Abstract
OBJECTIVE
To utilize diffusion tensor tractography and evaluate the integrity of the corticospinal tract in children with unilateral Sturge-Weber syndrome (SWS).
METHODS
Sixteen children (age: 1.5-12.3 years) with SWS involving one hemisphere and varying degrees of motor deficit, underwent magnetic resonance imaging (MRI) as part of a prospective clinical research study. Diffusion tensor imaging (DTI) was obtained and fiber tracking of the corticospinal tract was performed yielding average FA and ADC values along the pathway. These values were compared between the two hemispheres (affected vs. unaffected) and also correlated with the degree of motor deficits, after correction for age.
RESULTS
Cortico-spinal tract FA values on the side of the affected hemisphere were lower (p=0.008) and ADC values were higher (p=0.011) compared to the normal side. Furthermore, FA and ADC values on the side of the angioma did not show the normal age-related variations, which the contralateral corticospinal pathway values did demonstrate. Although none of the patients had severe hemipares, those with moderate motor deficit had increased ADC values, as compared to those with mild (p= 0.009) or no motor deficit (p= 0.045).
CONCLUSION
MRI with DTI shows abnormalities of the corticospinal tract in children with SWS even before severe motor impairment develops. Thus, DTI can be a clinically useful method to evaluate the integrity of the corticospinal tract in young children who are at risk for progressive motor dysfunction.
Keywords: diffusion tensor imaging, Sturge-Weber syndrome, corticospinal tract, motor impairment
INTRODUCTION
Sturge-Weber syndrome (SWS) is a sporadic neurocutaneous condition occurring with an estimated frequency of about 1 in 50,000. As the term “encephalofacial angiomatosis” implies, SWS comprises a combination of facial and leptomeningeal angiomas often associated with glaucoma. Neurological manifestations are quite varied and include seizures, hemiparesis, stroke-like episodes, and hemianopsia.[1,2] Additional complications include headache, developmental delay, learning disabilities and attention deficit. The pathogenesis likely involves chronic hypoxia of the involved cortex due to impaired venous flow or a “vascular steal” phenomenon.[3]
Patients with SWS may demonstrate a greater area of involved cortex by functional imaging modalities, such as positron emission tomography (PET), as opposed to structural imaging techniques.Functional neuroimaging in SWS has some predictive value, e.g., with respect to seizure severity.[4,5] However, neuroimaging has provided relatively little insight in predicting the degree of motor deficit. Although hemispheric volume loss seen on MRI correlates with the severity of hemiparesis,[6] motor deficit may develop before brain atrophy becomes apparent on conventional MRI.. Therefore, better techniques to identify and quantify motor tract changes as a result of disease progression would be clinically useful.
Novel imaging methods may detect and quantify subtle structural and functional brain abnormalities earlier than conventional imaging modalities. Diffusion tensor imaging (DTI) is an MRI technique which is able to assess the degree and direction of water diffusion, and individual white matter tracts in the brain can be isolated by using fiber tracking software.[7, 8] The degree of restriction to water diffusion is quantified as the apparent diffusion coefficient (ADC), and the preponderant directionality is characterized by the fractional anisotropy (FA). FA is a scalar index from 0 to 1 with 0 being isotropic diffusion and 1 representing diffusion in one direction only. Since water diffuses primarily along the direction of fiber bundles in white matter, DTI can be used to study the integrity of white matter. In a recent study using DTI in children with unilateral SWS, we reported decreased FA and increased ADC in the white matter of the affected hemisphere extending beyond abnormal cortex (identified by glucose metabolism PET).[9] In the present study, we have specifically evaluated the integrity of the corticospinal tract and hypothesized that abnormalities on diffusion tension tractography are correlated with degree of motor weakness in children with SWS.
METHODS AND MATERIALS
Patients
Sixteen children (9 girls, 7 boys; mean age: 5.5 years, age range of 1.5-12.3 years) with SWS and unihemispheric brain involvement underwent MRI with DTI as they participated in a prospective clinical research study. All subjects were studied according to the guidelines of the Human Investigations Committee at Wayne State University. The clinical characteristics of the patients are shown in Table 1. Motor deficit was classified as absent (0), mild (1), or moderate (2) by a clinical neurologist. Mild deficit was defined as motor strength of 4/5 on the Medical Research Council [MRC] scale. Patients with moderate impairment were characterized by increased tone, clonus or weakness of at 3/5 on the involved side. None of the children had severe hemiparesis (hemiplegia).
Table 1.
Age, motor involvement as well as fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values measured in the cortico-spinal tract ipsi- and contralateral to the angioma.
Motor category: 0 = no motor involvement; 1: mild motor involvement; 2: moderate motor involvement.
| Patient | Age (months) |
Motor category |
FA_ipsi | ADC_ipsi | FA_contra | ADC_contra | FA Ipsi/Contra |
ADC Ipsi/Contra |
MRI findings |
|---|---|---|---|---|---|---|---|---|---|
| AC | 148 | 1 | 0.5633 | 0.00077 | 0.5802 | 0.00073 | 0.97 | 1.05 | Angioma entire hemisphere Mild atrophy |
| ED | 33 | 1 | 0.4858 | 0.00093 | 0.5161 | 0.00083 | 0.94 | 1.12 | Angioma entire hemisphere Moderate atrophy |
| EC | 44 | 0 | 0.5393 | 0.00083 | 0.5548 | 0.0008 | 0.97 | 1.04 | Parietal, occipital angioma Mild atrophy |
| MF | 107 | 2 | 0.4885 | 0.00097 | 0.5606 | 0.00077 | 0.87 | 1.26 | Enlarged lateral ventricle, angioma entire hemisphere Moderate atrophy |
| EH | 97 | 1 | 0.5113 | 0.00083 | 0.5556 | 0.0008 | 0.92 | 1.04 | Angioma entire hemisphere |
| DH | 112 | 1 | 0.5449 | 0.00083 | 0.5529 | 0.00083 | 0.99 | 1 | Parietal , occipital angioma Mild atrophy |
| AH | 21 | 0 | 0.5092 | 0.00083 | 0.5367 | 0.0008 | 0.95 | 1.04 | Parietal, occipital angioma Mild atrophy and prominent transmedullary veins. |
| AJ | 111 | 1 | 0.4894 | 0.0008 | 0.5278 | 0.00077 | 0.93 | 1.04 | Angioma parietal, temporal and occipital Mild atrophy |
| AL | 48 | 1 | 0.5068 | 0.0008 | 0.5168 | 0.00083 | 0.98 | 0.96 | Enlarged lateral ventricle, moderate atrophy, posterior angioma, enlarged transmedullary veins |
| AM | 30 | 0 | 0.53 | 0.00087 | 0.49 | 0.00087 | 0.92 | 1 | Posterior angioma. |
| AP | 113 | 1 | 0.5189 | 0.00083 | 0.5806 | 0.0008 | 0.89 | 1.04 | Parietal, temporal, frontal angioma Moderate atrophy. |
| NS | 45 | 2 | 0.4708 | 0.00087 | 0.5066 | 0.0008 | 0.93 | 1.08 | Parietal, occipital angioma Moderate atrophy. |
| ES | 18 | 2 | 0.4821 | 0.00093 | 0.5413 | 0.00083 | 0.89 | 1.12 | Parietal, occipital angioma Moderate atrophy |
| AW | 23 | 1 | 0.4816 | 0.0008 | 0.5323 | 0.00083 | 0.9 | 0.96 | Parietal, occipital angioma, enlarged ventricle on affected side, mild atrophy, prominent transmedullary veins. |
| JW | 98 | 1 | 0.6172 | 0.00077 | 0.5735 | 0.00077 | 1.08 | 1 | Parietal, occipital angioma, prominent lateral ventricle, Mild atrophy |
MRI data acquisition
All MR studies were carried out on a Sonata 1.5 T MR instrument (Siemens, Erlangen, Germany), using a standard head coil. During the scanning phase, children younger than 7 years of age were sedated with nembutal (3 mg/kg), followed by fentanyl (1 μg/kg). The MRI protocol included an axial 3D gradient echo T1 weighted (TR/TE: 20/5.6 ms, flip angle: 25°, voxel size 1×0.5×2 mm3), axial T2 weighted Turbo spin echo (TR/TE: 5020/106 ms, voxel size: 1×1×6 mm3), diffusion tensor imaging (DTI), susceptibility weighted imaging, followed by a post-gadolinium (0.1 mmol/kg) T1 weighted (see parameters above) acquisition. DTI acquisition was performed using single shot diffusion-weighted echo planar imaging. The acquisition parameters included: TR/TE = 6600/97 ms, Nex = 8, acquisition matrix = 128×128, bandwidth 95 KHz, FOV = 230×230×3 mm3, with two b values (0, 1000 s/mm2) applied sequentially in 6 non-collinear directions. The image data sets were transferred from the scanner to a PC workstation for post-processing.
DTI processing
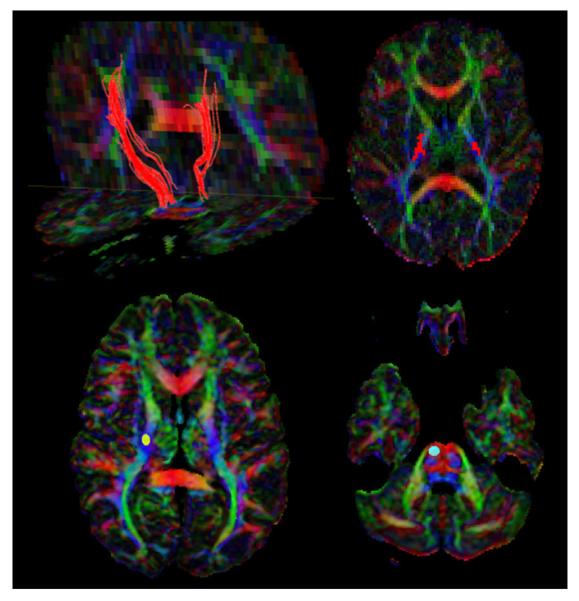
The entire DTI raw data set was examined in a slice-wise manner to exclude subject movement during the study. Fiber tracking was performed using DTIStudio software [10] based on Fiber Assignment by Continuous Tracking algorithm with an FA threshold of0.20, which has been reported to be optimal for tracking the corticospinal tract.[11] An angle threshold of 60 degrees was used. Brute-force fiber tractography and multiple region of interest (ROI) techniques were utilized to track the corticospinal tract.[12] In brief, a generous seed ROI was placed in an axial, direction coded DTI color map over the posterior limb of the internal capsule followed by a target ROI in the axial plane at the level of the pons, in order to retain all fibers that were generated by the first operation and went through the second ROI (Figure 1 lower panel). The resulting fiber bundle was in certain cases further purified by placing another ROI over the fibers in the white matter underlying the precentral gyrus (presumed motor cortex), which was identified as the gyrus anterior to the central sulcus defined by the “inverted omega sign” on axial MRI images.[13] Once a fiber tract was demonstrated in its entire length,(Figure 1 upper panel) the mean FA and ADC values for the entire tract were calculated using the statistical program incorporated in the DTI Studio software. To assess the reliability of fiber tracking, a separate investigator repeated measurements for this tract on both right and left sides with fiber tracking parameters held constant.
Figure 1.
Upper panel: DTI tractography, in a patient (A.M.) with moderate right-sided motor deficit, illustrating asymmetry of the corticospinal pathways. Coronal, directioncoded color DTI images howing a shorter and thinner left corticospinal tract.This is further reflected by the fewer number of voxels occupied by this tract in the posterior limb of internal capsule, as shown in the axial color DTI image. Lower panel: Axial, direction-coded color DTI map (red, left-right; blue, superior-inferior; green, anterior—posterior direction) of a patient showing placement of the ROI (region of interest) over the posterior limb of the right internal capsule and the right pons.
Statistical Analysis
Intrarater reliability was investigated by measuring the FA and ADC values twice for all the subjects. The absolute mean percentage differences for each measurement along the various tracts were calculated using the formula (measurement 1 — measurement 2)*100/average of two measurements. Interrater reliability was investigated by comparing values obtained by two independent investigators, who followed the same protocol, and the absolute mean percentage differences for each tract were measured using the same formula. FA and ADC values for the corticospinal tract ipsi- vs. contralateral to the angioma were compared by using a paired t-test. FA and ADC asymmetries, defined by ratios between values ipsi- and contralateral to the angioma, were compared between patients with left vs. right hemispheric involvement, using an unpaired t-test. Correlation between age and DTI variables was performed using the Pearson’s test. FA and ADC values were then compared among the three motor deficit groups (no deficit, mild and moderate deficit), using analysis of variance, with age as a co-variate (ANCOVA). P<0.05 was considered to be significant.
RESULTS
Intrarater and interrater reliability of fiber tracking
The intrarater variability was low overall, varying from 1.8 to 5.8% difference between the repeated measurements. Interrater variability had a percentage difference of 2.8 to 4.2%.
Changes in FA and ADC values
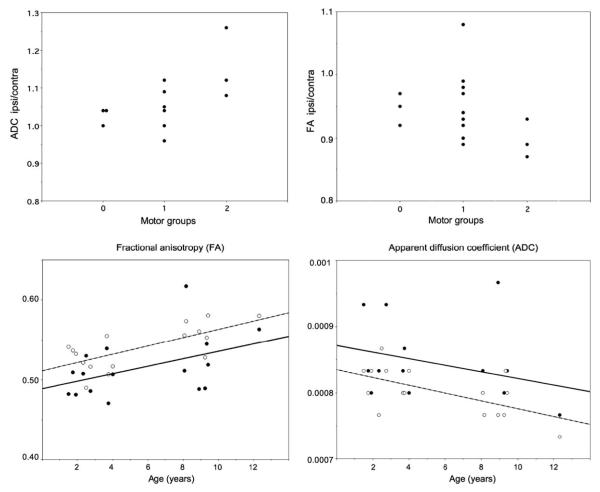
FA values in the corticospinal tract ipsilateral to the angioma were significantly lower (p=0.008, paired t-test), while ADC values were significantly higher (p=0.011) as compared to the contralateral side. Patients with left vs. right-sided hemispheric involvement showed no difference in FA or ADC asymmetries (FA: p=0.33, ADC: p=0.84). There was a strong positive correlation between the age of the patients and FA on the contralateral side (r=0.70, p = 0.002), consistent with previously described maturational changes [14,15], whereas the correlation was less robust on the ipsilateral side (r = 0.45, p = 0.08). Also, there was a negative correlation between age and ADC values in the contralateral hemisphere (r = - 0.6, p = 0.014), again, consistent with maturational changes, but not on the ipsilateral side (r = -0.3, p = 0.24). These changes are illustrated in Fig.2.
Figure 2.
Relationship between FA, ADC and extent of hemiparesis and correlation between FA, ADC and age of the patients.
DTI variables vs. motor deficit
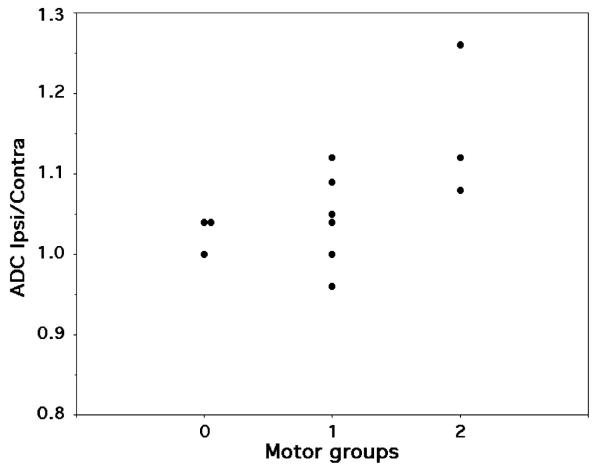
Of the total of 16 patients, 3 patients had no motor deficits, 10 had mild, while 3 children had moderate motor deficit on the side opposite to the leptomeningeal angioma. ANCOVA showed a significant overall difference of the ipsilateral ADC values among the three motor groups after adjustment for age (p = 0.03). Post hoc tests showed that patients with moderate motor involvement had higher ADC values as compared to those with mild (p= 0.009) and no motor deficit (p= 0.045)(Figure 3. ). In contrast, there was no significant difference of FA values among the three motor groups (p= 0.13), although there was a strong trend for lower FA values in patients with moderate motor deficit as compared to those with no deficit in post hoc comparisons (FA=0.48 vs. 0.54, p=0.051).
Figure 3.
ADC ratios (ipsilateral/contralateral) in the three motor groups. The ratios were highest in motor group 2. (r =0.53, p =0.036, Spearman’s test).
DISCUSSION
DTI tractography is an emerging imaging technique that can visualize and quantify subtle structural abnormalities of specific anatomic pathways. Diffusion abnormalities of the corticospinal tract have been reported in a variety of conditions including stroke, amyotrophic lateral sclerosis and congenital hemiparesis[16,17,18]. We have recently reported abnormal FA and ADC values, probably reflecting Wallerian degeneration, in the distal parts of the affected cortico-spinal tract in children who had undergone hemispherectomy.[19] The present study, albeit preliminary, shows that DTI with tractography is sensitive and can be used to detect abnormalities of the cortico-spinal tract, reflected by increased ADC and decreased FA on the affected side, in SWS patients with hemiparesis, even when it is not severe. Importantly, despite the limited number of cases, patients with moderate motor involvement showed more pronounced DTI abnormalities, reflected by decreased age-adjusted FA values, than those with no or mild motor involvement.
Two previous case reports have reported conflicting results of cerebral diffusion abnormalities in patients with SWS.In the first study, increased ADC values, associated with decreased N-acetyl aspartate on proton MR spectroscopy, were found in the affected posterior region, consistent with impaired brain tissue integrity in a 26-year old woman with SWS.[20] Increased ADC can be also the result of vasogenic edema possibly related to recent or ongoing seizures as well as ischemia.[21] In a second study, Siejens et al.[22] reported lack of abnormal FA or ADC in the frontal lobe gray matter of an 11-year old boy, despite mild atrophy and pial contrast enhancement in the frontal lobe. However, the white matter, let alone the corticospinal tract, was not evaluated in that study. In our recent study of 12 children with unilateral SWS, decreased FA and increased ADC values were found in not only the parieto-occipital white matter but also in adjacent central white matter regions which often showed deep transmedullary veins.[9] These findings, together with the results of the current study, support the notion that early white matter changes play an important role in the neurological manifestations of SWS.
Previous PET studies of glucose metabolism showed a correlation between abnormal glucose metabolism and the severity of epilepsy in SWS patients.[5] However, PET is not sensitive to detect white matter abnormalities due to low baseline glucose metabolic rates in this region. On the other hand, DTI has the ability to specifically assess various white matter tracts and detect subtle and early diffusion abnormalities. It should be pointed out that DTI variables undergo characteristic maturational changes in children: FA increases while ADC decreases with age in the brain of normal children.[14,15] We have found a similar correlation in the corticospinal tract contralateral to the angioma. However, age-related changes of DTI variables were much less apparent in the affected cortico-spinal tract, suggesting that the disease process interferes with normal white matter maturation in children with SWS. White matter integrity in SWS may be affected by ischemia causing axonal degeneration due to cortical injury, disruption of the myelination process, and/or ischemic damage in existing myelin. All these processes could be manifested in abnormal FA and ADC values in the affected cortico-spinal tract.
It should be also noted that white matter shows a left-right asymmetry of water diffusion even under normal conditions.[23,24] A recent study of right-handed children using diffusion MR tractography indeed showed a significant left>right asymmetry of FA values the cortico-spinal tract [25] with an average asymmetry of less than 3%. In our patient group with equal representation with right and left hemispheric involvement, we did not find any significant difference between these two subgroups in hemispheric asymmetries. Therefore, it is unlikely that the findings were substantially affected by the lateralization of the angioma.
All our patients had less than severe motor deficits, which showed a significant association with ADC values in the affected corticospinal tract. Thus, DTI appears to be a sensitive tool useful not only in the early detection of abnormalities in the corticospinal tract but may be useful to monitor change over a period of time with or without therapeutic intervention. Further studies addressing this are needed. Furthermore, it is well known that motor strength and ‘upper motor neuron pathways’ encompass more than the corticospinal pathway alone. [26] In this study we restricted ourselves to studying the corticospinal tract as it is a well delineated pathway that is amenable to tractography. We cannot dismiss the role of the ancillary pathways such as the rubrospinal, vestibulospinal, tectospinal and reticulospinal tracts, which are not as easy to measure with current tractography techniques but may be investigated in the future with probabilistic tractography.[27]
Conclusion
DTI tractography can detect structural changes in the ipsilateral corticospinal tracts of children with SWS with mild or moderate motor deficits. These mild diffusion abnormalities correlate with the severity of motor deficit. Longitudinal studies could be clinically useful to monitor changes in corticospinal tract over time and select patients who could benefit from early intervention to optimize motor outcome.
Acknowledgments
This work was supported by a grant from the NIH (NS41922, to Dr. Juhasz). The authors are grateful to Yang Xuan BS for acquiring the MRI data, and to Fangyu Peng MD, PhD and Anne Deboard RN for assisting sedation during the MRI studies.
References
- 1.Roach ES, Bodensteiner JB. Neurologic manifestations of Sturge-Weber syndrome. In: Bodensteiner JB, Roach ES, editors. Sturge-Weber syndrome. The Sturge-Weber Foundation; Mt Freedom, NJ: 1999. pp. 27–38. [Google Scholar]
- 2.Pascual-Castroviejo I, Diaz-Gonzalez C, Garcia-Melian RM. Sturge-Weber syndrome: study of 40 patients. Pediatr Neurol. 1993;9:283–8. doi: 10.1016/0887-8994(93)90064-j. [DOI] [PubMed] [Google Scholar]
- 3.Okudaira Y, Arai H, Sato K. Hemodynamic compromises as a factor in clinical progression of Sturge—Weber syndrome. Childs Nerv Syst. 1997;13:214–219. doi: 10.1007/s003810050070. [DOI] [PubMed] [Google Scholar]
- 4.Chugani HT, Mazziotta JC, Phelps ME. Sturge-Weber syndrome: a study of cerebral glucose utilization with positron emission tomography. J Pediatr. 1989;114:244–53. doi: 10.1016/s0022-3476(89)80790-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Asano E, Muzik O, Chugani DC, Juhasz C, Pfund Z, et al. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001;57:189–195. doi: 10.1212/wnl.57.2.189. [DOI] [PubMed] [Google Scholar]
- 6.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–70. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 7.Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–50. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- 8.Watts R, Liston C, Niogi S, Ulug AM. Fiber tracking using magnetic resonance diffusion tensor imaging and its applications to human brain development. Mental Retard Dev Disabil Res Rev. 2003;9:168–77. doi: 10.1002/mrdd.10077. [DOI] [PubMed] [Google Scholar]
- 9.Juhász C, Haacke M, Hu J, Xuan Y, Macke M, Behen ME, et al. Multimodality imaging of cortical and white matter abnormalities in Sturge-Weber syndrome. AJNR Am J Neuroradiol. 2007 (in press) [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DTI Studio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori S, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med. Sci. 2004;3:11–7. doi: 10.2463/mrms.3.11. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Zhang J, van Zijl PC, Mori S. Analysis of noise effects on DTI-based tractographyusing the brute-force and multi-ROI approach. Magn Reson Med. 2004;52:559–65. doi: 10.1002/mrm.20147. 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kumabe T, Nakasato N, Inoue T, Yoshimoto T. Primary thumb sensory cortex located at the lateral shoulder of the inverted omega-shape on the axial images of the central sulcus. Neurol Med Chir. (Tokyo) 2000;40:393–401. doi: 10.2176/nmc.40.393. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–58. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 15.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK, et al. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–8. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller M, Frandsen J, Anderson G, Gjedde A, Vestergaard-Poulsen P, Ostergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibertracking. J.Neurol.Neurosurg.Psychiatry. 2007;78:587–92. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sage CA, Peeters RR, Gorner A, Robberecht W, Sunaert S. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage. 2007;34:486–499. doi: 10.1016/j.neuroimage.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Hoon AH., Jr. Neuroimaging in cerebral palsy : Patterns of brain dysgenesis and injury. J.Child Neurol. 2005;20:936–939. doi: 10.1177/08830738050200120201. [DOI] [PubMed] [Google Scholar]
- 19.Wakamoto H, Eluvathingal TJ, Makki M, Juhasz C, Chugani HT. Diffusion tensor imaging of the corticospinal tract following cerebral hemispherectomy. J Child Neurol. 2006;21:566–71. doi: 10.1177/08830738060210071401. [DOI] [PubMed] [Google Scholar]
- 20.Cakirer S, Yagmurlu B, Savas MR. Sturge-Weber syndrome: diffusion magnetic resonance imaging and proton magnetic resonance spectroscopy findings. Acta Radiol. 2005;46:407–10. doi: 10.1080/02841850510021274. [DOI] [PubMed] [Google Scholar]
- 21.Provenzale JM, Petrella JR, Cruz LC, Jr, Wong JC, Engelter S, Barboriak DP. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2001;22:1455–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Sijens PE, Gietiling EW, Meiners LC, Sival DA, Potze JH, Irwan R, et al. Diffusion tensor imaging and magnetic resonance spectroscopy of the brain in a patient with Sturge-Weber syndrome. Acta Radiol. 2006;47:972–6. doi: 10.1080/02841850600849100. [DOI] [PubMed] [Google Scholar]
- 23.Buchel C, Raedler T, Sommer M, Sach M, Wieller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–51. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–23. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing—Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007 Feb 16; doi: 10.1093/cercor/bhm003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;143:263–79. doi: 10.1016/S0079-6123(03)43026-4. [DOI] [PubMed] [Google Scholar]
- 27.Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Johansen-Berg H, Howard RS, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129:1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]