Abstract
Mature immunologically competent dendritic cells are the most efficient antigen-presenting cells that powerfully activate T cells and initiate and sustain immune responses. Indeed, dendritic cells are able to efficiently capture antigens, express high levels of costimulatory molecules, and produce the combination of cytokines required to create a powerful immune response. They are also considered to be important in initiating autoimmune disease by efficiently presenting autoantigens to self-reactive T cells that, in this case, will mount a pathogenic autoimmune reaction. Triggering T cells is not a simple on–off procedure, as T cell receptor responds to minor changes in ligand with gradations of T cell activation and effector functions. These “misfit” peptides have been called Altered Peptide Ligands, and have been shown to have important biological significance. Here, we show that fully capable dendritic cells may present, upon natural antigen processing, a self-epitope with Altered Peptide Ligands features that can unexpectedly induce anergy in a human autoreactive T cell clone. These results indicate that presentation of a self-epitope by immunologically competent dendritic cells does not always mean “danger” and show a mechanism involved in the fine balance between activation and tolerance induction in humans.
Recognition of antigens presented by dendritic cells (DCs) is perceived as a “danger” by T cells, which therefore mount a vigorous immune response (1, 2). Indeed, DCs are the most efficient antigen-presenting cells (APC) able to activate even naive T cells (3, 4). A first signal, which originates from the engagement of the T cell receptor (TCR) with the MHC:peptide complex, is amplified by a so-called costimulatory signal that originates from the interaction of receptors on T cells such as CD28 with ligands such as CD80/86 on DCs (5). Together, these two kinds of signals can promote T cell cycle progression and IL-2 production. Engagement of the TCR with the MHC:peptide complex in the absence of a further signal is, by contrast, thought to lead to a TCR signaling insufficient for IL-2 production and, therefore, proliferation (6). This state of partial or insufficient stimulation is generally called “anergy,” and occurs when T cells recognize peptides presented by “nonprofessional” APC that lack costimulatory molecules. In this context, anergy is therefore defined by an impaired IL-2 production and enhanced IL-2 responsiveness (7). According to this model, a self-antigen presented by DCs should be able to break tolerance in self-reactive T cells, leading to an autoimmune process, whereas a self-antigen presented by APC lacking costimulatory ligands should induce T cell tolerance. However, it is apparent that this rule is not always followed. Indeed, we have previously shown that monocytes failed to stimulate human autoreactive T cells, whereas “nonprofessional” APC (thyroid epithelial cells) succeeded, by unmasking a “cryptic” epitope of the thyroid peroxidase (TPO) molecule (8).
This lack of T cell activation was likely because of the “absence” of the first signal, as the peptide recognized by the self-reactive T cell clone was not presented by monocytes. We could not, however, exclude that the relative poor ability of monocytes to activate T cells played a role in this process. We therefore used fully competent monocyte-derived DCs, which are known to be powerful APC (3), to test whether these cells could display the cryptic, highly stimulatory epitope or overcome the relative weakness of monocytes in T cell activation. As a third possibility, we had to evaluate whether DCs, upon processing of the TPO molecule, presented a self-peptide that delivered an inhibitory (or anergic) signal to T cells.
Materials and Methods
Human T Cells.
Human T cell clones specific for different epitopes of the TPO molecules have been previously described (8, 9). They were established from the thyroid infiltrate of a Graves' patient (10). The T cell clones described in this study are restricted by HLA DQB1*0602/DQA1*0102. They were maintained at 1 × 106/well in RPMI medium 1640 supplemented with 10% human AB serum, 100 units/ml penicillin, and 50 μg/ml streptomycin (complete medium). Cells were fed at regular intervals with recombinant human IL-2 (20 ng/ml, kindly donated by Hoffman–La Roche) and stimulated every 3 wk with irradiated (4,500 rad) allogeneic peripheral blood leukocytes and phytohemagglutinin (PHA).
DC Cultures.
Human monocytes from HLA DQB1*0602/DQA1*0102 donors were purified and cultured for 4–8 days at a concentration of 1 × 106/well in complete medium with 50 ng/ml granulocyte–monocyte colony-stimulating factor (PharMingen) and 10 ng/ml IL-4 (Sandoz Pharmaceutical), according to a well established procedure (11, 12). In some cases, after the soluble antigen TPO was added, DCs were further matured for 24 h with bacterial lipopolysaccharide (Sigma) at 10 ng/ml (13). Cells were washed extensively before use.
Induction of T Cell Anergy.
Anergy was induced in T cell clone 37 by using both T:T presentation and a more physiological approach, by prestimulating the T cells with live professional APC pulsed with antigens.
Briefly, 106/well T cells were incubated overnight with medium and peptides at 10 μM concentration in the absence of professional APC (T:T presentation), or with 105/well live HLA-matched DC prepulsed with antigens. The following day, T cells were washed and rested for up to 7 days. T cells at 104/well were then challenged with different doses of peptides presented by 1 × 103/well live HLA-matched DC in a standard 72-h proliferative assay in a round-bottom 96-well microtiter plate.
Establishment of TCR Transfectant.
To reconstitute the TCR expressed by T cell clone 37, we used the surface TCR−/− murine T cell hybridoma 58α−β−, which expresses intracellularly all of the CD3 subunits but not the TCRA and TCRB chains (14). A potential problem of reconstituting a human TCR into a murine T cell might arise from an altered interaction of the human TCR chains with the mouse CD3 subunits, causing a failure in TCR signaling. To overcome this problem, cDNA chimeras composed by the human DNA encoding the extracellular portion of the TCR and the murine DNA encoding the intracellular portion of the TCR were made. TCRA and TCRB of T cell clone 37 were amplified by Anchored-PCR (8). In the obtained sequence, the sense AV15S1-specific primer introduced EcoRI restriction site 3 bp upstream of the initiation codon for the AV construct, whereas the sense BV1S1-specific primer introduced EcoRI and MluI restriction sites 9 bp upstream of the initiation codon. The antisense primer for the TCRAV introduced SspI restriction site after the JC junction, without altering the amino acid sequence. The antisense primer for TCRB introduced a XhoI restriction site close to the JC junction, without altering the amino acid sequence. The murine TCRAC and TCRBC were amplified by using sense primers that introduced a SspI site (5′-CCAATATTCAGAACCCAGAACCTGCT-3′) and a XhoI site (5′-CCCTCGAGATCTGAGAAATGT-3′), respectively. The antisense primers, located on the 3′ untranslated regions, introduced a NotI restriction site for TCRAC (5′-GGAGCGGCCGCGTGAGGAGGACGGAAC-3′) and XbaI restriction site for TCRBC (5′-TTTGGATCCATCTATGGCCAGGG-3′). Human TCRAV-J was ligated with murine TCRAC in pBluescript II (Stratagene) opened with EcoRI and NotI. Human TCRB-D-J was ligated with murine TCRBC in pGEM-3Z (Promega) opened with EcoRI and XbaI. Chimeric TCRA construct was sequenced and excised from pBS II by EcoRI and NotI and ligated in the mammalian expression vector pFRCM that confers resistance to methotrexate. Chimeric TCRB construct was sequenced and excised from pGEM-3Z by MluI and XbaI and ligated in the mammalian expression vector pCMV4 that confers resistance to G418 (Neo). The murine T cell hybridoma 58α−β− was previously transfected with the expression vector RSV5T4 containing the human CD4 cDNA and conferring resistance to mycophenolic acid and called 54α−β− (15) (gift of O. Acuto, Institut Pasteur, Paris, France). A total of 1 × 107 cells/ml in PBS were transfected with about 30 μg of each plasmid DNA in Gene Pulse cuvettes and electroporated at 250V, 250 μF. After transfection, TCR expression was assessed by the reappearance of mouse CD3 (KT3 MoAb; Serotec) on the cell surface. TCR37.33 was a stable subclone with the high expression of CD3.
T Cell Proliferation Assay.
The reactivity of the TPO-specific human T cells clones was assessed by coculturing 1 × 104 T cells from each T cell clone with either 3 × 104 glutaraldehyde-fixed autologous EBV-transformed PBMC or 1 × 103/well live HLA-matched human DC as APC. APC were incubated with or without antigens for 1 h at 37°C before the incubation with the T cells.
Human purified TPO antigen (gift from B. Rapoport, Cedars-Sinai Medical Center, Los Angeles, CA) was used in the assay at 0.2 μg/ml. Peptides of the human TPO molecule P3 (TPO536-547 DPLIRGLLARPA), and P4 (TPO537-548 PLIRGLLARPAK, gifts from D. Wraith, University of Bristol, U.K.) were used at serial dilutions, as described in the Figs. The promiscuous myelin basic protein (MBP) peptide 13-32 has been used as irrelevant peptide. All T cell clones were tested at least twice, and all of the experiments showed similar profile of responsiveness. All of the proliferative assays were performed in triplicate for 72 h in a round-bottom 96-well microtiter plate, and the cells were pulsed with [3H]thymidine [1 μCi (1 Ci = 37 GBq)] during the last 8 h of culture.
Fluorescence-Activated Cell Sorter Staining.
Human T cells and TCR transfectants were collected in ice-cold PBS (supplemented with 1% FCS and 0.05% azide) and stained by using a mAb against human TCRBV1 (BL37.2-IgG1 rat, donated by F. Romagne, Immunotech, Marseille, France). The counterstain was a biotinylated goat-anti-rat serum (Southern Biotechnology Associates), followed by fluorescein-labeled PE-conjugated streptavidin (Southern Biotechnology Associates). Cells were double stained with fluorescein isothiocyanate-conjugated mouse anti human CD4 (IgG1; Sigma). Cells were than analyzed on a fluorescence-activated cell sorter (FACStar Plus; Becton Dickinson). TCRBV1 specificity of the BL37.2 mAb was confirmed by a rat anti human isotype control staining.
To calculate the extent of TCR down-regulation upon peptide stimulation, transfectant TCR37.33 was coincubated with an equal number of autologous EBV-transformed human B cells in presence of increasing concentrations of peptides, for 4 and 24 h. Cells were then washed, stained for expression of human TCRBV1, and fixed in 1% formaldehyde. TCR37.33 cells were tightly gated on forward scatter/side scatter to exclude the B cell subset.
Results
The Self-Antigen TPO Processed by Competent DCs Does Not Stimulate T Cell Clone 37.
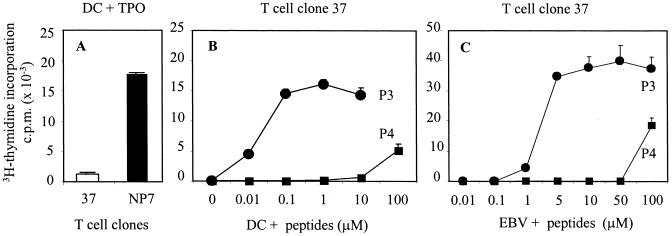
The immunodominant and cryptic epitope of the TPO antigen P3 () is recognized by the human T cell clone 37 and is presented upon endogenous processing by thyroid epithelial cells, but not by autologous monocytes exogenously loaded with the TPO antigen (8). To test whether more powerful APC, such as DCs, could stimulate these T cells better, we isolated HLA-matched DCs to present the TPO antigen to T cell clone 37. Despite the high levels of HLA and costimulatory molecules expressed by these DCs (data not shown), T cell clone 37 failed to proliferate in response to DCs loaded with the exogenous TPO (Fig.1A). Although the DCs did not present the immunogenic peptide recognized by clone 37, they induced vigorous proliferation in T cell clone NP-7, specific for peptide P4 (TPO 537–548). Thus, peptide P4, but not P3, is presented by DCs upon processing of the TPO antigen.
Figure 1.
Proliferation of human TPO-specific T cell clones in response to antigen and peptides. (A) Live DCs pulsed with 0.2 μg/ml purified human TPO antigen (gift from B. Rapoport, Cedars-Sinai Medical Center) do not induce proliferation in T cell clone 37, but induce a marked proliferation in T cell clone NP-7. Lower concentrations of TPO antigens were tested, producing comparable results. A challenge with 0.2 μg/ml of TPO, however, always resulted in a strong proliferation in clone NP-7. (B) In the same experiment, proliferation in T cell clone 37 is achieved with different concentrations of peptides P3 and P4 (gifts from D. Wraith, University of Bristol) presented by HLA-matched DCs and (C) autologous EBV-transformed B cell line. All results shown are representative of three different experiments.
Unlike exogenous TPO, peptide P3 even at concentrations as low as 0.01 μM induced T cell clone 37 to proliferate, proving that the T cells were capable of proliferating in response to antigens. Peptide P4, when presented by DCs, induced (in contrast) a modest proliferation in clone 37, and only at extremely high concentrations (100 μM). There is nothing peculiar about this peptide (P4), which led to a vigorous proliferation of clone NP-7 at 0.5 μM concentration (data not shown). When peptide P3 was presented to clone 37 by an autologous EBV-transformed B cell line, T cell proliferation was only induced at peptide concentrations two logs higher (1 μM) than that needed when DCs were used (Fig. 1C), proving the strong APC power of the DCs we prepared. This is of significance as it has been shown that DCs with inhibitory functions can be generated and they have a much-reduced ability to stimulate T cells (16–19). Although both peptides P3 and P4 are presented to T cell clones 37 and NP-7 in the context of DQB1*0602/DQA1*0102 (8), they bind to the HLA groove in a different register and display a different antigen conformation to the TCR (9). The TCR can interact with a spectrum of peptides as part of its ligand. The basis of this broad specificity for ligands was previously investigated by using synthetic peptides with amino acid substitutions in TCR binding residues, and it was shown that one TCR has the ability to interact productively with multiple different ligands (20). The analogs of an immunogenic peptide are the so-called “altered peptide ligands,” which are able to induce partial TCR activation, as shown in elegant studies performed on single peptide-specific T cell clones (21–23). In this view, peptide P4 appeared to be recognized by the TCR of clone 37 as a natural APL, able to engage with the TCR, and induce some proliferation only at high doses, regardless of the APC used.
Establishment of a TCR Transfectant with the Functional Characteristics of Clone 37.
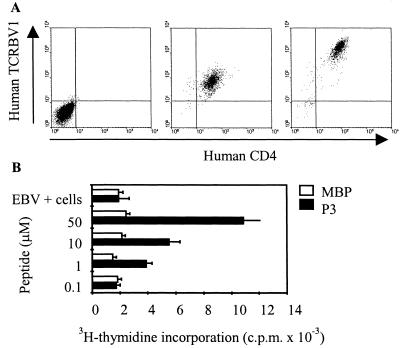
We next tried to isolate the MHC:peptide complex and dissect how its engagement with the TCR was taking place. For this purpose, we have transfected the human TCR of clone 37 and human CD4 in the TCR-deficient mouse hybridoma 58α−β− (14) (Fig. 2A Left). The resulting cell line would therefore be murine, except for the expression of the human TCR and human CD4, and express neither the murine nor the human CD28. Because human autologous EBV-transformed B cell line or human HLA-matched DCs were used as APC, the only engagements occurring between the two cell types would be through the MHC:peptide complex (on APC) and the TCR/CD4 (on T cell). This transfectant is therefore an excellent tool to assess how the MHC:peptide complexes triggered the TCR. Among many transfectants produced, 37.33 expressed stable levels of human CD4 and TCRBV1 (Fig. 2A Middle). Although the transfected molecules were always expressed at lower intensity than the parental human T cell clone 37 (Fig. 2A Right), TCR37.33 still retained the original peptide specificity (Fig. 2B).
Figure 2.
Reconstitution of the TCR expressed by clone 37 into a murine T cell hybridoma. The TCR-transfected clone 37.33 expressing the chimeric human/mouse TCR was stained by the anti human TCRBV1 (BL37.2 mAb) and anti human CD4 (Middle). The untransfected mouse cell line 58α−β− served as negative control (Left). (Right) The expressions of TCR and CD4 in the parental human T cell clone 37. (B) Peptide-specific responsiveness of clone 37.33 was measured by using graded doses of peptide P3 (black bars) and monitoring IL-2 release and subsequent CTLL-2 cell proliferation. The irrelevant MBP peptide 13-32 (white bars) induced no IL-2 release. The TCR-transfected clone 37.33 remained stable in culture for over 2 yr without losing peptide specificity. All results are representative of three different experiments.
A Nonstimulatory Concentration of Peptide P4 Induces a Transient TCR Down-Regulation.
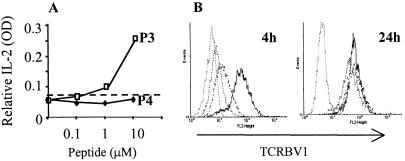
T cell responses following TCR interaction with APL have highlighted the complexity of the signaling system. The indications are that the TCR responds to minor changes in ligand with gradations in T cell activation and effector functions (24) and with specific changes in the upstream TCR signaling pathway (25). When challenged with increasing concentrations of peptides P3 and P4 presented by the autologous EBV-transformed B cell line, IL-2 release by TCR37.33 was observed only upon stimulation with P3 but not with P4 complexes (Fig. 3A). Surprisingly, a similar level of TCR down-regulation was achieved at 4 h upon challenge with the two peptides at 10 μM concentration (Fig. 3B). Marginal discrepancies in TCR down-regulation induced by the two peptides are sometimes detected, but no consistent pattern was observed, excluding that such differences may be the cause of the variation in the release of IL-2. The TCR down-regulation was however transient and at 24 h cells expressed normal levels of TCR (Fig. 3B). Thus, the two peptides are both capable of engaging the TCR, but whereas one MHC:peptide conformation (P3) induced a productive signal mirrored by the release of IL-2, the other (P4) induced a different or defective TCR signal.
Figure 3.
Engagement with the MHC:peptide complex down-regulates the TCR. TCR37.33 were incubated for 24 h with equal numbers of APC prepulsed with or without increasing concentration of peptides. (A) At each peptide concentration, the supernatants were collected after 24 h and the released IL-2 assessed by ELISA. Challenge with peptide P3 is represented by the open symbols, whereas peptide P4 is represented by the filled symbols. APC with the irrelevant MBP peptide at 10 μM did not induce any IL-2 release (broken line). (B) After 4 and 24 h, cells were stained for expression of human TCRBV1 and fixed in 1% formaldehyde. TCR37.33 cells were tightly gated on forward scatter/side scatter to exclude the APC. TCR expression was tested in TCR37.33 incubated with APC and the irrelevant MBP peptide 13–32 (solid line, mean fluorescence intensity 97.8), peptide P3 (dotted line, mean fluorescence intensity 8.76) or peptide P4 (dashed line, mean fluorescence intensity 12.19). Peptides were used at 10 μM. The gray line represents TCR37.33 stained with a rat anti human isotype control (mean fluorescence intensity 5.8). The data are representative of at least four different experiments.
Peptide P4 Induces T Cell Anergy.
We therefore addressed the question as to whether the impaired TCR signal observed upon challenge with P4 could induce T cell anergy in the parental human T cell clone 37. T cells were exposed to the two peptides in the absence of APC for 24 h, relying on the well-known effect of T:T presentation (7, 26–28) and to medium alone as control. The following day, T cells were washed and rested, and, at this stage, similar levels of TCR expression were detected in all groups of T cells (data not shown). T cells were then rechallenged with peptides presented by live DCs. T cells pretreated with medium proliferated vigorously in response to increasing concentrations of P3 (Fig. 4). Proliferation was also observed in response to P4, but only at the high concentration of 100 μM. T cells pretreated with 10 μM of the immunodominant peptide P3 were, on the contrary, completely anergized and unable to respond to a further challenge with P3. Only modest proliferation (<10% of controls) was observed upon rechallenge with P4 at 100 μM. Most surprising, pretreatment with 10 μM of P4, a dose that never induced proliferation in clone 37, led to complete anergy upon rechallenge with either peptide P3 or P4 presented by DCs. In all cases, the T cells were viable, as indicated by proliferation to exogenous human IL-2. In some experiments, T cells were rested for up to 7 days, producing overlapping results (data not shown).
Figure 4.
Anergy induction in the absence of costimulation. Human T cell clone 37 was incubated in the absence of APC with 10 μM of peptide P3, 10 μM of peptide P4, and medium alone. After 24 h, T cells were washed, rested, and challenged with DCs prepulsed with increasing concentrations of peptides P3 and P4. T cells preincubated with medium proliferated in response to peptide P3 and a high dose (100 μM) of peptide P4. Similar results were obtained if T cells were preincubated with the irrelevant MBP peptide or rested for up to 7 days (data not shown). T cells pretreated with peptides P3 or P4 in the absence of APC did not respond to a further peptide stimulation (Middle and Right). T cell proliferation to exogenous IL-2 confirmed that T cells were still viable. All results are representative of five different experiments.
DCs Present a Tolerogenic Peptide upon Processing of the TPO Self-Antigen.
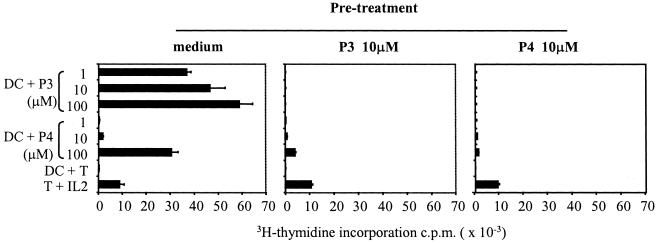
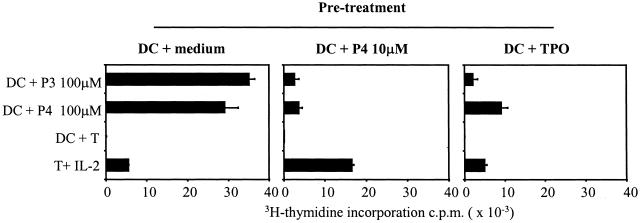
To approach more closely the in vivo situation, we next asked what the outcome is after engagement of clone 37 with DCs presenting peptide P4 or the naturally processed TPO antigen. Peptide P3 was excluded from this experiment, as it is an agonist epitope and its presentation by DC induced a powerful proliferation in clone 37 as shown in Fig. 1. The agonist peptide P3, however, can induce anergy in T cell clone 37 if used in T:T presentation (in absence of professional APC), as described in Fig. 4. The results in Fig. 5 (Middle) show that live DCs pulsed with 10 μM of the synthetic peptide P4 induced anergy in T cell clone 37. Most important, upon processing of the TPO molecule, live DCs presented peptides that were able to induce a significant anergy in T cell clone 37 (Fig. 5 Right). Indeed, after the initial challenge with DCs pulsed with TPO, which as in Fig. 1A produced no proliferation, T cells were only marginally (<10% of controls) able to proliferate to high concentrations of the immunodominant ligand P3. Proliferation to high concentration of P4 was also significantly reduced (<40% of controls). Because the initial pretreatment was made with a low concentration of peptide P4 (or naturally processed TPO antigen) that never induced proliferation of T cell clone 37, we excluded that the anergy obtained was simply because of temporary refractoriness of the proliferating T cells. In some cases, we also used DC treated with tumor necrosis factor or bacterial lipopolysaccharide during tolerance induction, or rested the anergized T cells for longer (up to 7 days), obtaining overlapping results.
Figure 5.
DCs loaded with antigen can induce anergy. Human T cell clone 37 was incubated with DCs loaded with peptide P4 (at 10 μM) or 0.2 μg/ml of purified human TPO antigen. After 24 h, T cells were washed, rested, and challenged with DCs prepulsed with high doses of peptides P3 and P4. T cells preincubated with DCs only proliferated in response to peptides P3 and P4. Similar results were obtained if T cells were preincubated with DCs pulsed with the irrelevant MBP peptide or rested for up to 7 days (data not shown). T cells pretreated with DCs pulsed with P4 were anergized. T cells pretreated with DCs pulsed with TPO were also anergized, although anergy was more evident to the immunogenic peptide P3 (<10% of control). Proliferation to 100 μM of P4 was also significantly reduced (<40% of control). Experiments were also performed after purification of the pretreated T cells, obtaining similar profiles. The data are representative of three different experiments.
Discussion
Dendritic cells are the most specialized APC, inducing T cell activation after antigen capture and migration in the regional lymph nodes (4, 29). DCs are thus the initiators of immune responses to foreign antigens (2, 30). They are also considered to be key players in the induction and maintenance of autoimmune reactions (31). In humans, the study of these cells has been facilitated by the use of monocyte-derived DCs as large and homogeneous population of cells can be prepared (11). Studies on these human monocytes-derived DCs as well as murine derived DCs have helped in understanding their origin and function (3, 32). It has become apparent that the term DCs defines a heterogeneous population of cells with characteristics ranging from extremely powerful to quite poor T cell activators. The latter have intrinsic down-modulatory activity, thus playing an important function in the homeostatic control of the immune system (16, 18, 19). Immunologically competent DCs, however, are always considered to be extremely powerful APC, as they express all of the required costimulatory molecules and release the appropriate cytokines necessary to initiate an immune response (3, 4, 32). In this context, it has to be reminded that the engagement of the TCR with a MHC:peptide complex delivers the main signal that leads to T cell activation (5). This primary signal is controlled by a delicate and flexible interaction between the TCR and MHC:peptide complex, and, indeed, several studies have shown that minor changes in the peptide recognized by the TCR can dramatically modify the pattern of T cell responsiveness (21, 33). These modified peptides, the so called “altered peptide ligands” proved also to be useful tools to probe the interactions between the TCR and the MHC:peptide complexes involved in the process of thymic education. Indeed, it has been shown that in the thymus, those peptides with the ability to induce positive selection are variants of the antigenic peptide and are identified as APL (34, 35). Our results, therefore, suggest how the self-reactive T cell clone 37 could have escaped thymic deletion. Indeed, positive selection might have occurred if peptide P4, a naturally occurring APL of TPO produced by professional antigen presenting cells, is presented in the thymus. “Naturally” occurring epitope variants, with APL characteristics, may have an important role in immune recognition and pathology has been demonstrated in viral infections (36, 37). In these latter situations, however, the virus mutates changing amino acids in critical TCR contact sites and thus leading to the generation of “naturally induced” APL.
In this study, we report that fully competent DCs that process the self-antigen TPO generate epitopes with altered peptide ligand characteristics for the dominant T cell clones infiltrating the thyroid of Graves' patients (8). Here we report that DCs can generate “tolerogenic” epitopes with APL characteristics upon processing of self-antigens. Our findings are not in contrast with the notion that DCs are potent inducers of adaptive immunity, but they highlight that presentation of a self-antigen by immunologically competent DCs does not always lead to “danger” but may lead to “safety” for the organism. In this case, the “danger” (activation) or the “safety” (anergy) are caused by the differential “editing” of the TPO antigen upon “exogenous” vs. “endogenous” processing (8). This is a novel concept, and represents the so-called “exception to the rule,” that could play an important role in pathological conditions. It has indeed to be stressed that the tolerogenic DCs described so far either constitute a separate differentiation pathway (38) or they have been exposed to cytokines that blocked the DCs maturative steps (12, 16). More recently, DCs that uptake apoptotic cells were described to induce peripheral tolerance to self (18, 39). In all cases, these tolerogenic DCs are either not fully matured or intrinsically incompetent in their APC function. In this paper we show that immunologically efficient DCs can generate epitopes such as P4, with characteristics of ALP, as we have evidence that P4 induces a different pattern of cytokine release compared with the cryptic epitope P3 in clone 37 (data not shown). The consequence of the presentation of APL by DCs is that these powerful antigen presenting cells are able to induce and maintain self-reactive T cells in an anergic status, thus defining a novel way to induce peripheral tolerance of self-reactive T cells recognizing cryptic self-epitope (8). It has to be remembered that because cryptic epitopes have been considered to have a key role in driving autoimmune diseases (40), our study has a specific relevance to autoimmune diseases. In conclusion, these results suggest an alternative role for fully capable DCs not simply as powerful “initiators” of immune responses, but also as effective creators of immune tolerance for self-reactive T cells.
Acknowledgments
We thank Dr. Oreste Acuto for the gift of the 58α−β− cell line expressing the human CD4, Dr. Basil Rapoport for the gift of the human purified TPO, and Prof. David Wraith for synthesizing the TPO peptides. This work has been supported by European Economic Community Biomed Grant BMH4-98-3703, the Arthritis Research Campaign. S.Q. is a recipient of a Career Development Research Fellowship from the Wellcome Trust.
Abbreviations
- DC
dendritic cell
- APC
antigen-presenting cell
- TCR
T cell receptor
- TPO
thyroid peroxidase
- APL
altered peptide ligands
- PHA
phytohemagglutinin
- MBP
myelin basic protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190204697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190204697
References
- 1.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 2.Gallucci S, Lolkema M, Matzinger P. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Sallusto F, Lanzavecchia A. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone J A. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 6.Gimmi C D, Freeman G J, Gribben J G, Gray G, Nadler L M. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz R H. J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quaratino S, Feldmann M, Dayan C M, Acuto O, Londei M. J Exp Med. 1996;183:349–358. doi: 10.1084/jem.183.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quaratino S, Thorpe C J, Travers P J, Londei M. Proc Natl Acad Sci USA. 1995;92:10398–10402. doi: 10.1073/pnas.92.22.10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayan C M, Londei M, Corcoran A E, Grubeck-Loebenstein B, James R F L, Rapaport B, Feldmann M. Proc Natl Acad Sci USA. 1991;88:7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel A S, Quaratino S, Douek D C, Londei M. Eur J Immunol. 1997;27:26–34. doi: 10.1002/eji.1830270105. [DOI] [PubMed] [Google Scholar]
- 13.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature (London) 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 14.Letourneur F, Malissen B. Eur J Immunol. 1989;19:2269–2274. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- 15.Blank U, Boitel B, Mege D, Ermonval M, Acuto O. Eur J Immunol. 1993;23:3057–3065. doi: 10.1002/eji.1830231203. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A H. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 17.Urban B C, Ferguson D J, Pain A, Willcox N, Plebanski M, Austyn J M, Roberts D J. Nature (London) 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 18.Sauter B, Albert M L, Francisco L, Larsson M, Somersan S, Bhardwaj N. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman R M, Turley S, Mellman I, Inaba K. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evavold B D, Sloan-Lancaster J, Wilson K J, Rothbard J B, Allen P M. Immunity. 1995;2:655–663. doi: 10.1016/1074-7613(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 21.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta F C, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 22.Sloan Lancaster J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 23.Sloan Lancaster J, Shaw A S, Rothbard J B, Allen P M. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 24.Evavold B D, Sloan-Lancaster J, Allen P M. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 25.Kersh E, Shaw A, Allen P. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 26.Lamb J R, Feldmann M. Nature (London) 1984;308:72–74. doi: 10.1038/308072a0. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz R H. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi G, Sidhu S, Batchelor R, Lechler R. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludewig B, Odermatt B, Ochsenbein A F, Zinkernagel R M, Hengartner H. Immunol Rev. 1999;169:45–54. doi: 10.1111/j.1600-065x.1999.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 31.Green E A, Flavell R A. Immunol Rev. 1999;169:11–22. doi: 10.1111/j.1600-065x.1999.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 32.Mellman I, Turley S J, Steinman R M. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 33.Kersh G J, Allen P M. Nature (London) 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 34.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 35.Jameson S C, Bevan M J. Curr Opin Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- 36.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Nature (London) 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 37.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, et al. Nature (London) 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 38.Suss G, Shortman K. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang F P, Platt N, Wykes M, Major J R, Powell T J, Jenkins C D, MacPherson G G. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]