Abstract
In the nose, odorants are detected on the cilia of olfactory sensory neurons (OSNs), where a cAMP-mediated signaling pathway transforms odor stimulation into electrical responses. Phosphodiesterase (PDE) activity in OSN cilia was long thought to account for rapid response termination by degrading odor-induced cAMP. Two PDEs with distinct cellular localization have been found in OSNs: PDE1C in cilia; PDE4A throughout the cell but absent from cilia. We disrupted both genes in mice and performed electroolfactogram analysis. Unexpectedly, eliminating PDE1C did not prolong response termination. Prolonged termination occurred only in mice lacking both PDEs, suggesting that cAMP degradation by PDE1C in cilia is not a rate-limiting factor for response termination in wildtype. Pde1c−/− OSNs instead displayed reduced sensitivity and attenuated adaptation to repeated stimulation, suggesting potential roles for PDE1C in regulating sensitivity and adaptation. These observations provide new perspectives in regulation of olfactory transduction.
INTRODUCTION
In vertebrates, olfactory sensory neurons (OSNs) use a cyclic AMP (cAMP) second messenger-mediated signal transduction pathway to convert odor stimulation into electrical signals. Olfactory signal transduction takes place on the cilia extending from the dendritic knob of the OSN1, 2. Odor exposure leads to elevated activity of Adenylyl Cyclase III (ACIII), resulting in a rapid rise of the cAMP concentration in the cilia. cAMP directly opens olfactory cyclic nucleotide-gated (CNG) ion channels, resulting in influx of Ca2+ and Na+ and membrane depolarization3, 4. Ca2+ can then open Ca2+-activated Cl− channels, which results in efflux of Cl− and further depolarization, contributing to the generation of action potentials5–8.
In cyclic nucleotide-mediated signal cascades, the magnitude and duration of second messenger signals are determined by the activities of two opposing enzymes: the cyclase for the production and the phosphodiesterase (PDE) for the degradation of cyclic nucleotides. In olfactory transduction, PDE activity in the cilia has long been thought to account for rapid termination of the OSN response by degrading odor-induced cAMP9, 10, leading to the closure of CNG channels. Two distinct cyclic nucleotide PDEs, PDE1C11, 12 and PDE4A13, 14, have been identified in mammalian OSNs. PDE1C is a Ca2+/calmodulin-stimulated PDE11; PDE4A is Ca2+ insensitive but has substrate specificity for cAMP15. Immunohistochemical staining revealed that the PDE1C protein is selectively enriched in the cilia, while the PDE4A protein is distributed throughout the cell, including the dendritic knob from where the cilia emanate, but is absent from the cilia13, 14, 16. PDE1C was therefore hypothesized to be critical for rapid termination of the OSN response due to its cilial localization and Ca2+ dependency, while PDE4A was not expected to affect OSN responses as it is excluded from the cilia.
To assess the function of PDE1C and PDE4A in olfactory transduction, we disrupted both the Pde1c and the Pde4a gene in mice and conducted electrophysiological analysis of OSN responses using electroolfactogram (EOG). Loss of PDE1C was predicted to prolong response termination. Contrary to the expectation, eliminating PDE1C alone resulted in accelerated termination of the EOG response. Prolonged response termination occurred only in mice lacking both PDE1C and PDE4A, revealing that the activity of either PDE1C in the cilia or PDE4A outside of the cilia is sufficient to allow rapid termination of the EOG response. These results suggest that in wildtype OSNs cAMP degradation by PDE1C in cilia is not a rate-limiting factor for response termination. We found that Pde1c−/− OSNs instead display reduced sensitivity to odors and attenuated adaptation to repeated stimulation.
RESULTS
Generation of Pde1c−/− and Pde4a−/− mice
We took a loss-of-function approach to examine the role of phosphodiesterases in olfactory transduction. We generated mouse lines in which the Pde1c and Pde4a genes are disrupted individually and in combination (Supplementary Fig. 1 online). This genetic approach allows specific and complete elimination of the targeted PDE activity, a condition not achievable with current pharmacological inhibitors. Both individual knockout mice and Pde1c−/−;Pde4a−/− double knockout mice show apparently normal growth rate and feeding, nursing, and mating behaviors.
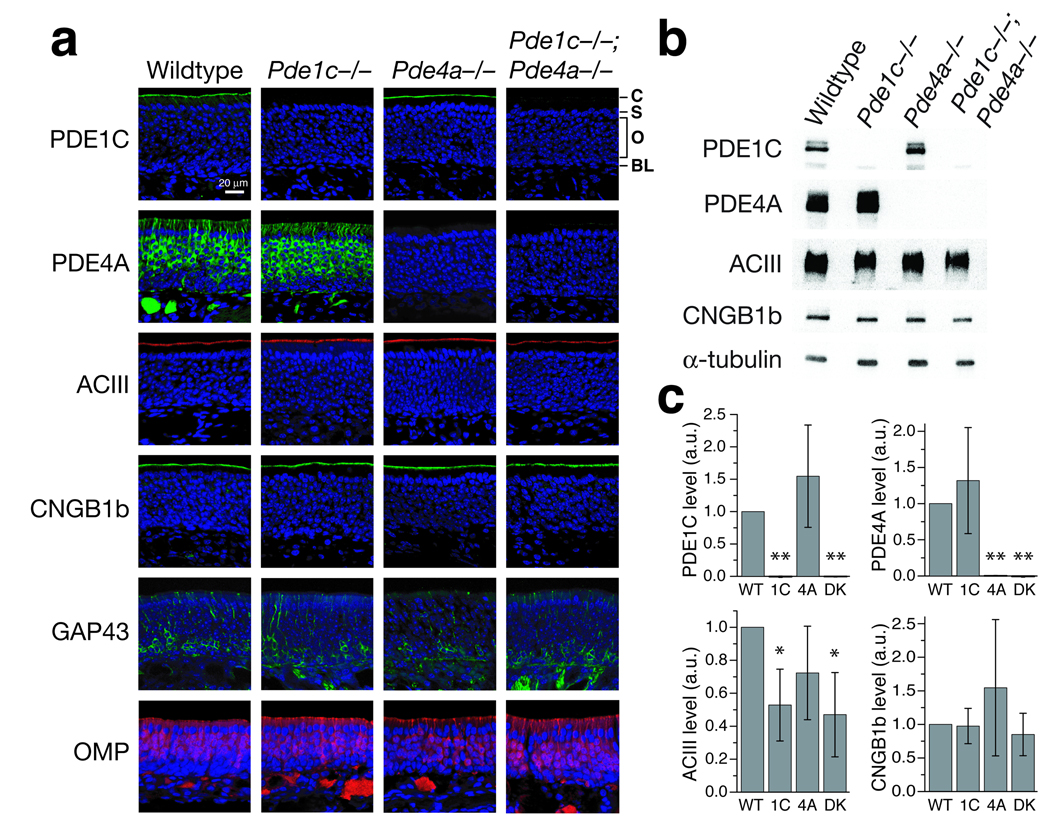
We confirmed the loss of PDE1C and PDE4A proteins in the relevant knockout strains by immunohistochemistry (Fig. 1a). In wildtype olfactory epithelium (OE), PDE1C is detected primarily in the cilial layer, whereas PDE4A is detected in OSN cell bodies, dendrites, and axons. PDE1C and/or PDE4A immunoreactivity is absent in the relevant mutant mice. The thickness of the OE at similar nasal positions is comparable between all genotypes, and all genotypes show similar immunostaining for other olfactory transduction components, including ACIII and the CNG channel subunit CNGB1b, and for GAP43 (immature OSN marker) and OMP (mature OSN marker) (Fig. 1a). The loss of either PDE does not affect the localization of the other PDE, i.e. in Pde1c−/− mice, the localization of PDE4A protein is normal, and vice versa (Fig. 1a and Fig 3a). However, we found that there is a significant reduction in the level of ACIII protein in Pde1c−/− and Pde1c−/−;Pde4a−/− olfactory epithelium as examined by Western blotting (Fig. 1b). The level of CNGB1b protein in these mice appears unchanged.
Figure 1. Molecular characterization of Pde1c−/−, Pde4a−/−, and Pde1c−/−;Pde4a−/− mice.
(a) Immunostaining on sections of olfactory epithelium. Sections were counterstained with DAPI (blue) to label cell nuclei. C, cilial layer; S, supporting cell layer; O, olfactory sensory neuron layer; BL, basal lamina. Scale bar: 20 µm. (b and c) Western blot analysis of total OE proteins. (b) Example Western blots for PDE1C, PDE4A, ACIII, and CNGB1b on total OE proteins from all four genotypes. α-tubulin is used as a loading control. (c) Quantification of Western blotting. Values are normalized to α-tubulin staining and shown relative to wildtype in arbitrary units (a.u.). PDE1C is detected at similar levels in wildtype and Pde4a−/− mice (1.55 ± 0.79), but is undetectable in Pde1c−/− and Pde1c−/−;Pde4a−/− mice. PDE4A is detected at similar levels in wildtype and Pde1c−/− mice (1.32 ± 0.73), but is undetectable in Pde4a−/− and Pde1c−/−;Pde4a−/− mice. ACIII levels are significantly reduced in Pde1c−/− (0.52 ± 0.22) and Pde1c−/−;Pde4a−/− mice (0.47 ± 0.26), but not in Pde4a−/− mice (0.72 ± 0.28). CNGB1b levels are similar in all genotypes (Pde1c−/− , 0.97 ± 0.26; Pde4a−/−, 1.55 ± 1; Pde1c−/−;Pde4a−/−, 0.85 ± 0.31). Data are the average of 3 independent sets of mice, each set consisting of a mouse of each genotype. Error bars are 95% confidence intervals (CI). Statistical significance was determined by one-sample t-test. * P < 0.05, ** P < 0.01. WT, wildtype; 1C, Pde1c−/; 4A, Pde4a−/−; DK, Pde1c−/−;Pde4a−/−.
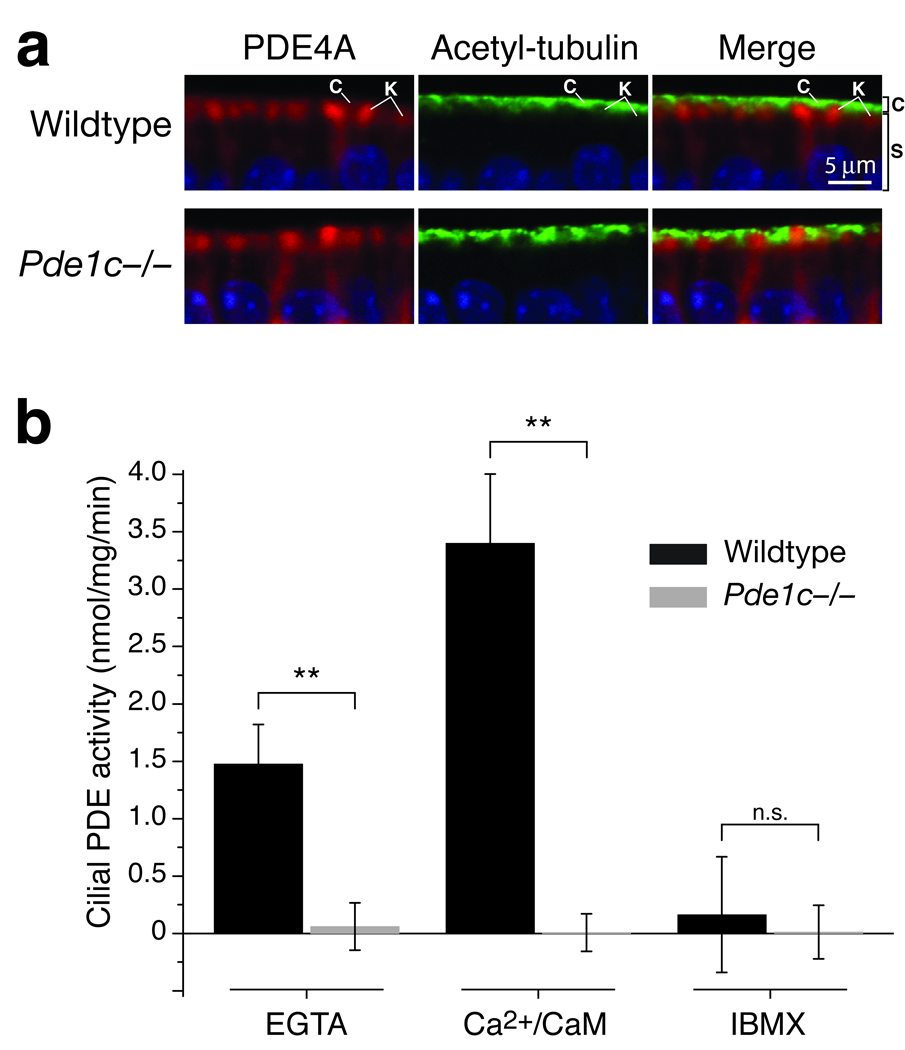
Figure 3. Knockout of PDE1C eliminates all PDE activity from OSN cilia.
(a) Co-immunostaining for PDE4A (red) and the cilia marker acetylated tubulin (green) on sections of OE from wildtype and Pde1c−/− mice. Shown are apical portions of the OE under high magnification. In both wildtype and Pde1c−/− mice, immunoreactivity for PDE4A and acetylated tubulin appears in distinct spatial domains. C, cilial layer; K, dendritic knobs; S, supporting cell layer. Sections are counterstained with DAPI. Scale bar: 5 µm. (b) PDE catalytic activity assay in cilia preparations from wildtype and Pde1c−/− mice. PDE activity in wildtype cilia was increased by addition of Ca2+/CaM and inhibited by addition of IBMX. Activity from Pde1c−/− cilia under all conditions was virtually undetectable, similar to wildtype cilia treated with IBMX. Error bars are 95% CI. Data for each genotype are the average of 5 independent preparations (mice). Each preparation was assayed in duplicate.
Pde1c−/− OSNs show smaller responses and faster termination
To determine the function of PDE1C in olfactory transduction, we recorded EOG signals from Pde1c−/− and wildtype mice in response to 100 ms odor pulses. The EOG has been widely used as a reliable and convenient means to assess OSN responses17, 18 and is thought to result primarily from the summed generator potentials of OSNs, although it may also have other components. We used two common odorants, amyl acetate and heptaldehyde, and analyzed four metrics of the EOG signal: the amplitude, the response latency, the rise time, and the rate of response termination. Similar results were obtained for both odorants. We present the data for amyl acetate in the main text, and provide quantification of the responses to heptaldehyde in Supplementary Table 1 online.
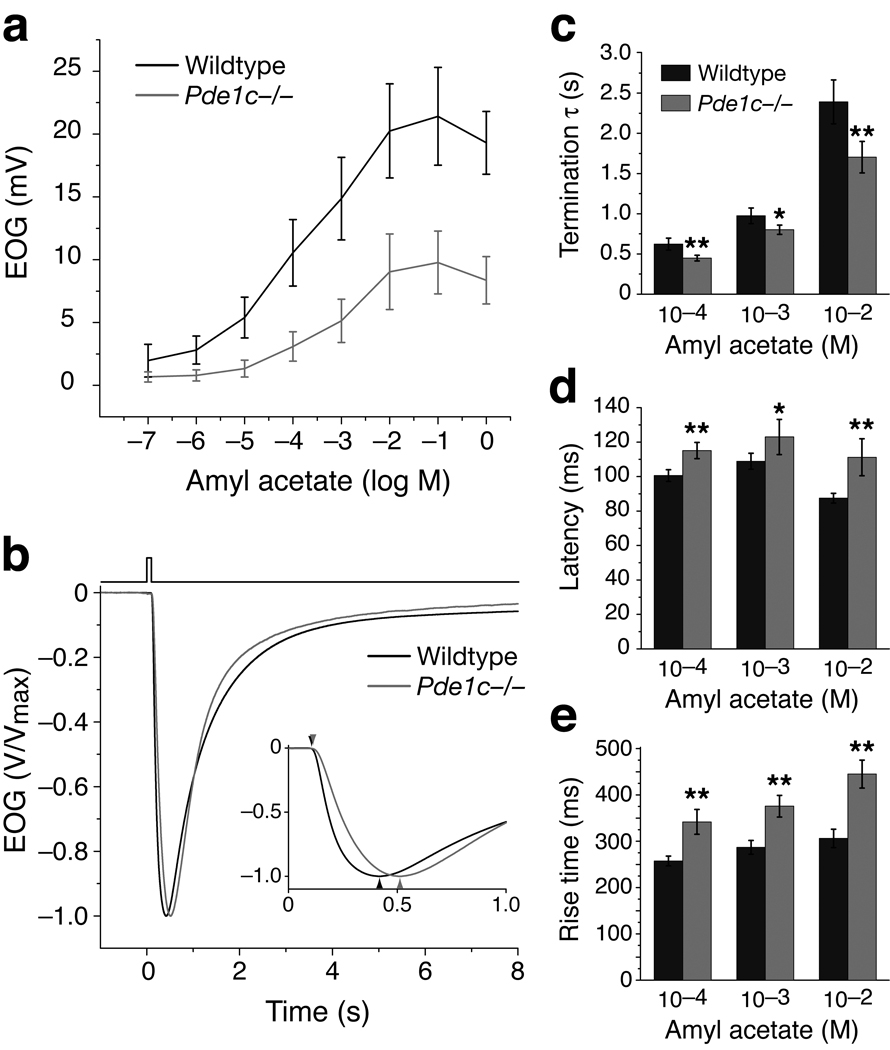
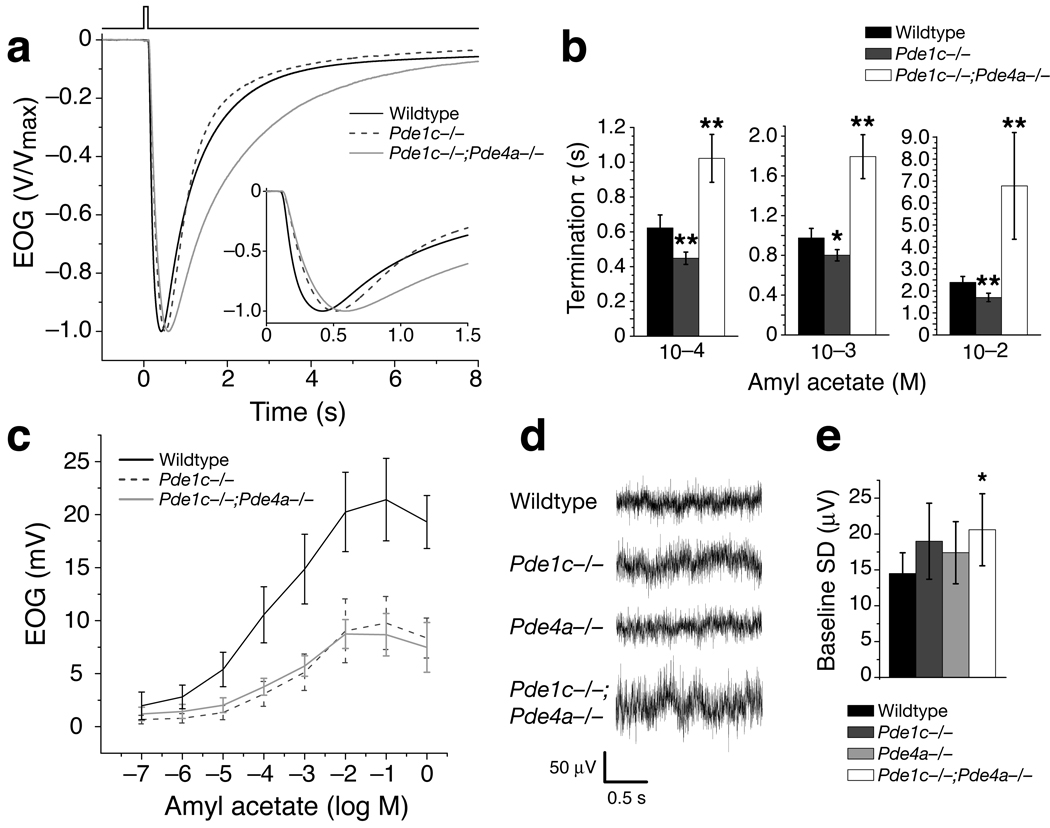
Eliminating PDE1C, the only PDE known in the cilia, was expected to prolong response termination. It may also allow for larger responses with quicker onset as cAMP concentrations could increase more rapidly when degradation of cAMP is reduced. Contrary to expectations, Pde1c−/− mice displayed faster response termination compared to the wildtype (Fig. 2b). The rate of response termination was quantified by calculating the time constant (τ) through fitting the decay phase of the EOG signal with a single exponential equation. The termination τ of Pde1c−/− mice were smaller than those of the wildtype for all odorant concentrations tested (Fig. 2c and Table 1, which lists data for 10−4, 10−3 and 10−2 M amyl acetate). Pde1c+/− heterozygous mice displayed responses no different from the wildtype (Supplementary Fig. 2 online).
Figure 2. Pde1c−/− OSNs display reduced EOG amplitude, faster response termination, and slower onset kinetics.
(a) Dose-response relations of EOG responses to amyl acetate from wildtype (n = 7) and Pde1c−/− (5) mice. Amyl acetate was delivered as 100 ms pulses. Concentrations on the X-axis are those of the liquid solution. Data points are linked with straight lines; error bars are SD. (b) EOG responses to a single 100 ms pulse of 10−3 M amyl acetate. Responses are normalized and averaged for comparison of response kinetics (Wildtype, n = 15; Pde1c−/−, 10). Inset shows the traces plotted on an expanded time axis. (c) Termination time constants (τ), determined by a single exponential fit to the decay phase of the EOG signal. For 10−4 M, 10−3 M, and 10−2 M, P = 9.7x10−4, 0.011, and 8.4×10−4; wildtype, n = 20; Pde1c−/−, n = 14. (d) Response latencies, defined as the time between the initiation of odor pulse and the start of the response (1% of peak amplitude). For 10−4 M, 10−3 M, and 10−2 M, P = 4.5×10−5, 0.011, and 5.1×10−5; wildtype, n = 15; Pde1c−/−, n = 10. (e) Response rise times, defined as the time from the start of the response to the peak. For 10−4 M, 10−3 M, and 10−2 M, P = 1.1×10−6, 9.0×10−7, 5.8×10−8; wildtype, n = 15; Pde1c−/−, n = 10. In (c–e), error bars are 95% CI. P values are from unpaired t-tests. *, P < 0.05; **, P < 0.01.
Table 1.
Analysis of EOG responses (Amyl Acetate stimulation)
| Wildtype | Pde1c−/− | Pde4a−/− | Pde1c−/−:Pde4a−/− | |||
|---|---|---|---|---|---|---|
| Maximum amplitude (mV) |
21.4 ± 3.9 (7) | 9.8 ± 2.5 (5) ** | 20.2 ± 2.1 (5) | 8.7 ± 1.4 (8) ** | ||
| Latency (ms) |
10−4 M | 100.6 ± 3.4 (15) | 115.1 ± 4.7 (10) ** |
102.6 ± 4.2 (14) | 116.4 ± 3.9 (17) ** |
|
| 10−3 M | 108.8 ± 4.7 (15) | 123.0 ± 10 (10) * |
110.7 ± 5.2 (14) | 125.0 ± 4.5 (17) ** |
||
| 10−2 M | 87.5 ± 2.8 (15) | 111.2 ± 11 (10) ** |
87.8 ± 2.3 (14) | 118.9 ± 8.7 (17) ** |
||
| Rise time (ms) |
10−4 M | 257 ± 11 (15) | 341 ± 27 (10) ** |
264 ± 10 (14) | 405 ± 13 (17) ** |
|
| 10−3 M | 287 ± 15 (15) | 376 ± 23 (10) ** |
289 ± 15 (14) | 441 ± 19 (17) ** |
||
| 10−2 M | 306 ± 20 (15) | 445 ± 30 (10) ** |
307 ± 14 (14) | 562 ± 25 (17) ** |
||
| Termination τ(s) |
10−4 M | 0.62 ± 0.07 (20) | 0.45 ± 0.04 (14) ** |
0.65 ± 0.09 (14) | 1.02 ± 0.14 (17) ** |
|
| 10−3 M | 0.97 ± 0.10 (20) | 0.80 ± 0.06 (14) * |
0.99 ± 0.13 (14) | 1.79 ± 0.22 (17) ** |
||
| 10−2 M | 2.39 ± 0.27 (20) | 1.70 ± 0.20 (14) ** |
2.27 ± 0.30 (14) | 6.78 ± 2.43 (17) ** |
||
| Paired– pulse: ratio of 2nd pulse to 1st pulse |
10−4 M | 0.48 ± 0.04 (13) | 0.61 ± 0.03 (9) ** |
0.48 ± 0.05 (8) | 0.44 ± 0.06 (8) | |
| 10−3 M | 0.39 ± 0.04 (13) | 0.51 ± 0.04 (9) ** |
0.39 ± 0.04 (8) | 0.34 ± 0.04 (8) | ||
| 10−2 M | 0.20 ± 0.03 (13) | 0.29 ± 0.04 (9) ** |
0.22 ± 0.05 (8) | 0.19 ± 0.03 (8) | ||
| Sustained pulse: % decline at 10 s |
10−4 M | 61.3 ± 4.6 (15) | 56.8 ± 3.5 (10) | 69.2 ± 2.6 (6) | 32.8 ± 12.2 (9) ** |
|
| 10−3 M | 54.7 ± 4.9 (15) | 53.4 ± 5.9 (10) | 56.4 ± 3.7 (6) | 26.9 ± 9.2 (9) ** |
||
| 10−2 M | 43.5 ± 4.1 (15) | 44.7 ± 4.9 (10) | 42.6 ± 6.7 (6) | 13.1 ± 6.1 (9) ** |
||
Odorant concentrations are for amyl acetate. For maximum amplitude, values are ± SD; for all others, values are ± 95% CI. The number of mice (n) is indicated in parentheses.
indicates P < 0.05 in an unpaired t–test compared to wildtype,
indicates P < 0.01.
Pde1c−/− mice also displayed significantly reduced EOG amplitudes with slower onset kinetics. The maximum EOG amplitude of Pde1c−/− mice (9.8 ± 2.5 mV, n = 5 mice) was approximately half of that of the wildtype (21.4 ± 3.9 mV, n = 7 mice) (Fig. 2a). Both the response latency, defined as the time between the initiation of the odor pulse and the start of the response (1% of EOG peak value), and the rise time, defined as the time from the start of the response to the peak, are significantly longer in Pde1c−/− mice (Fig. 2d,e and Table 1). The reduced response magnitude, slower onset kinetics, and faster response termination observed in Pde1c−/− mice are reminiscent of OSNs in a state of cellular adaptation.
PDE1C knockout eliminates all PDE activity from OSN cilia
Since Pde1c−/− mice did not show the expected prolonged response termination, it raised a question whether there is additional PDE activity in the Pde1c−/− cilia responsible for removal of cAMP. Our initial characterization of Pde1c−/− mice showed that PDE4A, the only other PDE found in OSNs, remained localized in the dendrite and cell body but not in the cilial layer (Fig. 1a). We further confirmed this observation with double-label immunofluorescence comparing anti-PDE4A staining with that for the cilia marker acetylated tubulin. PDE4A and acetylated tubulin antibodies labeled distinct spatial domains in the OE of both wildtype and Pde1c−/− mice (Fig. 3a).
We also carried out a PDE catalytic activity assay on preparations of OSN cilia (Fig. 3b). PDE activity in OSN cilia is known to be stimulated by Ca2+ and calmodulin (CaM)16, which has been attributed to the Ca2+/CaM sensitivity of PDE1C11. We measured PDE activity in wildtype and Pde1c−/− cilia preparations under three conditions: in a standard reaction buffer containing no Ca2+ (0 mM Ca2+ and 0.1 mM EGTA); in the reaction buffer supplemented with 200 µM Ca2+ and 50 nM CaM; and in the reaction buffer supplemented with a broad-range PDE inhibitor 3-isobutyl-1-methylxanthin (IBMX, 0.5 mM). Wildtype preparations exhibited PDE activity in the absence of Ca2+ (1.5 ± 0.33 nmol mg−1 min−1), reflecting the basal PDE activity in the cilia. When the buffer is supplemented with Ca2+ and CaM, wildtype cilial PDE activity is increased ~2.3-fold (3.4 ± 0.60 nmol mg−1 min−1), consistent with a previous report16. IBMX blocked all cilial PDE activity (0.17 ± 0.50 nmol mg−1 min−1). In Pde1c−/− cilia preparations, no basal PDE activity was detected in the standard reaction buffer (0.08 ± 0.21 nmol mg−1 min−1), nor did the addition of Ca2+/CaM elicit any PDE activity (0.002 ± 0.16 nmol mg−1 min−1). In both conditions the activity levels were similar to those when IBMX was added (0.02 ± 0.23 nmol mg−1 min−1 for Pde1c−/− cilia). We conclude that knocking out PDE1C eliminated all PDE activity from OSN cilia.
Pde4a−/− OSNs display no aberrant response properties
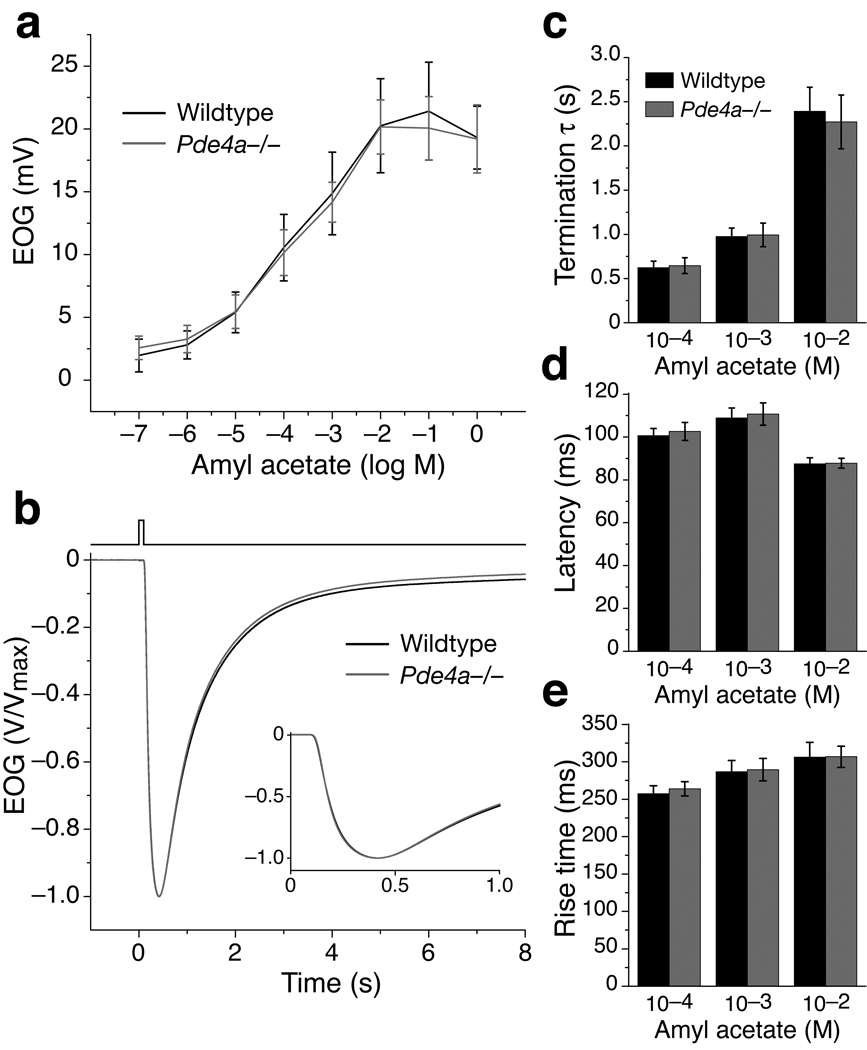
To address whether PDE4A influences OSN responses, we measured EOG signals in Pde4a−/− mice using the same odorants and stimulation protocols as for Pde1c−/− mice. No differences were observed between Pde4a−/−, Pde4a+/− and wildtype mice in any aspect of the EOG signal (Fig. 4 and Supplementary Fig. 2). These results are consistent with the idea that because PDE4A is excluded from the cilia, its impact on olfactory transduction may be minimal under most circumstances.
Figure 4. Pde4a−/− OSNs display no aberrant EOG response properties.
Data for wildtype mice is re-plotted from Figure 2 for reference. (a) Dose-response relationship of EOG responses to amyl acetate from Pde4a−/− mice (n = 5). Error bars are SD. (b) Normalized and averaged EOG responses from Pde4a−/− mice (n = 14) to a single 100 ms pulse of 10−3 M amyl acetate. Inset shows the traces plotted on an expanded time axis. (c) Termination τ for responses to three amyl acetate concentrations. For 10−4 M, P = 0.70; 10−3 M, P = 0.82; 10−2 M, P = 0.57. (d) Response latencies. For 10−4 M, P = 0.47; 10−3 M, P = 0.61; 10−2 M, P = 0.87. (e) Response rise times. For 10−4 M, P = 0.39; 10−3 M, P = 0.81; 10−2 M, P = 0.97. In (c–e), error bars are 95% CI. All P values are from unpaired t-tests, n = 14 for Pde4a−/−. No significant differences (P < 0.05) are found in any parameters.
Pde1c−/−;Pde4a−/− OSNs show prolonged response termination
Previous electrophysiological experiments showed that treatment of OSNs with IBMX prolonged OSN responses to odors, indicating degradation of cAMP by PDE activity is essential for rapid response termination9. However, neither Pde1c−/− nor Pde4a−/− mice displayed prolonged termination of EOG signals, suggesting that in these mutant mice the rate of cilial cAMP removal is still adequate to allow rapid response termination. As IBMX is a broad-range PDE inhibitor, treatment would inhibit simultaneously both PDE1C and PDE4A, a situation which would reflect a Pde1c−/−;Pde4a−/− double knockout.
We generated Pde1c−/−;Pde4a−/− mice and performed EOG recordings. These mice displayed a significantly prolonged response termination for all odorant concentrations tested (Fig. 5a,b and Table 1). This result suggests that removal of cAMP from the cilia is substantially impaired when both PDEs are not functional, consistent with previous studies using IBMX to eliminate all PDE activity9. In addition, Pde1c−/−;Pde4a−/− mice displayed significantly reduced EOG amplitudes and slower onset kinetics compared to the wildtype (Fig. 5c). The reduction in EOG amplitudes and the extended latency of responses in the double knockout mice are comparable to those of Pde1c−/− mice. The increase in the response rise time in double knockout mice is even greater than that in Pde1c−/− mice (Supplementary Fig. 3 online).
Figure 5. Pde1c−/−;Pde4a−/− double knockout mice display prolonged response termination and increased baseline noise.
Data for wildtype and Pde1c−/− mice are re-plotted from Figure 2 for reference. (a) Normalized and averaged EOG responses from Pde1c−/−;Pde4a−/− (n = 17) mice to a single 100 ms pulse of 10−3 M amyl acetate. Inset shows the traces plotted on an expanded time axis. (b) Termination τ for responses to three amyl acetate concentrations. Responses from Pde1c−/−;Pde4a−/− mice have significantly longer termination τ compared to wildtype. Error bars are 95% CI. For 10−4 M, P = 8.3×10−6; 10−3 M, P = 3.5×10−8; 10−2 M, P = 5.2×10−4; all unpaired t-tests with the wildtype, n = 17 for Pde1c−/−;Pde4a−/−. (c) Dose-response relationship for amyl acetate from Pde1c−/−;Pde4a−/− mice (n = 8). Error bars are SD. (d) Representative baseline traces (filtered DC - 1 kHz) from each genotype. (e) Baseline noise is quantified as the standard deviation in the voltage from 2 s of recording (sampling rate 2 kHz) without stimulation. Pde1c−/−;Pde4a−/− mice display significantly increased baseline noise compared to wildtypes. Error bars are 95% CI. Compared with wildtype: Pde1c−/−, P = 0.144; Pde4a−/−, P = 0.278; Pde1c−/−;Pde4a−/−, P = 0.048; all unpaired t-tests, n = 12 for wildtype, n = 10 for Pde1c−/−, n = 7 for Pde4a−/−, n = 11 for Pde1c−/− ;Pde4a−/−. * indicates P < 0.05, ** indicates P < 0.01.
Given the additive effect of the double knockout on response kinetics, we further examined Pde1c−/−;Pde4a+/−, Pde1c+/−;Pde4a−/−, and Pde1c+/−;Pde4a+/− mice. We did not observe any significant genetic dosage effects, as Pde1c−/−;Pde4a+/− mice show responses similar to Pde1c−/− mice, and both Pde1c+/−;Pde4a−/− and Pde1c+/−;Pde4a+/− mice show responses similar to the wildtype (Supplementary Fig. 2 and Supplementary Table 2 online).
Pde1c−/−;Pde4a−/− mice also displayed significantly larger baseline noise of the EOG signal than the wildtype (Fig. 5d,e). The baseline noise was quantified as the standard deviation in the voltage during 2 s of baseline recording (2 kHz sampling rate). The increase in baseline noise likely reflects an increase in sporadic opening of CNG channels due to spontaneously generated cAMP, which cannot be quickly removed from the cilia of double knockout mice. Pde1c−/− mice displayed only a trend of increased baseline noise; the difference from wildtype is not significant.
OSN adaptation is differently affected in the mutant mice
Adaptation, the phenomena of decreasing responses either to repeated stimuli or during sustained stimuli, is a characteristic feature of OSN responses. Both forms of adaptation are thought to rely on different but overlapping sets of mechanisms19, 20. Due to its Ca2+/CaM sensitivity, PDE1C has long been hypothesized to contribute to OSN adaptation11, 12, 20. Ca2+, which enters cilia as part of the OSN response, can bind CaM and subsequently elevate PDE1C activity. If the stimulation continues or a subsequent stimulation occurs while PDE1C activity is elevated, the amount of cAMP available to open the CNG channel would be reduced, leading to smaller responses (i.e. adaptation). However, it has been previously reported that OSNs display a similar extent of adaptation to repeated stimulation when stimulated with intracellular delivery of either cAMP or non-hydrolyzable cAMP analogs, suggesting that the combined activity of all PDEs does not play a role in adaptation to repeated stimulation21. The function of individual PDEs in OSN adaptation has not been directly examined.
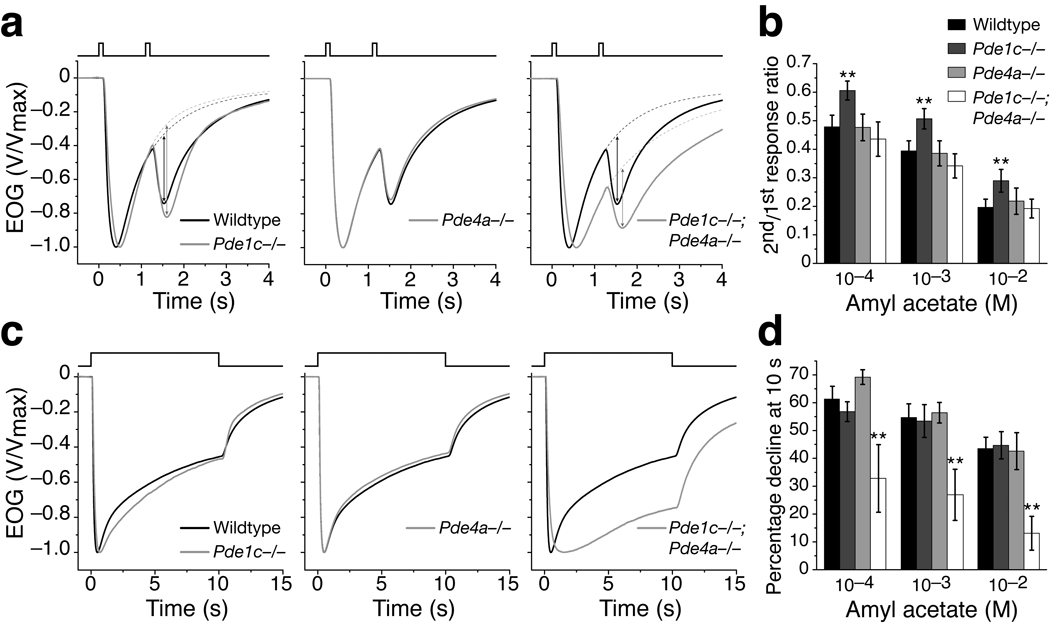
To assess the role of PDE1C and PDE4A in OSN adaptation to repeated odor exposure, we recorded EOG signals from mice bearing single or combined mutations of Pde1c and Pde4a using a paired-pulse protocol, which consisted of two identical 100 ms odor pulses separated by a 1 s interpulse interval (Fig. 6a). The extent of adaptation is quantified as the ratio of the 2nd “net” peak amplitude (see Methods for details) to the 1st peak amplitude18. Pde1c−/− mice still displayed adaptation, but with significant attenuation. The ratios of 2nd to 1st pulse response were significantly larger compared to the wildtype for all concentrations tested (Fig. 6b). These data suggest PDE1C likely contributes to OSN adaptation to repeated stimulation.
Figure 6. OSN adaptation is differently affected in Pde1c−/− and Pde1c−/−;Pde4a−/− mice.
(a) Normalized and averaged EOG responses from wildtype (n = 13), Pde1c−/− (9), Pde4a−/− (8), and Pde1c−/−;Pde4a−/− (8) mice to two 100 ms pulses of 10−3 M amyl acetate separated by a 1 s interval. The contribution of the residual 1st response was removed from the recorded 2nd response to obtain the net 2nd response peak amplitude (see Methods), as indicated by the arrows on the Pde1c−/− and Pde1c−/−;Pde4a−/− traces. (b) Ratio of 2nd net peak amplitude to the 1st peak amplitude. For Pde1c−/− compared to wildtype, P = 2.6×10−4, 3.9×10−4, and 0.0011 for 10−4, 10−3, and 10−2 M respectively. For Pde4a−/− compared to wildtype, P = 0.95, 0.77, and 0.42. For Pde1c−/−;Pde4a−/− compared to wildtype, P = 0.23, 0.071, and 0.85. (c) Normalized and averaged EOG responses to a 10 s pulse of 10−3 M amyl acetate from wildtype (n = 15), Pde1c−/− (10), Pde4a−/− (6), and Pde1c−/−;Pde4a−/− (9) mice. (d) Response decline during stimulation, quantified by the percent reduction in the peak amplitude at the 10 s time point. For Pde1c−/− compared to wildtype, P = 0.18, 0.74, and 0.73 for 10−4, 10−3, and 10−2 M respectively. For Pde4a−/− compared to wildtype, P = 0.056, 0.67, and 0.80. For Pde1c−/−;Pde4a−/− compared to wildtype, P = 4.5×10−5, 8.3×10−6, and 1.7×10−8. In (b) and (d), error bars are 95% CI and P values are from unpaired t-tests. * indicates P < 0.05, ** indicates P < 0.01.
Paired-pulse protocols with interpulse intervals of 2, 4, 6, and 8 s were also examined (Supplementary Fig. 3 online). As the interpulse interval lengthened, the response amplitude to the 2nd pulse progressively recovered. When the interpulse interval was 4 s or longer, there were no differences in the ratios of 2nd to 1st pulse responses between Pde1c−/− and wildtype mice, indicating the influence of PDE1C on adaptation is limited to a short period following stimulation.
Pde4a−/− mice displayed adaptation indistinguishable from wildtype (Fig. 6a,b). Interestingly, Pde1c−/−;Pde4a−/− mice also did not show a deficit in adaptation, despite the fact that Pde1c−/− mice have attenuated adaptation. This observation in double knockouts is consistent with the previous report that OSNs did not display apparent deficits in adaptation to repeated stimulation when all PDE activity is bypassed21.
To assess the role of PDE1C and PDE4A in OSN adaptation during sustained odor exposure, we recorded EOG signals in response to a 10 s odor pulse (Fig. 6c). During the pulse, the EOG amplitude progressively declined in all genotypes. The extent of adaptation was quantified as the percentage of amplitude decline relative to the peak amplitude at the 10 s time point. Neither Pde4a−/− nor, interestingly, Pde1c−/− displayed deficits in adaptation during sustained stimulation, although the longer response latency and rise time are still evident in Pde1c−/− mice (Fig. 6c,d). In contrast, however, Pde1c−/−;Pde4a−/− double knockout mice display significantly less decline during the course of stimulation (Fig. 6c,d). For 10−3 M amyl acetate (Fig. 6c), the response of Pde1c−/−;Pde4a−/− mice declined approximately 27%, whereas wildtype and single knockout mice declined approximately 55%. This decreased response decline is consistent with the idea that cilial cAMP removal is substantially impaired when both PDE1C and PDE4A are eliminated.
DISCUSSION
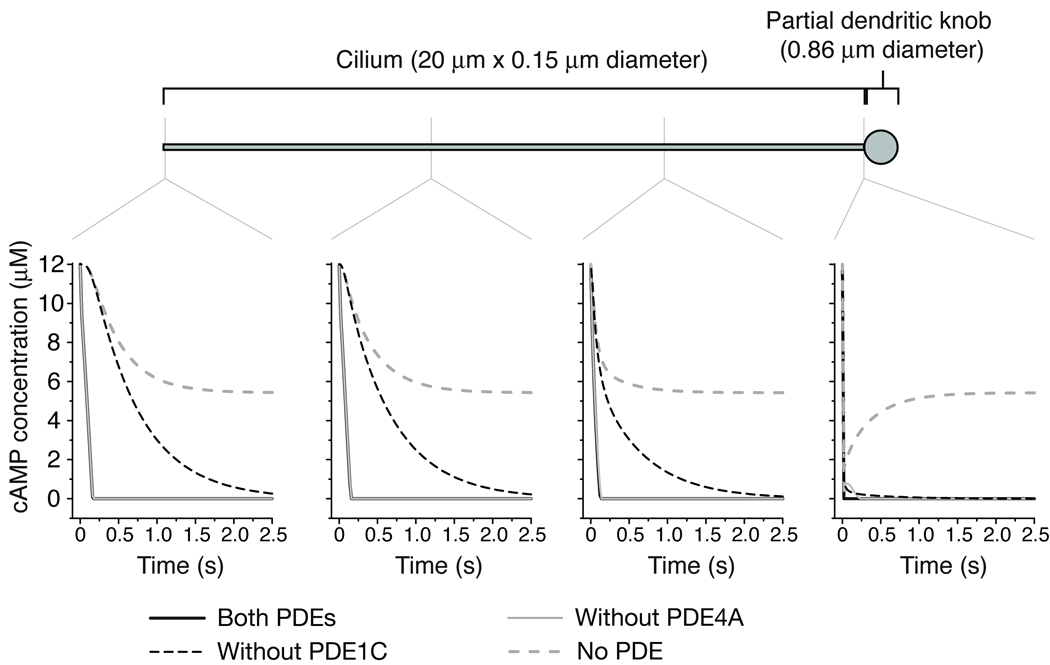
Rapid response termination after a stimulus is critical to allow sensory neurons to represent the temporal information of the stimulus, and to be prepared for subsequent stimulation. In OSNs, rapid response termination has long been thought to rely substantially on degradation of cAMP in the cilia. However, Pde1c−/− OSNs, despite the lack of all cilial PDE activity, do not display prolonged termination. This result suggests that other mechanism(s) must be able to efficiently remove cAMP from cilia in the absence of cilial degradation. The most reasonable mechanism is that cAMP diffuses from the cilia into the dendritic knob and dendrite, where it can be degraded by PDE4A. These two mechanisms for cilial cAMP removal—degradation in the cilia and diffusion away from the cilia followed by degradation in the dendrite—are illustrated in Supplementary Fig. 5. Indeed, cAMP diffusion in olfactory cilia has been measured to be nearly as fast as diffusion in water (2.7×10−6 cm2 s−1, ref. 22). Computer modeling using this value together with published values of PDE enzyme activities shows that diffusion of cAMP from the cilia followed by degradation in the knob can occur within the time scale of termination of EOG responses (Fig. 7, and Supplementary note online). cAMP degradation (or sequestration) in the dendritic knob is required for efficient flux of cAMP away from the cilia, since the dendritic knob is not large enough to be an effective sink for cAMP (given the knob volume is approximately equal to the total volume of all the cilia of an OSN). In the absence of both cilial and dendritic degradation (as in Pde1c−/−;Pde4a−/− OSNs), removal of cilial cAMP is severely hindered, leading to prolonged response termination.
Figure 7. Computer modeling of degradation and diffusion of cilial cAMP.
A cilium is attached to a “mini-knob”, which has approximately 1/12 of the volume of a 2 µm diameter dendritic knob (an estimation of the volume of the knob that would be available to a single cilium). The cilium is initially filled with 12 µM cAMP, a concentration that saturates the olfactory CNG channels. Four scenarios were modeled: with activities of both PDEs (modeling wildtype); without PDE4A activity; without PDE1C activity; and without any PDE activity (modeling Pde1c−/−;Pde4a−/− OSNs). Diffusion of cAMP is included in all scenarios. The change in cAMP concentrations is shown at four points along the model cilium for each scenario. Note that the black lines (wildtype modeling) are obscured by the light gray lines (modeling without PDE4A). For further details see the Supplementary note online.
In OSNs, rapid response termination depends on the closing of both CNG channels and Ca2+-activated Cl− channels. Removal of odor-evoked cAMP, in addition to feedback inhibition by Ca2+/CaM18, 23, leads to closing of CNG channels, while removal of cilial Ca2+ leads to closing of the Cl− channels24. The slowest of these processes would be the rate-limiting factor for response termination. When cAMP can be degraded in the cilia (as in wildtype or Pde4a−/− OSNs), cAMP removal will be faster than when cAMP can only be removed by diffusion to and degradation in the dendrite (as in Pde1c−/− OSNs). Our modeling suggests the difference between these rates can be several-fold (Fig. 7 and Supplementary note). It is then notable that in Pde1c−/− OSNs the termination of EOG responses is even accelerated (Fig. 2). The olfactory transduction system appears to tolerate such a range in the rate of cilial cAMP removal, suggesting that it is not a rate-limiting factor in the termination of OSN responses under normal circumstances, and that other termination mechanisms, namely Ca2+/CaM inhibition of CNG channels18, 23 and Ca2+ extrusion24, predominantly determine the kinetics of response termination. Only when cilial cAMP removal is severely hampered, as when the activities of both PDE1C and PDE4A are eliminated, does cAMP removal become rate-limiting.
In wildtype OSNs, the majority of cAMP generated in the cilia, either spontaneously or from odor stimulation, is likely degraded by PDE1C. This scenario is consistent with the fact that Pde4a−/− mice display no response deficits. In this case, PDE4A in the dendritic knob functions as a fail-safe for response termination. PDE4A may also prevent odor-evoked cAMP from escaping the dendrite and influencing other cellular processes. That PDE4A may function to spatially restrict cAMP to the cilia is reminiscent of the role of PDE4 enzymes in maintaining cAMP signaling domains in cardiac myocytes (for review, see ref. 15). Techniques to image cAMP in the living cells25 may be useful to further examine cAMP regulation in OSNs.
Although no other PDEs have been reported in canonical OSNs (PDE2 has been found in guanylyl cyclase D-positive neurons in the OE14, 26, 27), OSNs might express other PDE(s) that may account for eventual termination of OSN responses in Pde1c−/−;Pde4a−/− mice. Our cilial PDE activity assays and those of others16 indicate that the activity of unknown PDEs, if any, is unlikely to have a significant effect on olfactory signal transduction.
Pde1c−/− OSNs displayed an unexpected reduction in sensitivity to odor, a paradoxical phenotype for removal of an enzyme that negatively regulates signal transduction. The reduced sensitivity in Pde1c−/− OSNs comes, at least in part, from the decrease in the levels of ACIII protein (Fig. 1b), as mice heterozygous for Adcy3 (coding for ACIII) show reduced EOG amplitudes28. The cause of the decreased ACIII level in Pde1c−/− OSNs remains unclear. The reduced sensitivity in Pde1c−/− OSNs could also be caused by negative regulation of the activities of signal transduction components. This negative regulation of transduction components is likely to occur even in the absence of odor stimulation. It is well established that the PDE inhibitor IBMX can induce responses in a large portion of OSNs, including 40 – 60% of OSNs in rats and mice7, 29, suggesting that in resting OSNs there is continued production and degradation of cAMP in the cilia, even though they often display low spontaneous electrical activity7, 30, 31. PDE1C, presumably due to its basal catalytic activity, is likely to account for degradation of spontaneously produced cAMP. In Pde1c−/− OSNs, the lack of cAMP degradation in cilia would transiently increase cilial cAMP and subsequently cilial Ca2+ levels in resting OSNs. Excess Ca2+ is expected to negatively regulate the signal transduction pathway20, targeting ACIII19, 32 and the CNG channel23. Regardless of the exact mechanism(s), either reduced ACIII levels, negative feedback on transduction components, or both, the Pde1c−/−phenotype nevertheless suggests that cilial PDE activity may indirectly aid in maintaining the signal transduction machinery in a state that is primed for odor response.
It is worth noting that several previously unexplained features of odor responses from IBMX-treated OSNs9 can be interpreted after considering the PDE knockout phenotypes. When bathed in low concentrations of IBMX, OSNs did not show prolonged response termination, but rather showed smaller response amplitudes and slowed onset kinetics9, reminiscent of the Pde1c−/− phenotype and highlighting that a basal level of PDE activity may be necessary to maintain OSN sensitivity. When bathed in high concentrations of IBMX, OSNs showed prolonged response termination with reduced response amplitude9, reminiscent of the Pde1c−/−;Pde4a−/− double knockout phenotype.
We also found that PDE1C likely contributes to OSN adaptation to repeated stimulation. Until recently, adaptation to repeated stimulation was attributed mainly to the Ca2+/CaM feedback inhibition on the CNG channel33–36. However, our recent work suggested that Ca2+/CaM-mediated desensitization of the CNG channel has little effect on adaptation to repeated stimulation, but rather contributes to rapid response termination18. An increase in cilial Ca2+ as part of the OSN response, in addition to feedback on the CNG channel, likely elevates PDE1C activity through binding with CaM, potentially leading to smaller responses to subsequent stimuli. Pde1c−/− OSNs exhibit attenuated adaptation in a paired-pulse stimulation paradigm, consistent with the lack of elevated PDE activity from Ca2+/CaM feedback. It is worth to note that as Pde1c−/− OSNs show reduced sensitivity to odors that is reminiscent of already adapted OSNs, the phenotype of attenuated adaptation might also result from a lower ability to further adapt. Interestingly, adaptation to repeated stimulation in Pde1c−/−;Pde4a−/− double knockout mice is not significantly different from the wildtype. This result is consistent with a previous report showing that when the effects of all PDEs were bypassed pharmacologically, OSNs adaptation was not affected21. That eliminating PDE4A masks the adaptation deficit from the loss of PDE1C suggests that the mechanisms by which adaptation is achieved when both PDEs are eliminated (either by genetic or pharmacological means) are substantially different from the wildtype situation, even though the apparent adaptation phenotype is similar. When both PDEs are eliminated, greater recruitment of the remaining adaptation mechanisms, potentially due to additional Ca2+ entering the cilia during the prolonged response termination phase, may account for the apparently normal adaptation, whereas in wildtype mice, Ca2+/CaM stimulated PDE1C activity is likely a significant contributor to adaptation.
Response to a sustained stimulation can be viewed as an integration of responses to a series of short stimulations, with the final response determined by a composite of adapted activation and termination mechanisms. Pde1c−/− OSNs display a response decline comparable to wildtype OSNs during a sustained stimulation. This result could be superficially understood as the attenuated adaptation to repeated stimulation (i.e. larger subsequent responses) and the faster termination counteracting during sustained stimulation, producing an intermediate response. Therefore, the apparently normal phenotype of adaptation during sustained stimulation in Pde1c− /− OSNs is likely determined by a different composition of mechanisms than in wildtype OSNs. It is interesting to compare the phenotype of the Pde1c−/− mice to the CNGB1ΔCaM mice, which carry a mutation that renders the olfactory CNG channel resistant to Ca2+/CaM-mediated desensitization18. In CNGB1ΔCaM OSNs, the response decline during sustained stimulation is attenuated, consistent with the reduced ability of these OSNs to terminate the response in combination with the normal adaptation to repeated stimulation. Pde1c−/−;Pde4a−/− OSNs do display a profound deficit in adaptation during sustained stimulation, again consistent with the response being a composite of the phenotypes of prolonged response termination and wildtype-like adaptation to repeated stimulation in these neurons.
METHODS
For all experiments, mice were handled and euthanized with methods approved by the Animal Care and Use Committees of The Johns Hopkins University.
Gene targeting
For Pde1c, the targeting vector was designed to delete exons 5–8 (6.7 kilobases genomic DNA on chromosome 6), which encodes the N-terminal portion of the predicted catalytic domain, and to insert a stop codon in the remainder of exon 5 through homologous recombination in embryonic stem cells. For Pde4a, the targeting vector was designed to delete exons 9–14 and part of exon 15 (9.9 kilobases genomic DNA on chromosome 9), which encode the entire predicted catalytic domain. Further details of the generation of these knockout mice are provided in Supplementary Figure 1.
Immunohistochemistry
Anesthetized mice were perfused transcardially with 1X PBS followed by 4% (wt/vol) paraformaldehyde. Olfactory tissues were dissected and postfixed for 2 hrs at 4°C followed by cryoprotection in 30% (wt/vol) sucrose overnight at 4°C. Cryosections were cut 14 µm thick and stored at −80°C. Tissue sections were incubated overnight at 4°C with primary antibodies in 1x PBS containing 0.1% (vol/vol) Triton X-100 and 1% (vol/vol) normal donkey serum. After washing, the sections were incubated with secondary antibodies conjugated to either Alexa-488, Alexa-546, or Alexa-647 (Molecular Probes) and imaged by confocal microscopy (LSM 510 Meta, Zeiss) using either a 63x (Fig. 1a) or 100x (Fig. 3a) objective. Images were acquired using the Zeiss LSM software v. 4.2 at 1024 ×1024 pixels resolution, 12 bit-depth, and cropped using Adobe Photoshop. No adjustments to contrast or brightness were made. Primary antibodies were used at the following dilutions: anti-PDE1C213, 14 (kindly provided by Dr. J. Beavo), 1:500; anti-PDE4A13 (kindly provided by Dr. J. Cherry), 1:200; anti-CNGB1b18, 23, 1:200; anti-ACIII (Santa Cruz SC-588), 1:200; anti-OMP (kindly provided by Dr. F. Margolis), 1:1000; anti-Gap43 (Chemicon MAB347), 1:500; anti-acetylated tubulin (Sigma T7451), 1:500.
Western blotting
Olfactory epithelium was dissected and homogenized in 2x Laemmli buffer and stored at −80°C. Tissue homogenates were subjected to SDS-PAGE followed by transfer onto PVDF membrane. Membranes were incubated with blocking buffer (5% (wt/vol) non-fat dry milk in 20 mM Tris, 150 mM NaCl, and 0.1% (vol/vol) Tween-20) for 1 hr at room temperature and then incubated overnight at 4°C with primary antibodies at proper dilutions in blocking buffer. Membranes were then washed with TBST (20 mM Tris, 150 mM NaCl, and 0.1% Tween-20) followed by incubation with secondary antibodies conjugated to HRP in blocking buffer for 1 hr at room temperature. The blot was visualized with ECL Plus reagent (GE Life Sciences) with detection on a Typhoon 9410 Variable Mode Imager. Primary antibodies were used at the following dilutions: anti-PDE1C, 1:5000; anti-PDE4A, 1:5000; anti-CNGB1b, 1:2000; anti-ACIII, 1:1000; anti-alpha tubulin (Sigma), 1:10,000.
Preparation of OSN cilia and PDE assay
Cilia from OE were isolated by the calcium-shock method37 with slight modifications. Anesthetized mice were perfused transcardially with 1X PBS to minimize blood contamination. The olfactory turbinates were dissected into a buffer containing (in mM) 120 NaCl, 5 KCl, 1.2 MgCl2, 10 HEPES, pH 7.4 and 0.75 mg/ml DTT on ice. CaCl2 stock solution was then added to a final concentration of 10 mM Ca2+. The samples were gently rocked at 4°C for 20 min, followed by centrifugation at 500 rcf for 5 min to pellet de-ciliated epithelia. The supernatant was centrifuged at 18,500 rcf for 20 min to pellet cilia. The cilia pellet was resuspended in BHB buffer (50 mM BES, 0.1 mM EGTA, pH 7.2) plus 0.08 mg/ml DTT and 1x Complete EDTA-free Protease Inhibitor Cocktail (Roche). Protein concentration was determined by the BCA Protein Assay (Pierce).
The PDE activity assay is adapted from Borisy et al., (1992)16. Four µg of OSN cilial protein was incubated with 80,000 cpm 3H-cAMP (GE Life Sciences) for 20 min at 37°C in each of three conditions: 1) in the reaction buffer alone (50 mM BES, 5 mM MgCl2, 0.3 mg/ml BSA, 0.1 mM EGTA, 10 µM unlabelled cAMP); 2) the reaction buffer supplemented with 200 µM Ca2+ and 50 nM CaM; and 3) the reaction buffer supplemented with 0.5 mM IBMX (3-isobutyl-1-methylxanthin, ICN Biomedicals). The reaction was stopped by heating to 100°C for 1.5 min and followed by immediate cooling on ice. Subsequently, 1 unit of 5’ nucleotidase (Sigma) was added to the reaction (in order to convert the PDE product AMP to adenosine), and the reaction was further incubated at 37°C for 30 min. The reaction was then applied to a 0.5×4 cm column containing 1 ml QAE-Sephadex A25 (Sigma) pre-equilibrated with BHB buffer. The flow-through from two 1.5 ml washes with BHB buffer was collected and mixed with 10 ml of Ultima Gold XR scintillation fluid (Packard Bioscience) followed by assessment of the radioactivity by scintillation counting.
Electroolfactogram
EOG recording was performed essentially as described38. The mouse was sacrificed by CO2 asphyxiation and decapitated. The head was cut sagittally to expose the medial surface of the olfactory turbinates. The recording electrode, a Ag-AgCl wire in a capillary glass pipette filled with Ringer solution (in mM: 135 NaCl, 5 KCl, 1 CaCl2, 1.5 MgCl, 10 HEPES, pH 7.4) containing 0.5% agarose, was placed on the surface of the OE and connected to a differential amplifier (DP-301, Warner Instruments). EOG signals were recorded from the surface of turbinate IIB and acquired and analyzed with AxoGraph software (Axon Instruments) on a Macintosh computer. The signals were filtered DC-1 kHz and recorded at a sampling rate of 1 kHz. The recorded signals were further low-pass filtered at 25 Hz during analysis. Vapor-phase odorant stimuli were generated by placing 5 ml of odorant solution in a sealed 60 ml glass bottle. This vapor is delivered by a Picospritzer (Parker Hannifin) as a pulse injected into a continuous stream of humidified air flowing over the tissue sample. All EOG recordings were conducted at room temperature and in mice older than 6 weeks.
For analysis of paired-pulse responses, as the response to the 1st odor pulse has not decayed to baseline at the time the 2nd pulse is given, the recorded 2nd response reflects a sum of the residual 1st response and the “net” response to the 2nd pulse. The net 2nd response peak amplitude was determined by first fitting a trace to the decay phase of the 1st response, and subsequently subtracting the value of this trace at the peak time of the 2nd response from the 2nd pulse peak amplitude18.
Statistical analysis
All statistical significance was determined by unpaired t-test.
Computational Modeling
Computational modeling was performed using the Virtual Cell software from the National Resource for Cell Analysis and Modeling (http://www.vcell.org) (see Supplementary Note online for details).
Supplementary Material
Acknowledgements
We thank Dr. J. Beavo (Univ. of Washington) for antibody to PDE1C2, Dr. J. Cherry (Boston Univ.) for antibody to PDE4A, and Dr. F. Margolis (Univ. of Maryland) for antibody to OMP. We also thank Drs. L. Brand, R. Cone, S. Hattar, R. Kuruvilla, T. Leinders-Zufall, R. Reed, J. Reisert, and Y. Song for suggestions and comments on experiments and the manuscript, and members of the Hattar-Kuruvilla-Zhao tri-lab of the Department of Biology, Johns Hopkins University for discussion. This work was supported by NIH/NIDCD grant DC009735.
REFERENCES
- 1.Firestein S, Shepherd GM, Werblin FS. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. J Physiol. 1990;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorantinduced currents in salamander olfactory receptor cells. J Physiol. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 4.Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- 5.Kurahashi T, Yau KW. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- 6.Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- 7.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 8.Reisert J, Bauer PJ, Yau KW, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol. 2003;122:349–363. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firestein S, Darrow B, Shepherd GM. Activation of the sensory current in salamander olfactory receptor neurons depends on a G protein-mediated cAMP second messenger system. Neuron. 1991;6:825–835. doi: 10.1016/0896-6273(91)90178-3. [DOI] [PubMed] [Google Scholar]
- 10.Boekhoff I, Breer H. Termination of second messenger signaling in olfaction. Proc Natl Acad Sci U S A. 1992;89:471–474. doi: 10.1073/pnas.89.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan C, et al. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan C, Zhao AZ, Bentley JK, Beavo JA. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J Biol Chem. 1996;271:25699–25706. doi: 10.1074/jbc.271.41.25699. [DOI] [PubMed] [Google Scholar]
- 13.Cherry JA, Davis RL. A mouse homolog of dunce, a gene important for learning and memory in Drosophila, is preferentially expressed in olfactory receptor neurons. J Neurobiol. 1995;28:102–113. doi: 10.1002/neu.480280109. [DOI] [PubMed] [Google Scholar]
- 14.Juilfs DM, et al. A subset of olfactory neurons that selectively express cGMPstimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 16.Borisy FF, et al. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JW, Scott-Johnson PE. The electroolfactogram: A review of its history and uses. Microsc Res Tech. 2002;58:152–160. doi: 10.1002/jemt.10133. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, et al. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leinders-Zufall T, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. J Neurosci. 1999;19:RC19. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]
- 21.Boccaccio A, Lagostena L, Hagen V, Menini A. Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase. J Gen Physiol. 2006;128:171–184. doi: 10.1085/jgp.200609555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J. 1999;76:2861–2867. doi: 10.1016/S0006-3495(99)77440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 24.Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willoughby D, Cooper DM. Live-cell imaging of cAMP dynamics. Nat Methods. 2008;5:29–36. doi: 10.1038/nmeth1135. [DOI] [PubMed] [Google Scholar]
- 26.Leinders-Zufall T, et al. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, et al. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 28.Wong ST, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 29.Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 30.Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol. 1989;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firestein S, Werblin F. Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science. 1989;244:79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- 32.Wei J, et al. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in Neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen TY, Yau KW. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotideactivated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 34.Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- 35.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 36.Munger SD, et al. Central role of the CNGA4 channel subunit in Ca2+-calmodulindependent odor adaptation. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anholt RR, Aebi U, Snyder SH. A partially purified preparation of isolated chemosensory cilia from the olfactory epithelium of the bullfrog, Rana catesbeiana. J Neurosci. 1986;6:1962–1969. doi: 10.1523/JNEUROSCI.06-07-01962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, et al. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.