Abstract
One of the first steps in the acquisition of a new motor skill is the formation of motor memories. Here we tested the capacity of transcranial DC stimulation (tDCS) applied over the motor cortex during motor practice to increase motor memory formation and retention. Nine healthy individuals underwent a crossover transcranial magnetic stimulation (TMS) study designed to test motor memory formation resulting from training. Anodal tDCS elicited an increase in the magnitude and duration of motor memories in a polarity-specific manner, as reflected by changes in the kinematic characteristics of TMS-evoked movements after anodal, but not cathodal or sham stimulation. This effect was present only when training and stimulation were associated and mediated by a differential modulation of corticomotor excitability of the involved muscles. These results indicate that anodal brain polarization can enhance the initial formation and retention of a new motor memory resulting from training. These processes may be the underlying mechanisms by which tDCS enhances motor learning.
INTRODUCTION
The ability to learn an unlimited repertoire of motor skills is one of the main characteristics of human beings. Recent studies have suggested that the primary motor cortex (M1) is crucially involved in this ability by forming and retaining short-term memory representations of recently practiced movements (Classen et al. 1998; Hadipour-Niktarash et al. 2007; Molina-Luna et al. 2008; Muellbacher et al. 2002). In particular, it has been shown that although disruption of M1 activity can interfere with retention of practiced motor tasks (Hadipour-Niktarash et al. 2007; Muellbacher et al. 2002), enhancing M1 excitability with transcranial DC stimulation can improve it (Reis et al. 2009). However, it is not clear whether this beneficial effect on retention is mediated by a larger amount of motor memory formation or changes in retention mechanisms.
One of the initial steps in the acquisition of new complex motor skills is the formation of motor memories (Classen et al. 1998; Muellbacher et al. 2002). Classen et al. (1998) used transcranial magnetic stimulation (TMS) over M1 to ascertain how simple motor memories are formed. In this paradigm, the preferred direction of TMS-evoked thumb movements is measured before and after performance of motor training. In this manner, it is possible to observe that training results in changes in the direction of TMS-evoked movements, indicative of formation of new motor memories containing the kinematic details of the practiced motions retrieved by the magnetic stimulation. This phenomenon is mediated by N-methyl-d-aspartate (NMDA), muscarinic, adrenergic and GABAergic neurotransmission (Butefisch et al. 2000; Sawaki et al. 2002b, 2003) and can be enhanced by dopaminergic (Floel et al. 2005a,b) and adrenergic agents (Butefisch et al. 2002; Sawaki et al. 2002a), d-amphetamine, congruent action observation (Celnik et al. 2006; Stefan et al. 2008), and hebbian-type noninvasive stimulation (Butefisch et al. 2004).
Transcranial DC stimulation (tDCS) is a noninvasive, painless cortical stimulation technique (Nitsche and Paulus 2000, 2001; Nitsche et al. 2003a,b, 2005). Whereas anodal tDCS results in increased cortical excitability without direct neuronal depolarization, cathodal tDCS decreases excitability (Nitsche and Paulus 2000; Nitsche et al. 2003; Purpura and McMurtry 1965). Interestingly, anodal tDCS appears to have a facilitatory effect on different forms of learning in healthy individuals (Antal et al. 2004; Floel et al. 2008; Kincses et al. 2004; Nitsche et al. 2003c; Reis et al. 2009) and in stroke (Hummel et al. 2005) and Parkinson patients (Boggio et al. 2006; for a review see Wu et al. 2007). However, it is not clearly understood what specific process mediates the enhanced learning when tDCS is applied during training. For instance, it is possible that the performance improvement described after learning a motor task under the influence of tDCS is mediated by increased memory formation, stronger retention of the formed memories, or better recall. Antal et al. (2004) and Nitsche et al. (2003c) previously showed performance improvements during early learning, which suggests that tDCS may influence memory formation. In this study, we used a well-established paradigm that assesses the formation and retention of a motor memory without engaging voluntary recall to test the hypothesis that anodal tDCS over M1 enhances these processes relative to sham stimulation. In addition, in three separate controls we determine 1) the polarity specificity of the effects by testing cathodal stimulation, 2) the longevity of the effects by periodically reassessing the effects of stimulation and training, and 3) the association between motor practice and stimulation by testing the effects of anodal tDCS without training.
METHODS
Subjects
Nine right-handed (self-assessed) healthy individuals with no history of neurological or psychiatric conditions (mean ± SD: 30 ± 9 yr old, six females) participated in the study. All subjects denied the use of acute or chronic CNS-acting medication, drinking alcohol in the previous 24 h, and reported to have had ≥5 h of sleep the previous night. All subjects signed informed consent approved by Johns Hopkins Medical Institution Institutional Review Board and in accordance with the Declaration of Helsinki.
Experimental design
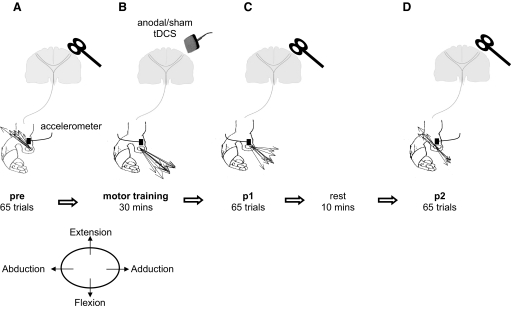
All subjects participated in two randomly ordered sessions separated by ≥5 days and designed to evaluate the effect of anodal tDCS on the encoding of motor memories (Fig. 1) (Classen et al. 1998).
FIG. 1.
Schematic representation of the main experiment setup. A: pre. Transcranial magnetic stimulation (TMS)–evoked movement directions were derived from the first-peak acceleration in the 2 major axes of the movement (extension/flexion and abduction/adduction) measured by an accelerometer mounted on the proximal phalanx of the thumb. Black arrows indicate the direction of individual TMS-evoked thumb movements (in this case extension and abduction). B: motor training. Pre was followed in 2 separate sessions randomly ordered by 30 min of motor training with either sham or anodal transcranial DC stimulation (tDCS) being applied over the left primary motor cortex (M1). Voluntary thumb movements were performed in a direction opposite to the baseline TMS-evoked movement direction (in this case: flexion and adduction). C: post 1 (p1). The direction of TMS-evoked thumb movements was determined as previously measured in pre. D: post 2 (p2). The subjects rested for 10 min and the direction of TMS-evoked thumb movements was again determined.
RECORDINGS AND STIMULATION PROCEDURES.
Subjects sat comfortably in a chair while the right forearm was restrained in a semipronated position with a molded cast with the four fingers in a slightly extended position and the thumb left entirely free. Using a two-dimensional accelerometer (Kistler Instruments, Amherst, NY) placed on the proximal phalanx of the thumb, movement directions were determined and calculated from the first peak acceleration vector in both the vertical axis (extension/flexion) and the horizontal axis (adduction/adduction) (Fig. 1) (Classen et al. 1998). Electromyographic (EMG) activity was recorded through pairs of disposable electrodes placed over the right extensor pollicis brevis and right flexor pollicis brevis muscles. EMG signals were recorded, amplified, and filtered (bandwidth 5 Hz to 1 kHz) with a Viking IVP (Nicolet, Viasys Healthcare). All signals were sampled at 2 kHz, visually displayed on-line, and stored for off-line analysis using a custom LabVIEW program (National Instruments, Austin, TX).
TRANSCRANIAL MAGNETIC STIMULATION.
Transcranial magnetic stimulation (TMS) was delivered using a 70-mm loop-diameter figure-of-eight coil (Magstim BiStim2, Whitland, South West Wales, UK) placed over the left M1 to optimally activate the corticospinal tract and elicit focal isolated and directionally consistent thumb movements. Using a frameless neuronavigation system (BrainSight, Rogue Research, Montreal, Quebec, Canada) we coregistered the subjects' heads to a standard magnetic resonance image and marked this “hot spot.” We decided to use BrainSight because this is an accurate way to ensure the TMS coil remains in the same position within the X, Y, and Z directions during and between each session. Resting motor threshold for the muscle agonist to the training (see following text) was determined at this position and defined as the minimum TMS intensity that evoked motor-evoked potentials (MEPs) of 50 μV in ≥5 of 10 trials (Rossini et al. 1994). Muscle relaxation was monitored by visual feedback of the EMG recording.
TRANSCRANIAL DC STIMULATION.
Transcranial DC stimulation (tDCS) was delivered through two sponge electrodes (surface area: 25 cm2) embedded in a saline-soaked solution. Depending on the session, the anode or cathode was positioned on the marked motor “hot spot” and the other electrode on the skin overlying the contralateral supraorbital region. During training, 1-mA anodal tDCS was delivered for about 30 min in the corresponding session and ≤30 s in the sham session using a Phoresor ΙΙ Auto (Model PM850; IOMED, Salt Lake City, UT). At the onset and offset of tDCS, the current was increased or decreased in a ramplike fashion, a method shown to achieve good blinding level (Gandiga 2006).
EXPERIMENTAL PROCEDURE.
The formation of a motor memory was assessed using a previously described protocol (Butefisch et al. 2000; Celnik et al. 2006; Classen et al. 1998; Stefan et al. 2008). At the beginning of each session, 65 TMS stimuli were delivered at 0.1 Hz over the hot spot to determine the baseline TMS-evoked thumb movement direction (Pre; Fig. 1). Subjects subsequently performed motor training, consisting of voluntary thumb brisk movements paced by an audio metronome at 1 Hz for 30 min in a direction opposite to the baseline TMS-evoked direction. For example, if the principal baseline direction was extension and abduction, then subjects were instructed to perform repetitive flexion and adduction movements (Figs. 1 and 2). The participants were instructed to relax and let the thumb return to its original position after each motion. This was ensured by monitoring on-line EMG and acceleration signals and providing verbal feedback when needed. Consistency and accuracy of training movements were quantified off-line by calculating the angular variance and compound acceleration of the first peak acceleration vector (Table 1) (Stefan et al. 2008).
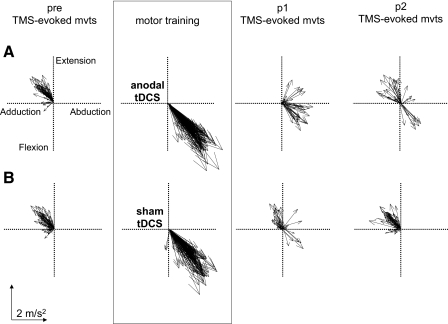
FIG. 2.
Intervention-dependent changes in TMS-evoked thumb movement directions in a representative subject. Each line represents the first-peak acceleration direction vector of a single thumb movement. The pre block is characterized by predominantly extension/adduction movement directions in both sessions. A: after motor training with anodal tDCS the direction of TMS-evoked thumb movements (p1) changed to a direction similar to training (flexion/abduction). This was partially maintained in p2. B: after training with sham tDCS the direction of TMS-evoked thumb movements within p1 did not have such a dramatic change with only a small proportion of movements moving in a direction similar to motor training. For p2 all movements were in a direction similar to the pre block.
TABLE 1.
Baseline measures
| Parameter | Sham | Anodal | Paired t-Test: Sham × Anodal | |||
|---|---|---|---|---|---|---|
| A. Psychological measures | ||||||
| Attention, VAS | 4.7 ± 0.4 | 4.6 ± 0.4 | P = 0.5, t = 0.7 | |||
| Fatigue, VAS | 2.3 ± 0.6 | 2.8 ± 0.7 | P = 0.5, t = 0.6 | |||
| Pain, VAS | 1.4 ± 0.2 | 2.0 ± 0.6 | P = 0.38, t = 0.9 | |||
| B. Motor training kinematics | ||||||
| Compound acceleration, m/s2 | 1.07 ± 0.13 | 1.06 ± 0.23 | P = 0.6, t = 0.5 | |||
| Angular variance, deg | 17.2 ± 2.0 | 21.0 ± 3.0 | P = 0.26, t = 1.2 | |||
| C. Measures of corticomotor excitability preceding interventions | ||||||
| Motor threshold (agonist muscle), % | 45.0 ± 2.0 | 43.0 ± 2.0 | P = 0.13, t = 1.6 | |||
| MEPantagonist, mV | 2.9 ± 0.7 | 3.1 ± 0.6 | P = 0.9, t = 0.14 | |||
| MEPagonist, mV | 1.3 ± 0.4 | 1.4 ± 0.4 | P = 0.9, t = 0.05 | |||
Psychological measures. Values (means±SE) depict subject's choice in a visual analog scale (VAS), where 1 represents poorest attention, maximal fatigue and pain, and 7 represents maximal attention, least fatigue, and least pain. Motor training kinematics. Units (means±SE) are in meters per second squared for compound acceleration and degrees for angular variance. Measures of corticomotor excitability preceding interventions. Units (means±SE) are the percentage of stimulator's output for motor threshold and millivolts for MEP amplitudes elicited in muscles acting as antagonist and agonist to the trained thumb movements. P and t values originate from paired t-tests performed on the sham and anodal sessions.
In the main experiment, while subjects performed the motor training, one of two interventions were delivered in separate sessions: 1) anodal tDCS applied over the active M1 for a total of 30 min and 2) sham tDCS applied in an identical manner to the anodal session except that stimulation lasted for only 30 s at the beginning of training.
At the end of each intervention, 65 TMS-evoked thumb movement directions were determined again as done at baseline for about 11 min [post 1 (p1)]. Then, subjects rested for 10 min and another 65 TMS pulses were applied [post 2 (p2); Fig. 1].
At the end of each session, subjects reported their alertness, attention, and perceived pain of tDCS using a self-scored visual analog scale (VAS), where 1 represents poorest attention, maximal fatigue, and maximal pain and 7 represents maximal attention, least fatigue, and least pain (Table 1) (Stefan et al. 2005).
Controls
DURATION OF MOTOR MEMORIES.
To assess the longevity of the effects observed with anodal tDCS, three subjects that took part in the main experiment were exposed to an additional block of TMS 50 min after the cessation of anodal tDCS and training (p3).
SPECIFICITY OF POLARITY EFFECTS ON MOTOR TRAINING.
To determine whether tDCS effects were polarity specific, three subjects who completed the main experiment underwent a third session where training was performed under the influence of cathodal tDCS applied over the active M1 as done in the anodal session.
COMBINATION OF TRAINING AND TDCS.
To test whether stimulation alone could elicit changes in TMS-evoked movement directions, three additional subjects (two females, mean age 24 ± 3 yr) were tested using a similar experimental protocol, although they received anodal tDCS for 30 min without performance of motor training.
Data analysis
TRAINING TARGET ZONE.
We defined a training target zone (TTZ) as a window of ±20° centered on the mean direction of the performed training movements (Butefisch et al. 2000; Celnik et al. 2006; Stefan et al. 2008). The percentage of TMS-evoked thumb movements falling within the TTZ, the primary outcome measure, was calculated for pre, p1, and p2.
TMS-EVOKED MOVEMENT DIRECTION DISTANCE RELATIVE TO TRAINING DIRECTION.
For each session we calculated the mean angular direction of the training movements. Then, the angular direction of each individual TMS-evoked movement was subtracted from this mean value (Classen et al. 1998). For example, if the mean training direction was 320° and a movement in pre had an angular direction of 150° then the deviation from training would be 170° (Fig. 1). These values were averaged for each subject for pre, p1, and p2.
COMPOUND ACCELERATION VECTOR.
The mean magnitude of the first-peak acceleration in the extension/flexion direction during pre, p1, and p2 was calculated (Stefan et al. 2008). Since the principal training direction differed across subjects, we inverted the values so that all subjects had an extension movement within the pre block (Stefan et al. 2008).
CORTICOMOTOR EXCITABILITY.
We calculated the MEP amplitudes in muscles acting as either agonist or antagonist of the trained movement direction for each subject. Amplitudes were measured as peak-to-peak for each individual MEP and then averaged for pre, p1, and p2. Ratios between post and pre amplitudes for the agonist and antagonist muscles were calculated.
Statistical analysis
Separate repeated-measures ANOVAs (ANOVARM) were used for the percentage of movements falling in TTZ, deviation from the training direction, and compound acceleration with factors block (pre, p1, p2) and session (sham, anodal). ANOVARM was also used for the MEP ratio with factors muscle (agonist, antagonist), block (p1, p2), and session (sham, anodal). Measures of baseline corticomotor excitability (motor threshold, TMS stimulus intensity, and MEPagonist and MEPantagonist amplitudes), attention, fatigue, pain of tDCS, and training movement kinematics (angular variance and compound acceleration) were analyzed with separate paired t-tests. When significant differences were found, post hoc analysis was performed using paired t-test. Data are expressed as means ± SE and effects were considered significant if P ≤ 0.05.
RESULTS
Summary
At baseline, TMS-evoked movements were consistent across sessions. Subjects were then trained in the opposite direction for 30 min (Fig. 2). Both, anodal and sham sessions showed training-induced effects where the proportion of TMS-evoked movements reflected the practiced movement direction. However, this effect was more prominent in the anodal session immediately after the training (p1) and lasted longer (p2) relative to sham (Fig. 2).
Training parameters
Subjects' ratings of attention, fatigue, and pain were comparable across sessions [separate paired t-tests: t(8) ≥ 0.9, P ≤ 0.38; Table 1]. For the sham session five of nine subjects believed they had received real stimulation and seven of nine subjects felt that way in the anodal session. Motor training kinematics measured as compound acceleration and angular variance did not differ across sessions [separate paired t-test: t(8) ≤ 1.2, P ≥ 0.13; Table 1]. Preceding interventions, motor thresholds, and both MEPantagonist and MEPagonist amplitudes were comparable across sessions [separate paired t-test: t(8) ≤ 1.6, P ≥ 0.13; Table 1].
Effects of tDCS on motor memory formation
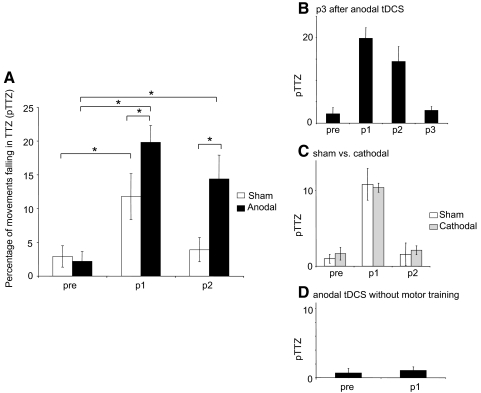
ANOVARM revealed a significant main effect for block [F(2,16) = 21.7, P < 0.001], session [F(1,8) = 16, P = 0.004], and block × session interaction [F(2,16) = 3.6, P = 0.05] for the percentage of movements falling within TTZ, the primary outcome measure. Paired t-tests showed that in both sham and anodal sessions there was a significant increase in the percentage of movements falling in the TTZ from pre to p1 [from 2 ± 1 to 12 ± 4% in sham, t(8) = 2.9, P = 0.02; and from 2 ± 1 to 17 ± 4% in anodal, t(8) = 7.4, P < 0.001; Fig. 3 A]. However, only in the anodal session was this effect significantly maintained at p2 [11 ± 4%, t(8) = 2.9, P = 0.002; Fig. 3A]. In addition, compared with the sham the anodal session had significantly more movements falling in the TTZ within p1 [t(8) = 2.6, P = 0.015] and p2 [t(8) = 3.6, P = 0.004].
FIG. 3.
Percentage of movements falling in the training target zone (pTTZ). A: with anodal tDCS applied during training there was a significant increase in movements falling within the TTZ during p1 and p2. Asterisks denote P ≤ 0.02. B: 50 min after the cessation of anodal tDCS (p3) TMS-evoked movement directions returned to premotor training values (n = 3). C: TMS-evoked movement directions were similar between sham and cathodal sessions at p1 and p2 times (n = 3). D: application of anodal tDCS for 30 min without motor training did not elicit changes in pTTZ. Data are means ± SE.
TMS-evoked movement direction distance relative to training direction
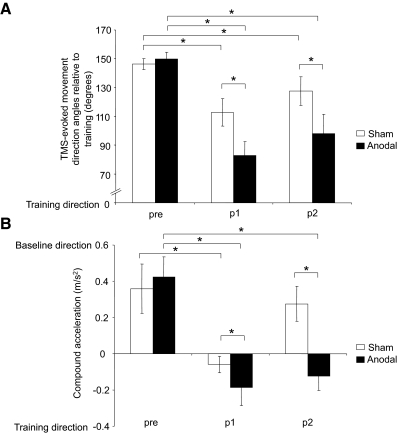
ANOVARM revealed a significant main effect for block [F(2,16) = 23.6, P < 0.001], session [F(1,8) = 16.8, P = 0.003], and block × session interaction [F(2,16) = 5.4, P = 0.016]. Post hoc paired t-tests showed that both sham and anodal sessions had a significant decrease in the relative angular distance from pre to p1 and p2 [sham: pre (146 ± 4°) to p1 (112 ± 9°) and p2 (128 ± 10°), t(8) = 4, P = 0.002, t(8) = 2.3, P = 0.03; and anodal: pre (150 ± 4°) to p1 (83 ± 10°) and p2 (98 ± 14°), t(8) = 8.4, P < 0.001, t(8) = 4.6, P = 0.001; Fig. 4 A]. However, the TMS-evoked movement directions in the anodal intervention were significantly closer to the training direction than sham during p1 [t(8) = 3.4, P = 0.005] and p2 [t(8) = 2.7, P = 0.02; Fig. 4A].
FIG. 4.
Angular distance and compound acceleration of TMS-evoked movements. A: angular distance of TMS-evoked movements relative to training during pre, p1, and p2. In comparison to sham, anodal tDCS led to a greater reduction in the angular distance between training movements and those evoked by TMS at p1 and p2 times. Please note that at pre, both sessions have similar angular difference from the trained direction. B: average compound acceleration during pre, p1, and p2. Anodal tDCS led to a greater change in direction of compound acceleration during p1 and p2 relative to sham. Asterisks, P ≤ 0.03. Data are means ± SE.
Compound acceleration vector
ANOVARM revealed a significant main effect for block [F(2,16) = 10, P = 0.001], session [F(1,8) = 5.4, P = 0.05], and block × session interaction [F(2,16) = 6, P = 0.01]. Paired t-tests revealed that in both sham and anodal sessions there was a significant change in direction of compound acceleration from pre to p1 [from pre (0.36 ± 0.14 m/s2) to p1 (−0.06 ± 0.05 m/s2) in sham, t(8) = 2.5, P = 0.02; and from pre (0.42 ± 0.11 m/s2) to p1 (−0.19 ± 0.1 m/s2) in anodal, t(8) = 3.6, P = 0.003; Fig. 4B]. However, only in the anodal session was this effect significantly maintained at p2 [−0.12 ± 0.08 m/s2, t(8) = 3, P = 0.009; Fig. 4B]. In addition, there was a significantly greater change in compound acceleration vector in the anodal session for p1 and p2 relative to sham [t(8) = 2.4, P = 0.02, t(8) = 2.7, P = 0.02, respectively; Fig. 4B].
Corticomuscular excitability
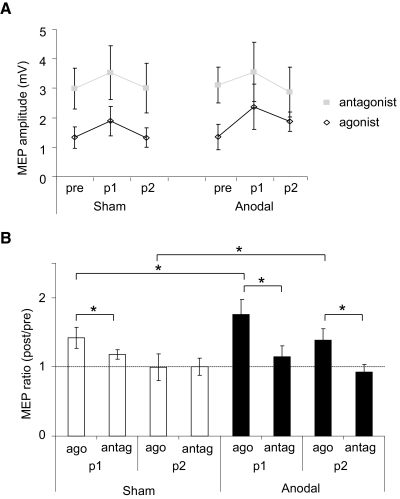
ANOVARM revealed a significant main effect of session [F(1,8) = 7.1, P = 0.03], block [F(1,8) = 20, P = 0.002], and muscle [F(1,8) = 41, P < 0.001] in assessment of MEP amplitudes. In addition, there were significant interactions between session and muscle [F(1,8) = 10.2, P = 0.01] and block and muscle [F(1,8) = 7, P = 0.03]. Paired t-tests showed that within the sham session there was a significant difference between the increase of the MEPagonist ratio (post/pre) (1.4 ± 0.15) compared with the MEPantagonist ratio (1.18 ± 0.07) for p1 only [t(8) = 2.4, P = 0.02; Fig. 5 B], whereas in the anodal session there was a significant difference at p1 [MEPagonist (1.8 ± 0.2), MEPantagonist (1.14 ± 0.16), t(8) = 5.5, P = 0.002] and p2 measures] MEPagonist (1.38 ± 0.17), MEPantagonist (0.93 ± 0.11), t(8) = 2.8, P = 0.04; Fig. 5B]. In addition, relative to sham the anodal session showed a significantly greater increase in the MEPagonist ratio for p1 [t(8) = 2, P = 0.05] and p2 [t(8) = 2, P = 0.05].
FIG. 5.
Corticomotor excitability, measured by the motor-evoked potential (MEP) amplitude for the agonist and antagonist muscles involved in motor training. A: changes in the MEP amplitude recorded from the training antagonist (closed squares) and agonist (open diamonds) muscles. MEP amplitudes increased with both sham and anodal tDCS. B: MEP amplitude ratio (post/pre) significantly increased in the MEPagonist muscle compared with the MEPantagonist during p1 for both sham and anodal tDCS. For anodal tDCS this difference was maintained during p2. For both p1 and p2, anodal tDCS resulted in a greater increase in the MEPagonist ratio. Asterisks, P ≤ 0.05. Data are means ± SE.
Control results
Only the anodal group showed a clear training effect during p2, suggesting that the motor memory had been retained for a longer period (∼30 min). To test whether this effect was still present 50 min after the cessation of training, three subjects were given an additional block of TMS (p3, Fig. 3B). The percentage of movements falling in the TTZ were not significantly different between pre and p3 [2.2 ± 2 and 3 ± 1%, respectively; paired t-test: t(2) = 0.6, P = 0.6]. Similarly, at p3 there were no significant differences in MEPagonist and MEPantagonist ratios [1.1 ± 0.03 and 0.9 ± 0.08 mV, respectively; paired t-test: t(2) = 2.1, P = 0.16], indicating a return to similar excitability to the pre measure.
To determine whether the previously described anodal tDCS effects were polarity specific, three subjects participated in a third session in which cathodal stimulation was applied over M1 during training. Here, the percentage of movements falling in the TTZ were not significantly different from sham stimulation in either p1 or p2 [cathodal: 10.4 ± 0.6 and 2.1 ± 0.6%, respectively, ANOVARM: session: F(1,2) = 0.3, P = 0.3; block: F(1,2) = 53, P = 0.001; session × block F(2,4) = 0.6 P = 0.6; Fig. 3C]. MEP ratios were also statistically similar across sham and cathodal sessions for p1 [MEPagonist (1.39 ± 0.03), MEPantagonist (1.1 ± 0.09)] and p2 measures [MEPagonist (1.0 ± 0.04), MEPantagonist (1.0 ± 0.1); ANOVARM: session: F(1,2) = 0.3, P = 0.66; block: F(1,2) = 23, P = 0.04; muscle: F(1,2) = 50, P = 0.019; all interactions: F(1,2) ≤ 5.6, P ≥ 0.14].
Finally, to determine whether anodal tDCS without training could elicit changes in TMS-evoked movement directions we studied an additional group of three subjects. In this group there was no significant difference in the percentage of movements falling in the TTZ between pre (1 ± 0.7%) and p1 [1 ± 0.5%, paired t-test: t(2) = 0.5, P = 0.7; Fig. 3D]. Similarly, there was no significant difference between MEPagonist and MEPantagonist muscle ratios [1.09 ± 0.02 and 1.1 ± 0.07, respectively; paired t-test: t(2) = 0.3, P = 0.8].
DISCUSSION
The main finding of the present study was that anodal transcranial DC stimulation (tDCS) over the primary motor cortex, engaged in generating the training movements, enhanced the encoding and retention of motor memories. This effect was reflected by 1) changes in all kinematic measures (movements falling in the training zone, angular differences, and compound muscle accelerations), 2) longer-lasting effects relative to training alone, 3) the required association of training and stimulation, and 4) the polarity specificity.
Previous studies have shown that performing simple motor training results in the formation of motor memories containing kinematic details of the practiced motions (Butefisch et al. 2002, 2004; Celnik et al. 2006; Classen et al. 1998; Floel et al. 2005a,b; Stefan et al. 2008). Consistent with these, we found that motor training with sham tDCS increased the probability of TMS-evoked movements falling in the TTZ, decreased the net TMS-evoked movement direction distance relative to the training direction, and changed toward the direction of the practiced movements the net acceleration of all TMS-evoked thumb movements. However, when the same group of participants performed the motor training under the influence of anodal tDCS they experienced larger gains in all measures in p1 and maintained the effects into p2. These findings appear to be polarity specific, since training during cathodal tDCS resulted in effects similar to those during training with sham and required the association of physical practice with anodal stimulation because tDCS alone did not result in any kinematic change.
Since subjects performing the training combined with anodal tDCS have persistence of the effects 30 min after the completion of training (p2)—longer motor memory retention—we decided to investigate the longevity of the motor memory formed. With this aim, we retested a subgroup of participants 50 min after the cessation of training and stimulation (p3). Across all measures, performance returned to pretraining values, indicating that any changes within the motor cortex representation had reverted to baseline. This type of motor memory encoding enhancement has been previously observed with d-amphetamine (Butefisch et al. 2001; Sawaki et al. 2002a), hebbian-type TMS stimulation during training (Butefisch et al. 2004), and congruent action observation (Celnik et al. 2006; Stefan et al. 2008). Of note, because ratings of attention, fatigue, and pain of the tDCS were similar across sessions, it is unlikely that these results could directly be explained by unspecific psychological differences across sessions.
Similar to previous studies, we found that training resulted in a specific increase in the excitability of the training muscles (Butefisch et al. 2004; Celnik et al. 2006; Classen et al. 1998). Interestingly, anodal tDCS increased the excitability of the muscle agonist to the training without a similar change in the antagonist muscle, suggesting that tDCS had a training-specific effect rather than a global increase in corticomotor excitability. These results, together with the findings that the no-training plus tDCS control group had minimal changes in the excitability of either muscle, suggest that the effect of tDCS on cortical excitability is more prominent on neuronal populations engaged in motor actions.
Cathodal tDCS, although known to reduce the excitability of the motor cortex at rest (Nitsche et al. 2003a; Purpura and McMurtry 1965), in the present study did not influence corticomotor excitability relative to sham. It is possible that this lack of effect was due to the performance of motor training, which elicits increased excitability (Butefisch et al. 2002, 2004; Celnik et al. 2006; Classen et al. 1998; Floel et al. 2005a,b; Stefan et al. 2008), thus overiding or masking the effect of cathodal tDCS.
The mechanism behind the current enhancement of motor memory encoding through anodal tDCS is unknown. However, this form of motor memory encoding is influenced by NMDA-receptor function (Butefisch et al. 2000) and it has been indirectly shown that within humans the mechanisms underlying the effect of tDCS involves the modulation of NMDA receptors (Nitsche et al. 2003d). Therefore it is plausible that the observed results are due to anodal tDCS increasing the efficacy of NMDA-receptor function, possibly through an increase in intracellular calcium concentration (Bennett 2000).
A previous study by Rosenkranz et al. (2000) explored the effect of tDCS during a paradigm identical to that of the present study by applying tDCS only during the last 10 min of training. The authors found that both anodal and cathodal tDCS had a negative effect on the formation and retention of a motor memory. We believe that the stark difference in results may be due to the differing tDCS protocols. Work by Iezzi et al. (2008) and Siebner et al. (2004) have shown that a TMS protocol can be either facilitatory or inhibitory, depending on the prior state of the system. It is possible that applying tDCS after 20 min of training has an effect different from that of tDCS at the onset of training due to the preconditioning of the motor cortex resulting from training. Alternatively, it is possible that the lack of findings in the Rosenkranz investigation was due to a shorter stimulation period.
Noninvasive cortical stimulation with tDCS elicits behavioral gains during implicit motor learning (Nitsche et al. 2003d), visuomotor processing (Antal et al. 2004; Reis et al. 2009), and probabilistic learning (Kincses et al. 2004) in healthy volunteers. In addition, it has been shown to improve motor performance in stroke (Hummel et al. 2005) and Parkinson disease patients (Boggio et al. 2006). More recently, Reis et al. (2009) reported that tDCS in association with training enhances learning of a novel skill by modulation of short-term retention. The present study supports these observations and suggests that anodal tDCS enhances both the process of encoding and retention of simple motor memories. In addition, we can rule out changes in memory-recall mechanisms since the behavioral measures obtained with the TMS paradigm used here do not require voluntary engagement of recall processes.
One of the initial steps in the development of more complex skills is the encoding of motor memories (Classen et al. 1998; Molina-Luna et al. 2008). It has been suggested that the motor cortex may encode the memory trace of a skill (Monfils et al. 2005) because changes in corticomotor organization occur during training (Karni et al. 1995; Kleim et al. 2004; Nudo et al. 1996a,b,c) and skill learning is associated with an enlargement of representations of trained body parts (Pascual-Leone et al. 1995). Therefore this study, showing that anodal tDCS over M1 enhances the motor memory formed after simple repetitive practice, may provide evidence as to how this form of stimulation results in the behavioral gains observed in previous studies (Antal et al. 2004; Kincses et al. 2004; Nitsche et al. 2003d; Reis et al. 2009).
In summary, we showed that anodal tDCS enhances the effects of simple repetitive training on the formation and retention of motor memories. This effect was polarity specific, relied on the interaction between motor training and tDCS, and lasted longer than performance of training without stimulation. The observed changes in the motor cortex representation may play an important role in the acquisition of more complex skills. The current results provide a rationale for the possible mechanism that may underlie the behavioral gains observed with the application of tDCS in previous studies. It also supports the view that such noninvasive cortical stimulation can be a useful tool in the rehabilitation of patients with brain damage.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants R01 HD-053793 and R21 HD-060169.
REFERENCES
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann KP, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci 19: 2888–2892, 2004. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Alonso-Alonso M, Mansur CG, Rigonatti SP, Schlaug G, Pascual-Leone A, Fregni F. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil 85: 927–930, 2006. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Sawaki L, Waldvogel D, Classen J, Kopylev L, Cohen LG. Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol 51: 59–68, 2002. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: 3661–3665, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol 91: 2110–2116, 2004. [DOI] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. Neuroimage 29: 677–684, 2006. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998. [DOI] [PubMed] [Google Scholar]
- Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol 58: 121–130, 2005a. [DOI] [PubMed] [Google Scholar]
- Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology 65: 472–474, 2005b. [DOI] [PubMed] [Google Scholar]
- Floel A, Rosser N, Michka O, Knecht S, Breitenstein C. Noninvasive brain stimulation improves language learning. J Cogn Neurosci 20: 1415–1422, 2008. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117: 845–850, 2006. [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27: 13413–13419, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128: 490–499, 2005. [DOI] [PubMed] [Google Scholar]
- Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, Berardelli A. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol 100: 2070–2076, 2008. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995. [DOI] [PubMed] [Google Scholar]
- Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia 42: 113–117, 2004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci 24: 628–633, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM, Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage 40: 1748–1754, 2008. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist 11: 471–483, 2005. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553: 293–301, 2003a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation: technical, safety and functional aspects. Suppl Clin Neurophysiol 56: 255–276, 2003b. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114: 2220–2222; author reply 2222–2223, 2003c. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, Meyer BU. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clin Neurophysiol 115: 2419–2423, 2004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: 1899–1901, 2001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626, 2003d. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568: 291–303, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794, 1996b. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28: 166–185, 1965. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett 15: 61–63, 2009. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Butefisch CM, Kopylev L, Davis B, Cohen LG. Cholinergic influences on use-dependent plasticity. J Neurophysiol 87: 166–171, 2002a. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Cohen LG, Classen J, Davis BC, Butefisch CM. Enhancement of use-dependent plasticity by D-amphetamine. Neurology 59: 1262–1264, 2002b. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Werhahn KJ, Barco R, Kopylev L, Cohen LG. Effect of an alpha(1)-adrenergic blocker on plasticity elicited by motor training. Exp Brain Res 148: 504–508, 2003. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 31: 3379–3385, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Classen J, Celnik P, Cohen LG. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci 27: 730–738, 2008. [DOI] [PubMed] [Google Scholar]
- Wu AD Functional neuroimaging and repetitive transcranial magnetic stimulation in Parkinson's disease. Rev Neurol Dis 4: 1–9, 2007. [PubMed] [Google Scholar]