Abstract
Influence of subcortical inhibition on barrel cortex receptive fields. By the time neural responses driven by vibrissa stimuli reach the barrel cortex, they have undergone significant spatial and temporal transformations within subcortical relays. A major regulator of these transformations is thought to be subcortical GABA-mediated inhibition, but the actual degree of this influence is unknown. We used disinhibition produced by GABA receptor antagonists to unmask the excitatory sensory responses that are normally suppressed by inhibition in the main subcortical sensory relays to barrel cortex; principal trigeminal (Pr5) and primary thalamic (VPM) nuclei. We found that, within subcortical relays, inhibition only slightly suppresses short-latency receptive field responses, but robustly suppresses long-latency center and surround receptive field responses. However, the long-latency subcortical effects of inhibition are mostly not reflected in the barrel cortex. The most robust effect of subcortical inhibition on barrel cortex responses is to transiently suppress the receptive field responses of high-frequency sensory stimuli. This transient adaptation caused by subcortical inhibition recovers within a few stimuli and gives way to a steady-state adaptation that is independent of subcortical inhibition.
INTRODUCTION
Inhibition mediated by GABAA and GABAB receptors is an important regulator of sensory responsiveness along ascending sensory pathways to the cortex. In the rodent vibrissa system, sensory inputs from the whiskers travel via trigeminal axons to the trigeminal complex where the principal nucleus (Pr5) gives rise to the lemniscal pathway projecting to the ventroposterior medial nucleus (VPM) of the thalamus. In turn, VPM gives rise to the thalamocortical pathway projecting to barrel cortex. Along these pathways, the excitation driven by sensory inputs is strongly controlled by inhibition, but the actual impact of subcortical inhibition on responses ultimately reaching the cortex is not known.
Within the trigeminal complex, few GABAergic interneurons are found in Pr5; most are found in the caudalis (Sp5c) and interpolaris (Sp5i) subnuclei (Avendano et al. 2005). Recent evidence indicates that a large portion of Pr5 inhibition originates from cells located in Sp5i (Furuta et al. 2008). Thus the responsiveness and receptive fields of Pr5 cells to whisker stimuli are thought to be controlled by inhibitory projections from Sp5i and excitatory projections from Sp5c (Furuta et al. 2008). However, the impact of GABA blockers (disinhibition) within Pr5 on sensory responses and receptive fields has not been tested previously.
Within the somatosensory thalamus, no inhibitory neurons are found in the VPM of rodents (Barbaresi et al. 1986; Harris and Hendrickson 1987; Spacek and Lieberman 1974). Hence the source of VPM inhibition is the reticular nucleus of the thalamus (nRt). The impact of disinhibition within VPM on sensory responses and receptive fields has been previously tested (Castro-Alamancos 2002a; Hartings and Simons 2000; Lee et al. 1994). Abolishment of VPM inhibition enlarges receptive fields, increases the duration of evoked responses, and, to some extent, relieves sensory adaptation in VPM cells. However, it is not known how the changes in VPM responsiveness caused by disinhibition affect sensory responses in barrel cortex and how thalamic disinhibition affects the excitatory input to VPM cells from the cortex (corticothalamic).
By the time ascending responses to vibrissa stimuli reach the cortex, they have undergone significant spatial and temporal transformations (for reviews, Castro-Alamancos 2004; Kleinfeld et al. 2006; Moore 2004; Simons et al. 1995), but it is not known which aspects of these transformations are caused by subcortical inhibition. This study set out to determine which aspects of sensory stimulation are suppressed by GABA-mediated inhibition in subcortical structures. The approach was to test the impact of Pr5 and VPM GABAA and GABAB receptor blockade (disinhibition) on sensory responses within those nuclei and their main target, the barrel cortex; sensory responses were compared before and during disinhibition in the same cells. By unmasking the excitatory responses that are normally suppressed by inhibition, disinhibition reveals the effect that inhibition has on sensory stimuli. Using this strategy, we tested the impact of subcortical disinhibition on responses driven by multi- and single-whisker stimulation of the center and surround receptive field. In addition, we tested how disinhibition affected responses driven by a high-frequency stimulus train. We found that Pr5 and thalamic disinhibition had strong effects within subcortical nuclei, particularly, on long-latency responses, but surprisingly few of these effects are reflected in the barrel cortex.
METHODS
Surgery
Spague-Dawley rats (300–350 g) were used in this study and cared for in accordance with National Institutes of Health guidelines for laboratory animal welfare. All experiments were approved by the Drexel University Institutional Animal Care and Use Committee. Rats were anesthetized with urethane (1.5 g/kg ip) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2%). A unilateral craniotomy extended over a large area of the parietal cortex. Small incisions were made in the dura as necessary. Body temperature was automatically maintained constant with a heating pad at 37°C. The level of anesthesia was monitored with field recordings and limb-withdrawal reflexes and kept constant at about stage III/3 using supplemental doses of urethane.
Electrophysiology
In every experiment, a tungsten electrode was lowered into the depth of the barrel cortex (0.6–1 mm) contralateral to the stimulated whiskers to record field potential (FP) and multiunit activity. To conduct single-unit recordings in the barrel cortex, a high-impedance (5–30 MΩ) glass electrode filled with artificial cerebrospinal fluid (ACSF) was lowered into the vicinity of the FP electrode to perform single-unit recordings from cells located in layers 2/3 and 4 (200–950 μm). Cortical cells were classified as fast spiking or regular spiking based on spike width as previously described (Hirata and Castro-Alamancos 2008). However, because the population of fast-spiking cells was small and did not differ in the effects of disinhibition from regular-spiking cells, we combined both groups of cells into one group.
To conduct single-unit recordings in VPM, a high-impedance (5–30 MΩ) glass electrode filled with ACSF was inserted contralateral to the stimulated whiskers at approximately the following coordinates from bregma (in mm): posterior = 3.5, lateral = 3, depth = 5–6. VPM cells were identified by their short latency to a whisker deflection (<7 ms) and, in some cases, by tracing the track of the recording electrode in histological sections confirming that the electrode was inside VPM as previously described (Aguilar and Castro-Alamancos 2005).
To conduct single-unit recordings in Pr5, a tungsten electrode (5–15 MΩ) was inserted ipsilateral to the stimulated whiskers at a 15° angle (with respect to the vertical plane) in the anteroposterior direction. The coordinates for electrode insertion were approximately: posterior, 7; lateral, 3. The electrode was lowered around 9 mm. The insertion of the electrode in Pr5 was confirmed by tracing the track of the electrode in histological sections.
Whisker stimulation
Sensory stimulation consisted of independently deflecting six individual whiskers using six different whisker stimulators. After isolating a unit, the whiskers were stimulated using a hand-held probe. The whisker that produced the shortest latency and strongest response was considered the principal whisker (PW). This whisker and up to five additional whiskers surrounding it, called adjacent whiskers (AWs), were selected for stimulation (Aw1–Aw5). Each of the selected whiskers was placed in an independent whisker stimulator by inserting it into a glass micropipette (1/0.5 mm OD/ID) that was glued to the membrane of a miniature speaker. Each whisker was inserted into the micropipette for ∼5 mm, leaving ∼10 mm from the end of the micropipette to the skin. Application of a 1-ms square current pulse to the speaker deflected the micropipette and the whiskers inside. The resulting whisker deflection is a very low-amplitude (∼2°) and very high-velocity (∼1,000°/s) stimulus. The whisker stimulators were oriented in the preferred direction to produce the largest response as determined with the hand probe. Each of the six whisker stimulators was driven by counter/timer boards controlled with LabVIEW software (National Instruments, Austin, TX).
Whisker stimulation was delivered according to the following protocols. A trial consisted of an initial 2 s without whisker stimulation followed by stimulation delivered to each whisker at 2-s intervals (the order of whisker stimulation was randomly selected). The first whisker was stimulated 2 s after the trial began, the second whisker was stimulated 4 s after the trial started, and so on, so that the sixth (last) whisker stimulus was delivered 12 s after the start of the trial. Thus a single trial contained stimuli for all six whiskers and lasted a total of 14 s. Whisker stimuli consisted of 10 stimuli delivered at 10 Hz. When all whiskers were stimulated simultaneously (ALL) each trial lasted 5 s. Every trial was repeated 30 times to derive peristimulus time histograms (PSTHs) and to average FP responses. In most experiments, protocols for single-whisker stimulation and simultaneous multiwhisker stimulation were combined in the same trial so that stimulation of each individual whisker was followed (3 s after the last whisker) by stimulation of the six whiskers together in the same trial.
Thalamic radiation (corticothalamic) stimulation
The electrode used to stimulate corticothalamic fibers in the thalamic radiation was placed, as previously described (Hirata et al. 2006), at approximately: posterior = 2.5, lateral = 4.5, depth = 3.5–5. Due to the synaptic facilitation of corticothalamic synapses reaching VPM, low-frequency corticothalamic stimulation is ineffective at driving spikes in VPM cells, but high-frequency stimulation (>2 Hz) readily discharges VPM cells (see Castro-Alamancos 2004). Thus the thalamic radiation stimulating electrode and the VPM recording electrode were diligently aligned so that stimulation of the thalamic radiation resulted in virtually no short-latency (<7 ms) response at low frequency but a highly reliable (∼100%) response at 5–40 Hz. The intensity was kept <150 μA. For corticothalamic stimulation, a trial consisted of 3 s of no stimulation followed by a 10-pulse train delivered at 2, 5, 10, 20, and 40 Hz. Each trial lasted 11 s. PSTHs were created with a minimum of 20 trials.
Microdialysis
To apply drugs into Pr5, a microdialysis cannula (250 μm diam, 2-mm-long membrane) was placed around the following coordinates: posterior = 9.5, lateral = 2.5, depth = 6–8. The cannula entered straight into the brain (no angle), while the Pr5 recording electrode entered at an angle (i.e., 15° angle with respect to the vertical plane in the anteroposterior direction). To apply drugs into VPM, a microdialysis cannula (250 μm diam, 2-mm-long membrane) was placed around the following coordinates: posterior = 3, lateral = 2–3, depth = 4–6, as previously described (Hirata et al. 2006). The cannula entered into the brain at an angle (∼30°) from the midline, while the VPM recording electrode entered lateral to the cannula and parallel to the midline.
ACSF was continuously infused through the probe at 2–4 μl/min. ACSF contained (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 MgSO4 7H2O, 10 dextrose, and 1 CaCl2 2H2O. Bicuculline (BMI) and CGP35348 (CGP) were used at 100–300 and 5–10 mM, respectively. Based on experience, and a typical exchange of ∼10% (reverse dialysis recovery), the doses of drugs used here are 10 times those used during direct application in slices. Based on diffusion experiments in cortex and thalamus, using arrays of recording electrodes at different distances from the probe, we have estimated the spread of these drugs to be 1 mm (Aguilar and Castro-Alamancos 2005; Castro-Alamancos 2000; Hirata et al. 2006). In the trigeminal nucleus, the spread is expected to disinhibit Pr5 and part of the adjacent motor trigeminal nucleus but not the sensory spinal subnuclei, which lie much more posterior. In some experiments, we observed whisker movements after Pr5 disinhibition; those recording periods were discarded. In thalamus, the spread is expected to disinhibit both VPM and the medial sector of the posterior nucleus of the thalamus (POm).
Data analysis
Spontaneous cell firing was computed by counting the number of spikes during the 2- to 3-s period at the beginning of each trial and for a minimum of 30 trials. For the analyses shown, spontaneous firing was not subtracted from the evoked responses, but doing this had no effect on the statistical results shown here. Note that the spontaneous firing for a specific response time window is always equal or smaller than the smallest evoked response (e.g., stimulus 10 of Aw5).
If the data were considered normally distributed, according to the Shapiro–Wilk normality test, we used parametric statistics. Otherwise, we used non parametric tests (SPSS). In general, FP data were found to be normally distributed, but single-unit data were not. Thus FP data were first tested for a significant main effect using the repeated-measures ANOVA followed by multiple comparisons with Tukey's test. Nonparametric comparisons for single-unit data consisted of the Wilcoxon signed ranks test.
Histology
At the end of the experiments, the animals were given an overdose of sodium pentobarbital and either perfused through the heart with saline followed by paraformaldehyde (4%) or the brain was directly extracted and placed in the fixative. The brains were then sectioned in the coronal plane using a vibratome (80–100 μm) and processed for Nissl's staining. For the cells included in the study, subsequent analysis confirmed the location of electrode tracts within VPM and Pr5.
RESULTS
In the following experiments, we determined the effect of disinhibition on single- and multiwhisker stimulation measured during a short- and a long-latency response time window. Single-whisker stimulation was delivered to the center receptive field (PW) or the surround receptive field (5 different AWs). Multiwhisker stimulation involved simultaneous stimulation of the center and surround receptive field (6 whiskers together, ALL). The rationale for the short- and long-latency response windows is as follows. The short-latency (<8 ms) subcortical response window (Pr5 and VPM) corresponds to fast onset sharp responses driven by whisker stimuli during control conditions that can account for the sharp FP (and single unit) population response in barrel cortex, which has an onset of ∼5 ms and peaks <15 ms. The long-latency response window corresponds to responses that follow the short-latency responses ≤50 ms poststimulus.
Whisker stimulation consisted of a train of 10 stimuli delivered at 10 Hz. We choose this stimulation because it provides three different types of information about the effects of disinhibition. The first stimulus in the train (stimulus 1) is a low-frequency stimulus because it is delivered <1 Hz. The second and subsequent few stimuli (stimuli 2–5) are transition high-frequency stimuli because the effects of the 10-Hz stimulation are not stable (steady) during these stimuli. Finally, the last few stimuli in the train (stimuli 6–10) are steady-state high-frequency stimuli because the effects of 10-Hz stimulation have mostly stabilized. Evaluation of transition stimuli during a 10-Hz train is important because IPSPs in VPM are strongest during these stimuli and fade during the steady-state stimuli (Castro-Alamancos 2002a, 2004).
Effect of Pr5 disinhibition on Pr5 sensory responses
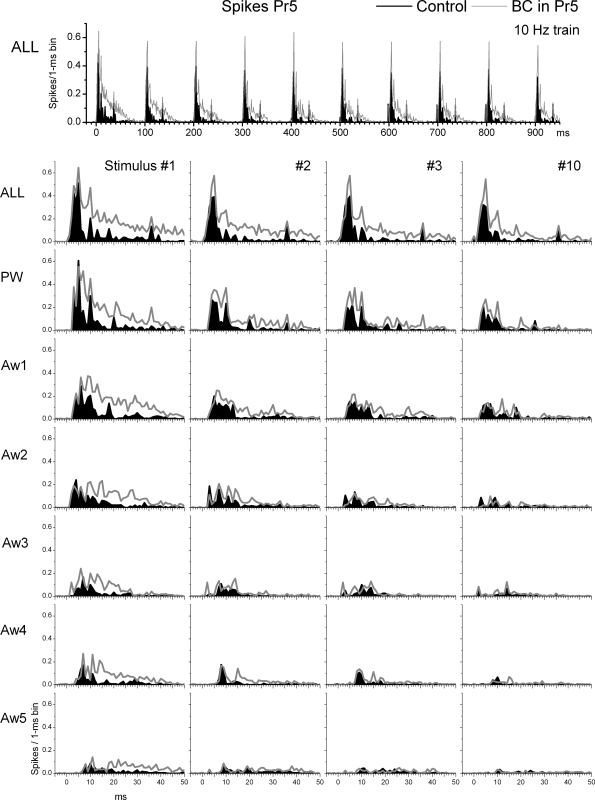
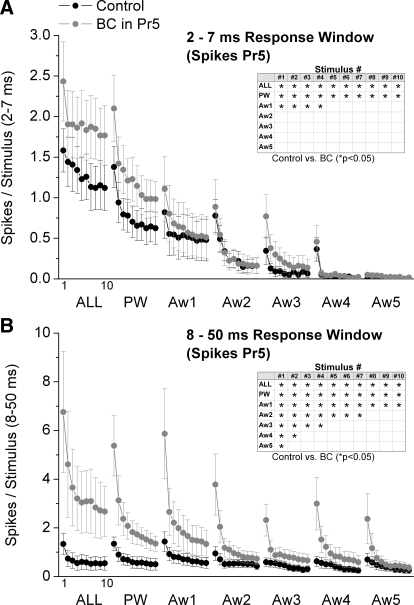
We tested the impact of Pr5 disinhibition on the spatial and temporal response properties of Pr5 cells by infusing bicuculline and CGP35348 into Pr5 using microdialysis. In the experiments, six whisker stimulators were used simultaneously to map the responses of the PW and five AWs (Aw1–Aw5) before and during drug applications. Figure 1 overlays population (n = 8 cells) PSTHs of responses evoked in Pr5 cells by a 10-Hz train (10 stimuli) of whisker deflections delivered to each of the six whiskers individually (single-whisker stimulation; PW, Aw1–Aw5) or simultaneously (multiwhisker stimulation; ALL) during control conditions (black traces) and during Pr5 disinhibition (gray traces). Figure 2 shows evoked responses (spikes per stimulus) measured during a short-latency (2–7 ms) or a long-latency (8–50 ms) time window poststimulus. The plots show responses evoked by each of the 10 stimuli in the 10-Hz train (stimuli 1–10) during single- or multiwhisker stimulation. For each stimulus number in the train, we made pair-wise statistical comparisons (Wilcoxon signed ranks test) between control and Pr5 disinhibition. Statistically significant (P < 0.05) effects of Pr5 disinhibition are marked with an asterisk in a table inset within each figure.
FIG. 1.
Effect of Pr5 disinhibition on Pr5 cell responses to whisker stimulation. Population peristimulus time histograms (PSTHs) show responses evoked by simultaneous multiwhisker (ALL) or single whisker stimulation of the PW and 5 AWs (Aw1–Aw5) during control (black traces) and during Pr5 disinhibition (BC; gray traces). The whisker stimulation consisted of 10 stimuli (1–10) delivered at 10 Hz. Bottom: only the responses to stimuli 1–3 and 10 of the 10-Hz train are shown.
FIG. 2.
Population data showing the effect of Pr5 disinhibition on short- and long-latency Pr5 cell responses. A: short-latency responses, measured during a 2- to 7-ms window poststimulus, evoked in Pr5 cells during control (black symbols) and during Pr5 disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1–Aw5). Inset: asterisk marks the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. B: the graph is organized as in A but shows long-latency responses measured during an 8- to 50-ms window poststimulus.
Pr5 responses under control conditions were similar to those previously described under the same conditions (Aguilar and Castro-Alamancos 2005). As shown in Fig. 1, during control conditions, low-frequency (stimulus 1) multiwhisker (ALL) or PW deflections evoke sharp short-latency responses. In addition, AWs also evoked significant responses that were smaller than the PW response. Ten-hertz whisker stimulation produced little sensory adaptation (depression) during multiwhisker stimulation (i.e., responses to stimuli 1 and 10 were similar). However, more adaptation was observed during single-whisker (PW or AW) stimulation. As noted later, sensory adaptation during 10-Hz stimulation is much stronger in VPM (see following text).
Pr5 disinhibition (gray traces) had variable effects on the spontaneous firing of Pr5 cells. When all cells are considered together, Pr5 disinhibition did not significantly increase the spontaneous firing of Pr5 cells (0.35 ± 0.26 vs. 1.3 ± 0.7 Hz; P = 0.15, n = 8 cells). However, the variability reflected two distinct populations. Half of the cells had very little spontaneous firing during control and were not affected by Pr5 disinhibition (0.02 ± 0.01 vs. 0.01 ± 0.01 Hz; n = 4), whereas the other half showed some increase in spontaneous firing (0.67 ± 0.5 vs. 2.6 ± 1.2; n = 4). Regarding sensory responses, Pr5 disinhibition significantly enhanced short-latency (2–7 ms) multiwhisker, PW and Aw1 responses evoked by stimuli 1–4 (Fig. 2A). In addition, Pr5 disinhibition enhanced short-latency multiwhisker and PW responses evoked by stimuli 5–10 (Fig. 2A). There were no effects on short-latency responses evoked by Aw2–Aw5.
Pr5 disinhibition had stronger effects on long-latency (8–50 ms) responses. Pr5 disinhibition significantly enhanced long-latency (8–50 ms) multi- and single-whisker responses evoked by stimulus 1 (Fig. 2B). As the number of pulses in the 10-Hz train increased, the number of AWs that were affected by Pr5 disinhibition decreased so that by stimulus 10, only one AW (Aw1), the PW and multiwhisker responses were enhanced (Fig. 2B).
Based on the effects of Pr5 disinhibition on Pr5 cells, we deduce the following effects of inhibition within Pr5. During single-whisker stimulation of the center receptive field (PW), Pr5 inhibition suppresses low frequency, transition, and steady-state short- and long-latency responses. In essence, all center field responses are suppressed in Pr5 by inhibition. Moreover, these same effects are produced by Pr5 inhibition during simultaneous multiwhisker stimulation of the center and surround receptive field (ALL). However, during single-whisker stimulation of the surround receptive field (AWs), Pr5 has few effects on short-latency responses but strong effects on long-latency responses. Pr5 inhibition suppresses low-frequency and transition short-latency responses but only for the strongest AW (Aw1). In addition, Pr5 inhibition suppresses low-frequency long-latency responses of every AW, transition long-latency responses for three of the five AWs, and steady-state responses of only one AW. Thus the impact of Pr5 inhibition on the long-latency surround fades as the high-frequency stimulus train progresses.
In conclusion, Pr5 inhibition suppresses all aspects of center receptive fields. Also, Pr5 inhibition strongly suppresses the long-latency responses of the surround receptive field, particularly, for low-frequency stimuli. Thus the effect on the surround diminishes as the number of stimuli in a high-frequency train increases.
Effect of thalamic disinhibition on VPM sensory responses
We next tested the impact of thalamic disinhibition on the spatial and temporal response properties of VPM cells by infusing bicuculline and CGP35348 into VPM using microdialysis as described previously (Aguilar and Castro-Alamancos 2005; Castro-Alamancos 2002a). The experiments and analyses were conducted similar to the Pr5 experiments described in the preceding text.
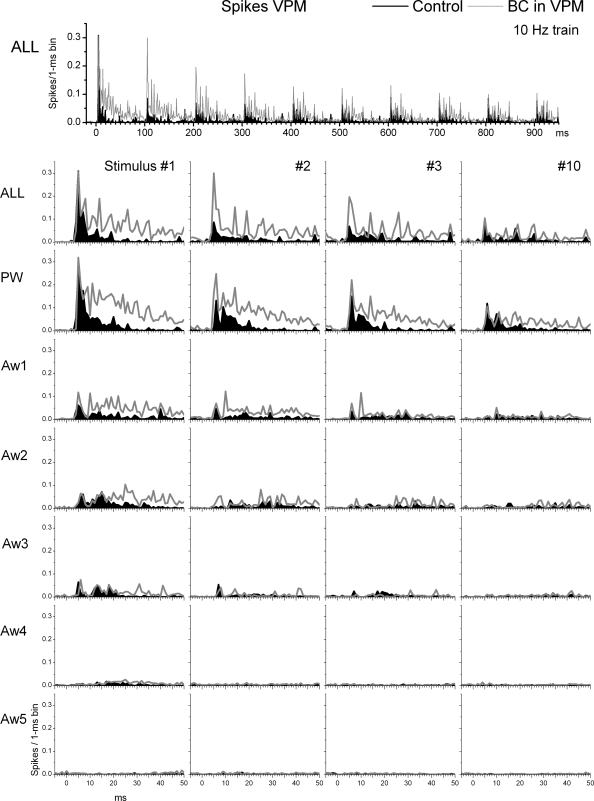
VPM responses under control conditions were similar to those previously described under the same conditions (Aguilar and Castro-Alamancos 2005; Castro-Alamancos 2002a; Hirata et al. 2006). As shown in Fig. 3, during control conditions, low-frequency (stimulus 1) multiwhisker (ALL) or PW deflections evoke sharp short-latency responses. In addition, several of the AWs produced responses that were smaller than the PW response. Ten-hertz whisker stimulation produced robust sensory adaptation that lead to significantly smaller responses. Thalamic disinhibition significantly increased the spontaneous firing of VPM cells from 1 ± 0.3 to 2.5 ± 0.6 Hz (P < 0.01, n = 16 cells).
FIG. 3.
Effect of thalamic disinhibition on ventroposterior medial nucleus (VPM) cell responses to whisker stimulation. Population PSTHs show responses evoked by simultaneous multiwhisker (ALL) or single whisker stimulation of the PW and 5 AWs (Aw1–Aw5) during control (black traces) and during thalamic disinhibition (BC; gray traces). The whisker stimulation consisted of 10 stimuli (1–10) delivered at 10 Hz. Bottom: only the responses to stimuli 1–3 and 10 of the 10-Hz train are shown.
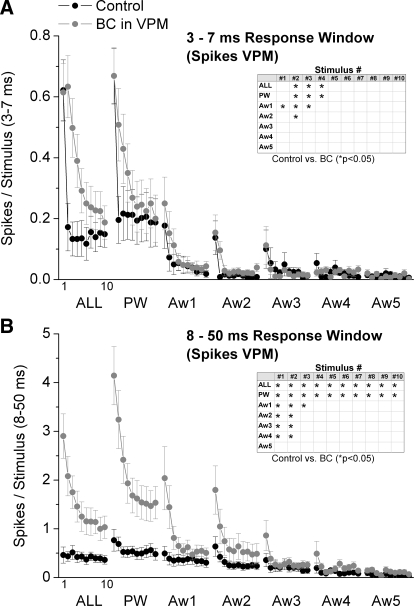
Regarding sensory responses, thalamic disinhibition had rather small effects on short-latency (3–7 ms) responses but strong effects on long-latency (8–50 ms) responses evoked by whisker stimulation. For stimulus 1 in the train (i.e., low-frequency stimulus), only the first AW (Aw1) showed a small but significant increase in short-latency (3–7 ms) response probability during disinhibition (Fig. 4 A), which is consistent with a slight enlargement of the receptive field. However, thalamic disinhibition affected more significantly short-latency responses to stimuli 2–4 and had no effect on stimuli 5–10. For stimulus 2, thalamic disinhibition enhanced multiwhisker, PW, Aw1, and Aw2 short-latency responses. Thus there was a significant enlargement of the receptive field for stimulus 2. Finally, for stimuli 3 and 4, thalamic disinhibition enhanced only multiwhisker and PW short-latency responses indicating no effect on the size of the receptive field.
FIG. 4.
Population data showing the effect of thalamic disinhibition on short- and long-latency VPM cell responses. A: short-latency responses, measured during a 3- to 7-ms window poststimulus, evoked in VPM cells during control (black symbols) and during thalamic disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1–Aw5). Inset: asterisk marks the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. B: the graph is organized as in A but shows long-latency responses measured during an 8- to 50-ms window poststimulus.
In contrast to the rather small effects on short-latency responses, thalamic disinhibition had strong effects on long-latency responses (8–50 ms). For stimuli 1 and 2, thalamic disinhibition enhanced multiwhisker, PW and AW responses of four of the five AWs (Aw1–4). Thus there was a large increase in the receptive field for the first two stimuli. For stimulus 3, thalamic disinhibition enhanced multiwhisker, PW and Aw1 long-latency responses. Finally, for stimuli 4–10, thalamic disinhibition enhanced only multiwhisker and PW long-latency responses.
Based on the effects of thalamic disinhibition on VPM cells, we deduce the following effects of inhibition within VPM. During single-whisker stimulation of the center receptive field (PW), thalamic inhibition suppresses short- and long-latency responses differently. For short-latency responses, only transition stimuli are inhibited, whereas for long-latency responses, all stimuli (low frequency, transition, and steady state) are inhibited. In essence, only transition short-latency center field responses are suppressed in VPM by thalamic inhibition, whereas all long-latency center field responses are inhibited. Moreover, these same effects are produced by thalamic inhibition during simultaneous multiwhisker stimulation of the center and surround receptive field (ALL). However, during single-whisker stimulation of the surround receptive field (AWs), thalamic inhibition suppresses low-frequency short-latency responses of only the strongest AW (Aw1), and some transition responses of the two strongest AWs. In addition, thalamic inhibition suppresses low frequency and some transition long-latency responses of four of the five AWs.
In conclusion, thalamic inhibition suppresses all aspects of long-latency center receptive field responses, but only transition stimuli of short-latency center receptive field responses. In addition, thalamic inhibition has little effect on short-latency surround receptive fields but suppresses long-latency surround receptive field responses, particularly, for low-frequency and the first transition stimulus.
Effect of thalamic disinhibition on VPM corticothalamic responses
In the preceding text, we described the effects of thalamic disinhibition on sensory evoked responses in VPM. Next, we describe the effects of thalamic disinhibition on corticothalamic responses evoked in VPM cells by stimulating the thalamic radiation. Corticothalamic responses are of interest in this context because corticothalamic feedback driven by whisker stimuli generates long-latency responses in VPM similar to those shown in the preceding text to be unmasked by thalamic disinhibition (Temereanca and Simons 2004). Thus we tested if thalamic disinhibition unmasks corticothalamic responses.
Single-unit recordings were obtained from VPM cells, and a stimulating electrode was placed in the thalamic radiation to evoke corticothalamic responses. A hallmark of the corticothalamic pathway reaching VPM is that it produces strong frequency-dependent facilitation (for review, see Castro-Alamancos 2004). Accordingly, stimulating and recording electrodes were carefully aligned so that low-intensity stimulation (<150 μA) elicited virtually no response during low-frequency stimulation (0.1 Hz) but a near 100% firing during 10-Hz stimulation (for additional details, see Hirata et al. 2006).
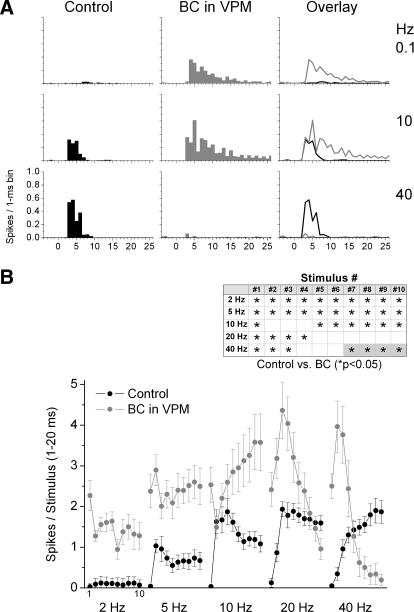
Once the recording and stimulating electrodes had been aligned, the thalamic radiation was stimulated with 10 pulses at different frequencies (0.1, 2, 5, 10, 20, and 40 Hz) for a minimum of 20 trials for each frequency. This protocol was repeated at least twice for every frequency during control conditions and then again twice after application of the drugs via microdialysis. Figure 5 A shows population PSTHs of steady-state (10th stimulus in a train) corticothalamic responses before and during thalamic disinhibition. Figure 5B shows population data (n = 8 cells) of corticothalamic responses corresponding to the number of spikes evoked during a 20-ms window after each of the 10 stimuli in a train delivered at different frequencies during control conditions and during thalamic disinhibition. During control conditions, responses to low-frequency stimulation were mostly absent for all cells. As the stimulation frequency increases, corticothalamic responses became apparent, showing strong frequency-dependent facilitation as previously described (Hirata et al. 2006). There is virtually no facilitation at 2 Hz; facilitation develops at 5 Hz and is strongest at 10 Hz and higher frequencies. Within a 10-stimulus train, facilitation starts by the second stimulus and is fairly steady for the last five stimuli (Fig. 5B). During thalamic disinhibition, there were two major effects. First, low-frequency responses were strongly enhanced. Thus responses to all 10 stimuli in a train at 2 and 5 Hz are significantly enhanced by thalamic disinhibition. Second, there were complex effects of thalamic disinhibition during facilitation. Steady-state facilitated responses (i.e., last 5 stimuli in a train), evoked at 5 and 10 Hz, were further enhanced by disinhibition. However, the last five stimuli in a train at 20 and 40 Hz did not reach a steady facilitated state; instead these responses began to depress after reaching peak facilitation during stimuli 2–4. This depression phenomenon appears to be related to the ability of high-frequency corticothalamic stimulation (facilitation) to trigger epileptic discharges (leading to postdischarge depression) and will not be further addressed in this study. It is worth noting that epileptic discharges were not evoked during thalamic disinhibition when high-frequency whisker stimulation was used. In conclusion, the main point of the present results in relation to ascending sensory information is that, just like for low-frequency sensory stimulation, low-frequency corticothalamic responses are robustly enhanced by disinhibition. Thus corticothalamic feedback from ascending sensory inputs may partly explain the enhanced long-latency VPM responses during thalamic disinhibition described in the preceding text (Figs. 3 and 4).
FIG. 5.
Effect of thalamic disinhibition on VPM cell responses to corticothalamic stimulation. A: population PSTHs show responses evoked by the 10th stimulus in a 10-pulse train delivered at 0.1, 10, and 40 Hz during control (black) and during thalamic disinhibition (BC; red). The responses shown in the left and middle panels are overlaid in the right panel. B: corticothalamic responses, measured during a 1- to 20-ms window poststimulus, evoked in VPM cells during control (black symbols) and during thalamic disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to corticothalamic stimulation delivered to the thalamic radiation at 2, 5, 10, 20, and 40 Hz. Inset: asterisk makrs the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. The gray color fill highlights that the effect of disinhibition was to significantly suppress, rather than enhance, the evoked response.
Effect of Pr5 disinhibition on cortical sensory responses
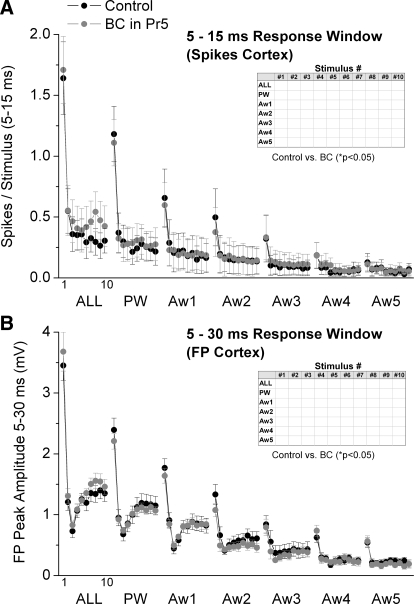
As described in the preceding text (Figs. 1 and 2), Pr5 disinhibition leads to significant effects on Pr5 cell responses evoked by whisker stimulation. We next tested if the Pr5 effects translated into cortical effects by recording single-unit (n = 13 cells) and FP (n = 12) responses from electrodes in barrel cortex. Figure 6 A shows cortical single-unit responses (spikes per stimulus) measured during a 5- to 15-ms time window poststimulus, and Fig. 6B shows the peak amplitude of FP responses measured during a 5- to 30-ms time window poststimulus during control and Pr5 disinhibition. Despite the strong effects of Pr5 disinhibition on Pr5 cells, we found that cortical single-unit and FP responses were not significantly affected by Pr5 disinhibition. Moreover, Pr5 disinhibition did not significantly affect the spontaneous firing of cortical cells (1.97 ± 0.7 vs. 2.13 ± 0.8 Hz; n = 13 cells). In conclusion, none of the significant effects produced by Pr5 disinhibition on Pr5 cells transformed cortical responses. Thus changes in Pr5 receptive fields caused by inhibition are not translated into changes in cortical receptive fields.
FIG. 6.
Population data showing the effect of Pr5 disinhibition on barrel cortex single-unit and FP responses. A: single-unit barrel cortex responses, measured during a 5- to 15-ms window poststimulus, evoked during control (black symbols) and during Pr5 disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1–Aw5). Inset: asterisk marks the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. B: the graph is organized as in A but shows the negative peak FP amplitude for barrel cortex responses measured during a 5- to 30-ms window poststimulus.
Effect of thalamic disinhibition on cortical sensory responses
As described in the preceding text (Figs. 3 and 4), thalamic disinhibition leads to significant effects on VPM cell responses evoked by whisker and corticothalamic stimulation. We next tested if the thalamic effects translated into cortical effects by recording single-unit (n = 8) and FP (n = 16) responses from electrodes located in barrel cortex. Thalamic disinhibition did not significantly affect the spontaneous firing of cortical cells (1.8 ± 0.3 vs. 2 ± 0.4 Hz; n = 8 cells).
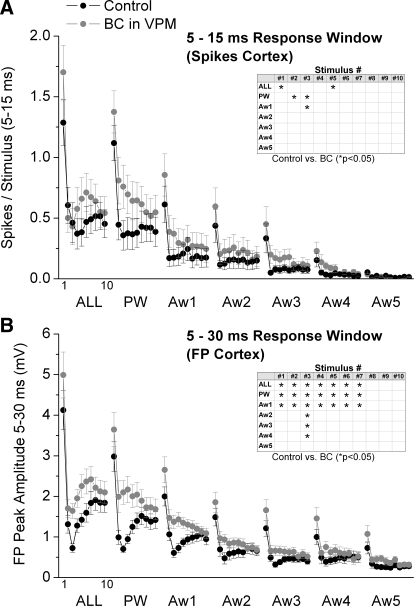
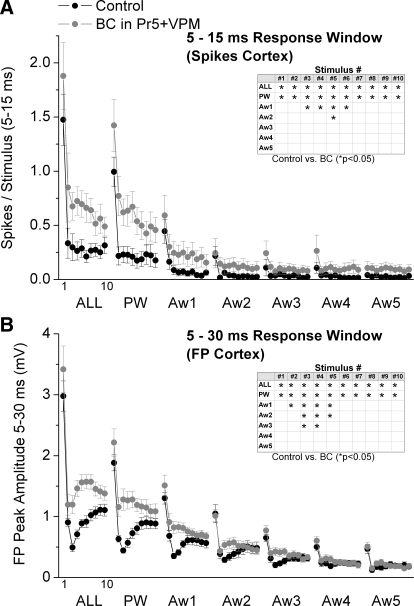
Regarding sensory responses, Fig. 7 A shows cortical single-unit responses (spikes per stimulus) measured during a 5- to 15-ms time window poststimulus, and Fig. 7B shows the peak amplitude of FP responses measured during a 5- to 30-ms time window poststimulus during control and thalamic disinhibition. In addition, population PSTHs and FP averages of the effects of thalamic disinhibition on cortical single-unit and FP responses are shown in Figs. 8 and 9, respectively. Despite the strong effects of thalamic disinhibition on VPM cell responses, we found that cortical FP and single-unit responses were only slightly affected. Thalamic disinhibition significantly enhanced single-unit cortical responses evoked by multiwhisker stimulation, but only for stimuli 1 and 5. In addition, PW responses to stimuli 2 and 3, and Aw1 responses to stimulus 3 were also significantly enhanced (Fig. 7A). More robust effects were observed on cortical FP responses, so that multiwhisker, PW and Aw1 responses evoked by stimuli 1–7 were enhanced by disinhibition. In addition, the responses to stimulus 3 from four of the five AWs (Aw1–Aw4) were also enhanced by inhibition.
FIG. 7.
Population data showing the effect of VPM disinhibition on barrel cortex single-unit and FP responses. A: single-unit barrel cortex responses, measured during a 5- to 15-ms window poststimulus, evoked during control (black symbols) and during VPM disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1–Aw5). Inset: asterisk marks the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. B: the graph is organized as in A but shows the negative peak FP amplitude for barrel cortex responses measured during a 5- to 30-ms window poststimulus.
FIG. 8.
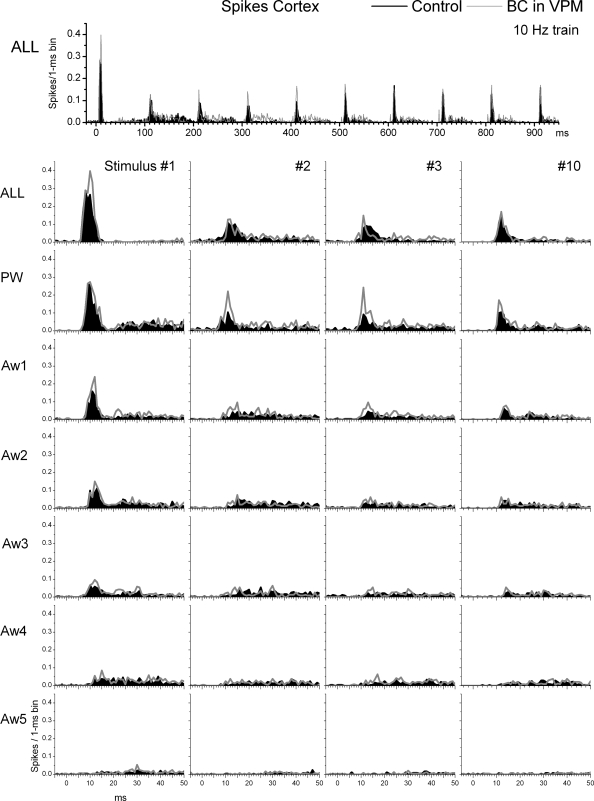
Effect of thalamic disinhibition on single-unit responses in barrel cortex to whisker stimulation. Population PSTHs show responses evoked by simultaneous multiwhisker (ALL) or single whisker stimulation of the PW and 5 AWs (Aw1–Aw5) during control (black traces) and during thalamic disinhibition (BC; gray traces). The whisker stimulation consisted of 10 stimuli (1–10) delivered at 10 Hz. Bottom: only the responses to stimuli 1–3 and 10 of the 10-Hz train are shown.
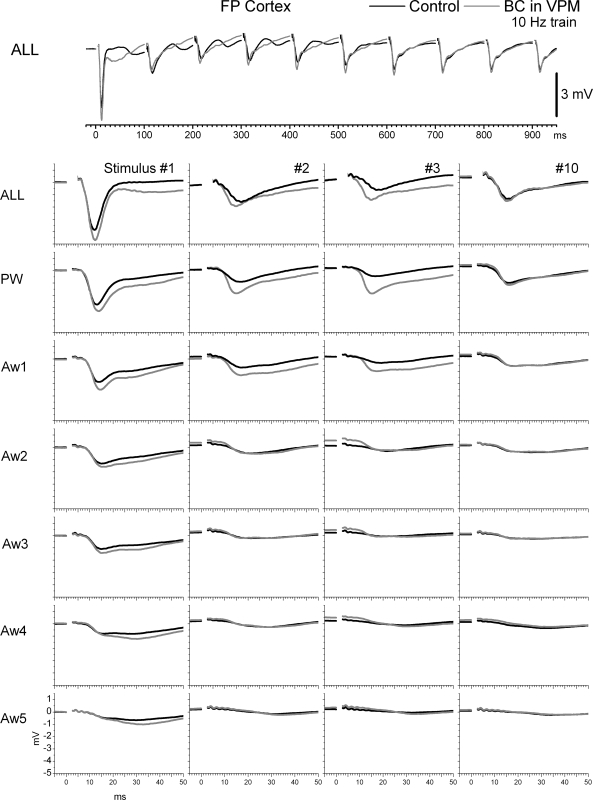
FIG. 9.
Effect of thalamic disinhibition on FP responses in barrel cortex to whisker stimulation. Average FPs show responses evoked by simultaneous multiwhisker (ALL) or single whisker stimulation of the PW and 5 AWs (Aw1–Aw5) during control (black traces) and during thalamic disinhibition (BC; gray traces). The whisker stimulation consisted of 10 stimuli (1–10) delivered at 10 Hz. Bottom: only the responses to stimuli 1–3 and 10 of the 10-Hz train are shown.
In conclusion, compared with the effects of thalamic disinhibition within the thalamus, there were only three subtle effects in the cortex that were most obvious in FP responses. First, FP responses (and to some extent single-unit responses) evoked by a 10-Hz train of multiwhisker or single-whisker stimulation reveal a characteristic profile (Fig. 7B) consisting in a strong response to the first stimulus, followed by a robust depression to the succeeding few stimuli (2–5) usually peaking by stimulus 3, and followed by some recovery of the transient depression, so that a steady-state adaptation is reached for the last several stimuli in the train. We will refer to this transient (reversible) depression of responses within a train of stimuli as transient adaptation. The main effect of thalamic disinhibition was to reduce the transient adaptation produced by the initial stimuli in a 10-Hz train, while having no effect on steady-state adaptation. Second, in both FP and single-unit responses, the late part of the sharp short-latency responses (<15 ms) evoked by multiwhisker stimulation, and to a less extent by PW and Aw1 stimulation, are enhanced (see PSTH for ALL stimulus 1 in Fig. 8). However, longer latency spikes (>15 ms) are not affected by thalamic disinhibition. The enhancement of the late component of the short-latency response is likely driven by the enhancement of long-latency responses seen in VPM after thalamic disinhibition (see Figs. 3 and 4B). Because cortical inhibition is still intact, it continues to effectively suppress the longer latency activity unmasked by thalamic disinhibition. Third, there was little evidence of a large increase in the receptive field size (surround) of cortical responses after thalamic disinhibition. Such effect is rather small during low-frequency or steady-state stimulation, involving only one AW, and mostly visible in FP responses. The surround enhancement is more pronounced for some transition stimuli (e.g., stimulus 3 of FP responses).
Effect of simultaneous Pr5 and thalamic disinhibition on cortical sensory responses
Considering the meager effects of thalamic disinhibition on cortical responses, we next tested if simultaneous Pr5 and thalamic disinhibition produces stronger effects than thalamic disinhibition alone by recording single-unit (n = 11) and FP (n = 16) responses from electrodes located in barrel cortex. Pr5+thalamic disinhibition tended to increase the spontaneous firing of some cortical cells, but this was not consistent among the population of recorded cells and the effect was not statistically significant (2.1 ± 0.8 vs. 3.9 ± 1.9 Hz; n = 11 cells).
Regarding sensory responses, Fig. 10 A shows cortical single-unit responses (spikes per stimulus) measured during a 5- to 15-ms time window poststimulus, and Fig. 10B shows the peak amplitude of FP responses measured during a 5- to 30-ms time window poststimulus during control and Pr5+thalamic disinhibition. Pr5+thalamic disinhibition significantly enhanced single-unit and FP cortical responses evoked by multiwhisker and PW stimulation for stimuli 1–10 of the 10-Hz train. In addition, the responses of some of the AWs to transition stimuli (between 3 and 6) were also enhanced by disinhibition.
FIG. 10.
Population data showing the effect of Pr5 plus thalamic disinhibition on barrel cortex single-unit and FP responses. A: single-unit barrel cortex responses, measured during a 5- to 15-ms window poststimulus, evoked during control (black symbols) and during Pr5 plus thalamic disinhibition (BC; gray symbols). The x axis shows the responses evoked by each of the 10 stimuli in the 10-Hz train. Each block of 10 stimuli correspond (left to right) to multiwhisker stimulation delivered simultaneously to all 6 whiskers (ALL), single-whisker stimulation of the PW and of each of the 5 AWs (Aw1–Aw5). Inset: asterisk marks the responses that showed a significant effect of disinhibition as determined with a pair-wise comparison. B: the graph is organized as in A but shows the negative peak FP amplitude for barrel cortex responses measured during a 5- to 30-ms window poststimulus.
In general, the effects produced by Pr5+thalamic disinhibition were similar but more robust than those produced by thalamic disinhibition alone. First, as mentioned for thalamic disinhibition alone, a main effect of Pr5+thalamic disinhibition was to reduce transient adaptation of PW and multiwhisker responses to transition stimuli (stimuli 2–6) in a 10-Hz train. This indicates that subcortical inhibition produces transient adaptation in the center receptive field. Second, as with thalamic disinhibition alone, the late part of the sharp short-latency FP and single-unit responses (<15 ms) were slightly but significantly enhanced for center receptive field stimuli (i.e., multiwhisker and PW). This indicates that subcortical inhibition suppresses the throughput of the center receptive field. Third, there was little evidence of a large increase in the receptive field size in cortex after Pr5+thalamic disinhibition. Thus AW responses were only enhanced for a few whisker stimuli in the middle of the train (transition stimuli), but not for either low-frequency stimuli (stimulus 1) or steady-state 10-Hz stimuli (stimulus 10). Therefore the main effect of subcortical inhibition on the surround receptive field is to produce transient adaptation during high-frequency stimulation. In conclusion, subcortical inhibition affects cortical receptive fields by slightly reducing the overall throughout of the center receptive field and by producing transient adaptation of high-frequency stimuli in both center and surround receptive fields.
DISCUSSION
What sensory stimuli are suppressed by Pr5 inhibition?
The main source of GABA-mediated inhibition in Pr5 originates in the spinal subnuclei (Avendano et al. 2005; Furuta et al. 2008). Our results reveal the main effects of this inhibitory input on Pr5 cell receptive fields. Thus at short latencies, Pr5 inhibition slightly suppresses multiwhisker (simultaneous center plus surround) and center receptive field responses of all the stimuli tested (low frequency, transition, and steady state). Pr5 inhibition also suppresses the short-latency response of the strongest whisker in the surround during low-frequency and transition stimuli. At long latencies, Pr5 inhibition produces a strong suppression of multiwhisker (simultaneous center plus surround) and center receptive field responses of all the stimuli tested (low frequency, transition, steady state). In addition, Pr5 inhibition suppresses the long-latency response of each of the five whiskers in the surround during low-frequency stimulation, some (3 or 4 of 5) whiskers in the surround during transition stimulation, and the strongest whisker in the surround during steady-state stimulation. Thus the impact of Pr5 inhibition on the long-latency surround fades as the high-frequency stimulus train progresses. In conclusion, the main effect of the internucleus inhibition in Pr5 is to suppress long-latency receptive field responses, and this effect fades as the number of stimuli in a high-frequency train increases.
What sensory stimuli are suppressed by VPM inhibition?
The main source of GABA-mediated inhibition in VPM is nRt (Barbaresi et al. 1986; Harris and Hendrickson 1987). Our results reveal the main effects of this inhibitory input on VPM cell receptive fields. At short latencies, VPM inhibition suppresses multiwhisker (simultaneous center plus surround) and center receptive field responses of only transition stimuli in a high-frequency train. VPM inhibition also slightly suppresses the short-latency response of the strongest whisker in the surround during low-frequency and a few of the transition stimuli. At long latencies, VPM inhibition suppresses multiwhisker (simultaneous center plus surround) and center receptive field responses of all the stimuli tested (low frequency, transition, steady state). In addition, VPM inhibition suppresses the long-latency response of most of the whiskers in the surround (4 of 5) during low-frequency and the first transition stimulus. In conclusion, the main effect of VPM inhibition is to transiently suppress short-latency responses in a high-frequency train and to suppress long-latency receptive field responses of low-frequency stimuli.
These results indicate that subcortical inhibition is not the main cause of the restricted short-latency receptive fields in Pr5 or VPM because only one AW in the surround was enhanced by disinhibition at short latencies, and the effect was rather small. However, subcortical inhibition strongly suppresses long-latency center and surround receptive field responses in Pr5 and VPM, which raises the question about the source of the unmasked excitatory input. There are multiple possible origins of the responses unmasked in Pr5 and VPM during disinhibition. In Pr5, some AW responses may originate within Pr5 itself from primary afferents, others may originate from spinal subnuclei and others from corticofugal feedback (Furuta et al. 2008; Minnery and Simons 2003). In VPM, some AW responses may be relayed from Pr5, others directly from spinal subnuclei, and others from corticothalamic feedback (Lee et al. 1994; Minnery et al. 2003; Temereanca and Simons 2004; Timofeeva et al. 2004). In fact, we show here that a main effect of thalamic disinhibition is to enhance low-frequency corticothalamic responses, which could easily cause part of the long-latency receptive field enhancement observed in VPM. Moreover, during control conditions, the receptive fields in VPM are more restricted than those in Pr5. Thus absence of thalamic inhibition may lead to larger receptive fields in VPM that are equivalent to those in Pr5.
How are cortical receptive fields influenced by subcortical inhibition?
Initially we tested the effect of Pr5 disinhibition on cortical responses and found that the robust effects observed on Pr5 cell responses were not reflected in the cortex. Even when inhibition was abolished in both Pr5 and thalamus, cortical responses were still not extensively affected. Based on the effects of disinhibition in both Pr5 and VPM, we conclude that subcortical inhibition slightly suppresses multiwhisker (simultaneous center plus surround) and center receptive field responses of all stimuli tested (low frequency, transition and steady state). In addition, subcortical inhibition suppresses between one and three whiskers in the surround but only during transition stimuli.
Cortical layer IV surround receptive fields are thought to originate from either thalamocortical (Goldreich et al. 1999; Kwegyir-Afful et al. 2005; Simons and Carvell 1989) and/or intracortical sources (Armstrong-James et al. 1991; Fox et al. 2003). We found that a rather large enlargement of the subcortical long-latency receptive fields meant almost nothing at the level of cortex. This is likely because subcortical inhibition mostly affects long-latency but not short-latency receptive fields. Subcortical long-latency responses suffer from two major deficiencies in their ability to drive cortical responses compared with short-latency responses. First, they are much more dispersed than short-latency responses and therefore are unable to drive effective cortical responses, which depend on tight presynaptic synchrony (Bruno and Sakmann 2006). Second, the long-latency responses unmasked by subcortical disinhibition are still subjected to suppression by local intact inhibition within both the thalamus and cortex during Pr5 disinhibition, and the cortex during Pr5+thalamic disinhibition.
Subcortical inhibition had a very consistent suppressing effect on FP cortical responses evoked by transition stimuli; note that these FP cortical responses are very closely correlated with subthreshold PSPs of nearby cells (Hirata and Castro-Alamancos 2008). This transient adaptation mediated by subcortical inhibition is likely due to the fact that IPSPs in VPM are most robust during transition stimuli in a 10-Hz train and fade during steady-state stimuli (Castro-Alamancos 2002a). Therefore it is logical that transition stimuli were unmasked in VPM and cortex by subcortical disinhibition. Importantly, while subcortical disinhibition significantly relieved the suppression during transition high-frequency stimuli, it did not relieve steady-state adaptation. Under our conditions, steady-state adaptation is somewhat apparent for single-whisker responses in Pr5 and is much stronger for both single- and multiwhisker responses in VPM and barrel cortex of both center and surround receptive fields. In general, disinhibition had little effect on these steady-state adaptations. Thus while GABA-mediated inhibition is a major factor in regulating sensory responsiveness within the subcortical nuclei, it is clear that there are other factors responsible for sensory response transformations such as, synaptic depression of ascending excitatory connections (Castro-Alamancos 2002a,b, 2004; Castro-Alamancos and Oldford 2002), and the inhibitory effects of other neurotransmitters, such as glycine in Pr5 (Avendano et al. 2005; Furuta et al. 2008).
Regarding the relevance of our findings to behavior, we speculate that long-latency receptive field changes that may occur in subcortical structures during different behavioral states will probably have little impact on cortical receptive fields. Thus only changes in short-latency subcortical receptive fields will be effectively reflected in cortical responses. Also, the transient and steady-state adaptations we observe within a stimulus train may occur during long bouts of active whisking at ∼10 Hz in behaving animals. If this is the case, the initial few cycles within a whisking bout will be critically influenced by the state of subcortical inhibition, whereas later cycles within a whisking bout (steady state) will be more dependent on the state of synaptic depression in ascending excitatory connections.
Conclusions
We found that inhibition has significant effects within the two main relay nuclei providing sensory information to the barrel cortex (Pr5 and VPM). Pr5 inhibition mainly suppresses long-latency receptive field responses, and this effect fades as the number of stimuli in a high-frequency train increases. VPM inhibition transiently suppresses short latency responses in a high-frequency train and also suppresses long-latency receptive field responses of low-frequency stimuli. Interestingly, the effects of inhibition within subcortical nuclei have relatively small effects on barrel cortex sensory responses. The main effects of subcortical inhibition on cortical receptive fields are to slightly suppress the overall throughput of center receptive field and to transiently suppress high-frequency stimuli for the center and part of the surround receptive field.
GRANTS
This work was supported by the National Institutes of Health.
REFERENCES
- Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci 25: 10990–11002, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Callahan CA, Friedman MA. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol 303: 193–210, 1991. [DOI] [PubMed] [Google Scholar]
- Avendano C, Machin R, Bermejo PE, Lagares A. Neuron numbers in the sensory trigeminal nuclei of the rat: A GABA- and glycine-immunocytochemical and stereological analysis. J Comp Neurol 493: 538–553, 2005. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382: 305–326, 1986. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622–1627, 2006. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA Origin of synchronized oscillations induced by neocortical disinhibition in vivo. J Neurosci 20: 9195–9206, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol 87: 946–953, 2002b. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–331, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Wright N, Wallace H, Glazewski S. The origin of cortical surround receptive fields studied in the barrel cortex. J Neurosci 23: 8380–8391, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschenes M. Inhibitory gating of vibrissal inputs in the brain stem. J Neurosci 28: 1789–1797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Kyriazi HT, Simons DJ. Functional independence of layer IV barrels in rodent somatosensory cortex. J Neurophysiol 82: 1311–1316, 1999. [DOI] [PubMed] [Google Scholar]
- Harris RM, Hendrickson AE. Local circuit neurons in the rat ventrobasal thalamus–a GABA immunocytochemical study. Neuroscience 21: 229–236, 1987. [DOI] [PubMed] [Google Scholar]
- Hartings JA, and Simons DJ. Inhibition suppresses transmission of tonic vibrissa-evoked activity in the rat ventrobasal thalamus [In process citation]. J Neurosci 20: RC100, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Cortical transformation of wide-field (multiwhisker) sensory responses. J Neurophysiol 100: 358–370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Kwegyir-Afful EE, Bruno RM, Simons DJ, Keller A. The role of thalamic inputs in surround receptive fields of barrel neurons. J Neurosci 25: 5926–5934, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. II. Differential effects of GABAA and GABAB receptor antagonists on responses of VPM neurons. J Neurophysiol 71: 1716–1726, 1994. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Bruno RM, Simons DJ. Response transformation and receptive field synthesis in the lemniscal trigeminothalamic circuit. J Neurophysiol 90: 1556–1570, 2003. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol 89: 40–56, 2003. [DOI] [PubMed] [Google Scholar]
- Moore CI Frequency-dependent processing in the vibrissa sensory system. J Neurophysiol 91: 2390–2399, 2004. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Jones EG, Diamond IT. Neuronal integration in the somatosensory whisker/barrel cortex. In: Cerebral Cortex. The Barrel Cortex of Rodents. New York: Plenum, 1995, vol. 11, p. 263–298.
- Spacek J, Lieberman AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat 117: 487–516, 1974. [PMC free article] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron 41: 639–651, 2004. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Lavallee P, Arsenault D, Deschenes M. Synthesis of multiwhisker-receptive fields in subcortical stations of the vibrissa system. J Neurophysiol 91: 1510–1515, 2004. [DOI] [PubMed] [Google Scholar]