Abstract
The oxidative addition and reductive elimination reactions of H2 on unsaturated transition-metal complexes are crucial in utilizing this important molecule. Both biological and man-made iron catalysts use iron to perform H2 transformations, and highly unsaturated iron complexes in unusual geometries (tetrahedral and trigonal planar) are anticipated to give unusual or novel reactions. In this paper, two new synthetic routes to the low-coordinate iron hydride complex [LtBuFe(μ-H)]2 are reported. Et3SiH was used as the hydride source in one route by taking advantage of the silaphilicity of the fluoride ligand in three-coordinate LtBuFeF. The other synthetic method proceeded through the binuclear oxidative addition of H2 or D2 to a putative Fe(I) intermediate. Deuteration was verified through reduction of an alkyne and release of the deuterated alkene product. Mössbauer spectra of [LtBuFe(μ-H)]2 indicate that the samples are pure, and that the iron(II) centers are high-spin.
Keywords: Iron, hydride, dihydrogen, oxidative addition, deuteration
Introduction
The oxidative addition of H2 to transition metal complexes to form hydride complexes is now a well known reaction.[1] It has been studied in detail in part due to chemists’ desire to understand the mechanism of homogeneous catalytic transformations of H2 such as hydrogenation.[2] The interaction of H2 with transition metal complexes during oxidative addition is usually thought to proceed through a dihydrogen complex, followed by scission of the H-H bond to give a dihydride complex.[3-7]
The standard organometallic description of the first part of this reaction is that the σ orbital of H2 interacts with an empty d orbital (Figure 1a). [1,8] This model has been supported by the spectroscopic and crystallographic characterization of hundreds of dihydrogen complexes, which typically have diamagnetic transition metal sites from strong-field ligands. [6] The subsequent H2 cleavage formally oxidizes the metal by two electrons (Figure 1b).
Figure 1.
Interaction of dihydrogen orbitals with metal d orbitals. In the standard model, an empty d orbital lobe forms a σ interaction with the H-H bonding orbital, and two lobes of a filled d orbital donate into the empty σ* orbital of H2.
The Holland research group has focused its organometallic chemistry efforts on complexes that have a high-spin electronic configuration.[9] We use bulky β-diketiminate ligands that are weak-field π-donors to enforce a low coordination number. High-spin complexes like these with 5 or more d electrons have no empty d orbitals, so the model above does not apply unless a spin-state change or other electronic reorganization can occur.
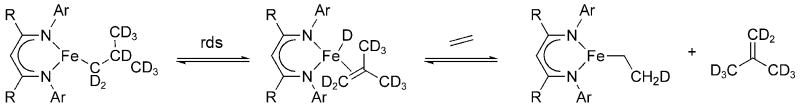
Recent computational and experimental work has been instrumental in demonstrating the importance of spin-state changes in organometallic chemistry. [10] [11] [12] [13] [14] [15] [16] One of our goals with β-diketiminate complexes is to discover new reactions that do not fit the usual organometallic mold and that may involve spin-state changes or use singly-occupied orbitals as acceptors (2-center/3-electron interactions). For example, alkyl complexes LRFeCH2CH2R (LR = LMe or LtBu, Figure 2) readily undergo β-hydride elimination upon mild heating.[17] As shown in Scheme 1, the transient hydride complex is trapped by the addition of another alkene. The β-hydride elimination mechanism was confirmed by the use of isotope labeling, activation parameters, and an H/D kinetic isotope effect (KIE) of 2.2.[17] Therefore, a pathway exists for high-spin complexes to undergo β-hydride elimination in the absence of completely empty d orbitals. Based on these ideas, we have also been interested to find examples of oxidative addition and reductive elimination, especially involving the fundamentally interesting molecule H2.
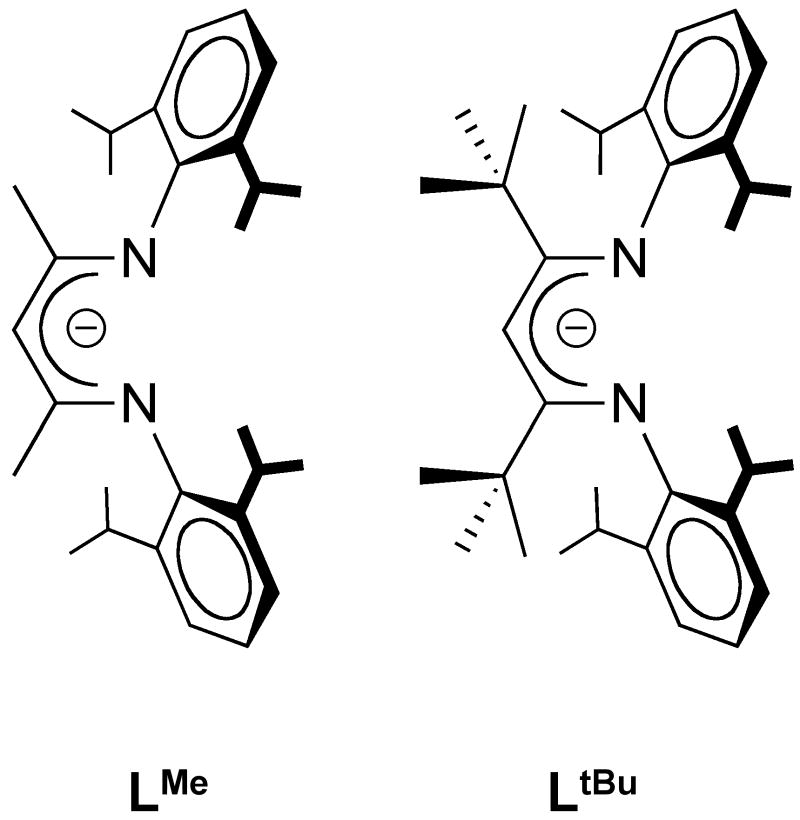
Figure 2.
β-diketimate ligands LR, where R indicates the substituent on the 2 and 4 positions of the C3N2 backbone.
Scheme 1.
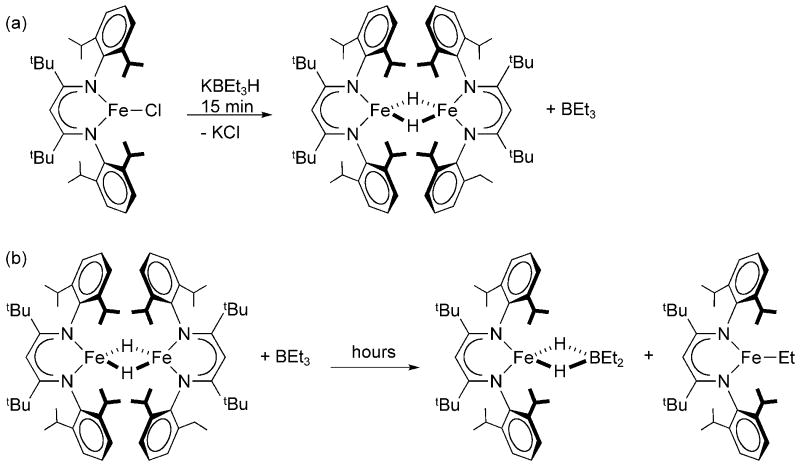
In previous work, we isolated a dimeric hydride complex, [LtBuFe(μ-H)]2, from the reaction of LtBuFeCl with KBEt3H (Scheme 2a). [18] This was the first iron hydride complex with a coordination number less than five, and the only other one known is its close analogue [LMeFe(μ-H)]2.[19] [LtBuFe(μ-H)]2 is curious because the hydride bridges hold the two iron(II) ions exceptionally close to one another (2.624(2) Å). [18] Despite the dimeric structure in the solid state, in solution the great steric interference between the diketiminate ligands on the two iron atoms leads the molecule to partially dissociate into monomers LtBuFeH, which were observed by 1H NMR spectroscopy. [18] Both the monomer and dimer show 1H NMR spectra with broadened resonances over a large chemical shift range, which are characteristic of paramagnetic molecules. Therefore, the available data suggested a high-spin electronic configuration for the iron(II) centers in [LtBuFe(μ-H)]2, but were not definitive because an excited electronic state might be accessed at room temperature.
Scheme 2.
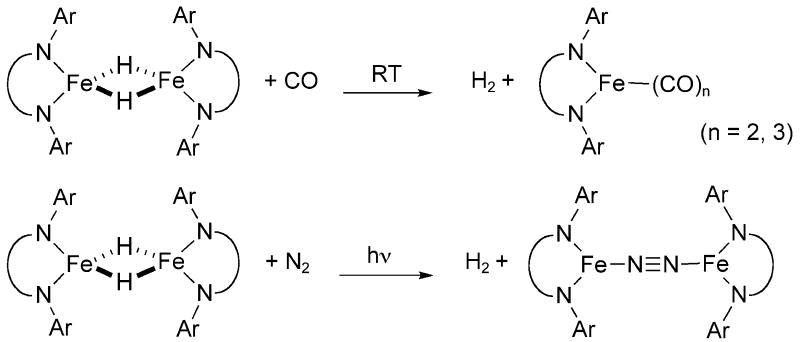
The reactivity of the dimeric hydride complex, [LtBuFe(μ-H)]2, has been examined with a range of substrates in solution.[18-21] The most relevant reaction to this work is the addition of strong field ligands such as CO to the hydride complex, which resulted in reductive elimination of H2 to form Fe(I) products (Scheme 3). [19] This observation shows that the reductive elimination of H2 from [LtBuFe(μ-H)]2 is facile in the presence of strong field ligands. However, photolysis was required to eliminate H2 in the presence of weaker ligands. For example, photolysis of the hydride complex under N2 atmosphere resulted in loss of H2 and formation of the dinitrogen complex, LtBuFeNNFeLtBu. [19] The intermediate species in this process are unknown, but it is conceivable that a highly unsaturated “LtBuFe” species is formed; then this iron(I) intermediate would be trapped by N2. The prospect of trapping this species with H2 inspired us to evaluate the oxidative addition of H2 to highly unsaturated iron(I) species in the absence of N2.
Scheme 3.
The hydride complex also reacts with boranes R3B to give LtBuFe(μ-H)2BR2 and LtBuFeR (Scheme 2b). [21] While the mechanistic study of this reaction was interesting, [21] the reactivity of [LtBuFe(μ-H)]2 towards boranes posed a practical problem. The synthesis of [LtBuFe(μ-H)]2 utilized KBEt3H as the hydride source, giving BEt3 as a byproduct. The desired hydride complex was the kinetic product of the reaction (formed within 15 minutes), but [LtBuFe(μ-H)]2 immediately began to react with the BEt3 byproduct to give the thermodynamic product LtBuFe(μ-H)2BR2 over several hours. Therefore, our samples of crude [LtBuFe(μ-H)]2 were invariably contaminated with the dihydridoborate complex, and the separation of the two complexes by crystallization was laborious. [19] Therefore, another motivation for the studies below was the development of a new synthetic route to [LtBuFe(μ-H)]2. Since [LtBuFe(μ-H)]2 demonstrated the ability to reductively eliminate H2, we decided to explore the microscopic reverse, oxidative addition of H2 to Fe(I), as a possible synthetic route. Here we report two new independent syntheses of [LtBuFe(μ-H)]2 and provide further characterization of the complex using Mössbauer spectroscopy.
Results and Discussion
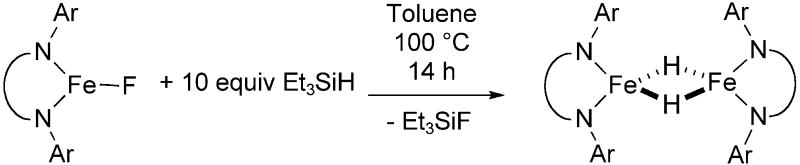
Synthesis of [LtBuFe(μ-H)]2 from a Silane
We have previously shown that LtBuFeF undergoes reactions with silylated substrates, eliminating Et3SiF and leaving the formerly silicon-bound group on iron.[22] This methodology was used to generate [LtBuFe(μ-H)]2 from LtBuFeF using Et3SiH as a hydride source (Scheme 4). A slurry of pink LtBuFeF in toluene was treated with 10 molar equivalents of Et3SiH and was heated overnight at 100 °C to produce a red-brown solution. Removal of the volatile components and crystallization from a saturated Et2O solution at -45 °C yielded [LtBuFe(μ-H)]2 in 83% yield. The identity of the product as the desired hydride complex was established through 1H NMR spectroscopy in C6D6, which was compared to the literature spectrum.[18]
Scheme 4.
The synthesis of [LtBuFe(μ-H)]2 via Et3SiH as a hydride source utilizes the formation of the strong Si-F bond as a thermodynamic driving force for the reaction. This method was inspired by Roesky, who used Et3SiH as hydride source to generate a β–diketiminate zinc-hydride dimer from the corresponding zinc fluoride complex.[23] However, a stoichiometric amount of Et3SiH was used in the zinc system while an excess was needed for the Fe system. Repeated attempts to generate [LtBuFe(μ-H)]2 using only 2 equivalents of Et3SiH resulted in incomplete conversion, as evidenced by a small amount of LtBuFeF remaining in the 1H NMR spectrum. The use of excess Et3SiH is not a problem as it is easily removed during the workup.
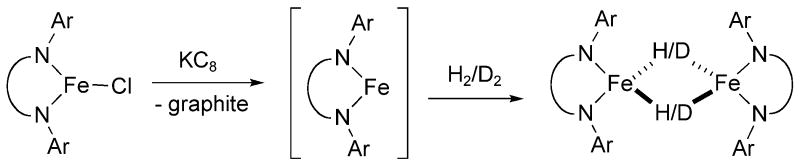
Synthesis from Dihydrogen
In another method, [LtBuFe(μ-H)]2 can be synthesized directly from LtBuFeCl using H2 as the hydride source (Scheme 5). Reduction of LtBuFeCl in Et2O with potassium graphite (KC8) under argon gave a dark green mixture. The mixture was degassed and placed under an atmosphere of purified H2, and the mixture turned red-brown. (The reaction is complete within 5 min of addition, as shown by a small-scale experiment in a J. Young NMR tube in C6D6.) After 18 h, H2 was removed, and the mixture was filtered through Celite to yield a red-brown solution. The red-brown solution was identified as [LtBuFe(μ-H)]2 by 1H NMR spectroscopy in C6D6.[18] Crystalline [LtBuFe(μ-H)]2 was obtained in 58% yield by cooling a saturated Et2O solution to -45 °C. [LtBuFe(μ-D)]2 was obtained in 62% yield through the same reaction conditions by substituting D2 for H2. Characterization of [LtBuFe(μ-D)]2 by 1H NMR spectroscopy in C6D6 revealed that the deuteride and the hydride complexes have identical 1H NMR spectra. The hydrides are not visible by 1H NMR spectroscopy, probably due to the proximity to the paramagnetic Fe atoms. Therefore, a different method was required to quantify the amount of deuteration in [LtBuFe(μ-D)]2.
Scheme 5.
We took advantage of the high-yield reaction of [LtBuFe(μ-H)]2 with 3-hexyne to form the three-coordinate Fe vinyl complex, LtBuFeC(Et)=CHEt.[18] Subsequently, a sample of LtBuFeC(Et)=CHEt was quenched with H2O, and the volatile components were separated and examined by GC-MS. As expected, the parent ion of 3-hexene (m/z 84) was observed. The experiment was repeated with [LtBuFe(μ-D)]2 to give LtBuFeC(Et)=CDEt, leading to 3-hexene-d1. The same analysis as above gave a mass spectrum with the parent ion at m/z 85 and a small peak at m/z 84. The relative intensities of the peaks showed that the sample of [LtBuFe(μ-D)]2 was 89% deuterated.
The absence of N2 is crucial for the success of the reaction of LtBuFeCl with KC8 and H2 to give [LtBuFe(μ-H)]2. If any N2 was present, the previously characterized dinitrogen complex, LtBuFeNNFeLtBu, was isolated. [24] Morris and co-workers have documented the parallels between H2 and N2 binding to unsaturated metal complexes. [25-27] They found that the fragments that bind N2 to give a M-N2 complex with an N-N stretching frequency below 2060 cm-1 did not form a stable H2 complex; rather, they oxidatively add H2 to give a dihydride species. A few complexes that deviate from the trend have been reported.[28-32] In the system examined here, the parallel is that two “LtBuFe” fragments can either cooperatively bind N2 in LtBuFeNNFeLtBu or cooperatively add H2 to give [LtBuFe(μ-H)]2. The N-N stretching frequency in LtBuFeNNFeLtBu is 1778 cm-1,[24] so the observation of H2 oxidative addition fits the established trend. We note that Mössbauer spectroscopy and DFT computations on LtBuFeNNFeLtBu show that the N2 ligand is reduced by two electrons (N22−).[33] Therefore, the iron(I) LtBuFe fragment gives exceptionally strong backbonding, and it is reasonable that the same bimetallic system oxidatively adds H2 and binds N2.
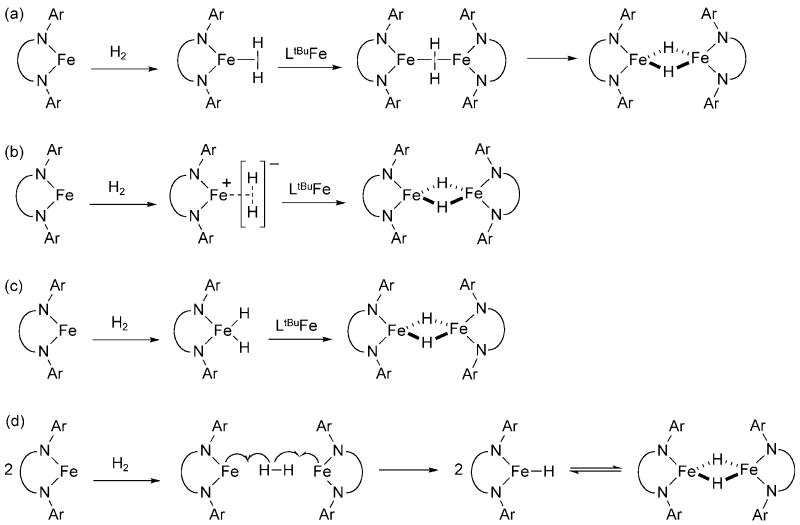
There are several possible mechanisms through which [LtBuFe(μ-H)]2 might be formed (Scheme 6). In each mechanism, we assume that LtBuFeCl is first reduced by KC8 to yield KCl and “LtBuFe” (see below). In Scheme 6a, the LtBuFe fragment binds H2 to form LtBuFe(H2), and subsequently attracts another LtBuFe fragment to give [LtBuFe(μ-H)]2. The π–backbonding from two Fe(I) centers results in homolytic cleavage of the H-H bond. However, it is possible that H2 is reduced by one or two electrons in the mononuclear intermediate. A one electron reduction of H2 would form a Fe(II) intermediate, LtBuFe(H2), with an H2− ligand (Scheme 6b). Though the radical anion H2− is unprecedented as a ligand, previous studies on alkyne and N2 binding to the LtBuFe fragment gave evidence for transfer of a single electron from the metal to the π-acceptor ligand. [33] [34] Alternatively, oxidative addition of H2 to the LtBuFe fragment would give the iron(III) intermediate LtBuFeH2 (Scheme 6c). Either mononuclear complex could interact with an additional LtBuFe fragment to give [LtBuFe(μ-H)]2. Finally, the reaction of two LtBuFe species simultaneously with H2 is possible (Scheme 6d). This reaction would be topologically similar to the homolytic cleavage of H2 and alkanes by rhodium porphyrins, which has been demonstrated to be termolecular [35-37]. The monomeric hydride complexes would then dimerize to give [LtBuFe(μ-H)]2.
Scheme 6.
Possible mechanisms for addition of H2 to the putative “LtBuFe” fragment. The diketiminate ligand is simplified in these pictures for clarity.
It is most likely that reaction with H2 with LtBuFeCl does not precede reduction, as LtBuFeCl showed no reactivity with H2 in the absence of a reducing agent. Reduction of a red solution of LtBuFeCl with KC8 in the absence of N2 or H2 produced a dark green mixture, for which the 1H NMR spectrum in C6D6 changed to have many peaks over the course of 3 h, accompanied by a pronounced color change from dark green to brown. These observations suggest the formation of a reactive intermediate, which unfortunately we have not been able to isolate or further characterize in Et2O, toluene, or pentane.
In order to test the reactivity of H2 toward a more stable high-spin Fe(I) complex with a very labile KCl ligand, we treated the iron(I) species LtBuFe(μ-Cl)K(18-crown-6)[24] with H2. Surprisingly, LtBuFe(μ-Cl)K(18-crown-6) did not react with H2 over 2 d at room temperature in C6D6, as judged by 1H NMR spectroscopy. In addition, this isolable Fe(I) complex was not very reactive with N2 as only partial conversion to LtBuFeNNFeLtBu was observed after 3 d. The low reactivity of LtBuFe(μ-Cl)K(18-crown-6) provides insight into the pathway of hydride formation: we surmise that the incoming H2 molecule must have access to the site occupied by the chloride ligand in LtBuFe(μ-Cl)K(18-crown-6), and that coordination of the (18-crown-6)KCl ligand is strong enough to inhibit the formation of essential LtBuFe(H2) or LtBuFe(N2) intermediates.
To our knowledge, the reaction reported here is the first example of binuclear oxidative addition of H2 to give a bridging diiron-hydride complex. There are a few other examples of binuclear oxidative addition of H2 to give a four-coordinate metal with two bridging hydride ligands. Bach and coworkers synthesized [(dtbpe)Ni(μ-H)]2 by the reduction of (dtbpe)NiCl2 with excess Mg under a H2 atmosphere.[38] [(dtbpe)Ni(μ-H)]2 was also prepared independently from a Ni(0) complex by treating (dtbpe)Ni-benzene with H2.[38] Schwartz and Andersen isolated a series of [P2PtH]2 complexes, where P2 is a chelating phosphine ligand,[39]by treating P2PtCl2 complexes with sodium amalgam under an atmosphere of H2. However, 1H NMR experiments show that these [P2PtH]2 complexes have terminal hydride ligands unlike [(dtbpe)Ni(μ-H)]2, which has bridging hydride ligands. These systems, like ours, utilize a reducing agent to open a coordination site for H2 and to reduce the metal to a low oxidation state that is more amenable to oxidative addition. Note that there are other complexes that utilize H2 as a hydride source for the synthesis of four-coordinate dinuclear bis(μ-hydride) complexes; however, these hydride complexes are generated via the hydrogenolysis of alkyl, aryl, or allyl metal complexes.[40-47]
Characterization
The stretching frequency, νFe-H, of the Fe-H bond in [LtBuFe(μ-H)]2 could not be assigned in previous reports due to the lack of a deuterated isotopomer. [18,19] The isolation of [LtBuFe(μ-D)]2 led to the examination of the IR spectra of [LtBuFe(μ-H)]2 and [LtBuFe(μ-D)]2 for a band that shifts between the two isotopomers. However, these complexes have identical IR spectra, and the spectra are featureless in the hydride stretch region between 1700 cm-1 and 2300 cm-1. The reason for the apparently low oscillator strength of the Fe-H stretching modes is not clear. We note that the hydride formulation is not in question, based on the X-ray crystal structure, [18] the insertion reactivity, [19] and the reaction with ligands to form H2. [19]
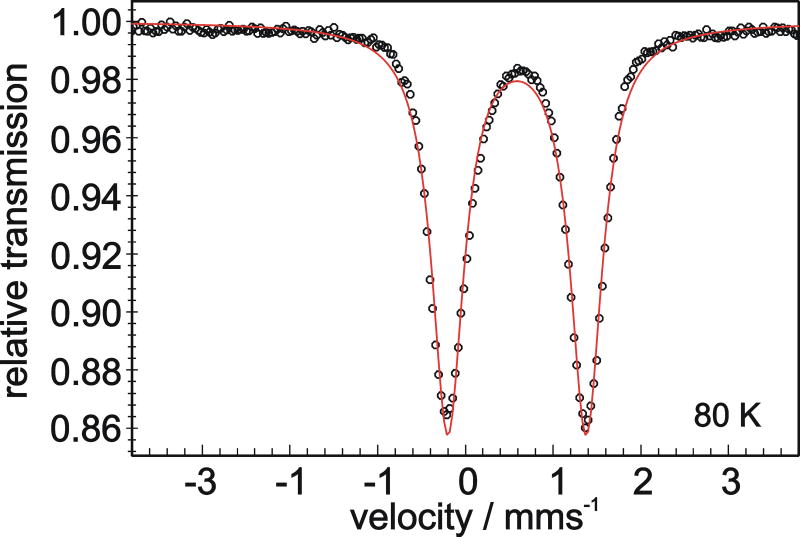
The purity and electronic structure of solid [LtBuFe(μ-H)]2 was evaluated using Mössbauer spectroscopy. The zero-field Mössbauer spectrum of solid [LtBuFe(μ-H)]2 at 80 K is shown in Figure 3. The spectrum exhibits one quadrupole doublet with δ = 0.59 mm/s and ΔEQ = 1.58 mm/s. The isomer shift is very close to the range (0.62-0.86 mm/s) observed in other high-spin Fe(II) diketiminate complexes.[33,34,48-52] High-spin, tetrahedral iron(II) sites in iron-sulfide clusters have similar parameters (δ = 0.6-0.7 mm/s and ΔEQ = 2-3 mm/s).[53,54] Low-spin octahedral iron sites have very different isomer shifts and quadrupole splittings: δ = 0.3-0.45 mm/s and ΔEQ < 1.5 mm/s.[54] The intermediate-spin (S = 1) iron(II) hydride complex [Fe(dppe)2H]+ has been characterized by Mössbauer spectroscopy, giving δ = 0.23 mm/s and ΔEQ = 1.53 mm/s.[55] The much higher isomer shift in [LtBuFe(μ-H)]2 (0.59 mm/s) strongly supports the assignment of the spin state at iron as high-spin (S = 2), and is consistent with the paramagnetically shifted 1H NMR spectrum.
Figure 3.
Mössbauer spectrum of solid [LtBuFe(μ-H)]2 at 80 K. The black circles are the data, and the red line represents a simulated spectrum using the parameters given in the text.
Concluding Remarks
Two new synthetic routes to [LtBuFe(μ-H)]2 were developed. In one, [LtBuFe(μ-H)]2 was synthesized from LtBuFeF using Et3SiH as a hydride source, utilizing the formation of the strong Si-F bond as the driving force for the reaction. [LtBuFe(μ-H)]2 was also synthesized via the binuclear oxidative addition of H2 to a low-coordinate Fe(I) intermediate. This reaction results in the homolytic binuclear cleavage of the H-H bond. These new syntheses were adapted to enable the isolation of the first low-coordinate iron deuteride complex, [LtBuFe(μ-D)]2.
These results show a new example of a high-spin complex that undergoes an organometallic reaction that is typically viewed through “2-electron” mechanisms. The oxidative addition of H2 is facile, and complements a number of recently reported ligand-induced reductive elimination reactions. [19] Continued research will address the mechanisms of these reactions.
Experimental
General Considerations
All manipulations were performed under a nitrogen atmosphere (or argon atmosphere where specified) by Schlenk techniques or in an M. Braun glovebox maintained at or below 1 ppm of O2 and H2O. NMR data were recorded on a Bruker Avance 500 spectrometer (500 MHz). All peaks in the NMR spectra are referenced to residual protiated solvents (benzene δ 7.16 ppm; toluene δ 2.08 ppm; cyclohexane δ 1.38 ppm). Infrared spectra (450-4000 cm-1) were recorded on KBr pellet samples in a Shimadzu FTIR spectrophotometer (FTIR-8400S) using 32 scans at 2 cm-1 resolution. GC-MS was performed using a Shimadzu QP2010 system with electron impact ionization. Pentane, hexane, tetrahydrofuran (THF), diethyl ether, and toluene were purified by passage through activated alumina and “deoxygenizer” columns from Glass Contour Co. (Laguna Beach, CA). Deuterated solvents were first dried over CaH2, then over Na/benzophenone, and then vacuum transferred into a storage container. Before use, an aliquot of each solvent was tested with a drop of sodium benzophenone ketyl in THF solution. Glassware was dried at 150 °C overnight, and Celite was dried overnight at 200 °C under vacuum. Ultra-high purity H2 was purchased from Air Products and was dried by passage through a column of activated alumina. D2 was purchased from Sigma-Aldrich and was dried by passage through a column of activated alumina. Et3SiH was stored under N2 over 4 Å molecular sieves. LtBuFeCl,[56] LtBuFeCH3[48] and LtBuFeF[22] were prepared by published procedures.
Synthesis of [LtBuFe(μ-H)]2 from Et3SiH
LtBuFeF (400 mg, 0.694 mmol) was added to a flask with a Teflon pin closure. Toluene (40 mL) was added to the flask to produce a pink slurry. Et3SiH (1.10 mL, 6.89 mmol, 9.9 equiv) was added to the flask via syringe. The flask was sealed, and the mixture was heated and stirred at 100 °C overnight. The solution changed color from pink to red-brown over the course of the reaction (~14 h). The solution was cooled to room temperature and the volatile components were removed under reduced pressure to yield a red-brown residue. The residue was dissolved in Et2O (70 mL) and was filtered through Celite. This solution was concentrated to 35 mL and was cooled to -45 °C to yield 243 mg of red-brown crystalline product. The mother liquor was concentrated to 5 mL and was cooled to -45 °C to produce a second crop of crystals (79 mg). The total yield was 322 mg (83%). 1H NMR (500 MHz, C6D6): 118, 70, 42, 24, 22, 20, 15, 12, 10, 6, 3, 1, -2, -3, -5, -8, -11, -12, -15, -16, -17, -23, -27, -28, -32, -38, -52, -58, -111, -115, -124 ppm. The complexity of the spectrum is attributed to hindered rotations and partial dissociation into monomeric LtBuFeH, as discussed previously. [18] IR (KBr pellet): 3057 (w), 3020 (w), 2962 (s), 2939 (s), 2869 (s), 1579 (w), 1539(m), 1485(s), 1475 (s), 1433 (s), 1385 (s), 1362 (s), 1312 (s), 1273 (m), 1253(m), 1215 (m), 1201 (m), 1184 (m), 1155 (w), 1120 (m), 1099 (m), 1072 (w), 1055 (w), 1022 (w), 935 (w), 887 (w), 843 (w), 800 (w), 779 (s), 756 (m), 711 (m), 667 (w) cm-1.
Synthesis of [LtBuFe(μ-H)]2 from H2
In an N2-filled glove box, LtBuFeCl (334 mg, 0.563 mmol, 1 equiv) was added to a flask with a Teflon pin closure and was dissolved in Et2O (40 mL) to produce a bright red solution. The flask was sealed, and the solution was degassed. In an argon-filled glove box, KC8 (86.5 mg, 0.640 mmol, 1.08 equiv) was added to the solution which resulted in an immediate color change from red to dark green. The flask was sealed, and the mixture was degassed again. The dark green mixture was stirred for 1 h. The mixture was frozen, and purified H2 (1 bar) was added to the frozen reaction mixture. H2 was removed after stirring overnight, and the mixture was filtered through Celite to yield a red brown solution. This solution was concentrated to 12 mL and cooled to -45 °C to yield 149 mg of red-brown crystalline product. The mother liquor was concentrated to 2 mL and was cooled to -45 °C to produce a second crop of crystals (32 mg). The total yield was 181 mg (58%). Synthesis of [LtBuFe(μ-D)]2 from D2 used the same method, and gave a yield of 62%.
Mössbauer spectroscopy
Mössbauer data were recorded on a spectrometer with alternating constant acceleration. The minimum experimental line width was 0.24 mm/s (full width at half-height). The sample temperature was maintained constant in an Oxford Instruments Variox cryostat. The γ-ray source was ca. 0.6 GBq 57Co/Rh. Isomer shifts are quoted relative to iron metal at 300 K. The zero-field spectra were simulated by using Lorentzian doublets.
Acknowledgments
This work was funded by the National Institutes of Health (GM065313). We thank Eckhard Bill (Max-Planck-Institut für Bioanorganische Chemie, Mülheim an der Ruhr, Germany) for collection and fitting of the Mössbauer spectrum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crabtree RH. The Organometallic Chemistry of the Transition Metals. Wiley; Hoboken, NJ: 2005. [Google Scholar]
- 2.Cotton FA, Wilkinson G, Murillo CA, Bochmann M. Advanced Inorganic Chemistry. Wiley; New York, NY: 1999. [Google Scholar]
- 3.Deutsch PP, Eisenberg R. Chem Rev. 1988;88:1147–1161. [Google Scholar]
- 4.Crabtree RH. Acc Chem Res. 1990;23:95–101. [Google Scholar]
- 5.Heinekey DM, Oldham WJ., Jr Chem Rev. 1993;93:913–926. [Google Scholar]
- 6.Kubas GJ. Metal Dihydrogen and σ-Bond Complexes: Structure, Theory and Reactivity. Kluwer Academic / Plenum Publishers; New York, NY: 2001. [Google Scholar]
- 7.McGrady GS, Guilera G. Chem Soc Rev. 2003;32:383–392. doi: 10.1039/b207999m. [DOI] [PubMed] [Google Scholar]
- 8.Morris RH. Non-Classical Hydrogen Bonding Along the Pathway to the Heterolytic Splitting of Dihydrogen. In: Peruzzini M, Poli R, editors. Recent Advances in Hydride Chemistry. Elsevier; New York: 2001. pp. 1–38. [Google Scholar]
- 9.Holland PL. Acc Chem Res. 2008;41:905–914. doi: 10.1021/ar700267b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey JN, Poli R, Smith KM. Coord Chem Rev. 2003;238-239:347–361. [Google Scholar]
- 11.Poli R, Harvey JN. Chem Soc Rev. 2003;32:1–8. doi: 10.1039/b200675h. [DOI] [PubMed] [Google Scholar]
- 12.Veige AS, Slaughter LM, Lobkovsky EB, Wolczanski PT, Matsunaga N, Decker SA, Cundari TR. Inorg Chem. 2003;42:6204–6224. doi: 10.1021/ic0300114. [DOI] [PubMed] [Google Scholar]
- 13.Poli R. J Organomet Chem. 2004;689:4291–4304. [Google Scholar]
- 14.Carreon-Macedo J-L, Harvey JN. J Am Chem Soc. 2004;126:5789–5797. doi: 10.1021/ja049346q. [DOI] [PubMed] [Google Scholar]
- 15.Harvey JN. Phys Chem Chem Phys. 2007;9:541. doi: 10.1039/b614390c. [DOI] [PubMed] [Google Scholar]
- 16.Besora M, Carreon-Macedo J-L, Cowan AJ, George MW, Harvey JN, Portius P, Ronayne KL, Sun X-Z, Towrie M. J Am Chem Soc. 2009;131:3583–3592. doi: 10.1021/ja807149t. [DOI] [PubMed] [Google Scholar]
- 17.Vela J, Vaddadi S, Cundari TR, Smith JM, Gregory EA, Lachicotte RJ, Flaschenriem CJ, Holland PL. Organometallics. 2004;23:5226–5239. [Google Scholar]
- 18.Smith JM, Lachicotte RJ, Holland PL. J Am Chem Soc. 2003;125:15752–15753. doi: 10.1021/ja038152s. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Sadique AR, Smith JM, Dugan TR, Cowley RE, Brennessel WW, Flaschenriem CJ, Bill E, Cundari TR, Holland PL. J Am Chem Soc. 2008;130:6624–6638. doi: 10.1021/ja710669w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadique AR, Gregory EA, Brennessel WW, Holland PL. J Am Chem Soc. 2007;129:8112–8121. doi: 10.1021/ja069199r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Brennessel WW, Holland PL. Organometallics. 2007;26:3217–3226. doi: 10.1021/om7003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vela J, Smith JM, Yu Y, Ketterer NA, Flaschenriem CJ, Lachicotte RJ, Holland PL. J Am Chem Soc. 2005;127:7857–7870. doi: 10.1021/ja042672l. [DOI] [PubMed] [Google Scholar]
- 23.Hao H, Cui C, Roesky HW, Bai G, Schmidt H-G, Noltemeyer M. Chem Commun. 2001:1118–1119. [Google Scholar]
- 24.Smith JM, Sadique AR, Cundari TR, Rodgers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J Am Chem Soc. 2006;128:756–769. doi: 10.1021/ja052707x. [DOI] [PubMed] [Google Scholar]
- 25.Morris RH, Earl KA, Luck RL, Lazarowych NJ, Sella A. Inorg Chem. 1987;26:2674–2683. [Google Scholar]
- 26.Jessop PG, Morris RH. Coordination Chemistry Reviews. 1992;121:155–284. [Google Scholar]
- 27.Morris RH. Inorg Chem. 1992;31:1471–1478. [Google Scholar]
- 28.Earl KA, Jia G, Maltby PA, Morris RH. J Am Chem Soc. 1991;113:3027–3039. [Google Scholar]
- 29.Leal AJ, Tenorio MJ, Puerta MC, Valerga P. Organometallics. 1995;14:3839–3847. [Google Scholar]
- 30.King WA, Luo X-L, Scott BL, Kubas GJ, Zilm KW. J Am Chem Soc. 1996;118:6782–6783. [Google Scholar]
- 31.Reid SM, Neuner B, Schrock RR, Davis WM. Organometallics. 1998;17:4077–4089. [Google Scholar]
- 32.Seino H, Arita C, Nonokawa D, Nakamura G, Harada Y, Mizobe Y, Hidai M. Organometallics. 1999;18:4165–4173. [Google Scholar]
- 33.Stoian SA, Vela J, Smith JM, Sadique AR, Holland PL, Münck E, Bominaar EL. J Am Chem Soc. 2006;128:10181–10192. doi: 10.1021/ja062051n. [DOI] [PubMed] [Google Scholar]
- 34.Stoian SA, Yu Y, Smith JM, Holland PL, Bominaar EL, Münck E. Inorg Chem. 2005;44:4915–4922. doi: 10.1021/ic050321h. [DOI] [PubMed] [Google Scholar]
- 35.Sherry AE, Wayland BB. Journal of the American Chemical Society. 1990;112:1259–1261. [Google Scholar]
- 36.Wayland BB, Ba S, Sherry AE. Journal of the American Chemical Society. 1991;113:5305–5311. [Google Scholar]
- 37.Wayland BB, Ba S, Sherry AE. Inorganic Chemistry. 1992;31:148–150. [Google Scholar]
- 38.Bach I, Goddard R, Kopiske C, Seevogel K, Pörschke K-R. Organometallics. 1999;18:10–20. [Google Scholar]
- 39.Schwartz DJ, Andersen RA. J Am Chem Soc. 1995;117:4014–4025. [Google Scholar]
- 40.Brown RK, Williams JM, Fredrich MF, Day VW, Sivak AJ, Muetterties EL. Proc Natl Acad Sci USA. 1979;76:2099–2102. doi: 10.1073/pnas.76.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teller RG, Williams JM, Koetzle TF, Burch RR, Gavin RM, Muetterties EL. Inorg Chem. 1981;20:1806–1811. [Google Scholar]
- 42.Fryzuk MD. Can J Chem. 1983;61:1347–1351. [Google Scholar]
- 43.Fryzuk MD, Jones T, Einstein FWB. Organometallics. 1984;3:185–191. [Google Scholar]
- 44.Goeden GV, Huffman JC, Caulton KG. Inorg Chem. 1986;25:2484–2485. [Google Scholar]
- 45.Bennett BL, Roddick DM. Inorg Chem. 1996;35:4703–4707. [Google Scholar]
- 46.MacAdams LA, Buffone GP, Incarvito CD, Golen JA, Rheingold AL, Theopold KH. Chem Commun. 2003:1164–1165. doi: 10.1039/b301943h. [DOI] [PubMed] [Google Scholar]
- 47.Monillas WH, Yap GPA, Theopold KH. Angew Chem Int Ed Engl. 2007;46:6692–6694. doi: 10.1002/anie.200701933. [DOI] [PubMed] [Google Scholar]
- 48.Andres H, Bominaar EL, Smith JM, Eckert NA, Holland PL, Münck E. J Am Chem Soc. 2002;124:3012–3025. doi: 10.1021/ja012327l. [DOI] [PubMed] [Google Scholar]
- 49.Holland PL, Cundari TR, Perez LL, Eckert NA, Lachicotte RJ. J Am Chem Soc. 2002;124:14416–14424. doi: 10.1021/ja025583m. [DOI] [PubMed] [Google Scholar]
- 50.Vela J, Stoian S, Flaschenriem CJ, Münck E, Holland PL. J Am Chem Soc. 2004;126:4522–4523. doi: 10.1021/ja049417l. [DOI] [PubMed] [Google Scholar]
- 51.Eckert NA, Stoian S, Smith JM, Bominaar EL, Münck E, Holland PL. J Am Chem Soc. 2005;127:9344–9345. doi: 10.1021/ja0436704. [DOI] [PubMed] [Google Scholar]
- 52.Cowley RE, Elhaik J, Eckert NA, Brennessel WW, Bill E, Holland PL. J Am Chem Soc. 2008;130:6074–6075. doi: 10.1021/ja801375g. [DOI] [PubMed] [Google Scholar]
- 53.Beinert H, Holm RH, Münck E. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 54.Münck E. Physical Methods in Bioinorganic Chemistry. 2000:287–319. [Google Scholar]
- 55.Franke O, Wiesler BE, Lehnert N, Peters G, Burger P, Tuczek F. Z Anorg Allg Chem. 2006;632:1247–1256. [Google Scholar]
- 56.Smith JM, Lachicotte RJ, Holland PL. Chem Commun. 2001:1542–1543. doi: 10.1039/b103635c. [DOI] [PubMed] [Google Scholar]