Abstract
A fundamental question is how the CNS controls the hand with its many degrees of freedom. Several motor cortical areas, including the dorsal premotor cortex (PMd) and primary motor cortex (M1), are involved in reach to grasp. Although neurons in PMd are known to modulate in relation to the type of grasp and neurons in M1 in relation to grasp force and finger movements, whether specific parameters of whole hand shaping are encoded in the discharge of these cells has not been studied. In this study, two monkeys were trained to reach and grasp 16 objects varying in shape, size, and orientation. Grasp force was explicitly controlled, requiring the monkeys to exert either three or five levels of grasp force on each object. The animals were unable to see the objects or their hands. Single PMd and M1 neurons were recorded during the task, and cell firing was examined for modulation with object properties and grasp force. The firing of the vast majority of PMd and M1 neurons varied significantly as a function of the object presented as well as the object grasp dimension. Grasp dimension of the object was an important determinant of the firing of cells in both PMd and M1. A smaller percentage of PMd and M1 neurons were modulated by grasp force. Linear encoding was prominent with grasp force but less so with grasp dimension. The correlations with grasp dimension and grasp force were stronger in the firing of M1 than PMd neurons and across both regions the modulation with these parameters increased as reach to grasp proceeded. All PMd and M1 neurons that signaled grasp force also signaled grasp dimension, yet the two signals showed limited interactions, providing a neural substrate for the independent control of these two parameters at the behavioral level.
INTRODUCTION
When reaching to grasp an object, human and nonhuman primates characteristically preshape the hand to match object properties, such as size and shape (Castiello et al. 1993, 1998; Jeannerod 1984; Mason et al. 2004; Paulignan et al. 1991; Roy et al. 2002; Santello and Soechting 1998; Wing et al. 1986). A prominent view is that the necessary visuomotor or sensori-motor transformations required to shape the hand are mediated by parieto-frontal circuits involving the posterior parietal cortex, ventral premotor (PMv/area F5), and the primary motor cortex (M1) (Castiello and Begliomini 2008; Jeannerod et al. 1995; Raos et al. 2006; Rizzolatti et al. 1988). In this dorsolateral circuit, object representations are hypothesized to be encoded in anterior intraparietal sulcus (AIP) in object-centered coordinates. In PMv these representations are hypothesized to be transformed into movement-based representations that are used to specify and control the specific grasp required (Castiello and Begliomini 2008; Murata et al. 2000; Taira et al. 1990). The visuomotor transformations for the reach are thought to use a dorsomedial circuit consisting of the occipital parietal sulcus (area VGA) to the dorsal premotor cortex (PMd/area F2) (Galletti et al. 2003; Tanne-Gariepy et al. 2002). For both of these circuits the transformation continues in M1, generating the descending motor commands for the execution of the required reach and grasp. However, some authors have argued against such dedicated reaching and grasping circuits (Grol et al. 2007).
Elucidating the individual contributions of the frontal motor areas is essential to fully understanding the cortical networks involved in prehension. This also will require discerning the reach and grasping signals encoded in the firing of neurons within each of these regions. The firing of the majority of PMv neurons is correlated with specific goal-related hand motor behaviors such as grasping and manipulating objects as opposed to movements of single digits (Murata et al. 1997; Rizzolatti et al. 1987, 1988; Umilta et al. 2007). The discharge of PMv neurons and the local field potentials exhibit specificity for the type of grasp, with precision grip well represented (Murata et al. 1997; Rizzolatti et al. 1988; Spinks et al. 2008). In comparison with M1 neurons, the firing of PMv neurons is tuned for grasp earlier and more consistently throughout the entire act of reach-to-grasp (Umilta et al. 2007). It has been proposed that PMv provides a vocabulary of elementary motor actions that reflects the type of grasp needed to interact with an object of a particular size, shape, or other relevant property (Castiello and Begliomini 2008; Raos et al. 2006; Rizzolatti et al. 1988).
The contribution of PMd neurons to grasping has been less well studied. Dorsal premotor neurons are highly modulated in relation to parameters of reach (Caminiti et al. 1991; Fu et al. 1993; Kalaska et al. 1997; Messier and Kalaska 2000; Wise et al. 1997). However, PMd also has a representation of distal movements (Dum and Strick 2002; He et al. 1995; Raos et al. 2004; Stark et al. 2007). Similar to neurons in PMv, the firing of PMd neurons are tuned to the type of prehension used to grasp an object (Raos et al. 2004). It has been hypothesized that PMd neurons maintain, in memory, a motor representation of the grasp used throughout the behavior (Raos et al. 2006). However, studies have not examined what specific parameters of object and/or hand shape are found in the firing of PMd neurons.
The hand area of M1 plays a major role in the control of hand and finger movements. M1 is a major source of the corticospinal tract that controls the hand and fingers, including the monosynaptic cortico-motoneuronal projection with direct access to hand and finger motoneurons (Bortoff and Strick 1993; Maier et al. 1993; Palmer and Ashby 1992; Rathelot and Strick 2006, 2009). The importance of M1 in prehension is further shown by the deleterious effects of lesions/inactivation of M1 (Brochier et al. 1999; Murata et al. 2008; Schieber and Poliakov 1998), the extensive activation observed during functional imaging (Ehrsson et al. 2000; Kuhtz-Buschbeck et al. 2008; Takasawa et al. 2003), and the alterations produced by transcranial magnetic stimulation (TMS) (Chouinard et al. 2005; Lemon et al. 1995; Schabrun et al. 2008). Furthermore, M1 neurons are highly active during both precision and power grasping (Gardner et al. 2007,Maier et al. 1993; Umilta et al. 2007; Wannier et al. 1991). The discharge of M1 neurons modulates in relation to multiple finger movements, the populations of neurons related to different fingers overlap extensively, and the projections from M1 diverge to multiple muscles (Poliakov and Schieber 1999; Rathelot and Strick 2006; Schieber 1991; Schieber and Hibbard 1993). These provide a neural substrate that could contribute to control of overall hand shape. M1 neurons have been shown to respond to properties of objects such as the texture and weight (Picard and Smith 1992) and the local field potentials in M1 vary with grasp configuration (Spinks et al. 2008), but the parameters of hand shaping represented by M1 neurons remains to be determined.
The discharge of M1 neurons also modulates with grasp force, although there are conflicting results on the degree of encoding of grasp force in the firing of PMd neurons during precision grip. Some studies found no correlation (Boudreau et al. 2001) and others found context-dependent correlations (Hepp-Reymond et al. 1999) between PMd firing rates and grasp force level. The discharge of M1 neurons modulates with the force during precision grip, although several studies have described inverse relationships (Hepp-Reymond 1988; Maier et al. 1993; Wannier et al. 1991). Functional imaging studies provide evidence that activation increases with grip force level (Dettmers et al. 1995; Ehrsson et al. 2002; Kuhtz-Buschbeck et al. 2008; Ward et al. 2008). During reach to grasp that included whole hand grasping, the discharge of M1 and PMd neurons modulate during application of grasp force (Gardner et al. 2007; Raos et al. 2004; Stark et al. 2007; Umilta et al. 2007). However, studies of the modulation of PMd and M1 neurons during whole hand grasp in an explicitly controlled task as well as varied grasp force are lacking.
This study investigated hand shaping and grasp force during whole hand prehension with three specific goals. The first goal was to investigate which specific parameters of object/hand shape are encoded in the firing of PMd and M1 neurons during reach to grasp. The second goal was to examine the contribution of whole hand grasp force to the firing of PMd and M1 neurons. The third goal was to assess the relationship between hand shaping and grasp force signals in neuronal firing. Behavioral studies support the hypothesis that these two aspects of prehension are controlled independently (Biegstraaten et al. 2006; Jackson and Shaw 2000; Mason et al. 2004, 2006). There is also some evidence for independent control in several regions in the CNS. For example, hand shape and grasp force signals are signaled relatively independently in the simple spike firing of Purkinje cells (Mason et al. 2006). A temporal dissociation in the control of hand shaping and grasp force has been identified in the AIP by TMS (Davare et al. 2007). Inactivation of F5 or AIP in the monkey results in deficits in hand preshaping while leaving grip force essentially intact (Fogassi et al. 2001; Gallese et al. 1994). Therefore the third goal assessed the degree to which these two parameters are independently present in the firing of PMd and M1 neurons.
To address these three goals, two rhesus monkeys were trained to reach to and grasp a set of objects with explicit grasp force requirements. Detailed kinematic analyses of the wrist, hand, and finger movements during the same task in the same animals have been previously published (Mason et al. 2004, 2006; Theverapperuma et al. 2005). The discharge of both PMd and M1 cells was predominantly related to properties of the object grasped with fewer neurons modulated by grasp force.
METHODS
Behavioral task
The experimental protocol was approved and monitored by the University of Minnesota Institutional Animal Care and Use Committee and conformed to the “Guiding Principles in the Care and Use of Animals” of the American Physiological Society. The details of the behavioral paradigm and many of the experimental procedures were included in recent publications (Mason et al. 2004, 2006; Theverapperuma et al. 2005). Therefore only a brief description of these methods is provided in this report.
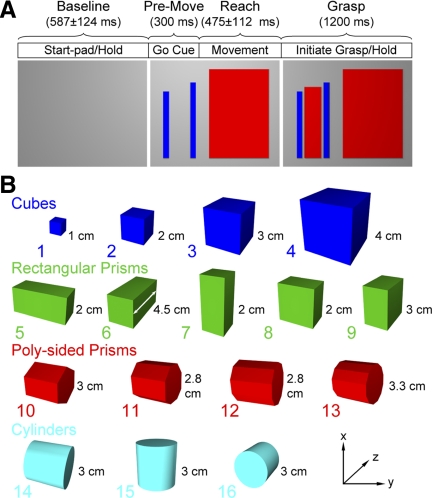
Two rhesus monkeys (monkey G: female, 5.2 kg; monkey L: male, 6.8 kg) were trained with their heads fixed to reach to and grasp objects at a specified grasp force level (Fig. 1 A). Trials were initiated when the monkey placed its hand on a 5 × 5-cm start-pad located by the animal's side. The monkey was required to maintain its hand on the start pad for a randomized period of 450–750 ms. A “go” cue (blue vertical bars), presented on a 15-in computer monitor at eye level, signaled the monkey to reach (∼15 cm) and grasp the target object. The required grasp force range to be generated and maintained during the grasp was specified by the heights of two vertical bars. The left bar provided the lower and right bar the higher of the required force range. The monkeys were trained to grasp the objects with an overhand power grasp while allowing considerable freedom in placing their fingers in contact with the force sensor to meet the force level requirements. Visual feedback of the applied force was provided by a vertically displaced red indicator bar. The monkeys received a juice reward if they successfully initiated (within a 0.5-s window) and maintained the specified grasp force level for 1.5 s.
FIG. 1.
A: the reach to grasp task timeline (top) and the corresponding computer monitor display sequence (bottom). The monkey began with its hand on the start-pad while viewing a blank monitor (left). A large red rectangle on the monitor provided a “go” cue to initiate the reach-to-grasp (middle). Simultaneously, blue bars signaled the level of grasp force required at the target object. The central red slider provided feedback on the exerted grasp force (right). The task epochs were 1) baseline epoch during the initial start-pad hold, 2) premovement epoch starting 300 ms before movement onset, 3) reach epoch beginning on movement onset and ending with grasp initiation, and 4) grasp epoch beginning with grasp initiation and maintaining the grasp (1,200 ms). B: 4 object classes (cubes, rectangular prisms, poly-sided prisms, and cylinders) were presented with the x-y plane parallel to the frontal plane of the monkey. Grasp dimensions measured along the z-axis (e.g., as shown by the white line segment on object 6) were 1, 2, 2.8, 3, 3.3, 4, or 4.5 cm. The cubes had volumes of 1, 8, 27, and 64 cm3, the rectangular solids had a volume of 18 cm3 (3 were 4.5 × 2 × 2 cm and 2 were 2 × 3 × 3 cm), and the poly-sided prisms and cylinders were 3 × 3 cm (length × diameter). The poly-sided prisms had 6, 8, 10, or 12 sides.
This study examined the encoding of object properties in the absence of direct vision of the objects and hand. As described in our earlier study (Mason et al. 2004), the animals were highly trained and could recognize the unseen objects through touch. The animals were not allowed to see the target object or their hand but were allowed to touch objects before the initiation of each block of trials when objects were changed.
The 16 objects (Fig. 1B), mounted on a fixed metal shaft, were made of Lexan painted black and categorized within four geometric classes (cubes, rectangular prisms, poly-sided prisms, and cylinders). A Force Sensing Resistor (FSR 1.27 cm diam, Interlink Electronics, Camarillo, CA) was positioned on the face of each object opposite the monkey. Monkey G, which performed the task with the right hand only, was required to exert one of five instructed levels of anterior-posterior (AP) grasp forces on the object (0.2, 0.4, 0.6, 0.8, and 1.0 N, each level having an allowable target force range of ±0.1 N). On grasp initiation, the monkey was allowed a 500-ms window within which to acquire the target force level (e.g., reach target or correct for overshooting target force). As shown in Figs. 4 and 5, the animals rarely under or overshot the desired grasp force. The monkey was required to maintain the target force level within the upper and lower tolerance range for 1.5 s before receiving a juice reward. Monkey L was trained to exert five force levels with its right hand but only three of the five force levels (0.2, 0.6, and 1.0 ± 0.1 N) when performing the task with the left hand. Only three of the five force levels were used for the left hand to ensure the maximum number of objects (blocked trials) could be completed within a recording session. Objects were presented in random order with five repetitions for each of the force levels generated pseudorandomly within each block. For monkey L, object 1 (smallest cube) was not used for the right hand because the animal had difficulty mastering the grasp of this object at all five force levels. Behavioral data collected included the force generated on the target object as well as the time of the reach onset (i.e., time of lift-off from the start-pad) and grasp onset (i.e., time of contact with object). The details of the data collection from the video-based tracking system used for the collection of the kinematic data have been published (Mason et al. 2004, 2006; Theverapperuma et al. 2005).
Surgical preparation, recording, and histological procedures
After achieving an acceptable level of competency in task performance, an 18-mm circular, chronic recording chamber was surgically implanted over the cortex contralateral to the monkey's working hand (Mason et al. 2006). The stereotaxic coordinates of the center of the chamber for monkey G was 15 mm anterior to interaural zero and 18 mm (left side) lateral to the midline. The chamber placement coordinates for monkey L were 15 mm (left side) and 20 mm (right side) anterior to interaural zero and 18 mm (left and right side) lateral to the midline. At the same surgery, a head-holding system was fixed to the skull. Parylene-coated tungsten microelectrodes (3–5 MΩ) were inserted approximately vertically to the cortex using a microdrive with an X-Y micropositioner mounted directly to the chamber.
The action potentials of isolated premotor cortex (PM) and M1 neurons were digitized, the time of occurrence was stored (16 kHz), and the instantaneous firing frequency was calculated based on fractional intervals, binned at 16.67 ms—the same bin width used for the video-based motion analysis (Mason et al. 2004, 2006; Theverapperuma et al. 2005). The cells were tested for the presence of proprioceptive and cutaneous receptive fields (Mason et al. 2006). Specifically, proprioceptive responses were identified by passively moving limb segments and fingers about each joint in isolation and noting changes in cell activity during the imposed movements. In addition, cutaneous receptive fields were tested by stroking, lightly probing, and/or applying air puffs to the hand and arm. Neurons were further categorized into PM or M1 cells based on histology and intracortical microstimulation (ICMS) response threshold (Tanne-Gariepy et al. 2002; Weinrich and Wise 1982). Specifically, the animal was examined visually and manually for evoked limb movements or muscle twitches over a stimulation amplitude range of 5–60 μA during ICMS (35 ms duration train of 12 cathodal pulses, each pulse: 200 μs duration, at 333 Hz). Recording sites that evoked a response with <25 μA ICMS were identified as within M1, whereas sites requiring larger stimulation intensities were identified as PM.
To identify the recording locations, electrolytic lesions were made after completing the recordings in each chamber (Mason et al. 2006; Roitman et al. 2005). After completion of all recordings, each animal was initially anesthetized with ketamine (20 mg/kg, IM) and xylazine (0.4 mg/kg, IM), followed by a lethal dose of sodium pentobarbital (150 mg/kg, IP). Intracardiac perfusion with saline containing heparin was followed by perfusion with Zamboni's fixative (Mason et al. 2006; Roitman et al. 2005). After removal of the cerebral hemispheres, 50-μm sagittal sections were cut with a freezing microtome and stained with thionin. Cell recording locations were determined from the recovered recording tracks and electrolytic lesions.
Analysis of cell firing
For each trial, four task-related epochs were identified (baseline, premovement, reach, and grasp). The baseline epoch began when the monkey's hand touched the start-pad and ended with the presentation of the go-cue. The duration of the baseline epoch varied from trial to trial. The premovement epoch was defined as the 300 ms before reach onset, rather than the timing of the go cue, because the latter was not recorded in both animals. Reach onset was determined as the time the hand lifted from the start-pad force sensor (i.e., peak force deceleration less than −0.3 N). The reach epoch ended and initiation of the grasp epoch began when the force sensor on the target was first activated (object force sensor >0.1 N). The grasp epoch was limited to the initial 1,200 ms of grasp initiation and object hold.
All statistical analyses of firing were based on single trials, not averaged data, so that the data analyses accurately reflect the variability caused by the signal from that of the noise (Howell 2008) and to provide consistency with our study of Purkinje cell firing during this same task (Mason et al. 2006). Averaging firing across trials will inflate R2 values because the natural variation across trial repetitions (i.e., noise) is effectively aggregated within the estimate of the variability caused by the signal (Kenney 1979). Therefore the R2 values reported in this study may appear smaller than studies using average firing, but the R2 values must be understood in the context that all analyses were based on single trial data.
The first analysis of the firing was based on the task epochs. To determine whether a cell was task related, a paired Student's t-test evaluated whether the firing in the premovement, reach, and grasp epochs was different from baseline firing (Mason et al. 2006). For cells defined as task related, an ANOVA was done on the mean firing rates during the premovement, reach and grasp epochs with object or object grasp dimension and with force level as treatment factors (α = 0.05). As expected, grasp aperture, defined as the distance between the thumb and index finger, was found to vary linearly with the object grasp dimension (e.g., the grasp width of the object) (Mason et al. 2004). Grasp dimension provided a method of estimating one aspect of object shape as a single continuous variable.
Whereas the epoch-based ANOVA analysis provided evidence of significant differences in discharge across the independent variables, the post hoc analyses provided insight into the nature of this relationship (e.g., whether linear). The post hoc linear regression was based on the individual trial firing rates at each level of grasp dimension or grasp force. Replication across force levels and grasp dimension required use of a “lack-of-fit” test based on estimates of the deviations-from-linearity (Zar 1999). If the deviation from linearity was not significant (P > 0.05), the relationship between cell firing and force and grasp dimension was considered linear.
Temporal regression analysis
The epoch-based analysis was performed over relatively long duration periods. To provide a more precise measure of the timing of the cell correlation with either hand shape or grasp force, a bin by bin multiple regression analysis was computed (Fu et al. 1995, 1997; Mason et al. 2006). The temporal regression analysis was based on individual trials to insure high power statistical results (e.g., larger sample size) and a log transform of the firing was used so as to insure homogeneity of variance (Ashe and Georgopoulos 1994; Howell 1987). The firing, F, in each 16.67-ms bin (ti) was regressed to the variables
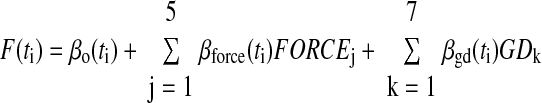 |
(1) |
in which FORCE is the grasp force level the monkeys needed to maintain during the task, and GD represents the objects' grasp dimension. The paradigm required that the monkey maintain the desired force level during the hold period or the trial was aborted. Therefore during the grasp epoch, the desired grasp force level and the actual grasp force exerted by the monkey were equivalent (Figs. 4 and 5, top row force plots). Total and partial (object grasp dimension and force) R2 values were calculated using a multiple regression analysis (Edwards 1979) and tested for significance (α = 0.05). A time bin was considered significant only if the model or partial R2s remained significant for at least five consecutive time bins (i.e., 83 ms). The overall trend in significant R2s across the cell population was determined using the percentage of cells with significant regressions within each time bin. The temporal regressions were computed in absolute time rather than normalized time to remain consistent with previously published findings of simple spike firing for the same task (Mason et al. 2006). Because the trials were aligned on grasp initiation, variability in movement duration across trials may be introduced in the temporal regressions, specifically early in the task (premovement and reach). However, the results of the temporal regression are consistent with those of the epoch-based analysis, suggesting the variability in movement times had limited effect on the temporal regression.
RESULTS
Behavior and kinematic analysis
Analysis of the wrist, hand, and finger movements for this same task and monkeys was published previously (Mason et al. 2004, 2006; Theverapperuma et al. 2005). As detailed in the previous publications, monkey G grasped objects 2–16 using a power grasp with a palm-finger opposition and used a precision grasp with the smallest cube (object 1). An analysis of precision versus power grip was therefore not possible because it would be based on insufficient data (i.e., the animals used a precision grasp with only 1 object). For the right hand, monkey L used an overhand power grasp with a thumb-finger opposition for objects 2–16. For the left hand, monkey L was trained on all 16 objects and used a precision grasp with the smallest cube. These studies also showed that reach kinematics remained constant across objects and grasp force levels, implying that modulation in the firing of the motor cortical cells was not related to variations in the reach. Hand shaping began with the initiation of reach and continued throughout the reach. Hand shape throughout the reach and grasp epochs was found to match object properties (e.g., size) but showed no significant relation to or interaction with the grasp force. For example, the shaping of the hand throughout the reach did not change with grasp force. Nor did arm kinematics vary with grasp force.
Task-related modulation and cell locations
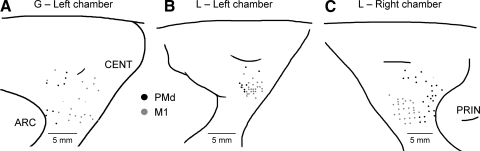
The histology results indicated that cell recordings for both monkeys were generally located throughout the precentral gyrus, medial to the genu of the arcuate sulcus (Fig. 2). The classification of a cell as being within the PM (black) or M1 (gray) was based on the histology, and when available, ICMS results. The vast majority of the PM cells were located in the PMd where grasp-related neurons are located (Raos et al. 2004; Stark et al. 2007) and, therefore we will refer to the cell location as PMd.
FIG. 2.
A–C: cortical surface maps of reconstructed locations of microelectrode penetrations for the left motor cortical chamber in monkey G (A) and the left (B) and right (C) in monkey L. Histology and intracortical microstimulation (ICMS) results were used to classify dorsal premotor cortex (PMd; black, ≥25 μA ICMS) and and primary motor cortex (M1; gray, <25 μA ICMS) cell recording sites. ARC, arcuate; CENT, central; PRIN, principle.
Two hundred twelve PMd and M1 neurons were recorded in the two monkeys. A total of 170 cell recordings (35 for monkey G; 35 and 100 for monkey L, left and right chambers, respectively) were analyzed in this report, retaining only those neurons in which 4 or more of the 16 objects were successfully completed for five force levels (or 3 force levels for monkey L, left hand) and with at least five repetitions per treatment combination (e.g., a minimum of 60–100 trials per cell). The majority of these cells (156/170) showed significant differences in firing relative to baseline for at least one of the three epochs (paired t-test) and were classified as task-related.
The majority of the task-related cells responded primarily to proprioceptive manipulations (76%, PMd; 59% M1), with fewer cells having primarily cutaneous fields (24%, PMd; 41% M1). Most of the cells had receptive fields relating to the hand (48%, PMd; 82% M1), with fewer relating to the arm (24%, PMd; 14% M1) and/or shoulder areas (43%, PMd; 16% M1).
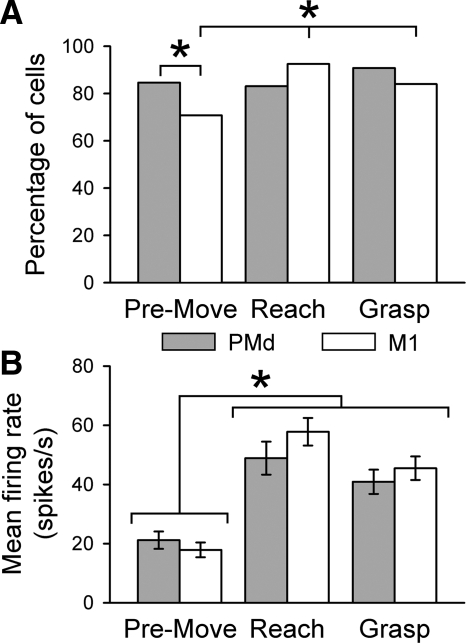
Task-related modulation was very common in the two cortical areas, including 91% (58/64) of the cells in PMd and 92% (98/106) of the cells in M1 (Fig. 3 A). Significantly more M1 task-related cells were modulated during the reach (92%, 98/106) and grasp (84%, 89/106) epochs than during the premovement (71%, 75/106) epoch (χ2 = 16.9, df = 2, P < 0.05). In contrast, the number of task-related PMd cells was constant across epochs (χ2 = 1.7, df = 2, P > 0.05). The only difference in the task-related cells between the two areas was during the premovement epoch when significantly more PMd cells were modulated than M1 cells (χ2 = 4.34, df = 1, P < 0.05). Mean firing rates also differed across epochs (Fig. 3B) with significantly higher firing rates during reach and grasp than during premovement [F(2,165) = 10.73 and 24.48, P < 0.05 for PMd and M1 cells, respectively]. However, there was no difference in the average firing of task-related cells in PMd and M1 for the three epochs [F(1,424) = 0.93, P > 0.05]. In summary, PMd and M1 cells have similar firing rates across epochs and similar percentages of cells modulated during the behavioral epochs of reach and grasp. The only notable difference was that during the premovement epoch a greater percentage of PMd cells were task-related than in M1.
FIG. 3.
A and B: the percentage of cells (A) with significant mean firing rates (B) across epochs for PMd (gray) and M1 (white) cells. The total number of task-related cells was based on significant increases in firing relative to the baseline (paired t-test, P < 0.05 during ≥1 epoch). *Significant χ2 at P < 0.05 (A) and significant F at P < 0.05 (B).
Firing patterns in PMd and M1 during reach to grasp
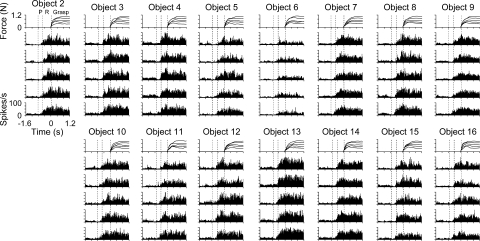
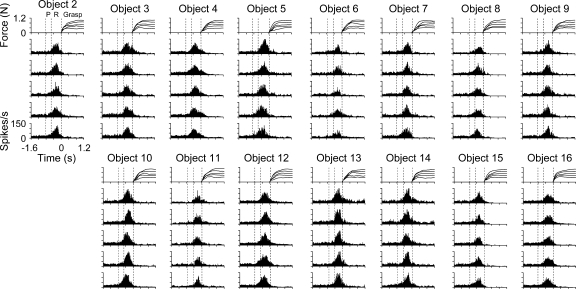
The firing patterns for two individual M1 cells are shown in Figs. 4 and 5. Each neuron was successfully isolated and discriminated for 15 objects at each of the five force levels with at least five repetitions for a total of ≥375 trials. Histograms of PMd and M1 firing were constructed to qualitatively assess the discharge modulation in relation to the task epochs, objects, and grasp force. Several aspects of the cell discharge modulations are shown by these examples. First, most PMd and M1 cells had significant modulation in their firing for more than one task epoch. Only a few cells had significant firing restricted to a single epoch (premovement: 2, reach: 11, or grasp: 2 cells). Cell L061 (Fig. 4), for example, showed significant differences in firing relative to baseline during premovement, reach, and grasp epochs (t1,467 = 19.06, −52.53, and −52.90, respectively, P < 0.05). Similarly, for cell L048, significant modulation occurred during each epoch (t1,467 = −13.59, −67.25, and 29.64, respectively, P < 0.05), although the modulation in firing was predominantly related to reach (Fig. 5).
FIG. 4.
Example of a task-related M1 cell (L061) with significant object-related firing. Histograms and force profiles represent the averaged cell firing rate and grasp force levels, respectively, across all trial repetitions for each object (2–16) and force level (1–5 N). Histograms are ordered with the repetitions of the lowest force (0.2 N) on the bottom and highest force (1.0 N) on the top for each object. In this and subsequent figures, all data were aligned on grasp initiation (time = 0). Three vertical dashed lines represent the average onset times for the premovement (P), reach, and grasp epochs.
FIG. 5.
Example of a task-related M1 cell (L048) with significant object- and force-related firing. Conventions as in Fig. 4.
The second aspect of discharge modulation in PMd and M1 was that the temporal profiles were consistent across objects and grasp force. In cell L061 (Fig. 4), for which monkey L completed all objects except object 1, the firing rate increased during reach with peak firing just before or at the onset of grasp initiation. The firing slightly decreased in amplitude after grasp onset and was relatively sustained as the grasp force was held at target level. For cell L048 (Fig. 5), the temporal firing pattern consisted of an increase in firing followed by a decrease in firing during the reach epoch. The amplitude of the firing differed markedly for objects 6 and 13, yet the basic temporal pattern was preserved. A significant change in firing amplitude across objects was found during the premovement, reach, and grasp epochs [F(14,393) = 115.92, 14.58, and 12.37, respectively, P < 0.05]. Similar observations (i.e., relatively fixed modulation patterns that varied in amplitude with object) were noted for most PMd and M1 cells. Significant object-related firing was found for 149 (54/58 PMd and 95/98 M1) of the 156 task-related cells (ANOVA, P < 0.05).
The third property of the discharge was that some cells were modulated by grasp force. For example, there was an increase in firing with grasp force level (bottom-to-top histograms) during the reach and grasp epochs for cell L048 (Fig. 5; e.g., longer duration and increased firing with increased grasp force) but not during premovement. This increase was statistically significant [F(4,393) = 3.68 and 160.06, for reach and grasp epochs, respectively, P < 0.05; F(4,393) = 0.89, P > 0.05, for premovement epoch], but the overall effect size was small (R2 = 0.015 for reach and R2 = 0.024 for grasp epoch). A post hoc regression analysis confirmed that firing increased linearly with grasp force during both reach and grasp epochs (lack-of-fit test, P > 0.05). This example reflects the limited, yet significant, grasp force coding observed in the population (Fig. 8D). Examples of more robust encoding of grasp force can be seen in Figs. 7E and 10E. However, cell L061 (Fig. 4) showed no significant modulation with grasp force during any of the epochs [F(4,438) = 0.64, 0.13, and 0.44, for premovement, reach, and grasp, respectively, P > 0.05]. These findings warranted a more detailed analysis of cell discharge as a function of object properties and grasp force levels across epochs.
Modulation with grasp dimension and grasp force
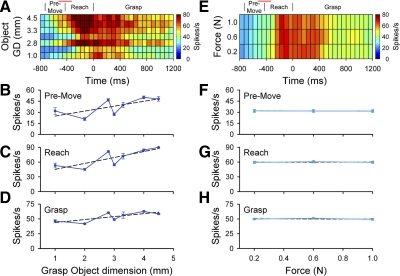
As described above, PMd and M1 cells were significantly modulated by object. Given the importance of grasp aperture in prehension, we examined the relation between cell firing and grasp dimension. An example of a PMd neuron with significant differences in firing across object grasp dimension is shown in Fig. 6, A–D. The grasp dimension was a significant factor for premovement, reach, and grasp epochs [F(6,249) = 15.79, 26.10, and 15.07, respectively, P < 0.05]. A post hoc analysis showed that firing was not linearly related to object grasp dimension during the premovement, reach, and grasp epochs [lack-of-fit test, F(5,263) = 13.68, 18.04, and 10.09, respectively, P < 0.05], suggestive of a more complex relation between firing and grasp dimension. For this cell, there was no significant difference in the firing as a function of the grasp force level for any of the epochs [F(2,249) = 0.26, 0.39, and 1.03, respectively, P > 0.05; Fig. 6, E–H]. No significant interaction between object grasp dimension and grasp force was found (P > 0.05).
FIG. 6.
A and B: example of a PMd cell (L553) with object grasp dimension related modulation. Color plots for the mean firing rate by object grasp dimension (A) and grasp force (E). B–H: mean firing rates and SE across object grasp dimension (B–D) and force level (F–H) during the premovement (B and F), reach (C and G), and grasp epochs (D and H). Post hoc linear regressions indicated that cell firing was not linearly related to object grasp dimension during the 3 epochs. Vertical lines above the color plots represent the average onset times for the premovement, reach, and grasp epochs with the SD of reach onset indicated by a horizontal bar at the top.
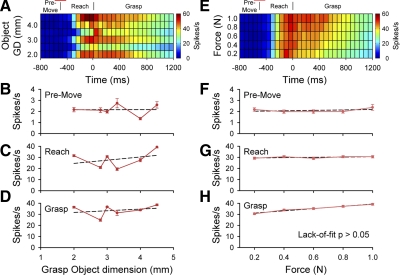
An M1 cell with significant differences in the mean firing rate as a function of object grasp dimension is shown in Fig. 7. Significant differences in the firing rate was noted across object grasp dimension for each epoch [F(5,243) = 2.52, 30.00, and 24.00, respectively, P < 0.05; Fig. 7, B–D]. The relation between firing and object grasp dimension was not linear [lack-of-fit test, F(4,462) = 3.2, 36.12, and 25.85 for premovement, reach, and grasp, respectively, P < 0.05]. A significant force effect was found during the grasp epoch [F(4,438) = 15.97, P < 0.05], and a post hoc regression indicated that firing rates increase linearly with the grasp force level [lack-of-fit test, F(3,463) = 0.43, P > 0.05; Fig. 7H]. No significant force-related effects were noted during the premovement and reach epochs [F(4,438) = 0.26 and 0.81, P > 0.05; Fig. 7, F and G). There was no significant interaction between object grasp dimension and grasp force (P > 0.05).
FIG. 7.
A and B: example of an M1 cell (L052) with object grasp dimension and force-related modulation. Color plots for the mean firing rate by object grasp dimension (A) and grasp force levels (E). B–H: mean firing rates and SE across object grasp dimension (left) and force level (right) during the premovement (B and F), reach (C and G), and grasp (D and H). Post hoc linear regressions indicated that cell firing was not linearly related to the object grasp dimension. Cell firing was linearly related to grasp force during the grasp epoch. Conventions as in Fig. 6.
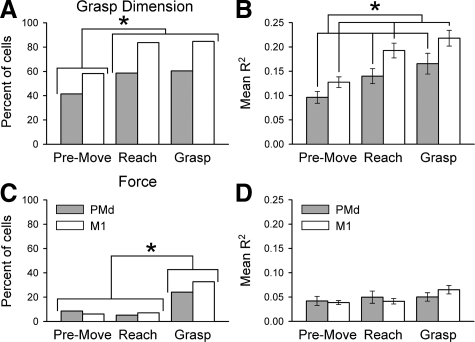
For the population of cells, most PMd (93%, 53/58) and M1 (97%, 95/98) task-related cells were significantly modulated by grasp dimension during at least one epoch (Fig. 8A). Almost all task-related cells with object grasp dimension signaling (117/156) were modulated during at least two or more epochs. Overall, the correlation of the firing with grasp dimension was greater for M1 neurons. Across epochs, the percentage of cells with significant modulation with grasp dimension (Fig. 8A), and the average R2s for the fit (Fig. 8B) were greater for M1 than PMd [percentage, χ2 = 20.76, df = 1, P < 0.05; R2 grasp dimension F(1,311) = 9.03, P < 0.05]. For PMd and M1, the percentage of cells with significant modulation with grasp dimension increased as reach to grasp progressed from premovement (41%, 24/58 and 58%, 57/98) to reach (59%, 34/58 and 84%, 82/98) and grasp (60%, 35/58 and 85%, 83/98; χ2 = 24.4, P > 0.05). The average R2s for grasp dimension differed across epochs [F(2,311) = 8.88, P < 0.05], with the R2s significantly increasing from the premovement to the reach and grasp epochs (post hoc Bonferroni adjusted, P < 0.017), and this was consistent across PMd and M1 cells [i.e., no interaction effect F(2,311) = 0.20, P > 0.05]. Finally, post hoc linear regressions testing for “lack-of-fit” showed that a modest fraction of the cells modulated with object grasp dimension had firing rates linearly related to grasp dimension during the premovement (PMd: 33%, 8/24; M1: 25%, 14/57), reach (PMd: 6%, 2/34;M1: 16%, 13/82), and grasp (PMd: 20%, 7/35;M1: 12%, 10/83) epochs.
FIG. 8.
Modulation related to object grasp dimension was the dominant parameter encoded in PMd and M1 cells. Percentage of PMd (gray) and M1 (white) cells with significant object grasp dimension (A) and force (C) effects across epochs. Corresponding R2 for object grasp dimension (B) and force (D) parameters. *Significant χ2 at P < 0.05 (A and C) and significant F at P < 0.05 (B).
A second analysis assessed whether other measures, categorical or continuous, provided additional or better information compared with object grasp dimension. Therefore in addition to object grasp dimension, the firing was modeled with object volume and object class in separate regressions (ANOVA, α = 0.05). When comparing object grasp dimension, object volume, and object class, the percentage of significantly modulated cells was 53 (31/58), 52 (30/58), and 44% (26/58) for PMd and 75 (74/98), 75 (73/98), and 67% (65/98) for M1, respectively (based on the average over the 3 epochs). Although there were significant differences in the fraction of cells modulated by these three properties (χ2 = 9.78, df = 2, P < 0.05), this is not unexpected because the firing of a much smaller number of cells was modulated in relation to object class than grasp dimension or object volume (pairwise contrasts, class and grasp dimension, χ2 = 9.70, df = 1, P < 0.05; class and volume, χ2 = 3.22, df = 1, P < 0.05, grasp dimension vs. volume, χ2 = 1.6, df = 1, P > 0.05). Most important was the almost complete overlap of the cells significantly modulated by object grasp dimension and volume (95% of the cells modulated by grasp dimension were also modulated by object volume). Similarly, there was also a nearly complete overlap for cells modulated by object grasp dimension and class (95%) and object volume and class (94%). Therefore the analysis based on object volume or class did not provide additional information on the parameters of object shape represented in these neurons.
Only a minority of PMd (33%, 19/58) and M1 (40%, 39/98) task-related cells modulated with grasp force (Fig. 8C). Significantly more cells were modulated in response to grasp dimension than grasp force for both cortical areas (PMd, χ2 = 57, df = 1, P < 0.05; M1, χ2 = 180, df = 1, P < 0.05). More cells with force-related modulation were recruited during the grasp epoch compared with premovement and reach epochs (χ2 = 32.09, df = 2, P < 0.05) when force was actively applied to the object. Of the 19 PMd cells with force-related modulation, 14 of these cells modulated with force during the grasp epoch. Of the 39 M1 cells with force related modulation, 32 of these cells modulated with force during the grasp epoch. No differences in cell percentages were noted between PMd and M1 cells across epochs (χ2 = 0.06, df = 1, P > 0.05) or within epochs (χ2 = 1.17, df = 2, P > 0.05, no interaction effect). However, the average R2 for the fit to grasp force did not differ across epochs [F(2,61) = 1, P > 0.05] or for PMd and M1 cells [F(1,61) = 0.01, P > 0.05; Fig. 8D]. Cells with grasp force modulation had predominantly linearly related firing with grasp force during premovement (PMd: 60%, 3/5; M1: 67%, 4/6), reach (PMd: 67%, 2/3; M1: 86%, 6/7), and grasp (PMd: 100%, 14/14; M1: 84%, 27/32). All cells with significant force-related modulation also had a significant grasp dimension effect. However, only a few cells (1/53 PMd and 8/95 M1) had a significant grasp dimension by force interaction effect. The interaction effect was primarily observed during the grasp epoch (PMd 1/1 and M1 7/8).
The linear regression analysis showed that a smaller fraction of PMd and M1 cells were significantly modulated by grasp force than grasp dimension. It is possible that the lower recruitment levels and smaller R2s observed for grasp force may be compensated by larger modulation levels in the firing rate such that the effect size was greater. To examine this possibility, effect size was defined as the percent change in the mean firing rate between lowest and highest factorial levels (i.e., difference between grasp dimensions or grasp force levels with the lowest and highest mean firing rates). Overall, the percent change in firing was greater for grasp dimension than force-related modulation [F(1,261) = 53.32 and F(1,109) = 7.93, P < 0.05; grasp dimension 54 ± 20 and 48 ± 18; force 31 ± 17 and 33 ± 13 spikes/s for PMd and M1, respectively]. No differences were noted across epochs [F(2,261) = 5.63, P < 0.05 but no significant post hoc Bonferroni multiple comparison, and F(2,109) = 0.09, P > 0.05; premovement 49 ± 21 and 43 ± 18; reach 48 ± 21 and 46 ± 18; grasp 52 ± 22 and 46 ± 18 spikes/s for PMd and M1 cells, respectively] or cell types [F(1,370) = 0.30, P > 0.05; PMd 50 ± 21 and M1 45 ± 18 spikes/s]. Therefore for the three measures examined (percent cells with significant modulation, model R2, and effect size), cell firing was more strongly correlated with grasp dimension than grasp force.
Timing of the grasp dimension and grasp force modulations
The epoch-based analyses showed that PMd and M1 cell discharge modulated in relation to object grasp dimension and grasp force. However, the firing histograms indicated that the cell firing changed within epochs as well as during the transitions between epochs (Figs. 4 and 5). Furthermore, brief periods of cell modulation could be masked by averaging the firing rate over the entire grasp segment (1,200 ms). Therefore a linear multiple regression model (Eq. 1) relating cell firing to grasp dimension and grasp force over time was used to more precisely define the timing of the modulations.
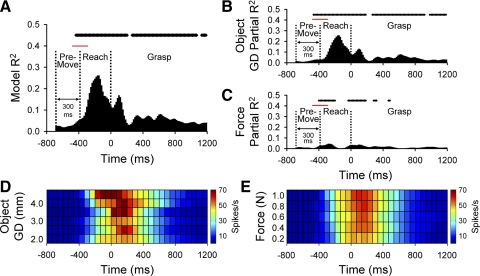
An example of the temporal regression analysis for a PMd cell is shown in Fig. 9. The R2model (Fig. 9A) as a function of time was significant for most time bins (top raster plot, P < 0.05) during the reach and grasp epochs but not during the premovement epoch. The R2model increased during the reach, attaining peak amplitude mid-reach. A secondary peak in R2model occurred during grasp initiation followed by a brief segment of nonsignificant R2model ∼300 ms after grasp initiation. The R2model was again significant during the remainder of the grasp epoch. Examination of the model's partial R2 for object grasp dimension, R2gd (Fig. 9B) and grasp force level, R2force (Fig. 9C) indicated that most of R2model was related to the object grasp dimension rather than the grasp force level. The difference was further exemplified by the color plots of the mean firing rates over time for grasp dimension and grasp force–related firing (Fig. 9, D and E, respectively). Firing increased monotonically and linearly with increased grasp force levels [F(4,438) = 2.50, P < 0.05, post hoc lack-of-fit test, F(3,463) = 0.82, P > 0.05; Fig. 9E], although the overall change in firing was small. In contrast, firing showed greater differences in both onset and magnitude across object grasp dimension (Fig. 9D).
FIG. 9.
A–C: example of a PMd cell (L056) with predominant object-related encoding. The model (A) and partial R2 profiles for grasp dimension (B) and grasp force (C) parameters. Above each bar plot a raster display indicates times at which the discharge was significantly correlated with the model parameter. A time bin was considered significant only if the model or partial R2s remained significant for 83 ms. D and E: color plots of the firing rates over time, averaged across objects (D) and force levels (E). Vertical dashed lines in the bar plots represent the average onset times for the premovement, reach, and grasp epochs with the SD indicated by a horizontal bar at the top.
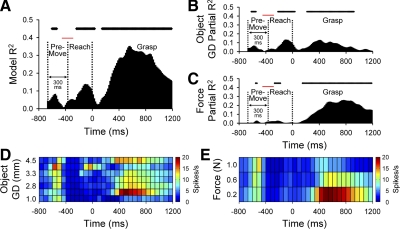
An example of the temporal regression analysis for an M1 cell is shown in Fig. 10. The R2model (Fig. 10A) as a function of time was significant for some of the premovement epochs and most of the reach and grasp epochs (top raster plot, P < 0.05). The R2model had relative peaks during the premovement, at the end of the reach, and during the grasp force hold. A closer examination of R2gd (Fig. 10B) and grasp force level, R2force (Fig. 10C) indicated that most of R2model was related to the object grasp dimension during the premovement and reach epochs but largely related to the force during the grasp epoch. Firing decreased linearly with grasp force [F(2,249) = 31.52, P < 0.05, post hoc lack-of-fit test, F(1,267) = 1.53, P > 0.05, Fig. 10E].
FIG. 10.
A–C: example of an M1 cell (L518) with object- and force-related encoding. The model (A) and partial R2 profiles for grasp dimension (B) and grasp force (C). D and E: color plots of the firing rates over time, averaged across grasp dimensions (D) and force as levels (E). Conventions as in Fig. 9.
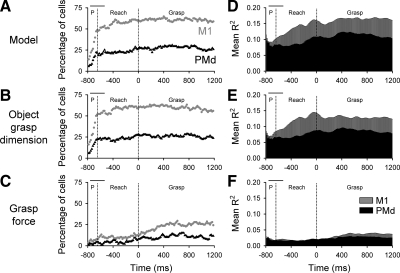
Across the PMd and M1 cell populations, the firing of more M1 (gray) than PMd (black) cells had significant R2model and R2gd (Fig. 11, A and B) during all three epochs. A greater fraction of M1 cells had a significant R2force for all epochs compared with PMd cells and this difference was most pronounced during grasp (Fig. 11C). The mean grasp R2force exhibited a similar trend (Fig. 11F). The model and grasp dimension R2s for M1 cells increased throughout reach, peaking at grasp onset, and remaining relatively high during the grasp epoch (Fig. 11, D and E). The model and grasp dimension mean R2s for PMd cells also increased during reach and then plateau, but the increase was much less pronounced than observed for M1 cells.
FIG. 11.
M1 cells (gray) were more actively engaged during reach-to-grasp than PMd cells (black). A–C: the percentage of cells with significant R2model (A) for monkeys G and L and corresponding percentages of partial R2gd (B) and R2force (C) parameters. D–F: averaged R2model (D) and corresponding averaged partial R2gd (E) and R2force (F) parameters. First vertical line represents the average onset time for the premovement (P; ±SD) and the 2nd line represents the onset of grasp.
DISCUSSION
Firing modulation in PMd and M1 with grasp dimension
The first major finding of this study is that both PMd and M1 cells are significantly modulated by grasp dimension. As described in previous studies, a large percentage of task-related neurons in PMd (93%) and M1 (97%) modulate in relation to the object grasped (Raos et al. 2004; Stark et al. 2007). This study extends those findings by showing that a specific parameter of hand shape, grasp dimension, is signaled in the firing of PMd and M1 neurons. This modulation occurred during the premovement, reach, and grasp epochs, consistent with a role for PMd and M1 in both the planning and execution of hand shape.
The control of grip aperture is a critical parameter of prehension. Grip aperture increases during the first half of reach, followed by gradual closure of the grip to match the object (Castiello et al. 1998; Jeannerod 1981, 1984; Paulignan et al. 1991; Santello and Soechting 1997, 1998). For both PMd and M1, there was an increase in the modulation of firing with grasp dimension as the task evolved that was evident in both the epoch-based (percentage of cells modulated, R2, and effect size) and temporal regression analyses. We hypothesize that this temporal progression reflects the matching of the grip aperture to the grasp dimension that occurs during the second half of the reach and during the grasp period.
The modulation with grasp dimension was more prevalent in M1 than PMd neurons. This suggests that grasp dimension is strongly represented in the final stages of motor cortical processing of hand shape. Intrinsic hand muscles receive the strongest cortical input just before contact, as fingers close around the object, and at grasp initiation just as contact with the object is achieved (Lemon et al. 1995). Similarly, peak grasp dimension encoding (temporal R2) occurred shortly before and just after the transition from reach to grasp epochs in M1 and, to a lesser extent, in PMd. These findings are consistent with the hypothesis that a critical transition from reach to grasp-related processing occurs within M1 during the late-reach phase of reach to grasp (Lemon et al. 1995).
Parameters of hand shape (or their correlates) in addition to grasp dimension are likely to be represented in the firing of PMd and M1 neurons. The activity of PMd neurons are modulated during grasping and exhibit preference for different grips (Raos et al. 2004; Stark et al. 2007). Similarly, PMv neurons encode goal-related hand motor behaviors such as grasping and manipulating objects as opposed to movements of single digits (Murata et al. 1997; Raos et al. 2006; Rizzolatti et al. 1988). However, our analysis of other possible parameters of hand shape, including object volume and shape, did not provide any additional insights into what those parameters may be. Other object factors known to influence hand shaping and grasp such as fragility, texture, and weight have been studied (Savelsbergh et al. 1996; Weir et al. 1991a,b).
Whether there are underlying parameters of motor control that produce the strong correlation between the firing of PMd and M1 neurons and object properties is a critical question. Not only does hand shape change with the different objects, but so do patterns of muscle activation (Brochier et al. 2004). These patterns of muscle activation change in time during reach to grasp, not unlike the firing properties of PMd and M1 neurons in this study. Specifically, the transition from reach to grasp in the firing might be related to muscles involved with extending and then abducting the fingers, such as the extensor digitorum communis (Brochier et al. 2004). However, although this study was not designed to dissociate kinematic versus muscle activity, it is unlikely that these cells purely encode muscle activity given the limited grasp force-related effects. Another likely candidate is the kinematics of the individual fingers because movements of the fingers highly modulate cells widely distributed in M1 (Hamed et al. 2007; Poliakov and Schieber 1999; Schieber 2002; Schieber and Poliakov 1998). Elucidating the degree to which finger kinematics explain the correlations with object properties will require a study in which the movements of individual digits are monitored during reach to grasp.
The modulation of firing with object properties and hand shape were not due to visual inputs because the animals did not have vision of their hand or objects during reach-to-grasp. The animals did have a priori knowledge of which object was being presented (Mason et al. 2004). Also, during the initial training, monkeys were allowed to use visually guided reach to complete the task. This was sufficient for the monkeys to achieve the appropriate shaping of the hand without vision of the hand or object (Mason et al. 2004). Furthermore, hand shaping synergies in the monkey do not rely on vision (Mason et al. 2004). This is consistent with human subjects, because the removal of continuous vision of the hand or object has little affect on hand preshaping during reach (Santello 2002; Santello et al. 2002; Schettino et al. 2003; Winges et al. 2003). Furthermore, grasp neurons in both PMv and PMd maintain object-related encoding during reach to grasp, regardless of the presence or absence of visual feedback (Raos et al. 2004, 2006). Therefore much of the evolution of the hand shape during reach to grasp can be achieved via feedforward control and/or proprioceptive/tactile feedback.
Firing modulation with grasp force
The second major finding is that the firing of only a fraction of PMd and M1 cells was modulated by grasp force, and this was largely during the grasp epoch when force was actively applied to the object. During the premovement and reach epochs, grasp force was significantly modulated in only a small fraction of the cells in either area. A larger fraction of the cells were modulated (∼26% in PMd and ∼34% in M1) during grasp, but the overall effect size remained smaller than for grasp dimension. The fraction of PMd cells responsive to force magnitude during the grasp is similar to that found in other premotor areas in response to predictable force/pulse perturbations during a precision grasp including ∼33% in PMd, 28% in the supplementary motor area (SMA), and 38% in the ventral cingulate motor area (CMAv) (Boudreau et al. 2001; Cadoret and Smith 1997). However, the fraction of M1 cells was considerably lower than previously found for precision grasp (61% in caudal zone and 54% in rostral zone) (Boudreau and Smith 2001; Picard and Smith 1992) or during precision step-tracking/ramp-hold grasp (50–62% in premotor and motor areas) (Hepp-Reymond et al. 1994, 1999; Maier et al. 1993).
The relatively limited grasp force modulation in M1 neurons may be somewhat surprising. One possibility is that this small fraction of cells encoding grasp force could have a disproportionate influence. However, firing rates and depth of modulation as a function of grasp force were not disproportionately large or different. Another factor is that modulation of M1 neurons with force is not a simple proportional linear relation. For example, in M1 the best modulation can occur at smaller grip forces (Evarts et al. 1983; Hepp-Reymond et al. 1978), and an inverse relation between grip force and firing has been commonly reported (Hepp-Reymond 1988; Maier et al. 1993; Wannier et al. 1991). Other studies emphasized the encoding of dynamic aspects of precision grasp force in M1 (Boudreau and Smith 2001; Hepp-Reymond et al. 1999; Picard and Smith 1992). However, this study found that the firing correlations with static, whole hand, grasp force over a wide range were present in a minority of cells and the average effect size was small.
Recent TMS studies question a primary role for M1 in specifying and generating grip force. TMS of M1 does not result in marked disruption of grip force. The major effect of applying TMS during reach to grasp was to delay the onset of load force from the time of initial digit contact (Lemon et al. 1995). The overall profiles of the grip and load forces were only minimally altered. Virtual lesions of M1 induced by TMS do not alter the preload duration, maximal grip force, the overall grip force profile, or the ability to execute the grasp task (Chouinard et al. 2005; Schabrun et al. 2008). The main effects of virtual lesions include a 40-ms lag in the grip force relative to the grasp force and the loss of force scaling based on information obtained from previous trials. Therefore M1 does not seem essential to the specification of grip force, consistent with the limited force signaling observed in the neural firing.
For PMd, the relatively low percentage of cells modulated by grasp force and the corresponding modest R2 values are not unexpected. Studies of force coding in the firing of PMd neurons during arm or wrist movements show that limb kinetics are poorly represented (Xiao et al. 2006). Recent TMS studies in humans provide further evidence that PMd is unlikely a major contributor to the specification of grasp (load) force (Chouinard et al. 2005; Davare et al. 2006). Similarly, PMv is an unlikely site because inactivation in the monkey and human introduces deficits in hand preshaping (Gallese et al. 1994) while leaving grip force adjustments intact (Davare et al. 2006; Fogassi et al. 2001).
The small numbers of PMd and M1 cells involved in the encoding of grasp force raises the question of how and where the grasp force is specified in the motor system. One possible explanation is that the recording locations in this study were relatively anterior in M1 and not in the bank of the central sulcus (Fig. 2). Force-related modulation during limb tasks is more prominent in posterior M1, particularly in the bank of the central sulcus (Kalaska and Hyde 1985; Kalaska et al. 1989; Sergio et al. 2005), and recordings in this region may uncover stronger encoding of grasp force. Human functional imaging studies report positive relationships between activation and grip-force magnitude in M1 or M1/S1 (Dettmers et al. 1995; Ehrsson et al. 2002; Kuhtz-Buschbeck et al. 2008; Thickbroom et al. 1998; Ward et al. 2008). Imaging studies also suggest that the control of grip forces is widely distributed in the CNS including not only frontal and cingulate motor cortical areas but also regions in the parietal, frontal, and occipital cortex as well as in the thalamus, basal ganglia, and cerebellum (Bursztyn et al. 2006; Kuhtz-Buschbeck et al. 2008; Vaillancourt et al. 2003). However, the cerebellum is an unlikely site because previous findings from the same monkeys showed limited specificity for grasp force in the simple spike firing of Purkinje cells (Mason et al. 2006). Furthermore, limb kinetics has little influence on the discharge of Purkinje cells during arm movements (Pasalar et al. 2006). Therefore the structures or networks responsible for the generation and scaling of grasp forces remains to be determined.
The monkey controlled the grasp force using visual feedback. This type of feedback is not representative of natural prehensile activity. Controlling grasp force using visual feedback engages a more widely distributed network of cortical and subcortical regions than does internally guided force control from memory (Vaillancourt et al. 2003). The increase in the number of PMd and M1 cells with force modulation during the grasp epoch, when the visual feedback was provided, may be the result of the engagement of this larger and more distributed network.
Relationship of PMd to M1
Whether looking at task (e.g., firing relative to baseline) or specific parameters (e.g., grasp dimension and grasp force), the firing of PMd and M1 neurons show similar properties. This includes the dominant encoding of grasp dimension over grasp force. Furthermore, the temporal profile of the grasp dimension signals, although less pronounced in PMd, largely mirrored that of M1. Encoding of similar parameters would provide for an efficient information exchange between PMd and M1. The similarity in encoding of grasp-related information in PMd and M1 confirms functional MRI (fMRI) results in humans (Begliomini et al. 2007) and is consistent with the concept that PMd controls grasp, at least in part, through its direct connections with the M1 (Castiello and Begliomini 2008; Raos et al. 2004).
Independent control of hand shaping kinematics and grasp force
Individual PMd and M1 neurons signal both object properties and grasp force. These signals are encoded largely independently, that is a change in cell modulation related to grasp force has no relation to a change in modulation related to object grasp dimension. The encoding of multiple parameters in the firing of individual neurons is common in the CNS, including motor systems (Johnson and Ebner 2000; Johnson et al. 2001). However, different structures use different methods to signal and combine motor parameters such as direction, speed, and position. The seemingly ambiguous encoding of direction, speed and amplitude in PMd and M1 neurons during arm movements is resolved using a temporal parcellation scheme in which parameters are encoded at different times during movements (Fu et al. 1995; Johnson and Ebner 2000). In contrast, Purkinje cells of the cerebellum signal similar parameters simultaneously, presumably allowing the motor system to derive an aggregate parameter, such as velocity (Fu et al. 1997; Johnson and Ebner 2000; Roitman et al. 2005). The strategy for signaling grasp dimension and grasp force for PMd and M1 neurons described here is the same as found for cerebellar Purkinje cells in the same task (Mason et al. 2006). What is not clear is whether these signals are combined to represent an aggregate parameter of grasping and if so, where in the CNS this combination occurs (e.g., downstream of PMd and M1). One view would be to keep these signals separate as suggested by the behavioral evidence (Biegstraaten et al. 2006; Jackson and Shaw 2000; Mason et al. 2004, 2006). Maintaining separate processing and control of grasp kinematics and kinetics would allow for the accommodation of a large repertoire of prehensile behaviors.
GRANTS
This study was supported in part by National Institutes of Health Grants F32 NS-047798, R01 NS-31350, and T32 DA-022616-01 and the Minnesota Medical Foundation.
Acknowledgments
The authors thank M. McPhee for assistance with graphics.
Present address of C. R. Mason: Department of Physical Therapy, Angelo State University, San Angelo, TX 76909.
REFERENCES
- Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex 4: 590–600, 1994. [DOI] [PubMed] [Google Scholar]
- Begliomini C, Caria A, Grodd W, Castiello U. Comparing natural and constrained movements: new insights into the visuomotor control of grasping. PLoS ONE 2: e1108, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegstraaten M, Smeets JB, Brenner E. The relation between force and movement when grasping an object with a precision grip. Exp Brain Res 171: 347–357, 2006. [DOI] [PubMed] [Google Scholar]
- Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci 13: 5105–5118, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau MJ, Brochier T, Pare M, Smith AM. Activity in ventral and dorsal premotor cortex in response to predictable force-pulse perturbations in a precision grip task. J Neurophysiol 86: 1067–1078, 2001. [DOI] [PubMed] [Google Scholar]
- Boudreau MJ, Smith AM. Activity in rostral motor cortex in response to predictable force-pulse perturbations in a precision grip task. J Neurophysiol 86: 1079–1085, 2001. [DOI] [PubMed] [Google Scholar]
- Brochier T, Boudreau MJ, Pare M, Smith AM. The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res 128: 31–40, 1999. [DOI] [PubMed] [Google Scholar]
- Brochier T, Spinks RL, Umilta MA, Lemon RN. Patterns of muscle activity underlying object-specific grasp by the macaque monkey. J Neurophysiol 92: 1770–1782, 2004. [DOI] [PubMed] [Google Scholar]
- Bursztyn LL, Ganesh G, Imamizu H, Kawato M, Flanagan JR. Neural correlates of internal-model loading. Curr Biol 16: 2440–2445, 2006. [DOI] [PubMed] [Google Scholar]
- Cadoret G, Smith AM. Comparison of the neuronal activity in the SMA and the ventral cingulate cortex during prehension in the monkey. J Neurophysiol 77: 153–166, 1997. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Johnson PB, Galli C, Ferraina S, Burnod Y. Making arm movements within different parts of space: the premotor and motor cortical representation of a coordinate system for reaching to visual targets. J Neurosci 11: 1182–1197, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U, Begliomini C. The cortical control of visually guided grasping. Neuroscientist 14: 157–170, 2008. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennett K, Chambers H. Reach to grasp: the response to a simultaneous perturbation of object position and size. Exp Brain Res 120: 31–40, 1998. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennett KM, Stelmach GE. Reach to grasp: the natural response to perturbation of object size. Exp Brain Res 94: 163–178, 1993. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25: 2277–2284, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Clerget E, Thonnard JL, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci 27: 3974–3980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci 26: 2260–2268, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815, 1995. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682, 2002. [DOI] [PubMed] [Google Scholar]
- Edwards AL Multiple regression and correlation. In: Multiple Regression and the Analysis of Variance and Covariance, edited by Freedman JC, Lindzey G, Thompson RF. New York: W. H. Freeman, 1979, p. 38–52.
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision-versus power-grip tasks: an fMRI study. J Neurophysiol 83: 528–536, 2000. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Kuhtz-Buschbeck JP, Forssberg H. Brain regions controlling nonsynergistic versus synergistic movement of the digits: a functional magnetic resonance imaging study. J Neurosci 22: 5074–5080, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Fromm C, Kroller J, Jennings VA. Motor cortex control of finely graded forces. J Neurophysiol 49: 1199–1215, 1983. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain 124: 571–586, 2001. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol 73: 836–854, 1995. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol 78: 478–491, 1997. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol 70: 2097–2116, 1993. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport 5: 1525–1529, 1994. [DOI] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res 153: 158–170, 2003. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Ro JY, Babu KS, Ghosh S. Neurophysiology of prehension. II. Response diversity in primary somatosensory (S-I) and motor (M-I) cortices. J Neurophysiol 97: 1656–1670, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol MJ, Majdandzic J, Stephan KE, Verhagen L, Dijkerman HC, Bekkering H, Verstraten FA, Toni I. Parieto-frontal connectivity during visually guided grasping. J Neurosci 27: 11877–11887, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed SB, Schieber MH, Pouget A. Decoding M1 neurons during multiple finger movements. J Neurophysiol 98: 327–333, 2007. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci 15: 3284–3306, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res 128: 123–133, 1999. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC Functional organization of motor cortex and its participation in voluntary movements. In: Comparative Primate Biology, edited by Steklis HD, Irwin J. New York: Liss, 1988, vol. 4, p. 501–624.
- Hepp-Reymond MC, Husler EJ, Maier MA, Ql HX. Force-related neuronal activity in two regions of the primate ventral premotor cortex. Can J Physiol Pharmacol 72: 571–579, 1994. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wyss UR, Anner R. Neuronal coding of static force in the primate motor cortex. J Physiol 74: 287–291, 1978. [PubMed] [Google Scholar]
- Howell DC Statistical Methods for Psychology (2nd ed.). Boston, MA: Duxbury Press, 1987.
- Howell DC Fundamental Statistics for the Behavioral Sciences. Belmont, CA: Thomson Wadsworth, 2008.
- Jackson SR, Shaw A. The Ponzo illusion affects grip-force but not grip-aperture scaling during prehension movements. J Exp Psychol Hum Percept Perform 26: 418–423, 2000. [DOI] [PubMed] [Google Scholar]
- Jeannerod M Intersegmental coordination during reaching at natural visual objects. In: Attention and Performance IX, edited by Long J, Baddeley A. Hillsdale, NJ: L. Erlbaum Associates, 1981, p. 153–169.
- Jeannerod M The timing of natural prehension movements. J Mot Behav 16: 235–254, 1984. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: 314–320, 1995. [PubMed] [Google Scholar]
- Johnson MT, Ebner TJ. Processing of multiple kinematic signals in the cerebellum and motor cortices. Brain Res Rev 33: 155–168, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Mason CR, Ebner TJ. Central processes for the multiparametric control of arm movements in primates. Curr Opin Neurobiol 11: 684–688, 2001. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud'homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Hyde ML. Area 4 and area 5: differences between the load direction-dependent discharge variability of cells during active postural fixation. Exp Brain Res 59: 197–202, 1985. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol 7: 849–859, 1997. [DOI] [PubMed] [Google Scholar]
- Kenney DA Correlation and Causality. New York: Wiley, 1979.
- Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage 40: 1469–1481, 2008. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. J Neurosci 15: 6145–6156, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Bennett KM, Hepp-Reymond MC, Lemon RN. Contribution of the monkey corticomotoneuronal system to the control of force in precision grip. J Neurophysiol 69: 772–785, 1993. [DOI] [PubMed] [Google Scholar]
- Mason CR, Hendrix CM, Ebner TJ. Purkinje cells signal hand shape and grasp force during reach-to-grasp in the monkey. J Neurophysiol 95: 144–158, 2006. [DOI] [PubMed] [Google Scholar]
- Mason CR, Theverapperuma LS, Hendrix CM, Ebner TJ. Monkey hand postural synergies during reach-to-grasp in the absence of vision of the hand and object. J Neurophysiol 91: 2826–2837, 2004. [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol 84: 152–165, 2000. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol 78: 2226–2230, 1997. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601, 2000. [DOI] [PubMed] [Google Scholar]
- Murata Y, Higo N, Oishi T, Yamashita A, Matsuda K, Hayashi M, Yamane S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J Neurophysiol 99: 773–786, 2008. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol 448: 397–412, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9: 1404–1411, 2006. [DOI] [PubMed] [Google Scholar]
- Paulignan Y, Jeannerod M, MacKenzie C, Marteniuk R. Selective perturbation of visual input during prehension movements. 2. The effects of changing object size. Exp Brain Res 87: 407–420, 1991. [DOI] [PubMed] [Google Scholar]
- Picard N, Smith AM. Primary motor cortical responses to perturbations of prehension in the monkey. J Neurophysiol 68: 1882–1894, 1992. [DOI] [PubMed] [Google Scholar]
- Poliakov AV, Schieber MH. Limited functional grouping of neurons in the motor cortex hand area during individuated finger movements: a cluster analysis. J Neurophysiol 82: 3488–3505, 1999. [DOI] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Gallese V, Fogassi L. Functional properties of grasping-related neurons in the dorsal premotor area F2 of the macaque monkey. J Neurophysiol 92: 1990–2002, 2004. [DOI] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Murata A, Fogassi L, Gallese V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol 95: 709–729, 2006. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71: 491–507, 1988. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Fogassi L, Luppino G, Matelli M, Ponzoni-Maggi S. Neurons related to goal-directed motor acts in inferior area 6 of the macaque monkey. Exp Brain Res 67: 220–224, 1987. [DOI] [PubMed] [Google Scholar]
- Roitman AV, Pasalar S, Johnson MT, Ebner TJ. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci 25: 9244–9257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AC, Paulignan Y, Meunier M, Boussaoud D. Prehension movements in the macaque monkey: effects of object size and location. J Neurophysiol 88: 1491–1499, 2002. [DOI] [PubMed] [Google Scholar]
- Santello M Kinematic synergies for the control of hand shape. Arch Ital Biol 140: 221–228, 2002. [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Patterns of hand motion during grasping and the influence of sensory guidance. J Neurosci 22: 1426–1435, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Matching object size by controlling finger span and hand shape. Somatosens Mot Res 14: 203–212, 1997. [DOI] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Gradual molding of the hand to object contours. J Neurophysiol 79: 1307–1320, 1998. [DOI] [PubMed] [Google Scholar]
- Savelsbergh GJP, Steenbergen B, van der Kamp J. The role of fragility information in the guidance of the precision grip. Hum Mov Sci 15: 115–127, 1996. [Google Scholar]
- Schabrun SM, Ridding MC, Miles TS. Role of the primary motor and sensory cortex in precision grasping: a transcranial magnetic stimulation study. Eur J Neurosci 27: 750–756, 2008. [DOI] [PubMed] [Google Scholar]
- Schettino LF, Adamovich SV, Poizner H. Effects of object shape and visual feedback on hand configuration during grasping. Exp Brain Res 151: 158–166, 2003. [DOI] [PubMed] [Google Scholar]
- Schieber MH Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. J Neurophysiol 65: 1381–1391, 1991. [DOI] [PubMed] [Google Scholar]
- Schieber MH Motor cortex and the distributed anatomy of finger movements. Adv Exp Med Biol 508: 411–416, 2002. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science 261: 489–492, 1993. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci 18: 9038–9054, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Paquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol 94: 2353–2378, 2005. [DOI] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci 28: 10961–10971, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Asher I, Abeles M. Encoding of reach and grasp by single neurons in premotor cortex is independent of recording site. J Neurophysiol 97: 3351–3364, 2007. [DOI] [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res 83: 29–36, 1990. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Oku N, Osaki Y, Kinoshita H, Imaizumi M, Yoshikawa T, Kimura Y, Kajimoto K, Sasagaki M, Kitagawa K, Hori M, Hatazawa J. Cerebral and cerebellar activation in power and precision grip movements: an H2 15O positron emission tomography study. J Cereb Blood Flow Metab 23: 1378–1382, 2003. [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103, 2002. [DOI] [PubMed] [Google Scholar]
- Theverapperuma LS, Hendrix CM, Mason CR, Ebner TJ. Finger movements during reach-to-grasp in the monkey: amplitude scaling of a temporal synergy. Exp Brain Res 169: 433–448, 2005. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Mastaglia FL. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res 121: 59–64, 1998. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Brochier T, Spinks RL, Lemon RN. Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J Neurophysiol 98: 488–501, 2007. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol 90: 3330–3340, 2003. [DOI] [PubMed] [Google Scholar]
- Wannier TM, Maier MA, Hepp-Reymond MC. Contrasting properties of monkey somatosensory and motor cortex neurons activated during the control of force in precision grip. J Neurophysiol 65: 572–589, 1991. [DOI] [PubMed] [Google Scholar]
- Ward NS, Swayne OB, Newton JM. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging 29: 1434–1446, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci 2: 1329–1345, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir PL, MacKenzie CL, Marteniuk RG, Cargoe SL. Is object texture a constraint on human prehension?: kinematic evidence. J Mot Behav 23: 205–210, 1991a. [DOI] [PubMed] [Google Scholar]
- Weir PL, MacKenzie CL, Marteniuk RG, Cargoe SL, Frazer MB. The effects of object weight on the kinematics of prehension. J Mot Behav 23: 192–204, 1991b. [DOI] [PubMed] [Google Scholar]
- Wing AM, Turton A, Fraser C. Grasp size and accuracy of approach in reaching. J Mot Behav 18: 245–260, 1986. [DOI] [PubMed] [Google Scholar]
- Winges SA, Weber DJ, Santello M. The role of vision on hand preshaping during reach to grasp. Exp Brain Res 152: 489–498, 2003. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. [DOI] [PubMed] [Google Scholar]
- Xiao J, Padoa-Schioppa C, Bizzi E. Neuronal correlates of movement dynamics in the dorsal and ventral premotor area in the monkey. Exp Brain Res 168: 106–119, 2006. [DOI] [PubMed] [Google Scholar]
- Zar JH Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1999.