Abstract
In a seminal report in 1999, Schenk and colleagues demonstrated that vaccination of a mouse model of Alzheimer’s disease (AD) with amyloid-β1–42 peptide (Aβ1–42) and adjuvant resulted in striking mitigation of AD-like pathology – giving rise to the field of AD immunotherapy. Later studies confirmed this result in other mouse models of AD and additionally showed cognitive improvement after Aβ vaccination. Based on these results, early developmental clinical trials ensued to immunize AD patients with Aβ1–42 plus adjuvant (so-called “active” Aβ immunotherapy; trade name AN-1792; Elan Pharmaceuticals, Dublin, Ireland). However, the phase IIa trial was halted after 6 % of patients developed aseptic meningoencephalitis. Despite occurrence of this adverse event, many individuals demonstrated high serum antibody titres to Aβ and histological evidence of clearance of the hallmark AD pathology, β-amyloid plaques. While raising justifiable safety concerns, these important results nonetheless demonstrated the feasibility of the active Aβ immunotherapy approach. This review focuses on alternative approaches to active Aβ vaccination that are currently in various stages of development – from pre-clinical studies in animal models to current clinical trials. Specifically, the focus is on those strategies that target inflammatory and immune aspects of AD, and can therefore be classified as immunotherapeutic in a broad sense.
Keywords: Alzheimer’s disease, vaccination, AN-1792, immunization, inflammation, cytokine
INTRODUCTION
In 1999, the Alzheimer’s disease (AD) field paused to assimilate findings that suggested an exciting new therapeutic approach. Dale Schenk and colleagues at Elan Pharmaceuticals sought to exacerbate AD-like pathology in the PDAPP mouse model of AD [1], which overexpresses mutant human amyloid precursor protein (APP). APP is the parent molecule that gives rise to the amyloid β (Aβ) peptides that form the β-amyloid plaques pathognomonic of AD. They administered peripheral injections of Aβ emulsified in complete Freund’s adjuvant. Instead of their predicted outcome, they found that the treated mice had brains virtually devoid of β-amyloid plaques. These animals demonstrated high titres of antibodies directed against Aβ, and the authors noted major histocompatibility complex class II (MHC II)-positive microglia that co-localized with Aβ deposits, suggesting that the Aβ vaccine promoted productive clearance of β-amyloid plaques [2]. Additional studies from independent groups both confirmed these findings in other AD mouse models and demonstrated correlation with reduced behavioral impairment [3, 4]. Based on these encouraging results, Elan Pharmaceuticals and Wyeth partnered to develop an Aβ vaccine indicated for use in humans: AN-1792. The formulation initially consisted of a synthetic Aβ1–42 peptide with QS-21 adjuvant, and this was later modified to include polysorbate 80 with the aim of increasing peptide solubility [5]. They began a phase I trial in December 1999 with a cohort of 80 participants and did not report significant adverse events. With this encouraging safety profile, they undertook a phase IIa trial to evaluate efficacy as an AD therapeutic [6].
IS THERE A NEED FOR ALTERNATIVE AD IMMUNOTHERAPEUTIC APPROACHES?
During the initiation of these early developmental clinical trials, a number of scientists brought into question the safety of a therapeutic approach that relied on inducing autoimmunity. After all, socalled “active” Aβ immunotherapy provoked the immune system to target the body’s own peptide, even if that peptide had become pathogenic. Yet, the phase I trial was successfully completed without adverse events, suggesting that the vaccine was relatively safe. This led the Elan/Wyeth team to begin their phase IIa trial in October 2001, which was powered to detect drug efficacy in mitigating AD cognitive symptoms. However, that trial was halted in January 2002 when approximately 6 % of patients developed brain inflammation consistent with aseptic meningoencephalitis [6]. The occurrence of this serious adverse event prompted a call for Elan/Wyeth to publish results of the trial [7]. The following year, Nicoll and colleagues presented a case report of a 72-year-old woman who had a 5-year history of probable AD and who had received the AN-1792 vaccine and responded by elevated Aβ antibody titres [8]. Upon histologic examination, her brain showed “patchy” evidence of β-amyloid plaques and some plaques had atypical punctate immunoreactivity for Aβ. These puncta often co-localized with “activated” phagocytic microglia, suggesting that, much as in the original reports in transgenic AD mice [2, 9], the vaccine promoted microglial clearance of Aβ. Yet, this first case report of an AD patient who received AN-1792 also showed evidence of brain infiltrating CD4+ T lymphocytes consistent with aseptic meningoencephalitis. This finding led to the conclusion that AN-1792 promoted Aβ-reactive autoimmune T cells, presumed to be of the pro-inflammatory T helper type 1 (Th1) subtype, that invaded the CNS and mediated brain inflammation [10]. Such an effect may have been due to use of the Th1-promoting adjuvant (QS-21). This was a departure from the adjuvant used in mice (complete Freund’s adjuvant, which is not approved for human use) that promoted an anti-inflammatory Th2 response [11, 12]. It is interesting to note that another group confirmed generation of Aβ-specific antibodies after AN-1792 vaccination in 20 of 30 patients [13], and further showed that those patients who responded in terms of Aβ-specific antibody titres demonstrated significantly slower rates of cognitive decline and an increase in activities of daily living compared to patients who did not generate antibodies [14]. Interestingly, even two patients who experienced transient episodes of AN-1792-related aseptic meningoencephalitis showed these beneficial effects.
However, a recent study that examined long-term effects (after a 6-year follow-up of 80 patients) of AN-1792 did not find evidence for halting of progressive AD-type disease despite generation of Aβ-specific antibodies and clear evidence of β-amyloid plaque removal [15]. There are numerous reasons that may explain why AD patients receiving AN-1792 did not demonstrate durable improvement in cognitive function. For example, it is plausible that Aβ vaccination was “too little, too late”, and elevated Aβ levels/cerebral amyloidosis had already set into motion a pathogenic cascade (known as the amyloid cascade hypothesis [16]) such that removal of Aβ from the equation at a late stage would have little if any effect [17]. Another explanation is that targeting Aβ removal alone is not sufficient to produce a therapeutic effect. Yet, if these findings are representative, they call into question the use of an Aβ vaccine to treat established disease, and suggest that a preventative vaccine may be a more viable approach. Further, these results show that active Aβ vaccination did have a clear effect on interrupting its biologic target (i.e., β-amyloid), and therefore suggest that other AD therapeutics that are safe and aimed at targeting immune/inflammatory aspects of the disease may be efficacious, in principle.
ACTIVE Aβ VACCINE ALTERNATIVE APPROACHES: Aβ-SPECIFIC ANTIBODY STRATEGIES
Passive Aβ Vaccination
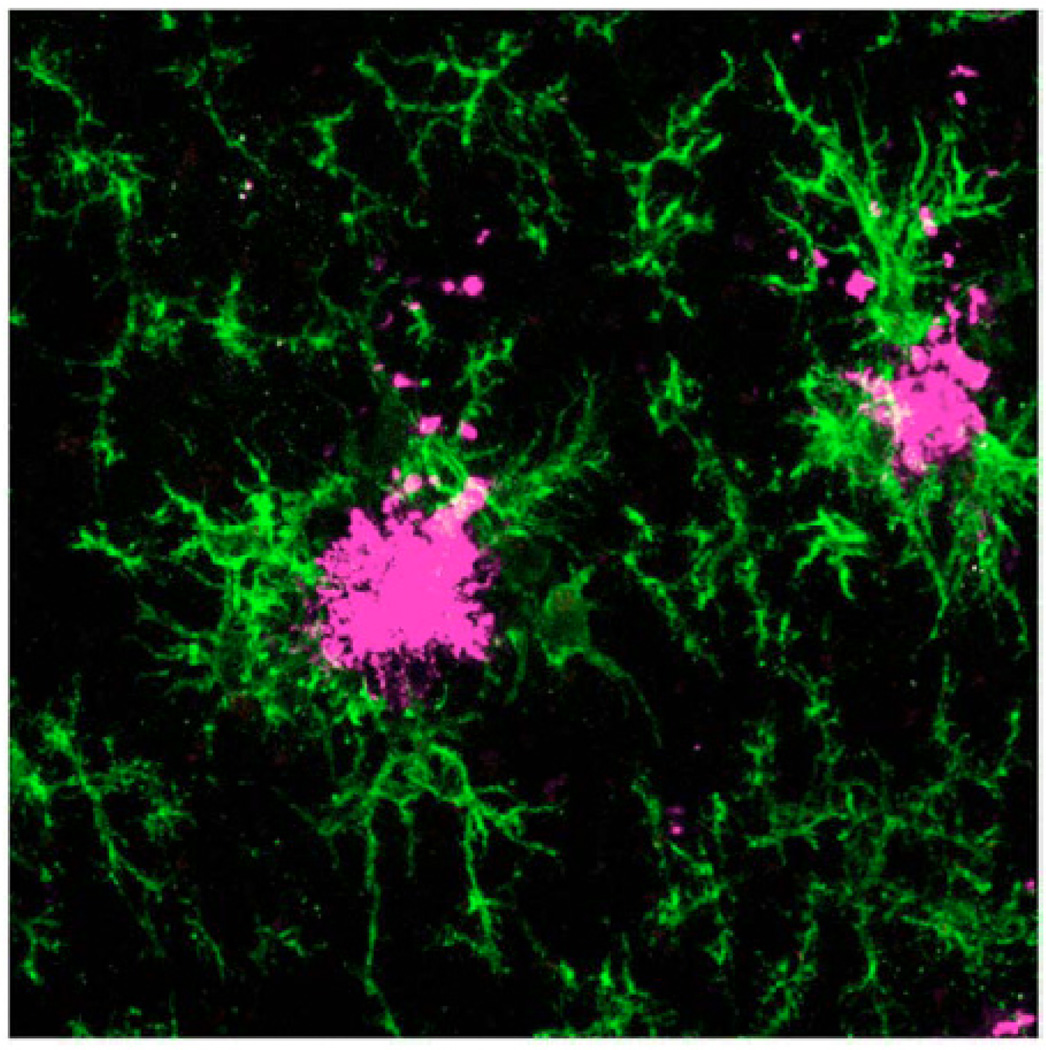
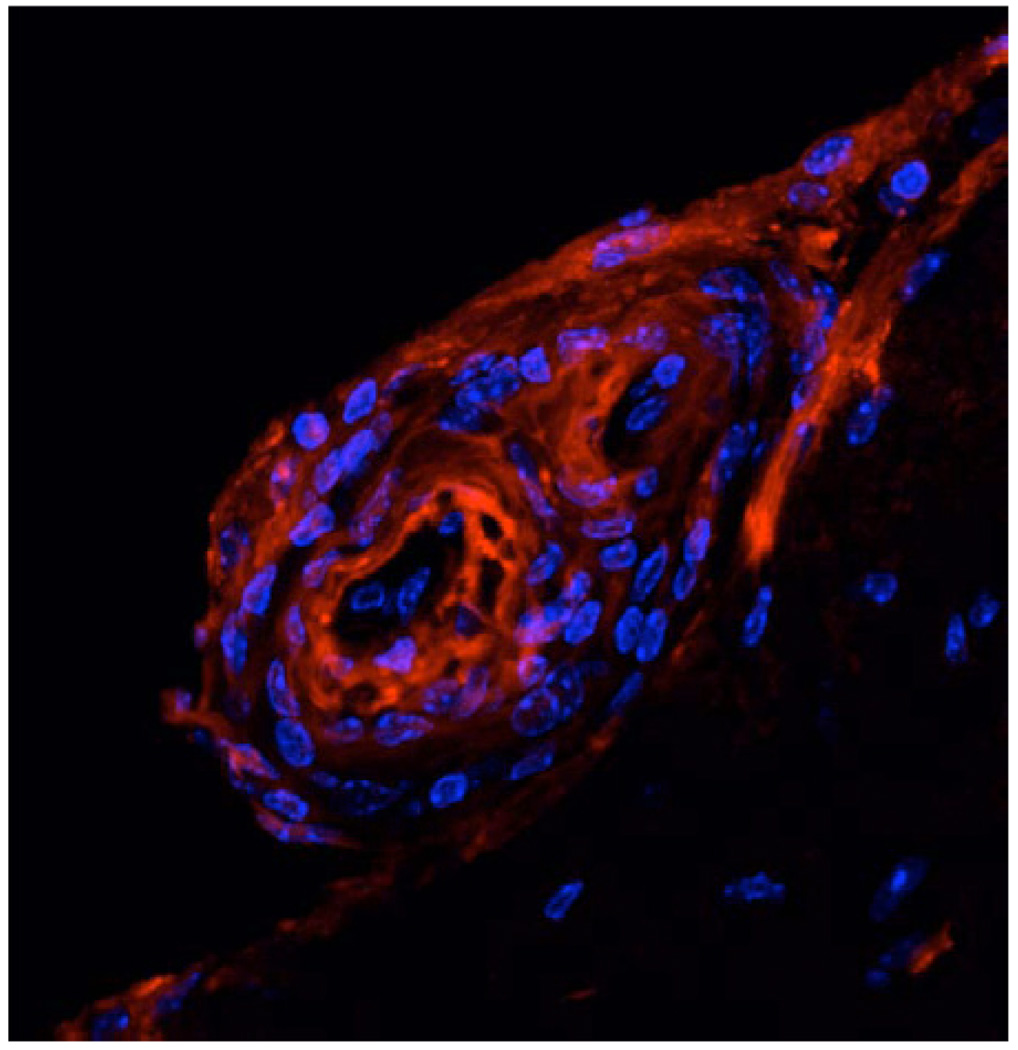
Shortly after the original Schenk et al. report [2], two others showed that passive transfer of Aβ monoclonal antibodies from vaccinated mice to AD model mice reduced cerebral amyloidosis [9, 18]. These reports showed a clear requirement for Aβ antibodies in mediating the beneficial effects of Aβ vaccination on reduced cerebral amyloidosis. Such effects were suggested to be mediated at least partially by reactive microglia that became activated to engulf antibody-decorated Aβ via Fc receptor-mediated phagocytosis [9]. While activated microglia are often found in close apposition to β-amyloid plaques (Fig. 1), they are not efficient Aβ phagocytes [19]. Bard and colleagues [9], however, suggested that the “passive” Aβ vaccine coaxed these cells into phagocytosing and clearing antibody-opsonized plaques. Further, such passive transfer of Aβ antibodies would theoretically circumvent the potentially unsafe and damaging autoaggressive CD4+ T cell response that was presumed to have mediated aseptic meningoencephalitis in a small percentage of AN-1792 recipients. Thus, so-called “passive” Aβ vaccination emerged as an early alternative to “active” Aβ immunotherapy. Interestingly, more recent studies using both “active” and “passive” Aβ immunotherapy in the 3x Tg-AD mouse model of AD [20] reported amelioration of behavioral deficits, clearance of cerebral amyloidosis, and reduction of soluble hyperphosphorylated tau protein (thought to be an early event in formation of neurofibrillary tangles leading to neuronal injury and demise in AD) [21, 22]. However, a safety concern has emerged from studies in passively vaccinated mouse models of AD – the presence of small cerebral bleeds, termed “microhemorrhages” [23–25]. These small bleeds may be especially relevant in AD, where 83% of cases have deposits of β-amyloid in cerebral vessels, a pathology referred to as cerebral amyloid angiopathy (CAA) [26], which is recapitulated in certain AD mouse models (Fig. 2). However, whether these microhemorrhages will be adverse in this therapeutic approach is unclear at this time, and the Elan/Wyeth team is currently pursuing this alternative approach (trade name bapineuzumab, or AAB-001). We eagerly await results from “passive” Aβ immunization early developmental clinical trials.
Fig. (1).
Confocal microscopy reveals reactive microglia (green signal) and Aβ deposits (magenta signal) in an 18-month-old Tg2576 AD model mouse. Reproduced from Town et al., 2008 [79]. Note the tight association between microglia and Aβ plaques.
Fig. (2).
Confocal microscopy showing vascular Aβ deposits (red signal) and cell nuclei stained with DAPI (blue signal) in an 18-month-old Tg2576 AD model mouse Reproduced from Town et al., 2008 [79].
Aβ Vaccines with Alternative Adjuvants and/or Routes of Administration
AN-1792 may have provoked brain inflammation in a small subset of treated individuals owing to the use of a pro-inflammatory Th1 adjuvant (QS-21) [27]. It is possible that this design issue promoted an autoaggressive CD4+ T cell response in some patients. If this were the case, then strategies that introduced the Aβ immunogen with alternative adjuvants (perhaps anti-inflammatory Th2 adjuvants) or no adjuvant and/or relied on a different route of administration (i.e., not intraperitoneal, intramuscular, or intravenous)may be both efficacious and safe. Some of the earliest work focusing on Aβ vaccines without adjuvant and relying on a different route of administration came from Cindy Lemere, Harold Weiner, and Dennis Selkoe. They showed that full-length Aβ1–40 administered intra-nasally 1) produced significant Aβ antibody titres and 2) reduced cerebral Aβ levels/plaques in the PDAPP mouse model of AD [28–30]. The antibodies produced were largely of the IgG1 and IgG2b isotypes, suggesting a predominately Th2 response in this paradigm, and antibodies were directed against amino acids 1–15 of Aβ [28], now widely recognized as the B cell epitope.
We have recently developed a transcutaneous Aβ vaccine that relies on full-length Aβ1–42 plus cholera toxin (a strong mucosal Th2 adjuvant [31]) administered to the skin. This Aβ vaccine does not simply provide for a less invasive, more convenient route of administration; we also demonstrated that it targets a population of skin-resident innate immune cells with unique immunomodulatory potential, called Langerhans cells (LCs). Transcutaneous Aβ immunotherapy targets LCs to promote a robust humoral Th2 response as indicated by Aβ-specific antibodies primarily of the IgG1 isotype. Further, studies in splenocytes showed a largely Th2 response profile, as indicated by high levels of the prototypical Th2 cytokine, interleukin-4, following Aβ1–42 recall stimulation ex vivo. As in vivo proof-of-principle, we transcutaneously vaccinated the PSAPP mouse model of AD, and noted brain-to-blood efflux of Aβ [18, 32] and reduction of cerebral Aβ levels/plaques by approximately 50% [33]. Importantly, this form of Aβ immunotherapy does not induce microhemorrhage, which has been reported after “passive” Aβ immunization in AD mice as mentioned above [23–25]. We also did not detect brain inflammation analogous to aseptic meningoencephalitis, but it should be noted that only one report of “active” Aβ vaccination (where the formulation was modified to include pertussis toxin, widely used in mouse models of multiple sclerosis to induce brain T cell infiltration [10]) showed this effect [34] – and therefore the mouse models of AD do not seem to recapitulate this AN-1792-related adverse event well.
Immunotherapy with Alternative Aβ Epitopes and DNA-Based Approaches
The aseptic meningoencephalitis that occurred in a subgroup of AD patients receiving the AN-1792 vaccine may also be due to use of full-length Aβ1–42 peptide, which contains both T and B lymphocyte epitopes. While the B cell epitope (the first 11 to 15 amino acids of the peptide [11, 35]) is important for generation of therapeutic Aβ antibodies, it has been hypothesized that the T cell epitope (amino acids 15–42 of the peptide [27, 36]) may have provoked an autoaggressive T cell response that led to aseptic meningoencephalitis in some AN-1792-vaccinated patients. Lemere and colleagues noted that their intra-nasal vaccine using E. coli LT or LT(R192G) as mucosal adjuvants produced strong Aβ antibody titres that were directed against amino acids 1–15 [37], leading the authors to pursue Aβ1–15 in lieu of full-length peptide as immunogen. However, the authors later showed that intra-nasal Aβ1–15 plus the above adjuvants was not effective as a “priming” immunogen, but did show promise as a “boosting” immunogen in an Aβ1–40/42 “primed” and Aβ1–15 “boosted” regimen in mice [38]. In an effort to increase the immunogenicity of the Aβ1–15 peptide, the authors designed a “dendrimeric” vaccine in which they fused 16 copies of Aβ1–15 peptide on a branched lysine core and administered this via subcutaneous, transcutaneous, and intranasal routes with the adjuvant LT(R192G). This dendrimeric Aβ vaccine produced Aβ-specific (directed against amino acids 1–7) antibodies mainly of the IgG1 and IgG2b isotypes, suggesting a humoral Th2 response [39]. It is interesting to note that while the authors used the so-called B cell epitope, a T cell response may still have been generated (likely owing to the use of the Th2-promoting LT(R192G) adjuvant), as Th2 cell help to B cells is required for IgG1 antibody production that the authors detected. They did not, however, report evidence of a Th1 cell response that may underlie the pro-encephalitogenic adverse event observed after AN-1792 treatment in AD patients.
David Cribbs and Michael Agadjanyan have taken a different approach by designing a DNA-based prototype epitope vaccine that fuses the pan human leukocyte antigen DR-binding protein (PADRE) synthetic antigen with the immunodominant Aβ1–15 B cell epitope. The PADRE epitope is a logical choice because it is a non-self epitope (and therefore would be unlikely to induce autoimmunity) that promotes T cell help to antibody-producing B cells. They were able to demonstrate high-titre Aβ-specific antibodies when immunizing BALB/c mice [35]. Further, formulation of the PADRE-Aβ1–15 DNA epitope vaccine with the adjuvant Quil A resulted in a strong humoral Th2 response as indicated by high titres of Aβ-specific antibodies of the IgG1 isotype [40]. The authors have more recently modified their PADRE DNA-based Aβ vaccine to include three copies of the Aβ1-11 epitope fused to PADRE plus the Th2 adjuvant macrophage-derived chemokine (MDC/CCL22). Using this pMDC-3Aβ1–11-PADRE vaccine, they showed that immunization of 3x Tg-AD mice produced a robust Th2 response and high-titre Aβ antibodies [41]. Importantly, this DNA epitope vaccine mitigated accumulation of cerebral Aβ pathology, reduced gliosis, and prevented behavioral deficits in aged mice without increasing cerebral microhemorrhages [41]. These results suggest that it is possible to generate a strong humoral Th2-type response without producing adverse cerebral microhemorrhages, which may or may not be a safety concern in AD patients as mentioned above.
ACTIVE Aβ VACCINE ALTERNATIVES THAT DO NOT RELY ON Aβ-SPECIFIC ANTIBODIES
Non-Steroidal Anti-Inflammatory Drugs
Whereas Aβ vaccines in all of their forms rely on Aβ-specific antibodies that are thought to promote productive removal of β-amyloid plaques by acutely activating microglial Aβ clearance mechanisms (see, for example [9]), an alternate approach has been to dampen the chronic, low-level, microglial activation that occurs in AD. Evidence from neuropathologic studies supports the notion that non-steroidal anti-inflammatory drugs (NSAIDs, such as ibuprofen and naproxen) reduce numbers of activated microglia. For example, MacKenzie and Munoz examined brains of cognitively normal people who had been prescribed NSAIDs for one year or longer as a treatment for osteoarthritis or rheumatoid arthritis [42]. They found that numbers of activated microglia were reduced in NSAID users compared to non-users, showing a direct biologic effect of NSAIDs on reducing brain inflammation.
There now exist at least 25 studies that have reported on the relationship between NSAIDs and risk for AD in humans (see [43] for a review). Many of the early studies in the 1990s examined inflammatory conditions such as arthritis, for which NSAIDs are commonly indicated, and found an inverse association with AD. Based on these studies, it was suggested that history of arthritis was a surrogate for NSAID exposure, leading later investigations to focus specifically on NSAID use. There have been at least 12 non-prospective studies to date, 10 of which concluded that AD cases were less likely to have been using these agents whereas two studies concluded that there was no association [43]. Christine Szekely and Peter Zandi performed a systematic review of these studies (which met stringent inclusion criteria), with a resulting meta-analysis for 8 non-prospective studies (1,833 AD cases and 13,780 controls) that showed a 53% AD risk reduction in those study participants who reported using non-asprin NSAIDs compared with non-users [43, 44]. They also performed a meta-analysis for 5 prospective studies (836 AD cases and 16,294 controls) and found a 29% risk reduction [43, 44]. Interestingly, AD risk reduction was more pronounced for longer (≥ 2 years) duration of NSAID use, where a risk reduction of 58% was evident [43]. Use of aspirin was also associated with 45% risk reduction for AD in non-prospective studies but did not reach statistical significance in prospective studies [43]. Unfortunately, the one randomized controlled clinical trial of NSAIDs in non-demented elderly did not show evidence for AD prevention [45]. However, this trial was prematurely halted (after 2 years of treatment and 2 years of follow-up) due to possible cardiotoxicity of certain NSAIDs and therefore should be interpreted with caution.
To determine if NSAIDs had a direct effect on AD-like pathology, Greg Cole and colleagues administered ibuprofen or curcumin (a naturally-occurring NSAID found in the curry spice tumeric) to the Tg2576 mouse model of AD (which overexpresses “Swedish” mutant human APP and develops cerebral amyloidosis with age), and found that both NSAIDs reduced cerebral β-amyloid plaques by approximately 50% [46, 47]. Additionally, either NSAID reduced brain inflammation (as determined by interleukin-1β, astrogliosis, and microgliosis analyses) and presence of dystrophic neurites in these AD mice [46, 47], and ibuprofen was further shown to reduce behavioral impairment in Tg2576 mice [48]. The beneficial effect of ibuprofen on reducing cerebral amyloidosis was also found in doubly-mutant human APP and presenilin-1 mice [49], and these authors further noted a pronounced effect of the nitric oxide-releasing NSAID (NCX-2216) on mitigating cerebral amyloidosis [49]. More recently, Wilcock and colleagues administered both “active” Aβ immunotherapy and NCX-2216 to the above doubly-transgenic mice to probe for a synergistic effect on reducing AD-like pathology. While the authors found that both monotherapies reduced cerebral Aβ levels/pathology, they did not find that co-treatment was able to further enhance the beneficial effects of Aβ immunotherapy [50]. Nonetheless, results from both human and mouse studies suggest that NSAIDs, if administered early enough and for a long enough duration, may slow the rate of AD progression.
Modulation of Microglial Activation States by CD40-CD40L Interruption
As evidenced from the postmortem studies in individuals taking NSAIDs that had reduced numbers of reactive microglia [42], and by the inverse risk relationship between NSAID use and AD, reducing brain inflammation by blunting the chronic, low-level activation of microglia that occurs in AD may be another therapeutic avenue. CD40 is a ∼50 KDa transmembrane glycoprotein that is a member of the tumor necrosis factor (TNF)/nerve growth factor receptor superfamily [51] and is expressed by microglia [52, 53], including microglia associated with β-amyloid plaques [54]. In 1999, we showed that ligation of microglial CD40 with its cognate ligand, CD40 ligand (CD40L, expressed by activated astrocytes associated with β-amyloid plaques [55]), synergistically activated microglia to produce TNF-α in response to low levels (in the nM range) of Aβ peptides. This form of microglial activation was deleterious, as it resulted in TNF-α-dependent neuronal injury. Further, when mice deficient in CD40L were crossed with the Tg2576 mouse model of AD, abnormal phosphorylation of tau (an index of neuronal stress) was reduced prior to amyloid deposition, suggesting that CD40-CD40L interaction is an early event in AD pathogenesis [56]. We later showed that both genetic and pharmacologic means of interrupting the CD40-CD40L interaction in mouse models of AD 1) reduced cerebral Aβ levels/plaques, 2) reduced astrogliosis and microgliosis, 3) increased brain-to-blood efflux of Aβ, and 4) shifted APP metabolism from amyloidogenic to non-amyloidogenic [57]. Reduction of AD-like pathology in the PSAPP mouse model of AD was also associated with reduced behavioral impairment following CD40L neutralizing antibody treatment commencing at an age when this AD mouse model already manifests extensive cerebral amyloidosis [58].
We proposed that CD40-CD40L interaction on microglia shifted these cells from an antiinflammatory, pro-phagocytic phenotype to a proinflammatory response endorsing antigen-presenting cell function [59]. In the presence of Aβ, CD40L-stimulated microglia in vitro 1) reduced Aβ phagocytosis/clearance, 2) up-regulated production of pro-inflammatory cytokines and 3) increased loading of MHC II with Aβ peptide [59].
These Aβ-MHC II complexes were functional, resulting in stimulation of pro-inflammatory cytokines including interferon-γ and interleukin-2 in T cell co-culture assays. Thus, it seems that interrupting CD40-CD40L signaling on microglia may shift the activation profile of these cells from a chronic, low-level pro-inflammatory state (as they seem to exist surrounding β-amyloid plaques) to a productive anti-inflammatory and pro-phagocytic state supporting Aβ clearance [19, 60, 61].
Further evidence for a beneficial role of interrupting CD40-CD40L signaling in mitigating AD-like pathology comes from our recent work showing that administration of human umbilical cord blood cells (HUCBCs) to a doubly-transgenic mouse model of AD reduces AD-like pathology. HUCBC administration also reduces vascular Aβ deposits, astrogliosis, and circulating levels of soluble CD40L, suggesting promotion of antiinflammatory responses. The beneficial effects of HUCBC treatment depend on functional CD40, as CD40 deficient mice do not respond with reduced AD-like pathology after treatment. These effects also likely rely upon promoting increased microglial phagocytosis of Aβ, as primary microglia isolated from HUCBC-treated AD mice demonstrate increased phagocytosis of Aβ, and sera from HUCBC-infused AD mice significantly increases microglial Aβ phagocytosis and inhibits CD40 expression [62]. Recently, we have combined “active” Aβ immunotherapy and CD40-CD40L blockade approaches (by both genetic and pharmacologic means) in mouse models of AD. Results showed additional benefit in reducing cerebral amyloidosis for combined approaches, and we did not detect potentially damaging inflammatory responses or cerebral microhemorrhage in this paradigm [63]. There are likely other molecular manipulations that will produce a similar state change in microglial activation profiles, including strategies that 1) increase microglial cGMP levels [64–66], 2) promote activity of the membranebound protein-tyrosine phosphatase CD45 [67, 68], or 3) increase microglial responsiveness to transforming growth factor-β (TGF-β) [69], to name just a few. While such strategies are not currently being tested in humans, it will be important to pursue such alternative immunotherapeutic strategies in early developmental clinical trials.
Promoting Aβ Clearance by Blood-Borne Macrophages
Pioneering studies in the late 1980s and early 1990s from Henryk Wisniewski suggested for the first time that brain-resident microglia, while competent to engulf Aβ in vitro, were not capable of phagocytosing/clearing Aβ deposits in vivo (at least not in the absence of genetic/pharmacologic manipulation). He concluded this based on ultra-structural studies of microglia and β-amyloid plaques in AD patient brains, where he never found Aβ deposits within microglial lysosomes. However, in the rare comorbidity of stroke and AD in elderly patients, Wisniewski noted brain-infiltrating macrophages that contained β-amyloid fibrils [70–72]. Thus, the suggestion arose early that, while microglia did not have Aβ clearance aptitude, peripheral macrophages (Greek etymology: “big eater”) were competent Aβ phagocytes. Yet, it would take another 15 years before modern cellular/molecular biology technique was applied to address this hypothesis. Specifically, Mathias Jucker and Serge Rivest generated bone marrow chimeras (where peripheral immune cells – but not CNS resident microglia – were marked by green fluorescent protein [73] or thymidine kinase [74]) in transgenic mouse models of AD. They found that a small percentage – only about 1% – of total CD11b+/Iba1+ cells were of peripheral origin, and only about 20% of β-amyloid plaques were associated with such blood-borne macrophages [75].
These results begged the question of whether a relatively small percentage of brain infiltrating monocytes/macrophages played a functional role in restricting cerebral amyloidosis. This issue has been at least partially addressed in a recent study from Joseph El Khoury, who crossed an AD mouse model with an animal deficient in CC-chemokine receptor 2. These bigenic mice have markedly diminished recruitment of brain resident microglia/peripheral macrophages to sites of β-amyloid plaques and demonstrate heavier β-amyloid burden than AD model mice alone [76]. While this study does not definitively establish the provenance of the amyloid-clearing cells, it none-theless demonstrates the importance of brain innate immunity in restricting cerebral amyloidosis. An additional report has shown that depletion of CD11c+ cells using a CD11c-diphtheria toxin transgenic mouse bone marrow chimera in an AD mouse model opposes the beneficial effect of T cell-directed immunotherapy, suggesting that peripheral innate immune cells are required for β-amyloid clearance [77]. These studies, by negatively impacting brain penetration/Aβ homing of these peripheral innate immune cells, lead us to deduce that such cells are an important antiamyloid force. However, if these cells are to be targeted as a therapeutic modality, then strategies for increasing both brain infiltration and amyloid clearance potential of blood-borne macrophages need to be developed.
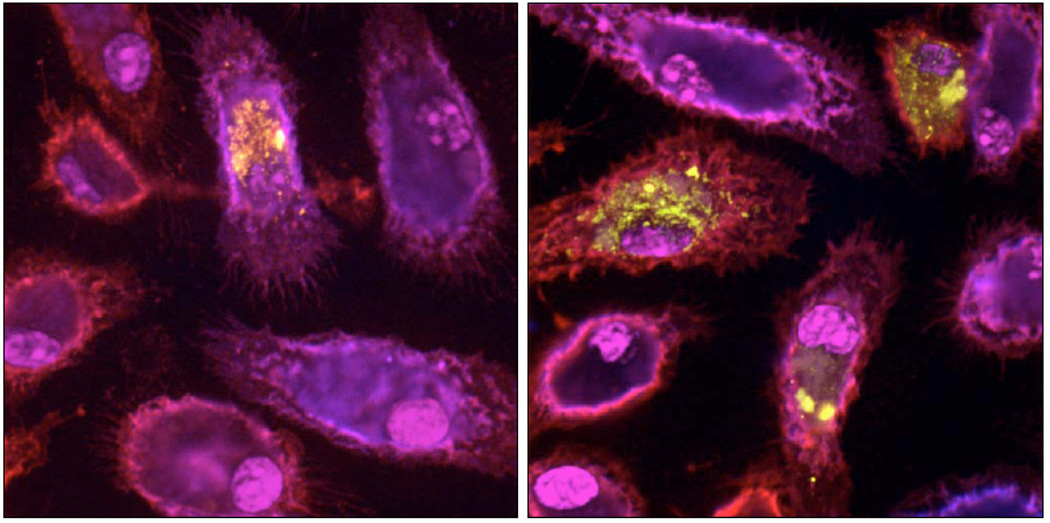
We have recently described one such strategy based on inhibiting TGF-β/Smad 2/3 signaling in blood-borne macrophages. Specifically, we crossed a mouse that expresses a dominant-negative TGF-β receptor type II (required for all TGF-β signaling) transgene under regulatory control of the CD 11c promoter (CD11c-DNR mice) (expressed by peripheral macrophages but only by a small percentage of “dendritic cell-like” microglia [78, 79]) with two widely used transgenic mouse models of AD and assayed AD-like pathology and behavioral impairment in aged (16–18 month-old) mice [79]. Our initial hypothesis was that inhibiting innate immune TGF-β signaling would hyper-activate microglia [80], thereby causing neuroinflammation and bystander injury in the context of AD-like pathology [69, 81]. Surprisingly, we found the converse; there was a marked reduction in cerebral amyloidosis (> 90% reduced β-amyloid plaques by some measures) and a reduction in behavioral impairment in crossed mice by some measures. We were further surprised when we observed an increase in antiinflammatory interleukin-10 levels and CD45+CD11b+CD11c+Ly-6C- cells in brains of crossed mice – which morphologically and phenotypically most closely resembled an “antiinflammatory” subset [82, 83] of brain infiltrating macrophages. Further, a portion of these cells could be found associated with β-amyloid plaques, and some of these cells co-localized with and contained Aβ deposits. We further showed that cultured macrophages from CD 11c-DNR mice had ∼3-fold greater capacity to engulf pre-aggregated Aβ1–42 peptides (Fig. 3) [79]. These results demonstrate in pre-clinical models that blood-borne macrophages can be promoted to enter the brain and to restrict β-amyloid plaques without producing a potentially damaging neuroinflammatory re sponse. We are currently pursuing small molecule inhibitors of Smad 2/3 signaling as potential pharmacologic agents for use in AD patients.
Fig. (3).
Confocal images of cultured peripheral macrophages immunolabeled with CD 11b (red signal) and CD11c (blue signal) markers, and nuclear counter-stained with DAPI (magenta signal). Cells have been pulse-chased with labeled Aβ1–42 peptide (yellow signal), and wild-type cells are shown on the left while CD11c-DNR cells (lacking TGF-β/Smad 2/3 signaling) are shown on the right. Reproduced from Town et al., 2008 [79].
CONCLUDING REMARKS
In the late 1990s, the work of Dale Schenk and colleagues sent a powerful message to the AD research community: the immune system could be harnessed to militate against AD. It is important to place this work in context because only a decade before “brain inflammation” was regarded by many as epiphenomenon – a symptom of AD that was not relevant to disease pathobiology or treatment. The Elan team demonstrated that generation of Aβ antibodies resulted in clearance of cerebral amyloid in mouse models that was likely dependent on microglial phagocytosis of antibody-opsonized Aβ deposits. Unfortunately, translation of these pre-clinical findings into humans was hindered by a severe adverse reaction (aseptic meningoencephalitis) in a small percentage of patients receiving the vaccine. Despite this major setback, it is becoming clear that the Aβ immunotherapy approach specifically – and AD immunotherapy in general (as discussed in this review) – is a valuable therapeutic avenue that needs to be pursued. By developing a better understanding of the mechanisms responsible for both the beneficial and potentially detrimental effects of AD immunotherapy, we will be better able to harness the immense potential of this therapeutic modality.
ACKNOWLEDGEMENTS
The author thanks C. Szekely for helpful discussion and for critically reading this manuscript. T. Town is supported by an Alzheimer’s Association grant (IIRG0514993), a NIH/NIA “Pathway to Independence” award (4R00AG029726-02), and by Cedars-Sinai Medical Center, Department of Neurosurgery faculty start-up funds. T. Town is the inaugural holder of the Ben Winters Chair in Regenerative Medicine.
ABBREVIATIONS
- 3x Tg-AD
A transgenic mouse model of AD that over-expresses mutant forms of human amyloid precursor protein, human presenilin-1, and human tau
- Aβ
Amyloid-beta peptide
- AD
Alzheimer’s disease
- AN-1792
Elan/Wyeth trade name for their “active” Aβ immunotherapy vaccine
- APP
amyloid precursor protein
- β-amyloid
deposited form of Aβ
- BALB/c
an albino strain of laboratory mouse
- bapineuzumab
Elan/Wyeth trade name for their “passive” Aβ immunotherapy vaccine currently in clinical trials, also known as AAB-001
- CAA
cerebral amyloid angiopathy
- CD
cluster of differentiation
- CD 11c-DNR
a mouse that expresses a dominant-negative TGF-β receptor type II (required for all TGF-β signaling) transgene under regulatory control of the innate immune cell CD 11c promoter
- CD40L
cognate ligand for the CD40 receptor
- cGMP
cyclic guanosine monophosphate
- Fc
Fragment, crystallizable region of an antibody
- HUCBCs
human cord blood cells
- Iba1
ionized calcium-binding adaptor molecule 1
- IgG
immunoglobulin G
- LC
Langerhans cell
- LT
Escherichia coli enterotoxin
- Ly
lymphocyte antigen
- MDC/CCL22
macrophage-derived chemokine
- MHC II
major histocompatibility complex II
- NCX-2216
a nitric oxide-releasing NSAID
- NSAIDs
non-steroidal antiinflammatory drugs
- PADRE
pan human leukocyte antigen DR-binding protein
- PDAPP
a transgenic mouse model of AD that over-expresses mutant human APP
- PSAPP
a transgenic mouse model of AD that over-expresses mutant human APP and presenilin-1
- QS-21
Stimulon QS-21 adjuvant
- Quil A
Quillaia A adjuvant
- Smad
mothers against decapentaplegic
- Tg2576
a transgenic mouse model of AD that over-expresses mutant human APP
- TGF-β
Transforming growth factor-beta
- Th1
“Pro-inflammatory” T lymphocyte helper type 1 response
- Th2
“Anti-inflammatory” T lymphocyte helper type 2 response
- TNF-α
Tumor necrosis factor-alpha
REFERENCES
- 1.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnsonwood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoyazavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D. Alzheimer-type neuropathology in transgenic mice overexpressing V717f beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 2.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 3.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St. George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 4.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 5.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 6.Check E. Nerve inflammation halts trial for Alzheimer's drug. Nature. 2002;415:462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
- 7.Bishop GM, Robinson SR, Smith MA, Perry G, Atwood CS. Call for Elan to publish Alzheimer's trial details. Nature. 2002;416:677. doi: 10.1038/416677d. [DOI] [PubMed] [Google Scholar]
- 8.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 9.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 10.Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer's disease. Neuromolecular Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- 11.Town T, Tan J, Sansone N, Obregon D, Klein T, Mullan M. Characterization of murine immunoglobulin G antibodies against human amyloid-beta(1–42) Neurosci. Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- 12.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, Mori T, Smeed R, Crawford F, Klein T, Tan J, Mullan M. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer's beta-amyloid(1–42) J. Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 13.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat. Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 14.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 15.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebocontrolled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 17.St. George-Hyslop PH, Morris JC. Will anti-amyloid therapies work for Alzheimer's disease? Lancet. 2008;372:180–182. doi: 10.1016/S0140-6736(08)61047-8. [DOI] [PubMed] [Google Scholar]
- 18.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Town T, Nikolic V, Tan J. The microglial ″activation″ continuum: from innate to adaptive responses. J. Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 21.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 24.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J. Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J. Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 27.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with betaamyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann. NY Acad. Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 29.Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer's disease. DNA Cell Biol. 2001;20:705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- 30.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazdeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloidbeta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann. Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 31.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 32.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloidbeta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 33.Nikolic WV, Bai Y, Obregon D, Hou H, Mori T, Zeng J, Ehrhart J, Shytle RD, Giunta B, Morgan D, Town T, Tan J. Transcutaneous betaamyloid immunization reduces cerebral beta-amyloid deposits without T cell infiltration and microhemorrhage. Proc. Natl. Acad. Sci. USA. 2007;104:2507–2512. doi: 10.1073/pnas.0609377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with amyloidbeta peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003;126:285–291. doi: 10.1093/brain/awg031. [DOI] [PubMed] [Google Scholar]
- 35.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 36.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemere CA, Spooner ET, Leverone JF, Mori C, Clements JD. Intranasal immunotherapy for the treatment of Alzheimer's disease: Escherichia coli LT and LT(R192G) as mucosal adjuvants. Neurobiol. Aging. 2002;23:991–1000. doi: 10.1016/s0197-4580(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 38.Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA. Abeta1-15 is less im-munogenic than Abeta1-40/42 for intranasal immunization of wild-type mice but may be effective for ″boosting″. Vaccine. 2003;21:2197–2206. doi: 10.1016/s0264-410x(02)00754-5. [DOI] [PubMed] [Google Scholar]
- 39.Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, Bitan G, Lemere CA. Dendrimeric Abeta1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol. Aging. 2007;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS ONE. 2008;3:e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenzie IRA, Munoz DG. Nonsteroidal Anti-Inflammatory Drug Use and Alzheimer-Type Pathology in Aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- 43.Szekely CA, Town T, Zandi PP. NSAIDs for the chemoprevention of Alzheimer's disease. Subcell. Biochem. 2007;42:229–248. doi: 10.1007/1-4020-5688-5_11. [DOI] [PubMed] [Google Scholar]
- 44.Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, Goodman SN. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepi-demiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 45.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch. Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim GP, Chu T, Yang FS, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashe K, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol. Aging. 2001;22:983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 49.Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007;144:950–960. doi: 10.1016/j.neuroscience.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 52.Havenith CE, Askew D, Walker WS. Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T-cells. Glia. 1998;22:348–359. [PubMed] [Google Scholar]
- 53.Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, Yu H, Humphrey J, Mullan M. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J. Neuroimmunol. 1999;97:77–85. doi: 10.1016/s0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 54.Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer's disease and other neurological diseases. Brain Res. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 55.Calingasan NY, Erdely HA, Altar CA. Identification of CD40 ligand in Alzheimer's disease and in animal models of Alzheimer's disease and brain injury. Neurobiol. Aging. 2002;23:31–39. doi: 10.1016/s0197-4580(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 56.Tan J, Town T, Paris D, Mori T, Suo ZM, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 57.Tan J, Town T, Crawford F, Mori T, Delle-Donne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat. Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 58.Todd Roach J, Volmar CH, Dwivedi S, Town T, Crescentini R, Crawford F, Tan J, Mullan M. Behavioral effects of CD40-CD40L pathway disruption in aged PSAPP mice. Brain Res. 2004;1015:161–168. doi: 10.1016/j.brainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur. J. Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- 60.Town T, Tan J, Mullan M. CD40 signaling and Alzheimer's disease pathogenesis. Neurochem. Int. 2001;39:371–380. doi: 10.1016/s0197-0186(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 61.Tan J, Town T, Mullan M. CD40-CD40L interaction in Alzheimer's disease. Curr. Opin. Pharmacol. 2002;2:445–451. doi: 10.1016/s1471-4892(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 62.Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J. CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation. Neurobiol. Dis. 2008;29:336–353. doi: 10.1016/j.nbd.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paris D, Town T, Parker TA, Tan J, Humphrey J, Crawford F, Mullan M. Inhibition of Alzheimer's beta-amyloid induced vasoactivity and proinflammatory response in microglia by a cGMP-dependent mechanism. Exp. Neurol. 1999;157:211–221. doi: 10.1006/exnr.1999.7055. [DOI] [PubMed] [Google Scholar]
- 65.Paris D, Town T, Parker T, Humphrey J, Mullan M. beta-Amyloid vasoactivity and proinflammation in microglia can be blocked by cGMP-elevating agents. Ann. NY Acad. Sci. 2000;903:446–450. doi: 10.1111/j.1749-6632.2000.tb06397.x. [DOI] [PubMed] [Google Scholar]
- 66.Paris D, Town T, Mullan M. Novel strategies for opposing murine microglial activation. Neurosci. Lett. 2000;278:5–8. doi: 10.1016/s0304-3940(99)00901-5. [DOI] [PubMed] [Google Scholar]
- 67.Tan J, Town T, Mori T, Wu YJ, Saxe M, Crawford F, Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J. Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan J, Town T, Mullan M. CD45 inhibits CD40L-induced microglial activation via negative regulation of the Src/p44/42 MAPK pathway. J. Biol. Chem. 2000;275:37224–37231. doi: 10.1074/jbc.M002006200. [DOI] [PubMed] [Google Scholar]
- 69.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 70.Wisniewski HM, Wegiel J, Wang KC, Kujawa M, Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can. J. Neurol. Sci. 1989;16:535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- 71.Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta Neuropathol. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- 72.Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- 73.Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 75.Jucker M, Heppner FL. Cerebral and peripheral amyloid phagocytes--an old liaison with a new twist. Neuron. 2008;59:8–10. doi: 10.1016/j.neuron.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates pro gression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 77.Butovsky O, Kunis G, Koronyo-Hamaoui M, Schwartz M. Selective ablation of bone marrow-derived dendritic cells increases amyloid plaques in a mouse Alzheimer's disease model. Eur. J. Neurosci. 2007;26:413–416. doi: 10.1111/j.1460-9568.2007.05652.x. [DOI] [PubMed] [Google Scholar]
- 78.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, Steinman RM, McEwen BS. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J. Comp. Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 79.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan J, Town T, Saxe M, Paris D, Wu YJ, Mullan M. Ligation of microglial CD40 results in p44/42 mitogen-activated protein kinase-dependent TNF-alpha production that is opposed by TGF-beta 1 and IL-10. J. Immunol. 1999;163:6614–6621. [PubMed] [Google Scholar]
- 81.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 82.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 83.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]