Abstract
Purpose
Ischemia-reperfusion injury induced by the Pringle maneuver is a well-known problem after liver surgery. The aim of this study was to monitor metabolic changes in the pig liver during warm ischemia and the following reperfusion preceded by ischemic preconditioning (IPC).
Methods
Eight Landrace pigs underwent laparotomy. Two microdialysis catheters were inserted in the liver, one in the left lobe and another in the right lobe. A reference catheter was inserted in the right biceps femoris muscle. Microdialysis samples were collected every 30 min during the study. After 2 h of baseline measurement, IPC was performed by subjecting pigs to 10 min of ischemia, followed by 10 min of reperfusion. Total ischemia for 60 min was followed by 3 h of reperfusion. The samples were analyzed for glucose, lactate, pyruvate, and glycerol. Blood samples were drawn three times to determine standard liver parameters.
Results
All parameters remained stable during baseline. Glycerol and glucose levels increased significantly during ischemia, followed by a decrease from the start of reperfusion. During the ischemic period, lactate levels increased significantly and decreased during reperfusion. The lactate–pyruvate ratio increased significantly during ischemia and decreased rapidly during reperfusion. Only minor changes were observed in standard liver parameters.
Conclusions
The present study demonstrated profound metabolic changes before, during, and after warm liver ischemia under the influence of IPC. Compared with a similar study without IPC, the metabolic changes seem to be unaffected by preconditioning.
Keywords: Warm liver ischemia, Portal triad clamping, Preconditioning, Metabolic changes, Microdialysis
Introduction
Major operative blood loss and transfusion requirements are related to increased morbidity, mortality, and recurrence after hepatectomy for malignant disease [1–3]. Because the liver receives about a quarter of the total cardiac output, different techniques are used to reduce perioperative hemorrhage. Portal triad clamping by Pringle’s maneuver (PM) is often applied to reduce blood loss during liver resection. However, portal triad clamping predisposes the remaining liver to ischemia-reperfusion (I/R) injury, ranging from local damages to severe injury, which in turn may result in liver failure [4].
During recent years, several studies have shown that ischemic preconditioning (IPC), defined as a brief period of ischemia followed by a short interval of reperfusion before a prolonged ischemic period, confers a state of protection in several organs, including the liver, resulting in improved organ tolerance to longer subsequent episodes of ischemia [5–7]. The exact mechanisms by which IPC confers protection is not fully understood [8, 9].
Microdialysis is a method that provides the opportunity for continuously monitoring metabolic changes in the liver and other tissues [10–13]. A number of different metabolites can be measured to monitor hepatic metabolism. In particular, glucose, lactate, pyruvate, and glycerol have been evaluated. In a previous study, we described profound metabolic changes in the pig liver during and after warm ischemia measured by microdialysis [14].
The aim of the present study was to use microdialysis to monitor metabolic changes in the pig liver during warm ischemia, followed by reperfusion in a group of animals, which had been subjected to IPC.
Methods
A total of 8 female pigs (Danish Landrace/Yorkshire; Påskehøjgård Centre, Aarhus, Denmark) weighing approximately 60 kg were used for the experiments. The research procedure was conducted under a local project license (registration number: 2002-561-574) in accordance with the Danish regulations on animal experiments.
The animals were overnight fasted before the experiment. After premedication with an intramuscular injection of midazolam 0.4 mg/kg and ketamine 4 mg/kg, the pigs were intubated and mechanically ventilated with a mixture of air, oxygen, and 1.5% isoflurane. Fentanyl was administered intravenously at 10 ml/h, as well as saline at 12.5 ml/kg/h.
The left carotic artery and the left jugular vein were exposed and catheters were inserted. Samples for blood gas analysis were drawn hourly from the left carotic artery and analyzed for pO2, pCO2, pH, arterial base excess, and lactate concentration on an ABL615 apparatus (Radiometer, Copenhagen, Denmark); blood pressures were also measured in this vessel. Fluid and drugs were administered through the left jugular vein, and blood samples for measurements of alanine aminotransferase (ALT), alkaline phosphatase (AP), bilirubin, prothrombin time (PT), and total leukocytes (TL) were also drawn from this vessel. Animals were placed on a heating blanket and rectal temperature was taken and maintained at 37.5°C. Finally, a urinary catheter was inserted.
Pigs were humanely killed with an overdose of saturated potassium chloride while under anesthesia at the end of each experiment.
A midline laparotomy was performed and the liver was mobilized. Structures in the portal triad were exposed. Two microdialysis catheters (CMA 60 Microdialysis Catheter, Stockholm, Sweden) were inserted and fixed in the liver, one in the left lateral lobe and another in the right lateral lobe. A reference catheter was inserted in the right biceps femoris muscle. Each microdialysis catheter was connected to a microinfusion pump (CMA 107: CMA Microdialysis AB) and perfused with Ringer’s chloride at a flow rate of 0,3 μl/min. After insertion of the catheters, a “washout period” of 1 h was used to flush the dialyses probes and allow the liver tissue to recover from cellular damage due to the implantation procedure.
Microdialysis samples were collected every 30 min during the experiment. Ischemic preconditioning was performed by subjecting pigs to 10 min of hepatic ischemia, followed by 10 min of reperfusion. Ischemia was achieved by using the portal triad clamping, that is, the PM. Total ischemia for 60 min was followed by 3 h of reperfusion (Fig. 1).
Fig. 1.
The experimental design schematically
A total of 12 samples were collected. The dialysate in collected samples was analyzed for metabolites of the carbohydrate and lipid metabolism (glucose, lactate, pyruvate, and glycerol), using a CMA 600 microdialysis analyzer (CMA Microdialysis AB), and the lactate–pyruvate ratio was calculated. Between events in the monitored tissue and collected dialysate, there was a time delay of approximately 30 min REF. Baseline was an average of sample points 1–5. Sample point 6 represents time of preconditioning. Sample points 7 and 8 express the ischemic period. Sample point 9 represents the reperfusion period, and an average of sample points 10–12 represents the postreperfusion period (Fig. 1).
During the experiment ALT, AP, bilirubin, PT, and total leukocyte (TL) were measured three times, at the baseline, at the end of ischemia, and at the end of reperfusion.
Statistical methods
Statistical analyses were performed by SPSS® 10.0 programs (SPSS Inc., Chicago, IL). The results are expressed as mean ± SEM. Comparisons of data within a group and between groups were performed by nonparametric Kruskal–Wallis test, followed by the Mann–Whitney test. A P value <0.05 was considered significant.
Results
Lactate, glucose, pyruvate, and glycerol all remained stable during the baseline period before preconditioning.
Glycerol
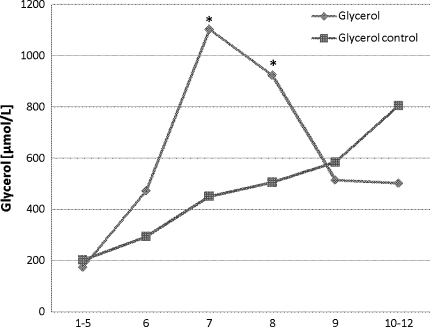
Glycerol level increased significantly (P < 0.01) from baseline during preconditioning and peaked during ischemia. At the end of ischemia, glycerol levels decreased rapidly but did not reach baseline levels. An insignificant increase in glycerol levels in the reference tissue was observed from the time of ischemia toward the end of the experiment (Fig. 2).
Fig. 2.
Glycerol levels in the whole liver at the time of (1–5) baseline, (6) preconditioning, (7) ischemia, (8) ischemia, (9) reperfusion, and (10–12) postreperfusion. * P < 0.05 compared with baseline
Lactate
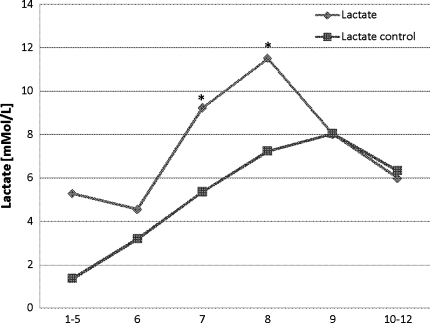
After initiating ischemia, lactate levels increased significantly (P < 0.01) from baseline levels and reached highest levels at the end of total ischemia. During reperfusion, lactate decreased over the next hour without normalization. Lactate levels in the reference catheter showed an insignificant increase from the time of preconditioning until the end of experiment (Fig. 3).
Fig. 3.
Lactate levels in the whole liver at the time of (1–5) baseline, (6) preconditioning, (7) ischemia, (8) ischemia, (9) reperfusion, and (10–12) postreperfusion. * P < 0.05 compared with baseline
Pyruvate
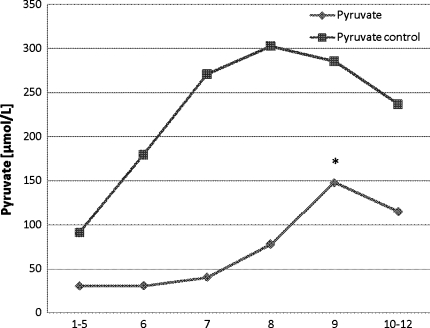
At the end of ischemia, an increase in pyruvate levels was observed. This increase continued significantly (P < 0.05) during the reperfusion period before levels declined at the end of the experiment. From the start of the experiment, a gradual increase was observed in pyruvate levels in the reference tissue and a slight decrease was seen during the reperfusion period (Fig. 4).
Fig. 4.
Pyruvate levels in the whole liver at the time of (1–5) baseline, (6) preconditioning, (7) ischemia, (8) ischemia, (9) reperfusion, and (10–12) postreperfusion. * P < 0.05 compared with baseline
Lactate–pyruvate ratio
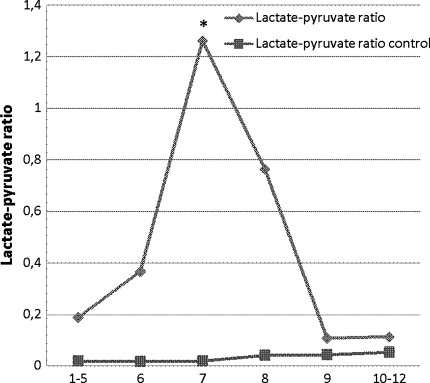
Immediately after initiating ischemia, the lactate–pyruvate ratio increased significantly (P < 0.01), followed by an immediate decrease from the time of reperfusion to baseline levels. The lactate–pyruvate ratio in the reference tissue remained low and stable during the whole of the experiment (Fig. 5).
Fig. 5.
Lactate–pyruvate ratio in the whole liver at the time of (1–5) baseline, (6) preconditioning, (7) ischemia, (8) ischemia, (9) reperfusion, and (10–12) postreperfusion. * P < 0.05 compared with baseline
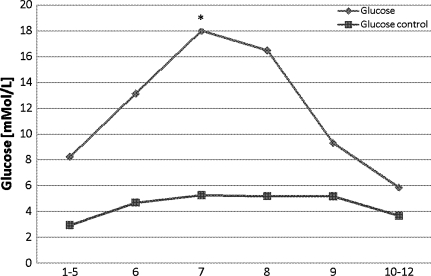
Glucose
Significantly higher levels of glucose (P < 0.05) were observed from the time of preconditioning and ischemia. This increase continued during the ischemic period. From the time of reperfusion, glucose levels decreased to baseline levels. In the reference catheter, glucose levels showed a slight but insignificant increase during the ischemic period until 1 h after reperfusion (Fig. 6).
Fig. 6.
Glucose levels in the whole liver at the time of (1–5) baseline, (6) preconditioning, (7) ischemia, (8) ischemia, (9) reperfusion, and (10–12) postreperfusion. * P < 0.05 compared with baseline
Transaminase levels
Only minor and insignificant variations were observed in ALT, AP, bilirubin, PT, and TL during the experiment (Table 1).
Table 1.
Transaminase levels
| Time 1 Baseline |
Time 2 Ischemia |
Time 3 Reperfusion |
|
|---|---|---|---|
| ALT (U/l) | 51 (±18) | 56 (±12) | 66 (±19) |
| AP (U/l) | 114 (±23) | 128 (±35) | 130 (±12) |
| Bilirubin (μmol/l) | 4 (±1) | 5 (±1) | 5 (±1) |
| PT | 0.96 (±0.08) | 0.93 (±0.12) | 0.84 (±0.13) |
| TL (109/l) | 16.71 (±6.2) | 21.07 (±7.21) | 21.26 (±7.42) |
Liver parameters: Alanine aminotransferase (ALT), alkaline phosphates (AP), bilirubin, prothrombin time (PT), and total leukocytes (TL)
Discussion
In the present study, we have investigated the metabolic changes in the pig liver before, during, and after warm liver ischemia preceded by IPC. We demonstrated pronounced metabolic changes in glycerol, lactate, pyruvate, and glycerol levels determined by microdialysis and only minor changes in transaminase levels.
The main purpose of vascular occlusion is to decrease blood loss during liver resection. However, portal triad clamping by the PM not only produces ischemia but also leads to I/R injury when the blood flow to the liver is re-established. I/R injury can in some cases lead to either an uneventful postoperative course or postoperative death [15–17]. To avoid this situation, strategies for early detection of biochemical changes and potential minimization of the degree of I/R injury are important. We considered microdialysis to be an appropriate method to continuously obtain information about metabolic changes in the liver during ischemia before preconditioning. By monitoring glucose, lactate, pyruvate, and glycerol levels, we achieved an insight in molecules reflecting oxygen supply as well as cell membrane damage.
Glycerol is an integrate component of the cell membrane and, hence, an indicator of cell membrane disintegration [12, 18]. From the time of preconditioning, we observed a significant increase in glycerol levels before a decrease at the end of the ischemic period. This variation could be an expression of cellular disintegration because we observed similar variations in glycerol levels in a previous study on warm ischemia without preconditioning [14]. This is also in agreement with a study by Nowak et al. [12] on cold ischemia.
When total ischemia is performed and the oxygen supply is decreased, the organism changes to anaerobic metabolism and lactate is produced from pyruvate to maintain anaerobic glycolysis. In the aforementioned study [14], we observed a significant increase in lactate levels immediately after initiating ischemia. The same significant increase was observed in this study after preconditioning. In agreement with the change to anaerobic metabolism during ischemia, we had expected a decrease in pyruvate levels in the ischemic period. However, we did not see a decrease from the time of ischemia, but a steady state was observed during the ischemic period before a slight increase from time of reperfusion. This deviation could not be explained by collateral blood flow because all ligaments were transected to avoid this. Furthermore, the lactate–pyruvate ratio, which is a sensible marker for a low oxygen supply [19], increased significantly immediately after initiating ischemia as well as decreased rapidly after reperfusion.
The impaired blood flow to the liver and tissues is normally associated with a decreased delivery of glucose [20, 21]. However, we observed a significant increase in glucose levels from the start of ischemia. This increase could be explained by gluconeogenesis as part of anaerobic metabolism, but this is unlikely in the present study because we demonstrated an increase in lactate levels and a steady state in pyruvate levels at the same time. More likely, the increase in glucose levels results from the hepatic cells responding to activation from nucleotides as shown by Guinzberg et al. [22].
Transaminase levels are sensitive markers of hepatocyte injury [23]. In a study by Clavien et al. [24], IPC was found to confer protection to the liver as demonstrated by a decrease in AST and ALT levels 24 h after patients underwent surgery compared with patients who were not sustained to IPC. In our study, the hepatic injury was minimal as evaluated by changes in transaminase levels. Despite this, we demonstrated profound metabolic changes during and after warm ischemia preceded by IPC, changes that were similar to the ones we demonstrated in a previous study before, under, and after ischemia without IPC [14].
In conclusion, we have shown that microdialysis is a sensitive method for detecting rapid changes in metabolism in the liver during warm ischemia under the influence of ischemic preconditioning. Compared with a similar study without IPC, metabolism judged by glycerol, lactate, pyruvate, and glucose levels seems to be unaffected by IPC, at least when hepatic I/P injury is mild.
References
- 1.Jarnagin WR, Gonen M, Fong Y, Dematteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/00000658-200210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyrniotis V, Arkadopoulos N, Kehagias D, Kostopanagiotou G, Scondras C, Kotsis T, Tsantoulas D. Liver resection with repair of major hepatic veins. Am J Surg. 2002;183:58–61. doi: 10.1016/S0002-9610(01)00827-3. [DOI] [PubMed] [Google Scholar]
- 3.Hanazaki K, Monma T, Hiraguri M, Ohmoto Y, Kajikawa S, Matsushita A, Nimura Y, Koide N, Adachi W, Amano J. Cytokine response to human liver ischemia-reperfusion injury during hepatectomy: marker of injury or surgical stress? Hepatogastroenterology. 2001;48:188–192. [PubMed] [Google Scholar]
- 4.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 5.Lee WY, Lee SM. Ischemic preconditioning protects post-ischemic oxidative damage to mitochondria in rat liver. Shock. 2005;24:370–375. doi: 10.1097/01.shk.0000175895.33415.cd. [DOI] [PubMed] [Google Scholar]
- 6.Peralta C, Hotter G, Closa D, Gelpi E, Bulbena O, Rosello-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 7.Kerem M, Bedirli A, Ofluoglu E, Deniz K, Turkozkan N, Pasaoglu H, et al. Ischemic preconditioning improves liver regeneration by sustaining energy metabolism after partial hepatectomy under ischemia in rats. Liver Int. 2006;26:994–999. doi: 10.1111/j.1478-3231.2006.01330.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishii S, Abe T, Saito T, Tsuchiya T, Kanno H, Miyazawa M, et al. Effects of preconditioning on ischemia/reperfusion injury of hepatocytes determined by immediate early gene transcription. J Hepatobiliary Pancreat Surg. 2001;8:461–468. doi: 10.1007/s005340100010. [DOI] [PubMed] [Google Scholar]
- 9.Hawaleshka A, Jacobsohn E. Ischaemic preconditioning: mechanisms and potential clinical applications. Can J Anaesth. 1998;45:670–682. doi: 10.1007/BF03012100. [DOI] [PubMed] [Google Scholar]
- 10.Rojdmark J, Blomqvist L, Malm M, Ms-Ray B, Ungerstedt U. Metabolism in myocutaneous flaps studied by in situ microdialysis. Scand J Plast Reconstr Surg Hand Surg. 1998;32:27–34. doi: 10.1080/02844319850158921. [DOI] [PubMed] [Google Scholar]
- 11.Nowak G, Ungerstedt J, Wernerman J, Ungerstedt U, Ericzon BG. Metabolic changes in the liver graft monitored continuously with microdialysis during liver transplantation in a pig model. Liver Transpl. 2002;8:424–432. doi: 10.1053/jlts.2002.32943. [DOI] [PubMed] [Google Scholar]
- 12.Nowak G, Ungerstedt J, Wernerson A, Ungerstedt U, Ericzon BG. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J Hepatobiliary Pancreat Surg. 2003;10:200–205. doi: 10.1007/s00534-002-0760-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. doi: 10.1001/archsurg.1996.01430160100022. [DOI] [PubMed] [Google Scholar]
- 14.Kannerup AS, Funch-Jensen P, Gronbaek H, Jorgensen RL, Mortensen FV. Metabolic changes in the pig liver during warm ischemia and reperfusion measured by microdialysis. J Gastrointest Surg. 2008;12:319–326. doi: 10.1007/s11605-007-0359-9. [DOI] [PubMed] [Google Scholar]
- 15.Belghiti J, Di CI, Sauvanet A, Uribe M, Fekete F. A ten-year experience with hepatic resection in 338 patients: evolutions in indications and of operative mortality. Eur J Surg. 1994;160:277–282. [PubMed] [Google Scholar]
- 16.Elias D, Desruennes E, Lasser P. Prolonged intermittent clamping of the portal triad during hepatectomy. Br J Surg. 1991;78:42–44. doi: 10.1002/bjs.1800780115. [DOI] [PubMed] [Google Scholar]
- 17.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller M. Science, medicine, and the future: microdialysis. BMJ. 2002;324:588–591. doi: 10.1136/bmj.324.7337.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillered L, Persson L. Theory and practice of microdialysis—prospect for future clinical use. Acta Neurochir Suppl (Wien) 1999;75:3–6. doi: 10.1007/978-3-7091-6415-0_1. [DOI] [PubMed] [Google Scholar]
- 20.Krejci V, Hiltebrand L, Buchi C, Ali SZ, Contaldo C, Takala J, et al. Decreasing gut wall glucose as an early marker of impaired intestinal perfusion. Crit Care Med. 2006;34:2406–2414. doi: 10.1097/01.CCM.0000233855.34344.29. [DOI] [PubMed] [Google Scholar]
- 21.Hovda DA, Lee SM, Smith ML, Von SS, Bergsneider M, Kelly D, et al. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma. 1995;12:903–906. doi: 10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- 22.Guinzberg R, Cortes D, Az-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Pina E. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab. 2006;290:E940–E951. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- 23.Iu S, Harvey PR, Makowka L, Petrunka CN, Ilson RG, Strasberg SM. Markers of allograft viability in the rat. Relationship between transplantation viability and liver function in the isolated perfused liver. Transplantation. 1987;44:562–569. doi: 10.1097/00007890-198710000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]