Abstract
Calcium influx associated with the opening of N-methyl-d-aspartate (NMDA) receptor channels is the major signal triggering synaptic and developmental plasticity. Controlling the NMDA receptor function is therefore critical for many functions of the brain. We explored the mechanisms of synaptic activation of the NMDAR glycine site by endogenous coagonist using whole cell voltage-clamp recordings from hippocampal neurons in mixed cultures, containing both neurons and glial cells, and, under more physiological conditions, in hippocampal slices. Here we show that the glycine site of the NMDA receptor at hippocampal synapses, both in culture and acute brain slices, is not saturated by the ambient coagonist concentration and is modulated through activity-dependent coagonist release. Augmentation of the NMDA receptor-mediated synaptic responses by local glutamate-induced coagonist release is spatially restricted and determined by spatiotemporal summation of synaptic events at neighboring synaptic inputs on a single dendritic branch. Therefore different spatiotemporal patterns of presynaptic activity could be translated into different levels of the NMDAR activation in specific afferent projections. These results suggest that the NMDA receptor glycine site may serve as a detector of the spatiotemporal characteristics of presynaptic activity patterns.
INTRODUCTION
Fast excitatory synaptic transmission, underlying interneuronal communication in the brain, implicates activation of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors by synaptically released neurotransmitter glutamate (Cowan et al. 2001). Unlike AMPA receptors, which are fully functional regardless of the membrane polarization level, NMDA receptors are normally blocked by external Mg2+ at the resting membrane potential. Sufficient levels of synaptic activity, leading to substantial postsynaptic depolarization, relieve the potential-dependent Mg2+ block of NMDA receptors, thus allowing them to conduct ionic currents. NMDA receptors are highly permeable for Ca2+, and therefore their channel opening is associated with the intracellular flux of Ca2+ ions. Many fundamental biological processes such as neural development, synaptic plasticity, learning, and memory depend on activation of NMDA receptors at central synapses by glutamate (Iwasato et al. 2000; Malenka and Bear 2004; Nakazawa et al. 2004). A combination of the two characteristic features of NMDA receptors, the requirement for presynaptic neurotransmitter release and postsynaptic depolarization in order for the NMDA channel to open, allows them to mediate coincidence detection during simultaneous activity of pre- and postsynaptic neurons and time-locked Ca2+ influx, often needed for such plastic changes to occur (Nakazawa et al. 2004).
NMDA receptors are heterotetramers composed of NR1 and one or more of four different NR2 subunits (NR2A–NR2D) (Thomas et al. 2006). In addition to glutamate binding, activation of NMDA receptors also requires occupation of their glycine binding site by an endogenous coagonist (Johnson and Ascher 1987; Kleckner and Dingledine 1987). NMDA receptors become activated when glutamate binds to NR2, while glycine binds to NR1 subunits (Clements and Westbrook 1991; Kuryatov et al. 1994; Laube et al. 1997). Because glycine concentration in the cerebrospinal fluid is in a micromolar range (McGale et al. 1977), it was suggested previously that the glycine site might be saturated by endogenous agonist under baseline conditions (Kemp et al. 1988). However, later experiments demonstrated that glycine transporters at synapses in neonatal rat hypoglossal motoneurons are capable of decreasing the concentration of glycine in the synaptic cleft to subsaturating levels (Berger et al. 1998). Moreover, it has been shown that both exogenously applied glycine and pharmacological blockade of glycine transporter 1 could lead to potentiation of the NMDA receptor-mediated synaptic currents in hippocampal (Bergeron et al. 1998; Wilcox et al. 1996) and prefrontal cortical neurons (Chen et al. 2003), indicating the lack of saturation of the NMDA receptor glycine site under baseline conditions. On the other hand, the glycine site of the NMDA receptor at the cerebellar mossy fiber to granule cell synapses was found to be saturated by endogenous coagonist (Billups and Attwell 2003). Taken together, these findings indicate that specific features of synaptic organization could determine whether intrasynaptic coagonist concentration could be lowered below the saturation level. It remains unknown, however, whether synaptic activity-dependent release of endogenous coagonist of the NMDA receptor glycine site may modulate the level of NMDAR activation when its glycine site is not saturated. We therefore asked what the synaptic rules are that determine degree of the glycine site occupation and, consequentially, NMDAR activation.
We found that the spatiotemporal integration of synaptic events in a segregated group of inputs converging on a single hippocampal neuron can lead to the release of the endogenous coagonist of the NMDA receptor glycine site. These observations provide support to the notion that different spatiotemporal patterns of network activity could be translated into different levels of NMDA receptor activation by activity-dependent modulation of the NMDAR glycine site occupancy.
METHODS
Primary hippocampal cell culture and immunocytochemistry
Hippocampi were dissected from neonatal Sprague Dawley rats (P1–P3) and cultured as previously described (Liu et al. 1999). The tissue block containing CA1 area of the hippocampus was processed following the culture protocol after cutting out the dentate gyrus and CA3 region. The neuronal and glial elements were defined by immunocytochemistry in multiple batches of hippocampal culture after fixation in 4% paraformaldehyde. Primary antibodies against βIII Tubulin (1/200; Promega) and glial fibrillary acidic protein (GFAP) (1/100; Promega) were applied to identify neuronal and glial element, respectively, following pretreatment of cultured cells with 0.5% Triton X-100 for 30 min at room temperature. After incubation in medium containing fluorescence-conjugated secondary antibodies (Alexa 488, 546, or 633; 1/400; Molecular Probes, Eugene, OR), the cell culture was scanned using two laser sources alternately under the Olympus confocal microscope (Fluoview software, V2.0). The Massachusetts Institute of Technology's Committee on Animal Care approved all experiments involving animals.
Electrophysiological recordings in culture
Whole cell patch-clamp recordings were obtained from cultured neurons at room temperature (22–23°C). The patch electrodes (2–3 MΩ resistance) contained (in mM) 125 Cs gluconate, 15 HEPES, 9 NaCl, 1 CaCl2, 10 BAPTA, 10 TEA, 4 Mg-ATP, and 0.3 Na-GTP (adjusted to pH 7.2 with CsOH). The sodium channel blocker QX-314 (2 mM) was added to the patch electrode solution in the experiments involving stimulation of a local group of neighboring synapses (see following text; all experiments shown in Fig. 3). Series resistance was monitored throughout experiment and was typically in the range 10–15 MΩ. The experimental chamber (0.2 ml) was continuously perfused (0.2 ml/min) with solution containing (in mM) 153 NaCl, 3 KCl, 1.2 CaCl2, 1.2 MgCl2, 1 Na2HPO4, 0.2 NaH2PO4, 10 glucose, and 0.1 picrotoxin (adjusted to pH 7.4 with NaOH). During paired recordings and burst excitatory postsynaptic current (EPSC) recordings, extracellular Ca2+ concentration was reduced to 0.8 mM to decrease the network activity under conditions of blocked GABAA receptor-mediated inhibition. The synaptic conductance ratio at +40 and −70 mV was used to make possible the comparison between single quanta EPSCs, evoked EPSCs, and burst EPSCs. The conductance values (G, nS) were obtained by normalizing the EPSC charge transfer by the driving force (holding potentials at which synaptic events were recorded) (Liu 2004). The NMDA/AMPA ratio was used as a measure of changes in NMDA receptor-mediated synaptic responses instead of analyzing NMDA receptor (NMDAR) currents in isolation, following pharmacological blockade of other synaptic conductances because activation of AMPA receptors might be needed for activity-dependent release of endogenous agonist of the NMDAR glycine site. The iontophoretic techniques used in this study were previously described (Murnick et al. 2002). A high-resistance electrode containing sodium glutamate was carefully positioned near the dendritic surface. TTX (1 μm) was added to the external solution in the experiments with iontophoretically applied glutamate. The responses were evoked by glutamate pulses (16 nA, duration of pulses ranged from 0.5 to 64 ms) with interpulse intervals of 4 s.
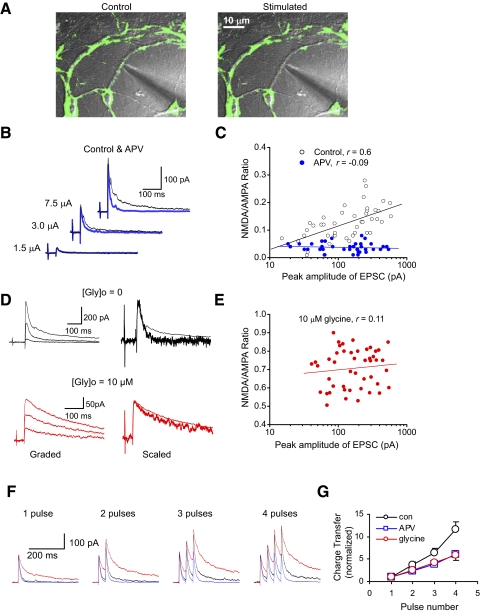
FIG. 3.
Spatiotemporal summation of responses at neighboring synapses is required to activate the release of endogenous coagonist. A: microscopic photographs showing that focal electrical stimulation of synapses, preloaded with fluorescent dye FM1-43, led to destaining of the area in close proximity to the stimulating electrode. B: traces demonstrate synaptic responses evoked by focal stimuli of increasing intensity under control conditions (black traces) and in the presence of APV (blue traces). In all experiments shown, the sodium channel blocker QX-314 (2 mM) was added to the patch electrode solution. The experiments illustrated in this figure were performed on hippocampal cultures. C: a plot of the NMDA/AMPA amplitude ratios as a function of the peak AMPAR EPSC amplitude (recorded at +40 mV) under control conditions (black symbols, correlation coefficient, r = 0.6) and in the presence of APV (n = 13 neurons; correlation coefficient, r = −0.09). The NMDR-mediated component of the EPSC was measured 50 ms after the peak. D, top: examples of EPSCs evoked by focal stimuli of increasing intensity without glycine in the external medium (left). The smallest and the largest EPSCs have been scaled by their peak amplitude (right). Note the slower decay of the larger response, indicating increased NMDAR current. Bottom: same experiments as on top, but in the presence of 10 μM glycine. E: no correlation was observed between the NMDA/AMPA amplitude ratios and the peak AMPAR EPSC amplitude in the presence of a saturating concentration of glycine (10 μM, n = 6 neurons; correlation coefficient, r = 0.11). F: the effects of temporal summation of the focal EPSCs on the NMDAR-mediated current. The EPSCs were evoked with different number of presynaptic stimuli (varied from 1 to 4) with an interpulse interval of 40 ms under control conditions (black traces), in the presence of glycine (red traces) and in the presence of APV (blue traces) at +40 mV. The trains were delivered at a frequency of 0.125 Hz. G: the EPSC charge transfer as a function of presynaptic stimuli number under control conditions (black symbols) and in the presence of either APV (blue symbols) or glycine (red symbols). The responses during trains of presynaptic stimulation were normalized by synaptic charge transfer in response to a single pulse. The increase in synaptic charge transfer during repetitive stimulation was significantly diminished in the presence of either the NMDAR antagonist APV (n = 9) or exogenously added glycine (10 μM; n = 11).
Electrical stimulation of local group synapses on a single dendritic branch
The stimulation was performed with a fine-tipped electrode filled with extracellular solution (10–12 MΩ resistance) in the presence of a low concentration of TTX (10–30 nM) in perfusion medium. The latter efficiently suppressed the induced network activity that could otherwise prevent reliable quantification of the NMDAR and AMPAR current components in locally induced evoked EPSCs. From 15 to 30 EPSCs were evoked and averaged at each stimulation intensity tested. Synaptic responses to three to five different stimulation intensities were collected and analyzed for each individual stimulation site. The AMPAR EPSC amplitude was measured as the difference between the mean current during a prestimulus baseline and the mean current over a 3-ms window at the peak of the response. The NMDAR-mediated component of the EPSC was determined at a holding potential of +40 mV as the difference between the baseline and the mean current over a 10 ms window measured 50 ms after the peak. The short trains of stimulation pulses were delivered at a frequency of 0.125 Hz. To evaluate the locality of stimulation, the synapses were preloaded for ∼1 min with the styryl dye FM1-43 (Biotium, Richmond, CA). The FM1-43-loading solution contained (in mM) 39 NaCl, 90 KCl, 30 glucose, 25 HEPES, 0.01 kynurenic acid, and 0.01 mM FM1-43. The activity-dependent destaining of FM1-43-loaded synapses was visualized under a confocal microscope (Olympus Fluoview).
Electrophysiological recordings in hippocampal slices
Hippocampal slices (350–400 μm) were prepared from 3- to 5-wk-old male Sprague Dawley rats with a vibratome. Slices were continuously superfused in solution containing (in mM) 120 NaCl, 3.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.0 NaH2PO4, 10 glucose, and 0.05 picrotoxin and equilibrated with 95% O2-5% CO2 (pH 7.3–7.4). Low magnesium solution contained 0.04 mM magnesium and 3.0 mM calcium with the other components remaining unchanged. Whole cell recordings of synaptic currents were obtained from CA1 pyramidal neurons under visual guidance either at room temperature (22–23°C) or at 35–36°C, which is specified in the description of specific experiments. The patch electrodes contained the same intracellular solution as in the experiments in culture. Miniature EPSCs (mEPSCs) were recorded in the presence of 0.5 μM TTX. The stimulating electrode (consisting of a glass pipette) was positioned to stimulate the Schaffer collaterals. “Weak” responses were evoked by low-intensity stimulation pulses (8–10 μA, 200 μs-long, at 0.1-Hz frequency). To evoke large compound responses, the bipolar stimulation electrodes were positioned to stimulate Schaffer collaterals with stronger stimulation pulses (30–200 μA, 0.2 ms, 0.1 Hz).
RESULTS
Glycine site of the NMDA receptor is not saturated under baseline conditions
To explore the mechanisms of synaptic activation of the NMDAR glycine site, we obtained whole cell voltage-clamp recordings from hippocampal neurons in mixed cultures, containing both neurons and glial cells. The latter was confirmed by immunostaining studies with antibodies against neuronal cell type- and glia-specific markers (βIII tubulin and GFAP, respectively; Fig. 1 A), indicating that a physical substrate for neuronal-glial interactions may exist in our hippocampal cultures. To evaluate whether the glycine site of the NMDA receptor is maximally activated by endogenous coagonist in the absence of evoked synaptic activity, we tested the effects of glycine added to the external medium on miniature excitatory postsynaptic currents (mEPSCs). The NMDAR-mediated component of the mEPSC, as measured 15 ms after the peak at a holding potential of +40 mV, was minimal without added glycine, suggesting that the concentration of the endogenous coagonist in the medium was low in the presence of TTX (1 μM) which blocked spontaneous action potential firing, while exogenously applied glycine produced dose-dependent enhancement of the NMDAR-mediated synaptic responses (Fig. 1, B–D). Glycine was effective in concentrations as low as 100 nM (n = 5, t-test, P < 0.001 vs. mEPSCs in medium without added glycine), with 1 μM glycine producing saturated NMDAR mEPSCs (n = 7, P = 0.7 for the effects of 1 μM vs. 10 μM glycine, n = 4). The addition of glycine to the external medium had no effect on the AMPA receptor mEPSCs recorded at –70 mV (Fig. 1, B and C). Consistent with these observations, the ratio of synaptic conductances (see methods) at positive (G40 mV) and negative (G-70 mV) holding potentials progressively increased as a function of glycine concentration (Fig. 1D). Thus the glycine site of the NMDA receptor is not saturated by endogenous coagonist under baseline conditions (Berger et al. 1998; Bergeron et al. 1998; Wilcox et al. 1996; but see Billups and Attwell 2003).
FIG. 1.
The glycine site of the N-methyl-d-aspartate (NMDA) receptor in hippocampal neurons is not saturated under baseline conditions. A, left and middle: immunohistochemistry for glia-specific marker, glial fibrillary acidic protein (GFAP) and neuronal cell type-specific marker, βIII tubulin, respectively. Right: GFAP and βIII tubulin images combined, demonstrating the presence of neurons and glia in our hippocampal cultures. B: effects of exogenously applied glycine on miniature excitatory postsynaptic currents (mEPSPs) recorded at –70 mV (bottom) and +40 mV (top). Traces are averages of 50–150 mEPSCs recorded at each holding potential before (black) and after (red) addition of glycine to the external medium. C: dose-response plot for the effects of different concentrations of glycine on the peak amplitude of the AMPAR mEPSCs at –70 mV (open symbols) and NMDA receptor (NMDAR) mEPSPs at +40 mV (black symbols) normalized to the control amplitude values obtained without added glycine. NMDA receptor-mediated component of the mEPSC, was measured 15 ms after the peak at +40 mV (marked with dashed lines in B). D: summary plot showing the ratios of synaptic conductances at +40 mV (G40 mV) and –70 mV (G-70 mV). The ratio increases as a function of added glycine concentration. Data points show means ± SE.
Synaptic activity levels determine the degree of the glycine site occupation
The basal concentration of endogenous coagonist is insufficient for the NMDAR activation (see preceding text). Does the degree of NMDAR activation depend on the level of synaptic activity? To address this question, we compared the conductance ratio G40 mV/G-70 mV under three different levels of synaptic activation without glycine in the culture medium (Fig. 2): low, represented by spontaneous mEPSCs reflecting release of single quanta of glutamate; medium, represented by multiquantal EPSCs recorded in synaptically connected pairs of neurons; and strong, represented by EPSCs resulting from spontaneous bursts of action potentials in many presynaptic neurons leading to the appearance of large-amplitude responses in the recorded cell (Liu 2004). We found that the G40 mV/G-70 mV ratio of mEPSCs in hippocampal cultures was close to 1, both in the absence or presence of the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV, 50 μM; n = 6; Fig. 2, A and D; P = 0.31 for the effects of APV on mEPSCs), confirming insignificant activation of NMDA receptors during single quanta synaptic events. The stimulus-evoked multiquantal EPSCs obtained in the course of paired recordings had a significantly greater G40 mV/G−70 mV ratio of 1.75 ± 0.33 (n = 13, P < 0.05 vs. mEPSCs), which was reduced to ∼1 by APV (Fig. 2, B and D). Finally, the high level of synaptic activation, provided by spontaneous burst EPSCs, was associated with the largest increase in the NMDA receptor-mediated component of synaptic responses (2.4 ± 0.13, n = 22, P < 0.05 vs. EPSCs obtained with paired recordings), also blocked by APV (Fig. 2, C and D).
FIG. 2.
Activity-dependent release of endogenous coagonist of the NMDA receptor glycine site determines the level of NMDAR activation. A, left: a schematic representation of a single synaptic connection. Middle: effects of the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV) on mEPSCs recorded at –70 mV (bottom) and +40 mV (top) without added glycine. Traces are averages of 50–150 mEPSCs recorded at each holding potential before (black trace) and after (blue trace) addition of 50 μM APV to the external medium. Right: averaged mEPSCs recorded at each holding potential before (black trace) and after (red trace) addition of 1 μM glycine. All experiments illustrated in this figure were performed on hippocampal cultures. B, left: a schematic representation of multiquantal release in a synaptically-connected pair of cultured neurons. Middle and right: evoked EPSCs recorded in synaptically connected pairs of neurons at –70 mV (bottom) and +40 mV (top) before and after addition of APV (blue trace) or glycine (red trace), respectively. Traces are averages of 15–30 evoked responses. C, left: a schematic representation of multiple fiber activation during spontaneous burst EPSCs. Middle and right: spontaneous burst excitatory postsynaptic currents (EPSCs) recorded in cultured neurons at –70 mV (bottom) and +40 mV (top) before and after addition of APV (blue trace) or glycine (red trace), respectively. D: summary plot of the G40 mV/G-70 mV ratios for 3 different levels of synaptic activation (mEPSCs, evoked “paired” EPSCs, and burst EPSCs) under control conditions and in the presence of APV without added glycine in external solution. The number of experiments for each group is shown in brackets. E: ratios of G40 mV/G-70 mV values before and after addition of glycine (Ratiocon and Ratioglycine, respectively) in all 3 experimental groups. F: continuous traces, containing burst EPSCs, recorded from 2 neurons at 2 holding potentials of –70 and +40 mV in the presence of 0.8 mM Ca2+ and 3.0 mM K+. Two neurons exhibited synchronously occurring burst EPSCs. Burst EPSCs were recorded without TTX in the external solution. G: burst EPSCs recorded in a pair of neurons at 2 holding potentials in modified external medium (with increased Ca2+ and/or increased K+ concentrations). H: G40 mV/G-70 mV ratios plotted as a function of the burst size (as in G) under control conditions and in the presence of APV (without added glycine).
We probed the level of activation of the NMDAR glycine site by endogenously released coagonist under different patterns of synaptic activity by calculating the G+40mV/G–70mV ratio before and after addition of a saturating concentration of glycine (1 μM, Fig. 1C; Rcon and Rglycine, respectively) in all three experimental groups (see preceding text). Consistent with the notion that the higher levels of synaptic activity may lead to the enhanced occupation of the NMDAR glycine site due to release of endogenous coagonist, thus partially occluding the effects of exogenously applied glycine, the Rcon/Rglycine ratio was found to be the lowest under conditions of low synaptic activity (mEPSCs) and the largest during the burst EPSCs (Fig. 2, A—C and E). Interestingly, even with the strongest synaptic input, the NMDA receptor glycine site was still unsaturated as even the largest synaptic responses still could be potentiated by exogenous glycine (although to a significantly lesser extent). Thus increased synaptic recruitment during bursting could result in a higher concentration of endogenous co-agonist, leading to a higher degree of NMDAR activation. To test this possibility, we studied the relationship between the G40 mV/G−70 mV ratio of burst EPSCs and the size of the burst. The size of the burst EPSC and the NMDA receptor current were monitored with simultaneous whole cell recordings from two neurons within the network with one neuron held at a holding potential of –70 mV, whereas the second recorded neuron was depolarized to +40 mV (Fig. 2F). Increasing [Ca2+]o from 0.8 to 2.0 mM resulted in an increase in the size of the burst EPSC at –70 mV. G40 mV/G−70 mV in these experiments increased accordingly (P < 0.05 vs. control, medium size burst). Conversely, decreasing the size of the burst by adding 8.0 mM potassium [K+]o to the external solution resulted in the significantly diminished G40 mV/G−70 mV ratio (Fig. 2, G and H; P < 0.05, data from 6 paired recordings). The conductance ratio remained unchanged in the presence of the NMDA receptor antagonist APV (Fig. 2H, data from 4 paired recordings). The G40mV/G−70mV ratio was remaining at ∼1.0 in the presence of APV, and it was no longer enhanced above this value by burst firing, supporting the notion that the ratios >1.0 represent an NMDAR-mediated component. These results, confirming that the NMDAR glycine site is not saturated under baseline conditions, demonstrate that an increase in neuronal network activity, resulting in a synchronous recruitment of many convergent synaptic inputs, may lead to the enhancement of the NMDAR-mediated synaptic responses through increased activation of the NMDAR glycine site.
Spatiotemporal summation of synaptic events at neighboring synaptic inputs mediates the release of endogenous coagonist
If the recruitment of multiple synaptic inputs is required for the accumulation of coagonist, providing conditions for the NMDAR activation, the activated synapses might need to be spatially close to each other to enable such coagonist buildup. To test this possibility, we activated a small group of synaptic terminals with low-intensity electric pulses (1.0–10 μA) delivered through small-tipped stimulation pipette positioned at the surface of a dendritic branch. The experiments were performed in the presence of a low concentration of TTX (10–30 nM) to block bursting synaptic inputs and reverberating synaptic activity induced by focal electric stimulation. To evaluate the locality of stimulation under these conditions, synaptic terminals in cultured neurons were preloaded with the fluorescent styryl dye FM1-43 (see methods for details). Repetitive electrical stimulation with low-intensity pulses (1–2 μA, 1-ms duration) resulted in destaining of the area surrounding the stimulating electrode, while more distant sites remained stained (Fig. 3A, n = 9). Spatially restrained destaining pattern indicates that electric stimulation resulted in a local activation of neighboring synapses in a small region of a given dendritic branch. Low-intensity electric pulses, delivered with such local stimulation techniques, evoked small-sized EPSCs at a holding potential of –70 mV, with the failure rate approaching 50% (data not shown). Identical to spontaneous single quanta mEPSCs, the minimal stimulation-evoked EPSCs exhibited negligible NMDAR component at +40 mV (Fig. 3B; no added glycine). Increase in stimulation intensity led to larger EPSCs, presumably due to recruitment of an increasing number of activated synapses at the stimulation position. The size of the EPSCNMDA (measured 50 ms after the peak), however, increased disproportionally, so that the NMDA/AMPA amplitude ratio positively correlated with the amplitude of the AMPAR EPSCs (correlation coefficient, r = 0.6, P < 0.01). The correlation was abolished by the NMDAR antagonist APV (50 μM; r = −0.09, P = 0.55; Fig. 3C). Such dependence of the NMDA/AMPA ratio on the number of activated synapses could be observed in the absence of exogenous glycine, but it disappeared in the presence of [Gly]o = 10 μM (Fig. 3, D and E; r = 0.11, P = 0.46), suggesting that the observed augmentation of the NMDA receptor responses was mediated by the increases in a concentration of the endogenous coagonist.
Can temporal summation of the local synaptic events also lead to increased activation of the NMDAR glycine site? To address this question, we varied the number of presynaptic pulses (from 1 to 4 stimuli) delivered to a single dendritic branch with a short interpulse interval (40 ms) at a constant stimulation intensity using the focal stimulation techniques (see preceding text, also methods; Fig. 3F). To quantify the effects of temporal summation, the EPSC charge transfer during trains of stimuli was normalized by the charge transfer underlying the EPSC evoked with a single presynaptic pulse. The normalized values of the AMPAR-mediated EPSC charge transfer (recorded in the presence of 50 μM APV) have increased as a function of the stimulus number, indicating the presence of synaptic frequency facilitation. However, the normalized EPSC size has increased disproportionally when the EPSCs were recorded without APV in the external medium. As frequency facilitation equally affects the amplitude of the AMPA and NMDA receptor-mediated components of the EPSC, the latter finding suggests that activation of NMDA receptors was enhanced in the course of repetitive stimulation (Fig. 3, F and G; n = 11, ANOVA, P < 0.001 for control vs. APV). When the NMDAR glycine site was maximally occupied by glycine (10 μM) added to the medium, this disproportional increase in the EPSC charge transfer was diminished (Fig. 3G), confirming that the enhancement of the EPSC charge transfer during repetitive stimulation was caused by the increase in NMDAR activation. These findings suggest that different temporal patterns of presynaptic activity could be translated into different levels of the NMDAR activation.
Where does the coagonist originate? Is it released from presynaptic terminals or from the other sources? To address this question, we directly activated AMPA and NMDA receptors on a single dendritic branch, using focally applied iontophoretic glutamate pulses. To avoid possible contributions of the network effects in these experiments, all iontophoretic glutamate applications were performed with 1 μM TTX in the external solution. Under these conditions, applied glutamate activates small areas (∼1 μm2) with short iontophoretic pulses (0.5–1.0 ms) but covers larger regions with prolonged pulse duration (see methods) (see also Murnick et al. 2002). The evoked currents recorded at −70 mV were largely mediated by AMPA receptors, whereas at +40 mV, they resulted from the activation of both AMPA and NMDA receptors (Fig. 4, A and B). Similar to spontaneously occurring single-quanta synaptic events (mEPSCs) and EPSCs evoked with minimal electrical stimulation (Figs. 2, A and D, and 3A), currents induced by very short iontophoretic pulses of glutamate in the absence of added glycine did not exhibit the NMDAR-mediated component at +40 mV (Fig. 4A, top). However, longer pulses of glutamate resulted in the appearance of a slower decaying phase, blocked by APV, and therefore mediated by NMDA receptors. The addition of glycine to the control external solution resulted in potentiation of the NMDAR currents (Fig. 4A, bottom). The amplitude of the NMDAR-mediated component increased monotonously with the duration of iontophoretic glutamate pulses (Fig. 4C). The NMDA/AMPA amplitude ratio was positively correlated with the duration of iontophoretic pulses (n = 6, r = 0.79, P = 0.001), but addition of glycine in a saturating concentration (10 μM) to the external solution abolished this correlation (Fig. 4C, n = 4, r = −0.22, P = 0.23). These data suggest that activation of glutamate receptors, located on neurons or glial cells, might trigger the release of endogenous coagonist, resulting in the enhancement of NMDAR function.
FIG. 4.
Modulation of the NMDA receptor function through local glutamate-induced coagonist release is spatially restricted. A: traces are averages of 3 responses induced by iontophoretic glutamate pulses of increasing duration (indicated for each set of traces) at holding potentials of −70 and +40 mV in hippocampal neurons in culture. The currents were recorded under control conditions (black traces) and then in the presence of APV (50 μM, blue traces; top) without added glycine or in the presence of glycine (10 μM, red traces; bottom). B: a schematic representation of the experimental design showing the position of the recording and iontophoretic pipettes. To evoke responses, the iontophoretic pipette containing sodium glutamate was positioned near the surface of a dendritic branch. C: the NMDA/AMPA amplitude ratios as a function of the iontophoretic pulse duration under control conditions (n = 6, open symbols) and in the presence of either APV (n = 3, blue symbols) or 10 μM glycine (n = 4, red symbols). The peak amplitude of the AMPAR-mediated iontophoretic current was recorded at −70 mV; NMDAR-mediated current, recorded at +40 mV, was measured 50 ms after the peak. D: example responses to 16-nA, 32-ms-long pulses of glutamate were recorded at 2 holding potentials, −70 and +40 mV without added glycine. mEPSCs were recorded in the same experiment over 4-s window following the iontophoretic current at both holding potentials. This has been repeated 15–20 times to collect 30–150 mEPSCs. E, left:: iontophoretically-induced currents at –70 and +40 mV under control conditions (black traces) and in the presence of 50 μM APV (blue traces). Right: averaged mEPSCs recorded over 4-s window after the iontophoretic current at –70 and +40 mV under control conditions (black traces) and in the presence of APV (blue traces). F: summary plot of the G40 mV/G-70 mV ratios calculated for iontophoretically induced currents and mEPSCs (as in D and E, n = 5 experiments) under control conditions and in the presence of APV without added glycine in external solution.
Next, we explored whether the glutamate-induced release of coagonist might be restricted to a group of closely located synaptic inputs. We found that long iontophoretic pulses of glutamate, while producing large NMDAR-mediated currents at +40 mV, did not lead to the appearance of the NMDAR component of mEPSCs recorded in the same experiment over 4-s window following the evoked response (Fig. 4, D–F; n = 5, significant effect of APV on G+40mV/G-70mV ratio for iontophoretic EPSCs, P = 0.01; but no effect of APV on G+40mV/G-70mV ratio for mEPSCs, P = 0.4). As mEPSCs originate from many synaptic inputs, the majority of which is not directly affected by the intophoretic pulses of glutamate, these findings further support the notion that transient elevation of glutamate concentration in a highly restricted area within the neuronal network may trigger local release of the endogenous coagonist of the NMDAR glycine site leading to selective activation of NMDARs at neighboring synapses.
Astrocytes provide the likely source of endogenous coagonist released during synaptic activation
What is the source of endogenous coagonist released by synaptic activity? Previous studies suggested a role for astrocytes, a type of glial cells ensheathing synapses in the CNS, in regulation of the NMDA receptor activity at hippocampal synapses (Yang et al. 2003). Consistent with the role of astrocytes in NMDAR function, we found that NMDAR-mediated responses could not be observed in the absence of a close interaction between neurons and glial cells. Thus long iontophoretic pulses of glutamate, inducing large NMDAR currents when delivered to a dendritic branch in the absence of exogenous coagonist, failed to induce NMDAR currents when applied to an outside-out patch of membrane pulled from the same neuron, held 100 μm above the dendritic branch (Fig. 5, A and B, n = 7). Addition of 1 μM glycine to the external solution resulted in the appearance of a slow-decaying current component, demonstrating that the membrane patch contained NMDA receptors.
FIG. 5.
Astrocytes are the likely source of endogenous coagonist released by activity. A: a microscopic photograph showing the iontophoretic pipette position relative to the apical dendrite in hippocampal culture. Inset: the position of the iontophoretic pipette relative to the outside-out membrane patch pulled in the same experiment. B: delivery of the iontophoretic glutamate pulses of increasing duration (8–32 ms) to single dendritic branches at +40 mV in the absence of the exogenously applied glycine resulted in large NMDAR-mediated currents (left). Delivery of the identical glutamate pulses to the outside-out patches excised from the same neuron did not induce the NMDAR-mediated response. In these experiments, the outside-out membrane patch has been held 100 μm above the dendritic branch (middle). In the presence of 1 μM glycine in the external medium, glutamate pulses could evoke again a slow-decaying current component (right, n = 7 experiments). C: iontophoretically induced currents (16-ms pulses) at –70 and +40 mV under control conditions (black traces) and in the presence of 50 μM APV (blue traces). D, left: 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM) blocked both NMDAR- and AMPAR-mediated currents evoked by local glutamate pulses (16-ms-long) in the absence of glycine (n = 7). Right: the addition of glycine to the external medium rescued the NMDAR current recorded at +40 mV. E: NMDAR-mediated currents were partially blocked in the presence of 20 μM NBQX (n = 4, P = 0.027). F: the effects of a decreased extracellular Ca2+ concentration (0.2 mM; n = 3) and the inhibitor of glial glycine transporter GlyT1, NFPS (5 –10 μM; n = 5) on the iontophoretic glutamate-induced currents (evoked by 16-ms pulses) at +40 mV. Blue traces show responses in the presence of APV (50 μM). G: summary of the experiments as in D–F showing the NMDA response amplitude under different experimental conditions as a fraction of control amplitude.
If activity-dependent release of the endogenous coagonist by astrocytes, triggered by activation of astrocytic glutamate receptors, underlies the observed increases in NMDAR activation, then such enhancements should not be seen when glial AMPARs are pharmacologically blocked. In agreement with this prediction, we found that an antagonist of the AMPARs, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM), blocked NMDA receptor-mediated currents evoked by local iontophoretic pulses of glutamate applied to a dendritic branch in the absence of added glycine (Fig. 5, C and D; n = 7, P = 0.004 vs. control). Subsequent addition of glycine to the external medium (still containing CNQX) rescued the NMDAR current (Fig. 5D, CNQX + glycine, n = 7). CNQX, however, could directly antagonize NMDA receptors by acting competitively at the NMDAR glycine site (Lester et al. 1989). We therefore repeated these experiments with the more selective AMPA receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) (20 μM). The size of the NMDAR currents was significantly reduced in the presence of the antagonist (Fig. 5E; P = 0.027, n = 4), suggesting that activation of glial AMPA receptors is necessary for the NMDA receptor activation. NMDAR currents were not modified by a decrease of [Ca]o from 1.2 to 0.2 mM (Fig. 5, F and G; n = 3, P = 0.3 vs. control). This implies that the coagonist release by astrocytes under these conditions was not strongly Ca2+-dependent. Finally, confirming previous observations (Chen et al. 2003; Martina et al. 2004), we found that inhibition of the glial glycine transporter GlyT1 (predominantly expressed in glia) with N-[3-([1,1-biphenyl]-4-yloxy)-3-(4-fluorophenyl)propyl]-N-methylglycine (NFPS) (5–10 μM) led to a significant potentiation of the NMDAR-mediated current (Fig. 5, F and G; n = 5, P = 0.01). Albeit not ruling out the role of d-serine, the latter finding indicates that endogenously released glycine may serve as a coagonist of the NMDAR glycine site at the studied synapses.
Occupation of the NMDA receptor glycine site in hippocampal slices is also determined by synaptic activity levels
Finally, we investigated whether the effects seen in cell cultures could also be observed under more physiological conditions in hippocampal slices. To parallel experiments in culture, we recorded NMDAR-mediated synaptic currents under different levels of synaptic activation at room temperature (22°C-23°C). Thus we recorded spontaneous mEPSCs and stimulation-induced responses, evoked by stimuli of different intensity, from CA1 pyramidal neurons at –70 mV in the low magnesium solution before and after addition of 20 μM d-serine (Fig. 6, A–C and E). d-serine, another NMDA receptor glycine site agonist, was used instead of glycine in these experiments to avoid any interference from the glycine transporters. It has been demonstrated previously at hypoglossal motoneuronal synapses in slices that although the concentration of endogenous coagonist around synaptic NMDA receptors is below the concentration required to saturate their glycine site, the lack of saturation could not be easily detected with exogenous glycine because it could be rapidly taken away in slices by the glycine transporter (Berger et al. 1998). In contrast to mEPSCs recorded in hippocampal culture, we found that mEPSCs in slices exhibited the NMDA receptor-mediated current component prior to the addition of the exogenous coagonist, indicating tonic activation of the NMDAR glycine site by endogenous coagonist during baseline synaptic transmission (Fig. 6A). Nevertheless, the amplitude of the NMDAR component of the mEPSC was significantly increased in the presence of 20 μM d-serine (P = 0.004, n = 8). This finding indicates that, although the basal level of coagonist present in the synaptic cleft might be higher in slices than in cultures, the ambient concentration of coagonist in hippocampal slices does not reach levels required to saturate the NMDAR glycine site. Similar to the results in culture, the magnitude of potentiation of the NMDAR-mediated current and the size of synaptic input (the amplitude of the AMPAR current) were negatively correlated (Fig. 6D; r = −0.53, P = 0.01), as the addition of exogenous coagonist had a lesser effect on the NMDAR-mediated component of EPSCs evoked by stronger stimuli (P = 0.001 for d-serine vs. control when “weak” stimulation was used, n = 8; P = 0.33 for the EPSCs evoked by stronger stimuli, n = 5; Fig. 6E). It indicates that for the increased synaptic inputs (for larger AMPAR EPSCs), the ability of exogenous d-serine to potentiate NMDA responses was diminished, demonstrating that stronger presynaptic stimuli might lead to the increased occupancy of the NMDAR glycine site by the endogenous agonist released in the course of synaptic activation. This observation is fully consistent with the results of our experiments in cultured hippocampal neurons, confirming that the NMDAR glycine site occupancy in slices of the hippocampus could be determined by the level of synaptic activity.
FIG. 6.
Activation level of the NMDAR glycine site at the Schaffer collateral-CA1 synapses in hippocampal slices is determined by synaptic strength and functional astrocytes are required for the activity-dependent augmentation of the NMDAR-mediated synaptic currents. A, left: averaged mEPSCs (30–50 events) in control (black), low magnesium solution (gray), and the low magnesium solution in the presence of 20 μM d-serine (red). Right: amplitude of the NMDAR mediated current measured at 15 ms after the AMPAR current peak in the low magnesium solution before and after addition of d-serine in individual experiments. B, left: averaged EPSCs (30–40 traces) evoked by “weak” stimulation (resulting in responses with the peak amplitude <70 pA) in control (black), low magnesium solution (gray), and the low magnesium solution in the presence of 20 μM d-serine (red). Right: amplitude of the NMDAR-mediated current measured at 50 ms after the AMPAR current peak in the low magnesium solution before and after addition of d-serine in individual experiments. C, left: averaged EPSCs evoked by strong stimulation (resulting in responses with the amplitude >70 pA) in control (black), low magnesium solution (gray), and in the low magnesium solution in the presence of 20 μM d-serine (red). Right: amplitude of the NMDAR mediated current in the low magnesium solution before and after addition of d-serine in individual experiments. Recordings in A–C were performed at room temperature (22–23°C). D: magnitude of potentiation of the NMDAR current by d-serine (NMDAd-serine/NMDAcontrol) is plotted as a function of the AMPAR current amplitude (mEPSCs, open squares, n = 8 neurons; “weak” evoked EPSCs, solid circles, n = 8 neurons; EPSCs evoked with strong stimulation, open circles, n = 5 neurons). Correlation coefficient, r = −0.53. E: summary plot of the NDMAR-to-AMPAR current ratio before and after addition of d-serine under different levels of synaptic activation at 22–23°C. Data points are means ± SE. F: magnitude of potentiation of the NMDAR current by d-serine (NMDAd-serine/NMDAcontrol) at 35–36°C is plotted as a function of the AMPAR current amplitude at –70 mV (mEPSCs, open squares, n = 7 neurons; “weak” evoked EPSCs, solid circles, n = 8 neurons; EPSCs evoked with strong stimulation, open circles, n = 6 neurons). Correlation coefficient, r = −0.57. G: summary plot of the NDMAR-to-AMPAR current ratio before and after addition of d-serine under different levels of synaptic activation at 35–36°C. Data points are means ± SE. H: hippocampal slices were preincubated for ≥90 min in the external medium containing gliotoxin, l-α-aminoadipic acid (1 mM). Left: averaged EPSCs (10–25 traces) evoked by “weak” stimulation in CA1 neurons (resulting in responses with the peak amplitude <50 pA) at holding potentials of –70 mV (bottom) and +50 mV (top) under control conditions (black trace) and in the presence of 20 μM d-serine (red trace). Right: amplitude of the NMDAR-mediated current measured at 50 ms after the peak at a holding potential of 50 mV before and after addition of d-serine in individual experiments. I, left: averaged EPSCs evoked by strong stimulation (resulting in responses with the peak amplitude >50 pA) at holding potentials of –70 mV (bottom) and +50 mV (top) under control conditions (black trace) and in the presence of 20 μM d-serine (red trace). Right, amplitude of the NMDAR-mediated current measured at 50 ms after the peak at a holding potential of 50 mV before and after addition of d-serine in individual experiments. J: magnitude of potentiation of the NMDAR current by d-serine (NMDAd-serine/NMDAcontrol) is plotted as a function of the AMPAR current amplitude (“weak” evoked EPSCs, solid circles, n = 7 neurons; EPSCs evoked with strong stimulation, open circles, n = 5 neurons). Correlation coefficient, r = 0.17. All data points were obtained in experiments on slices pretreated with L-α-aminoadipic acid (1 mM). K: Summary plot of the NDMAR-to-AMPAR current ratio before and after addition of d-serine under different levels of synaptic activation. The experiments were performed at 35–36°C. Data points are means ± SE.
We have also performed experiments in hippocampal slices at physiological temperatures (35–36°C), recording spontaneous mEPSCs and stimulation-induced responses, evoked by stimuli of different intensity in CA1 neurons. Similar to the results in slices obtained at room temperature, the magnitude of potentiation of the NMDAR-mediated current and the size of synaptic input (the amplitude of the AMPAR current) were negatively correlated (Fig. 6F; r = −0.57), as added d-serine produced a lesser augmentation of the NMDAR-mediated component of EPSCs evoked by stronger stimuli (t-test, P < 0.01 for d–serine vs. control when mEPSCs were recorded, n = 7; P < 0.05 for d–serine vs. control when “weak” stimulation was used, n = 8; P = 0.2 for the EPSCs evoked by stronger stimuli, n = 6; Fig. 6G). It confirms, that the level of the NMDAR glycine site activation in hippocampal neurons under more physiological conditions (slices, physiological temperature) could still be determined by the level of synaptic activity, and therefore our observations are likely to be functionally relevant.
If release of endogenous coagonist from glial cells is required for activity-dependent potentiation of NMDAR currents, then such augmentation of NMDA receptor-mediated synaptic currents in slices should be suppressed when astrocytes are functionally inactivated. We addressed this possibility experimentally, performing recordings in hippocampal slices preincubated for ≥90 min in the external solution containing gliotoxin, l-α-aminoadipic acid (1 mM). The ability of L-α-aminoadipate to induce selective ablation or functional inactivation of astrocytes have been repeatedly demonstrated (e.g., Huck et al. 1984; Khurgel et al. 1996). It has been shown also that even at relatively high concentrations (≤10 mM), the l-α-adipate-induced damage was limited to the glial cells (Casper and Reif-Lehrert 1983). In our experiments, we found that in slices pretreated with gliotoxin, the inverse dependence of the potentiation of the NMDAR EPSC by exogenous d-serine on the size of synaptic input (observed in untreated slices, Fig. 6, D and F) was abolished (Fig. 6J, r = 0.17; t-test, P = 0.3 for d–serine versus control when “weak” stimulation was used, n = 7, Fig. 6, H and K; P = 0.5 for the EPSCs evoked by stronger stimuli, n = 5, Fig. 6, I and K). This indicates that after the treatment the level of NMDA receptor activation was determined by the ambient concentration of endogenous coagonist, while its activity-dependent release was suppressed, thus supporting the notion that astrocytes might be the source of endogenous agonist acting at the glycine site of the NMDA receptors.
DISCUSSION
Our present observations suggest that the NMDAR glycine site at hippocampal synapses, both in mixed primary cultures and brain slices, is not saturated under baseline conditions and, therefore activity-dependent release of endogenous coagonist can modulate the level of NMDAR activation. Although the lack of saturation of the NMDA receptor glycine site at central synapses has already been demonstrated (Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2004; Thomson et al. 1989; Wilcox et al. 1996), the previous studies were focusing on the possibility of the glycine site modulation by exogenously applied coagonist either under steady-state conditions or during low-level baseline synaptic transmission. The mechanisms and conditions of modulation of the NMDAR glycine site occupancy by synaptic activity remained elusive. Our study provides functional evidence that the occupancy of the NMDA receptor glycine site could be determined by the spatiotemporal characteristics of afferent activity. Moreover, the activity-dependent release of the NMDAR glycine site coagonist was found to be spatially restricted, providing conditions for coordinated increases in activation of NMDA receptors at closely located synapses. Such synaptic mechanisms could mediate coincidence detection during simultaneous activity of groups of pre- and postsynaptic neurons and, thus contribute to the induction of pathway-specific synaptic plasticity during learning or development.
Endogenous coagonist, released in an activity-dependent manner, likely originated from glial cells, as we found that NMDA receptor-mediated responses could not be observed without physical proximity between neurons and glia in culture, while activity-dependent augmentation of NMDA receptor-mediated synaptic currents at CA3–CA1 synapses in hippocampal slices was suppressed when astrocytes were functionally inactivated. Astrocytes (but not another type of glial cells, oligodendrocytes) highly express the glycine transporter 1, GlyT1, which cotransports two Na+ ions and one Cl− ion (Billups and Attwell 2003). It has been demonstrated previously that glycine could be accumulated or released by glia, depending on physiological conditions (Aubrey et al. 2005; Roux and Supplisson 2000). Moreover, it has been suggested that activation of glial AMPA receptors by synaptically released glutamate could promote reverse transport conditions for the Na+-coupled glycine transporter by raising locally the intraglial Na+ concentration (Attwell et al. 1993; Marcaggi and Attwell 2004; Roux and Supplisson 2000). The reversed glycine uptake would increase the extracellular glycine concentration from a subsaturating level (∼100 nM) to the low micromolar range (Attwell et al. 1993). There is substantial evidence that astrocytes are responsive to exogenously applied glutamate (Lalo et al. 2006; Mennerick et al. 1996; Zhou and Kimelberg 2001) as well as glutamate released by the Schaffer collateral stimulation (Diamond et al. 1998; Ge et al. 2006; Lüscher et al. 1998). These previous findings, based on direct electrophysiological recordings from astrocytes, indicate that astrocytes possess AMPARs and, perhaps, NMDARs (Lalo et al. 2006; Schipke et al. 2001). Although not ruling out completely the possibility of coagonist release from neurons, this validates the proposed model that activation of glial glutamate receptors may lead to the coagonist release under physiological conditions (Attwell et al. 1993; Roux and Supplisson 2000).
Another gliotransmitter, d-serine, can also function as an endogenous activator of the NMDAR glycine site (Martineau et al. 2006; Mothet et al. 2000; Panatier et al. 2006; Wolosker et al. 1999; Zhang et al. 2008) and is released by astrocytes through Ca2+- and SNARE-dependent exocytosis in response to activation of glial glutamate receptors (Mothet et al. 2005). Our findings are consistent with the notion that activation of astrocytic glutamate receptors by synaptically released glutamate may trigger the release of the endogenous coagonist of the NMDAR glycine site. Previous observations, indicating that normal NMDA receptor EPSCs could be observed in hippocampal slices in the presence of NBQX, might be explained by the effects of coagonist that is ambiently present in the external medium in slice experiments. While blocking glial AMPA receptors might prevent an upregulation of the NMDAR function through activity-dependent coagonist release, the tonic coagonist concentration would be sufficient to fully support the activation of NMDA receptors under baseline conditions. In our experiments, mEPSCs recorded in cultured hippocampal neurons in a continuously perfused experimental chamber did not exhibit the NMDA receptor-mediated component without added glycine, indicating that an ambient coagonist concentration was very low under such experimental conditions. Therefore blocking AMPA receptors with NBQX, preventing coagonist release, resulted in a significant decrease in the size of the NMDA receptor-mediated currents. Endogenous coagonist of the NMDAR glycine site may be nearly instantaneously released in response to presynaptic stimuli in quantities corresponding to degree of presynaptic activation, reflecting spatial summation of the events at neighboring glutamatergic synapses, possibly due to glutamate spillover from closely located synaptic inputs (Kullmann and Asztely 1998), and the temporal pattern of synaptic activation.
Alternatively, the disproportional increases in NMDAR-mediated signaling could be explained by a greater degree of glutamate spillover when multiple presynaptic terminals are recruited. NMDA receptors have higher affinity for glutamate than AMPA receptors and could respond to low glutamate concentrations, which are not sufficient to activate AMPA receptors (Kullmann and Asztely 1998). If the glutamate binding site of the NMDA receptor is far from being saturated by synaptically released glutamate, there could be a supralinear increase in the NMDAR current due to accumulation of glutamate in response to stimuli of increasing intensity. Under this scenario, the NMDAR-mediated responses could, in principle, be augmented without increases in the glycine site occupancy. However, this possibility is unlikely because we found that a positive correlation between the NMDA/AMPA responses ratio and the number of activated synapses, observed in the absence of exogenously applied glycine, was eliminated by addition of a saturating concentration of glycine to the external medium. This observation supports a role for the endogenously released coagonist of the NMDAR glycine site in activity-dependent modulation of the NMDAR function.
The functional consequences of reduction in the affinity of the NMDA receptor for endogenous coagonist were previously demonstrated in the experiments on genetically modified mice with point mutations of the glycine site of the NR1 subunit (Kew et al. 2000). Thus mice carrying Grin1D481N mutation, in which the NMDAR1 glycine affinity was decreased fivefold, were viable but had deficits in theta burst-induced LTP in the hippocampus and spatial learning. The increases in NMDAR activation, associated with GlyT1 blockade, were also found to be functionally relevant, as inhibition of GlyT1 led to significant augmentation of NMDAR-dependent LTP in CA1 area of the hippocampus (Martina et al. 2004).
Taken together, our present results suggest a role for the NMDAR glycine site in synaptic computation. Spatiotemporal integration of incoming signals would lead to coordinated increases in activation of NMDA receptors in a local group of synapses. Although synapse independence was shown to increase the computing power of a neuronal network (Barbour 2001), there might be a need for the cellular mechanism mediating synchronous plastic modifications in a group of neighboring synapses. This could optimize conditions for the encoding and retaining specific information about afferent activity patterns. Thus maximal activation of NMDARs at closely located synaptic contacts, soaked in the coagonist released in an activity-dependent fashion, at depolarized membrane potentials would permit the induction of NMDAR-dependent synaptic plasticity, while the same synapses could still retain the ability to function independently at the resting membrane potential.
GRANTS
This work was supported by grants from the National Institutes of Health to G. Liu and V. Y. Bolshakov, National Basic Research Program of China 2006CB3031 and NSFC 30630026 to G. Liu, the National Alliance for Research on Schizophrenia and Depression to V. Y. Bolshakov and Whitehall Foundation to V. Y. Bolshakov.
Acknowledgments
We thank K. Tully for comments on the manuscript and B. Li for preparing and maintaining hippocampal cultures.
REFERENCES
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron 11: 401–407, 1993. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ, Clements JD. Dynamics of forward and reverse transport by the glial glycine transporter, glyt1b. Biophys J 89: 1657–1668, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B An evaluation of synapse independence. J Neurosci 21: 7969–7984, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol 80: 3336–3340, 1998. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc Natl Acad Sci USA 95: 15730–15734, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or D-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fiber to granule cell synapse. Eur J Neurosci 18: 2975–2980, 2003. [DOI] [PubMed] [Google Scholar]
- Casper DS, Reif-Lehrer L. Effects of alpha-aminoadipate isomers on the morphology of the isolated chick embryo retina. Invest Ophthalmol Vis Sci 24: 1480–1488, 1983. [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine transporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol 89: 691–703, 2003. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 7: 605–613, 1991. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Sudhof TC, Stevens CF. Synapses. Baltimore, MD: Johns Hopkins Univ. Press, 2001.
- Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron 21: 425–433, 1998. [DOI] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science 312: 1533–1537, 2006. [DOI] [PubMed] [Google Scholar]
- Huck S, Grass F, Hörtnagl H. The glutamate analogue a-aminoadipic aid is taken up by astrocytes before exerting its gliotoxic effect in vitro. J Neurosci 4: 2650–2657, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature 406: 726–731, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531, 1987. [DOI] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-d-aspartate receptor complex. Proc Natl Acad Sci USA 85: 6547–6550, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V, Richards JG, Trube G, Fischer G, Montkowski A, Hundt W, Reinscheid RK, Pauly-Evers M, Kemp JA, Bluethmann H. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci 20: 4037–4049, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia 16: 351–358, 1996. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241: 835–837, 1987. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci 21: 8–14, 1998. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron 12: 1291–1300, 1994. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26: 2673–2683, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor sub-units: analysis of the glutamate binding site on the NR2B subunit. Neuron 18: 493–503, 1997. [DOI] [PubMed] [Google Scholar]
- Lester RA, Quarum ML, Parker JD, Weber E, Jahr CE. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-d-aspartate receptor-associated glycine binding site. Mol Pharmacol 35: 565–570, 1989. [PubMed] [Google Scholar]
- Liu G Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7: 373–379, 2004. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron 22: 395–409, 1999. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron 21: 435–441, 1998. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia 47: 217–225, 2004. [DOI] [PubMed] [Google Scholar]
- Martineau M, Baux G, Mothet JP. D-serine signalling in the brain: friend and foe. Trends Neurosci 29: 481–491, 2006. [DOI] [PubMed] [Google Scholar]
- Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol 557: 489–500, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGale EHF, Pye IF, Stonier C, Hutchison EC, Aber GM. Studies of the inter-relationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. J Neurochem 29: 291–297, 1977. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Benz A, Zorumski CF. Components of glial responses to exogenous and synaptic glutamate in rat hippocampal microcultures. J Neurosci 16: 55–64, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 97: 4926–4931, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA 102: 5606–5611, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnick JG, Dube G, Krupa B, Liu G. High-resolution iontophoresis for single-synapse stimulation. J Neurosci Methods 116: 65–75, 2002. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 5: 361–372, 2004. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125: 775–784, 2006. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron 25: 373–383, 2000. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-d-aspartate receptors. FASEB J 15: 1270–1272, 2001. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Krupp JJ, Bagley EE, Bauzon R, Heinemann SF, Vissel B, Westbrook GL. Probing N-methyl-d-aspartate receptor desensitization with the substituted-cysteine accessibility method. Mol Pharmacol 69: 1296–1303, 2006. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Walker VE, Flynn DM. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature 338: 422–424, 1989. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol 76: 3415–3424, 1996. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96: 13409–13414, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA 100: 15194–15199, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gong N, Wang W, Xu L, Xu TL. Bell-shaped D-serine actions on hippocampal long-term depression and spatial memory retrieval. Cereb Cortex 10: 2391–2401, 2008. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci 21: 7901–7908, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]