Abstract
Central pain syndrome (CPS) is a debilitating condition that affects a large number of patients with a primary lesion or dysfunction in the CNS. Despite its discovery over a century ago, the pathophysiological processes underlying the development and maintenance of CPS are poorly understood. We recently demonstrated that activity in the posterior thalamus (PO) is tightly regulated by inhibitory inputs from zona incerta (ZI). Here we test the hypothesis that CPS is associated with abnormal inhibitory regulation of PO by ZI. We recorded single units from ZI and PO in animals with CPS resulting from spinal cord lesions. Consistent with our hypothesis, the spontaneous firing rate and somatosensory evoked responses of ZI neurons were lower in lesioned animals compared with sham-operated controls. In PO, neurons recorded from lesioned rats exhibited significantly higher spontaneous firing rates and greater responses to noxious and innocuous stimuli applied to the hindpaw and to the face. These changes were not associated with increased afferent drive from the spinal trigeminal nucleus or changes in the ventroposterior thalamus. Thus CPS can result from suppressed inputs from the inhibitory nucleus zona incerta to the posterior thalamus.
INTRODUCTION
Central pain is defined as “pain initiated or caused by a primary lesion or dysfunction in the CNS” (Merskey and Bogduk 1994). The diversity of clinical characteristics led to the designation of this condition as central pain syndrome (CPS). The pain is most often steady and unrelenting and has been described “as if knives heated in Hell's hottest corner were tearing me to pieces” (Head and Holmes 1911). It has no cure and is often resistant to conventional pharmacological treatment (Baastrup and Finnerup 2008). CPS can result from a variety of conditions, and these may produce lesions at any level along the spinal cord or the brain. The most common conditions are spinal cord injuries, multiple sclerosis (MS), and cerebrovascular lesions (stroke). The prevalence of CPS in these conditions is alarmingly high: a majority of spinal cord injury patients, almost 30% of MS patients, and nearly 10% of stroke patients suffer from CPS (Boivie 2005; Bonica 1991; Yezierski 2000).
Many of the earliest reported cases of CPS involved damage to the thalamus (Dejerine and Roussy 1906; Edinger 1891; Head and Holmes 1911). As a result, thalamic lesions were thought to be required for development of pain, and the syndrome was referred to for decades by the misleading term “thalamic pain.” Research since that time has established that CPS can result from damage to any structure along spino-thalamo-cortical pathways that convey pain and temperature information (Boivie 2005; Bowsher 1996; Finnerup et al. 2003; Kim et al. 2007; MacGowan et al. 1997; Peyron et al. 2000; Schmahmann and Leifer 1992).
Immediately following spinal cord injury or stroke, somatosensation, including pain, is reduced (hypoalgesia). In most patients, pain starts within a few weeks after the original insult and includes both increased pain with noxious stimulation (hyperalgesia) and pain in response to previously innocuous stimuli (allodynia). Perhaps most debilitating—and puzzling—is the presence, in the majority of patients, of spontaneous pain (Baliki et al. 2007; Boivie 2005; Greenspan et al. 2004; Tasker 1991). Spinal cord lesions typically produce particularly painful CPS symptoms with unremitting pain that can be diffuse and bilateral and may extend, “below-lesion,” to locations caudal to the spinal injury (Defrin et al. 2002a; Stormer et al. 1997; Yezierski 2000).
The delayed expression of CPS and the diffuse localization of painful symptoms suggest that the pathophysiology does not reflect only direct effects at the denervated spinal segments. Rather these features of CPS strongly suggest the occurrence of maladaptive plasticity in supraspinal structures at which inputs from various body parts converge.
One hypothesis that remains in favor, almost a century since it was first formulated, is that CPS results from abnormally suppressed inhibition in the thalamus (Head and Holmes 1911). Unfortunately, the consensus appears to end there, as there are conflicting hypotheses regarding the mechanisms and site of operation of this disinhibition (see Boivie 2005; Canavero and Bonicalzi 2007).
Here we demonstrate, in a rodent model of CPS (Wang and Thompson 2008), that central pain is associated with suppressed inputs from the inhibitory nucleus zona incerta to the posterior thalamus.
METHODS
Spinal lesions
All procedures were conducted in accordance with Animal Welfare Act regulations and PHS guidelines. Strict aseptic surgical procedures were used, in accordance with the guidelines of the International Association for the Study of Pain, and approved by the University of Maryland School of Medicine Animal Care and Use Committee. Thirty-four adult female Sprague-Dawley rats weighing 250–300 g were used. Animals were anesthetized with ketamine/xylazine (100/8 mg/kg ip), and the animals were placed on a thermoregulated heating pad. A laminectomy to expose the spinal cord was performed, and a quartz-insulated platinum electrode (5 μm tip) was targeted to the spinothalamic tract (STT), based on stereotaxic coordinates, on one side of the spinal cord. DC current (10 μA for 10 s, repeated 4 times) was passed through the electrode to produce an electrolytic lesion (∼0.6 mm3). Sham surgery was performed without performing laminectomy. The analgesic buprenorphine (0.05 mg/kg) was administered every 12 h for 24 h postoperatively (2 doses total).
Behavioral testing
Animals were tested on three consecutive days before the spinal lesion surgery, at day 3 postsurgery, at day 7 postsurgery, and at weekly intervals thereafter. To minimize the animals' anxiety, they were habituated for 2 wk before behavioral testing and were trained to stand upright with the forepaws on the experimenter's hand.
Hindpaw mechanical withdrawal thresholds
Calibrated von Frey filaments (Stoelting) were applied in ascending order to the hindpaw. We applied the filaments to the dorsal surface of the paws based on studies demonstrating that the dorsal approach more reliably and consistently detects threshold changes (Ren 1999). Mechanical withdrawal threshold was defined as the force at which the animal withdrew to three of the five stimuli delivered.
Face mechanical withdrawal thresholds
We tested face withdrawal thresholds in the same animals tested for hindpaw withdrawal and at the same time points described in the preceding text. von Frey filaments were applied in ascending order to the middle of the vibrissae pad between the second and third row of vibrissae. Mechanical withdrawal threshold was defined as the force at which the animal withdrew the face to three of the five stimuli delivered.
For both hindpaw and vibrissae withdrawal thresholds, Friedman test, followed by Mann Whitney U test (MWU), was used to compare pre- and postlesion data, and a P < 0.05 was considered significant. For all behavioral experiments, to determine the sample size, a power analysis was performed using α = 0.05 and power = 0.85. When behavioral testing was complete and animals showed significant hyperalgesia in the hindpaws and face (confirmed CPS), the animals were used for electrophysiological studies.
Cold withdrawal thresholds
Animals were tested for cold hyperalgesia 1 day before and 14 days after sham or lesion surgery. The animals were allowed to stand upright with their forepaws on the experimenter's hand, resting the ventral surface of either the ipsilateral or contralateral hindpaw on a thermoregulated cold plate (2°C). The experimenter—blind to the treatment—measured the latency to hindpaw withdrawal. The measurement was repeated three times for each hindpaw with ≥5 min between trials. MWU test was used to compare withdrawal latencies between animals receiving sham or lesion surgery at day 14. To compare between pre- and postsurgery withdrawal latencies, Kruskall-Wallis test was used, and P < 0.05 was considered significant. In preliminary experiments in control animals, we established that there is no significant adaptation in responses to these cold stimuli.
Reversal of mechanical hyperalgesia
Buprenorphine hydrochloride (Hospira) was administered (ip) at three different concentrations (10, 30, and 75 μg/kg) to rats with behaviorally confirmed mechanical hyperalgesia ≥14 days after spinal surgery. Twenty minutes after administration, mechanical withdrawal thresholds to von Frey filaments were assessed at the hindpaws and the vibrissae pad and compared with thresholds obtained before the administration of the drug. Behavioral testing was also repeated 24 h later to confirm that the effects of buprenorphine were reversible.
In vivo experiments
EXTRACELLULAR RECORDING.
At least 14 days after surgery, rats were anesthetized with urethan (1.5 g/kg ip) and prepared for extracellular recordings as previously described (Masri et al. 2006, 2008). We selected urethan as an anesthetic because it is the only anesthetic that has no, or negligible, effects on glutamatergic and GABAergic transmission and therefore produces only minimal disruption of signal transmission in the neocortex (Sceniak and Maciver 2006). With respect to the spinal lesion site, we recorded from the contralateral thalamus and from the ipsilateral spinal trigeminal nuclei. The recordings were obtained through quartz-insulated tungsten electrodes (2–4 MΩ). In the posterior thalamus (PO), we recorded from neurons with receptive fields in the vibrissae as determined by manual stimulation using a wooden probe; we also included in our analyses PO neurons with receptive fields in the vibrissae that received convergent inputs from the hindpaw. In ventral posteromedial thalamus (VPM), spinal trigeminal subnucleus caudalis (SpVc), and spinal trigeminal subnucleus interpolaris (SpVi), we recorded from neurons with receptive fields in the face, and in ventral posterolateral thalamus (VPL), we recorded from neurons with hindpaw receptive fields. We recorded from well-isolated units, digitized (40 kHz) the waveforms through a Plexon (Dallas, TX) data-acquisition system, and sorted units off-line with Plexon's off-line sorter, using dual thresholds and principal component analyses. We generated autocorrelograms with Neuroexplorer software (Littleton, MA) to confirm that we obtained recordings from single units.
MECHANICAL STIMULATION.
We recorded spontaneous activity and responses to a series of mechanical stimuli with forces that spanned the innocuous and noxious range (6–200 g, tip diameter = 0.8 mm) using an electronic von Frey anesthesiometer (IITC). Stimuli were applied (10 times each in a randomized order) to the receptive field of each recorded neuron, ipsilateral to the lesion site. We computed mean firing rate during spontaneous activity periods and during application of each level of mechanical stimulation. We used repeated-measures ANOVA followed by Dunnett's post hoc test to determine the lowest mechanical force that produced neuronal firing that differed significantly (P < 0.05) from spontaneous activity.
VIBRISSAE STIMULATION.
We stimulated vibrissae with air puffs delivered through a tube as in our previous studies (Masri et al. 2008). We exported time stamps of well-isolated units and of stimulus triggers to Matlab (MathWorks, Natick, MA) for analyses using custom-written algorithms. We constructed peristimulus time histograms (PSTHs, 1-ms bins) and defined significant stimulus-evoked responses as PSTH bins the response magnitude of which significantly exceeded (99% confidence interval) spontaneous activity levels, computed from a 200-ms period preceding the stimuli. We performed statistical analyses in STATA (StataCorp LP), and assessed, in individual neurons, changes occurring in sensory-evoked activity using the MWU test; P < 0.05 was considered significant.
BURST ANALYSIS.
We identified bursts of action potentials as clusters of at least three spikes with interspike intervals of ≤4 ms in which the first spike in the burst has a preceding interspike interval of ≥100 ms (Guido et al. 1995; Lu et al. 1992; Sherman 1996).
For identification of recording, stimulation, and spinal lesion sites, we marked recording sites by placing electrolytic lesions (5 μA for 10 s) at the end of each experiment, then deeply anesthetized the rats and perfused them transcardially with buffered saline followed by 4% buffered paraformaldehyde. We obtained coronal brain and spinal sections (70 μm thick) and Nissl-stained the sections to identify recording and lesion sites.
RESULTS
Animal model of central pain syndrome
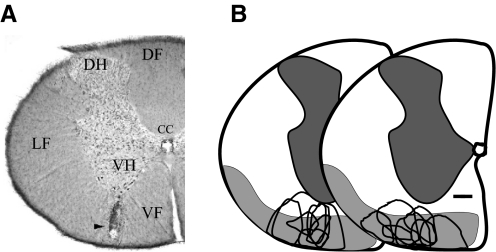
We and others have previously shown that CPS develops in rats following lesions of the spinal cord (Endo et al. 2008; Mills et al. 2001; Siddall et al. 1995; Wang and Thompson 2008). Because the spinothalamocortical system is affected in all central pain patients (see introduction), we experimentally produced lesions that included the STT. Rats (n = 18) received a single, unilateral electrolytic lesion in the anterolateral quadrant of the spinal cord at lower cervical to upper thoracic levels (Fig. 1A). In rats, STT afferents to medial and lateral thalamic nuclei travel in the ventral and ventrolateral funiculi of the spinal cord (Giesler et al. 1981). We therefore targeted our lesions to these spinal cord regions (Fig. 1B).
FIG. 1.
Spinal cord lesions. A: coronal section through the cervical spinal cord showing a representative lesion site (arrowhead) involving the spinothalamic tract (STT). B: a line drawing summarizing the location and size of spinal lesions in animals with mechanical hyperalgesia (unfilled areas, n = 18). Shaded areas represent the location of ascending STT axons, adapted from Fig. 5 in Giesler et al. (1981). CC, central canal; DH, dorsal horn; DF, dorsal funiculus; LF, lateral funiculus; VH, ventral horn; VF, ventral funiculus. Scale bar: 300 μm.
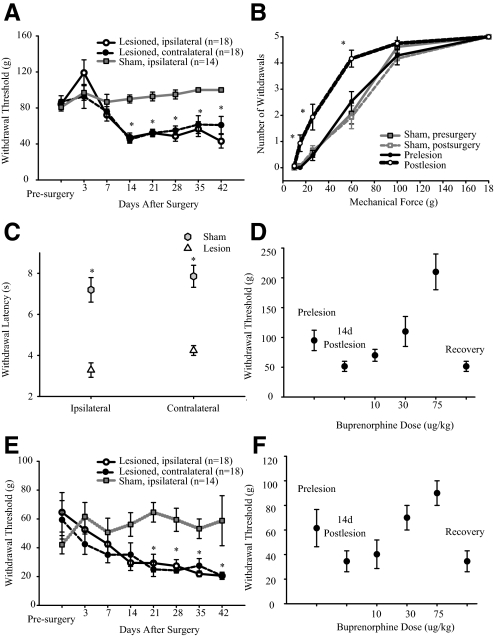
In spinal-lesioned animals—and in contrast to animals receiving sham surgery—hindpaw withdrawal thresholds to mechanical stimulation of the dorsal paw surface transiently increased (hypoalgesia) immediately after the lesion. Subsequently, significant reductions in thresholds (hyperalgesia) were evident bilaterally by 14 days postlesion (Fig. 2A). Mechanical thresholds decreased from 84 ± 22 to 47 ± 21 g (median: 83.4-60 g; P = 0.007, Friedman) on the ipsilateral hindpaw and from 86 ± 29 to 44 ± 17 g (median: 83.4-60; P = 0.007) on the contralateral hindpaw. The reduction in withdrawal threshold was evident in 94% (16/18) of spinal-lesioned animals and persisted for the duration of the experiments (≤42 days; Fig. 2A). Animals that did not develop hyperalgesia after spinal cord lesion were excluded from the study (n = 2). Sham surgery had no effect on mechanical withdrawal thresholds on both the ipsilateral (Fig. 2A) and the contralateral hindpaw (data not shown). Stimulus-response curves constructed from results obtained 14 days after surgery demonstrate a leftward shift in mechanical thresholds after spinal lesion (P < 0.02, MWU; Fig. 2B). The transient hypoalgesia seen immediately after lesion surgery, followed by bilateral hyperalgesia, is consistent with findings in human patients suffering from CPS following spinal injury (Boivie 2005; Bowsher 1995; Finnerup et al. 2003).
FIG. 2.
Behavioral assessment of spinal-lesioned and sham-operated animals. A: hindpaw (dorsal surface) mechanical withdrawal thresholds decrease over time and hyperalgesia develops bilaterally after spinal lesions. B: stimulus-response curves collected before and 14 days after surgery. C: animals with spinal lesions responded at significantly shorter latencies to cold stimuli applied to the dorsal surface of both hindpaws. D: systemic administration of the opiodergic drug buprenorphine hydrochloride, 14 days after spinal lesions, resulted in a dose-dependent reversal of ipsilateral hindpaw hyperalgesia in spinal-lesioned animals (n = 4). Data obtained from the contralateral hindpaw show similar reversal of hyperalgesia. E: mechanical hyperalgesia also develops bilaterally on the vibrissae pad. F: vibrissae pad mechanical hyperalgesia is reversed in a dose-dependent manner by systemic administration of opioidergic drug 14 days after spinal lesions (n = 4). Data obtained from the contralateral vibrissaae pad show similar reversal of hyperalgesia. All values means ± SE; *, statistically significant difference, P < 0.05.
We also tested responses to cold stimuli (see methods) because these stimuli reliably and selectively activate nociceptors and because cold hyperalgesia is a common complaint of CPS patients (Defrin et al. 2002b; Finnerup et al. 2003). Compared with sham operated control animals (n = 6), animals with spinal lesions (n = 7) displayed significantly lower withdrawal thresholds to cold stimuli applied to either hindpaw (Fig. 2C). Thresholds ipsilateral to the lesion site were sham: mean = 7.2 ± 1.5 s, median = 7.0; lesioned: mean = 3.3 ± 0.9 s, median = 3.3; P = 0.003, MWU. Thresholds contralateral to the lesion site were sham: mean = 7.9 ± 1.3 s, median = 7.9; lesion: mean = 4.2 ± 0.6 s, median = 4.3; P = 0.003, MWU; Fig. 2C. Withdrawal thresholds to cold stimuli were also significantly different between pre- and postsurgery in animals with spinal lesions (presurgery ipsilateral hindpaw: mean = 6.8 ± 1.1 s, median = 7; P = 0.009; presurgery contralateral: mean = 7.4 ± 1.1 s, median = 7; P = 0.008, Kruskall-Wallis test), but there was no difference between pre and post surgery values in animals receiving sham surgery (presurgery ipsilateral hindpaw: mean = 7.1 ± 1.0 s, median = 7; P = 0.7; presurgery contralateral: mean = 7.4 ± 1.1 s, median = 7; P = 0.8, Kruskall-Wallis test).
Histological analyses revealed that lesions at any level between C6 and T9 produced similar CPS-like behavioral effects, consistent with findings in humans that CPS can result from lesions anywhere along the spinothalamocortical pathway (see preceding text). As with lesions in CPS patients, our experimental lesions are unlikely to completely ablate or selectively involve the STT. Rather, as in humans, we find that lesions that involve the STT result in CPS. A summary of the location and size of all lesion sites is shown in Fig. 1B. In all animals with behaviorally confirmed CPS, the lesions affected parts of the STT.
To confirm that withdrawal from mechanical stimuli reflects a response to painful stimuli, we administered buprenorphine—a centrally acting opiate—to four animals with confirmed hyperalgesia. This resulted in a dose-dependent reversal in the reduction of hindpaw withdrawal thresholds on the ipsilateral hindpaw (Fig. 2D). Data obtained from the contralateral hindpaw show similar reversal of hyperalgesia. The effects of buprenorphine were reversible and withdrawal thresholds returned to preadministration levels at subsequent tests. Thus as in humans with CPS, centrally acting opiates can acutely reverse hyperalgesia, although opiates offer no long-term benefit to these patients (Attal et al. 2002; Eide et al. 1995).
Signs of central pain were not limited to the hindpaws as hyperalgesia extended to include the face. Figure 2E shows that mechanical thresholds for face withdrawal began to decrease 3 days after surgery and were significantly lower than prelesion values at 21 days postlesion (ipsilateral: 29 ± 18 g, median = 26, P = 0.007, Friedman; contralateral: 25 ± 15 g, median 26, P = 0.007, Friedman). Similar to its effects on hindpaw hyperalgesia, buprenorphine reversed the reduction in ipsilateral face withdrawal thresholds in spinal-lesioned animals in a dose-dependent, reversible manner (Fig. 2F). Data obtained from the contralateral face show similar reversal of hyperalgesia.
Consistent with previous reports in experimental animals with CPS (Defrin et al. 2002a; Stormer et al. 1997; Yezierski 2000), these findings demonstrate that animals with spinal lesions present with diffuse, bilateral mechanical hyperalgesia that involves dermatomes below and above the lesion site. Furthermore, spinal-lesioned animals are significantly hyperalgesic to cold stimuli ipsilateral to the lesion site. Therefore this model of central pain recapitulates clinical characteristics of CPS, as described in the preceding text, and allowed us to directly test the mechanisms responsible for CPS.
Mechanisms of CPS
As discussed in the preceding text, the delayed expression of CPS and the diffuse localization of painful symptoms suggest that the pathophysiology does not reflect only direct effects at the denervated spinal segments. Rather these features of CPS strongly suggest the occurrence of maladaptive plasticity in supraspinal structures at which inputs from various body parts converge. Here we test the hypothesis that one of the structures in which maladaptive plasticity occurs is the medial thalamus, whose activity is regulated by inhibitory inputs from the zona incerta (ZI).
Suppressed neuronal activity in ZI
The ZI is aptly named: the function of this “zone of uncertainty,” situated ventral to the thalamus, has been debated since Auguste Forel first described it (Forel 1877). ZI receives dense nociceptive inputs through the STT (Craig 2004; Shammah-Lagnado et al. 1985) and has been implicated in a variety of pain-related functions (Porro et al. 2003; Yen et al. 1989). A striking feature of ZI is its target specificity (Bartho et al. 2002; Mitrofanis 2005): In all sensory systems, it provides inhibitory inputs exclusively to “higher-order” thalamic nuclei (e.g., posterior nucleus in the somatosensory system and the inferior pulvinar in the visual system). ZI afferents avoid first-order thalamic nuclei (e.g., ventroposterior in the somatosensory system and the lateral geniculate in the visual system).
ZI sends a dense GABAergic projection on the posterior nucleus of the thalamus (PO) (Bartho et al. 2002; Power et al. 1999), a nucleus critically involved in nociceptive processing (Apkarian and Shi 1994; Casey 1966; Poggio and Mountcastle 1960; Zhang and Giesler 2005). We recently showed that ZI exerts potent feed-forward and tonic inhibition to PO neurons (Trageser and Keller 2004; Trageser et al. 2006; also see Lavallee et al. 2005). Because of these properties of ZI, we hypothesized that responses in ZI are affected in animals with behaviorally confirmed CPS. To test this hypothesis, we recorded spontaneous and stimulus-evoked activity from well-isolated neurons in the ventro-lateral sector of ZI (n = 17 from spinal-lesioned rats and n = 18 from sham-operated controls). We have recently shown that essentially all cells in this sector are inhibitory neurons that project to PO (Trageser et al. 2006). We tested responses of ZI neurons to stimulation of the mystacial vibrissae—the whiskers on rats' face—because of the large somatotopic representation of the vibrissae in ZI (Nicolelis et al. 1992), because of the reliability in which controlled stimuli can be applied, and because of the presence of lesion-induced hyperalgesia in the vibrissae pad.
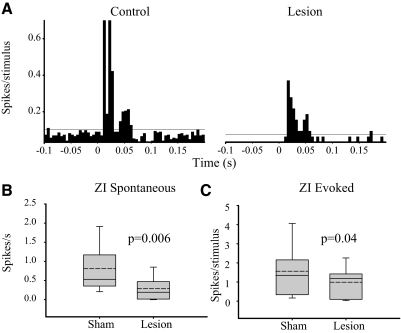
Figure 3A compares activity recorded from a ZI neuron in a sham-operated rat (control) with activity recorded from a rat with a spinal lesion and behaviorally confirmed hyperalgesia. In the neuron recorded from the spinal-lesioned rat, the spontaneous firing rate (1.6 Hz) is noticeably lower than in the neuron from the sham-operated rat (12 Hz). The magnitude of the vibrissae-evoked response in the spinal-lesioned rat (0.04 spike/stimulus) is also markedly lower than in the sham-operated control (1.5 spike/stimulus). As a group, ZI neurons from spinal-lesioned rats have significantly lower spontaneous firing rates (0.29 ± 0.28 vs. 0.80 ± 0.70 Hz in shams, P = 0.006, Fig. 3B), and lower vibrissae-evoked responses (1.0 ± 0.7 spike/stimulus vs. 1.4 ± 1.3 spike/stimulus in shams, P = 0.04, Fig. 3C). There was no significant difference in onset latency of ZI neurons between CPS animals and sham operated controls (sham: mean = 8.5 ± 2.7 ms, median = 8; CPS: mean = 9.8 ± 2.0 ms, median = 9, P = 0.9, MWU).
FIG. 3.
Spinal lesions result in suppression of spontaneous and sensory-evoked responses of zona incerta (ZI) neurons. A: peristimulus time histogram (PSTH, 1-ms bins) demonstrate that spontaneous and sensory-evoked activity in a ZI neuron recorded from a spinal-lesioned rat are markedly lower than in a control neuron. Horizontal lines represent 99% confidence interval. Group data demonstrate significant decreases in spontaneous in animals with central pain syndrome (CCPS, n = 17) when compared with sham-operated controls (n = 18; B) and stimulus-evoked (C) activity of ZI neurons from rats with CPS (n = 17) when compared with sham-operated controls (n = 18).
Abnormally high neuronal activity in PO
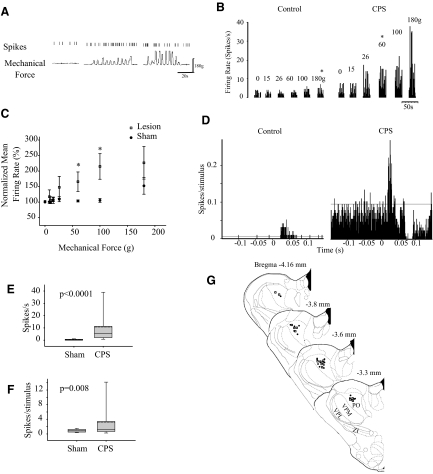
Because ZI normally exerts potent tonic and feed-forward inhibition on PO (see preceding text), we postulated that suppressed ZI activity would result in abnormally high activity in PO. To test this hypothesis, we recorded spontaneous and stimulus-evoked responses from PO neurons in spinal-lesioned rats with confirmed mechanical hyperalgesia (animals with CPS, n = 15) and from sham-operated controls (n = 20). In CPS animals, 12 of the 15 (80%) PO neurons recorded received convergent inputs from both the hindpaw and vibrissae pad. In sham-operated controls, 10 of 20 (50%) PO neurons recorded possessed receptive fields in both the hindpaw and vibrissae pad. Recordings from a representative PO neuron from an animal with CPS are shown in Fig. 4A. We applied mechanical stimuli to the dorsal surface of the hindpaw, ipsilateral to the lesion site, with the use of an electronic anesthesiometer; force traces are shown at the bottom. Note that this neuron responds with a larger number of spikes to increasing mechanical forces. The relationship between stimulus intensity and firing rate is shown in Fig. 4B for PO neurons from a sham-operated rat (“control”) and from a spinal-lesioned rat with confirmed CPS (“CPS”). The control PO neuron fired spontaneously at low rates that were not significantly modulated by mechanical stimuli until the force applied exceeded 180 g (P = 0.019, ANOVA); this force was identical to the one required to evoke a withdrawal response from the same animal during behavioral test sessions. In contrast, the PO neuron recorded from the rat with CPS exhibited significantly higher spontaneous activity and responded to increasing mechanical forces with increasing firing rates. The threshold for significant responses (60 g, P < 0.0001) corresponded to the behavioral withdrawal threshold, and both behavioral and electrophysiological thresholds were markedly lower than in the control animal.
FIG. 4.
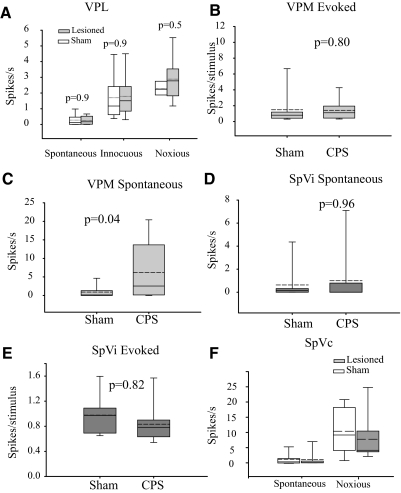
Neuronal activity in posterior thalamus (PO) is enhanced in animals with central pain. A: representative example of responses recorded from a PO neuron in an animal with CPS. Time stamps of action potentials (top trace) recorded during spontaneous activity and during application (randomized order) of various mechanical forces (bottom trace) to the dorsal surface of the hindpaw, using an electronic anesthesiometer. B: in a PO neuron from a sham-operated control, spontaneous firing rate is low, and responses significantly exceed this spontaneous activity level only when strong stimuli (>180 g) are applied. This threshold is identical to the behavioral withdrawal threshold in this animal. In a neuron from the spinal-lesioned animal with confirmed CPS, spontaneous activity is higher, and both electrophysiological and behavioral thresholds are considerably lower (60 g). C: group data showing that the activity of PO neurons is significantly higher in animals with CPS (n = 12) in response to mechanical hindpaw stimulation when compared with sham-operated controls (n = 10). D: the activity of PO neurons in animals with CPS (n = 15) is also higher in response to vibrissae stimulation when compared with sham operated controls (n = 20). PSTHs computed for PO neurons recorded from a sham-operated and a spinal-lesioned animal. Note the large increase in spontaneous and evoked activity. Horizontal lines represent 99% confidence interval. Spontaneous activity (E) and vibrissae-evoked activity (F) are enhanced in PO neurons recorded from spinal-lesioned animals. G: location of PO neurons recorded from sham operated controls (n = 20, •) and CPS animals (n = 15, □). Drawings were modified from the Paxinos and Watson atlas (Paxinos and Watson 1998). *, statistically significant difference, P < 0.05.
Stimulus-response curves constructed from neuronal responses to mechanical stimuli applied to the hindpaw show a significant leftward shift for spinal-lesioned animals compared with shams, consistent with the leftward shift observed for the behavioral responses (sham threshold = 180 g; CPS threshold = 60 g, Fig. 4C). As a group, spontaneous activity in PO neurons of animals with CPS was ∼30-fold higher than in sham-operated animals (sham: mean = 0.37 ± 0.40 Hz, median = 0.2, n = 12; CPS: mean = 10.7 ± 16.6 Hz, median = 5.6, n = 15; P < 0.0001, MWU, Fig. 4E). In all cases, the force required to elicit a significant change from baseline (spontaneous) neuronal firing correlated with the force needed to elicit paw withdrawal in both sham-operated and spinal-lesioned animals.
To test if behavioral hyperalgesia in the face is also reflected in abnormal PO activity, we recorded, in the same set of neurons, responses to stimulation of the vibrissae, using air puffs delivered to the vibrissae pad. The location of all recorded PO neurons is shown in Fig. 4G. Peristimulus histograms (PSTHs) were constructed for each cell to compute response magnitude. Typical of PO neurons in control animals (Diamond et al. 1992b; Lavallee et al. 2005; Trageser and Keller 2004), the unit depicted in Fig. 4D (control) had a long onset latency (21 ms) and exhibited low spontaneous activity and low-magnitude responses to vibrissae stimuli. In contrast, the unit recorded from an animal with CPS responded robustly to vibrissae stimuli and had a markedly high spontaneous firing rate. As a group, evoked response magnitudes of PO neurons from spinal-lesioned animals were significantly higher than those of control animals (sham: mean = 0.86 ± 0.39 spikes/stimulus, median = 0.60; CPS: mean = 3.2 ± 5.6, median = 1.2, P = 0.008, MWU, Fig. 4F). There was no significant difference in onset latency between CPS animals and sham operated controls (sham: mean = 16.7 ± 6.5 ms, median = 14; CPS: mean = 17.0 ± 7.1 ms, median = 14; P = 0.9, MWU).
Taken together, these results demonstrate that spinal lesions produce abnormally high spontaneous firing and enhanced PO responses to stimuli from dermatomes both below and above the lesion site. The finding that thresholds for PO responses decrease in parallel with hindpaw withdrawal thresholds suggest that the two phenomena are causally related, and that changes in PO responses may be responsible for behavioral hyperalgesia.
Neuronal activity in the VP thalamus
As described in the preceding text, ZI specifically targets “higher-order” thalamic nuclei, while avoiding “first-order” thalamic nuclei such as the somatosensory VPM and the VPL (Bartho et al. 2002). Because VPM and VPL activity is not regulated by ZI inputs, we predicted that activity in these nuclei would not be affected in animals with CPS.
To test this prediction, we first recorded from VPL neurons that respond to hindpaw stimulation (n = 12 each from spinal-lesioned rats and sham-operated controls). An innocuous mechanical stimulus (50-g force, which does not evoke a hindpaw withdrawal; see preceding text) evoked responses with similar magnitudes in cells from both control and lesioned rats (sham: 1.70 ± 1.39 Hz, median = 1.18; CPS: 1.79 ± 1.32 Hz, median = 1.5; P = 0.9, MWU, Fig. 5A). Similarly, a noxious mechanical stimulus (120 g) evoked similar responses in both groups of animals (sham: 2.28 ± 0.43 Hz, median = 0.12; CPS: 2.87 ± 1.31 Hz, median = 0.17; P = 0.5). There was also no difference between spontaneous firing rates of neurons recorded from spinal-lesioned animals and those of sham-operated controls (sham: 0.28 ± 0.35 Hz; CPS: 0.26 ± 0.26 Hz; P = 0.9 MWU, Fig. 5A). The finding that activity in VPL neurons was unaffected in CPS is consistent with the hypothesis that suppressed inhibitory inputs from ZI to PO is causally related to CPS.
FIG. 5.
A: spontaneous and sensory evoked responses of neurons in ventral posterolateral thalamus (VPL) are not significantly different between sham-operated (n = 12) controls and animals with CPS (n = 12). B: sensory-evoked responses in VPM are not significantly different between sham (n = 10) and animals with CPS (n = 13). C: in posteromedial thalamus (VPM), spontaneous activity was higher in animals with CPS, compared with controls. In spinal trigeminal subnucleus interpolaris (SpVi), there were no significant differences between CPS (n = 11) and sham operated controls (n = 10) in either sensory evoked responses (D) or spontaneous activity (E). F: the activity of neurons in SpVc is not significantly different between sham-operated controls (n = 15) and animals with CPS (n = 17).
We also recorded from VPM neurons that respond to innocuous vibrissae stimulation (n = 10 from spinal-lesioned rats and n = 13 from sham-operated controls). Consistent with our prediction, group comparisons revealed no significant difference in the magnitude of evoked responses in VPM (sham: 1.4 ± 2.0 spike/stimulus, median = 1.0; CPS: 1.1 ± 1.3 spike/stimulus, median = 0.4; P = 0.8, MWU, Fig. 5B). There was, however, a modest increase in spontaneous firing rates in neurons recorded from spinal-lesioned animals (sham: 1.4 ± 2.2 Hz, median = 0.2; CPS: 6.2 ± 7.5 Hz, median = 2.6; P = 0.04, MWU, Fig. 5C). (Compare the 30-fold increase in spontaneous activity of PO neurons with the 4-fold increase in VPM.) We are currently exploring the hypothesis that the increase in spontaneous activity of VPM neurons reflects changes in corticothalamic activity. Nevertheless, the finding that evoked activity in VPM neurons was unaffected in CPS is consistent with our hypothesis that abnormalities in ZI are responsible for the development of CPS.
Burst activity in thalamus
It has been suggested previously that CPS, in both humans and animal models, is associated with abnormally high incidence of bursting activity in lemniscal thalamic nuclei (Lee et al. 2005; Lenz et al. 1989; Vierck et al. 1990; Weng et al. 2003; but see Dostrovsky 2007). We therefore reasoned that the increased spontaneous activity in VPM might be due to an increase in the incidence of burst firing in this nucleus, as proposed previously for VPL (Wang and Thompson 2008). To test this prediction, we analyzed the incidence of bursts during spontaneous activity and in response to vibrissae stimuli. Criteria for defining a burst in extracellular recordings are described in methods. In spinal-lesioned rats, there was a large and significant increase (P = 0.02, χ2 test) in the percentage of VPM neurons that emitted bursts of three or more action potentials (sham: 23%; CPS: 70%). However, in bursting cells, the mean frequency of bursts was only marginally, and not significantly (P = 0.06, MWU), higher in spinal-lesioned rats (sham: mean = 0.02 ± 0.05 Hz, median = 0; CPS: mean = 0.04 ± 0.05 Hz, median = 0.02). Further, the percentage of VPM neurons that respond with bursts to vibrissae stimulation was not significantly different between the two groups (sham: 44%, CPS: 38%; P = 0.44, χ2 test). Nor was there a difference in the frequency of stimulus-evoked bursts between sham-operated and spinal-lesioned animals (sham: mean = 0.01 ± 0.03 Hz, median = 0; CPS: mean = 0.03 ± 0.06 Hz, median = 0.002, P = 0.5, MWU).
We performed similar analyses of spike bursts on PO neurons, and found no significant difference in either the percentage of spontaneously bursting cells (sham: 50%; CPS: 47%; P = 0.90, χ2 test) or in the frequency of spontaneous bursts (sham: mean = 0.04 ± 0.08 Hz, median = 0.002; CPS: mean = 0.04 ± 0.06 Hz, median = 0, P = 0.80, MWU). Similarly, neither the percentage of cells that burst in response to vibrissae stimuli (sham: 40%; CPS: 53%; P = 0.30, χ2 test), nor the incidence of evoked bursts (sham: mean = 0.02 ± 0.04 Hz, median = 0; CPS: mean = 0.02 ± 0.04 Hz, median = 0.004, P = 0.5, MWU), are significantly different in rats with CPS.
These findings indicate that the incidence of spike bursts—as defined in this study—are only marginally affected in VPM neurons and remain unchanged in VPL and PO neurons. Thus our findings do not resolve the current controversy regarding the causal role of spike bursts in CPS (see Dostrovsky 2007).
Brain stem activity unaffected in CPS
The changes in activity of PO neurons in CPS animals may reflect an increase in peripheral afferent input, rather than—or in addition to—a reduction in inhibition from ZI. To test this possibility, we recorded from the spinal trigeminal nucleus (SpV), the main source of afferent inputs to PO (Veinante et al. 2000). Spontaneous firing rates of vibrissae responsive neurons in the SpVi were not significantly different between neurons recorded from sham-operated and spinal-lesioned animals (sham: mean = 0.68 ± 1.8 Hz, median = 0.17, n = 11; CPS: mean = 0.99 ± 2.1 Hz, median = 0, n = 10; P = 0.96, MWU, Fig. 5D). There was also no significant difference in the magnitude of response to vibrissae stimuli (sham: mean = 2.27 ± 2.07 spike/stimulus, median = 1.47; CPS: mean = 2.03 ± 1.18 spike/stimulus, median = 1.65; P = 0.82, MWU, Fig. 5E).
To investigate if changes in PO reflect peripheral changes in nociceptive afferents from the face, we recorded from neurons in the SpVc. Spontaneous firing rates of neurons in SpVc of spinal-lesioned animals were not significantly different from neurons recorded from sham-operated controls (sham: mean = 1.8 ± 2.5 Hz, median = 0.5, n = 12; CPS: mean = 2.3 ± 5.0 Hz, median = 0.3, n = 13; P = 0.65, MWU, Fig. 5F). In addition, responses of these neurons to noxious mechanical stimuli applied to the face were not significantly different between the two groups (sham: mean = 10.4 ± 7.2 spike/stimulus, median = 8.4; CPS: mean = 10.5 ± 11.3 spike/stimulus, median = 4.0; P = 0.53, MWU, Fig. 5F). These findings indicate that changes in PO activity in spinal-lesioned rats are not due to increased noxious or innocuous afferent inputs from SpVi or SpVc.
DISCUSSION
The rat model used in our study (Wang and Thompson 2008) recapitulates several diagnostic signs of CPS: immediately following lesions that involve the STT, the rats display transient mechanical hypoalgesia below and unilateral to the lesion, and, within 2–3 wk, they developed bilateral hyperalgesia below the lesion. In addition, cold hyperalgesia appeared within 14 days in the hindpaw ipsilateral to the lesion. The time course of these changes, the presence of bilateral mechanical hyperalgesia, and the reversal of hyperalgesia by a centrally acting opiate, are all consistent with pathophysiology in supraspinal structures.
In humans, CPS is most commonly expressed with hyperalgesia below and at the level of the spinal lesion and only rarely in spinal segments above the lesion (Canavero and Bonicalzi 2007; Tasker et al. 1982). In contrast, our spinal lesions resulted in hyperalgesia that extended to the face, and the appearance, in PO, of neurons with receptive fields that included both the limbs and the face. These differences might reflect evolutionary differences in the functional organization and innervation of the thalamus in rodents and primates (Jones 2007). All rats that developed CPS had lesions within the STT, consistent with the obligatory role of insults to the spinothalamocortical pathway in the pathophysiology of CPS (Canavero and Bonicalzi 2007). It is important to note that the spinal lesions affected only a fraction of the STT (Fig. 1), such that noxious stimuli could be relayed to brain centers via the spared fibers.
Our overarching hypothesis was that CPS can result from abnormal inhibitory inputs from ZI to PO (the incerto-thalamic pathway). Consistent with this hypothesis, we found a significant suppression of both spontaneous and evoked activity in inhibitory neurons in ZI and abnormally high spontaneous and evoked activity of neurons in PO in animals with CPS. The positive association between behavioral and neurophysiological thresholds in rats with CPS is consistent with a causal role for suppressed incerto-thalamic inputs in CPS.
Reduced inhibition in PO
A role for the incerto-thalamic pathway in CPS is also consistent with our previous findings demonstrating that in normal rats, ZI regulates both spontaneous and evoked activity of PO neurons (Trageser and Keller 2004; Trageser et al. 2006; see also Lavallee et al. 2005). Along with an increase in spontaneous and evoked activity of PO neurons, we previously reported that inactivating ZI results in reduced response latency in a subset of PO neurons (Trageser and Keller 2004). In the present study, we did not observe an effect on response latency of PO neurons. This suggests that the effects of spinal lesions on incerto-thalamic inhibition are less pronounced than those produced by direct, large lesions of the ZI.
We considered the possibility that there is a reduction in other inhibitory inputs to PO. There are no GABAergic interneurons within PO (Barbaresi et al. 1986) and, therefore all GABAergic inhibition is mediated by extrinsic afferents. An important source of these afferents is the GABAergic reticular nucleus of the thalamus (TRN), which has been hypothesized to play a role in CPS (Boivie 2005; Foix et al. 1922). TRN does not receive ascending sensory inputs, and its major source of excitatory input is from somatosensory cortex (Liu and Jones 1999). Further, GABAergic terminals in PO that originate from ZI differ from those of TRN origin by their larger size, the presence of multiple release sites, and multiple filamentous contacts, all features suggesting that ZI exerts significantly more potent inhibition on PO (Bartho et al. 2002; Bokor et al. 2005). Moreover, we find that responses evoked with innocuous stimuli in VPM and VPL—nuclei that receive inhibition exclusively from TRN—are unaffected by spinal lesions, which argues against a role for TRN in our model of CPS.
The anterior pretectal nucleus (APT) also sends dense GABAergic inputs exclusively to higher-order thalamic nuclei, and it too can regulate the activity of PO neurons (Bokor et al. 2005). Further, both ZI and APT receive dense nociceptive inputs through the STT (Apkarian and Hodge 1989; Craig 2004; Shaw and Mitrofanis 2001), and both ZI and APT have been implicated in a variety of pain-related functions (Porro et al. 2003; Villarreal and Prado 2007; Villarreal et al. 2004; Yen et al. 1989). These findings suggest that APT might also be involved in the pathogenesis of CPS. Therefore we consider ZI/APT the most parsimonious candidates providing inhibitory regulation of PO. We are currently exploring the prediction that APT activity is also suppressed in CPS.
Additional CPS mechanisms
In addition to PO, ZI and APT innervate higher-order thalamic nuclei that have been traditionally implicated in nociceptive processing, including the medial dorsal nucleus, the central median nucleus, nucleus submedius, and the parafasicular nucleus (Bushnell and Duncan 1989; Craig and Burton 1981; Dostrovsky and Guilbaud 1990; Power and Mitrofanis 2002). It is therefore likely that neuronal activity in these nuclei is also enhanced in animals with CPS, a prediction we are currently testing. Together with PO, these nuclei project to cortical areas associated with the experience of pain (reviewed in Jones 2007), and abnormalities in their neuronal activity—caused by reduced inhibition from ZI/APT—may play an integral part in the development or maintenance of CPS.
Abnormalities in PO activity may also result from trans-synaptic changes to the membrane properties of these neurons, such as changes in the expression of channels or ionic pumps. In dorsal horn neurons of animals with neuropathic pain, such changes resulted in the reversal of normally inhibitory anionic currents into excitatory currents (Coull et al. 2003). Similar changes in PO neurons might explain the disinhibition we observed. The absence of changes in response latency might be attributed to the presence of shunting inhibition, which would remain unaffected by the postulated anionic disturbances.
Recordings of field potentials in brain slices taken from animals with CPS after spinal lesions reveal that bursting discharges are evoked in VPL in response to local electrical stimulation (Wang and Thompson 2008). The absence of abnormal VPL responses in the present experiments might be explained by differences in the nature of the stimulus or by the urethan anesthetic. Further experiments are planned to test these possibilities.
ZI also projects to the somatosensory cortex (Nicolelis et al. 1995). ZI projections on the neocortex are sparse in adult animals, and they preferentially target cortical layer I (Dammerman et al. 2000). Thus cortical influences on PO are unlikely to be affected by suppression of ZI input to the cortex. Superior colliculus, and other structures inhibited by ZI/APT, provide relatively sparse inputs to PO, and are not thought to significantly shape its response properties (Ling et al. 1997).
It is possible that enhanced PO responses in CPS are due to enhanced excitatory inputs rather than or in addition to reduced inhibition. The main sources of excitation to PO are the spinal trigeminal nuclei, the spinal dorsal horn, and the somatosensory cortex (Diamond et al. 1992a; Pierret et al. 2000; Zhang and Giesler 2005). We found that neuronal responses in SpVi and SpVc were not affected by spinal lesions and thus are unlikely to contribute to changes in PO. We did not directly test the contribution of peripheral inputs from the dorsal horn or of cortical inputs to PO, and thus their involvement in CPS remains a possibility. Similarly, the contribution of “modulatory” inputs, such as cholinergic or serotonergic afferents, cannot be excluded (Varela and Sherman 2007). However, these spinal, cortical, and modulatory inputs also project on the VPM and VPL nuclei, and evoked responses in these nuclei remain unchanged in animals with CPS. Therefore we conclude that reduced inhibition from ZI/APT on PO and related nuclei is an important mechanism in this model of CPS.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-051799 and NS-31078 to A. Keller, NS-055896 to S. M. Thompson, and F32-NS-064775 to R. L. Quiton. Support was provided also by a UMB Center for Persistent Pain grant to R. Masri and by the Christopher and Dana Reeve Foundation to A. Keller.
Acknowledgments
We are indebted to Drs. R. Dubner, J. D. Greenspan, and G. Schoenbaum for valuable discussions and suggestions.
REFERENCES
- Apkarian AV, Hodge CJ. Primate spinothalamic pathways. III. Thalamic terminations of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 288: 493–511, 1989. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 14: 6779–6795, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Guirimand F, Brasseur L, Gaude V, Chauvin M, Bouhassira D. Effects of IV morphine in central pain: a randomized placebo-controlled study. Neurology 58: 554–563, 2002. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs 22: 455–475, 2008. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Spontaneous pain and brain activity in neuropathic pain: functional MRI and pharmacologic functional MRI studies. Curr Pain Headache Rep 11: 171–177, 2007. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382: 305–326, 1986. [DOI] [PubMed] [Google Scholar]
- Bartho P, Freund TF, Acsady L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci 16: 999–1014, 2002. [DOI] [PubMed] [Google Scholar]
- Boivie J Central pain. In: Wall and Melzack's Textbook of Pain, edited by McMahon S, Koltzenburg M. Oxford, UK: Churchill Livingstone, 2005, p 1057–1074.
- Bokor H, Frere SG, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsady L. Selective GABAergic control of higher-order thalamic relays. Neuron 45: 929–940, 2005. [DOI] [PubMed] [Google Scholar]
- Bonica JJ History of pain concepts and pain therapy. Mt Sinai J Med 58: 191–202, 1991. [PubMed] [Google Scholar]
- Bowsher D Central pain. Pain Rev 2: 175–186, 1995. [Google Scholar]
- Bowsher D Central pain: clinical and physiological characteristics. J Neurol Neurosurg Psychiatry 61: 62–69, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 78: 415–418, 1989. [DOI] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V. Central Pain Syndrome: Pathophysiology, Diagnosis and Management. New York: Cambridge Univ Press, 2007.
- Casey KL Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29: 727–750, 1966. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938–942, 2003. [DOI] [PubMed] [Google Scholar]
- Craig AD Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477: 119–148, 2004. [DOI] [PubMed] [Google Scholar]
- Craig ADJ, Burton H. Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol 45: 443–466, 1981. [DOI] [PubMed] [Google Scholar]
- Dammerman RS, Flint AC, Noctor S, Kriegstein AR. An excitatory GABAergic plexus in developing neocortical layer 1. J Neurophysiol 84: 428–434, 2000. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Pain following spinal cord injury. Spinal Cord 40: 96–97; author reply 98–99, 2002a. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain 125: 501–510, 2002b. [DOI] [PubMed] [Google Scholar]
- Dejerine J, Roussy G. Le syndrome thalamique. Rev Neurol 15: 521–532, 1906. [Google Scholar]
- Diamond ME, Armstrong-James M, Budway MJ, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol 319: 66–84, 1992a. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol 318: 462–476, 1992b. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO The thalamus and human pain. In: Central Neuropathic Pain: Focus on Poststroke Pain, edited by Henry JL, Panju A, Yashpal K. Seattle: IASP Press, 2007, p. 101–112.
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain 40: 93–104, 1990. [DOI] [PubMed] [Google Scholar]
- Edinger L Giebt es central entstehende Schmerzen? Dtsch Z Nervenheilkd 1:262–282, 1891. [Google Scholar]
- Eide PK, Stubhaug A, Stenehjem AE. Central dysesthesia pain after traumatic spinal cord injury is dependent on N-methyl-d-aspartate receptor activation. Neurosurgery 37: 1080–1087, 1995. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain 138: 292–300, 2008. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory function in spinal cord injury patients with and without central pain. Brain 126: 57–70, 2003. [DOI] [PubMed] [Google Scholar]
- Foix C, Thevenard A, Nicolesco M. Algie faciale dórigine bulbo-trigéminale au cours de la syringomyélie. Troubles sympathiques concomitants. Douleur á type cellulaire. Rev Neurol 29: 990–999, 1922. [Google Scholar]
- Forel A Untersuchungen über die haubenregion und ihre oberen verknüpfungen im gehirne des menschen und einiger saügethiere, mit beiträgen zu den methoden der gehirnuntersuchung. Arch Psychiat Nervenkr 7:393–495, 1877. [Google Scholar]
- Giesler GJJ, Spiel HR, Willis WD. Organization of spinothalamic tract axons within the rat spinal cord. J Comp Neurol 195: 243–252, 1981. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Ohara S, Sarlani E, Lenz FA. Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals. Pain 109: 357–366, 2004. [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Vaughan JW, Godwin DW, Sherman SM. Receiver operating characteristic (ROC) analysis of neurons in the cat's lateral geniculate nucleus during tonic and burst response mode. Vis Neurosci 12: 723–741, 1995. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 34: 102–254, 1911. [Google Scholar]
- Jones EG The Thalamus. Cambridge, MA: Cambridge Univ. Press, 2007.
- Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci 27: 4995–5004, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallee P, Urbain N, Dufresne C, Bokor H, Acsady L, Deschenês M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci 25: 7489–7498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol 94: 1676–1687, 2005. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496: 357–360, 1989. [DOI] [PubMed] [Google Scholar]
- Ling CY, Schneider GE, Northmore D, Jhaveri S. Afferents from the colliculus, cortex, and retina have distinct terminal morphologies in the lateral posterior thalamic nucleus. J Comp Neurol 388: 467–483, 1997. [PubMed] [Google Scholar]
- Liu XB, Jones EG. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J Comp Neurol 414: 67–79, 1999. [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J Neurophysiol 68: 2185–298, 1992. [DOI] [PubMed] [Google Scholar]
- MacGowan DJ, Janal MN, Clark WC, Wharton RN, Lazar RM, Sacco RL, Mohr JP. Central poststroke pain and Wallenberg's lateral medullary infarction: frequency, character, and determinants in 63 patients. Neurology 49: 120–125, 1997. [DOI] [PubMed] [Google Scholar]
- Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J Neurophysiol 100: 681–689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R, Trageser JC, Bezdudnaya T, Li Y, Keller A. Cholinergic regulation of the posterior medial thalamic nucleus. J Neurophysiol 96: 2265–2273, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of Chronic Pain. Seattle: IASP Press, 1994.
- Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma 18: 1091–1105, 2001. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience 130: 1–15, 2005. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brain stem. Brain Res 577: 134–141, 1992. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Development of direct GABAergic projections from the zona incerta to the somatosensory cortex of the rat. Neuroscience 65: 609–631, 1995. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic, 1998.
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 30: 263–288, 2000. [DOI] [PubMed] [Google Scholar]
- Pierret T, Lavallee P, Deschenês M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J Neurosci 20: 7455–7462, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio GF, Mountcastle VB. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Central nervous mechanisms in pain. Bull Johns Hopkins Hosp 106: 266–316, 1960. [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Lui F, Giuliani D, Pellegrini M, Baraldi P. Independent time courses of supraspinal nociceptive activity and spinally mediated behavior during tonic pain. Pain 104: 291–301, 2003. [DOI] [PubMed] [Google Scholar]
- Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J Comp Neurol 404: 554–565, 1999. [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Ultrastructure of afferents from the zona incerta to the posterior and parafascicular thalamic nuclei of rats. J Comp Neurol 451: 33–44, 2002. [DOI] [PubMed] [Google Scholar]
- Ren K An improved method for assessing mechanical allodynia in the rat. Physiol Behav 67: 711–716, 1999. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethan on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865–3874, 2006. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomic correlates. Arch Neurol 49: 1032–1037, 1992. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Negrao N, Ricardo JA. Afferent connections of the zona incerta: a horseradish peroxidase study in the rat. Neuroscience 15: 109–134, 1985. [DOI] [PubMed] [Google Scholar]
- Shaw VE, Mitrofanis J. Lamination of spinal cells projecting to the zona incerta of rats. J Neurocytol 30: 695–704, 2001. [DOI] [PubMed] [Google Scholar]
- Sherman SM Dual response modes in lateral geniculate neurons: mechanisms and functions. Vis Neurosci 13: 205–213, 1996. [DOI] [PubMed] [Google Scholar]
- Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport 6: 1241–1244, 1995. [DOI] [PubMed] [Google Scholar]
- Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, Aldinger W, Walker N, Zimmermann M, Paeslack V. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord 35: 446–455, 1997. [DOI] [PubMed] [Google Scholar]
- Tasker RR Meralgia paresthetica. J Neurosurg 75:168, 1991. [DOI] [PubMed] [Google Scholar]
- Tasker RR, Organ LW, Hawrylyshyn PA. The thalamus and Midbrain of Man: A Physiological Atlas Using Electrical Stimulation. Springfield, IL: C.C. Thomas, 1982.
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol 96: 1456–1463, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci 24: 8911–8915, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol 98: 3538–3547, 2007. [DOI] [PubMed] [Google Scholar]
- Veinante P, Jacquin MF, Deschenês M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol 420: 233–243, 2000. [DOI] [PubMed] [Google Scholar]
- Vierck CJJ, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci 10: 2077–2095, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal CF, Kina VA, Prado WA. Antinociception induced by stimulating the anterior pretectal nucleus in two models of pain in rats. Clin Exp Pharmacol Physiol 31: 608–613, 2004. [DOI] [PubMed] [Google Scholar]
- Villarreal CF, Prado WA. Modulation of persistent nociceptive inputs in the anterior pretectal nucleus of the rat. Pain 132: 42–52, 2007. [DOI] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci 28: 11959–11969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HR, Lenz FA, Vierck C, Dougherty PM. Physiological changes in primate somatosensory thalamus induced by deafferentation are dependent on the spinal funiculi that are sectioned and time following injury. Neuroscience 116: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- Yen CT, Fu TC, Chen RC. Distribution of thalamic nociceptive neurons activated from the tail of the rat. Brain Res 498: 118–122, 1989. [DOI] [PubMed] [Google Scholar]
- Yezierski RP Pain following spinal cord injury: pathophysiology and central mechanisms. Prog Brain Res 129: 429–449, 2000. [DOI] [PubMed] [Google Scholar]
- Zhang X, Giesler GJJ. Response characteristics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol 93: 2552–2564, 2005. [DOI] [PubMed] [Google Scholar]