Abstract
Background and Purpose
Prior estimates of the prevalence of silent cerebral infarction (SCI) on magnetic resonance imaging (MRI) in community-based samples have varied between 5.8 and 17.7% depending on age, ethnicity, presence of co-morbidities and imaging techniques. We document the prevalence and risk factors associated with SCI at midlife in the community-based Framingham sample.
Methods
2040 Framingham Offspring (53% F; mean age 62±9 yrs) who attended the 6th examination (1996–98), underwent volumetric brain MRI (1999–2005) and were free of clinical stroke at MRI, constituted our study sample. We examined the age- and sex-specific prevalence and the clinical correlates of SCI using multivariable logistic regression models.
Results
At least one SCI was present in 10.7% of participants. 84% had a single lesion. SCI were largely located in the basal ganglia (52%), other subcortical (35%) and cortical areas (11%). Prevalent SCI was associated with the Framingham Stroke Risk Profile score (OR: 1.27; 95% CI: 1.10 – 1.46); Stage I hypertension by JNC-VII criteria (OR:1.56; CI:1.15 – 2.11)), an elevated plasma homocysteine in the highest quartile (OR: 2.23; CI: 1.42 – 3.51), atrial fibrillation (OR: 2.16; CI: 1.07 – 4.40), carotid stenosis >25% (OR: 1.62; 1.13 – 2.34)) and increased carotid intimal-medial thickness above the lowest quintile (OR: 1.65; CI: 1.22 – 2.24).
Conclusion
The prevalence and distribution of SCI in the Framingham Offspring.is comparable to prior estimates. Risk factors previously associated with clinical stroke were also found to be associated with midlife SCI. Our results support current guidelines emphasizing early detection and treatment of stroke risk factors.
Index words: cerebral infarction, magnetic resonance imaging, risk factors, prevalence, hypertension
Introduction
Silent cerebral infarctions (SCI), also termed covert infarcts or simply magnetic resonance imaging (MRI) infarcts, are parenchymal lesions that have the MRI characteristics of prior infarcts but have not been associated in that individual with clinical signs or symptoms corresponding to a stroke.1 They were first described by Fisher in a cohort of 114 subjects who came to autopsy, 88 brains had at least one lacune in the absence of clinical deficits or a stroke history.2 In recent studies, the prevalence of SCI on MRI scans has ranged from 5.84 to 28%.3, 4 Reasons for this variation in prevalence may include differences in the definition of SCI, in MR imaging techniques, age, sex and risk factor profile of the population being studied.3–6
Risk factors associated with clinical stroke such as age, sex, diabetes mellitus (DM), atrial fibrillation (AF), systolic blood pressure (SBP) and cigarette smoking have also been associated with SCIs.1, 4–8However there is a paucity of information on the association between SCI and other circulating biomarkers (such as plasma homocysteine (tHcy) and cholesterol concentrations) or intermediate phenotypes (such as carotid artery intimal medial thickness and left ventricular mass).9–12 Hence, we determined the prevalence of SCI in the Framingham Offspring Study sample and related risk factors comprising the Framingham Stroke Risk Profile (FSRP) as well other important cerebrovascular risk factors to prevalent SCI.
Participants and Methods
Study Sample
The Framingham Study is a longitudinal community-based study that began in 1948 with the recruitment of the Original Cohort.13 Their offspring and offspring spouses were recruited in 1971 to form the Offspring Cohort.14 These Offspring have undergone serial clinical examinations every 4–8 years and are currently undergoing an eighth examination. At exam 7 (1998–2001) participants were invited to undergo brain MRI. Participants with claustrophobia, metal in the eyes or other body parts, valvular prosthesis, vascular clips, cardiac pacemakers cochlear implants, or other implanted device sensitive to strong magnetic fields were excluded. Since some participants had brain MRI before their 7th baseline examination we chose the 6th examination as the baseline for this study. Of 3532 participants who attended the sixth examination, 2100 underwent brain MRI. Participants with prevalent stroke or dementia or other neurological conditions that might confound the diagnosis of SCI on MRI were excluded. The remaining 2040 participants were evaluated for these analyses. This study was approved by the Institutional Review Board at Boston Medical Center and all participants gave informed consent.
MRI techniques
MRI techniques used in this study have been detailed elsewhere.15 Briefly, participants were evaluated with a 1 Tesla Siemens Magnetom. T2 weighted double spin-echo coronal weighted sequences were accquired in 4 mm contiguous slices from nasion to occiput with a repetition time (TR) of 2420 ms, echo time (TE) of TE1 20/TE2 90 ms, echo train length 8 ms, field of view (FOV) 22 cm and an acquisition matrix of 182 ×256 interpolated to a 256×256 with one excitation. All images were transferred to the centralized reading center at the University of California- Davis Medical Center and analyses were performed on QUANTA 6.2, a custom-designed image analysis package operating on a Sun Microsystems Ultra 5 workstation. The presence of MRI infarction was determined from the size, location and imaging characteristics of the lesion. The image analysis system allowed for superimposition of the subtraction image (PD-T2), the proton density image and the T2 weighted image at 3X magnification to assist in interpretation of lesion characteristics. Signal void, best seen on the T2 weighted image was interpreted to indicate a vessel. Only lesions larger than 3mm in size were considered infarcts. Lesions were also required to have cerebrospinal fluid density on subtraction images, and to be distinctly separate from the circle of Willis vessels for suspected basal ganglia infarcts. Investigators blinded to subject demographic and stroke risk factor data processed and analyzed these scans. Scans were evaluated by three different raters. Kappa values for agreement ranged between 0.73 and 0.90.15
Definition of MRI infarct as SCI; exclusion of clinical stroke
MRI infarcts in persons without a clinical stroke documented by the Framingham Stroke surveillance team, were considered SCI. Details regarding stroke surveillance and ascertainment have been detailed in prior publications.16 Information regarding stroke symptoms was elicited using a structured questionnaire administered by a physician at each examination cycle. If participants reported a history suggestive of stroke or transient ischemic attack (TIA) or demonstrated neurological abnormalities, they were evaluated further by a study neurologist. Additional surveillance was carried out through annual telephone health updates and by the daily monitoring of admissions at the only acute care hospital in Framingham, MA. All participants admitted to the hospital were evaluated by a study neurologist if cerebrovascular disease was suspected. Finally participants underwent neurology assessment if they, their family, primary care physicians or personnel in an ancillary study referred them for possible stroke. The final determination regarding the presence and absence of stroke, type and location was made at a Stroke Review by at least two neurologists with access to all Framingham and outside records including brain imaging. SCI can also be defined as: MRI infarcts in subjects known not to have suffered either a clinical stroke or TIA prior to MRI, since some persons with clinical TIA have MRI lesions seen acutely on diffusion-weighted MRI.17 We chose not to use this definition since the determination of whether or not a TIA occurred, although based on the same review process used for clinical stroke, is, being based on history alone, more subjective than stroke ascertainment. Finally SCI can be defined after exclusion of lesions that correspond anatomically to documented past clinical events, either completed stroke or TIA. This last definition would permit inclusion of SCI in persons who have multiple MRI infarcts but fewer clinical events. We did not use this method choosing instead to maintain blinded reading of MRI scans without reference to clinical data.
Independent Variables
We evaluated components of the Framingham Stroke Risk Profile (FSRP), other vascular risk factors and intermediate phenotypes. The FSRP is a validated instrument that estimates the 10-year probability of incident stroke. The component factors of the FSRP are age, SBP, antihypertensive therapy, DM, cigarette smoking, cardiovascular disease (CVD), AF and left ventricular hypertrophy (LVH) on electrocardiographic criteria.18, 19 SBP was recorded as the average of two physician generated measurements. Participants were dichotomized according to baseline use of antihypertensive drugs and current smoking or nonsmoking status. DM was defined as random blood glucose ≥200mg/dl (11mmol/dl), a previous diagnosis of diabetes or use of a hypoglycemic medication or insulin; prior CVD included coronary artery disease, congestive heart failure and peripheral vascular disease. AF and LVH data were obtained from a standard 12 lead electrocardiogram completed at or before the baseline examination.20
All participants underwent transthoracic B mode echocardiogram. Left ventricular internal dimensions, posterior ventricular wall thickness, and interventricular septum thickness were measured at end-diastole; these measurement were used to generate left ventricular mass (LVM) using formulae described elsewhere.21 LVM was indexed to subject height.
Carotid imaging was conducted using Toshiba Medical Systems 7.5 Mhz transducer for the common carotid artery. A 5.0 MHz transducer was used for the carotid bulb and internal carotid artery. All measurements were performed by a single trained sonographer blinded to clinical data. Two images each at the distal common carotid artery, the carotid bulb and the proximal 2 cms of the internal carotid artery were obtained. Carotid artery stenosis was defined as < 49% when velocities were <150 cms/s and ≥50% if the velocity was ≥150 cms/s. Measures of carotid intima media thickness (IMT) were generated at each of the three sites imaged. The maximal IMT at each site was defined as the mean of maximal IMT measurements of the near and far wall; common carotid artery IMT (CCAIMT) was therefore the mean of the IMTs for the left and right common carotid arteries.22
Total, low density lipoprotein and high density lipoprotein were obtained after a 12 hour fast using the Lipid Research Clinics method. Cholesterol levels were measured using Abel Kendall method23 while tHcy levels were measured by high-performance liquid chromatography with fluorimetric technique.24
Statistical Analysis
Our primary outcome variable was prevalent SCI on MRI scans obtained at the seventh biennial examination. Logistic regression adjusted, for age and sex, was used to relate the variables that comprise the FSRP, tHcy levels, left ventricular mass, and carotid IMT to the risk of prevalent silent infarcts. Variables with skewed distributions were log-transformed. In addition, for those variables, we compared the top quartile to the lower three quartiles. For continuous risk factors, results are presented as the effect of an increase of one standard deviation. We investigated potential effect modification by sex and age using models with interaction terms. All analyses were performed with PC-SAS software 9.1 (SAS Institute Inc).
Using statistical methods described elsewhere25 we calculated population attributable risks (PAR) for prevalent SCIs. The PAR is the proportion of disease in the population attributable to the risk factor studied.
Results
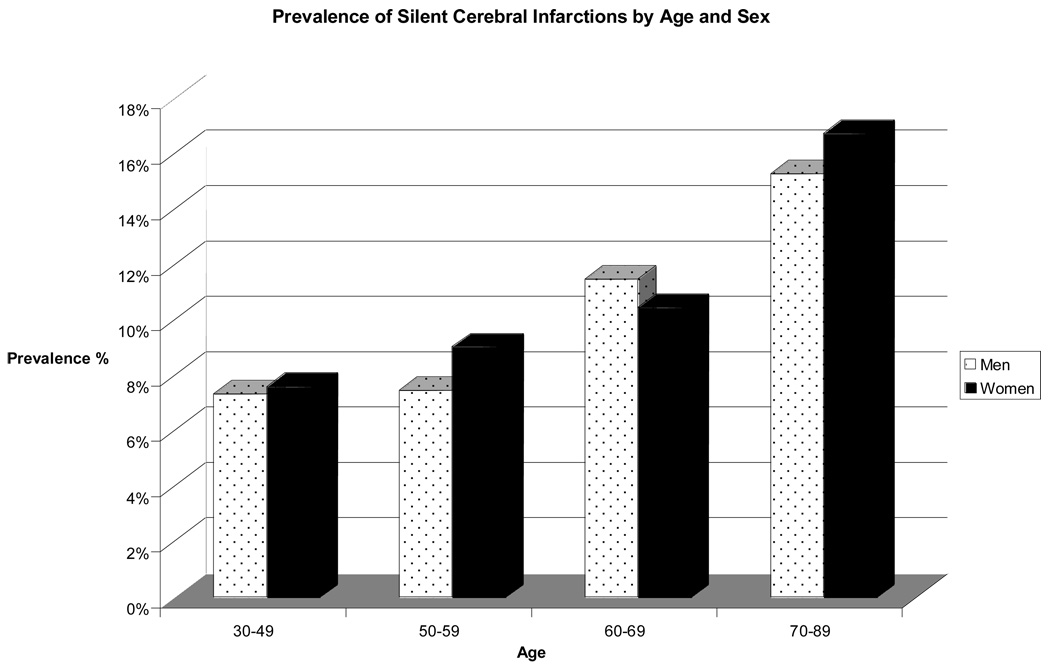
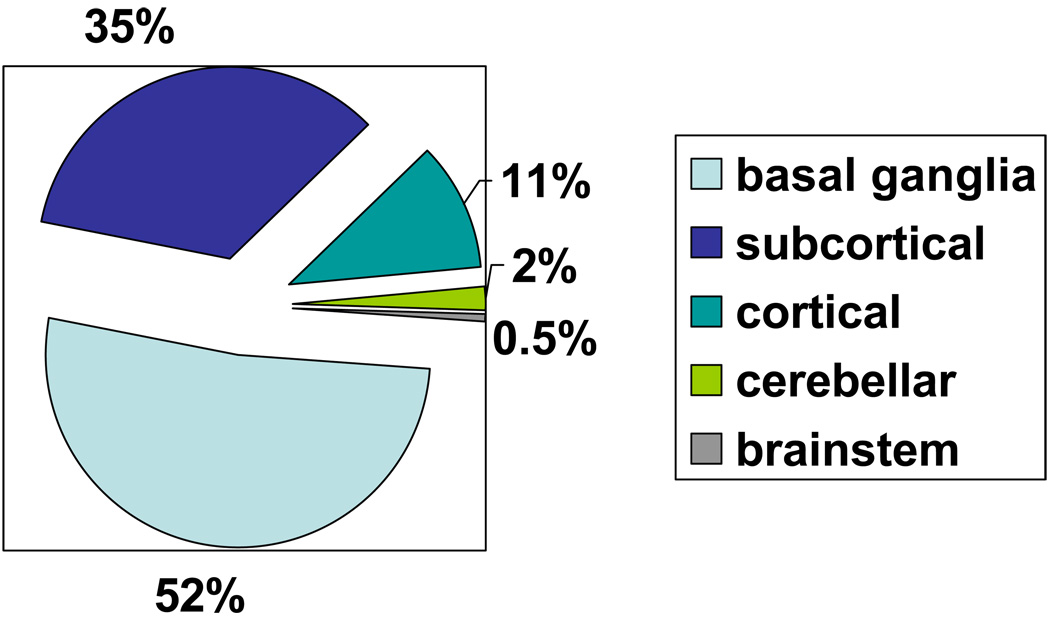
Demographic and risk factor prevalence data for the study sample and the subgroup of persons with SCI are shown in Table 1. SCI was present in 10.7% of 2040 Offspring studied. Participants with SCI were slightly older (mean age of 61 years versus 58 years for the entire sample) and had a higher SBP. Age- and sex-specific data on SCI prevalence are shown in Figure 1. The prevalence of SCI varied with age increasing from <8% in the 30–49 years age group to >15% at 70–89 years. Single SCI occurred in 185 participants (84.1%) and were most commonly located in the basal ganglia (51.9%). SCI were subcortical in 34.8% while 11% of lesions were cortical. Location data for prevalent SCI are presented in Figure 2.
Table 1.
Demographic characteristics and correlates, overall and by silent cerebral infarct (SCI) status:
| Variables | Overall (n=2040) | SCI present (n=220) | SCI absent (n=1820) |
|---|---|---|---|
| Male (%) | 47 | 49 | 47 |
| Age (years) at 6th examination | 58 (9) | 61 (9) | 58 (9) |
| College Degree (%) | 39 | 36 | 39 |
| APOE ε4 allele (%) | 22 | 23 | 22 |
| Age (in years) at MRI | 62 (9) | 64 (9) | 62 (9) |
| Framingham Stroke Risk Profile score * | 5.4 (6.0) | 7.6 (7.8) | 5.2 (5.7) |
| Systolic Blood Pressure (mm Hg) * | 126 (18) | 132 (19) | 126 (18) |
| Current smokers (%) | 14 | 15 | 13 |
| Hypertension (%) | 37 | 50 | 35 |
| Diabetes (%) | 8.7 | 12.7 | 8.2 |
| Left Ventricular Hypertrophy (%) | 0.3 | 0.5 | 0.3 |
| Cardio Vascular Disease (%) | 7.7 | 11.8 | 7.1 |
| Atrial fibrillation (%) | 2.2 | 5.0 | 1.9 |
| Plasma tHcy‡ (mmol/l)† | 8.9 (78.7) | 9.9 (44.3) | 8.8 (78.7) |
| tHcy – top quartile‡ | 25 | 35 | 24 |
| Total Cholesterol | 205 (37) | 209 (35) | 204 (37) |
| High Density Lipoprotein Cholesterol* | 51 (16) | 51 (16) | 52 (16) |
| Carotid Stenosis 25% + (%) | 15 | 24 | 14 |
| CCIMT†§ | 0.69 (1.41) | 0.72 (1.01) | 0.69 (1.41) |
| ICAIMT† ∥ | 0.79 (6.71) | 0.90 (2.97) | 0.78 (6.71) |
| LVM† # | 157 (307) | 159 (220) | 157 (307) |
| LVM_HGT† ** | 2.40 (4.29) | 2.45 (3.25) | 2.40 (4.29) |
mean (sd)
median (range)
plasma total homocysteine
common carotid artery intima media thickness
internal carotid artery intima media thickness
left ventricular mass
left ventricular mass adjusted for height
Figure 1.
Prevalence of Silent Cerebral Infarcts by Age and Sex
Figure 2.
Location information of silent cerebral infarctions
The results of logistic regression analyses are provided in Table 2. Neither age nor gender significantly modified the effect of any of the risk factors on prevalence of SCI. Additionally, we did not observe any significant age-sex interaction. The aggregate FSRP score was significantly related to prevalent SCI. Of the variables comprising the FSRP, AF, hypertension and SBP were each associated with an increased risk of SCI. Among variables that were not components of the FSRP, plasma tHcy and carotid artery stenosis ≥25% were associated with a higher risk of prevalent SCI. Plasma tHcy as a continuous variable, as well as a value in the highest quartile ( compared with the lower three quartiles) was related to prevalent SCI. Carotid artery stenosis ≥25%, higher CCAIMT, higher ICAIMT and the highest quartile of ICA IMT as compared to the lower three quartiles were also significantly associated with prevalent SCI.
Table 2.
Logistic regression odds-ratio relating various risk factors to prevalent silent cerebral infarcts
| Independent Variable | OR (95% CI) | P value |
|---|---|---|
| Framingham Stroke Risk Profile score* | 1.27 (1.10 – 1.46) | <.001 |
| Systolic Blood Pressure (mm Hg) * | 1.23 (1.07 – 1.42) | .005 |
| Current smokers | 1.34 (0.90 – 1.99) | .146 |
| Hypertension | 1.56 (1.15 – 2.11) | .004 |
| Diabetes Mellitus | 1.40 (0.90 – 2.17) | .135 |
| Left Ventricular Hypertrophy | 1.51 (0.17 – 13.04) | .709 |
| Cardio Vascular Disease | 1.38 ( 0.87 – 2.18) | .175 |
| Atrial fibrillation | 2.16 (1.07 – 4.40) | .033 |
| Plasma homocysteine (mmol/l) | 2.23 (1.42 – 3.51) | <.001 |
| tHcy – top quartile † | 1.67 (1.23 – 2.25) | <.001 |
| Total Cholesterol* | 1.10 (0.95 – 1.27) | .198 |
| High Density Lipoprotein* | 0.97 (0.82 – 1.13) | .664 |
| Carotid Stenosis 25% + | 1.62 (1.13 – 2.34) | .009 |
| CCIMT ‡ | 1.20 (1.02 – 1.40) | .026 |
| CCIMT – top quartile‡ | 1.11 (0.80 – 1.53) | .544 |
| ICAIMT*§ | 1.32 (1.13 – 1.54) | <.001 |
| ICAIMT – top quartile§ | 1.65 (1.22 – 2.24) | .001 |
| LVM_HGT*∥ | 0.88 (0.73–1.07) | .192 |
Effect of increase of 1 SD
plasma total homocysteine
Common carotid artery intima media thickness
Internal carotid artery intima media thickness
Left ventricular mass adjusted for height
The portion of disease in the population attributable to individual risk factors, the PAR, was 16.6% for hypertension, whereas it was 8.2% for carotid artery disease and elevated serum homocysteine, and 3.1% for atrial fibrillation.
Discussion
Among clinically stroke-free participants in this mid-life, community-dwelling cohort, 10.7% had SCIs on routine brain MRI. Whereas prior studies have also reported a significant association of hypertension and tHcy with prevalent SCI, we are the first study to demonstrate a significant relationship between AF and SCI. We are also the first study to evaluate the effect of LVM on SCI prevalence. The association of these risk factors for clinical stroke with subclinical SCI is not surprising but reinforces the importance of early detection and treatment of cardiovascular risk factors in midlife. This is especially true since SCI have been associated with an increased risk of incident stroke and cognitive impairment.26
Prior cohort studies have reported a prevalence of SCI on MRI4–6 27 varying from 11% in the younger participants (mean age: 63 years, range: 55–70 years) within the Atherosclerosis Risk in Communities (ARIC) study to 20% in the older participants of the Rotterdam Scan Study (RSS) and 28% in the Cardiovascular Health Study (CHS) sample; mean age was 76 years in both the CHS and RSS. 4, 6 However, autopsy data from 966 brains in the Hisayama Study evaluating asymptomatic community- dwelling adults (mean age = 74 years) disclosed a lower 12.9% prevalence of SCI, perhaps related to failure to count some SCI at autopsy, or to ethnic differences.27 MRI is reliably sensitive in identifying autopsy confirmed cerebral infarcts.28, 29 In our sample, the prevalence of SCI rises with increasing age in both men and women. which is consistent with prior studies‥4, 5, 27 Vermeer and colleagues reported a 30% higher overall prevalence of SCI in women compared to men in all age groups.5 However, we did not find a significant sex difference in the prevalence of SCI; neither did the ARIC and CHS studies.4
Studies have also differed on imaging criteria and technology. MRI scanners used in population studies have varied between field strengths of 1.0 to 1.5 Tesla, slice thicknesses of 5–8 mm and an interslice gap of zero to twenty percent.4, 5, 9 Prior studies share with our study well-defined MRI criteria for infarcts. However, our study was the only one to require a distinct separation between basal ganglial SCI and Circle of Willis vessels perhaps thereby improving diagnostic specificity. CHS additionally reported the the prevalence of lesions <3 mm and designated these as small infarct-like lesions; these lesions had a prevalence of 7.8% and were associated with memory loss.4 We did not assess SCI <3 mms in size.
We observed that more than four-fifth’s of our participants with SCI had a single lesion. In the Hisayama study 70% of participants with SCI had two infarcts or less while Kwon, et al found that 70% had a single lesion.12, 27 Lee et al described single lesion SCIs in 72%.3
We observed a similar distribution of SCI to that noted in prior studies; over half were in the basal ganglia, one-third were subcortical and one-tenth were cortical. In CHS, 72% were subcortical and in the RSS 80% were basal ganglia lesions. 4, 5 Lee, et al note that 38% were in the basal ganglia, a quarter were periventricular, 12 % thalamic and 9% pontine.3 It has been speculated that SCIs are silent (or rather produce subtle neurological damage) because they occur in clinically ineloquent areas of the brain;3 the predominance of lesions in the basal ganglia and subcortically rather than cortically appear to support this hypothesis.
The FSRP has been previously associated with incident clinical stroke, total brain volume and prevalent white matter hyperintensity volume.20, 30 We have now shown that the FSRP score was also associated with an increased risk of prevalent SCI. Additionally, the individual components of the FSRP were evaluated for their impact on SCI prevalence, but with the exception of SBP, hypertension and AF there were no other significant relationships. However, all the components of the FSRP were positively associated with an increased prevalence of SCI, although the relationships failed to reach statistical significance for some components. While prior studies have related the individual components of the FSRP to prevalent SCI, this is the first study to relate the aggregate score to prevalence of silent strokes.
Hypertension has consistently been implicated as a risk factor for SCI.1. Vermeer demonstrated a more than two-fold increase in SCI in the RSS while Lee and others showed a more than three-fold increase in SCI prevalence in hypertensives.3, 5 At autopsy Shinkawa, et al. found that SCI and non SCI brains differed significantly with regard to diastolic blood pressure measurements.27 In our study we demonstrated that hypertension and elevated SBP are important risk factors for SCI.
In our data, AF increased the risk of prevalent SCI more than two- fold. AF is known to be an important risk factor for clinical stroke, associated with a five-fold increase in stroke incidence.31 In prior studies of persons with AF and no known clinical stroke, the prevalence of SCI on CT scans, in studies that have varied in sample size, has ranged from 13 to 48%.7, 8, 32–34 In the Hisayama autopsy study persons with SCI at autopsy had a significantly higher frequency of AF prior to death, than persons without SCI (10.7% versus 3.9%).27 In a study of 994 healthy middle-aged adults undergoing a screening medical examination with brain MRI, Lee and colleagues did not observe an association between prevalent SCI and AF; this may be explained by the lower mean age (49 years) of their sample, over a decade younger than the Framingham Offspring at the time of MRI.3 The other large community based cohorts did not evaluate AF as an SCI risk factor, or found it non significant.4–6, 35 Risk factors for stroke generally and perhaps SCI in particular are also risk factors for AF. Thus hypertension, prior CVD and MI, and diabetes which predispose to AF also predispose to clinical stroke and, probably to SCI. In this way AF may be a concomitant outcome rather than the mechanism underlying the occurrence of SCI.
In this study carotid artery stenosis ≥25% was associated with an increased prevalence of SCI. In the CHS study, participants with carotid stenosis ≥75% had nearly twice the likelihood of prevalent SCI on MRI.36 Uehara and others evaluated MRIs in 219 stroke-free participants who had presented to the hospital with nonspecific neurological complaints. Carotid artery stenosis >25% on MR angiography was associated with a three fold increased risk of basal ganglial SCIs on multivariate analysis.37 It is known that carotid artery stenosis may serve as an embolic source for cerebrovascular lesions; Norris and Zhu report a direct relationship between degree of stenosis and the frequency of both overt and silent stroke in a population that included participants with carotid distribution transient ischemic attacks.38 The pathogenetic mechanism, however, is unclear. In 108 participants, convergent color Doppler revealed greater turbulence in internal carotid arteries with stenosis greater than 50% if the participants also had an SCI on brain MRI than in those without an SCI.39 Uehara however found that there was no statistically significant difference in the prevalence of SCIs in the territory served by the stenosed carotid as compared to that of the nonstenosed artery.37 This is similar to prior data from Framingham40 and to data from the Asymptomatic Carotid Atherosclerosis Study suggesting that carotid artery disease may be a marker for atherosclerotic disease rather than the direct proximate cause of SCI 41
In our study, common carotid artery intima media thickness (CCAIMT) was significantly associated with prevalence of SCI as was ICAIMT. CCAIMT is known to predict clinical vascular outcomes including stroke and myocardial infarction.42, 43 Prior studies have related CCAIMT and ICAIMT to clinical stroke.44 A single Japanese study observed an association between CCAIMT and prevalent MRI infarcts in 28 adults, but it was not clear whether the observed MRI infarcts were silent since no history regarding prior stroke had been obtained.11 In the CHS study, prevalent SCI prevalence was associated with both CCAIMT and ICAIMT.36
Two prior studies have evaluated the effect of elevated circulating tHcy concentrations on prevalent SCI. Matsui and colleagues examined 153 elderly community dwelling participants with cranial MRI. Individuals in the highest and middle tertiles of plasma tHcy had more than four times and nearly three times the risk of SCI, respectively, compared to persons in the lowest tertile.9 In the RSS, participants in the upper quintile had approximately 2.5 times the risk of SCI compared to persons in the lowest quintile. 10 The results of our study are consistent with the literature. We demonstrate an increased prevalence of SCI in association with the plasma homocysteine level; Elevated tHcy is known to be associated with an increased incidence of stroke and MI.44
Pathogenetic hypotheses for SCIs have included dysregulated cerebral blood flow from hypertension and vascular disease, large vessel atherothrombosis, as well as from cerebral emboli.1, 45 Risk factors that proved significant for prevalent SCI remain those that fit these mechanisms: hypertension, atrial fibrillation, hyperhomocysteinemia, and carotid artery disease.
The strengths of evaluating the prevalence and risk factors for SCI using data from the Framingham Offspring Study include the use of previously validated MRI imaging to assess the presence or absence of MRI infarcts and the well-validated ongoing surveillance for clinical stroke which minimizes the probability of clinically symptomatic events being misclassified as SCI. In the past, studies have used differing methods to determine participants’ clinical history with regard to stroke symptomatology. Some have relied on subject self report,3, 6 others both on self report and evaluation of participants’ medical records5 while a few have used periodic visits and surveillance of hospital admission.4, 27 In our study surveillance techniques include systematic history taking for symptoms suggestive of stroke or transient ischemic attack at each biennial health evaluation and active daily surveillance of admissions to the local hospital. ⋅
Participants in the Framingham Study are largely of European descent. This limits the generalizability of our findings to other ethnic groups. Ethnic differences have been described in other multi-ethnic cohorts such as the Northern Manhattan Study.46 A second limitation of this study is the lack of incidence data given that the participants underwent a single cranial MRI.
In conclusion, we document a 10.7% prevalence of SCI in a community- based sample. This is concerning since SCI have been related both to the risk of incident stroke and to cognitive impairment26. The significant relationship between hypertension, elevated serum homocysteine as well as carotid artery disease and prevalent SCI underscores the importance of current guidelines for the early diagnosis and prevention of hypertension and atherosclerosis and their risk factors. AF is also associated with SCI although our observational data cannot show if screening for and appropriately treating AF would reduce the population burden of SCI.
Acknowledgments
Supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195) and by grants from the National Institute on Aging ( R01 AG16495; AG08122) and from the National Institute of Neurological Disorders and Stroke ( R01 NS17950).
References
- 1.Masuda J, Nabika T, Notsu Y. Silent stroke: Pathogenesis, genetic factors and clinical implications as a risk factor. Curr Opin Neurol. 2001;14:77–82. doi: 10.1097/00019052-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CM. Lacunes: Small, deep cerebral infarcts. Neurology. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 3.Lee SC, Park SJ, Ki HK, Gwon HC, Chung CS, Byun HS, Shin KJ, Shin MH, Lee WR. Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension. 2000;36:73–77. doi: 10.1161/01.hyp.36.1.73-a. [DOI] [PubMed] [Google Scholar]
- 4.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, Anton-Culver H, O'Leary DH. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The cardiovascular health study. Chs collaborative research group. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 6.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 7.Silent brain infarction in nonrheumatic atrial fibrillation. Eaft study group. European atrial fibrillation trial. Neurology. 1996;46:159–165. doi: 10.1212/wnl.46.1.159. [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, Thadani V, Meyer ML, Bridgers SL. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. The veterans affairs stroke prevention in nonrheumatic atrial fibrillation investigators. Circulation. 1995;92:2178–2182. doi: 10.1161/01.cir.92.8.2178. [DOI] [PubMed] [Google Scholar]
- 9.Matsui T, Arai H, Yuzuriha T, Yao H, Miura M, Hashimoto S, Higuchi S, Matsushita S, Morikawa M, Kato A, Sasaki H. Elevated plasma homocysteine levels and risk of silent brain infarction in elderly people. Stroke. 2001;32:1116–1119. doi: 10.1161/01.str.32.5.1116. [DOI] [PubMed] [Google Scholar]
- 10.Vermeer SE, van Dijk EJ, Koudstaal PJ, Oudkerk M, Hofman A, Clarke R, Breteler MM. Homocysteine, silent brain infarcts, and white matter lesions: The rotterdam scan study. Ann Neurol. 2002;51:285–289. doi: 10.1002/ana.10111. [DOI] [PubMed] [Google Scholar]
- 11.Kamide K, Rakugi H, Nakano N, Ohishi M, Nakata Y, Takami S, Katsuya T, Higaki J, Ogihara T. Insulin resistance is related to silent cerebral infarction in patients with essential hypertension. Am J Hypertens. 1997;10:1245–1249. doi: 10.1016/s0895-7061(97)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Kwon HM, Kim BJ, Lee SH, Choi SH, Oh BH, Yoon BW. Metabolic syndrome as an independent risk factor of silent brain infarction in healthy people. Stroke. 2006;37:466–470. doi: 10.1161/01.STR.0000199081.17935.81. [DOI] [PubMed] [Google Scholar]
- 13.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: The Framingham study. Am J Public Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. Jama. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 17.Lamy C, Oppenheim C, Calvet D, Domigo V, Naggara O, Meder JL, Mas JL. Diffusion-weighted mr imaging in transient ischaemic attacks. Eur Radiol. 2006;16:1090–1095. doi: 10.1007/s00330-005-0049-5. [DOI] [PubMed] [Google Scholar]
- 18.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 20.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The Framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 21.Dhingra R, Ho Nam B, Benjamin EJ, Wang TJ, Larson MG, D'Agostino RB, Sr, Levy D, Vasan RS. Cross-sectional relations of electrocardiographic qrs duration to left ventricular dimensions: The Framingham Heart study. J Am Coll Cardiol. 2005;45:685–689. doi: 10.1016/j.jacc.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Nam BH, Wilson PW, Wolf PA, Levy D, Polak JF, D'Agostino RB, O'Donnell CJ. Association of c-reactive protein with carotid atherosclerosis in men and women: The Framingham heart study. Arterioscler Thromb Vasc Biol. 2002;22:1662–1667. doi: 10.1161/01.atv.0000034543.78801.69. [DOI] [PubMed] [Google Scholar]
- 23.Nam BH, Kannel WB, D'Agostino RB. Search for an optimal atherogenic lipid risk profile: From the Framingham study. Am J Cardiol. 2006;97:372–375. doi: 10.1016/j.amjcard.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 24.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Jacques PF, Selhub J, Seshadri S, Au R, Beiser A, Wolf PA. Homocysteine and cognitive performance in the Framingham offspring study: Age is important. Am J Epidemiol. 2005;162:644–653. doi: 10.1093/aje/kwi259. [DOI] [PubMed] [Google Scholar]
- 25.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 26.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 27.Shinkawa A, Ueda K, Kiyohara Y, Kato I, Sueishi K, Tsuneyoshi M, Fujishima M. Silent cerebral infarction in a community-based autopsy series in japan. The hisayama study. Stroke. 1995;26:380–385. doi: 10.1161/01.str.26.3.380. [DOI] [PubMed] [Google Scholar]
- 28.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 29.Chodosh EH, Foulkes MA, Kase CS, Wolf PA, Mohr JP, Hier DB, Price TR, Furtado JG., Jr Silent stroke in the nincds stroke data bank. Neurology. 1988;38:1674–1679. doi: 10.1212/wnl.38.11.1674. [DOI] [PubMed] [Google Scholar]
- 30.Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the copenhagen city heart study and the Framingham study. Stroke. 1994;25:802–807. doi: 10.1161/01.str.25.4.802. [DOI] [PubMed] [Google Scholar]
- 31.Wolf PA. Prevention of stroke. Lancet. 1998;352 Suppl 3:SIII15–SIII18. doi: 10.1016/s0140-6736(98)90089-7. [DOI] [PubMed] [Google Scholar]
- 32.Petersen P, Madsen EB, Brun B, Pedersen F, Gyldensted C, Boysen G. Silent cerebral infarction in chronic atrial fibrillation. Stroke. 1987;18:1098–1100. doi: 10.1161/01.str.18.6.1098. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg WM, Seeger JF, Carmody RF, Anderson DC, Hart RG, Pearce LA. Epidemiologic features of asymptomatic cerebral infarction in patients with nonvalvular atrial fibrillation. Arch Intern Med. 1990;150:2340–2344. [PubMed] [Google Scholar]
- 34.Kempster PA, Gerraty RP, Gates PC. Asymptomatic cerebral infarction in patients with chronic atrial fibrillation. Stroke. 1988;19:955–957. doi: 10.1161/01.str.19.8.955. [DOI] [PubMed] [Google Scholar]
- 35.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 36.Manolio TA, Burke GL, O'Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral mri findings to ultrasonographic carotid atherosclerosis in older adults : The cardiovascular health study. Chs collaborative research group. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 37.Uehara T, Tabuchi M, Mori E. Risk factors for silent cerebral infarcts in subcortical white matter and basal ganglia. Stroke. 1999;30:378–382. doi: 10.1161/01.str.30.2.378. [DOI] [PubMed] [Google Scholar]
- 38.Norris JW, Zhu CZ. Silent stroke and carotid stenosis. Stroke. 1992;23:483–485. doi: 10.1161/01.str.23.4.483. [DOI] [PubMed] [Google Scholar]
- 39.Ohyama M, Mizushige K, Ohyama H, Takahashi T, Hosomi N, Ichihara S, Kohno M. Carotid turbulent flow observed by convergent color doppler flowmetry in silent cerebral infarction. Int J Cardiovasc Imaging. 2002;18:119–124. doi: 10.1023/a:1014645621378. [DOI] [PubMed] [Google Scholar]
- 40.Wolf PA, Kannel WB, Sorlie P, McNamara P. Asymptomatic carotid bruit and risk of stroke. The Framingham study. JAMA. 1981;245:1442–1445. [PubMed] [Google Scholar]
- 41.Brott T, Tomsick T, Feinberg W, Johnson C, Biller J, Broderick J, Kelly M, Frey J, Schwartz S, Blum C, et al. Baseline silent cerebral infarction in the asymptomatic carotid atherosclerosis study. Stroke. 1994;25:1122–1129. doi: 10.1161/01.str.25.6.1122. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the carotid atherosclerosis progression study (caps) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 43.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 44.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 45.Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: A preliminary estimate. Cerebrovasc Dis. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- 46.Prabhakaran S, Wright CB, Yoshita M, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008 February 5;70(6):425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]