage, which has made identification of a precursor lesion elusive in the past.
1.1. A novel origin of pelvic serous carcinoma: the distal fallopian tube, STICs, and “p53 signatures”
Until recently, the only carcinogenic sequence that had been formulated for serous carcinoma was in the endometrium. Endometrial serous carcinoma, described in detail by Hendrickson and colleagues in 1983, was subsequently shown to be closely linked to high p53 accumulation – due to p53 mutations – and poor prognosis (Eifel et al., 1983; Kohler et al., 1992; Tashiro et al., 1997; Sherman et al., 1995).
This was followed by observations that many uterine serous carcinomas did not invade the underlying myometrium (Sherman et al., 1995; Ambros et al., 1995; Wheeler et al., 2000; Zheng et al., 1998). This tumor subtype, designated serous endometrial intraepithelial carcinoma, was presumed to be as early, non‐invasive, but potentially aggressive form of serous carcinoma, with the capacity to produce distant metastases in the absence of frank invasion.
Serous intraepithelial carcinomas (STIC) are also reported in the fallopian tube, but are rare as solitary entities, usually seen in association with more advanced fallopian tube or pelvic serous cancers (Bannatyne and Russell, 1981). One exception is the patient with a hereditary BRCA mutation. Because these individuals have a substantial lifetime risk of ovarian cancer, prophylactic removal of the fallopian tubes and ovaries is now the standard, and results in an 85% reduction in pelvic cancer risk. One prior report in 2000 identified a clear cell carcinoma in the fimbria of a woman with a hereditary BRCA1 mutation, a finding in contrast to the estimated low incidence of tubal carcinoma in the population (Hartley et al., 2000; Stewart et al., 2007). Subsequent studies, however, have shown that between 57 and 100% of prophylactic surgeries in this population reveal involvement of the fallopian tube, most at the fimbriated end, in both BRCA1 and BRCA2 carriers (Powell et al., 2005; Finch et al., 2006; Callahan et al., 2007; Leeper et al., 2002). These observations lead to the development of a proposed fallopian tube pathway to high‐grade serous malignancies. Thus, like the endometrium, STIC is an intraepithelial malignancy with potential for spread and directly precedes invasive serous carcinoma. STICs are composed of secretory cells showing significant atypia, such as loss of nuclear polarity, prominent nucleoli, and increased nuclear to cytoplasmic ratio. By immunohistochemistry, STICs, almost by definition, contain p53 mutations and in about 80% will be highlighted by nuclear accumulation of mutated p53 protein. They are also highly proliferative.
Important features of early serous cancer (STIC) in the distal fallopian tube are cytologic atypia, high proliferative index and strong staining for p53. The latter is strongly linked to accumulation of mutant p53 protein. Staining for p53 is negative in 10–15% of these cases because mutations in the gene can occur upstream of the protein segment targeted by the immunohistochemical analysis and such truncated mutated protein products will go undetected. In our experience with early serous cancers, all cases with thorough p53 sequencing scored positive for a mutation (Kindelberger et al., 2007; Carlson et al., 2008; Lee et al., 2007). In a recent study of pelvic serous carcinomas we found p53 mutations in 92% (Roh M and Miron A, unpublished data). This, combined with the prior experience of others, underscores the significance of p53 mutations in the pathogenesis of pelvic serous cancer and the earliest malignant change, the STIC. The question that has remained is whether p53 mutations are present prior to the development of malignancy.
Although STIC strongly links the fimbria to serous carcinogenesis in the women with BRCA mutations, it does not in itself provide any additional insight into mechanism. However, because mutations in p53 are considered integral to the development of both STICs and invasive carcinomas, prior investigators have immunostained the tubes and ovaries of woman with ovarian cancer or at high risk to search for this phenomenon in non‐malignant (dysplastic) epithelium. Such studies have met with mixed results. Hudson et al., identified p53‐immuno‐positive ovarian cortical inclusion cysts in women with ovarian cancer and speculated that these were precursor lesions (Hutson et al., 1995). Barakat et al. (2000) performed a similar analysis of ovaries from women with BRCA mutations who were undergoing risk‐reducing surgery, but found none. Recently Folkins et al. (2008) confirmed the findings of Barakat et al., reporting only a single focus of p53 positive ovarian surface epithelium in 75 cases analyzed from this population. Studies of the fallopian tubes have been more revealing. Piek et al. (2001) studied the fallopian tubes from BRCA+ women and reported higher proliferative rates in the tubal epithelium than controls and noted the presence of p53 positive “dysplastic” foci.
More recently, Lee et al. (2007) systematically analyzed a series of BRCA+ women and controls for p53 positivity. They required the presence of at least 12 consecutive p53 positive nuclei, a criterion that was revised to consecutive “secretory” cell nuclei, given that these cells sometimes intermixed with normal ciliated cells. The latter were not p53 positive, consistent with the concept that the secretory cells were uniquely susceptible to this process. These variable sized stretches of p53 positive cells were designated “p53 signatures”. Upon their discovery, the question that arose was whether p53 signatures were a credible precursor to pelvic serous cancer.
Prior studies had shown that serous carcinomas were imbued with secretory cell characteristics and Piek et al. (2001) noted that the p53 positive cells that they observed were secretory in type. Lee et al. (2007) confirmed this and noted further that the p53 signatures were most common in the fimbria, a site that Cass et al. (2005) and several others had confirmed to be the dominant site of origin for tubal carcinomas. Moreover, Lee et al. linked p53 signatures to expression of γ‐H2AX, which localizes to areas of DNA damage, and p53 mutations. Equally important, they noted that the p53 signatures were equally common in women with and without known BRCA mutations being present in approximately one‐third of each group. This finding was subsequently confirmed by another study and strongly suggested that the initial events of serous carcinogenesis – DNA damage of secretory cells and p53 mutations – were not influenced by known genetic risk factors (Folkins et al., 2008).
The possibility that a “generic” precursor to pelvic serous cancer arose in the tube was strengthened further in a recent study by Saleemuddin et al. (2008). In this study, BRCA+ women were segregated into those with and without p53 signatures. The former group had a significantly older age of first birth and lower parity, reproductive factors associated with higher lifetime ovulation events that segregate with ovarian cancer risk. Two final observations further solidified the relationship between p53 signatures and pelvic serous cancer. First, p53 signatures are sometimes seen in continuity with intraepithelial carcinomas and can share common p53 mutations. This is the most physically compelling evidence that the p53 signature precedes STIC. Second, intermediate lesions or – “tubal intraepithelial lesions in transition” (TILTs) – in which p53 positive epithelium displays a higher degree of proliferative activity but falls short of malignancy, have also been described (Jarboe et al., 2008). Together this body of evidence indicated that an Novel precursor to pelvic serous carcinoma resided in the distal fallopian tube and that it is the earliest lesion in a continuum of the tubal serous carcinogenic sequence (Figure 1) (Table 1).
Figure 1.
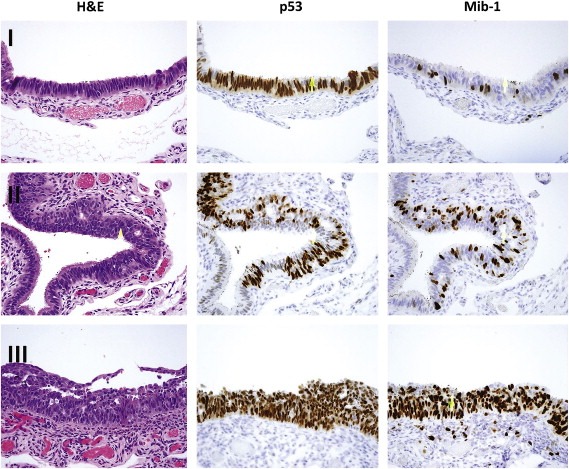
– Morphologic and immunohistochemical correlates of a serous carcinogenic sequence in the distal fallopian tube. Step I, the p53 signature characterized by normal appearing epithelium with nuclear p53 immunostaining and a low proliferative index. Step II, an intermediate lesion, with increased proliferation and mild epithelial atypia. Step III, a tubal intraepithelial carcinoma, with neoplastic epithelium and high proliferative index.
Table 1.
The link between a putative precursor (p53 signature), STIC and pelvic serous cancer.
| 1. Predilection for the distal fallopian tube (fimbria) |
| 2. Occurs in the setting of cellular DNA damage (γ‐H2AX+) |
| 3. Target the tubal secretory cell (HMFG2+/BCL2+/p73(−)/LHS28(−) |
| 4. Strong nuclear accumulation of p53 |
| 5. Ovarian cancer‐associated p53 mutations |
| 6. Documented continuity between the p53 signature and STIC |
| 7. Existence of intermediate forms with features of both p53 signature and STIC |
| 8. Shared risk factors (low parity, older age of first birth) |
1.2. The widening impact of the tubal serous carcinogenic sequence
The association of STIC with BRCA mutations has strongly endorsed the concept that this entity is the more common early malignancy in women with genetic risk. This association raised the obvious question of whether pelvic serous malignancies without a documented history of a BRCA mutation could be linked to the distal fallopian tube. Three recent studies have supported this pathway as an explanation for a significant proportion of pelvic serous carcinomas, including many fulfilling the criteria for either ovarian or primary peritoneal serous carcinomas. Kindelberger et al. (2007) showed that approximately one‐half of tumors classified as serous ovarian carcinomas co‐existed with a STIC. Carlson et al. (2008) similarly showed that complete examination of the fallopian tubes in women with primary peritoneal serous carcinomas disclosed a STIC in 47%. In both studies, p53 mutation analysis in the STICs and remote tumors disclosed the same mutations, genetically linking the two. Moreover, a recent study from another group that examined paired STICs and remote tumors supported a genetic link between STIC and remote tumors by shared anomalies in chromosomal number (Salvador et al., 2008).
The above studies suggest that a significant percentage of ovarian or peritoneal serous carcinomas could be explained by an origin in the distal fallopian tube. Figure 2 illustrates two potential scenarios, one arising from the ovary and the other from the distal tube. Because serous carcinomas presumed to arise from the tube and ovary share a similar immunophenotype, it is likely that the cell of origin is the same, either tubal epithelium or tubal‐like epithelium in the ovarian cortex.
Figure 2.
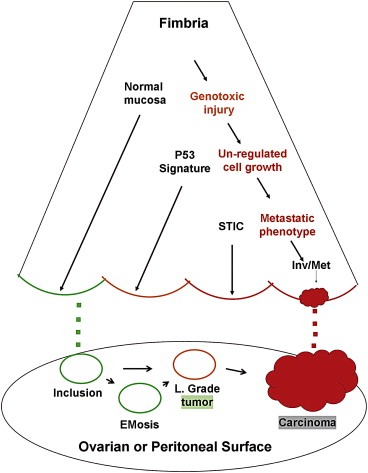
– Schematic of the distal fallopian tube and two pathways to pelvic serous cancer. In one pathway, passive transfer of normal cells from the fimbria (green dots) leads to ovarian cortical inclusions that are usually quiescent but may ultimately evolve into serous malignancy via endometriosis or low‐grade tumors. In the second pathway, several tubal mucosal epithelial entities, including normal secretory cells (green), p53 signatures (yellow), and tubal intraepithelial carcinoma (STIC, red) are linked by a series of connected biologic events including unrepaired genotoxic injury, unregulated cell growth, and eventually, acquisition of a metastatic phenotype. The latter permits spread to the ovarian or peritoneal surfaces (red dots).
1.3. Implications of early serous carcinogenesis and the feasibility of cancer prevention
One of the greatest accomplishments in gynecologic cancer prevention in the past 50 years has been the widespread use of the Papanicolaou smear and more recently, the discovery and development of a vaccine that promises to significantly reduce the incidence of high‐grade cervical cancer precursors and their malignant counterparts. The past and future success of this venture has depended heavily on two characteristics of cervical carcinogenesis: first, these tumors are preceded by precursor lesion that will remain non‐invasive for as long as 20 years, and second, they are inextricably linked to a subset of human papillomaviruses. Prevention of the precursor lesion and subsequent development of invasive squamous carcinoma of the cervix, long a function of the cumbersome combination of Papanicolaou smear surveillance and precursor removal, can now be achieved by vaccinating the individual prior to her (or his) exposure to the virus by sexual contact.
The precursor spectrum outlined above for pelvic serous cancer presents a completely different kind of challenge for preventing or interrupting the course of this malignancy. Serologic detection has yet to emerge as a viable approach to ovarian cancer detection, although it is reasonable to assume that endometrioid and mucinous tumors remain localized for a sufficient length of time that their early detection will prevent ovarian cancer deaths. In contrast, serous carcinomas virtually always involve the pelvic peritoneum, even when detected in low volume, meaning that early detection might still encounter incurable disease in the context of current chemotherapy. Nevertheless, in light of the above review, certain intriguing questions remain, including the following (Table 2).
What are the risk factors for the p53 signature? Preliminary evidence suggests that the p53 signature possesses risk factors in common with pelvic serous cancer. This is a compelling endorsement for epidemiologic studies that define the p53 signature as a surrogate risk factor for serous cancer.
Reproducing the p53 signature in vitro is an important goal, and one that may be accomplished in the context of successful efforts to cultivate tubal epithelium. This is important because the p53 signature is limited to a small number of cells that may be more difficult to study by conventional means.
What is the incidence of occult serous carcinoma in the fallopian tube in the general population? With the examination of the distal fallopian tubes in routine hysterectomies, we are occasionally seeing occult malignancies in otherwise healthy individuals, specifically those who do not have a history of ovarian cancer. Large scale histologic studies of these populations would determine the approximate frequency of early cancer in the population.
Could STICs be detected by imaging or biomarker surveillance? There is evidence from the BRCA+ population that STICs do not immediately exfoliate tumor cells with the capacity to spread and metastasize. It is conceivable that STICs, which are biologically abnormal and potentially detectible, could be identified at a time point when the risk of mortality was much lower, hence an argument for early detection. The challenge will be devising an effective detection strategy.
Table 2.
Important research questions regarding early serous carcinogenesis.
| 1. Defining the risk factors for the p53 signature. |
| 2. Reproducing the p53 signature and the tubal serous carcinogenic sequence in vitro. |
| 3. Determining the frequency of occult tubal serous carcinoma in the general population. |
| 4. Successful imaging or molecular‐based detection of early serous carcinomas prior to extra‐genital spread. |
1.4. Caveats
Despite the above advances, two realities must be emphasized. 1) The origin of a significant percentage of pelvic serous carcinomas remains unknown, even with protocols that focus on extensive pathologic analysis of fallopian tube. 2) The proportion of symptomatic pelvic serous carcinomas in women with a family history of BRCA mutations that are assigned to the ovary is much higher than that assumed from the evaluation of prophylactic salpingo‐oophorectomies in this population, which show a high percentage of tubal involvement (Schulte S, Folkins AK, Crum CP, unpublished data) (Piek et al., 2003). Evidence for p53 signatures elsewhere in the female genital tract – Müllerian inclusions in the ovary or peritoneum – is scant, yet the odds that a single source can be identified to explain all serous carcinomas would seem low. Nevertheless, the shared properties between serous cancer precursors and their malignant endpoints provide a framework on which to construct a tangible model for this disease. Targeting the precursor may not prove a viable strategy for reducing the risk of pelvic serous cancer, but it is a strategy that merits close examination.
Acknowledgements
This work was supported by grants from the NCI (P50 CA105009 [SPORE]: D. Cramer, PI), NCI KO8 CA108748 (R Drapkin, PI), NCI 1R21CA124688‐01A1 (CP Crum, PI), The Charlotte Geyer Foundation (CP Crum, PI), The Columbia Hospital For Women Research Foundation (CP Crum, PI), and the Francis Ward Paine and TSA Pemberton Funds from the Division of Women's and Perinatal Pathology, Brigham and Women's Hospital.
Crum Christopher P., (2009), Intercepting pelvic cancer in the distal fallopian tube: Theories and realities, Molecular Oncology, 3, doi: 10.1016/j.molonc.2009.01.004.
References
- American Cancer Society, 2007. Cancer Facts and Figures www.cancer.org [Google Scholar]
- Ambros, R.A. , Sherman, M.E. , Zahn, C.M. , Bitterman, P. , Kurman, R.J. , 1995 Nov. Endometrial intraepithelial carcinoma: A distinctive lesion specifically associated with tumors displaying serous differentiation. Hum. Pathol.. 26, (11) 1260–1267. [DOI] [PubMed] [Google Scholar]
- Bannatyne, P. , Russell, P. , 1981. Early adenocarcinoma of the fallopian tubes. A case for multifocal tumorigenesis. Diagn. Gynecol. Obstet.. 3, 49–60. [PubMed] [Google Scholar]
- Barakat, R.R. , Federici, M.G. , Saigo, P.E. , Robson, M.E. , Offit, K. , Boyd, J. , 2000 Jul 15. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 89, (2) 383–390. [DOI] [PubMed] [Google Scholar]
- Callahan, M.J. , Crum, C.P. , Medeiros, F. , Kindelberger, D.W. , Elvin, J.A. , Garber, J.E. , 2007 Sep 1. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol.. 25, (25) 3985–3990. [DOI] [PubMed] [Google Scholar]
- Carlson, J.W. , Miron, A. , Jarboe, E.A. , Parast, M.M. , Hirsch, M.S. , Lee, Y. , 2008 Sep 1. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol.. 26, (25) 4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass, I. , Holschneider, C. , Datta, N. , Barbuto, D. , Walts, A.E. , Karlan, B.Y. , 2005 Dec. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype?. Obstet. Gynecol.. 106, (6) 1327–1334. [DOI] [PubMed] [Google Scholar]
- Eifel, P.J. , Ross, J. , Hendrickson, M. , Cox, R.S. , Kempson, R. , Martinez, A. , 1983 Sep 15. Adenocarcinoma of the endometrium. analysis of 256 cases with disease limited to the uterine corpus: treatment comparisons. Cancer. 52, (6) 1026–1031. [DOI] [PubMed] [Google Scholar]
- Finch, A. , Shaw, P. , Rosen, B. , Murphy, J. , Narod, S.A. , Colgan, T.J. , 2006 Jan. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol. Oncol.. 100, (1) 58–64. [DOI] [PubMed] [Google Scholar]
- Folkins, A.K. , Jarboe, E.A. , Saleemuddin, A. , Lee, Y. , Callahan, M.J. , Drapkin, R. , 2008 May. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol.. 109, (2) 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, A. , Rollason, T. , Spooner, D. , 2000. Clear cell carcinoma of the fimbria of the fallopian tube in a BRCA1 carrier undergoing prophylactic surgery. Clin. Oncol. (R. Coll. Radiol.). 12, 58–59. [DOI] [PubMed] [Google Scholar]
- Hutson, R. , Ramsdale, J. , Wells, M. , 1995 Oct. P53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology. 27, (4) 367–371. [DOI] [PubMed] [Google Scholar]
- Jarboe, E. , Folkins, A. , Nucci, M.R. , Kindelberger, D. , Drapkin, R. , Miron, A. , 2008 Jan. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int. J. Gynecol. Pathol.. 27, (1) 1–9. [DOI] [PubMed] [Google Scholar]
- Kindelberger, D.W. , Lee, Y. , Miron, A. , Hirsch, M.S. , Feltmate, C. , Medeiros, F. , 2007 Feb. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol.. 31, (2) 161–169. [DOI] [PubMed] [Google Scholar]
- Kohler, M.F. , Berchuck, A. , Davidoff, A.M. , Humphrey, P.A. , Dodge, R.K. , Iglehart, J.D. , 1992 Mar 15. Overexpression and mutation of p53 in endometrial carcinoma. Cancer Res.. 52, (6) 1622–1627. [PubMed] [Google Scholar]
- Lancaster, J.M. , Powell, C.B. , Kauff, N.D. , Cass, I. , Chen, L.M. , Lu, K.H. , 2007 Nov. Society of gynecologic oncologists education committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol. Oncol.. 107, (2) 159–162. [DOI] [PubMed] [Google Scholar]
- Lee, Y. , Miron, A. , Drapkin, R. , Nucci, M.R. , Medeiros, F. , Saleemuddin, A. , 2007 Jan. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol.. 211, (1) 26–35. [DOI] [PubMed] [Google Scholar]
- Leeper, K. , Garcia, R. , Swisher, E. , Goff, B. , Greer, B. , Paley, P. , 2002 Oct. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol. Oncol.. 87, (1) 52–56. [DOI] [PubMed] [Google Scholar]
- Piek, J.M. , van Diest, P.J. , Zweemer, R.P. , Jansen, J.W. , Poort-Keesom, R.J. , Menko, F.H. , 2001 Nov. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol.. 195, (4) 451–456. [DOI] [PubMed] [Google Scholar]
- Piek, J.M. , Torrenga, B. , Hermsen, B. , Verheijen, R.H. , Zweemer, R.P. , Gille, J.J. , 2003. Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: a clinic-based study. Fam. Cancer. 2, (2) 73–78. [DOI] [PubMed] [Google Scholar]
- Powell, C.B. , Kenley, E. , Chen, L.M. , Crawford, B. , McLennan, J. , Zaloudek, C. , 2005 Jan 1. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J. Clin. Oncol.. 23, (1) 127–132. [DOI] [PubMed] [Google Scholar]
- Shih, I. , Kurman, R.J. , 2004 May. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol.. 164, (5) 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, G. , Stohr, R. , Cope, L. , Dehari, R. , Hartmann, A. , Cao, D.F. , 2005 Feb. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am. J. Surg. Pathol.. 29, (2) 218–224. [DOI] [PubMed] [Google Scholar]
- Sherman, M.E. , Bur, M.E. , Kurman, R.J. , 1995 Nov. P53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum. Pathol.. 26, (11) 1268–1274. [DOI] [PubMed] [Google Scholar]
- Stewart, S.L. , Wike, J.M. , Foster, S.L. , Michaud, F. , 2007 Dec. The incidence of primary fallopian tube cancer in the united states. Gynecol. Oncol.. 107, (3) 392–397. [DOI] [PubMed] [Google Scholar]
- Saleemuddin, A. , Folkins, A.K. , Garrett, L. , Garber, J. , Muto, M.G. , Crum, C.P. , 2008 Aug 20. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol. Oncol.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador, S. , Rempel, A. , Soslow, R.A. , Gilks, B. , Huntsman, D. , Miller, D. , 2008 Sep. Chromosomal instability in fallopian tube precursor lesions of serous carcinoma and frequent monoclonality of synchronous ovarian and fallopian tube mucosal serous carcinoma. Gynecol. Oncol.. 110, (3) 408–417. [DOI] [PubMed] [Google Scholar]
- Tashiro, H. , Isacson, C. , Levine, R. , Kurman, R.J. , Cho, K.R. , Hedrick, L. , 1997 Jan. P53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am. J. Pathol.. 150, (1) 177–185. [PMC free article] [PubMed] [Google Scholar]
- Wheeler, D.T. , Bell, K.A. , Kurman, R.J. , Sherman, M.E. , 2000 Jun. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am. J. Surg. Pathol.. 24, (6) 797–806. [DOI] [PubMed] [Google Scholar]
- Zheng, W. , Khurana, R. , Farahmand, S. , Wang, Y. , Zhang, Z.F. , Felix, J.C. , 1998 Dec. P53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am. J. Surg. Pathol.. 22, (12) 1463–1473. [DOI] [PubMed] [Google Scholar]


