Abstract
Mesencephalic trigeminal (M-V) neurons are primary somatosensory neurons with somata located within the CNS, instead of in peripheral sensory ganglia. In amphibians, these unipolar cells are found within the optic tectum and have a single axon that runs along the mandibular branch of the trigeminal nerve. The axon has collaterals in the brain stem and is believed to make synaptic contact with neurons in the trigeminal motor nucleus, forming part of a sensorimotor loop. The number of M-V neurons is known to increase until metamorphosis and then decrease, suggesting that at least some M-V neurons may play a transient role during tadpole development. It is not known whether their location in the optic tectum allows them to process both visual and somatosensory information. Here we compare the anatomical and electrophysiological properties of M-V neurons in the Xenopus tadpole to principal tectal neurons. We find that, unlike principal tectal cells, M-V neurons can sustain repetitive spiking when depolarized and express a significant H-type current. M-V neurons could also be driven synaptically by visual input both in vitro and in vivo, but visual responses were smaller and longer-lasting than those seen in principal tectal neurons. We also found that the axon of M-V neurons appears to directly innervate a tentacle found in the corner of the mouth of premetamorphic tadpoles. Electrical stimulation of this transient sensory organ results in antidromic spiking in M-V neurons in the tectum. Thus M-V neurons may play an integrative multisensory role during tadpole development.
INTRODUCTION
Mesencephalic trigeminal (M-V) neurons are primary somatosensory neurons with the unusual characteristic that their somata are located in the CNS rather than in peripheral sensory ganglia (Johnston 1909). Their location within the CNS suggests that they may directly integrate neural information originating both in the sensory periphery and from within the CNS, but the nature of this integration has remained unclear. M-V neurons are found in most jawed vertebrates, with the exception of lampreys and hagfishes (Butler and Hodos 2005). In amphibians, reptiles, fish, and birds M-V somata are confined to the optic tectum, whereas in mammals they can be found throughout the midbrain (Weinberg 1928). Functionally they are similar to primary trigeminal neurons, which normally reside peripherally in the trigeminal ganglion. Like trigeminal neurons, M-V cells are unipolar, having a single axon with a peripheral branch that originates in the mandibular division of the trigeminal nerve and has central collaterals that terminate in the motor nucleus of V (Hiscock and Straznicky 1982). Typically M-V neurons receive direct proprioceptive information from muscles and connective tissue in the jaw area and show several species-specific adaptations. For example, in sharks M-V cells respond directly to pressure in the teeth (Roberts and Witkovsky 1975), whereas in ducks they encode proprioceptive information from the beak (Manni et al. 1965). Because their central collaterals terminate in the motor nucleus of V, M-V cells are believed to be involved in a sensorimotor loop controlling biting, mastication, and other jaw movements (Luo and Dessem 1996). Recordings from rat M-V neurons show that these cells can generate rhythmic bursting when depolarized (Pedroarena et al. 1999), consistent with their role in regulating repetitive jaw motions. This ability to generate rhythmic oscillations has been shown to improve over development (Wu et al. 2001). Their centrally located somata have also been shown anatomically to receive synaptic inputs from several brain stem structures and to contain various classes of neurotransmitter receptors, suggesting that they may be able to integrate both central and peripheral neural information (Alley 1973; Copray et al. 1990).
In amphibians, M-V cells are most similar to Rohon–Beard neurons, a transient population of mechanosensory neurons located within the medulla and that disappear during metamorphosis (Lamborghini 1980, 1987; Roberts 1998). In Xenopus tadpoles M-V neurons are first detected around developmental stage 47, near the time when tadpoles begin to filter feed, and after the cells in the trigeminal ganglion have already formed (Kollros and Thiesse 1985). M-V cells steadily increase in number, peaking around the onset of metamorphosis, after which the number of cells begins to decline to adult levels (Kollros and Thiesse 1985). This pattern of increase and decrease in the number of M-V cells during development has also been reported in other species (Rogers and Cowan 1973). In adult Xenopus, some M-V neurons are known to have small dendritic arbors and axonal collaterals within the tectum itself, suggesting that they may also be acting as a type of tectal interneuron that integrates somatosensory information from the jaw with visual and other sensory input (Lowe and Russell 1984).
Because M-V cells are not clustered within a distinct nucleus in amphibian brains, characterizing their functional connectivity in vivo has been difficult. For example, it is not clear whether their location within the optic tectum means that they can be driven by visual input. Furthermore, the finding that the number of M-V neurons increases during early development and then decreases postmetamorphically suggests that some M-V neurons may exhibit unique functions during early development that are different from their adult function. Here we record from visually identified M-V neurons in Xenopus laevis tadpoles, using both whole-brain and in vivo preparations, to better understand the functional properties and connectivity of these neurons during Xenopus larval development.
METHODS
Whole cell electrophysiology
All experiments were done in accordance with Institutional Animal Care and Use Committee standards. Wild-type Xenopus laevis tadpoles were raised in 10% Steinberg's solution at 23° on a 12:12 light:dark cycle. Stages of development were identified according to Nieuwkoop and Faber (1956). We used stage 48–49 tadpoles for this set of experiments, which corresponds to 2.5–4 wk postfertilization. For whole-brain recordings, tadpole brains were prepared as described by Pratt and Aizenman (2007) and Wu et al. (1996). Briefly, tadpoles were anesthetized in 0.01% tricaine methanesulfonate (MS-222). Next, tadpoles were moved to a recording chamber where brains were dissected in HEPES-buffered extracellular saline (in mM: 115 NaCl, 2 KCl, 3 CaCl2, 3 MgCl2, 5 HEPES, 10 glucose; pH 7.25, osmolarity 255 mOsm) and pinned to a piece of submerged Sylgard. To access neurons in the optic tectum for whole cell recording, the ventricular membrane covering the tectum was carefully removed using a broken glass pipette as described by Pratt and Aizenman (2007).
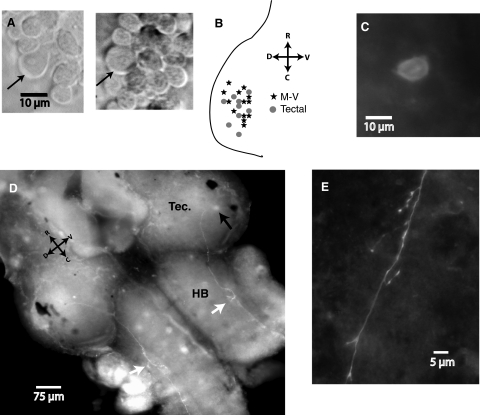
Tectal and M-V cells were visualized and identified using a Nikon (Tokyo, Japan) E600 FN light microscope with a ×60 fluorescent water-immersion objective and a Hamamatsu (Hamamatsu City, Japan) infrared (IR) charge-coupled detector camera; images were captured using Zarbeco (Randolph, NJ) software. With a ×60 objective it is possible to readily distinguish between principal tectal neurons and the sparse M-V neurons. M-V neurons possess three distinctive morphological features that make them readily identifiable: 1) M-V cells have noticeably larger somas that are more round and less oval shaped; 2) the somas of M-V neurons appear bright and lucent, whereas the tectal cells have a darker, grainy appearance; and 3) M-V cells have a visibly large nucleus. This does not rule out the possibility that there are other smaller M-V cells that we did not identify. We limited our recordings to M-V cells that had these three characteristics. Principal tectal neurons that we recorded from were from the same region of the tectum as M-V neurons. The approximate locations of a subset of recorded cells were plotted onto a tracing of the tectum (see Fig. 1 B) to show the general spatial distribution representative of the population of neurons included in this study.
FIG. 1.
Morphology and projection patterns of mesencephalic trigeminal (M-V) neurons in Xenopus tadpoles. A: 2 examples of infrared differential interference contrast images taken from different whole-brain preparations of living M-V neuron somata surrounded by principal tectal neurons. Arrow indicates an M-V neuron. Notice the M-V somata are bigger and rounder, with a less grainy look than that of the smaller neighboring principal tectal neurons. Also notice that the nucleus is visible in M-V neurons. B: a schematic representation of an optic tectal lobe with the location of representative M-V neurons (stars) and principal tectal neurons (filled dots) used in this study plotted onto it. Notice that locations of the 2 cell types significantly overlap. C: fluorescence image of an M-V neuron that had been filled with biocytin during recording, fixed, and subsequently labeled with Texas red–conjugated avidin. Notice the lack of dendritic branches, a characteristic of unipolar neurons. D: 2 biocytin-filled M-V neurons, one in each tectum, imaged at low power with the ventral side of the brain facing up, to display the projection of their axons. In both cases, the axons project slightly medially and ventrally within the tectum and then caudally down through the hindbrain. Notice that at very similar points in the hindbrain both axons send out several small axon collaterals, indicated with the white arrows. E: higher-power image of an M-V axon sprouting collaterals with terminals in the hindbrain.
Electrophysiological recordings were done using 8- to 12-MΩ glass micropipettes filled with K+-gluconate–based internal saline (in mM: 100 K-gluconate, 8 KCl, 5 NaCl, 1.5 MgCl2, 20 HEPES, 10 EGTA, 2 ATP, and 0.3 GTP; pH 7.2, osmolarity 255 mOsm). Recordings were obtained and quantified as described in Pratt and Aizenman (2007, 2008). Retinal ganglion cell (RGC) responses were activated by placing a bipolar stimulating electrode on the optic chiasm. The stimulating electrode consisted of two side-by-side, 25-μm platinum leads (part CE2C75; FHC, Bowdoin, ME). For hindbrain stimulation, the bipolar electrode was placed about 100 microns caudal to the tectum. For calculating statistical significance, we used nonparametric statistics (Mann–Whitney) unless otherwise indicated. All error bars are SE.
For in vivo recordings, tadpoles were anesthetized in 0.01% MS-222, then moved to a recording chamber, secured onto a Sylgard block submerged in HEPES buffered saline with 0.1 mM tubocurarine. To record from the optic tectum, a single shallow incision was made vertically to expose the tectal surface and the membrane covering the tectum was cleared away as described earlier. Whole-field visual responses were elicited by delivering a flash of white light through the microscope illumination system. Tentacle responses were evoked by placing a bipolar stimulating electrode against the skin in the ventral side of the tentacle.
Anatomy
For biocytin fills, 0.2% biocytin was added to the K+-gluconate–based internal saline. After whole cell recording, brains were immediately fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) with 5% sucrose overnight at 4°C. To remove residual paraformaldehyde, brains were rinsed in two successive PBS rinses, each rinse lasting about 1 day at 4°C. To fluorescently label biocytin we used a protocol slightly modified from that described by Campbell et al. (2005). Briefly, after PBS rinse, brains were incubated with Texas Red–conjugated avidin (2.6 μl/ml; Vector Laboratories, Peterborough, UK) in PBS with 0.3% Triton X-100 and 0.3% bovine serum albumin for about 4 h at room temperature and kept in the dark. Brains were then rinsed in PBS three times for 24 h at 4°C and mounted in Vectashield aqueous mounting media (Vector Laboratories) on a customized glass microscope slide and covered with cover glass. Biocytin-filled neurons were visualized using an Olympus inverted scope with a Texas Red filter (600- to 650-nm emission) with a ×60 or ×10 fluorescent objective. Images were captured with a digital camera and Openlab software.
RESULTS
Anatomy of M-V neurons in the developing optic tectum
We used an isolated brain preparation with the tectal surface exposed to map the distribution of M-V cells in stage 48–49 tadpoles. M-V cells were easily distinguished from principal tectal neurons using a ×60 objective and differential interference contrast optics. The cells were identified based on several distinct morphological features reported in the literature (Fig. 1A; Kollros and Thiesse 1985; Lewis and Straznicky 1979): 1) Neuronal somata are noticeably larger than those of principal tectal cells and have a more lucent and smooth appearance. 2) The shape of the soma is rounder and less oval shaped than that of principal tectal neurons. 3) The cell nucleus is visibly larger. M-V cells could be found throughout the optic tectum. Figure 1B shows the position relative to the tectal edge of several M-V and principal tectal cells that we recorded in this study. We found no obvious spatial organization, which is not surprising since the tectum has not yet formed layers or fully differentiated at this stage in development (Lazar 1973). However, during these developmental stages (48–49), there was a higher probability of finding M-V cells in the more rostrolateral part of the tectum. In general, we were able to identify an average of only two large M-V cells per tectal lobe. The most that was ever observed in one tectum was five. We were not able to identify M-V cells at earlier developmental stages. To determine the morphology of the cells, we filled them with biocytin while recording. Out of nine M-V cells filled, no defined dendrites could be observed and all had a single axon (Fig. 1C). This is consistent with prior anatomical observations from adult frogs and more developed tadpoles describing M-V cells as being unipolar with no, or very small, dendrites (Kollros and Thiesse 1985; Lewis and Straznicky 1979; Lowe and Russell 1984). The axons of the filled M-V cells were found to project caudally and slightly ventrally through the tectum and then project caudally through ipsilateral hindbrain (Fig. 1D). Once in the brain stem, the axons appear to send out many small collaterals (Fig. 1, D and E). Axons appear to continue caudally past the hindbrain to the edge of the brain section as far as we could follow them. In adult frogs M-V axons have been described as continuing through the trigeminal nerve and ultimately innervating the face area (Hiscock and Straznicky 1982; Lowe and Russell 1984). In comparison, principal tectal neurons are known to have an extensive dendritic arbor and axon that can project both within the tectum and to other brain areas (Wu and Cline 2003; Wu et al. 1999).
M-V cells display a greater level of intrinsic excitability than principal tectal neurons
Distinct morphological features prompted us to compare the physiology of the large M-V neurons to the physiology of nearby principal tectal neurons. We carried out whole cell voltage- and current-clamp recordings of M-V cells and nearby principal tectal neurons to characterize intrinsic excitability and expression of intrinsic currents, responses to optic nerve stimulation, in vivo visual stimuli, direct hindbrain stimulation, and, finally, responses to somatosensory stimulation.
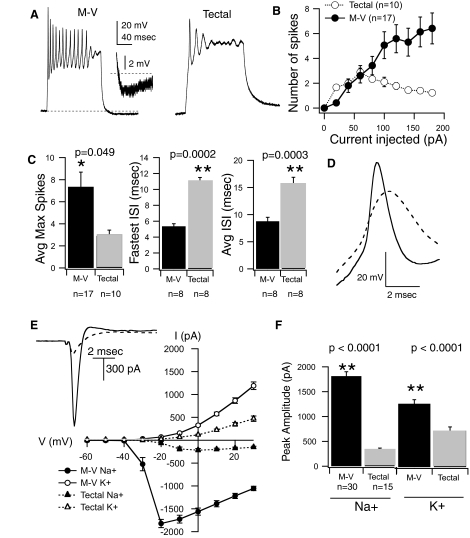
To characterize intrinsic excitability in both cell types we measured the number of action potentials a cell could generate in response to a series of intracellular injections of square current pulses, 200 ms in duration, of various amplitudes. Cells were routinely stepped from a baseline membrane potential of −60 mV, maintained by a small DC current injection. We observed that M-V cells were capable of firing more spikes than principal tectal neurons at most of the current injection levels tested (Fig. 2, A and B). In contrast to principal tectal neurons, the number of spikes generated by the M-V cells did not tend to plateau with increasing current injection amplitude. In principal tectal neurons the number of spikes often decreased at the higher current injection levels, a result of characteristically strong adaptation (Fig. 2B; Aizenman et al. 2003; Pratt and Aizenman 2007). Furthermore, the maximum number of spikes a cell could fire was significantly greater for the M-V cells (M-V = 7.4 ± 1.3 spikes, n = 17; tectal = 2.9 ± 0.5 spikes, n = 10; P = 0.049; Fig. 2C). The maximum number of spikes refers to the maximum number of spikes a neuron can fire independent of the current injection amplitude. The interspike interval (ISI), which is inversely proportional to spike rate, of M-V cells was about twice as fast as that of tectal neurons, regardless of the amount of current injected (average ISI for M-V cell = 8.8 ± 0.74 ms, n = 8; tectal = 15.6 ± 1.25 ms, n = 8; P = 0.0003; Fig. 2C). Therefore in response to DC injection, M-V cells not only fired more spikes, but could fire at significantly higher frequencies than principal tectal neurons. We also found differences in the spike form itself. On average, spikes recorded from M-V cells had significantly higher amplitude, faster rise time, and shorter half-width (Fig. 2D, Table 1). In a subset of M-V cells, we observed a slow, small afterhyperpolarization (AHP) following a depolarizing current injection (see Fig. 2A, inset). The AHP was never observed in principal tectal neurons.
FIG. 2.
Intrinsic membrane properties differ significantly between M-V and principal tectal neurons. A: example traces from whole cell current-clamp recordings of an M-V neuron (left) and a tectal neuron (right) that were held at −60 mV and given a square 100-pA current injection. Notice that the M-V neuron fired many more spikes compared with the tectal neuron. Tectal neurons tend to display strong accommodation, as demonstrated here by the inability of this tectal neuron to repolarize sufficiently. The inset shows a blow-up view of a small, slow afterhyperpolarization (AHP) seen in the M-V neuron. B: averaged input–output curves plotting the number of action potentials a neuron fired vs. amount of current injected. Notice that M-V neurons (filled circles) tended to fire more spikes than principal tectal neurons (empty circles) and that the number of spikes does not plateau with increasing current. C: averaged data showing that M-V neurons (black bars) fired significantly more spikes and at a higher frequency than principal tectal neurons (gray bars). D: averaged spike waveforms from multiple cells (solid line: M-V neurons; dashed line: principal tectal neurons). Notice that M-V action potential is greater in amplitude, peaks faster, and repolarizes faster and more completely than in principal tectal neurons. E: averaged current–voltage plots for M-V neurons (circles, solid lines, n = 30) and principal tectal neurons (triangles, dashed lines, n = 15). Voltage-gated Na+ current is inward (filled symbols). Voltage-gated K+ current is outward (empty symbols). Notice that M-V neurons display overall greater maximum Na+ and K+ currents. Inset, top left: examples of currents from an M-V neuron (solid line) and a principal tectal neuron (dashed line) in response to a voltage step that evoked their maximum (peak) Na+ current. F: averaged peak Na+ and K+ currents, regardless of the voltage step, for M-V neurons (black bars) and principal tectal neurons (gray bars). M-V neurons express larger peak Na+ and K+ currents. Notice also that the ratio of Na+ to K+ is larger for M-V neurons compared with tectal neurons.
TABLE 1.
Intrinsic properties of M-V and principal tectal neurons
| Property | M-V | Tectal |
|---|---|---|
| AP threshold, mV | −32.9 ± 2.0 (9) | −28.6 ± 0.78 (8) |
| AP height, mV | 49.7 ± 6.2 (9) | 38.12 ± 6.4 (8) |
| AP rise time, ms | 0.32 ± 0.11 (9) | 0.9 ± 0.06 (8) |
| AP rate of rise, mV/ms | 95.33 ± 3.01 (9) | 22.7 ± 2.04 (8) |
| AP half-width, ms | 1.2 ± 0.43 (9) | 3.26 ± 0.27 (8) |
| Resting membrane potential, mV | −60.6 ± 1.17 (20) | −47.9 ± 1.4 (10) |
| Input resistance, GΩ | 0.61 ± 0.03 (16) | 1.3 ± 0.08 (23) |
| Cell capacitance, pF | 16.6 ± 1.0 (16) | 13.6 ± 0.72 (16) |
Values are means±SE; n values are in parentheses.
Since the ability of a neuron to fire action potentials is largely dependent on the combined expression of its sodium and potassium currents, we expected that these currents might differ between the two cell types as well. To measure intrinsic currents the cell was held in voltage-clamp mode and stepped to increasingly more depolarized potentials, from −60 to +20 mV, and the mixed inward and outward currents evoked by each step were recorded (see methods; Fig. 2E, inset). In this preparation the inward and outward currents of tectal cells have previously been shown to be sufficiently temporally distinct to permit accurate separation of both Na+ and K+ peak current amplitudes (Aizenman et al. 2003; Pratt and Aizenman 2007). Currents can similarly be separated in M-V neurons. We find that in M-V neurons inward Na+ currents typically peak at around 1.25 ± 0.07 ms (n = 8) after the onset of the depolarizing step. If we use tetrodotoxin (TTX) to block Na+ channels and pharmacologically isolate the K+ current, we find that the K+ current starts to be activated at around 1.6 ± 0.05 ms (n = 3) in the voltage ranges at which the peak Na+ current is evoked. This occurs after the peak of the Na+ current. The K+ current reaches its maximal value at around 8.1 ± 0.25 ms after the onset of the depolarizing pulse. For this study, the amplitude of the K+ current was measured at the end of the voltage step, a few hundred milliseconds after the Na+ current is inactivated. At voltage levels at which the Na+ current is maximal, the amplitude of the K+ current is only as large as 4.7% of the Na+ current value. Taken together, these data suggest that not only are the peak of the Na+ current and the onset of the K+ current temporally distinct, but that at most, even if the currents were temporally overlapping, the measurement of the Na+ current would have an error of <5%. Thus the peak amplitudes of Na+ and K+ currents can be adequately separated and accurately measured in M-V neurons.
Using mixed-current traces evoked by depolarizing steps, current–voltage (I–V) graphs were generated for each cell type by plotting the peak inward and outward current as a function of voltage (Fig. 2E). We found that M-V cells expressed significantly larger maximum Na+ and K+ currents compared with their near-neighbor principal tectal cells (Fig. 2, E and F; Na+ max for M-V = 1,813 ± 89.8 pA, n = 30; for tectal cells = 332.6 ± 32 pA, n = 15; P < 0.0001; max K+ currents for M-V cells = 1,311.6 ± 85.6 pA, n = 30; for tectal cells, 754.8 ± 78, n = 15; P < 0.0001). The differences in current amplitudes between cell types cannot be accounted for by the fact that M-V neurons have larger cell bodies. Although the whole cell capacitance of M-V neurons was about 22% larger than that of principal tectal neurons (see Table 1), this small increase is not sufficient to account for the severalfold difference in Na+ and K+ current amplitudes. Accurately measuring voltage-dependent currents in whole cell recordings can be problematic due to suboptimal temporal and spatial control of the membrane voltage in the entire cell. In this preparation, however, we have found it feasible to measure voltage-gated Na+ and K+ currents since currents recorded from amphibian neurons are considerably slower and smaller than their mammalian counterparts. Furthermore, Xenopus tadpole neurons are small and compact, which may afford better spatial clamp (Aizenman et al. 2003; Pratt and Aizenman 2007). Nevertheless, to minimize some of these issues, we have restricted our analysis to describing only the maximum Na+ and K+ current recorded, regardless of the voltage step at which this maximum current occurred. In this way, we can be confident that we have captured its maximum current amplitude.
Passive properties also influence a neuron's intrinsic excitability. We found differences in both the input resistance and resting membrane potential. M-V cells had lower input resistances (M-V cell = 0.61 ± 1.0 GΩ, n = 16; tectal cell = 1.3 ± 0.08 GΩ, n = 23; P < 0.0001; see Table 1) and rested at more hyperpolarized membrane potentials (M-V cell = −60.6 ± 1.2 mV, n = 20; tectal cells = −48 ± 1.4 mV, n = 10; P < 0.0001; Table 1; reported membrane potential measurements are corrected for a 12-mV junction potential). These differences cannot account for the increase in excitability seen in M-V cells, since they would make the cell less excitable, not more.
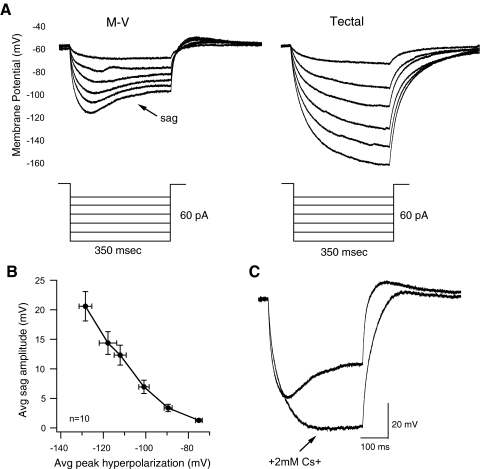
Ih is present in M-V cells
Ih is an inward mixed-cation current that is activated at hyperpolarized potentials and is present in various cell types with bursting phenotypes (Pape 1996). To find out whether either cell type expressed Ih, we held the cell at −60 mV in current-clamp and injected a series of square hyperpolarizing current injections of various amplitudes, each lasting 350 ms. The M-V cells expressed what appeared to be Ih in the form of a depolarizing “sag” during the hyperpolarizing pulse. This sag increased in magnitude with increasing hyperpolarizing steps (n = 11 cells; see example in Fig. 3 A, left). In contrast, none of the principal tectal neurons measured exhibited this sag (n = 22 cells; see example in Fig. 3A, right).
FIG. 3.
M-V neurons display an H-type current that is activated in response to hyperpolarizing steps. A: responses of an M-V neuron (left) and principal tectal neuron (right) held at −60 mV to hyperpolarizing steps of different amplitudes. Notice that the MV neuron displays a depolarizing potential or “sag” during the hyperpolarizing step, whereas the principal tectal neuron responses do not. Also, principal tectal neuron responses reach greater hyperpolarized potentials compared with those of M-V neurons, likely due to lack of the sag current. B: plot of average sag amplitude in M-V cells as a function of average peak hyperpolarization. Observe how the absolute amplitude of sag potential (the absolute difference, in mV, between initial peak hyperpolarization and the potential at which the sag plateaus) increases as the cell is stepped to more hyperpolarized potentials. C: an M-V neuron response to the most hyperpolarized step before and after washing in 2 mM Cs+ into the external media. Cs+ effectively abolishes the depolarizing sag and the resulting response looks more nearly similar to that of a principal tectal neuron.
In M-V neurons, for any given current injection, we observed a direct correlation between Ih amplitude and the initial peak hyperpolarized potential (Fig. 3B). Ih is known to be responsible for rebound spiking after the hyperpolarizing step in some neurons (Pape 1996). Although we did not see rebound spiking in the M-V cells on release from hyperpolarization, there was a small subthreshold rebound depolarization (Fig. 3A, left). To verify that this depolarizing sag is indeed Ih, we perfused Cs+ (2 mM CsCl)—which is known to block Ih—into the extracellular media. Cs+ completely blocked the depolarizing sag (Fig. 3C; n = 5), confirming that M-V cells express a classic Ih.
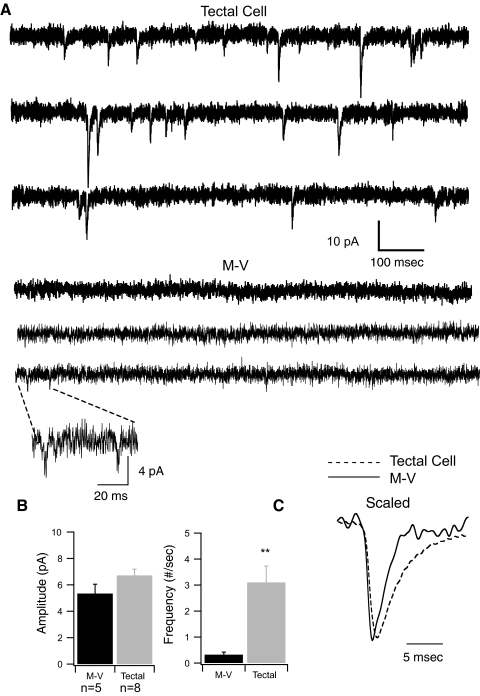
Spontaneous synaptic activity in M-V neurons
Prior electron microscopy (EM) studies had observed synaptic terminals in M-V neurons in frogs, but it was not clear whether these formed functional synapses (Munoz and Gonzalez 1990). We previously showed that spontaneous excitatory postsynaptic currents (sEPSCs) in stage 48–49 principal tectal neurons have relatively smaller amplitudes than those during earlier stages, but occur at a relatively higher frequency so that the overall amount of background synaptic drive received by principal tectal cells increases over development (Pratt and Aizenman 2007). We tested whether stage 48 M-V cells received an amount of synaptic drive similar to that of their nearby principal tectal neuron neighbors. Spontaneous synaptic events were recorded with the cell voltage clamped at −60 mV. Examples of spontaneous events from the two different cell types are shown in Fig. 4 A. It was clear that sEPSCs occurred much less frequently in the M-V cells compared with principal tectal neurons (M-V frequency = 0.33 ± 0.10 events/s, n = 5; tectal neurons = 3.1 ± 0.63 events/s, n = 8; P = 0.0016; Fig. 4B). Moreover, the frequency of events for M-V cells that we report here included only those neurons in which events could be detected. In about 50% of these cells, no spontaneous synaptic events were detected at all (see methods). This does not rule out the possibility that our detection method was not sufficiently sensitive to pick out very small events that may get lost in the noise. sEPSC amplitudes recorded from M-V and principal tectal neurons were not significantly different from one another overall (tectal cell average amplitude = 6.7 ± 0.48, n = 8; M-V = 5.3 ± 0.71, n = 5; P = 0.12). However, principal tectal cells tended to have a subpopulation of larger events, but due to the high frequency of smaller events the differences in sEPSC amplitudes for M-V neurons and principal tectal cells did not reach statistical significance. Events recorded from M-V cells also appeared to have a faster decay rate (Fig. 4C). The low frequency and faster kinetics of M-V sEPSCs are consistent with the fact that these cells do not have complex dendritic arbors and have synapses directly on the soma.
FIG. 4.
Voltage-clamp recordings of spontaneous postsynaptic currents. A, top 3 lines: traces showing spontaneous excitatory synaptic currents recorded in voltage clamp from one principal tectal neuron. Each line sweep has a 1-s duration. Bottom 3 sweeps are typical spontaneous events from an M-V neuron. Notice the paucity of events recorded from the M-V neuron compared with the principal tectal neuron. Inset: a scaled-up trace containing 2 small synaptic events. B: average synaptic amplitudes and frequencies for M-V (black bars) and principal tectal neurons (gray bars). C: scaled averaged synaptic waveforms from multiple neurons (dashed line: principal tectal neurons, n = 8; solid line: M-V neurons, n = 5). Notice that the rates of rise and decay appear faster for averaged M-V synaptic events.
Evoked visual responses in M-V cells
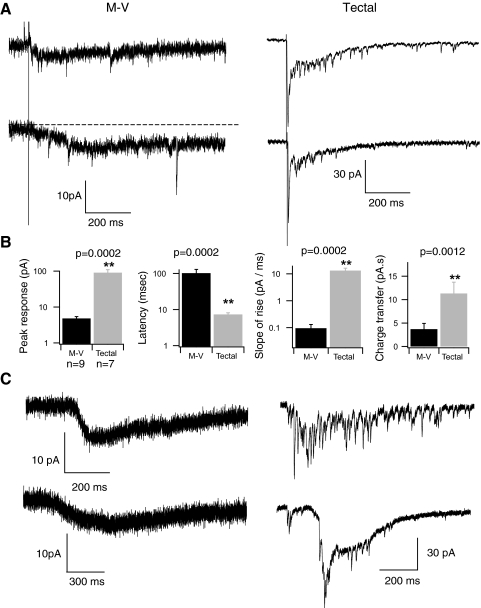
Although the above-cited recordings, together with anatomical evidence, suggest that the somata of M-V cells in the Xenopus optic tectum receive some synaptic contacts, the origin of the synapses remains unknown. Although M-V cells are primary somatosensory neurons, their physical location in the optic tectum raises the interesting possibility that they may be activated by visual input. Principal tectal neurons are known to receive direct glutamatergic synaptic input from retinal ganglion cells (RGCs). We compared responses to RGC axon stimulation in M-V and principal tectal cells by stimulating RGC axons directly in a whole-brain preparation (see methods). In response to RGC stimulation, principal tectal neurons usually exhibit not only a fast, inward monosynaptic response but also a secondary recurrent response generated by polysynaptic tectal–tectal connections (Fig. 5 A, right traces; Pratt et al. 2008). In contrast, none of the M-V cell responses displayed this biphasic response (Fig. 5A, left traces). Instead, optic nerve stimulation reliably produced responses in M-V neurons that were significantly smaller in amplitude (Fig. 5B; M-V peak amplitude = 4.8 ± 0.6 pA, n = 9; tectal cell = 90.5 ± 19.2, n = 7; P = 0.0002); had a longer latency to peak (M-V latency to peak = 101.7 ± 27.8 ms, n = 9, tectal = 7.3 ± 0.82, n = 7; P = 0.0002); and even though M-V responses had a noticeably slower decay, the maximum responses carried less charge than that of tectal cell responses (M-V average maximum total charge = 3.68 ± 1.2 pA·s, n = 9; tectal = 11.3 ± 2.4, n = 7; P = 0.0012). Occasionally during some of these slow, shallow responses, we observed what appear to be small, fast synaptic events (Fig. 5A) similar those we observed while recording spontaneous synaptic activity. Due to the small amplitude of the slow responses, it was difficult to accurately calculate their reversal potential. However, M-V responses did tend to become smaller when the cell was depolarized toward 0 mV, consistent with the fact that they are excitatory.
FIG. 5.
Visually driven responses in M-V and principal tectal neurons. A: 2 examples of maximal M-V (left) and principal tectal (right) responses to direct retinal ganglion cell (RGC) axon stimulation in a whole-brain preparation. B: averaged data obtained from direct RGC axon stimulation. Far left: tectal neurons display about a 10-fold greater peak response to RGC stimulation and (2nd from left) a much shorter latency to peak. Furthermore, the rise time of the response is much less for principal tectal neurons, as indicated by a larger slope (2nd from right). The overall average charge transferred in response to RGC stimulation is also significantly greater for principal tectal neurons compared with M-V neurons (far right). Black bars: M-V neurons; gray bars: principal tectal neurons. C: examples of maximal M-V and principal tectal neuron responses to a whole-field flash of white light recorded in vivo. Notice that these in vivo light-evoked responses have characteristics similar to the responses of direct RGC axon stimulation shown in A.
Tectal cells receive direct glutamatergic input from RGC axons (Wu et al. 1996). Blocking glutamatergic transmission eliminates the monosynaptic response as well as the recurrent synaptic activity (Pratt et al. 2008). To see if the M-V cell responses to RGC stimulation are mediated by glutamate we recorded responses in the presence of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor blockers d-2-amino-5-phosphonovaleric acid (d-APV, 50 μM) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 30 μM). We found that blocking glutamate receptors blocked the response to RGC stimulation in M-V cells (n = 5) and, as expected, in the principal tectal cells (data not shown). Although this experiment demonstrates that the M-V neuron response to RGC axon stimulation is dependent on glutamatergic synaptic transmission, it does not address whether the M-V cells are receiving direct input from RGCs or from nearby tectal neurons, since synaptic blockade inhibits both possible sources of input. Due to the slow kinetics and small amplitudes of the visual responses in M-V neurons, it was not possible to accurately calculate the onset time of the responses. This would have helped to determine whether these cells are activated monosynaptically by visual inputs.
M-V synaptic responses to RGC axon stimulation are slow, similar to those mediated by metabotropic glutamate receptors (mGluRs; Coutinho and Knopfel 2002). To test whether mGluRs may mediate, at least in part, M-V responses to RGC stimulation, we evoked responses in the presence of the mGluR receptor blocker α-methyl-4-carboxyphenylglycine (MCPG, 500 μM). This manipulation did not completely block the response in M-V cells, although it did decrease the maximum charge of the response by about 33% (average charge of M-V response in the presence of MCPG = 2.4 ± 0.9 pA·s, n = 4) and decreased the peak amplitude of the response by almost 40% (amplitude of M-V response in MCPG = 2.9 ± 0.6 pA, n = 4). Similarly, blocking mGluR receptors also decreased the maximum charge of principal tectal cell responses by 30% (average charge of tectal response in presence of MCPG = 8.0 ± 2.5 pA·s, n = 8). MCPG had no effect on the peak amplitude of the early monosynaptic component of the tectal response (average peak response of tectal neurons in the presence of MCPG = 87.3 ± 8.1 pA·s, n = 8) and thus the MCPG-induced reduction in total charge on principal tectal neurons likely resulted from reduction of polysynaptic activity. Based on the observation that M-V responses are slow and that MCPG decreases by the same amount the total charge of the response of both principal tectal neurons and M-V neurons, one possibility is that M-V cells receive their synaptic drive from nearby tectal neurons in the form of recurrent activity and not directly from the RGC axons. However, our data cannot rule out the existence of direct monosynaptic input onto M-V cells from RGC axons.
To address the possibility that optimal M-V responses to optic nerve stimulation may have been compromised by using a whole brain preparation, we recorded synaptic responses to whole-field visual stimuli in vivo (Fig. 5C). We found that in vivo visual responses were not statistically different from responses evoked by direct RGC axon stimulation (average area of M-V response to whole field flash = 4.4 ± 1.2 pA·s, n = 7; M-V response to RGC stimulation = 3.68 ± 1.2 pA·s, n = 9; P = 0.3; average peak amplitude of M-V response to whole field flash = 6.3 ± 1.9 pA, n = 7; amplitude of RGC response = 4.8 ± 0.6 pA, n = 9; P = 0.92). Together these data suggest that these large cells located in the optic tectum receive relatively modest but reliable synaptic drive in response to visual input.
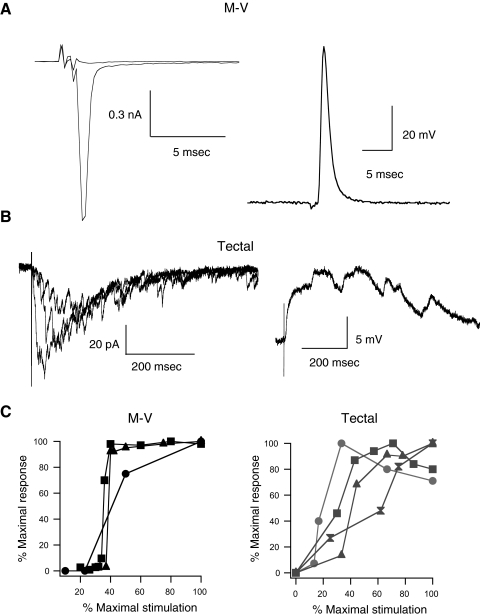
Hindbrain stimulation evokes an antidromic spike in M-V cells
One possible interpretation of our results is that the population of cells we are recording from might be very immature tectal neurons rather than M-V neurons. If M-V neurons are really a separate neuronal population composed of primary sensory trigeminal neurons, then it should be possible to antidromically activate their axon by stimulating in the sensory periphery. Based on our anatomical evidence (Fig. 1) and on published results (Hiscock and Straznicky 1982; Lowe and Russell 1984), the axon of M-V neurons travels along the trigeminal nerve, through the hindbrain, and connects to the cell soma in the tectum. Thus we first characterized whether an antidromic action potential could be evoked in M-V cells by directly stimulating the hindbrain. A bipolar stimulating electrode was placed in the hindbrain caudal to the tectum, about 100 μm from the edge of the tectum. Both contralateral and ipsilateral responses were tested. In five of five M-V cells recorded in voltage-clamp, ipsilateral but not contralateral hindbrain stimulation resulted in a large, fast, all-or-nothing inward current (Fig. 6 A, left). The nearly all-or-nothing nature of the response is illustrated in the input–output curves of those neurons (Fig. 6C, left). The average maximum response was 985 ± 154 pA (n = 5) and was similar in shape, but smaller in amplitude, to the depolarization-evoked voltage-gated Na+ currents (Fig. 2). In current-clamp mode, hindbrain stimulation led to a short-latency, antidromic action potential, which was also all-or-nothing. Since an antidromic spike does not involve synaptic transmission, it should persist in the presence of synaptic blockers. We found that glutamatergic synaptic blockade (dAPV and NBQX) had no effect on the response of the M-V cell to hindbrain stimulation (n = 4; data not shown). Perfusing in the sodium channel blocker TTX, however, completely abolished the response (n = 2; data not shown). These observations further confirm that M-V responses to hindbrain stimulation are not synaptic, but result from an antidromic spike activated by direct stimulation of the M-V cell axons. In contrast, the response observed in principal tectal cells to hindbrain stimulation was quite different. We never observed an antidromic spike in principal tectal cells, although both ipsi- and contralateral hindbrain stimulation resulted in a significant synaptic response (Fig. 6B). These responses were similar to responses to RGC stimulation: there was usually a faster monosynaptic component followed by recurrent activity. Furthermore, synaptic responses were graded and increased gradually as stimulus intensity was increased (Fig. 6, B and C). The optic tectum, like its mammalian homolog the superior colliculus, is known to receive inputs from multiple sensory modalities. Our findings suggest that even during early development, principal tectal cells can receive input from both visual and other sensory modalities that ultimately project to the tectum via various sensory nuclei in the hindbrain (Butler and Hodos 2005). Our findings also suggest that principal tectal neurons and M-V cells constitute different cell types that are integrated into the tectal circuitry in distinct ways.
FIG. 6.
Nonvisual inputs to M-V and principal tectal neurons. A: example of antidromic spiking recorded in voltage (left) and current-clamp (right) configurations in response to direct hindbrain stimulation. Notice the rapidly activated, all-or-nothing response with a very fast onset and lack of polysynaptic activity. These are all consistent with the response being an antidromic spike. B: example of a principal tectal neuron response to hindbrain stimulation in voltage clamp (left) and current clamp (right). Notice the graded nature of the voltage-clamp responses and that the tectal response includes a complex polysynaptic component. C: input–output curves in response to hindbrain stimulation for several M-V neurons (left) and principal tectal neurons (right). Responses are expressed as a percentage of the maximum response for each individual neuron to normalize the data. Again notice that the tectal neuron responses tend to be graded, whereas the M-V responses have a more sudden onset.
Ipsilateral tentacle stimulation evokes an antidromic spike in M-V cells
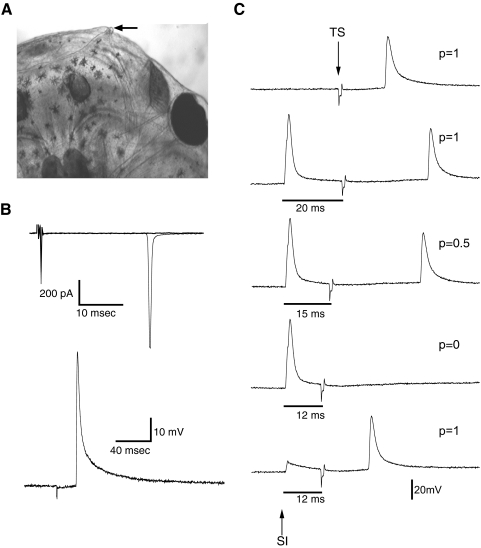
Once we characterized antidromic spikes in M-V evoked by hindbrain stimulation, we tested whether we could directly activate them by stimulating their peripheral terminals. In frogs and rodents, M-V cells have been shown to receive input from the mandibular branch of the trigeminal nerve. Xenopus tadpoles are unique in that they have a small specialized tentacle at either corner of their mouth. During the developmental stages studied here, the tentacle is just beginning to develop (Fig. 7 A). This tentacle is thought to function as a sensory organ that is highly innervated by nervous tissue originating from the mandibular branch of the trigeminal nerve (Ovalle et al. 1998). Furthermore, the jaw area in general also appears to be highly innervated by somatosensory nerve endings (Roberts 1998). We tested whether M-V cells receive input from the jaw and tentacle by recording from cells in vivo while using a stimulating electrode to map electrically evoked responses from the skin area around these structures. Electrical stimulation consisted of single pulses delivered by a bipolar stimulating electrode placed directly against the skin (see methods). Pulse amplitude ranged between 100 and 200 μA with a duration of 0.2 ms. We observed that electrical stimulation of the tentacle evoked an all-or-nothing response that was similar to what was observed during hindbrain stimulation, but occurring at a longer latency (Fig. 7B). Similarly, the response to tentacle stimulation was not affected by the presence of synaptic blockers (n = 5; data not shown), consistent with the idea that electrical stimulation of the tentacle is directly activating the neuron's axon antidromically. Responses in M-V cells could not be evoked by stimulating the skin in locations other than the tentacle, including those immediately adjacent to the tentacle, suggesting that the electrical stimulus remains fairly localized and that M-V neurons appear to selectively innervate the tentacle. Interestingly, stimulating the tentacle did not evoke responses in principal tectal neurons, but synaptic responses could be observed in these neurons in response to stimulation of the jaw area. However, these responses were not characterized further in this study.
FIG. 7.
Antidromic spikes in M-V neurons are evoked in vivo by direct electrical stimulation of the tentacle. A: overhead view of the anterior right portion of the head of a stage 49 Xenopus tadpole. Arrow pointing to small right tentacle located at the corner of the mouth. Through development, the tentacle will grow several millimeters in length. B: an example of an in vivo M-V neuron response to ipsilateral tentacle stimulation recorded in voltage clamp (top) and current clamp (bottom trace). The waveform of these responses matches those evoked by hindbrain stimulation in Fig. 6. C: collision test to confirm that tentacle-evoked responses are antidromic. Top trace: neuron spikes consistently in response to tentacle stimulation (TS). “p” indicates the probability of seeing a spike after a stimulus. Second trace from top: somatic current injection (SI) is added, preceding tentacle stimulation, in this case by 20 ms. Here, neuron spikes consistently in response to both stimuli. Third trace from top: now SI has been adjusted to precede TS by 15 ms. At this time interval, we observe a 50% decrease in probability of TS eliciting a spike. Fourth trace from top: when SI precedes TS by 12 s, a TS-evoked spike is never observed since it has essentially been occluded by the presence of the SI-generated spike in close temporal proximity. Bottom trace: if the strength of SI is reduced so that only a subthreshold depolarization occurs, even at a 12-ms time interval, TS successfully elicits a spike. These results are consistent with the fact that spikes evoked by tentacle stimulation are indeed antidromic.
To further confirm that responses in M-V cells to tentacle stimulation were antidromic spikes, we carried out a collision test. The collision test is a classic method for mapping efferent and afferent projections (Lipski 1981). In this test, two spikes are evoked at different sites in the same neuron, one at the cell soma and one at the axon terminal. As the temporal interval these two spikes is decreased, the somatic spike “collides” with the antidromic spike originating at the axon terminal and cancels it out (Lipski 1981). Here, we injected current directly into the soma of the M-V cell, then, at some interval after that, we electrically stimulated the tentacle, to evoke an action potential. If there were a direct projection from the tentacle to the soma, then we would expect, at a given time interval, the spike evoked by somatic injection to interfere, or collide, with the spike that originated at the tentacle. An example of this is illustrated in Fig. 7C. First, tentacle stimulation alone was shown to reliably activate an action potential with essentially no failures (Fig. 7C, top trace). Then we added a preceding somatic current injection pulse that reliably evokes a spike. The first time interval between the two stimulations was set far enough apart that we observed a spike in response to both stimulations every time without fail. An example of this response is shown in Fig. 7C, second trace. As the time interval between the somatic spike and the tentacle-evoked spike is decreased, the probability of seeing a spike from the tentacle stimulation also decreased. The interval was further reduced until a spike evoked by tentacle stimulation was never observed (Fig. 7C, fourth trace). If the somatic spike is truly canceling out the tentacle-evoked spike, then the tentacle-evoked spike should reappear if the somatic injection is reduced to subthreshold levels. This prediction was borne out and is shown in Fig. 7C, bottom trace. Although the specific interstimulus intervals varied from cell to cell, the collision test was effective in all cells where it was performed (n = 3). These experiments confirm that M-V cells are specialized primary somatosensory neurons that have an axon projecting from the tentacle to the soma in the optic tectum.
DISCUSSION
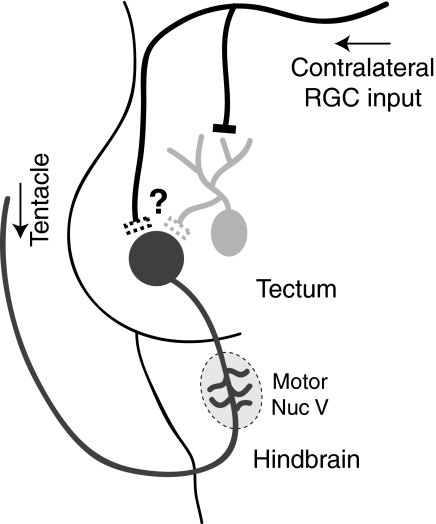
Our experiments confirm that M-V neurons in the Xenopus tadpoles are unipolar primary somatosensory neurons residing in the optic tectum. Their principal axon originates in the tentacle, a transient sensory structure located next to the mouth. M-V neurons can be distinguished morphologically and electrophysiologically from principal tectal neurons. Similarly to principal tectal neurons, M-V cells receive input from multiple sensory modalities, but are integrated differently into the tectal circuitry. M-V cell axons appear to be directly activated by peripheral somatosensory activation and are also synaptically activated in vivo and in vitro by visual input (Fig. 8). Taken together, these experiments suggest that M-V neurons may play an integrative, multisensory role during tadpole development.
FIG. 8.
Proposed wiring diagram and model of M-V neurons in Xenopus laevis tadpoles. M-V neurons are unipolar cells with their somata located within the optic tectum. They have a single axon that exits the tectum caudally, sprouting small collaterals within the hindbrain, likely making synapses onto the trigeminal motor nucleus. The axon then exits the brain via the trigeminal nerve and innervates the ipsilateral tentacle located at the corner of the mouth. Somatosensory information from the tentacle directly triggers an antidromic spike in the M-V axons that travels toward the brain, presumably causing neurotransmitter release in the hindbrain collaterals. M-V somata also receive slow visual input either directly via RGC axons or indirectly from principal tectal neurons via local projections. This input may serve to modulate release induced by tentacle stimulation or may facilitate oscillatory bursting behavior in more mature neurons.
Identification of M-V cells
Our anatomical observations coincide well with previous descriptions of M-V cells in Xenopus laevis. Like earlier descriptions of M-V cells, we observed that these cells have a relatively large, round soma, a large nucleus, and the soma appears lucent, not grainy (Kollros and Thiesse 1985; Lewis and Straznicky 1979). These morphological characteristics make M-V neurons easy to identify for electrophysiological recording. The position and number of cells observed here also correlate well with prior studies that quantify the number of M-V cells based on morphology. Previous studies report anywhere between 2 and 20 M-V neurons per tectum in stage 48–50 tadpoles and cells were reported not to be clustered within a distinct nucleus (Kollros and Thiesse 1985; Lewis and Straznicky 1979).
M-V cells have previously been described as unipolar, primary sensory neurons in amphibians (Kollros and Thiesse 1985), chickens (Rogers and Cowan 1973), and rodents (Croydon et al. 1999). This is consistent with our biocytin fills, which reveal that these cells do not have dendrites, but have a long process that projects caudally through the tectum and hindbrain. The finding that antidromic spikes could be evoked in the presence of synaptic blockers, together with results from the collision test, further confirm that these cells project all the way to the sensory periphery and are thus truly primary sensory neurons that are situated within the CNS. The finding that M-V cells are directly activated by electrical stimulation of the tentacle, which is known to be innervated by the mandibular branch of the trigeminal nerve (Ovalle et al. 1998), is also consistent with the fact that M-V neurons form part of the trigeminal system. Ideally, we would have been able to activate M-V neurons using actual somatosensory stimuli, such as touching or gentle pressure, rather than electrical stimulation. However, because the tentacle is so short in these young tadpoles (see Fig. 7A), we found that any attempts to use mechanical stimuli in our present experimental setup severely compromised the stability of our recordings. Thus it was not possible to do this experiment. Nonetheless, based on the well-described somatosensory function of M-V neurons in various other systems and the anatomical and electrophysiological evidence presented here that their peripheral axon innervates the Xenopus tentacle, it is likely that these neurons would normally be activated by some type of mechanical stimuli sensed by the tentacle. A more detailed parametric study of the range of mechanical stimuli that activate M-V neurons in tadpoles will thereby be left to a further dedicated study.
The observation that M-V cells directly innervate the tentacle is interesting in light of previous studies. In the Xenopus tectum, the number of M-V cells increases in number through stage 57–58, which is near the onset of the metamorphic climax (Nieuwkoop and Faber 1956). During metamorphosis, the number of M-V cells begins to decrease (Kollros and Thiesse 1985). Interestingly, the tentacle also begins to degenerate around this time, some of the tissue being resorbed into the skin around the jaw (Nieuwkoop and Faber 1956; Ovalle et al. 1998). This degeneration may explain the postmetamorphic decrease in the number of M-V cells. Thus during development at least a subset of M-V neurons constitute a transient population that can innervate a transient sensory organ. It is unclear whether M-V cells innervating the mouth and jaw later in development, and in adults, are ones that develop later or whether surviving ones reinnervate new target areas.
M-V cells are electrophysiologically distinct from principal tectal neurons
We found that M-V cells display much larger voltage-gated Na+ and K+ currents compared with nearby principal tectal cells. Furthermore, in these cells the peak amplitude of the Na+ current is much greater in magnitude than the peak amplitude of the K+ current, whereas principal tectal neurons typically express peak K+ currents that are significantly greater in amplitude than peak Na+ current. The increased Na+ to K+ current ratio is likely responsible for the ability of M-V neurons to fire multiple spikes at higher frequencies (Aizenman et al. 2003; Pratt and Aizenman 2007). Although M-V cells were able to generate more action potentials than principal tectal neurons in response to sustained current injection, we found a lot of variability in the number of spikes a given M-V cell could fire. It ranged from one spike to 18 spikes in a 200-ms current-injection step. One possibility is that this variability is due to the immature age of these M-V cells, which are just beginning to appear during these developmental stages. M-V cells also exhibit an H-type current, whereas tectal cells do not. Ih has been proposed to be a characteristic of neurons that burst (Pape 1996), although we did not observe bursting in these cells. Again, this lack of bursting might be explained by the fact that we are recording from immature M-V cells and perhaps they develop the ability to burst later in development, similarly to their mammalian counterparts (Wu et al. 2001).
Multisensory integration in M-V neurons
In these experiments, M-V neurons and principal tectal cells both appear to process multisensory information, but are integrated differently into the optic tectal circuitry. Here we show that M-V neurons receive somatosensory input in the form of action potentials resulting from electrical activation of the sensory periphery, while they receive visual input via synaptic contacts originating either from RGC terminals directly or indirectly via collaterals of principal tectal neurons (Fig. 8). In contrast, principal tectal cells receive both somatosensory and visual information in the form of graded synaptic input via separate pathways, suggesting that both cell types integrate multisensory information differently. M-V cells were found to have axonal collaterals within the hindbrain, consistent with anatomical observations proposing that M-V cells synapse onto trigeminal motor nuclei (Hiscock and Straznicky 1982). These collaterals may form the basis of a somatosensorimotor loop regulating movement of the tentacle or mouth.
What is the nature of the visual responses in M-V neurons? In contrast to principal tectal neurons, visual input to M-V neurons evokes a slow, low-amplitude response. Visual responses and all spontaneous synaptic activity in M-V neurons could be completely blocked by AMPA and NMDA receptor blockers. However, this does not conclusively prove that visual responses are mediated by these receptors, since M-V cells could receive visual input from principal tectal neurons and these blockers have been shown to block visual input to these neurons (Pratt and Aizenman 2007; Zhang et al. 1998). Based on the slow time course of M-V visual responses, however, it is unlikely that they are mediated by a direct activation of AMPA and NMDA receptors by RGC terminals, unless these are acting in a slow paracrine fashion via glutamate spillover (Diamond 2002). Alternatively, M-V cells could receive indirect recurrent input from principal tectal neurons. This recurrent activity reflects the activity of the tectal network (Pratt et al. 2008) and can be mediated by AMPA, NMDA, or mGlu receptors. Blockers of mGluRs also caused a significant reduction of visual responses in M-V neurons, but also reduced recurrent excitation in principal tectal cells, making it unclear whether the mGluRs are situated on the M-V cells or whether they are responsible for maintaining tectal network activity in principal neurons. Further experiments designed to elucidate the different neurotransmitter receptor subtypes expressed by M-V neurons should help to clarify these issues.
What is the functional significance of the visual input onto M-V neurons? M-V cells have been thought to regulate feeding behavior. In rats, bursting of M-V cells is thought to be involved in generating repetitive chewing motions by forming part of a somatosensorimotor loop. In Xenopus, their anatomical localization within the optic tectum led to the hypothesis that M-V cells may be involved in a motor-snapping response that is triggered by visual cues associated with food presentation (Lowe and Russell 1984). Interestingly, in Xenopus and Rana frogs M-V cells are first observed during the time when tadpoles begin to filter feed (Kollros and McMurray 1955) and in Xenopus this also coincides with a period in development when the tentacle first appears. One interpretation is that in tadpoles M-V cells form part of a feedback loop where somatosensory cues sensed by tentacle, together with slow modulatory visual inputs, help facilitate the characteristic opening and closing that occur as the tadpoles filter feed. Although the visual responses measured in M-V cells were usually too small to evoke action potentials, they may still affect the output of the M-V cell by bringing the membrane potential closer to spike threshold. In this way, visual input may be playing a modulatory role, enhancing somatosensory responses either by increasing spike frequency and probability or by facilitating neurotransmitter release presynaptically (Alle and Geiger 2008) in the hindbrain. Furthermore, as these cells acquire a bursting phenotype, visual input may also facilitate burst generation.
Conclusions
The optic tectum and its mammalian homolog the superior colliculus are brain areas important for multisensory integration and contain neurons responsive to a variety of sensory modalities. Here we describe, for the first time, the presence of functional, visually driven synaptic contacts in M-V neurons—a distinct population of primary somatosensory neurons located within the tectum. We also found that M-V cells appear to directly innervate the Xenopus tentacle, a transient sensory organ in the mouth area. Taken together these data suggest that M-V cells may play an integrative role in which visual input could modulate the tentacle-driven output of M-V neurons. Further experiments aimed at identifying the postsynaptic targets of M-V neurons in the hindbrain will further explore this possible modulatory role for visual activity in regulating motor output.
GRANTS
This work was supported by a National Research Service Award from the National Eye Institute to K. G. Pratt and by funds from the Klingenstein Foundation.
Acknowledgments
We thank members of the Aizenman lab and S. Udin for helpful discussion during the project, J. Sullivan for comments on the manuscript, and I. Sears for technical support.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. [DOI] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol 18: 314–320, 2008. [DOI] [PubMed] [Google Scholar]
- Alley KE Quantitative analysis of the synaptogenic period in the trigeminal mesencephalic nucleus. Anat Rec 177: 49–59, 1973. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. Hoboken, NJ: Wiley, 2005.
- Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology 146: 1163–1169, 2005. [DOI] [PubMed] [Google Scholar]
- Copray JC, Ter Horst GJ, Liem RS, van Willigen JD. Neurotransmitters and neuropeptides within the mesencephalic trigeminal nucleus of the rat: an immunohistochemical analysis. Neuroscience 37: 399–411, 1990. [DOI] [PubMed] [Google Scholar]
- Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist 8: 551–561, 2002. [DOI] [PubMed] [Google Scholar]
- Croydon A, Millar B, Linden R, Maden M. Mesencephalic innervation of the vibrissal follicle-sinus complex in the mouse embryo. Int J Dev Neurosci 17: 401–409, 1999. [DOI] [PubMed] [Google Scholar]
- Diamond JS A broad view of glutamate spillover. Nat Neurosci 5: 291–292, 2002. [DOI] [PubMed] [Google Scholar]
- Hiscock J, Straznicky C. Peripheral and central terminations of axons of the mesencephalic trigeminal neurons in Xenopus. Neurosci Lett 32: 235–240, 1982. [DOI] [PubMed] [Google Scholar]
- Johnston JB The radix mesencephalica trigemini. J Comp Neurol Psychol 19: 593–644, 1909. [Google Scholar]
- Kollros JJ, McMurray MV. The mesencephalic V nucleus in anurans. I. Normal development in Rana pipiens. J Comp Neurol 102: 47–63, 1955. [DOI] [PubMed] [Google Scholar]
- Kollros JJ, Thiesse ML. Growth and death of cells of the mesencephalic fifth nucleus in Xenopus laevis larvae. J Comp Neurol 233: 481–489, 1985. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE Rohon–Beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol 189: 323–333, 1980. [DOI] [PubMed] [Google Scholar]
- Lamborghini JE Disappearance of Rohon–Beard neurons from the spinal cord of larval Xenopus laevis. J Comp Neurol 264: 47–55, 1987. [DOI] [PubMed] [Google Scholar]
- Lazar G The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat 116: 347–355, 1973. [PMC free article] [PubMed] [Google Scholar]
- Lewis S, Straznicky C. The time of origin of the mesencephalic trigeminal neurons in Xenopus. J Comp Neurol 183: 633–645, 1979. [DOI] [PubMed] [Google Scholar]
- Lipski J Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Russell IJ. The relation between soma position and fibre trajectory of neurons in the mesencephalic trigeminal nucleus of Xenopus laevis. Proc R Soc Lond B Biol Sci 221: 437–454, 1984. [DOI] [PubMed] [Google Scholar]
- Luo P, Dessem D. Morphological evidence for recurrent jaw-muscle spindle afferent feedback within the mesencephalic trigeminal nucleus. Brain Res 710: 260–264, 1996. [DOI] [PubMed] [Google Scholar]
- Manni E, Bortolami R, Battistaazzena G. Jaw muscle proprioception and mesencephalic trigeminal cells in birds. Exp Neurol 12: 320–328, 1965. [DOI] [PubMed] [Google Scholar]
- Munoz M, Gonzalez A. Electron microscopic observations of the trigeminal mesencephalic nucleus in the frog, Rana ridibunda. J Hirnforsch 31: 341–348, 1990. [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). New York: Garland, 1956.
- Ovalle WK, Shinn SL, Nahirney PC. Ultrastructure of the larval tentacle and its skeletal muscle in Xenopus laevis. Tissue Cell 30: 216–225, 1998. [DOI] [PubMed] [Google Scholar]
- Pape HC Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327, 1996. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Pose IE, Yamuy J, Chase MH, Morales FR. Oscillatory membrane potential activity in the soma of a primary afferent neuron. J Neurophysiol 82: 1465–1476, 1999. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11: 467–475, 2008. [DOI] [PubMed] [Google Scholar]
- Roberts A Skin sensory systems of amphibian embryos and young larvae. In: Amphibian Biology, edited by Heatwole H. Chipping Norton, NSW, Australia: Surrey Beatty & Sons, 1998, p. 923–935.
- Roberts BL, Witkovsky P. A functional analysis of the mesencephalic nucleus of the fifth nerve in the selachian brain. Proc R Soc Lond B Biol Sci 190: 473–495, 1975. [DOI] [PubMed] [Google Scholar]
- Rogers LA, Cowan WM. The development of the mesencephalic nucleus of the trigeminal nerve in the chick. J Comp Neurol 147: 291–320, 1973. [DOI] [PubMed] [Google Scholar]
- Weinberg E The mesencephalic root of the fith nerve, a comparative anatomical study. J Comp Neurol 46: 249–405, 1928. [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science 274: 972–976, 1996. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Time-lapse in vivo imaging of the morphological development of Xenopus optic tectal interneurons. J Comp Neurol 459: 392–406, 2003. [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci 19: 4472–4483, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Hsiao CF, Chandler SH. Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. J Neurosci 21: 3729–3739, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature 395: 37–44, 1998. [DOI] [PubMed] [Google Scholar]