Abstract
The aim of this study was to investigate the effects of self-reinnervation of the medial (MG) and lateral gastrocnemius (LG) muscles on joint kinematics of the whole hindlimb during overground walking on surfaces of varying slope in the cat. Hindlimb kinematics were assessed (1) with little or no activity in MG and LG (short-term effects of self-reinnervation), and (2) after motor function of these muscles was presumably recovered but their proprioceptive feedback permanently disrupted (long-term effects of self-reinnervation). The stance phase was examined in three walking conditions: downslope (−50%, i.e. −26.6°), level (0%) and upslope (+50%, +26.6°). Measurements were performed prior to and at consecutive time points (between 1 and 57 weeks) after transecting and immediately suturing MG and LG nerves. It was found that MG-LG self-reinnervation did not significantly change hip height and hindlimb orientation in any of the three walking conditions. Substantial short-term effects were observed in the ankle joint (e.g., increased flexion in early stance) as well as in metatarsophalangeal and knee joints, leading to altered interjoint coordination. Hindlimb kinematics in level and upslope walking progressed back towards baseline within 14–19 weeks. Thus in these two conditions the cats were walking without any detectable kinematic deficits, despite the absence of length feedback from two major ankle extensors. This was verified in a decerebrate preparation for four of the five cats. In contrast, ankle joint kinematics as well as interjoint coordination in downslope walking gradually progressed towards, but never reached their baseline patterns. The short-term effects can be explained by both mechanical and neural factors that are affected by the functional elimination of MG and LG. Permanent changes in kinematics during downslope walking indicate the importance of proprioceptive feedback from the MG and LG muscles in regulating locomotor activity of ankle extensors. Full recovery of hindlimb kinematics during level and upslope walking suggests that the proprioceptive loss is compensated by other sensory sources (e.g. cutaneous receptors) or altered central drive.
Keywords: Locomotion, Kinematics, Stretch reflex, Nerve injury, Cat
Introduction
Surgical self-reinnervation, which involves transecting and suturing specific nerve branches, is a procedure applied in animal models to emulate a nerve lesion induced through trauma and its subsequent surgical repair in humans. This intervention involves a paralytic phase (i.e., no muscle excitation) followed by a recovery phase in which regenerating motoneuron axons re-occupy motor endplates in the reinnervated muscle (Sanes and Lichtman 1999). Subsequently, active motor function is returned to the muscle (Gregor et al. 2003). Although the motor site appears to recover fully, self-reinnervation results in a long-term (up to 36 months post surgery) loss of length feedback and in an attenuation of force feedback in the corresponding muscles in the cat (Cope and Clark 1993; Cope et al. 1994). Several studies have documented neuromuscular recovery at the muscle level after self-reinnervation (e.g., Gordon and Stein 1982b; Foehring et al. 1986), but very little is known about hindlimb kinematics following this procedure. Gait kinematics are the final and most general output variables of the motor system. Therefore, monitoring kinematics is an important clinical tool for patient diagnostics and evaluation of functional recovery after peripheral nerve injury or neurotomy (Simon et al. 1978; Lehmann et al. 1985).
Recently, it has been reported for a limited number of cats that ankle and knee kinematics remain altered 9 months following self-reinnervation of all triceps surae muscles (i.e., soleus, medial and lateral gastrocnemius), in particular during downslope walking (Abelew et al. 2000). Note the Abelew study focused on the long-term effects of self-reinnervation when neuromuscular recovery was thought to be complete, but proprioceptive feedback permanently disrupted (Cope et al. 1994). As downslope walking requires more pronounced muscle–tendon unit (MTU) lengthening of the ankle extensors in stance compared to level and upslope walking (Gregor et al. 2006), Abelew et al. (2000) explained these long-term deficits in joint angle kinematics by the loss of length feedback from the self-reinnervated muscles.
The present study extends the data set presented by Abelew et al. (2000) by (1) measuring kinematics of all hind-limb joints as opposed to ankle and knee only, (2) including consecutive time points (from 1 to 57 weeks) after the self-reinnervation surgery, (3) testing a greater number of cats allowing for a comprehensive statistical analysis of a more comprehensive data set, and (4) applying the self-reinnervation procedure only to medial (MG) and lateral gastrocnemius (LG) muscles. MG and LG were selected to test if the kinematic deficit found previously (Abelew et al. 2000) was a consequence mainly of the loss of proprioceptive feedback from soleus (SO). In some tasks, such as paw shaking in cats (Smith et al. 1980; Fowler et al. 1988; Prilutsky et al. 2005a), differential activation of the one-joint SO and the two-joint gastrocnemius muscles has been reported. In a previous study (Gregor et al. 2006), the results indicated that force feedback plays a larger role in the activity of the MG and LG muscles, while length feedback appears to contribute more to the activity of SO muscle. We hypothesized that the kinematic deficit during down-slope walking would be absent following self-reinnervation of only the MG and LG muscles if the loss of proprioceptive feedback from SO was responsible for the kinematic deficit reported in the study by Abelew et al. (2000).
Therefore, the aim of this report was to document the effects of MG-LG self-reinnervation on joint kinematics of the whole hindlimb (metatarsophalangeal, ankle, knee and hip) during overground slope walking in the cat. Specifically, extensive quantitative analysis was performed to examine hindlimb kinematics (1) with little or no activity in the MG and LG muscles (short-term effects of self-reinnervation of these muscles) and (2) after motor function of these muscles was presumably recovered but their stretch reflex disrupted (long-term effects of self-reinnervation). This is the first systematic report in which the short- and long-term effects of MG-LG self-reinnervation on gait kinematics have been assessed, including all joints of the hindlimb, within the same group of animals. This study is the first part of a larger project. Future studies will investigate the effects of self-reinnervation of cat MG and LG muscles on ground reaction forces, hindlimb kinetics, and patterns of muscle activity in slope walking, as well as the time course of histological changes in the self-reinnervated nerves and muscles after self-reinnervation. Preliminary results on joint kinematics have been presented in abstract form (Maas et al. 2004).
Methods
Animal care and training
All surgical and experimental procedures were in agreement with the “Principles of Laboratory Animal Care” (NIH publication No.86-23, revised 1985) and the University Institutional Animal Care and Use Committee. Adult female cats (Felis Domesticus, n = 5, mean body mass = 3.1 kg, SD 0.6) were selected on the basis of friendliness and attraction to food reward. All cats were housed in one large room of adequate space with access to food and water ad libitum.
Prior to the experimental measurements, each cat was trained to walk within a Plexiglas enclosed walkway (2.5 × 0.4 m) on a level surface (0%) as well as on up- and down-sloped surfaces (±50%, i.e. ±26.6°), using operant conditioning procedures involving food reward (e.g., Prilutsky et al. 2005b). The walkway surface was covered with a thin layer of non-slip rubberized material, to prevent paw slippage during slope walking. The mat over the force plate was isolated from the remainder of the walkway.
Hindlimb kinematics
The cats were measured in each slope condition prior to (i.e., baseline) and at consecutive time points after surgical self-reinnervation of the MG and LG muscles (see below). Prior to measurements, a reversible sedative-analgesic was administered (i.e., 60 µg/kg medetomidine; 60 µg/kg reversing agent atipamezole), both hindlimbs were shaved and external measurements of hindlimb segment lengths (i.e., pelvis, thigh, shank, tarsals and digits) were made. Refective markers (diameter = 6 mm) were placed over the segment endpoints of both hindlimbs: iliac crest (IC), greater trochanter (HIP), lateral femoral epicondyle (KNEE), lateral malleolus (ANKLE), fifth metatarsophalangeal (MTP) joint and the most distal part of the fifth digit (TOE). The marker positions were used to define joint angles (Fig. 1) for subsequent analysis. The cats walked within the walkway set at one of the three walking conditions (50% downslope, level or 50% upslope). Step cycles were accepted only if (1) the cat walked with an uninterrupted gait at a steady pace through the walkway, as assessed by the mean velocity of right and left IC markers and (2) if the stance time was between 400 and 650 ms, as speed of locomotion affects gait kinematics (e.g., Goslow et al. 1973; Prilutsky et al. 1994). Selection of step cycles with such a relatively narrow time range resulted in very similar stance times for each time window and each slope condition (see Table 1), as opposed to the range of stance times reported in previous studies (Carlson-Kuhta et al. 1998; Smith et al. 1998; Abelew et al. 2000). Paw contact and paw liftoff (i.e., the stance phase) were identified using ground reaction force data, obtained from a miniature force plate (Bertec Inc.) concealed in the walkway floor. Reaction force data were collected at a sampling rate of 360 Hz and filtered using a symmetric low-pass Butterworth filter (cut-off frequency of 25 Hz). In our follow-up studies, these ground reaction forces are used to calculate resultant moments of force at the joints (for preliminary results see Prilutsky et al. 2006a, b). Trials in which paw slippage occurred were not used for analysis.
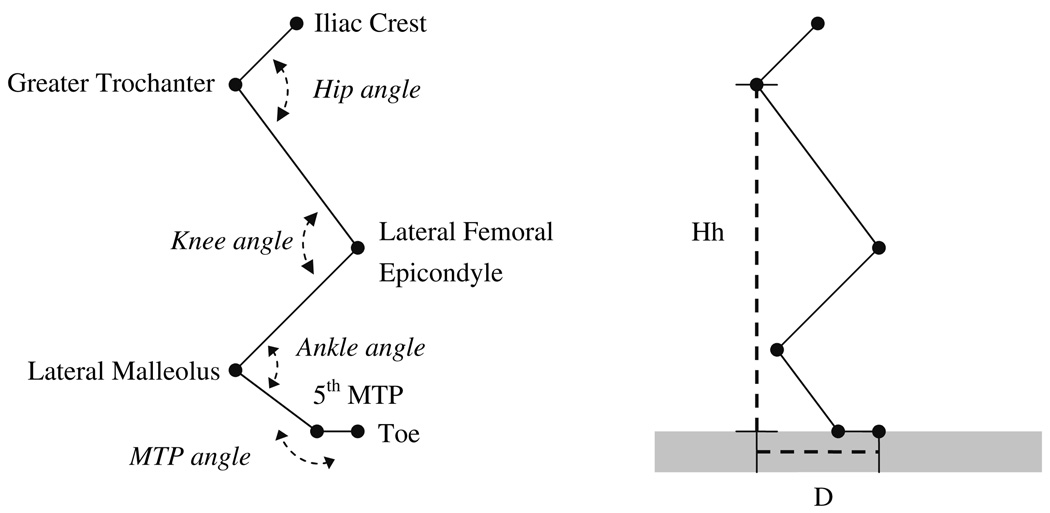
Fig. 1.
Definition of joint angles (left) and parameters for hindlimb orientation (right). The endpoints of the segments (i.e., pelvis, thigh, shank, tarsals, and digits) over which the reflective markers were placed are indicated: iliac crest, greater trochanter, lateral femoral epicondyle, lateral malleolus, fifth metatarsophalangeal joint (MTP) and the most distal part of the fifth digit (Toe). Joint angles were defined as the included angle between the adjacent segments, except for the MTP joint. Hh (hip height), the orthogonal distance from walkway to greater trochanter. D, distance from Hh line to the most distal part of the fifth digit (Toe) parallel to the walkway. D is measured at paw contact when the hindlimb has an anterior orientation (Da) and at paw liftoff when the hindlimb has a posterior orientation (Dp)
Table 1.
Step cycle parameters and hindlimb orientation for the three slope conditions before (control) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles
| Slope | Control | W1 | W2 | W3 | W4 | W5 |
|---|---|---|---|---|---|---|
| Cycle period (s) | ||||||
| Down | 0.78 (0.07) | 0.79 (0.06) | 0.78 (0.04) | 0.82 (0.16) | 0.76 (0.06) | 0.79 (0.07) |
| Level | 0.79 (0.07) | 0.83 (0.03) | 0.87 (0.09) | 0.84 (0.05) | 0.78 (0.04) | 0.79 (0.09) |
| Up | 0.80 (0.03) | 0.81 (0.05 | 0.85 (0.05) | 0.86 (0.02) | 0.79 (0.10) | 0.83 (0.07) |
| Speed (m/s) | ||||||
| Down | 0.50 (0.08) | 0.48 (0.06) | 0.50 (0.06) | 0.51 (0.19) | 0.49 (0.06) | 0.49 (0.08) |
| Level | 0.58 (0.09) | 0.50 (0.04) | 0.53 (0.05) | 0.53 (0.07) | 0.59 (0.09) | 0.59 (0.13) |
| Up | 0.52 (0.03) | 0.48 (0.03)# | 0.48 (0.05) | 0.45 (0.06)# | 0.50 (0.03) | 0.49 (0.04) |
| Stance time (ms) | ||||||
| Down | 498 (54) | 496 (35) | 503 (28) | 528 (120) | 503 (32) | 516 (50) |
| Level | 481 (39) | 510 (14) | 523 (31) | 522 (54) | 484 (37) | 478 (59) |
| Up | 529 (31) | 544 (22) | 567 (27) | 589 (23) | 530 (55) | 539 (38) |
| Stance (% of cycle) | ||||||
| Down | 64.0 (1.5) | 62.5 (1.5) | 64.6 (3.0) | 64.4 (4.2) | 66.2 (2.1) | 65.2 (2.7) |
| Level | 60.6 (0.6) | 61.7 (1.4) | 60.5 (2.8) | 62.4 (3.8) | 62.0 (2.0) | 61.0 (2.1) |
| Up | 65.8 (2.8) | 67.6 (2.9) | 66.7 (1.4) | 68.7 (2.1) | 66.8 (1.5) | 65.2 (0.9) |
| Swing (% of cycle) | ||||||
| Down | 36.0 (1.5) | 37.5 (1.5) | 35.4 (3.0) | 35.6 (4.2) | 33.8 (2.1) | 34.8 (2.7) |
| Level | 39.4 (0.6) | 38.3 (1.4) | 39.5 (2.8) | 37.6 (3.8) | 38.0 (2.0) | 39.0 (2.1) |
| Up | 34.2 (2.8) | 32.4 (2.9) | 33.3 (1.4) | 31.3 (2.1) | 33.2 (1.5) | 34.8 (0.9) |
| Da (cm) | ||||||
| Down | 15.7 (1.7) | 14.4 (2.3) | 15.6 (0.9) | 14.4 (1.9) | 15.3 (0.5) | 15.1 (0.9) |
| Level | 10.0 (0.8) | 10.4 (1.1) | 10.9 (0.8) | 10.8 (1.2) | 12.1 (2.0) | 10.6 (1.0) |
| Up | 7.0 (1.0) | 7.5 (1.1) | 7.7 (1.8) | 6.9 (2.5) | 7.8 (1.3) | 7.2 (1.8) |
| Dp (cm) | ||||||
| Down | 5.9 (2.9) | 5.9 (2.1) | 5.1 (2.1) | 6.5 (2.7) | 5.5 (2.2) | 6.3 (2.2) |
| Level | 15.1 (2.2) | 12.4 (1.1) | 13.9 (1.4) | 14.3 (0.9) | 13.4 (0.9) | 14.0 (1.8) |
| Up | 17.7 (0.9) | 16.7 (1.1) | 17.8 (1.2) | 17.7 (0.9) | 16.6 (2.1) | 17.7 (0.8) |
| Hh, PC (cm) | ||||||
| Down | 19.8 (1.3) | 19.0 (2.0) | 20.1 (2.0) | 20.2 (0.6) | 19.5 (1.5) | 20.0 (1.1) |
| Level | 22.2 (1.1) | 20.8 (1.7) | 22.2 (0.8) | 21.6 (1.3) | 21.7 (1.5) | 22.3 (0.6) |
| Up | 19.6 (0.9) | 18.5 (1.2) | 19.1 (1.4) | 18.7 (1.3) | 18.4 (1.2) | 19.5 (0.2) |
| Hh-min (cm) | ||||||
| Down | 19.0 (1.1) | 17.7 (2.4) | 18.9 (1.9) | 18.9 (1.1) | 18.5 (1.6) | 19.0 (1.0) |
| Level | 21.5 (1.0) | 19.9 (1.8) | 21.5 (0.8) | 21.2 (1.4) | 21.4 (1.5) | 21.9 (0.6) |
| Up | 19.0 (0.8) | 17.7 (1.6) | 18.5 (1.4) | 18.2 (1.5) | 18.1 (1.3) | 19.1 (0.1) |
| Number of step cycles | ||||||
| Down | 50 | 76 | 51 | 40 | 38 | 77 |
| Level | 43 | 95 | 92 | 55 | 48 | 72 |
| Up | 50 | 104 | 99 | 44 | 37 | 86 |
denotes a value significantly different from the control value. Values are shown as mean (SD). n = 5, except for W1 in upslope walking (n = 4). Hindlimb orientation parameters Da, Dp and Hh are defined in Fig. 1
Hip Height (Hh) at paw contact (PC) was equal to Hh at paw lift-off (PO) and, therefore, only one value is shown. Hh-min is the minimum hip height in stance
Hindlimb kinematics were calculated from the segment endpoint position data collected using either a six-camera Motus motion capture system (Peak Performance Technologies Inc., two cats) or a six-camera Vicon 460 motion capture system (VICON Inc., three cats). Digitized coordinates for a five-segment 2D model of the cat hindlimb including pelvis, thigh, shank, tarsals and digits were smoothed using a fourth-order, zero-lag Butterworth filter. For each marker, 98.5% of the power spectrum of the signal was used to determine the cut-off frequency (typically between 5 and 10 Hz). Knee joint position was estimated by triangulation using hip and ankle coordinates as well as thigh and shank segment lengths (Goslow et al. 1973; Fowler et al. 1993). Joint angles were defined as the included angle between the adjacent segments, except for the MTP joint (Fig. 1). Angular displacement for the MTP joint was measured using the plantar angle at the intersection of the metatarsal and phalangeal segmental lines on the cat’s paw (Trank and Smith 1996). Using these definitions to calculate angular displacements, flexion of hip, knee and ankle was defined by a decreasing joint angle while extension was defined by an increasing joint angle. For the MTP joint plantar flexion involved a decreasing joint angle and dorsal flexion involved an increasing joint angle. As plantar MTP flexion (until 180°) causes extension of the whole limb, this is further referred to as MTP extension.
Surgical self-reinnervation
After collecting baseline data, self-reinnervation of the MG and LG muscles within the right hindlimb was performed, using surgical procedures described previously (Cope et al. 1991; Huyghues-Despointes et al. 2003a). Surgery was performed under aseptic conditions using isoflurane anesthesia. A longitudinal incision was made in the popliteal region of the right hindlimb to expose the tibial nerve. The LG-SO branch was identified proximal to where it enters the muscle compartment. Using a dissecting scope the epineurium was then cut and the individual nerve bundles supplying LG (typically 3 or 4) were dissected free from the nerve bundle supplying SO. Each nerve bundle was identified using electrical stimulation via a bipolar hook electrode. Subsequently, the nerve branch to the MG was identified and the LG nerve bundles as well as the MG nerve were transected and immediately sutured using 10-0 non-absorbable nylon. The surgical intervention was immediately verified in the operating room by electrical stimulation of MG, LG and SO nerves branches proximal to the cut. SO muscle was clearly activated, but no response was observed in the MG and LG muscles. This indicated that soleus innervation was left intact. Subsequently, the opened fascia and skin were closed.
Assessment of reflexes after surgical self-reinnervation
After all kinematic data were collected, the effects of the MG and LG reinnervation on the integrity of both autogenetic and heterogenic reflex pathways of the reinnervated muscles as well as on the ipsilateral SO muscle were assessed using the method of Nichols (1989, 1999). In brief, after intercollicular decerebration the muscles and their corresponding tendons were dissected free from surrounding tissues. The distal tendons of undivided MG and LG were cut and connected to semiconductor myographs in series with linear motors. In some experiments, SO was prepared as well. Isometric force responses were measured at the tendon of one muscle, which was kept at a constant MTU length corresponding to an ankle angle of 90° and a knee angle of 110°. Reflexes were evoked by ramp-hold-release (ramp duration = 50 ms, amplitude = 2 mm, hold period = 350 ms) length perturbations. Stretches were applied while the muscle was in a passive state or while the muscle was excited by either a crossed-extensor reflex through electrical stimulation of the contralateral tibial nerve or by stimulatng the ipsilateral, caudal cutaneous sural nerve (40pps, 100 µs pulse width, 2x reflex threshold). The force responses of the reinnervated muscles were compared with their untreated counterparts in the contralateral limb. At the end of the experiment, the animals were euthanized with an overdose of pentobarbital sodium (Nembutal, 150 mg/kg) injected intravenously.
Treatment of data and statistics
The MG and LG muscles in the cat are predominantly active (Carlson-Kuhta et al. 1998; Smith et al. 1998; Gregor et al. 2006) and produce substantial forces (Walmsley et al. 1978; Fowler et al. 1993; Prilutsky et al. 1994) during the stance phase in walking. Our preliminary data also indicate that muscle activity of intact and self-reinnervated MG and LG as well as the ankle extension moment are negligibly low during most of swing (Prilutsky et al. 2006a, b). Accordingly, the major effects of self-reinnervation on kinematics were expected during the stance phase of the step cycle. Therefore, the current study has focused on hindlimb kinematics during stance.
All kinematic data were time-normalized with respect to stance duration. Linear interpolation between measured points was used to compute a value for each 0.5% of the stance phase. For the description of interjoint coordination (see Fig. 9), joint angles were normalized with respect to the range (i.e., minimum joint angle = 0%, maximum joint angle = 100%). All accepted step cycles were averaged within cats for each measurement day and each slope condition, grouped in post self-reinnervation time windows (see below) and then averaged across cats.
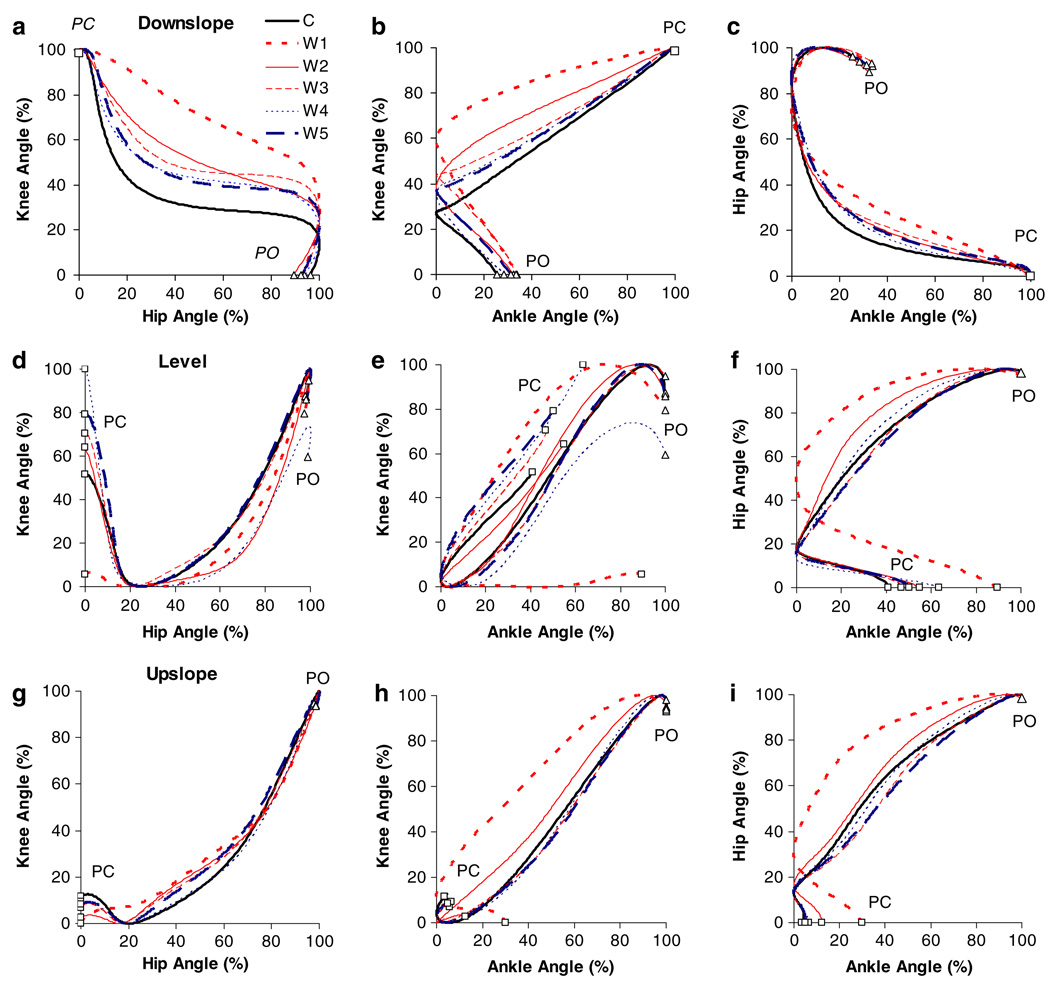
Fig. 9.
Mean hip–knee (left column), ankle–knee (middle column) and ankle–hip (right column) proWles during downslope (top row), level (middle row) and upslope (bottom row) overground walking before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles (n = 5, except for W1 in upslope walking n = 4). Paw contact, PC (open square), and paw lift-off, PO (open triangle), are indicated
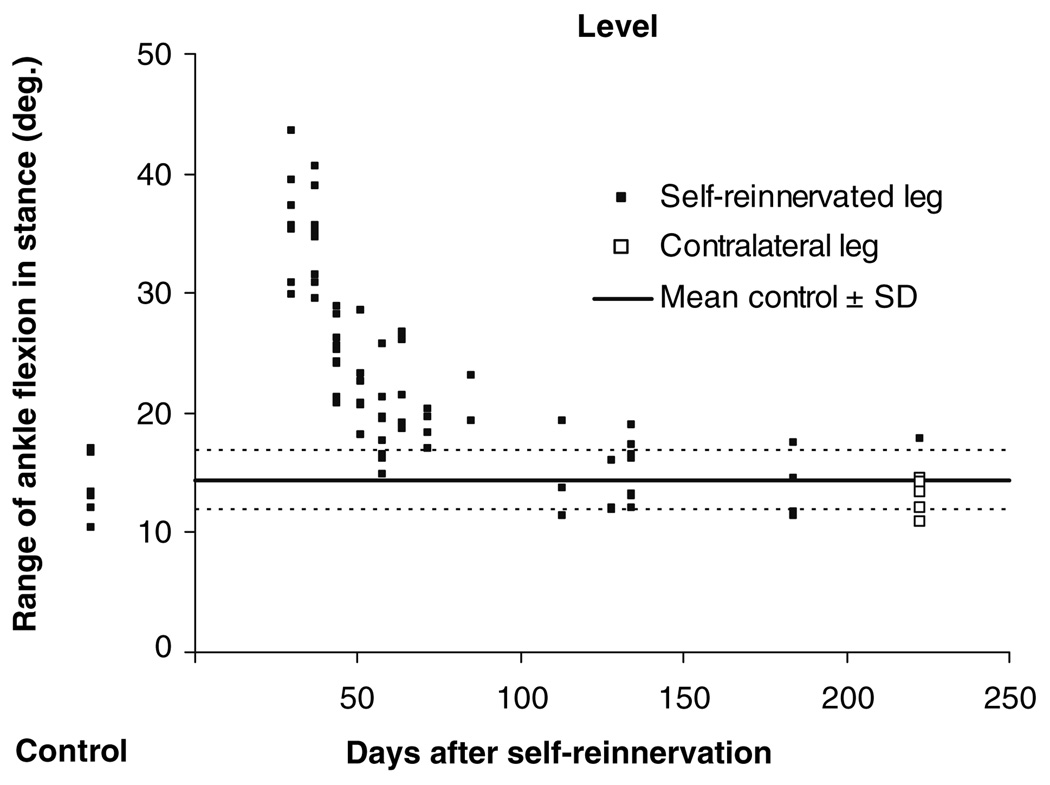
For two of the five cats, no data were collected before self-reinnervation. For those two cats, data from the left, contralateral, hindlimb recorded 6–13 months after self-reinnervation were used as controls. This was done because no statistically significant difference was found between pre-reinnervation data from the right hindlimb and data from the left hindlimb of the same animal collected 6–13 months after self-reinnervation of the right LG and MG in the three other cats (for exemplar data see Fig. 2).
Fig. 2.
The range of ankle flexion in stance during level walking for individual step cycles of one animal before self-reinnervation (Control) and at each measurement after self-reinnervation of the MG and LG muscles. For mean data of the same kinematic variable see Fig. 8h. Lines indicating the mean + and − one SD of the control data are plotted (1) to show recovery of the ankle joint parameter as well as (2) to compare pre self-reinnervation data of the ipsilateral leg (filled square) with data of the contralateral non-reinnervated hindlimb recorded in the last time window W5 (open square). With regard to the latter, it was not statistically different from the ipsilateral control. This was also the case for upslope and downslope walking
In general, all control data of the current study are similar to those recently reported (Gregor et al. 2006). Following MG-LG self-reinnervation, cat measurements were repeated weekly (two cats) or monthly (three cats) for a duration of 9–13 months. No physical training was applied in between measurements, but all cats could freely move in their housing facility. For statistical purposes, values of each date within certain post self-reinnervation time windows were averaged for those cats that have more than one measurement in that window. The following post self-reinnervation time windows were applied: W1: 0–7 weeks, W2: 8–13 weeks, W3: 14–19 weeks, W4: 20–31 weeks, W5: 32–57 weeks. The first post-op measurement was performed at 4, 14, 15, 41 and 49 days for the five cats respectively. (Note that for W1 in upslope walking, data for only four of the five cats were available.)
To evaluate the short-term effects of MG-LG self-reinnervation, measurements in W1 were compared to control values using paired t-tests. One-way ANOVAs for repeated measures were used to evaluate the recovery from self-reinnervation on the kinematic variables (i.e., W1–W5). One-sided Dunnett’s tests were applied to determine the significant differences between the control mean and the consecutive measurements after self-reinnervation. P values <0.05 were considered statistically significant. These statistical tests were all performed using the STATISTICA package (StatSoft, Inc.).
Results
Effects of MG-LG self-reinnervation on step cycle parameters and hindlimb orientation
Except for walking speed in upslope walking, no significant effects of self-reinnervation were found for the step cycle parameters of any condition at any time window (Table 1). In upslope walking, speed was slightly lower after self-reinnervation, but this was only significant for W1 and W3. In addition, the anterior placement of the paw at contact (Da) and the posterior position of the paw at lift-off (Dp) were not significantly altered after self-reinnervation (for the definition of Da and Dp see Fig. 1). A trend for a lower hip height (Hh) at PC as well as its minimum in stance (Hhmin) shortly (W1) after self-reinnervation was observed, suggesting a more crouched posture (Trank et al. 1996), but none of the differences were significant. For the subsequent time windows (W2–W5), however, Hh was very similar to the control values for all slope conditions.
Effects of MG-LG self-reinnervation on hindlimb joint kinematics
For all three walking conditions, the movement pattern in stance of the self-reinnervated hindlimb changed substantially 1–7 weeks after self-reinnervation (Compare C and W1 in Fig. 3, Fig. 4 and Fig. 5). The expected increase in ankle flexion in the beginning of stance after the functional elimination of two main ankle extensors was observed (see also Fig. 8g–i). However, the effects of self-reinnervation were not limited to the ankle joint. Changes in the MTP, knee and hip joints were observed as well. The mean short-term (W1) changes in whole hindlimb configuration are visualized with stick figures for the time instant in stance corresponding to peak ankle flexion (Fig. 6). Recovery was characterized by a progression of the affected kinematic parameters towards baseline. Figure 2 shows exemplar data of individual step cycles for the range of ankle flexion in stance during level walking before and at each measurement after MG-LG self-reinnervation indicating such a pattern of recovery. This figure also shows that data of the contralateral non-reinnervated hindlimb could be used as control (see “Methods”).
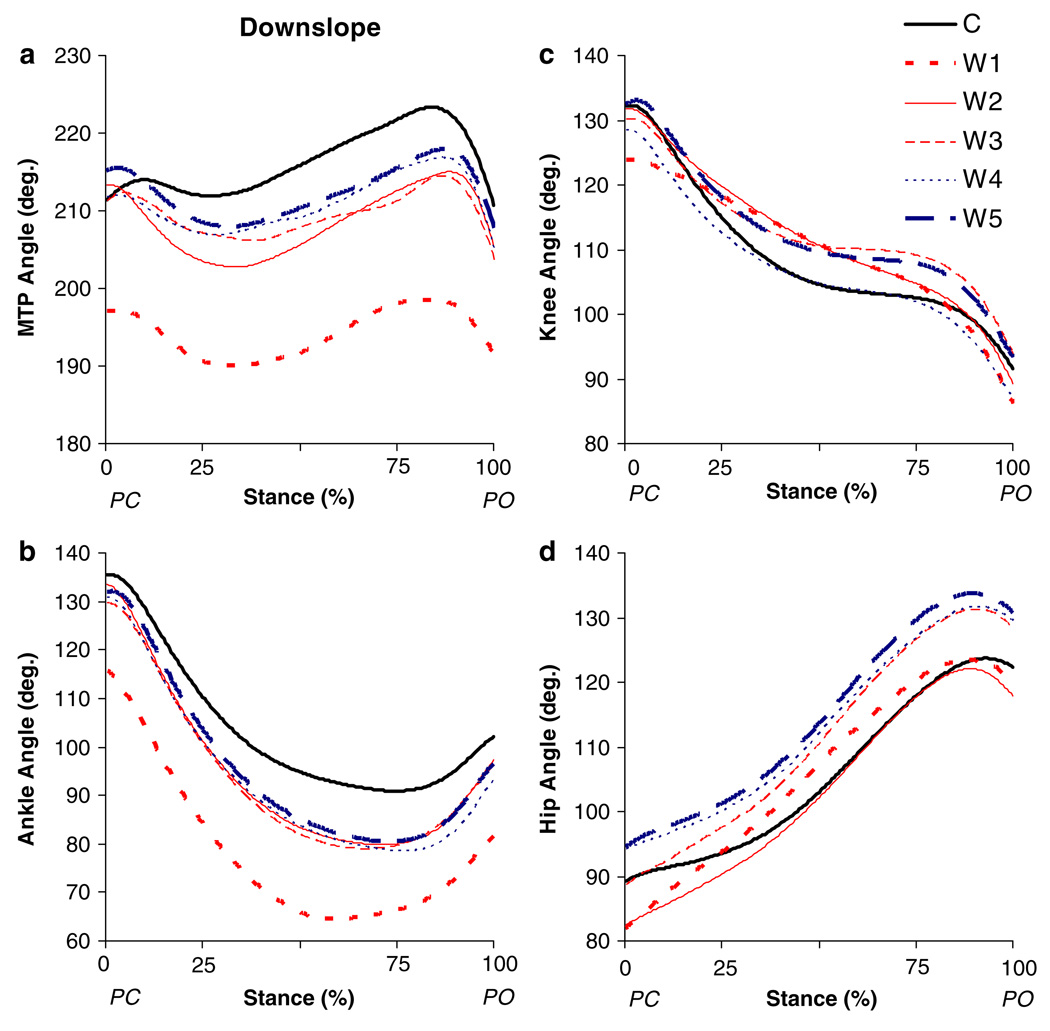
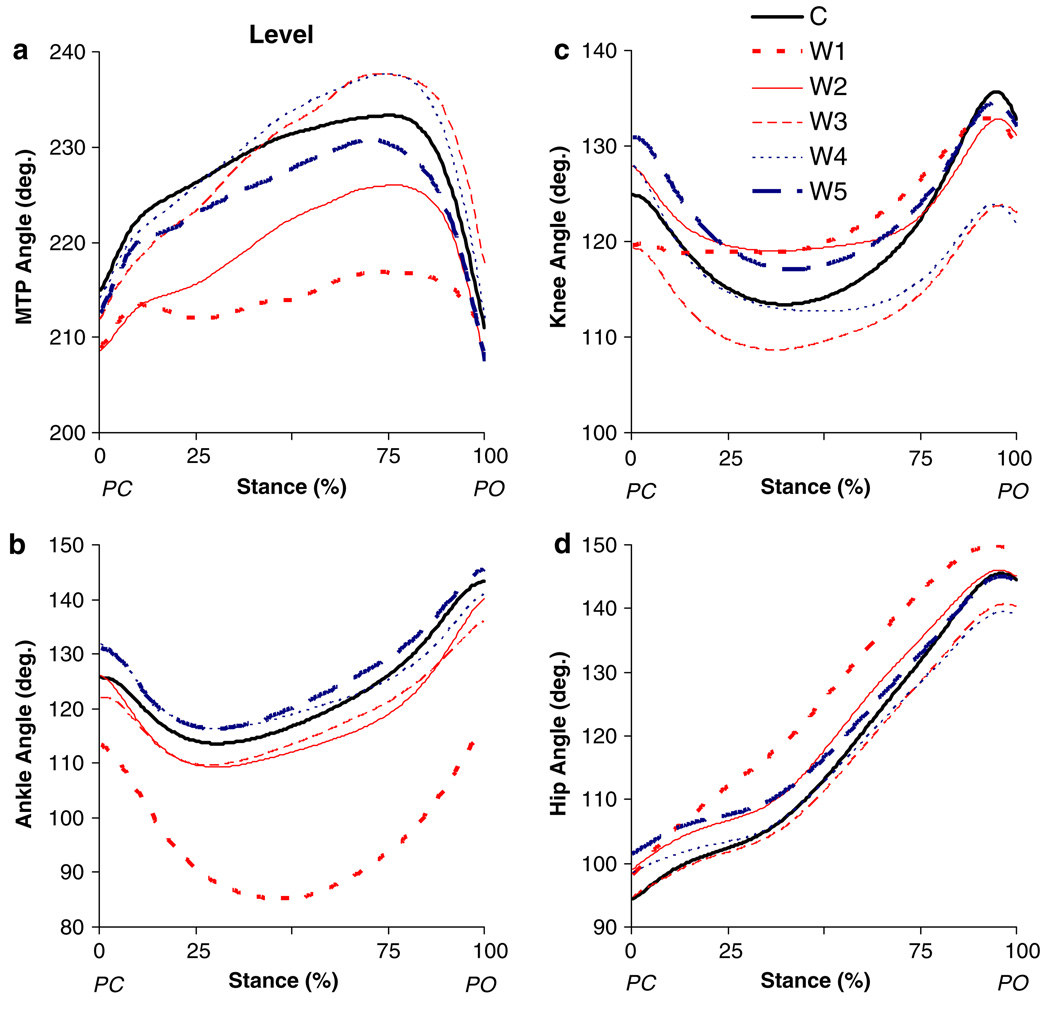
Fig. 3.
Mean angles for a MTP, b ankle, c knee and d hip joints as a function of normalized stance time during downslope overground walking before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles (n = 5). PC paw contact; PO paw lift-off
Fig. 4.
Mean angles for a MTP, b ankle, c knee and d hip joints as a function of normalized stance time during overground walking on a level surface before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles (n = 5). PC paw contact; PO paw lift-off
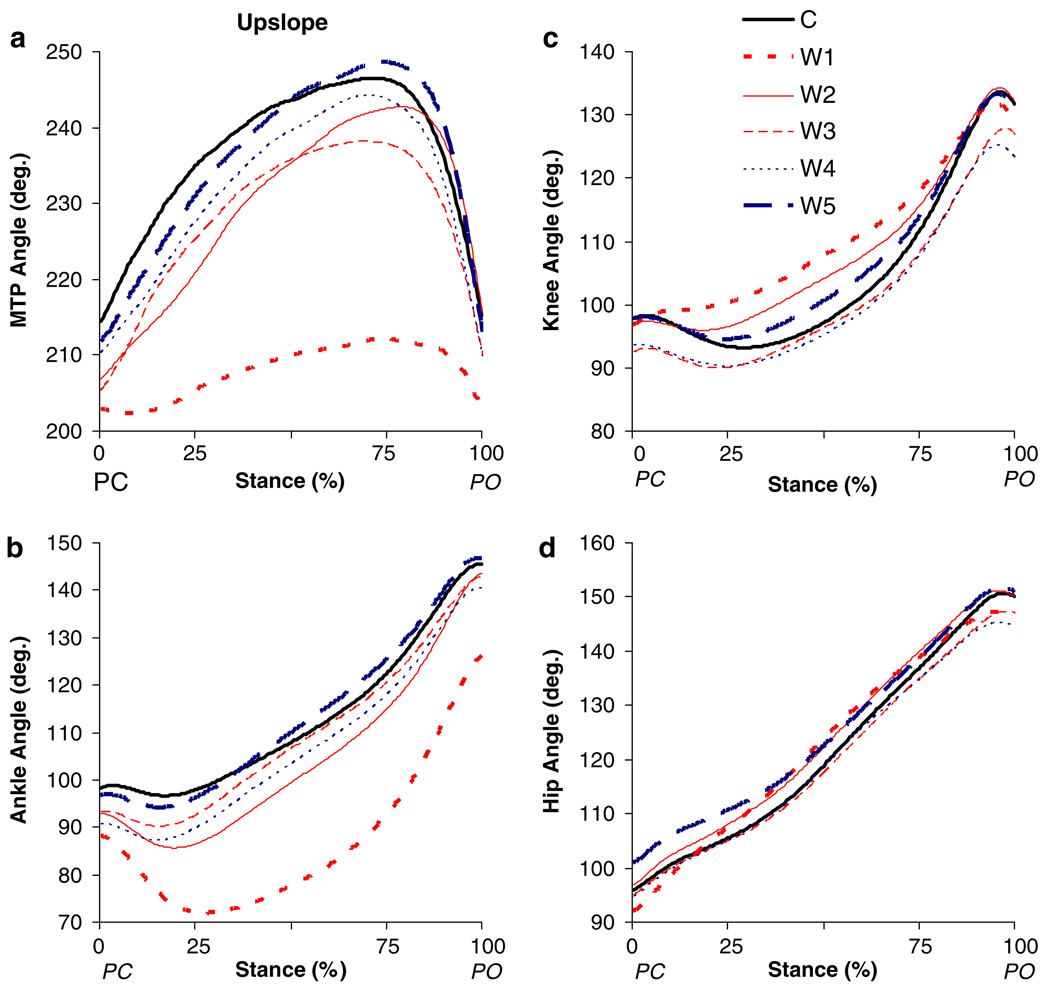
Fig. 5.
Mean angles for a MTP, b ankle, c knee and d hip joints as a function of normalized stance time during upslope overground walking before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles (n = 5, except for W1 n = 4). PC paw contact; PO paw lift-off
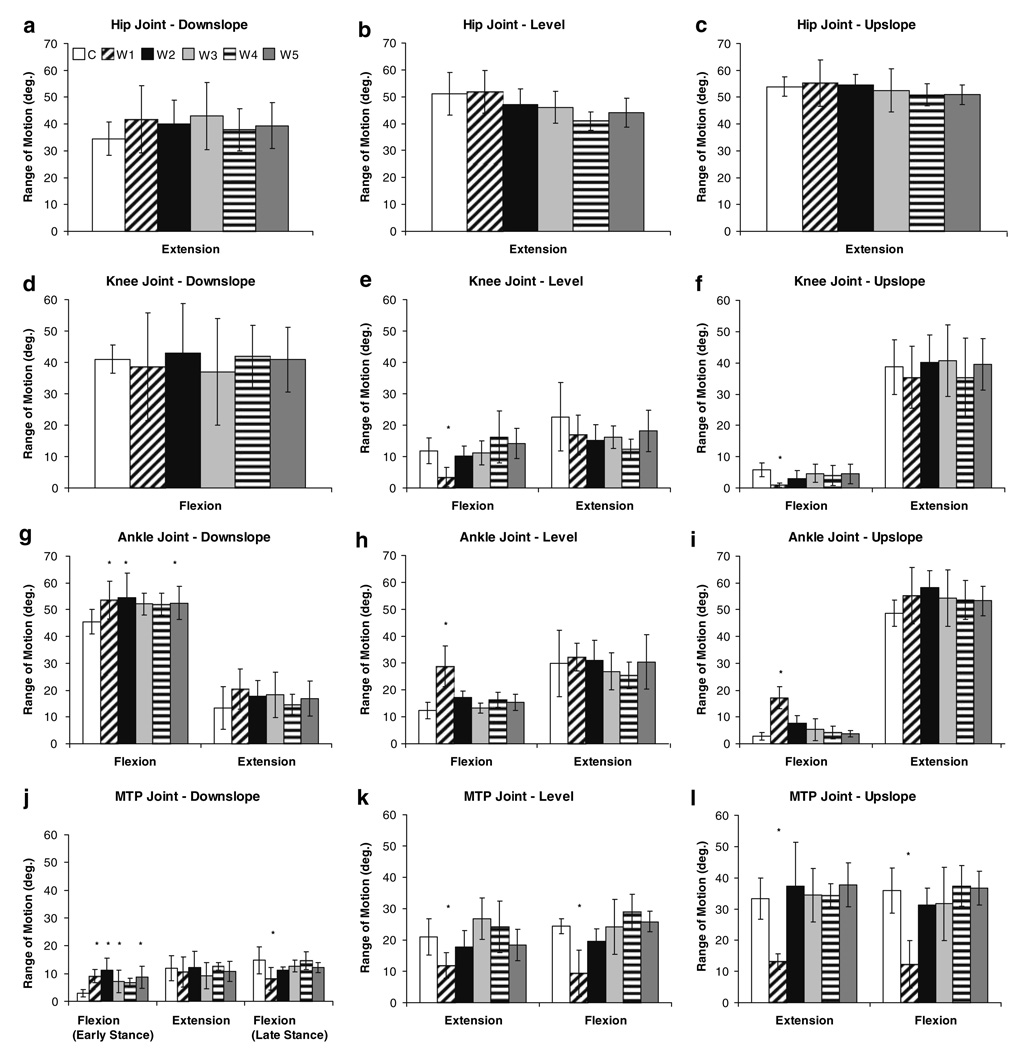
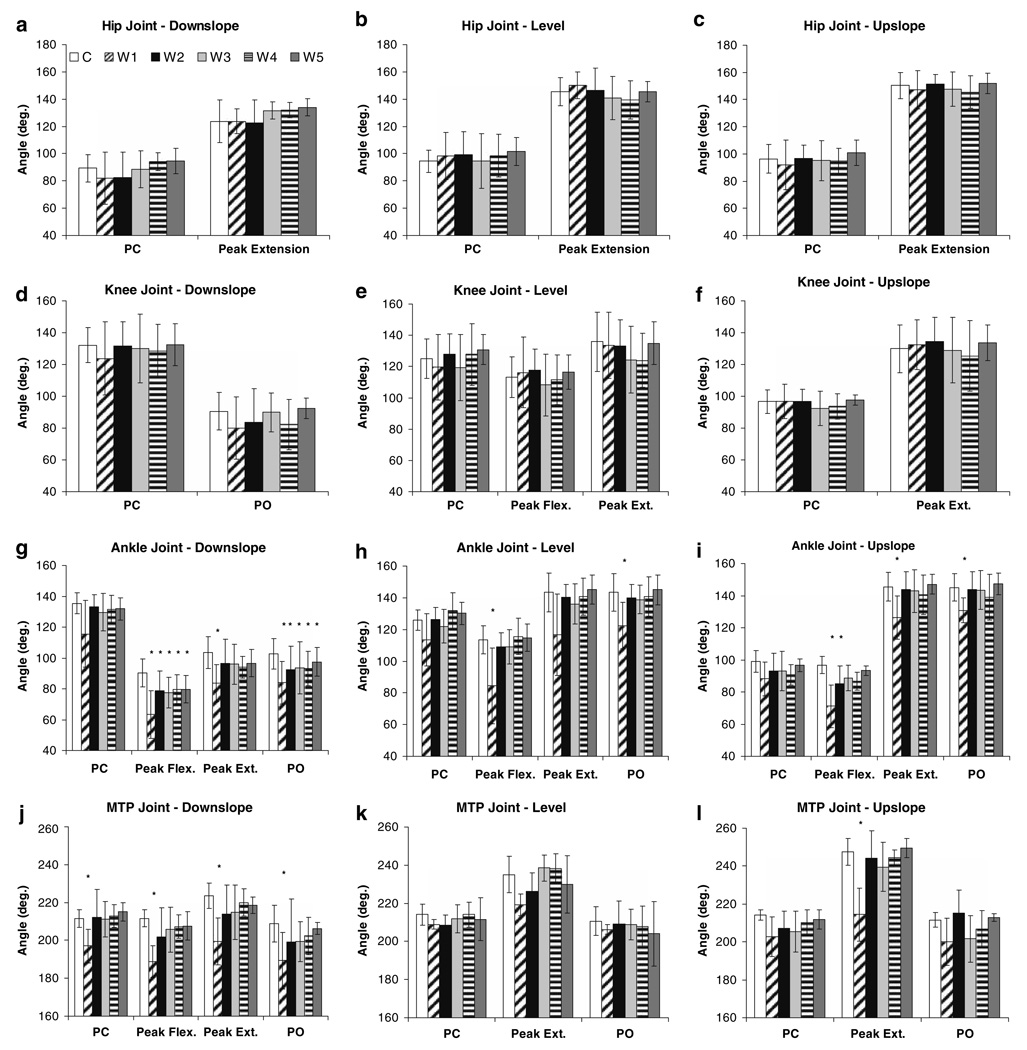
Fig. 8.
Ranges of motion during the extension and flexion phases in stance during downslope (left column), level (middle column) and upslope (right column) overground walking before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles. Note that for downslope walking no knee extension was observed (see also Fig. 3c) and that for the MTP joint (j) two flexion phases were found (see also Fig. 3a). Asterisk denotes a value significantly different from the control value. Values are shown as mean ± SD (n = 5, except for W1 in upslope walking n = 4)
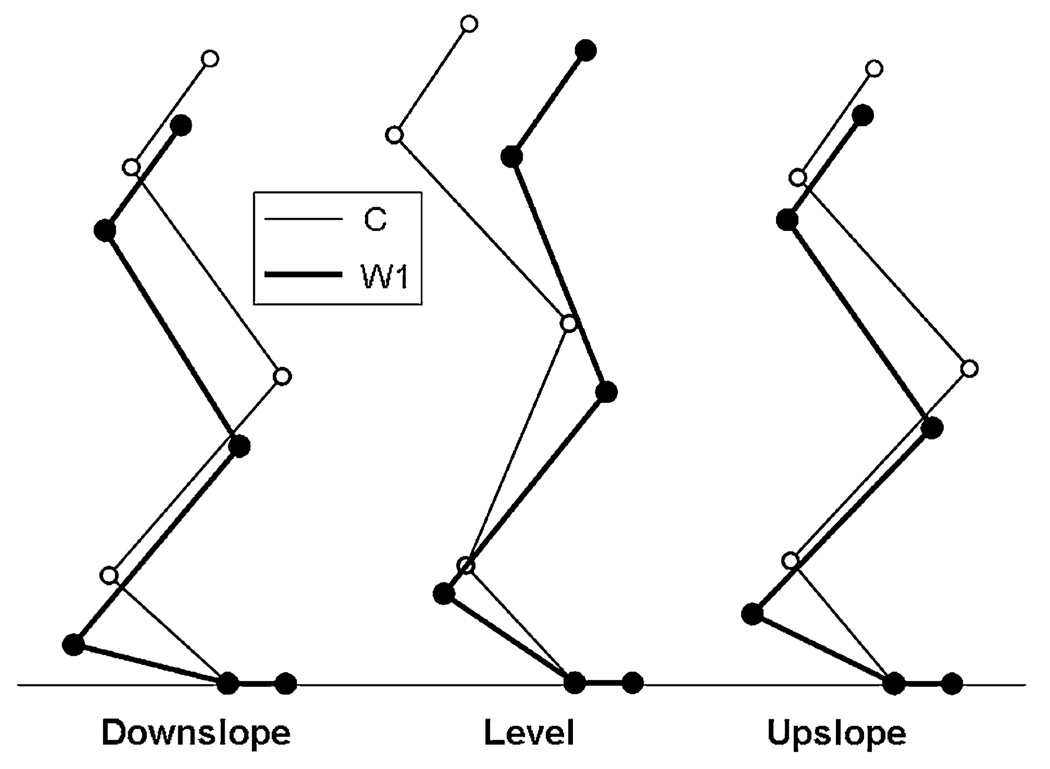
Fig. 6.
Stick figures of the hindlimb, reconstructed from the mean kinematics of all five cats, at the time instant in stance corresponding to peak ankle flexion before self-reinnervation (C, thin lines) and at the first time window (W1, thick lines) after self-reinnervation of the MG and LG muscles for downslope, level and upslope walking (for values of time instants see text)
Downslope walking
In the short-term (W1), self-reinnervation resulted in smaller ankle angles, which was significant for peak flexion, peak extension and angle at PO (Fig. 7g). The relative time of occurrence for peak ankle flexion shifted from latestance (72%, SD 11) towards mid-stance (63%, SD 10), but this was not significant (Fig. 3b). Also the range of ankle flexion (from 46° to 54°) and extension (from 13° to 20°) in stance increased (Fig. 8g), but statistical significance was found only for flexion. Self-reinnervation significantly decreased the MTP angle at all angular positions (Fig. 7j). In addition, the range of MTP flexion in early stance increased and the range of MTP flexion in late stance decreased, by 6° and 7° respectively (Fig. 8j). In contrast, no significant effects were found for any of the measured parameters of the hip and knee joints (Fig. 7a, Fig. 8a, and Fig. 7d, Fig. 8d).
Fig. 7.
Angular positions of all four joints at different points in stance during downslope (left column), level (middle column) and upslope (right column) overground walking before (C) and for the different time windows (W1–W5, see “Methods”) after self-reinnervation of the MG and LG muscles. When present, angles at paw contact (PC), paw lift-off (PO) as well as peak extension and flexion angles are indicated. As the PO data for hip and knee joints was similar to peak extension data (no significant effects for both parameters), only the latter is shown, except for the knee joint during downslope walking that did not involve knee extension. Asterisk denotes a value significantly different from the control value. Values are shown as mean ± SD (n = 5, except for W1 in upslope walking n = 4)
The values of some angular positions and ranges of motion that were significantly altered in W1 progressed back towards their control levels between W1 and W5, while other parameters remained significantly different (Fig. 7, Fig. 8). Dunnett’s test indicated significant differences between W2 and W5 and the control measurement for peak flexion angle and angle at PO of the ankle joint (Fig. 7g). The range of ankle flexion (Fig. 8g) was found to be significantly different from control in W2 to W5, but not in W3 (P = 0.06) and W4 (P = 0.06). The range of MTP flexion in early stance (Fig. 8j) was significantly different from control in all time points except W4 (P = 0.06). These apparently permanent effects of self-reinnervation on ankle and MTP joint kinematics in downslope walking can also be observed in Fig. 3.
Level walking
Peak ankle flexion (Fig. 7h) decreased (by 29°) and the range of ankle flexion (Fig. 8h) in stance increased significantly (by 16°) in the first time window after self-reinnervation (W1). Ankle angle at PC and PO as well as peak extension angle decreased, but was only significant at PO (Fig. 7h). In contrast to downslope walking, the time to peak ankle flexion increased (P < 0.05), i.e., from 32% (SD 4) to 46% (SD 5) of stance (Fig. 4b). Although no significant effects were found for the angular positions of the knee (Fig. 7e), range of knee flexion decreased significantly from 12° to 3° (Fig. 8e). For the MTP joint (Fig. 4a), significant differences were found for the range of extension and flexion, which decreased by 8° and 15°, respectively (Fig. 8k). In contrast, no changes in joint kinematics were identified for the hip (Fig. 7b, Fig. 8b). Further in the process of recovery (W2 → W5), the joint angle patterns in stance (Fig. 4) as well as the values of all angular parameters (Fig. 7, Fig. 8) progressed back towards baseline.
Upslope walking
Self-reinnervation resulted in a significant increase in peak ankle flexion as well as the time of its appearance in stance (from 17%, SD 2, to 28%, SD 5, of stance), but no significant change in its angle at PC (Fig. 5b, Fig. 7i). Accordingly, range of ankle flexion in early stance was altered substantially (Fig. 8i), i.e., from 3° to 22° (P < 0.05). In addition, ankle angle at PO and peak extension angle were significantly lower (Fig. 7i), but the range of ankle extension remained unchanged (Fig. 8i). Significant effects of self-reinnervation were found also for the range of knee flexion in early stance (from 6° to 2°, Fig. 8f), but not for the other knee (Fig. 7f, Fig. 8f) or hip (Fig. 7c, Fig. 8c) joint parameters. For the MTP joint (Fig. 5a), self-reinnervation resulted in a significant decrease in peak extension angle (Fig. 7l). As the MTP angle at PO and PC remained similar, the range of extension and flexion in stance decreased significantly as well (Fig. 8l). Similar to the recovery in level walking, hindlimb kinematics progressed back towards the level before self-reinnervation (Fig. 5). From W2 or W3, values of the angular parameters (Fig. 7, Fig. 8) were equal to control values and remained constant thereafter.
Effects of MG-LG self-reinnervation on interjoint coordination
Coordination between joints (i.e., hip–knee, ankle–knee and ankle–hip) was assessed using normalized (see “Methods”) angle–angle plots (Fig. 9). A positive slope represents simultaneous flexion or extension of each joint, while a negative slope represents movement of adjacent joints in the opposite direction (e.g., ankle flexion and knee extension). For all three walking conditions, self-reinnervation affected interjoint coordination in stance substantially (Fig. 9). These changes reflect the effects on the kinematics of individual joints, as described above.
Downslope walking
In W1 (0–7 weeks after self-reinnervation), the hip–knee diagram consisted of only two phases (Fig. 9a): hip extension–knee flexion followed by hip flexion–knee flexion. This was substantially different from the control pattern that was described by three phases. These angle–angle profiles gradually progressed towards, but never reached their base-line pattern and seem to be consistent from W3 (14–19 weeks) to W5 (32–57 weeks). Similar effects of self-reinnervation are observed in ankle–knee (Fig. 9b) and ankle–hip (Fig. 9c) coordination. For the former, the major short-term efffect (W1) was a decrease in the steepness of the positively sloped line in early stance, indicating an increase of ankle flexion relative to knee flexion. For ankle–hip coordination, self-reinnervation resulted in an increase of hip extension relative to ankle flexion in early stance. These data suggest that self-reinnervation of the MG and LG muscles causes a long-term disruption of interjoint coordination during downslope walking.
Level walking
Before self-reinnervation, early stance was characterized by hip extension–knee flexion–ankle flexion. The almost horizontal lines in both hip–knee (Fig. 9d) and ankle–knee diagrams (Fig. 9e) reflect the significant decrease in the range of knee flexion in early stance (from 12° to 3°) due to MG-LG self-reinnervation (Fig. 8e). Self-reinnervation altered only the slope of the ankle–hip diagram during ankle extension, i.e., it was more curved compared to the baseline pattern (Fig. 9f). Interjoint coordination gradually progressed towards baseline between W1 and W3. At the latter time window, all diagrams were indistinguishable from prereinnervation patterns and remained unchanged thereafter.
Upslope walking
The effects of MG-LG self-reinnervation on interjoint coordination during upslope walking were comparable to those of level walking. The first time window (W1) was characterized by knee extension instead of flexion in early stance, which resulted in an absence of the hip extension–knee flexion (Fig. 9g) as well as ankle flexion–knee flexion (Fig. 9h) phases. Also a more curved ankle extension–hip extension profile was found after self-reinnervation (Fig. 9i). Angle–angle patterns had returned back to their baseline patterns at W3.
Effects of MG-LG self-reinnervation on reflex integrity
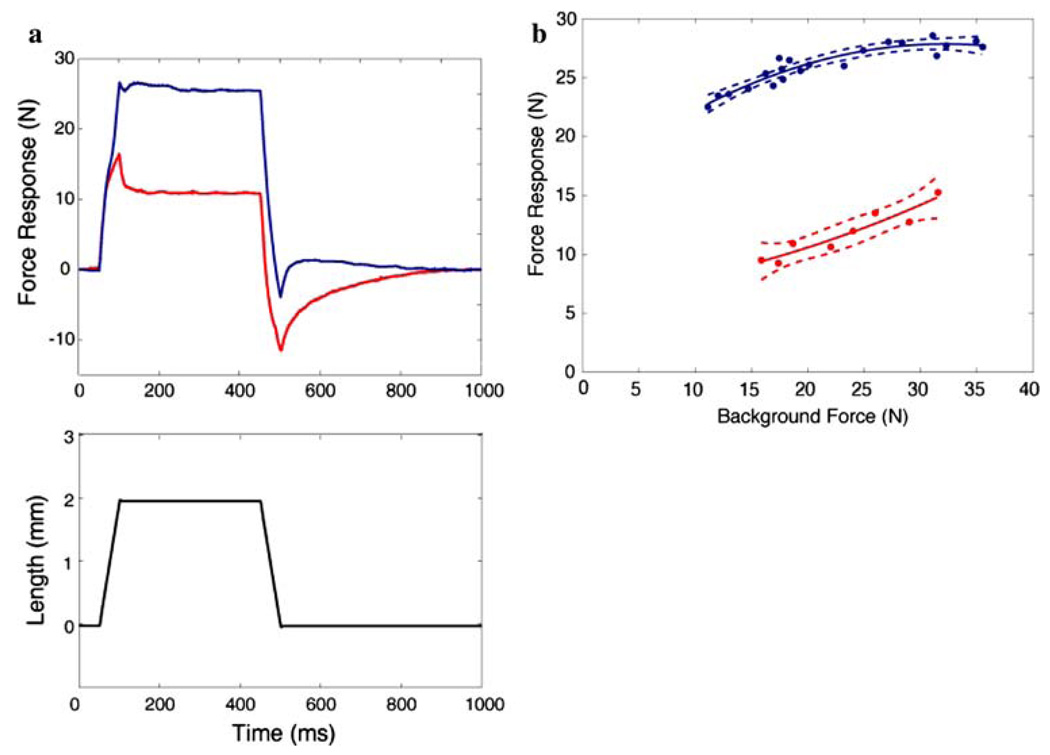
Stretch-evoked responses were measured in the undivided gastrocnemius muscles in all five animals. Four of the five experiments provided useful data. Crossed-extension reflexes provided a slowly decaying background force to obtain the force dependence of the responses. A loss of reflex action in the reinnervated muscles was considered to have occurred if at least two of the following were observed on the reinnervated side, namely, (1) a reduction in the magnitude of the force responses, (2) an increase in yielding and adaptation of these responses, and (3) an increase in force dependence (Nichols and Houk 1976; Huyghues-Des-pointes et al. 2003a). These points are illustrated in Fig. 10. In Fig. 10a, responses from the two muscles obtained at matching initial forces are shown superimposed. Besides a clearly reduced magnitude, the adaptation following the termination of the length ramp is also greater on the reinnervated side. Finally, the slope of the force–time trajectory is reduced toward the end of the ramp, indicating a reduction in stiffness beyond the short-range of the muscle. The responses obtained during the hold phase (absolute force minus background) are plotted as a function of background force during decaying crossed-extension reflexes in Fig. 10b. This figure illustrates that the magnitudes of responses on the reinnervated side are reduced over the entire range of forces by several-fold and are more force dependent. Stifiness remained relatively constant on the contralateral side. All three criteria were met for three of the animals. In one, activation was poor and therefore the comparison could not be made. In this animal however, reflexive responses could only be obtained on the contralateral side. In the three animals in which comparisons were also made between the SO muscles of the reinnervated and contralateral hindlimb, there was evidence that the stretch reflex was disrupted as well (data not shown). In two of the animals, all three criteria for the loss of reflex action were met. In the third, activation of the muscles was poor, but a stretch-evoked force response in SO was observed only on the contralateral side. This suggests that in these three animals length feedback was deficient in all three triceps surae muscles.
Fig. 10.
Comparison of stretch-evoked responses in reinnervated and contralateral gastrocnemius muscles. Data from one animal are shown, but similar results were found in three of the other four cats. a Super-imposed responses from the reinnervated (red, bottom trace) and contralateral (blue, top trace) muscles obtained at matching initial forces together with the imposed length changes. b The muscle responses obtained during the hold phase (absolute force minus background at t = 350 ms) plotted as a function of background force during decaying crossed-extension reflexes. The points were fitted with a second-order polynomial and dashed lines indicate the 95% confidence intervals. This figure clearly illustrates a loss of reflex action in the reinnervated muscle (for explanation of criteria see text)
Discussion
The ultimate goal of the present project is to understand the effects of self-reinnervation of the MG and LG muscles on the kinematics, kinetics, and muscle activity in overground walking on surfaces of varying slope in the cat. As a first step, this report documents both the short- and long-term effects of MG-LG self-reinnervation on joint kinematics of the whole ipsilateral hindlimb during such locomoting conditions.
It was found that self-reinnervation of the MG and LG muscles did not significantly change hip height and hind-limb orientation in any of the three walking conditions. However, substantial short-term effects were observed in the ankle joint as well as in MTP and knee joints, which led to changes in interjoint coordination. Recovery was characterized by a progression of hindlimb kinematics towards baseline, but the long-term effects of self-reinnervation were different for level and upslope walking compared to downslope walking. For the former two conditions, the cats were walking without any detectable kinematic deficits starting 14–19 weeks after surgery (W3). In contrast, joint kinematics as well as interjoint coordination in downslope walking gradually progressed towards, but never reached their baseline patterns within 57 weeks of observation.
Furthermore, reflex action was found to be absent in the reinnervated gastrocnemius muscles and, for some animals, also in the non-reinnervated ipsilateral SO. It should be kept in mind that these experiments assess the status only of length feedback. Positive force feedback, which can also contribute to muscular stiffness (Ross et al. 2003), is not expressed in the intercollicular decerebrate animal. Therefore, reductions in muscular stiffness in the intact animals could have been greater since force feedback is also disrupted by reinnervation (Cope et al. 1994). Disruption of reflex pathways in SO was not likely due to inadvertent crush injury of the nerve caused during the surgical procedures for MG-LG self-reinnervation, as (1) immediately after self-reinnervation of LG and MG in this study, electrical stimulation of the SO nerve elicited contraction of SO and (2) it has been shown that 1 year following crush injury the stretch reflex is fully restored (Cope and Clark 1993).
We hypothesized that the kinematic deficit during downslope walking would be absent following self-reinnervation of only the MG and LG muscles if the loss of proprioceptive feedback from SO was responsible for the kinematic deficit following self-reinnervation of all triceps surae muscles (Abelew et al. 2000). The fact that in the present study a permanent disruption in joint kinematics during down-slope walking was found suggests that this hypothesis should be rejected, which would indicate that the effects were mediated also by the loss of proprioceptive feedback from MG and LG. However, in three of the five animals the stretch reflex was disrupted in SO as well and, thus, the specific contribution of proprioceptive feedback from MG and LG could not be distinguished. The loss of reflex action in SO was not confirmed for all animals and not enough data are currently available to conclude that such a spillover occurs systematically. Our hypothesis can at this point, therefore, neither be rejected nor accepted.
Below, mechanical and neural mechanisms that are potentially responsible for the short- and long-term effects of MG-LG self-reinnervation are discussed.
Short-term effects of MG-LG self-reinnervation
Reinnervation of muscle fibers after nerve transection and suturing takes time. The proximal axons have to grow and replace the degenerated distal axons within the nerve to re-occupy motor-endplates (Sanes and Lichtman 1999). The first measurable signs of recovered motor innervation after self-reinnervation of cat triceps surae muscles, i.e. muscle contraction upon stimulation of the nerve proximal to the cut, have been reported to occur within 1–2 months (Gordon and Stein 1982a, b; Foehring et al. 1986). Rather normal bursts of MG muscle activity at regular intervals in the step cycle, as well as re-occupation of motor end-plates on the fibers of reinnervated muscles by axons of regenerating motoneurons have been found as early as 4–8 weeks post self-reinnervation (Gregor et al. 2003). Thus, in the initial weeks following surgical self-reinnervation the treated muscles remained essentially in a state of paralysis.
In intact cats, it has been shown that the LG and MG muscles exhibit a burst of activity just prior to paw contact, which continues for most of stance (Engberg and Lundberg 1969; English 1984; Whiting et al. 1984; Carlson-Kuhta et al. 1998; Smith et al. 1998; Gregor et al. 2006), resulting in substantial forces exerted at the distal tendons of these muscles (Fowler et al. 1993; Prilutsky et al. 1994; Kaya et al. 2003). The peak moments created by these forces at the ankle contribute about 45% to the peak ankle joint moment in level walking (Fowler et al. 1993). These results help explain our finding that in the first 7 weeks (W1) following the functional elimination of MG and LG the range of ankle flexion after paw contact was increased for all slope conditions (Fig. 8).
In the present study, MG-LG self-reinnervation also evoked short-term responses in the adjacent joints of the hindlimb, but no significant differences were found for the hip joint parameters. The decrease or absence of knee flexion can be considered as compensatory because it counteracts the effects of increased ankle flexion on hip height. Excessive lowering of the hip during stance would make it more diffcult for the contralateral hindlimb to clear the ground. There are several mechanisms by which such compensation might occur. The limited activity of LG and MG shortly after self-reinnervation may have an increased net knee extension effect, as these muscles do not only exert an extensor moment at the ankle joint but also a flexor moment at the knee. Note also that in the intact cat, a knee flexor moment precedes a knee extensor moment in stance (Gregor et al. 2006). However, this could be partially cancelled out by increased activity and force generation of the intact plantaris muscle, as recently reported after removal and temporarily weakening of gastrocnemius and/or soleus muscles (Misiaszek and Pearson 2002; Zhong et al. 2005). The plantaris has a similar flexion moment arm about the knee joint (Goslow et al. 1973; Prilutsky et al. 1996; Burkholder and Nichols 2004). The compensatory changes in knee angle can also be explained by attenuation of force feedback from the self-reinnervated ankle extensors. For both the stationary and the locomoting premammillary decerebrate cat, inhibitory force feedback from Golgi tendon organs between triceps surae and quadriceps muscles has been reported (Nichols 1994; Ross et al. 2003; Wilmink and Nichols 2003). Disruption of afferent pathways in the MG and LG muscles may potentially have resulted in increased activity of the knee extensors and, as a consequence, resulted in the more extended position of the knee joint.
For all slope conditions, lower absolute MTP angles (Fig. 3, Fig. 4, Fig. 5a) were found as well as less extension in early stance (Fig. 8). In contrast to the knee joint, this response of the MTP joint to MG-LG self-reinnervation decreases hip height and can thus not be considered as compensatory. Increased plantar flexion of the digits was also reported following denervation of the triceps surae (Wetzel et al. 1973). As in early stance the digits do not change orientation with regard to the walking surface (Smith et al. 1998), the effects on the MTP joint are most likely a result of a potentially decreased force exerted by the ankle extensors after MG-LG self-reinnervation and, as a consequence, a more horizontal orientation of the tarsals (Fig. 6).
Several previous studies have reported short-term changes in ankle angle due to different perturbations of the afferent and efferent pathways of the ankle extensors in cats, albeit for level walking only. An increase in the range of ankle flexion in early stance was found after denervation (Wetzel et al. 1973; Pearson et al. 1999) and weakening, using botulinum toxin (Misiaszek and Pearson 2002), of different combinations of ankle extensors. Pearson and colleagues (Pearson et al. 1999; Misiaszek and Pearson 2002) also reported a substantial increase in EMG activity of the intact synergist (i.e., MG). This was partly attributed to an increase in afferent feedback from muscle spindles, due to an enhanced stretch of the MTU. After denervation of LG, SO and plantaris muscles, a substantial part of recovery in ankle joint kinematics was found to occur during the first week, but full recovery was not yet achieved after 2 weeks (Pearson et al. 1999). Different from the present study, they started data collection as early as 5 h after the surgery and measurements were repeated on an almost daily basis, but for only 1–2 weeks. Due to the later start of post-op data collection (i.e., our measurements started when the animals had fully recovered from the surgery and could walk without pain medication) and less frequent repetition, these early changes were not captured in the present study. Nevertheless, substantial differences from the control data were still observed in the first time window of 0–7 weeks (W1).
Only a limited number of studies were encountered in the literature that reported kinematics of the whole hindlimb after surgical interventions of peripheral nerves and none were similar to the self-reinnervation procedure used in the present study. In agreement with the present results, Lin et al. (1997) reported an increased ankle flexion as well as less knee flexion, increased hip extension and decreased MTP extension in stance 2 weeks after transecting and suturing a 5 cm segment of the tibial nerve. Compensatory changes in knee and hip joints (i.e., increased flexion) have also been reported 25 days after denervation of the ankle flexors tibialis anterior and extensor digitorum longus (Carrier et al. 1997). This was accompanied by increased EMG magnitudes of knee and hip flexors, which as suggested above likely occurred in the present study for the knee and hip extensors. In chronic spinal cats, however, an increase in ankle flexion in early stance following denervation of LG and SO muscles was not compensated by changes in the knee and hip joints (Bouyer et al. 2001).
In conclusion, self-reinnervation of cat MG and LG muscles resulted in substantially altered hindlimb kinematics as well as interjoint coordination for all walking conditions. These short-term effects can be explained by both mechanical and neural factors that are affected by the temporary functional elimination of the MG and LG muscles.
Long-term effects of MG-LG self-reinnervation
The two major long-term findings of the present study are: (1) full recovery of hindlimb kinematics during level and upslope walking despite the absence of length feedback from two or possibly three major ankle extensors, and (2) a permanent disruption in joint kinematics and interjoint coordination of the whole hindlimb during downslope walking.
Mass of the whole gastrocnemius muscle (MG + LG) 9–13 months after self-reinnervation (24.0 g, SD 4.1) was found not to be statistically different from the contralateral controls (24.2 g SD 1.5, unreported data), indicating recovery of efferent pathways. This is in agreement with previous studies using similar procedures (Cope and Clark 1993; Cope et al. 1994). Other studies have reported essentially complete recovery of twitch and tetanus tension 6 months after self-reinnervation (Davis et al. 1978; Gordon and Stein 1982b). Preliminary findings have indicated close to control levels of MG and LG muscle activity in walking 3–4 months after self-reinnervation (Prilutsky et al. 2004). Therefore, it is not likely that the force generating capacity of these muscles plays a role in the long-term effects of MG-LG self-reinnervation. In contrast to motor recovery, we found that muscle self-reinnervation leads to a permanent disruption of length feedback (Fig. 10), which could be verified in four of the five cats. Such effects of self-reinnervation on sensory function, indicating functional deafferentation, have also been reported in previous studies (Cope and Clark 1993; Cope et al. 1994; Huyghues-Despointes et al. 2003a). Recent observations suggest that suppression by central neural circuits, i.e., via presynaptic and/or postsynaptic inhibition, prevents the recruitment of motoneurons in the presence of similar to normal afferent signals from muscle spindles (Haftel et al. 2005). However, future studies are needed to elucidate the underlying mechanisms of the disrupted length feedback after self-reinnervation.
The full recovery during level and upslope walking may be explained by the contractile conditions of the MG and LG muscles in these tasks. It has been shown that MTU lengthening and stretch velocity of MG and LG in early stance are rather small for level and upslope walking (Gregor et al. 2006), as well as that the muscle fascicles of MG are not lengthening, but shorten in that phase of the step cycle (Maas et al. 2005). For such muscle contractile conditions, only a modest contribution of length feedback is expected (Nichols and Houk 1976; Huyghues-Despointes et al. 2003a, b). On the other hand, upslope walking involves the highest ankle extensor moment as well as tendon force exerted by the MG and LG muscles (Herzog et al. 1993; Gregor et al. 2001, 2006; Kaya et al. 2003) and, thus, may be a condition that is dominated by force feedback. Recent results from intact cats indicated that force feedback may play a larger role than length feedback in modifying motor output of the MG and LG muscles under the conditions of the motor tasks used here (Gregor et al. 2006). Also in the locomoting premammillary decerebrate cat it was shown that the gastrocnemius muscles are a major source of positive force feedback (Ross et al. 2003). However, there is also evidence that force feedback in self-reinnervated muscles is attenuated (Cope et al. 1994), but this was not tested for the cats in this study. Collectively these data suggest that the local disruption of proprioceptive feedback has been compensated by other mechanisms, possibly by an increased use of other sensory sources (e.g., cutaneous receptors from the paw pad and length and force receptors of unaffected synergists) or by altered central drive.
The permanent changes in joint kinematics during down-slope walking suggest a profound contribution of length and force feedback from the MG and LG muscles. This can also be explained by the contractile conditions of MG and LG. It has been shown that the MG muscle contributes to the ankle extensor moment during downslope walking (Gregor et al. 2001; Kaya et al. 2003), but no such data are available for the LG. We have recently shown that walking down a slope is associated with lengthening contractions in the ankle extensors (Maas et al. 2005; Gregor et al. 2006), a muscle contractile condition in which the largest contribution of length feedback to muscle force output is found (Nichols and Houk 1976; Huyghues-Despointes et al. 2003a, b). The larger than normal ankle flexion in stance (e.g. Fig. 3) could therefore be the result of (1) less force exertion upon muscle lengthening due to the absence of autogenic length feedback from MG and LG, (2) the absence of excitatory heterogenic length feedback from MG and LG onto SO (Nichols 1989, 1999), and/or (3) less force exerted by the triceps surae due to the absence of positive force feedback (Ross et al. 2003). In addition, we found some evidence that the stretch reflex was disrupted in SO muscle as well, suggesting that in these animals length feedback was deficient in all three triceps surae muscles. Self-reinnervation of the MG and LG muscles could have caused a central suppression of the stretch reflex in the whole synergistic group of ankle extensors, but the exact mechanism is unclear. Not enough data are available to conclude that a suppression of feedback from a non-reinnervated synergist occurred in the present study. However, given that the actions of all three muscles of the triceps surae group are important during downslope walking, the data from the present study strengthen the conclusion from the study of Abelew et al. (2000) that proprioceptive feedback is important for coordination during active lengthening.
If reinnervation resulted in the loss of both length and force feedback in these experiments, as appears likely based on previous results (Cope et al. 1994), then the finding of residual deficits for downslope walking suggests that the absence of force feedback is more readily compensated than the absence of length feedback. Consideration of the different features of these two sources of sensory feedback provides a provisional explanation for this difference. Force feedback can increase in a muscle during shortening as well as lengthening contractions, as long as the central drive is increasing. The animal may increase motor performance during upslope walking by increasing central drive and therefore provide compensation for the absence of positive force feedback. In contrast, length feedback is much stronger during active lengthening than during active shortening of a muscle and, therefore, driven mainly by external forces and mechanical changes in a complex musculoskeletal system. Thus, active lengthening is not in general related simply to central drive. A longer period of training might be required for the animal to adapt by tuning intrinsic muscular properties. Furthermore, if disruption of cross-joint and inhibitory force feedback by the reinnervation results in preservation of normal changes in the length of the whole limb during locomotion (see above), then there might be little stimulus for the animal to adapt central drive.
In conclusion, the long-term effects of MG-LG self-reinnervation on hindlimb kinematics during walking are dependent on the slope of the walking surface. This can partially be explained by different contractile conditions of the MG and LG muscles between downslope walking and level as well as upslope walking. Most likely a combination of effects of changes in autogenic and heterogenic length feedback as well as positive force feedback due to self-reinnervation are involved. This indicates that proprioceptive feedback from individual muscles is important for the control of limb kinematics.
Acknowledgments
The authors thank Webb Smith for recruiting skilled students and managing the continuously growing data base and Dr. Guayhaur Shue for technical support. In addition, we thank Drs. Clotilde Huyghues-Despointes and Kyla Ross for assisting in terminal experiments to test the integrity of reflex pathways and Dr. Michael Kutner for statistical advice. This research was supported by National Institutes of Health Grants HD032571 and NS048844 as well as the Center for Human Movement Studies at the Georgia Institute of Technology.
Contributor Information
Huub Maas, School of Applied Physiology, Center for Human Movement Studies, Georgia Institute of Technology, Atlanta, GA, USA; Department of Physiology, Northwestern University Feinberg School of Medicine, 303 E. Chicago Avenue, Ward 5-660, Chicago, IL 60611, USA, e-mail: h-maas@northwestern.edu.
Boris I. Prilutsky, School of Applied Physiology, Center for Human Movement Studies, Georgia Institute of Technology, Atlanta, GA, USA
T. Richard Nichols, Department of Physiology, Emory University, Atlanta, GA, USA; Department of Biomedical Engineering, Emory University/Georgia Institute of Technology, Atlanta, GA, USA.
Robert J. Gregor, School of Applied Physiology, Center for Human Movement Studies, Georgia Institute of Technology, Atlanta, GA, USA Department of Physiology, Emory University, Atlanta, GA, USA.
References
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Bouyer LJG, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21:3531–3541. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ, Nichols TR. Three-dimensional model of the feline hindlimb. J Morphol. 2004;261:118–129. doi: 10.1002/jmor.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol. 1998;79:1687–1701. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Carrier L, Brustein E, Rossignol S. Locomotion of the hindlimbs after neurectomy of ankle flexors in intact and spinal cats: model for the study of locomotor plasticity. J Neurophysiol. 1997;77:1979–1993. doi: 10.1152/jn.1997.77.4.1979. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch refkexes. J Neurophysiol. 1994;71:817–820. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol. 1993;70:1787–1796. doi: 10.1152/jn.1993.70.5.1787. [DOI] [PubMed] [Google Scholar]
- Cope TC, Webb CB, Botterman BR. Control of motor-unit tension by rate modulation during sustained contractions in reinnervated cat muscle. J Neurophysiol. 1991;65:648–656. doi: 10.1152/jn.1991.65.3.648. [DOI] [PubMed] [Google Scholar]
- Davis LA, Gordon T, Hoffer JA, Jhamandas J, Stein RB. Compound action potentials recorded from mammalian peripheral nerves following ligation or resuturing. J Physiol. 1978;285:543–559. doi: 10.1113/jphysiol.1978.sp012588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969;75:614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- English AW. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J Neurophysiol. 1984;52:114–125. doi: 10.1152/jn.1984.52.1.114. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol. 1986;55:947–965. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- Fowler EG, Gregor RJ, Hodgson JA, Roy RR. Relationship between ankle muscle and joint kinetics during the stance phase of locomotion in the cat. J Biomech. 1993;26:465–483. doi: 10.1016/0021-9290(93)90010-c. [DOI] [PubMed] [Google Scholar]
- Fowler EG, Gregor RJ, Roy RR. Differential kinetics of fast and slow ankle extensors during the paw-shake in the cat. Exp Neurol. 1988;99:219–224. doi: 10.1016/0014-4886(88)90141-0. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Reorganization of motor-unit properties in reinnervated muscles of the cat. J Neurophysiol. 1982a;48:1175–1190. doi: 10.1152/jn.1982.48.5.1175. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J Physiol Lond. 1982b;323:307–323. doi: 10.1113/jphysiol.1982.sp014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow GE, Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141:1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Prilutsky BI, Nichols TR, Smith DW. EMG output in reinnervated medial gastrocnemius muscle during locomotion in the cat. Program No. 493.8. Abstract Viewer/Itinerary Planner. Washington: Society for Neuroscience; 2003. [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change and ankle extensor EMG patterns. J Neurophysiol. 2006;95:1397–1409. doi: 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Smith JL, Smith DW, Oliver A, Prilutsky BI. Hind-limb kinetics and neural control during slope walking in the cat: unexpected findings. J Appl Biomech. 2001;17:277–286. [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive aflerents. J Neurosci. 2005;25:4733–4742. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Leonard TR, Guimaraes AC. Forces in gastrocnemius, soleus, and plantaris tendons of the freely moving cat. J Biomech. 1993;26:945–953. doi: 10.1016/0021-9290(93)90056-k. [DOI] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of activation level. J Neurophysiol. 2003a;90:1537–1546. doi: 10.1152/jn.00718.2002. [DOI] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of movement history. J Neurophysiol. 2003b;90:1547–1555. doi: 10.1152/jn.00719.2002. [DOI] [PubMed] [Google Scholar]
- Kaya M, Leonard T, Herzog W. Coordination of medial gastrocnemius and soleus forces during cat locomotion. J Exp Biol. 2003;206:3645–3655. doi: 10.1242/jeb.00544. [DOI] [PubMed] [Google Scholar]
- Lehmann JF, Condon SM, Delateur BJ, Smith JC. Gait Abnormalities in Tibial Nerve Paralysis-a Biomechanical Study. Arch Phys Med Rehabil. 1985;66:80–85. [PubMed] [Google Scholar]
- Lin FM, Pan YC, Dinh TA, Sabbahi M, Shenaq S. Functional assessment of tibial-nerve recovery in the cat using gait analysis: preliminary study. J Reconstr Microsurg. 1997;13:177–183. doi: 10.1055/s-2007-1006402. [DOI] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Gregor RJ. In vivo fascicle length of cat medial gastrocnemius and soleus muscles during slope walking. XXth congress of the international society of biomechanics; Cleveland. 2005. p. 90. [Google Scholar]
- Maas H, Prilutsky BI, Welch T, Gregor RJ. Reinnervation of the gastrocnemius muscle in the cat: immediate and long-term effects in interjoint coordination. Program No. 180.6. Abstract viewer/itinerary planner. Washington: Society for Neuroscience; 2004. [Google Scholar]
- Misiaszek JE, Pearson KG. Adaptive changes in locomotor activity following botulinum toxin injection in ankle extensor muscles of cats. J Neurophysiol. 2002;87:229–239. doi: 10.1152/jn.00410.2001. [DOI] [PubMed] [Google Scholar]
- Nichols TR. The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J Physiol. 1989;410:463–477. doi: 10.1113/jphysiol.1989.sp017544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat. 1994;151:1–13. doi: 10.1159/000147637. [DOI] [PubMed] [Google Scholar]
- Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol. 1999;81:467–478. doi: 10.1152/jn.1999.81.2.467. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Fouad K, Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol. 1999;82:370–381. doi: 10.1152/jn.1999.82.1.370. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Gregor RJ, Nichols TR. Coordination of cat ankle extensors during the paw-shake before and after self-reinnervation of medial and lateral gastrocnemius muscles. Program No. 69.12. Abstract Viewer/Itinerary Planner. Washington: Society for Neuroscience; 2004. [Google Scholar]
- Prilutsky BI, Herzog W, Allinger TL. Force-sharing between cat soleus and gastrocnemius muscles during walking: explanations based on electrical activity, properties, and kinematics. J Biomech. 1994;27:1223–1235. doi: 10.1016/0021-9290(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Herzog W, Leonard T. Transfer of mechanical energy between ankle and knee joints by gastrocnemius and plantaris muscles during cat locomotion. J Biomech. 1996;29:391–403. doi: 10.1016/0021-9290(95)00054-2. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Maas H, Gregor RJ. In vivo fascicle velocity of cat gastrocnemius and soleus muscles during the paw-shake. XXth Congress of the International Society of Biomechanics; Cleveland. 2005a. [Google Scholar]
- Prilutsky BI, Maas H, Gregor RJ. Ankle joint moment during walking after self-reinntervation of selected ankle extensors in the cat. Blacksburg, Virginia: American Society of Biomechanics; 2006a. [Google Scholar]
- Prilutsky BI, Maas H, Nichols TR, Gregor RJ. Effects of self-reinnervation of selected cat ankle extensors on their activity and hindlimb mechanics in slope walking. Abstract Viewer/Itinerary Planner. Washington: Society for Neuroscience; 2006b. [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005b;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Ross KT, Huyghues-Despointes CMJ, Nichols TR. Heterogenic feedback among quadriceps and ankle extensors during spontaneous locomotion in premammillary cats. Program No. 276.11. Abstract Viewer/Itinerary Planner. Washington: Society for Neuroscience; 2003. [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Simon SR, Mann RA, Hagy JL, Larsen LJ. Role of posterior calf muscles in normal gait. J Bone Joint Surg. 1978;60:465–472. [PubMed] [Google Scholar]
- Smith JL, Betts B, Edgerton VR, Zernicke RF. Rapid ankle extension during paw shakes—selective recruitment of fast ankle extensors. J Neurophysiol. 1980;43:612–620. doi: 10.1152/jn.1980.43.3.612. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol. 1998;79:1702–1716. doi: 10.1152/jn.1998.79.4.1702. [DOI] [PubMed] [Google Scholar]
- Trank TV, Chen C, Smith JL. Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. J Neurophysiol. 1996;76:2316–2326. doi: 10.1152/jn.1996.76.4.2316. [DOI] [PubMed] [Google Scholar]
- Trank TV, Smith JL. Adaptive control for backward quadrupedal walking VI. metatarsophalangeal joint dynamics and motor patterns of digit muscles. J Neurophysiol. 1996;75:678–679. doi: 10.1152/jn.1996.75.2.678. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Wetzel MC, Gerlach RL, Stern LZ, Hannapel LK. Behavior and histochemistry of functionally isolated cat ankle extensors. Exp Neurol. 1973;39:223–233. doi: 10.1016/0014-4886(73)90225-2. [DOI] [PubMed] [Google Scholar]
- Whiting WC, Gregor RJ, Roy RR, Edgerton VR. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. J Biomech. 1984;17:685–694. doi: 10.1016/0021-9290(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Wilmink RJ, Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hind limb. J Neurophysiol. 2003;90:2310–2324. doi: 10.1152/jn.00833.2002. [DOI] [PubMed] [Google Scholar]
- Zhong H, Roy RR, Monti RJ, Edgerton VR. Mechanical responses of the cat plantaris muscle to chronic functional overload: in vivo and in situ measurements. Program No. 397.11. Abstract Viewer/Itinerary Planner. Washington: Society for Neuroscience; 2005. [Google Scholar]