Abstract
We report methods for the acquisition and analysis of optical images formed by thin films of twisted nematic liquid crystals (LCs) placed into contact with surfaces patterned with bio/chemical functionality relevant to surface-based assays. The methods are simple to implement and are shown to provide easily interpreted maps of chemical transformations on surfaces that are widely exploited in the preparation of analytic devices. The methods involve acquisition of multiple images of the LC as a function of the orientation of a polarizer; data analysis condenses the information present in the stack of images into a spatial map of the twist angle of the LC on the analytic surface. The potential utility of the methods is illustrated by mapping (i) the displacement of a monolayer formed from one alkanethiol on a gold film by a second thiol in solution, (ii) coadsorption of mixtures of amine-terminated and ethyleneglycol-terminated alkanethiols on gold films, which leads to a type of mixed monolayer that is widely exploited for immobilization of proteins on analytic surfaces, and (iii) patterns of antibodies printed onto surfaces. These results show that maps of the twist angle of the LC constructed from families of optical images can be used to reveal surface features that are not apparent in a single image of the LC film. Furthermore, the twist angles of the LC can be used to quantify the energy of interaction of the LC with the surface with a spatial resolution of <10 µm. When combined, the results described in this paper suggest non-destructive methods to monitor and validate chemical transformations on surfaces of the type that are routinely employed in the preparation of surface-based analytic technologies.
Keywords: liquid crystals, patterned chemistry, bioanalytics, chemically functionalized surfaces, imaging, printed antibodies
Introduction
Recent studies have demonstrated that a range of different molecular-level events at surfaces can be amplified into ordering transitions in thin films of nematic LCs, thus causing changes in the optical appearance of the LCs.1–17 The approach takes advantage of the long range ordering of molecules within LCs1–5 to amplify nanoscopic events at a surface into ordering transitions in the LC that occur on the optical (micrometer) scale. In the context of bioanalytic technologies, LC-based reporting of surface events may be useful for validation of surface chemical transformations that lie at the heart of the development and manufacture of surface array-based analytic devices. It also has potential merit as a means for target molecule detection because it does not rely upon complex instrumentation, and in the case of biomolecule detection, it obviates the need for labeling of the target molecules with radioactive or fluorescent probes.6,7 Whereas past studies have demonstrated that the ordering of LCs is sensitive to the presence of specific chemical functional groups,8 peptides,9 and proteins10,11 on surfaces, with few exceptions (see below), the interpretation of the optical appearance of the LC has been qualitative in nature. In this paper, we report an investigation that advances analytical methods based on LCs by providing simple and versatile procedures for quantitative and multiplexed measurements of the response of the LCs to molecular events on surfaces.
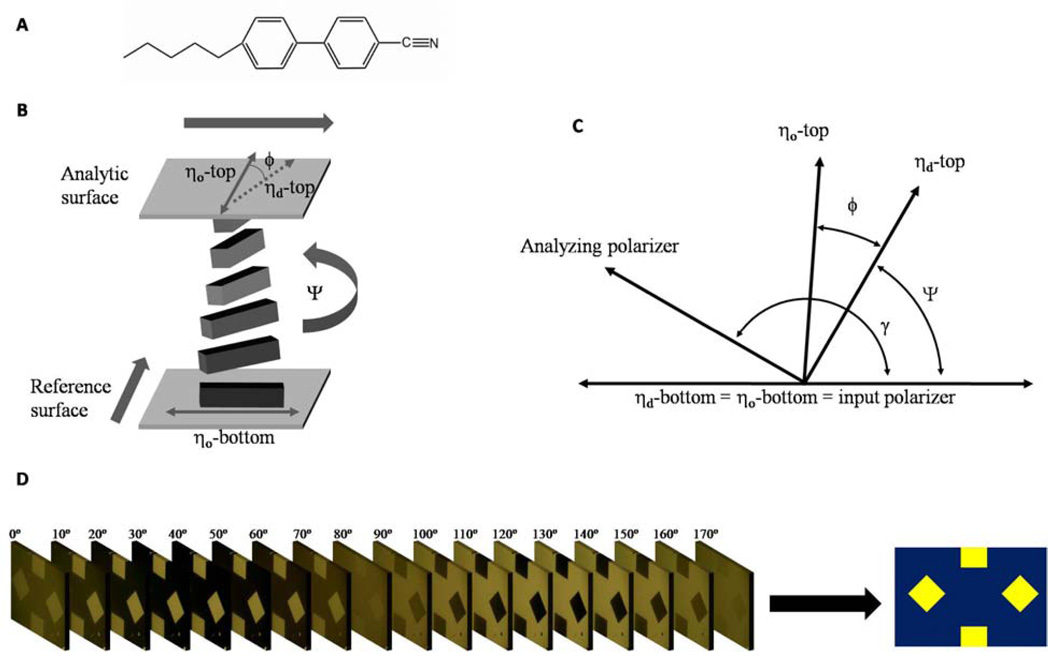
The approach reported herein uses LCs possessing a twist distortion (twisted nematic liquid crystals, TNLCs) to provide sensitive and quantitative analytic methods.12–14 In this approach, a nematic LC such as 4’-pentyl-4-cyanobiphenyl (5CB, Figure 1A) is confined between two surfaces that are structured so as to promote the LC to adopt mutually orthogonal orientations at the two confining surfaces (Figure 1B). The interactions of the LC with the two confining surfaces cause a twist distortion to form within the LC. The angle of the twist distortion (Ψ in Figure 1B) is determined by a competition between the elastic energy stored in the twisted state of the LC (which tends to minimize the twist angle) and the interaction energy of the LC with each surface (which promotes the orientation of the LC in preferred directions at the confining surfaces): A decrease in the interaction energy of the LC with one of the confining surfaces (hereafter referred to as the anchoring energy of a LC) leads to a decrease in the twist angle of the LC. In two past studies, TNLCs were used to quantitatively report the presence of peptides13 and peptide-antibody interactions.14 Those studies, however, involved time-consuming procedures and were limited by the need for macroscopic surface areas with uniform chemistry. Herein, we report methods that are easily automated and permit imaging of the spatial variation of the twist angle (and thus anchoring energy) of nematic LCs across patterned surfaces. This approach provides quantification of the orientational response of LCs to changes in surface chemistry and biomolecular interactions on surfaces patterned with microarrays.
Figure 1.
(A) Molecular structure of 5CB (B) Schematic illustration of TNLC. Bold arrows indicate the in-plane direction of deposition of the gold films that confine the LC. The angles indicated in the diagram are defined in the text. (C) Diagram of the angles used to analyze the TNLC between two polarizers. (D) A stack of images of a TNLC obtained using orientations of the analyzer ranging from 0 ° to 170 °. Image analysis condenses the information present in the stack of images into a single map of the twist angle over the entire surface.
Many techniques for the measurement of anchoring energies have been reported in the literature.17–21 The focus of these past studies, however, was directed to surfaces for electro-optic LC displays, and the methods are not easily applied to array-based surface analysis. The approach used in this paper is based on a methodology reported first by Clare et al.13 The method involves measurement of the intensity of light transmitted through a thin film of LC as a function of the orientation of the analyzer (Figure 1C and 1D). The measurement permits determination of the orientation of the LC at the analytic surface (top surface in Figure 1B), ηd, and the angular deviation of the orientation of the LC (defined as φ Figure 1B) from the so-called “easy axis”, ηo, of the LC at the analytic surface. The easy axis of the analytic surface is defined as the orientation assumed by the LC in the absence of a torque generated by twist in the LC. In this study, we define the easy axis by physical vapor deposition of thin gold films at an oblique angle of incidence,22 and by chemical functionalization of these gold films with ω-functionalized organothiols.23,24 For a LC film with a known twist elastic constant K22 and thickness d, the anchoring energy is calculated as
| (1) |
In our experiments, the anchoring energy of the LC on the reference surface is sufficiently high that the deviation of the orientation of the LC from the easy axis of the reference surface is negligible.
The methods reported by Clare et al permit the average angles Ψ and φ in Eq. (1) to be determined using mm-sized areas of surfaces. The essential advance reported in this paper is the development of generalized methods for data acquisition and automated analysis. These methods provide accurate measurements of the twist angle at each individual pixel of an image. There are several advantages to the methods reported here. First, because the output is a map of twist angle of the LC as a function of position, it is possible to characterize spatial variation in surface chemistry (including patterned surface chemistry such as might be used to construct a microarray) with a resolution better than 10 µm. Second, the methods of data acquisition and image analysis are easily automated, thus allowing rapid and routine analysis of surfaces for imperfections or desired patterns of chemistry. The methodology preserves the advantages of highly parallel data acquisition that are inherent to methods based on optical imaging. Third, the creation of a map of twist angle as a function of position on a sample allows rapid identification of heterogeneities in a surface that are not apparent from an analysis of individual images of the LC obtained using a given setting of polarizer and analyzer. Fourth, as mentioned above, the interactions of LCs with surfaces are sensitive to variations in the chemistry of surfaces that are difficult to detect by other methods (e.g., ellipsometry and surface plasmon reflectometry). We envisage that the methods reported in this paper will be useful for validation of chemically patterned surfaces used in surface-based analyses.
Materials and Methods
Materials
All materials were used as received, unless otherwise noted. Fisher’s Finest glass slideswere obtained from Fisher Scientific (Pittsburgh, PA). Gold (99.999% purity) was obtained from International Advanced Materials (Spring Valley, NY). Titanium (99.99% purity) was obtained from PureTech (Brewster, NY). Polished silicon wafers were purchased from Silicon Sense (Nashua, NH). Tetraethylene glycol-terminated thiol (HS(CH2)11EG4, referred to as EG4) and the corresponding amine-terminated thiol (HS(CH2)11EG4NH2, referred to as EG4N) as a hydrochloride salt were obtained from Prochimia (Gdansk, Poland). Pentadecanethiol, hexadecanethiol, 2-aminoethanethiol hydrochloride (AET) and N-hydroxysuccinimide (NHS) were obtained from Sigma-Aldrich (Mikwaukee, WI). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Hydrochloride (EDC) was obtained from Pierce Biotechnology (Rockford, IL). Liquid crystal 4’-pentyl-4-cyanobiphenyl (5CB) was obtained from EM Industries (New York, NY), sold under the trademark name Licristal (K15). Anhydrous ethanol containing 5% isopropyl alcohol and 5% methyl alcohol as denaturants was obtained from Sigma-Aldrich and purged with argon gas for 1 hour prior to use. Poly(dimethylsiloxane) (PDMS) elastomeric stamps were prepared using Sylgard 184 silicone elastomer kit obtained from Dow Corning (Midland, MI).
Protein solutions were prepared from phosphate buffered saline (PBS), pH 7.6 (Sigma-Aldrich, Cat #P3744). Biotinylated BSA was obtained from Pierce Biotechnology. Anti-biotin IgG and anti-rabbit IgG were obtained from Sigma-Aldrich.
Preparation of Gold Substrates
Glass slides, cleaned as described previously,13–15 were positioned within the chamber of an electron beam evaporator such that the incident angle of metal flux onto the substrate could be controlled. The incident angles (θi, with respect to the surface normal) were measured with a digital level, with an accuracy of ±0.5 °. All metal films were deposited at chamber pressures < 2 × 10−6 torr at deposition rates of 0.2 Å/s. A thin film of titanium (thickness of 42–60 Å) was deposited onto the glass substrate to serve as an adhesion layer between the glass and semitransparent film of gold (thickness of 105–140 Å). All gold substrates were used within 1 h of removal from the evaporator chamber.25
Formation of Patterned Self-Assembled Monolayers (SAMs)
A PDMS elastomeric stamp with recessed features (squares and diamonds measuring 1 mm on each side, with depths of ∼100 µm) was cast against a silicon master fabricated using standard photolithographic techniques. The master was silanized with (tridecafluoro-1,1,2,2,-tetrahydrooctyl)-1-trichlorosilane vapor (18 hours under vacuum) to aid in the release of the PDMS. After curing the PDMS for at least 18 h, the stamp was peeled from the master and ultrasonicated in a solution of 2:1 ethanol/water for 10 min × 3 cycles. The surfaces of the PDMS stamps were inked with a 2 mM ethanolic solution of hexadecanethiol for 30 s and then dried with a stream of nitrogen gas. The PDMS stamp was placed into conformal contact with an obliquely deposited gold slide for 2 min. The slide surface was rinsed with ethanol, and then either (i) immersed into an ethanolic solution of an organothiol, or (ii) aqueous solutions of organothiols were applied to bare regions of the gold films (squares and diamonds). In the latter case, the hydrophobic SAM formed from hexadecanethiol served to confine the aqueous drops to the bare regions of the gold film. After incubation, slides were rinsed sequentially with copious amounts of ethanol, water, and ethanol, and then dried under a stream of gaseous nitrogen.
Affinity Microcontact Printing
Patterned PDMS stamps with raised features (300 × 300 µm squares, with heights of ∼ 100 µm) were prepared in the manner described above. These stamps were functionalized with biotinylated BSA using a previously described procedure26 and incubated with 500 nM antibody in PBS for 2 h. The stamps were then rinsed sequentially with 0.05% v/v Triton X-100 in PBS for 10 s, PBS for 5 s, and water for 5 s followed by drying with a stream of nitrogen gas. The PDMS stamps were then contacted with gold films (deposited at an angle of 30 ° relative to the normal) that had been functionalized with 2-aminoethanethiol. The SAMs were rinsed with 0.01 M HCl and dried prior to contact printing. After 2 min of contact, the stamps were slowly removed from the gold films.
Fabrication of Optical Cells
Optical cells were fabricated by pairing an analytical surface with a reference surface such that the gold films were mutually oriented as depicted in Figure 1B. The reference surface was a gold film (deposited at an angle of 64 ° relative to the surface normal) that was functionalized with pentadecanethiol by immersion into a 1 mM ethanolic solution for 18 hr (unless otherwise stated). This type of surface strongly anchors the LC.12 The angle between the in-plane direction of gold deposition on the reference surface and analytic surface was 90 ° unless otherwise noted. The analytic surface and reference surface were spaced apart by 13 µm by using a thin film of Mylar. The cavity between the two surfaces was filled with 5CB heated into its isotropic phase (∼50 °C). The optical properties of the LC cell were measured after cooling the cell to 25 °C for 30 min.27
Measurement of the Twist Angle of the Liquid Crystal
We measured the twist angle of the LC by adapting previously reported methods.12–14,27,28 The optical cell containing the LC was placed between crossed polarizers with the input polarizer facing the reference surface. The easy axis of the LC on the reference surface was aligned to be parallel with the input polarizer using the technique described by Lien.29 Briefly, an iterative process was used to minimize the transmission of 546.5 nm light through the regions functionalized with hexadecanethiol on the analytic surface. This was accomplished by alternately rotating the sample between the stationary polarizers, followed by rotation of the analyzer. The transmission of light was minimized at each of the iterations. Three iterations were typically sufficient. Images were obtained using a polarized light microscope (BX60, Olympus) equipped with an X-Y translation stage and a digital camera (2.8 f-stop, 1/800 s shutter speed). Consistent settings of the light intensity were used (aperture set at one-half maximum, and lamp intensity set at two-tenths maximum) for individual samples. The lamp intensity was set at two-tenths of maximum to ensure that the images did not saturate during rotation of the analyzer. The analyzer was rotated at 10° increments, with images obtained at each analyzer position. The fraction of light transmitted through the optical cell, Twg, was fit to the function
| (2) |
where Ψ is the twist angle of the LC (Figure 1) and γ is the position of the analyzer relative to the input polarizer (γ = 0 – 170 °). The fit was performed at each pixel of the family of images of each optical cell using an algorithm implemented in MATLAB (version 7.3.0, 2006b) to yield a 2-dimensional matrix with elements representing the values of the twist angle at each pixel. A color map of twist angle was calculated by correlating a specific color with each value of the twist angle. Detailed procedures are reported in the Supporting Information.
Results and Discussion
Validation of Data Acquisition and Image Analysis using Surfaces that Generate Known Orientations of LCs
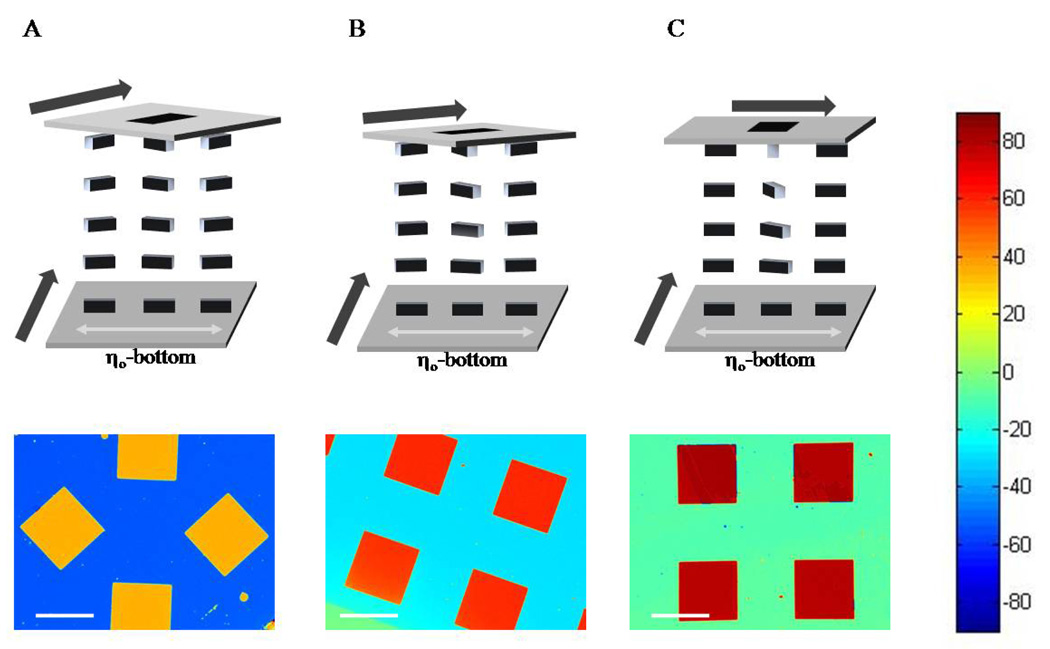
The first experiments described below were performed in order to validate the data acquisition and image analysis methodology. For these experiments, we used surfaces patterned with monolayers that gave rise to known orientations of the LC.30 The surfaces were prepared from semi-transparent gold films that were deposited by physical vapor deposition at an angle of incidence (measured from the surface normal) of either 64 ° (reference surface) or 49 ° (analytic surface). The surfaces were aligned with respect to each other so that the angle between the in-plane direction of deposition of gold on the reference surface and the analytic surface was nominally 45 °, 60 ° or 90 ° (Figure 2, top). These angles were estimated by eye – measurements reported below define the exact values. The reference surface was functionalized with a monolayer formed from pentadecanethiol by immersion into a 1 mM ethanolic solution for 10 min; these monolayers cause the easy axis of the LC on the surface to be perpendicular to the in-plane direction of gold deposition (see bottom surfaces in Figure 2).30 The analytic surfaces (top surfaces in Figure 2) were patterned with hexadecanethiol by using microcontact printing. The hexadecanethiol causes the easy axis of the LC to be parallel to the in-plane direction of deposition of the gold.30 The stamp used for printing was designed to leave square regions on the gold film that were not functionalized with hexadecanethiol. These square regions (1 mm on a side) were subsequently functionalized with pentadecanethiol by immersion of the entire analytic surface into an ethanolic solution of pentadecanethiol for 10 mins. We note that the shapes of the surface patterns in Figure 2A–C are not identical. The surface chemical functionalization, however, is the same on each surface. A film of LC was confined between the analytic and reference surfaces such that the thickness of the film of LC was nominally 13 µm.
Figure 2.
Schematic representation of TNLCs (top) and corresponding maps of twist angles of the LC (bottom) obtained using analytic surfaces patterned with hexadecanethiol (continuous area of surface) and pentadecanethiol (squares). Twist angles of the LC corresponding to each color are indicated by the color chart shown at the right side of the figure. Bold arrows in the top diagrams indicate the in-plane direction of deposition of the gold films that confine the LC. The angle between the direction of deposition of the gold films in the analytic (top) and reference (bottom) surfaces of the cell was (A) 45 °, (B) 60 °, and (C) 90 °. See text for details. Scale bars indicate 1 mm.
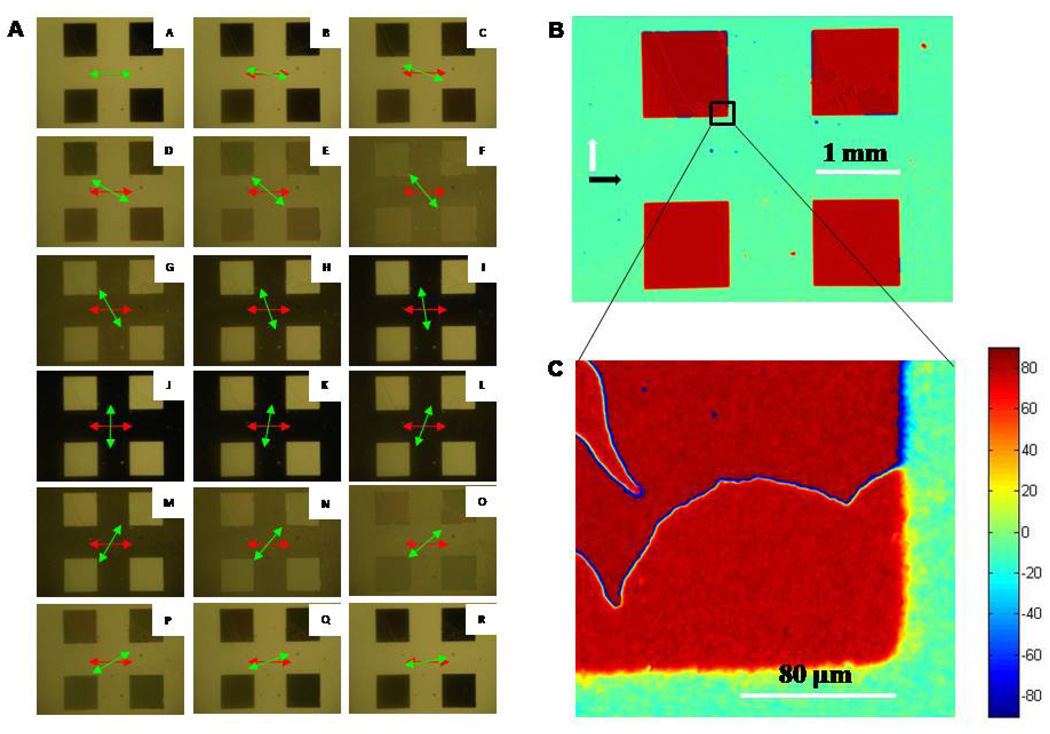
With the sample and input polarizer fixed in position as described in the Methods section, the analyzer was rotated in 10 ° increments. An image was captured at each position of the analyzer, for a total of 18 images. Figure 3A shows the set of 18 images obtained for the optical cell fabricated with a twist angle of approximately 45 °. The change in intensity of transmitted light (as a function of angle of the analyzer) was used to calculate the twist angle of the LC at each pixel of these images, as described in the Methods section. This result is shown in Figure 2A. Inspection of Figure 2A reveals that the LC in contact with the regions of the analytic surface functionalized with hexadecanethiol (blue areas) possessed an average twist of angle of ΨC16 = −52.4 ° ± 0.7 °. By assuming that the anchoring of the LC on both the reference and analytic surface was very strong (see below), the twist angle was used to calculate the angle between the in-plane direction of deposition of the gold films on the analytic surface and reference surface (δ) using the relation δ = 90 ° - |ΨC16|. This calculation yields δ = 37.6 ° ± 0.7° for this optical cell. The twist angle of the LC in contact with the surface formed from pentadecanethiol was calculated to be ΨC15 = 36.2 ° ± 0.7 °. These results, when combined, indicate that the angle between the azimuthal orientation of the LC on the pentadecanethiol and hexadecanethiol surfaces was 88.6 ° ± 1.0 °. We note that if the analytic and reference surfaces both possessed infinite anchoring energies, the angle between LC on the pentadecanethiol and hexadecanethiol regions should be 90 °. The above result (88.6 ° ± 1.0 °) indicates that the SAMs do indeed possess very high anchoring energies.
Figure 3.
(A) Images of TNLCs contacted with an analytic surface comprised of patterned pentadecanethiol (squares) and hexadecanethiol. Images A-R were obtained between two polarizers (transmission mode) as a function of the analyzer orientation (indicated by the green arrow). The input polarized orientation is indicated by the red arrow. (B) Map of twist angles obtained using a 4x objective lens. (C) Map of twist angle obtained using a 50x plan-view objective lens. Twist angles of the LC corresponding to each color are indicated by the color chart shown at the right side of the figure.
We also analyzed the optical cells prepared with nominal twist angles of 60 ° and 90 °. The maps of twist angles of the LC for these samples are shown in Figures 2B and 2C, respectively. The twist angle of the LC in the pentanethiol-functionalized squares was measured to be 61.0 ° ± 0.7 ° for the nominally 60 ° twisted optical cell and 81.2 ° ± 0.8 ° for the nominally 90° twisted optical cell. We measured the difference between twist angles in the pentanethiol-and hexadecanthiol-functionalized regions to be similar to that measured in the 45 ° twisted optical cell: − 88.6 ° ± 1.2 ° for the nominally 60 ° twisted optical and 87.8 ° ± 1.1 ° for the nominally 90 ° twisted optical cell. These results confirm strong anchoring of the LC on the surfaces used in these experiments.
The results above demonstrate accurate quantification of the twist angle of the LC at each pixel of the images obtained using the LCs, with each pixel representing an area of ∼6 µm × 6 µm over a field-of-view of 4 mm × 3 mm (image size of 640 × 480 pixels). At this resolution, individual pixels represent an average of the twist angle of the LC within the pixel area. As shown in Figure 3B and 3C, higher resolution maps are attainable when an objective lens of higher magnification is used for image acquisition (50x objective and 1600 × 1200 pixels). These high resolution maps of twist angles reveal defect lines (disclinations) in the LC. These defects lie between regions of the LC that differ in the handedness of the twist distortion, which is a common observation for LCs with twist angles approaching 90 °.31,32
Imaging of Displacement Reactions involving Alkanethiols
Heterogeneity and poor reproducibility of surface chemistry are key issues that currently limit the development of quantitative surface-based microarray technologies.33–35 Few techniques are sufficiently simple to allow routine validation of surface chemistry of the type typically used in multiplexed analytic technologies. As a model surface chemical transformation, we sought to determine if it would be possible to follow the displacement of a self-assembled monolayer formed from an alkanethiol on a surface by a second alkanethiol in solution by using methods based on TNLCs. This class of displacement reactions can limit the quality of chemical patterns that can be prepared by using SAMs formed from alkanethiols.36–39 In particular, we investigated the displacement of a microcontact printed SAM formed from hexadecanethiol by an ethanolic solution of pentadecanethiol. These two SAMs differ only by one methylene group, and thus serve to demonstrate the subtle variations in surface chemistry that can be reported by using LCs.
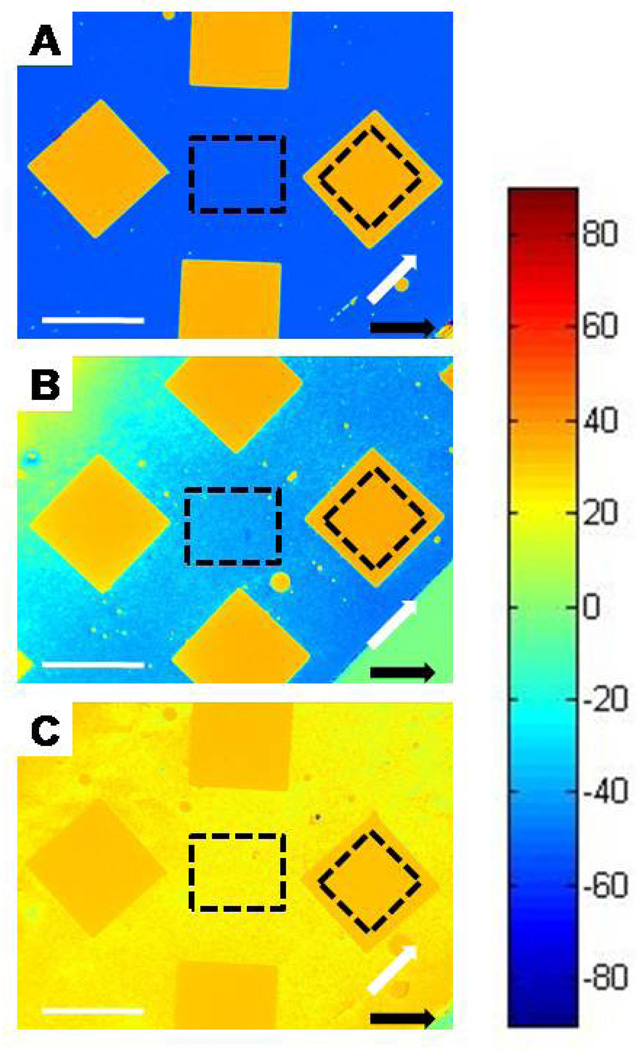
Using methods described above, we compared the twist angles of LCs in contact with analytic surfaces prepared by microcontact printing of hexadecanethiol and subsequent immersion into ethanolic solutions of pentadecanethiol for periods of 10 minutes, 2 hours, or 24 hours. Reference surfaces were prepared by immersion of gold films into ethanolic solutions of pentadecanethiol for the same period of time as the analytic surface with which they were paired. As shown in Figure 4A, after immersion of the microcontact printed surface into an ethanolic solution of pentadecanethiol for a period of 10 minutes, we observed orthogonal alignment of LC in the regions functionalized with pentadecanethiol (gold colored squares/diamonds) and in the regions microcontact printed with hexadecanethiol (blue background). A few small imperfections are evident in the area that was microcontact printed with hexadecanethiol These small circles are areas of the analytic surface that were not functionalized with hexadecanethiol due to particulate matter (dust) that prevented conformal contact of the PDMS stamp with the gold film. When the gold films patterned with hexadecanethiol were immersed in ethanolic solutions of pentadecanethiol for periods longer than 10 minutes, we observed a reduction in the twist of the LC on the areas of the surface where the hexadecanethiol had been stamped (Figure 4B). Inspection of Figure 4B also shows that regions of the surface that were microcontact printed with hexadecanethiol are no longer homogeneous. We calculated the average twist angle of the LC at the center of each sample that had been microcontact printed with hexadecanethiol (within the regions indicated by the dashed squares), and thereby determined that the twist angle changed from 88.6 ° to 74.4 ° to 8.7 ° (measured relative to the region formed from pentadecanethiol) following immersion in the solution of pentadecanethiol for 10 mins, 2 hrs and 24 hrs, respectively. In summary, these measurements of twist angles of the LC are consistent with the expected rapid formation of the SAM from pentadecanethiol on the bare gold regions of the microcontact printed surface, and a slow process of displacement of the microcontact printed hexadecanethiol by the pentadecanethiol in solution. We also note that microcontact printed monolayers can possess incomplete regions (defects), which may also be completed by adsorption of pentadecanethiol from solution.40,41 More broadly, these results demonstrate the use of TNLCs to report displacement reactions involving adsorbates on surfaces even in cases where the two competing species are similar in structure.
Figure 4.
Maps of twist angles of TNLCs in contact with analytic surfaces prepared by microcontact printing of hexadecanethiol, and subsequent immersion of the surfaces in pentadecanethiol solutions for (A) 10 mins, (B) 2hrs, and (C) 24 hr. The squares correspond to regions of the gold films on which hexadecanethiol was not microcontact printed. The direction of deposition of the gold on the analytic and reference surfaces is indicated by the white arrows and black arrows, respectively. Twist angles of the LC corresponding to each color are indicated by the color chart shown at the right side of the figure. Dashed squares indicate regions that were used to calculate average liquid crystal twist values. Scale bars represent 1 mm.
Imaging of Competitive Co-adsorption of Mixtures of Organothiols
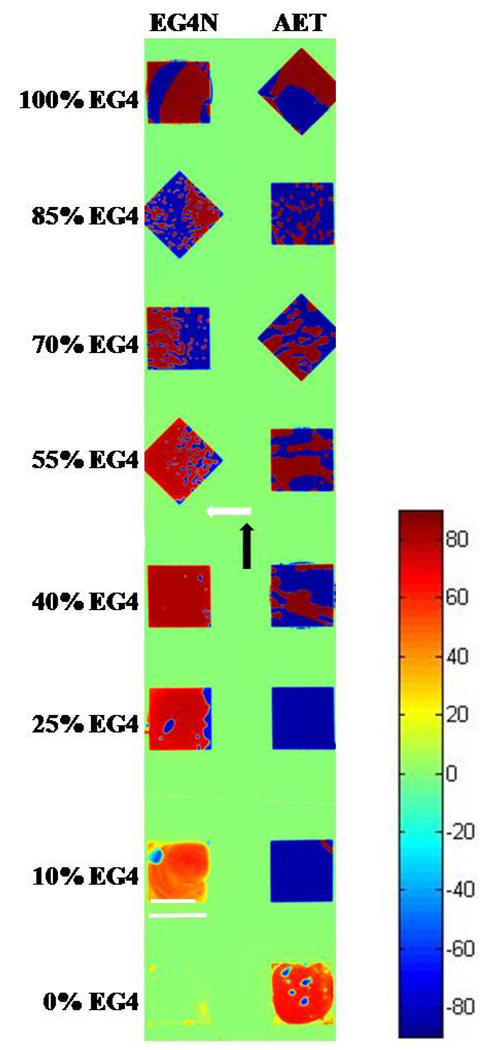
Mixed monolayers of alkanethiols are used widely to tune surface properties of gold films for bioanalytical assays. Commonly employed components of mixed SAMs formed on gold are EGn-terminated alkanethiols (which resist non-specific protein adsorption) and EGn-NH2-terminated alkanethiols (used for covalent attachment of biomolecules).42–44 Mixed monolayers of these13,14 and other organothiols45–53 are typically formed by competitive coadsorption from solution. The composition of the mixed monolayer is generally dictated by the kinetics of the reaction at the surface (at least, for short reaction times). The presence of adventitious adsorbates25 (as influenced, for example, by the age of the gold films) and other factors that are not well understood can impact the kinetics, and thus reproducible preparation of mixed monolayers requires careful control of experimental conditions. Precise control over surface composition of mixed monolayers is important for the development of quantitative analytic technologies.9 To explore the utility of methods based on TNLCs for the imaging of mixed monolayers, we microcontact printed hexadecanethiol onto gold films, as described above. The resulting bare gold regions (1mm × 1mm squares) were subsequently functionalized with monolayers of EG4 coadsorbed with either 2-aminoethanethiol*HCl (AET), or EG4N*HCl in various ratios (total organothiol concentration of 1 mM). We investigated both AET and EG4N as the second component because (i) AET and EG4N both possess primary amine groups, but AET is a smaller molecule than EG4N; we predicted lower levels of incorporation of AET into mixed monolayers as compared to EG4N, and (ii) we have previously used monolayers of AET in LC-based assays for biomolecules.26,54,55 We prepared mixtures of the thiols in a solvent comprised of water and ethanol (99:1). We used aqueous solutions (rather than the more commonly used anhydrous ethanol) in order to slow the rate of evaporation of the solutions (∼ 250 nL) deposited as droplets in the 1mm × 1mm squares on the surface. The analytical surface and reference surface were oriented such that the angle between the in-plane directions of gold deposition on the two surfaces was ∼90 °.
The spatial maps of the LC twist angles are shown in Figure 5. The continuous green region (twist angle of 1.9 ° ± 0.6 °) corresponds to the area of the surface where the hexadecanethiol was microcontact printed and indicates that the analytic and reference surfaces were oriented at 88.1 ° ± 0.6 ° with respect to each other. In most of the square-shaped regions of the surface, domains of opposite handed twist are evident as blue regions beside red regions. As noted above, domains of opposite twist are common in twisted LCs with twist angles approaching 90 °.31,32 Single component monolayers of EG4 are known to orient nematic 5CB with an easy axis that is perpendicular to the in-plane direction of gold deposition, evident in Figure 5 (top squares) as a twist angle of 87.9 ° ± 1.0 °.13 The orientation of the 5CB on the EG4 monolayer deviated from the orientation of the easy axis by an angle of φ = 0.2 ° ± 1.2 °, which indicates that the monolayer of EG4 caused strong anchoring of 5CB. A previous study concluded that SAMs of EG4 formed from aqueous solutions are more ordered than SAMs of EG4 deposited from ethanolic solutions.56 The result shown in Figure 5, when combined with the results of our past studies that used EG4 monolayers formed from ethanol,13 suggests that the crystalline order of the monolayer may influence the anchoring energy of the LC.
Figure 5.
Maps of twist angles of TNLCs on surfaces that were microcontact printed with hexadecanethiol and then functionalized with mixed monolayers (square regions) of either EG4 and AET or EG4 and EG4N. The composition of the mixed thiol solution used to form the monolayer is indicated to the left of the images. The direction of deposition of the gold on the analytic surface and reference surface is indicated by the white arrow and the black arrow, respectively. Twist angles of the LC corresponding to each color are indicated by the color chart shown at the right side of the figure. Scale bar (lower left) indicates 1 mm.
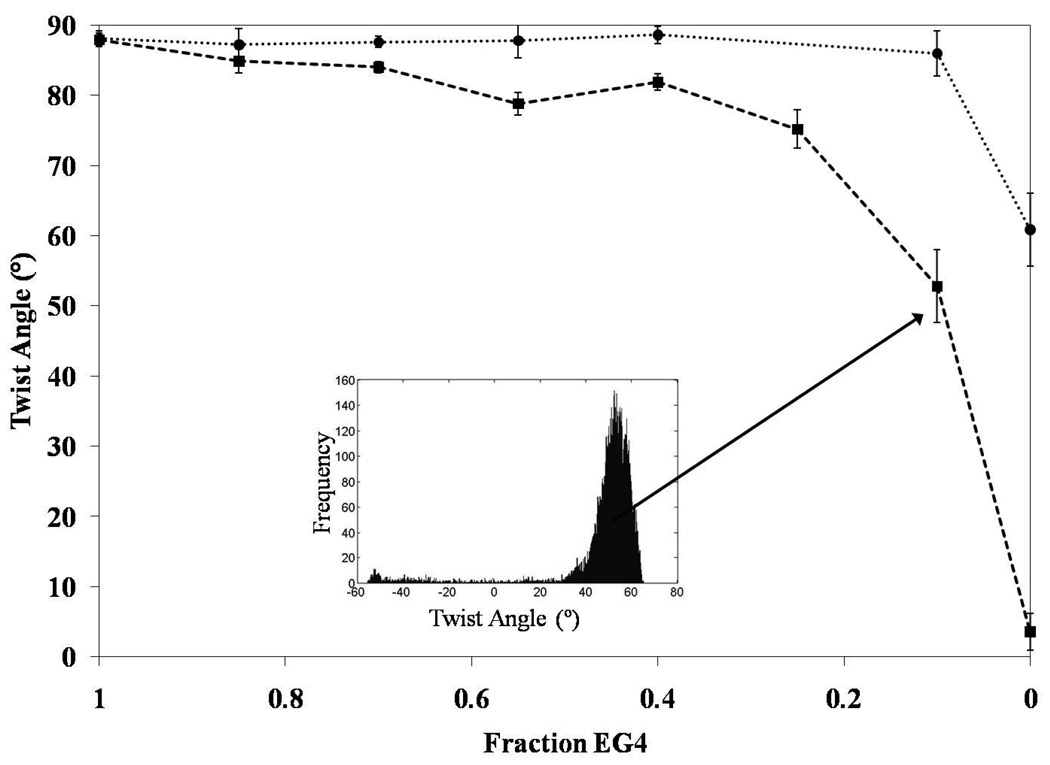
Inspection of Figure 5 reveals that incorporation of EG4N into EG4 monolayers leads to a decrease in the twist angle. We plot the twist angles of the red domains in Figure 6. Only domains with a positive twist angle were plotted for the sake of clarity, but the trend was identical regardless of the handedness of the twist. We measured a twist angle of 52.8 ° ± 5.2 ° for the mixed monolayer formed from the solution containing 90% EG4N and 10% EG4 - corresponding to a deviation of φ = 35.3 ° ± 5.2 ° from the easy axis. The large uncertainty in this measurement indicates a broad range of twist angles within the analytical area. This result highlights a useful attribute of the methodology reported in this paper, namely the ease with which heterogeneities can be detected over a large surface area. We quantified the distribution of twist angles by plotting a histogram of the twist angles within an analytic area. The inset of Figure 6 shows the histogram for the area functionalized with 90% EG4N. Our measurements of the mixed monolayers of AET and EG4 were strikingly different from the mixed monolayers containing EG4N: No change in twist angle was observed over the entire range of solution compositions for the mixed monolayers of AET and EG4 (Figure 6); Only for the pure component SAM formed from AET was the twist angle significantly less than 90 °. This result indicates the absence of measurable incorporation of AET into the SAM formed on the gold film. This result is consistent with our prediction that AET, in contrast to EG4N, does not compete effectively with EG4 during formation of the mixed SAMs due to its small size relative to EG4 (cohesive interactions within SAMs increase with size of the molecules forming the SAMs).48,57 As described in Supporting Information, measurements of twist angles of the LCs can be used to calculate maps of the anchoring energy of the LCs across surfaces.
Figure 6.
Twist angles of the LC calculated from the images shown in Figure 5. Data corresponding to the EG4/EG4N monolayers is represented by the filled squares; filled circles correspond to mixed monolayers of AET/EG4. The inset shows the distribution of twist angles within one of the analytic areas.
Imaging of Surfaces Presenting Affinity Microcontact Printed Antibodies
Previously, we reported that LCs can be used to image proteins captured and delivered to surfaces by using affinity microcontact printing.26,54,58 To demonstrate that twisted LC cells can be used in combination with affinity microcontact printing to achieve quantitative measurements of printed patterns of proteins, we covalently linked biotinylated BSA onto patterned PDMS stamps and used the stamps to capture anti-biotin IgG (with anti-rabbit IgG serving as a negative control). Captured IgG was then microcontact printed onto monolayers formed from AET on gold films deposited at an angle of 30 ° relative to the surface normal. Inspection of Figure 8A reveals that the analytic surface onto which anti-biotin IgG was printed caused the LC to adopt a complicated pattern of twist angles that radiated from a central point. In contrast, the analytic surface onto which the anti-rabbit control antibody was printed (Figure 7B) displayed uniform twist angles that were indistinguishable from background, indicating no measurable anti-rabbit IgG was transferred to the analytic surface.
Figure 7.
Maps of twist angles obtained using analytic surfaces on which anti-biotin IgG was affinity microcontact printed (A and C). The maps of twist angles shown in B and D are the results of control experiments performed using anti-rabbit IgG. For C and D, the antibody was rinsed with water prior to forming the TNLC cell. The direction of deposition of the gold film used for the analytic and reference surface is indicated by the white and black arrows, respectively. Twist angles of the LC corresponding to each color are indicated by the color chart shown at the right side of the figure. Scale bars represent 1 mm.
We speculated that the radial pattern evident in Figure 7A was caused by shear forces acting on the IgG molecules during the drying of the stamp under the gaseous stream of nitrogen (the stream was directed to the center of the stamp),54 and that a brief rinse of the analytic surface with water after contact printing might erase the radial pattern evident in Figure 7A. To test this proposition, we stamped anti-biotin IgG or anti-rabbit IgG onto an AET-functionalized surface, briefly rinsed the surface with deionized water adjusted to pH 5.0 with HCl, and then dried the surface under a stream of nitrogen gas. Figure 7C and 7D (control) show that this modified procedure does lead to elimination of the radial pattern. The above results illustrate the utility of methods based on TNLCs for imaging proteins printed on surfaces. In particular, the TNLC-based method enabled identification of the likely source of an initially complex response of the LC to the printed proteins. We note that analysis of individual optical images of the LC did not lead to an obvious suggestion regarding the possible origins of the complex patterns on the surface. The patterns of twist angle suggested the origin of the non-uniformity to be the gaseous stream used to dry the proteins on the stamp.
Conclusions
Overall, the results presented in this paper establish methods for data acquisition and image analysis that permit maps of bio/chemical functionality patterned on surfaces to be created using TNLCs. The methods involve the acquisition of a series of images (transmission mode between two polarizers) of a film of a TNLC that contacts the analytic surface: analysis of the stack of images yields maps of the twist angle of the LC across the surface. This analysis technique effectively condenses a large data set (stack of images) into a compact form (map of twist angle), revealing features on the surface that were not apparent in the individual images comprising the original stack. The utility of the approach for characterization of patterned surface chemistry was demonstrated by following (i) the displacement of a SAM formed from an alkanethiol on a gold film by a second alkanethiol in solution, (ii) coadsorption of mixtures of organothiols with chemical functionality relevant to bioanalytical surfaces, and (iii) the presence of antibodies printed onto surfaces. The twist angles of the LC can be used to quantify the energy of interaction of the LC with the surface with a spatial resolution of <10µm. These results, when combined, suggest that maps of twist angle created by TNLCs may provide the basis of methods that can be used to monitor and validate chemical modifications of surfaces that are employed in surface-based analytical technologies.
Acknowledgements
This research was partially supported by the National Science Foundation (DMR 0079983) and the National Institutes of Health (CA108467 and CA105730).
Footnotes
Supporting Information Available. Additional experimental procedures, and measurements of anchoring energies. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.de Gennes PG. The Physics of Liquid Crystals. 1st ed. London: Oxford University Press; 1974. [Google Scholar]
- 2.Cognard J. Mol. Cryst. Liq Cryst. Suppl. 1982;78:1–77. [Google Scholar]
- 3.Jerome B. Rep. Prog. Phys. 1991;54:391–451. [Google Scholar]
- 4.Luk Y-Y, Abbott NL. Science. 2003;301:623–626. doi: 10.1126/science.1084527. [DOI] [PubMed] [Google Scholar]
- 5.Shah RR, Abbott NL. J. Phys. Chem. B. 2001;105:4936–4950. [Google Scholar]
- 6.Skaife JJ, Abbott NL. Langmuir. 2001;16:3529–3536. [Google Scholar]
- 7.Skaife JJ, Abbott NL. Langmuir. 2001;17:5595–5604. [Google Scholar]
- 8.Skaife JJ, Brake JM, Abbott NL. Langmuir. 2001;17:5448–5457. [Google Scholar]
- 9.Luk Y-Y, Yang K-L, Cadwell K, Abbott NL. Surf. Sci. 2004;570:43. [Google Scholar]
- 10.Clare BH, Abbott NL. Langmuir. 2005;21:6451–6461. doi: 10.1021/la050336s. [DOI] [PubMed] [Google Scholar]
- 11.Luk Y-Y, Tingey ML, Dickson KA, Raines RT, Abbott NL. J. Am. Chem. Soc. 2004;126:9024–9032. doi: 10.1021/ja0398565. [DOI] [PubMed] [Google Scholar]
- 12.Gupta VK, Skaife JJ, Dubrovsky TB, Abbott NL. Science. 1998;279:2077–2080. doi: 10.1126/science.279.5359.2077. [DOI] [PubMed] [Google Scholar]
- 13.Clare BH, Guzman O, de Pablo JJ, Abbott NL. Langmuir. 2006;22:4654–4659. doi: 10.1021/la0535126. [DOI] [PubMed] [Google Scholar]
- 14.Clare BH, Guzman O, de Pablo JJ, Abbott NL. Langmuir. 2006;22:7776–7782. doi: 10.1021/la0604578. [DOI] [PubMed] [Google Scholar]
- 15.Govindaraju T, Bertics PJ, Raines RT, Abbott NL. J. Am. Chem. Soc. 2007;129:11223–11231. doi: 10.1021/ja073203x. [DOI] [PubMed] [Google Scholar]
- 16.Shah RR, Abbott NL. Langmuir. 2003;19:275–284. [Google Scholar]
- 17.Luk Y-Y, Tingey ML, Hall DJ, Israel BA, Murphy CJ, Bertics PJ, Abbott NL. Langmuir. 2003;19:1671–1680. [Google Scholar]
- 18.Akahane T, Kaneko H, Kimura M. Jpn. J. Appl. Phys. 1996;35:4434–4437. [Google Scholar]
- 19.Faetti S, Mutinati GC. Phys. Rev. E. 2003;68:026601. doi: 10.1103/PhysRevE.68.026601. [DOI] [PubMed] [Google Scholar]
- 20.Vilfan M, Copic M. Phys. Rev. E. 2003;68:031704. doi: 10.1103/PhysRevE.68.031704. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Yoon T-H, Kim JC. Phys. Rev. E. 2005;72:061705. doi: 10.1103/PhysRevE.72.061705. [DOI] [PubMed] [Google Scholar]
- 22.Skaife JJ, Abbott NL. Chem. Mater. 1999;11:612–623. [Google Scholar]
- 23.Gupta VK, Abbott NL. Science. 1997;276:1533–1536. [Google Scholar]
- 24.Shah RR, Abbott NL. J. Am. Chem. Soc. 1999;121:11300–11310. [Google Scholar]
- 25.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 26.Jang C-H, Tingey ML, Korpi NL, Wiepz GJ, Schiller JH, Bertics PJ, Abbott NL. J. Amer. Chem. Soc. 2005;127:8912–8913. doi: 10.1021/ja051079g. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca JG, Galerne Y. Appl. Phys. Lett. 2001;79:2910–2912. [Google Scholar]
- 28.Polossat E, Dozov I. Mol. Cryst. Liq. Cryst. 1996;282:223–233. [Google Scholar]
- 29.Lien A. Conference Record of the 1991 International Display Research Conference. 1991:192–194. [Google Scholar]
- 30.Gupta VK, Abbott NL. Phys. Rev. E. 1996;54:R4540. doi: 10.1103/physreve.54.r4540. [DOI] [PubMed] [Google Scholar]
- 31.Miyaji A, Yamaguchi M, Toda A, Mada H, Kobayashi S. IEEE Trans. Electron Devices. 1977;vol ED-23:811–815. [Google Scholar]
- 32.Choi S-W, Takanishi Y, Ishikawa K, Takezoe H. Appl. Phys. Lett. 2007:033115. [Google Scholar]
- 33.Schwartz DK. Annu. Rev. Phys. Chem. 2001;52:107–137. doi: 10.1146/annurev.physchem.52.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Himmelhaus M, Eisert F, Buck M, Grunze M. J. Phys. Chem. B. 2000;104:576–584. [Google Scholar]
- 35.Vanderah DJ, Parr T, Silin V, Meuse CW, Gates RS, La HY, Valincius G. Langmuir. 2004;20:1311–1316. doi: 10.1021/la035829g. [DOI] [PubMed] [Google Scholar]
- 36.Collard DM, Fox MA. Langmuir. 1991;7:1192–1197. [Google Scholar]
- 37.Baralia GG, Duwez A-S, Nysten B, Jonas AM. Langmuir. 2005;21:6825–6829. doi: 10.1021/la050245v. [DOI] [PubMed] [Google Scholar]
- 38.Xing YF, Li SFY, Lau AKH, O’Shea SJ. J. Electroanal. Chem. 2005;583:124–132. [Google Scholar]
- 39.Kakiuchi T, Sato K, Iida M, Hobara D, Imabayashi Si, Niki K. Langmuir. 2000;16:7238–7244. [Google Scholar]
- 40.Larsen NB, Biebuyck H, Delamarche E, Michel B. J. Am. Chem. Soc. 1997;119:3017–3026. [Google Scholar]
- 41.Akiyama M, Fujita M, Fujihira M. Chem. Lett. 2006;35:1112–1113. [Google Scholar]
- 42.Prime KL, Whitesides GM. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 43.Lahiri J, Isaacs L, Tien J, Whitesides GM. Anal. Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 44.Luk Y-Y, Kato M, Mrksich M. Langmuir. 2000;16:9604–9608. [Google Scholar]
- 45.Bain CD, Evall J, Whitesides GM. J. Am. Chem. Soc. 1989;111:7155–7164. [Google Scholar]
- 46.Bain CD, Whitesides GM. J. Am. Chem. Soc. 1989;111:7164–7175. [Google Scholar]
- 47.Laininis PE, Nuzzo RG, Whitesides GM. J. Phys. Chem. 1992;96:5097–5105. [Google Scholar]
- 48.Folkers JP, Laibinis PE, Whitesides GM. Langmuir. 1992;8:1330–1341. [Google Scholar]
- 49.Lee CY, Nguyen PCT, Grainger DW, Gamble LJ, Castner DG. Anal. Chem. 2007;79:4390–4400. doi: 10.1021/ac0703395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wink T, van Zuilen SJ, Bult A, van Bennekom WP. Analyst. 1997;122:43R–50R. doi: 10.1039/a606964i. [DOI] [PubMed] [Google Scholar]
- 51.Cotton C, Glidle A, Graham B, Cooper JM. Langmuir. 1998;14:5139–5146. [Google Scholar]
- 52.Riepl M, Ostblom M, Lundstrom I, Svensson SCT, van der Gon AWD, Schaferling M, Liedberg B. Langmuir. 2005;21:1042–1050. doi: 10.1021/la048358m. [DOI] [PubMed] [Google Scholar]
- 53.Iimura Y, Kobayashi N, Kobayashi S. Jpn. J. Appl. Phys., Part 1. 1995;34:1935–1936. [Google Scholar]
- 54.Tingey ML, Snodgrass EJ, Abbott NL. Adv. Mater. 2004;16:1331–1336. [Google Scholar]
- 55.Tingey ML, Wilyana S, Snodgrass EJ, Abbott NL. Langmuir. 2004;20:6818–6826. doi: 10.1021/la049728+. [DOI] [PubMed] [Google Scholar]
- 56.Canaria CA, So J, Maloney JR, Yu CJ, Smith JO, Roukes ML, Fraser SE, Lansford R. Lab Chip. 2006;6:289–295. doi: 10.1039/b510661c. [DOI] [PubMed] [Google Scholar]
- 57.EG4 also contains a longer hydrophobic chain than does AET. The solubility of EG4 in water is expected to be lower than AET. Lower solubility will thermodynamically favor surface adsorption of EG4 relative to AET.
- 58.Bernard A, Fitzli D, Sonderegger P, Delemarche E, Michel B, Bosshard HR, Biebuyck H. Nat. Biotechnol. 2001;19:866–869. doi: 10.1038/nbt0901-866. [DOI] [PubMed] [Google Scholar]