Abstract
Outbred, male Sprague-Dawley rats can be classified as either low or high cocaine responders (LCRs or HCRs, respectively) based on cocaine-induced locomotor activity in an open-field arena. This difference reflects cocaine’s ability to inhibit the striatal dopamine transporter and predicts development of sensitization. To investigate the relationship between initial cocaine locomotor responsiveness and cocaine reward, here we first classified rats as either LCRs or HCRs in a conditioned place preference (CPP) apparatus. Subsequently, we conducted cocaine conditioning trials, twice daily over four days with vehicle and cocaine (10 mg/kg, i.p. or 1 mg/kg, i.v.). When cocaine was administered by the i.p. route, similar to previous findings in the open-field, LCRs and HCRs were readily classified and locomotor sensitization developed in LCRs, but not HCRs. However, cocaine CPP was not observed. In contrast, when cocaine was administered by the i.v. route, the LCR/HCR classification not only predicted sensitization, but also CPP, with only LCR rats exhibiting sensitization and cocaine conditioning. Our findings show that the initial locomotor response to cocaine can predict CPP in male Sprague-Dawley rats under conditions when place conditioning develops, and that LCRs may be more prone to develop conditioning in the context of cocaine reward.
Keywords: cocaine, conditioned place preference, dopamine transporter, stimulant, locomotor activity, sensitization, reward, initial sensitivity, outbred rats, individual differences
1. Introduction
Individuals exhibit a wide range of initial responsiveness to both the therapeutic and reinforcing effects of stimulant drugs, and this differential responsiveness is due largely to phenotypic differences (see Lott et al., 2005; Volkow and Swanson, 2003). Individual variability in responsiveness to stimulants may influence subsequent risk for stimulant abuse and addiction. This relationship has been most clearly documented for alcohol where a low level of response to ethanol in young men is associated with an enhanced risk for alcoholism later in life (Schucket, 1994; Schucket et al., 2006). Utilizing animal models that are based on differential individual responsiveness to drug(s) may be an effective strategy for identifying the genes and cellular mechanisms that can contribute to vulnerability to drug addiction.
A number of animal models have exploited pre-existing (rather than drug-induced) differences among individuals that correlate with sensitivity to stimulant-induced effects. For example, outbred male Sprague-Dawley (S-D) and Wistar rats can be initially identified as low or high responders to novelty, LRs or HRs, respectively. The greater locomotor activity of HRs in an inescapable novel environment has been correlated with an enhanced vulnerability to self-administer the stimulants amphetamine and cocaine (Piazza et al., 1989, 2000; see Cain et al., 2004). It should be noted, however, that differential LR/HR novelty locomotor responsiveness was recently linked to different rates of learning the self-administration task, rather than cocaine intake per se (Mitchell et al., 2005). In addition, locomotor response to novelty is not always predictive of amphetamine- or cocaine-induced conditioned place preference (CPP) in rats, and mice categorized as LRs in a CPP chamber exhibit greater low dose cocaine-induced CPP than HRs (Brabant et al., 2005; Erb et al., 1994; Gong et al., 1996; Klebaur and Bardo, 1999; Shimosato and Watanabe, 2003).
Initial differences in cocaine-induced locomotor activation have also been reported and correlate well with several neurobiological and behavioral variables. For example, Cynomolgus monkeys that exhibit higher levels of cocaine-induced locomotor activity in a novel open-field arena are more likely to become subordinate in social groups, have lower numbers of basal ganglia dopamine D2 receptors and are more likely to self-administer cocaine (Morgan et al., 2000, 2002). In male S-D rats, acute cocaine-induced locomotor activity correlates positively with cocaine-induced enhancement of excitatory synaptic strength in the ventral tegmental area, which contains the cell bodies of the mesocorticolimbic DA neurons (Borgland et al., 2004).
In our laboratory, we have classified groups of outbred male S-D rats as either low or high cocaine responders (LCRs or HCRs, respectively), based on the median split of their open-field locomotor activity during the first 30 min after a cocaine injection (10 mg/kg, i.p.). In response to the initial dose of cocaine, HCRs exhibit significantly higher levels of locomotor activity and significant inhibition of in vivo striatal DA clearance by the dopamine transporter (DAT), relative to vehicle controls and LCRs (Briegleb et al., 2004; Gulley et al., 2003; Sabeti et al., 2002). With repeated cocaine administration, however, the responsiveness of the HCRs remains relatively constant whereas LCRs begin to exhibit marked cocaine-induced locomotor activity (i.e., locomotor sensitization) and significant inhibition of DA clearance (Sabeti et al., 2003). It is important to note that in our experiments S-D rats classified as LCRs and HCRs do not differ in novelty-induced locomotor activity when first placed in the open field apparatus (Briegleb et al., 2004; Gulley et al., 2003), and, conversely, S-D rats characterized by their response to a novel environment (HR/LR) do not consistently differ in their cocaine-induced locomotor activation (Gulley et al., 2003). These data suggest that the mechanisms underlying differential cocaine responsiveness in LCRs/HCRs and LRs/HRs may not be the same in S-D rats.
We designed the present study to determine what contribution, if any, individual differences in initial responsiveness to cocaine have on cocaine place conditioning in male S-D rats. The conditioned place preference (CPP) procedure is an animal model used to infer rewarding effects of drugs by measuring the capacity of a previously drug-paired environment to elicit and maintain approach behavior (for review, see Bardo and Bevins, 2000; Carr et al., 1989; Tzschentke, 1998). In this study, we first characterized outbred, male S-D rats as either LCRs or HCRs in a CPP apparatus and then subsequently conducted a series of cocaine (10mg/kg, i.p, or 1 mg/kg, i.v.) and vehicle place conditioning trials. We chose the CPP procedure because it can be adapted to induce cocaine CPP with daily injection of cocaine (Dong et al., 2004; Harris and Aston-Jones, 2003; Kotlinska and Biala, 1999; 2000; McGeehan and Olive, 2003; Tzschentke and Schmidt, 1997), the pattern of exposure we use in the laboratory to characterize rats as LCRs and HCRs and to measure the differential development of behavioral sensitization (e.g., Briegleb et al., 2004; Gulley et al., 2003; Sabeti et al., 2002; 2003).
2. Methods
2.1 Animals
Eighty male S-D rats (275–325g) were obtained from Harlan (Indianapolis, IN). Rats were housed in the testing room on a 12-h light/dark cycle (lights on at 7:30 AM) with ad libitum access to food and water. All experimental procedures were performed in agreement with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Colorado at Denver and Health Sciences Center – Downtown Denver campus.
2.2 Indwelling intravenous catheters
Half of the rats (n=40) were fit with chronic indwelling jugular venous catheters, constructed partly or entirely in the laboratory with parts from Plastics One, Inc (Roanoke, VA) using established procedures (Caine et al., 1993). All catheters featured an externalized access port protruding from the skin on the rat’s back and were implanted under ketamine (100 mg/kg, i.m.)/xylazine (10 mg/kg, i.m.) anesthesia. Catheters were flushed as needed with a sterile saline solution that contained 16.7 U/ml heparin sodium. Rats were allowed to recover from surgery for at least 5 days before the start of an experiment. Ten rats had catheter failures during the conditioning experiments (almost exclusively blockages). These data were excluded from the final analyses. One rat died during surgery.
2.3 Place conditioning apparatuses
Four commercially available CPP apparatuses were used in this study (Med Associates, St. Albans, VT). Each apparatus was housed inside a custom-made cabinet (internal dimensions approximately 105 × 57 × 56 cm) complete with a white noise speaker and ventilation fan. Each apparatus consisted of two distinct outer compartments (28 × 21 × 21 cm) -- one black with bar flooring and corn cob bedding and the other white with black stripes, mesh flooring and grey Tek-fresh™ bedding -- connected by a smaller gray compartment (12 × 21 × 21 cm). Fifteen infrared photobeams spaced at approximately 5 cm intervals throughout the apparatus (six in each outer compartment and three in the center compartment) recorded horizontal movement in the apparatus. Each compartment in the apparatus was illuminated with a 28 V, 100 mA bulb mounted on the compartment’s ceiling panel.
2.4 Characterizing the initial locomotor response to cocaine
In the first experiment, rats injected with cocaine (n=12) came from a single cohort and were tested at the same time in the laboratory. The median split of locomotor activity scores during the first 30 min after cocaine injection at session one (804) was used to classify these rats as either LCRs or HCRs. In subsequent experiments, cocaine-treated rats came from two (i.p. cocaine) and three (i.v. cocaine) cohorts tested at different times. Median locomotor activity scores for these groups were 1110 (n=12) and 775 (n=8) for rats injected i.p. with cocaine and 1112 (n=6), 581 (n=6), and 1055 (n=8) for rats infused i.v. with cocaine. Because locomotor activity levels vary between groups over time, LCR/HCR classification was made both within each distinct group tested (e.g., see Marinelli, 2005) and by treating the two i.p. cohorts and three i.v. cohorts as single populations (median locomotor scores were 927 for the 20 rats injected i.p. with cocaine and 894 for the rats infused i.v. with cocaine). Rats that were classified differently by these two methods (i.e., within and between groups) were excluded from the final data analysis (n=0 for i.p. cocaine and n=4 for i.v. cocaine).
2.5 Measuring initial response to cocaine and sensitization in the CPP apparatus
During the first experiment, we used the CPP apparatuses to measure locomotor activity only (i.e., place conditioning trials were not conducted). During this experiment, rats had free access to all three compartments of the place conditioning apparatus every time they were placed in the apparatus. Rats were first habituated to the CPP apparatus for 1 h. After 1 h, rats were removed and injected with either vehicle (saline “control rats”) or 10 mg/kg cocaine, i.p., and then returned to the chamber for an additional 30 min. Consecutive beam breaks were used as the measure of locomotor activity. Rats were tested in the CPP apparatus for their locomotor response to vehicle or cocaine once each day for seven days. Following a 24 h-period where rats remained in their home cages, a “reversal test” was performed. Here, the same testing procedure was used except that rats that received cocaine in sessions one to seven were administered vehicle and rats that received vehicle during sessions one to seven were administered cocaine.
2.6 Concurrent assessment of locomotor activity and CPP
In a second set of experiments, individual differences in cocaine-induced locomotor activity, sensitization, and CPP were assessed concurrently in the CPP apparatus. Session one was conducted as described above. Location within the apparatus during the first 15 min of this session was used as the rat’s baseline preference for the compartments within the CPP apparatus. Groups of rats spent nearly equal time in both conditioning compartments of the apparatus at baseline (see results). After 60 min in the apparatus, rats were injected with either vehicle (“control rats”) or cocaine by either the i.p. or i.v. route (10 mg/kg, i.p., or 1 mg/kg., i.v., 7-sec infusion), and locomotor activity was measured over the next 30 min. These data were used to categorize rats as either LCRs or HCRs. Over the next four days, rats were exposed to two place conditioning sessions each day, at least 4 h apart. Each daily conditioning session consisted of 30 min of restricted access in either the white or black compartment following exposure to either vehicle (i.p. or i.v.) or cocaine (10 mg/kg, i.p., or 1.0 mg/kg, i.v.). Rats were randomly assigned to receive cocaine in either the white or black compartment, and they received vehicle in the other. Also, rats received cocaine in either the morning or afternoon on a random daily basis. The next (sixth) day, rats were placed in the middle compartment and given free access to all three compartments of the apparatus for 1 h. A rat’s location in the apparatus during the first 15 min of this 1 h-period was used to measure post-conditioning place preference. After 1 h, rats were removed from the apparatus and given an injection of either vehicle or 10 mg/kg cocaine, i.p., (or infusion of vehicle or 1 mg/kg cocaine, i.v.) and then returned to the chamber for 30 min to measure locomotor response to either vehicle or cocaine.
2.7 Data analysis
Locomotor activity score was defined by total consecutive beam breaks and represents horizontal movement through the CPP apparatus. Non-consecutive beam breaks, which may be more likely to monitor stereotypy, were not analyzed. Place conditioning data are presented as a preference ratio, calculated as the time spent in the drug-paired chamber divided by the total time spent in drug- and vehicle-paired chambers.
Data are expressed as mean values ± standard error of the mean (SEM). Data were analyzed using SPSS for Windows, version 14.0 (SPSS Inc., Chicago, IL) using repeated measures analysis of variance (RMANOVA), one-way ANOVA, least significant difference (LSD) post hoc tests, and independent or paired samples t-tests.
2.7 Drugs
(−)-Cocaine HCl was obtained from the National Institute on Drug Abuse (RTI International, Research Triangle Park, NC). Cocaine was dissolved in a 0.9% sodium chloride solution. Heparin sodium (16.7 U/mL) was added to the solution for i.v., but not i.p., administration (both for drug and vehicle infusions).
3. Results
3.1 LCRs and HCRs are observed in a place conditioning apparatus
Figure 1 presents locomotor activity data from 16 rats injected i.p. with either saline vehicle (“control”; n=4) or cocaine (10 mg/kg; n=12) during each of seven once-daily sessions. In this experiment, the place preference chambers were used to measure locomotor effects of cocaine and development of cocaine sensitization, but not development of cocaine CPP (see Methods). Locomotor activity scores in the 30 min period after vehicle or cocaine injection were 638 ± 126 and 898 ± 654, respectively, but this difference was not significant. We then split locomotor activity scores from the 12 rats injected with cocaine at the median (804) to categorize rats as either LCRs or HCRs. Locomotor activity scores from these two groups (LCR, 619 ± 63; HCR, 1178 ± 138) were compared with each other and control rats. One way ANOVA revealed significant group differences [F(2,15) = 8.477, P = 0.004]. Post-hoc comparisons revealed significant differences between control and HCR (p = 0.007), and LCR and HCR (p = 0.003), but not control and LCR. There were no significant group differences in locomotor activity during the novelty (0 – 30 min) or habituation (30 – 60 min) components of session one.
Figure 1.
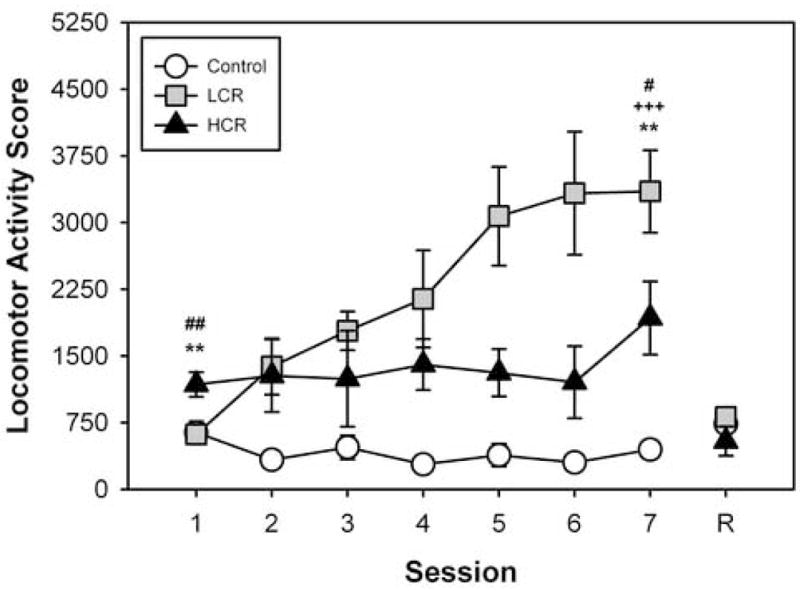
Repeated i.p. cocaine injections induce locomotor sensitization in LCRs, but not in HCRs, when locomotor activity is measured in a CPP apparatus. Vehicle or cocaine (10 mg/kg, i.p.) was administered once daily for seven days to the control and experimental groups, respectively. Ordinate: Locomotor Activity Score, representing consecutive beam breaks in the CPP apparatus during the 30-min post-injection interval. Abscissa: Session. On day 8 for a reversal test (R), rats in the control group (n=4) were given cocaine whereas LCRs (n=6) and HCRs (n=6) were given vehicle. Mean values ± SEM. +++, p < 0.001, control vs. LCR; #, p < 0.05, control vs. HCR; ##, p < 0.01, control vs. HCR; ** p<0.01, LCR vs. HCR. Although significant between group differences were revealed by ANOVA at sessions one, four, five, six and seven, only post-hoc comparisons at sessions one and seven are presented on the figure.
3.2 Locomotor sensitization develops in LCRs, but not HCRs, in the conditioning apparatus
After classifying rats as HCR/LCR (see Methods section 2.4) and comparing them with control rats, locomotor activity scores across the seven sessions were analyzed with RMANOVA. This analysis revealed a significant group by session interaction [F(12,78)=4.470, p < 0.001]. Subsequent analyses revealed that only LCRs showed a significant increase in locomotor activity across the seven sessions [Figure 1; from RMANOVA of LCR locomotor activity scores; F(6,30) = 12.183, P < 0.001]. Additionally, significant between-group differences were revealed by one-way ANOVA at sessions one, four, five, six and seven (p < 0.05 – 0.01). Post-hoc tests revealed that by session seven the relationship of between-group differences had changed, such that cocaine-elicited locomotor activity in LCRs was now greater than both cocaine-elicited locomotor activity in HCRs (3350 ± 464 vs. 1927 ± 411, respectively, p = 0.022) and control rats (3350 ± 464 vs. 447 ± 82, p < 0.001). There were no significant differences observed between groups on the reversal test conducted 24 h after session seven.
3.3 Locomotor sensitization develops in LCRs, but not HCRs, during a CPP experiment
In a second set of experiments, sensitization to cocaine-elicited locomotor activity and the development of CPP was measured simultaneously across six daily experimental sessions (see Methods). Figures 2A and C show the locomotor activity scores from sessions one and six, respectively, that were measured from control (n=4) and cocaine-injected rats (n=20; 10 mg/kg, i.p.). Mean locomotor activity scores from rats categorized as LCR (n=10) and HCR (n=10) were 538 ± 56 and 1687 ± 202, respectively. RMANOVA revealed a group by session interaction [F(2,21) = 4.363, P = 0.026]. Subsequent one-way ANOVA revealed significant group differences in locomotor activity scores between LCRs, HCRs, and control rats during session one [F(2,23) = 17.965, P < 0.001], during which HCRs exhibited significantly greater locomotor activity than LCRs (p < 0.001) and control rats (738 ± 106, p = 0.002). Locomotor activity scores from control rats and LCRs did not differ.
Figure 2.
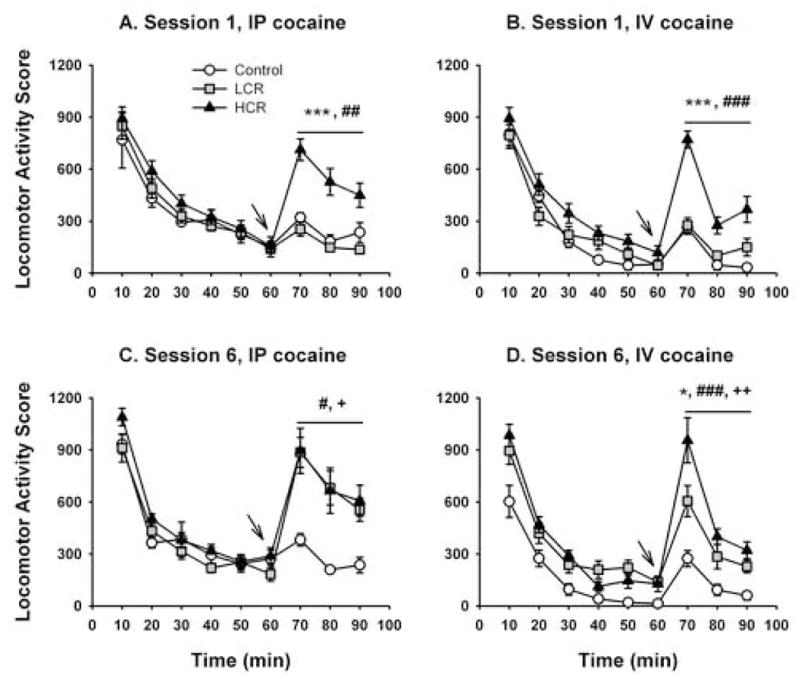
Time course of locomotor activity in the CPP apparatus during sessions one and six of an experiment in which locomotor activity was measured in a place conditioning apparatus before and after place conditioning trials with i.p. vehicle or cocaine (10 mg/kg; panels A and C) or i.v. vehicle or cocaine (1 mg/kg; panels B and D). A separate group of rats was either injected i.p. or infused i.v. with vehicle in both compartments of the conditioning apparatus as an additional experimental control (“control”). Ordinates: Locomotor Activity Score, representing consecutive beam breaks in the CPP apparatus during 10 min bins. Abscissa: Time, in minutes. Arrows at 60 min indicate the time at which cocaine or vehicle was administered to rats. Mean values ± SEM. +, p < 0.05, control vs. LCR; ++, p < 0.01, control vs. LCR; #, p < 0.05, control vs. HCR; ##, p < 0.01, control vs. HCR; ### p < 0.001, control vs. HCR; *, p < 0.05 LCR vs. HCR; ***, p < 0.001 LCR vs. HCR.
Only rats characterized as LCRs demonstrated sensitization to the locomotor effects of cocaine: paired-samples t-tests revealed significant differences in locomotor activity scores between sessions one and six for LCR rats [538 ± 55 vs. 2122 ± 221; t(9) = −6.953, p < 0.001], but not for HCRs or control rats. When locomotor activity scores were compared between groups at session six, both LCRs and HCRs exhibited significantly more locomotor activation than control rats, but LCRs and HCRs did not differ in the magnitude of locomotor activation elicited by cocaine [Figure 2C; overall ANOVA, F(2,23) = 4.238, P = 0.028; vehicle vs. LCR, p = 0.015; vehicle vs. HCR, p = 0.012). There were no significant between group differences revealed when locomotor activity scores from the novelty (0 – 30 min) or habituation (30 – 60 min) components of sessions one or six were analyzed with RMANOVA.
Figures 2B and D show locomotor activity scores from control (n=9) and cocaine-infused rats (n=16; 1 mg/kg, i.v.). RMANOVA revealed a group by session interaction [F(2,22) = 4.272, P = 0.027]. Subsequent one-way ANOVA revealed that locomotor activity scores from LCR (n=8), HCR (n=8), and control rats were significantly different both at session one [F(2,22) = 43.489, P < 0.001] and session six [F(2,22) = 17.933, P < 0.001]. At session one, the locomotor activity scores from control rats (339 ± 52) and LCRs (527 ± 62) were significantly different from HCRs (1414 ± 132; p < 0.001 and p < 0.001, respectively), but not from each other. By session six, locomotor activity scores from control rats (425 ± 61) were significantly different from both LCRs (1118 ± 180, p = 0.003) and HCRs (1676 ± 190, p < 0.001), and LCRs and HCRs differed from each other (p = 0.017). Once again, only rats characterized as LCRs showed marked and significant increases in the locomotor effects of cocaine between sessions one and six [t(7) = −4.198, p = 0.004]. One-way ANOVA of session one data also revealed a trend for group differences in the novelty component [0 – 30 min; HCR, 1748 ± 160; LCR, 1344 ± 123; control, 1419 ± 108; F(2,22) = 2.623, P = 0.095] and significant group differences in the habituation component [30–60 min; F(2,22) = 5.138, P = 0.015], in which HCRs exhibited more locomotor activity than control rats (532 ± 109 vs. 172 ± 46) but not more than LCRs (341 ± 80).
There were no significant differences in the 30 min post-injection locomotor activity scores measured in HCR rats treated with cocaine i.p. or i.v. (1687 ± 203 vs. 1414 ± 132, respectively) or in LCR rats treated with cocaine i.p. or i.v. (538 ± 56 vs. 527 ± 62, respectively). However, when data were analyzed in one-min bins starting three min before injection through 10 min after, RMANOVA revealed significant group by session interactions for both i.p. cocaine [F(24,240) = 3.117, P < 0.001] and i.v. cocaine [F(24,264) = 4.449, P < 0.001]. Subsequent ANOVAs and post hoc tests revealed that in HCR rats, locomotor activity scores first differed significantly from control one min after i.v. infusion of cocaine [ANOVA, F(2,61) = 6.616, P = 0.006; HCR vs. control, p = 0.002] and peaked by two minutes (Figure 3). In contrast, locomotor activity scores first differed significantly from control four minutes after i.p. injection of cocaine [ANOVA, F(2,21) = 14.039, P < 0.001; HCR vs. control, p = 0.002] and peaked by five minutes (Figure 3). A t-test performed on peak locomotor activity scores from HCRs revealed a statistical trend for higher peak locomotor activity after i.v. versus i.p administration of cocaine [131 ± 11 vs. 92 ± 15; t(16) = − 2.010, p = 0.062].
Figure 3.
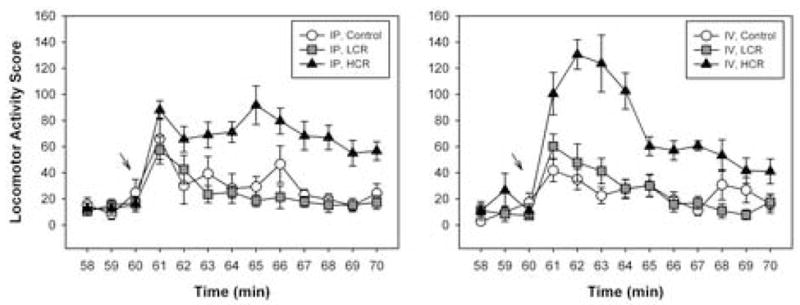
Time course of locomotor activity in the CPP apparatus during the three min prior to and 10 min after i.p. injection (left panel) or i.v. infusion (right panel) of cocaine or vehicle (“control”) during session one. Ordinates: Locomotor Activity Score, representing consecutive beam breaks in the CPP apparatus during one-min bins. Abscissa: Time, in minutes. Arrows at 60 min indicate the time at which cocaine or vehicle was administered to rats. Mean values ± SEM. Results of select post-hoc comparisons are presented in the text.
3.4 LCRs, but not HCRs, develop a CPP with intravenous cocaine
For rats injected i.p. with either vehicle or cocaine during conditioning trials, there were no significant group differences in CPP preference ratios either at baseline (“pre-conditioning; control, 0.49 ± 0.02; LCR, 0.52 ± 0.03; HCR, 0.51 ± 0.04) or on the post-conditioning test day (control, 0.55 ± 0.03; LCR, 0.53 ± 0.04; HCR, 0.55 ± 0.04). Figure 4 presents preconditioning and post-conditioning preference ratios for control rats and rats infused i.v. with 1 mg/kg cocaine during conditioning trials. When control rats (n=9) were compared to all cocaine-infused rats (n=16), the RMANOVA revealed an interaction between session and group [F(1,23) = 4.985, P = 0.036]. Subsequent post-hoc analyses revealed that only cocaine-conditioned rats increased time spent in the cocaine-paired compartment (paired samples t-test, t(15) = −2.810, p = 0.013); interestingly, the post-conditioning preference ratios did not differ between cocaine-conditioned and control rats. When control rats were compared to cocaine-conditioned rats classified as LCRs (n=8) or HCRs (n=8), again the RMANOVA revealed an interaction between session and group [F(2,22 = 4.183, P = 0.029]. One-way ANOVAs revealed between-group differences for post-conditioning preference ratios only [F(2,22) = 3.583, P = 0.047]. The postconditioning preference ratio for LCRs (0.67 ± 0.03) was significantly higher than the postconditioning preference ratios measured in control rats (0.50 ± 0.05, p = 0.021) and rats classified as HCRs (0.52 ± 0.06, p = 0.049). Only rats classified as LCRs showed a significant change in preference ratio [pre vs. post, t(7) = − 6.095, p < 0.001].
Figure 4.
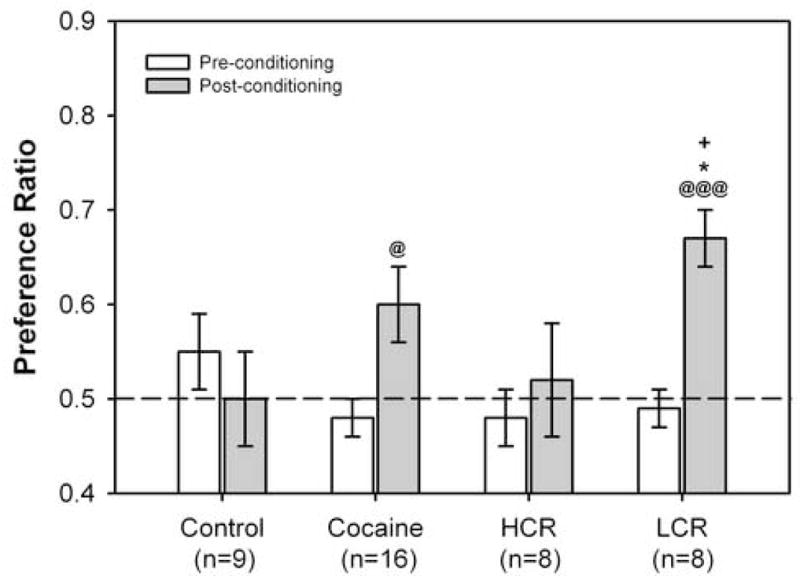
LCRs, but not HCRs, show CPP after i.v. cocaine conditioning. Pre- and post-conditioning preference ratios [time in drug paired compartment / (time in drug and vehicle paired compartments)] calculated after four twice-daily conditioning trials with once-daily i.v. infusions of cocaine (1 mg/kg) and vehicle (or vehicle and vehicle for control rats). For between-group post-hoc comparisons of post-conditioning preference ratios, +, p < 0.01, control vs. LCR; *, p < 0.05, LCR vs. HCR. For within-group comparisons of pre- versus post-conditioning preference ratios, @, p < 0.05; @@@, p < 0.001.
When we classified rats by their initial response to novelty (low responders and high responders, LRs and HRs, respectively) rather than by their response to cocaine, RMANOVA of pre and post-conditioning preference ratios revealed only a main effect of group [F(2,24) = 4.264, P = 0.026]. Rats classified as LRs tended to have higher preference ratios than HR rats both prior to (0.53 ± 0.02 vs. 0.45 ± 0.03) and after conditioning (0.66 ± 0.03 vs. 0.52 ± 0.05).
4. Discussion
We have previously demonstrated individual differences amongst outbred, male S-D rats in their initial locomotor responsiveness to a low i.p. dose of cocaine when behavior is measured in an open-field (Sabeti et al., 2002). Only rats categorized as LCRs under these conditions go on to exhibit cocaine-induced locomotor sensitization. Further, cocaine inhibition of in vivo striatal DA clearance by the DAT is initially no different from vehicle control in LCRs but emerges with repeated cocaine, together with the development of sensitization (Sabeti et al., 2002, 2003). We have ruled out several likely explanations for the differential responsiveness of LCRs and HCRs, including differences in brain cocaine levels and in cocaine’s affinity for DAT (Gulley et al., 2003). However, differences in rapid trafficking of DATs to and from the cell membrane appear to contribute, at least to the initial variability in acute cocaine-induced locomotor activation (Briegleb et al., 2004). Here, we extended these findings to show that 1) male S-D rats can be classified as LCRs/HCRs in a CPP apparatus and following i.v. cocaine administration; 2) this initial response to cocaine predicts the development of sensitization to cocaine’s locomotor effects when measured in this apparatus; and 3) the LCR/HCR classification predicts the development of cocaine CPP, in that LCRs, but not HCRs, develop a cocaine CPP with i.v. infusions of 1 mg/kg cocaine.
In the present study, parallel LCR/HCR differences in both initial cocaine responsiveness and development of behavioral sensitization were observed when rats were administered cocaine via either i.p. injection (10 mg/kg) or i.v. infusion (1 mg/kg). These results are not surprising given that equivalent peak levels of cocaine are achieved in striatum with these two dosing conditions (Orona et al., 1994). Interestingly, however, cocaine CPP developed only under the intravenous dosing conditions. That sensitization can occur in the absence of conditional responding is not surprising given our previous characterization of LCRs and HCRs, which suggests a predominately pharmacological sensitization involving increases in the ability of cocaine to inhibit DATs in the nucleus accumbens of LCRs and little involvement of contextual cues (Sabeti et al., 2003). This is also consistent with work from other laboratories. For example, locomotor sensitization develops with repeated cocaine exposure when either paired or unpaired with a neutral stimulus during a Pavlovian conditioning experiment, even though conditioned locomotion only develops in the paired group (Panlillio and Schindler, 1997).
We do not know why 10 mg/kg cocaine, i.p., failed to produce place conditioning in this experiment. This dose of cocaine produces robust CPP in S-D rats under some conditions (e.g., Cervo and Samanin, 1995; Kosten and Miserendino, 1998; Nomikos and Spyraki, 1988). In our laboratory, 10 mg/kg cocaine reliably produces small but statistically significant increases in CPP when cocaine and vehicle injections are administered on alternate days (unpublished observation). Consistent with our unpublished observations, in some reports 10 mg/kg cocaine, i.p., is a threshold dose with higher doses (e.g., 20 and 40 mg/kg) producing greater degrees of conditioning (e.g., O’Dell et al., 1996). It is important to note, however, that the CPP procedure we used in the present study differed from typical CPP procedures in that rats were not confined to the drug-paired chamber the first time they were treated with cocaine, but were allowed free access to all compartments so that we could classify them as LCR/HCR in the apparatus. This may have disrupted the relatively low level of cocaine CPP we see in our laboratory with 10 mg/kg i.p. cocaine under other conditions.
Cocaine place conditioning did develop, however, when cocaine was administered i.v., and under these conditions initial locomotor response to cocaine predicted cocaine CPP. Although locomotor activity levels recorded for 30 min after cocaine administration did not differ between rats injected with 10 mg/kg i.p. cocaine and rats infused with 1 mg/kg i.v. cocaine, differences were apparent at short times after cocaine administration. Specifically, locomotor activity differed from controls and peaked sooner for in rats infused i.v. with cocaine (1 – 2 min) compared with rats injected i.p. with cocaine (4 – 5 min). The more rapid increase in brain cocaine levels with intravenous administration could result in more pronounced signaling cascades. Consistent with this idea, psychomotor sensitization, inhibition of dopamine uptake, and expression of immediate early genes in the corticomesolimbic system are greater with more rapid experimenter-administered i.v. cocaine infusions (Samaha et al., 2002, 2004). When rats self-administer cocaine, rapid infusion speeds are more likely to lead to sensitization to cocaine’s reinforcing effects, although rapid infusion rates are not required for cocaine to function as a reinforcer (Liu et al., 2005). These authors have argued that both the acute reinforcing effects of cocaine and the development of cocaine sensitization are factors that contribute to addiction and that both should be incorporated into models that study this process.
To the extent that sensitivity to the locomotor-stimulating and direct rewarding effects of cocaine is correlated, it might seem unusual that the group with the higher initial locomotor response to cocaine demonstrated no significant preference. However, our data suggest that differences in the propensity to develop sensitization, rather than the magnitude of cocaine’s effects alone, may predict positive effects in the associative learning procedures used to model aspects of addiction. For example, Lewis rats are more likely than Fischer 344 rats to develop both a predominately pharmacological sensitization to the locomotor effects of cocaine and cocaine CPP across a range of cocaine doses (Kosten et al., 1994). Also, cocaine CPP can be induced with lower cocaine doses or fewer conditioning trials in rats that receive repeated experimenter-administered injections of cocaine prior to the start conditioning (Shippenberg and Heidbreder, 1995). A similar phenomenon has been demonstrated with the self-administration procedure (e.g., Schenk et al., 1993; Schenk and Partridge, 2000), and these data have been used to support the argument that pre-exposure to cocaine produces sensitization to the reinforcing effects of the drug. Our identification of a subgroup of rats that are more likely to develop both sensitization and CPP complements these findings and establishes a methodology with which to investigate genetic and other individual neurobiological differences that contribute to learning in the context of cocaine reward.
In summary, our data suggest that rats with low initial locomotor responsiveness to cocaine (LCRs) represent a phenotype more susceptible to development of cocaine locomotor sensitization and CPP. Our findings also emphasize the fact that using male S-D rats as a homogeneous population for studies with cocaine may obscure valuable insights and will likely yield more variable results. In our future studies with the CPP procedure we plan to further evaluate the role of cocaine dose and route of administration on the individual differences in reward sensitivity demonstrated in the present study. It will be important also to measure the direct reinforcing and motivational effects of cocaine in LCRs and HCRs using the drug self-administration procedure. Measuring both the rate of acquisition of various doses of cocaine, as well as responding under progressive ratio schedules of reinforcement, should further reveal if LCRs are also more susceptible than HCRs to abuse-related effects of cocaine.
Acknowledgments
This research was supported by United States Public Health Service grants DA DA004216, DA015050, and DA14389 from the National Institute on Drug Abuse, and GM008497 from the National Institute of General Medical Sciences.
Footnotes
Preliminary reports of these findings were presented at the 2005 and 2006 annual meetings of the Society for Neuroscience.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behav Brain Res. 2005;158:201–210. doi: 10.1016/j.bbr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology. 2004;29:2168–2179. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology. 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral neuroscience: a practical approach. Oxford UP; Oxford: 1993. pp. 117–143. [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Oxford: Clarendon Press; 1989. pp. 264–319. [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: Behavioral correlates in GluRA(−/−) mice. Proc Nat Acad Sci USA. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: Behavioral characteristics, cocaine pharmacokinetics and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kotlinska J, Biala G. Effects of the NMDA/glycine receptor antagonist, L-701,324, on morphine- and cocaine-induced place preference. Pol J Pharmacol. 1999;51:323–330. [PubMed] [Google Scholar]
- Kotlinska J, Biala G. Memantine and ACPC affect conditioned place preference induced by cocaine in rats. Pol J Pharmacol. 2000;52:179–185. [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott DC, Kim S-J, Cook EH, Jr, De Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Marinelli M. The many facets of the locomotor response to a novel environment test: theoretical comment on Mitchell, Cunningham, and Mark (2005) Behav Neurosci. 2005;119:1144–1151. doi: 10.1037/0735-7044.119.4.1144. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomologus monkeys (Macacac fascicularis) after group formations. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau OA, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nomikos GC, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in the rat. Psychopharmacology. 1996;123:144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Orona RA, Mayfield RD, Cline EJ, Zahniser NR. Repeated intravenous cocaine administration to rats produces behavioral sensitization without changing brain levels of cocaine. Neuorosci Lett. 1994;167:121–124. doi: 10.1016/0304-3940(94)91042-1. [DOI] [PubMed] [Google Scholar]
- Panlillio LV, Schindler CW. Conditioned locomotor-activating and reinforcing effects of discrete stimuli paired with intrperitoneal cocaine. Behav Pharmacol. 1997;8:691–698. doi: 10.1097/00008877-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere J-M, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rogue-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Samaha A-N, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Valadez A, McNamara C, House DT, Higley D, Bankson MG, Gibbs S, Horger BA. Development and expression of sensitization to cocaine’s reinforcing properties: role of NMDA receptors. Psychopharmacology. 1993;111:332–338. doi: 10.1007/BF02244949. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66:765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Pierson J, Danko GP, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1318. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J Neurosci Meth. 2003;128:103–110. doi: 10.1016/s0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: Pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Interactions of MK-801 and GYKI 52466 with morphine and amphetamine in place preference conditioning and behavioural sensitization. Behav Brain Res. 1997;84:99–107. doi: 10.1016/s0166-4328(97)83329-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]


