Abstract
Rationale
Preclinical evidence suggests that non-cannabinoid neurotransmitter systems are involved in the behavioral and physiological effects of cannabinoids, but relatively little research has been conducted in humans.
Objectives
The aims of this study were to assess whether oral Δ9-tetrahydrocannabinol (Δ9- THC) would function as a discriminative stimulus in humans and to examine the substitution profile of drugs acting at opioid, GABA and dopamine systems.
Methods
Healthy subjects who reported moderate cannabis use were enrolled. Subjects learned to identify when they received oral 25 mg Δ9-THC or placebo under double-blind conditions. Once subjects acquired the discrimination (i.e., ≥ 80% drug-appropriate responding for four consecutive sessions), multiple doses of Δ9-THC, the GABAA positive modulator triazolam, the μ-opioid agonist hydromorphone and the dopamine reuptake inhibitor methylphenidate were tested to determine if they shared discriminative-stimulus effects with the training dose of Δ9-THC.
Results
Eight subjects (N=8) accurately discriminated Δ9-THC and completed the study. The training dose of Δ9-THC functioned as a discriminative-stimulus and produced prototypical subject-rated drug effects. All of the drugs tested produced significant effects on the self-report questionnaires, but only Δ9-THC substituted for the training dose.
Conclusion
These results suggest that the discriminative-stimulus effects of Δ9-THC in humans are not directly mediated through central neurotransmitter systems acted upon by the drugs tested in this study.
Keywords: drug-discrimination, subjective effects, Δ9-THC, triazolam, hydromorphone, methylphenidate, marijuana, cannabis
Introduction
The central effects of cannabis (Cannabis sativa or Cannabis indica) appear to be mediated primarily through cannabinoid (CB) receptors of the endogenous cannabinoid system. Two CB-specific receptors have been identified (Matsuda et al., 1990; Munro et al., 1993), and there is evidence of at least one additional subtype (Breivogel et al., 2001; Hajos et al., 2001). The CB1 receptor subtype is primarily located in the central nervous system (Pertwee, 1997). The CB2 receptor subtype was initially thought to be located exclusively in the periphery, particularly in the immune system (Kaminski et al., 1992), although recent data have demonstrated the functional expression of CB2 receptors in neurons and glial cells in the brain (Onaivi et al., 2006). Nonetheless, several lines of evidence indicate that the central effects of cannabis and cannabinoids can be attributed to their actions at CB1 receptors. The in vivo potencies of various cannabinoid ligands have been correlated with their binding affinities at CB1 receptors (Compton et al., 1993), the distribution of CB1 receptors in the CNS corresponds well with the effects of cannabis and cannabinoid ligands in animals and humans (Breivogel and Childers, 1998) and CB1-selective antagonists, such as SR 141716A block the effects of cannabinoids in animals (reviewed in Chaperon and Thiebot, 1999) and of cannabis in humans (Gorelick et al., 2006; Huestis et al., 2001, 2007). Many of the phytocannabinoids present in cannabis bind to CB1 receptors and are biologically active, however, Δ9-tetrahydrocannabinol (Δ9-THC) is widely held as the primary active constituent of cannabis. In support of this notion, there appear to be only subtle differences in the clinical effects of Δ9-THC and cannabis (Chait and Zacny, 1992; Hart et al., 2002; Wachtel et al., 2002).
Although the endocannabinoid system, especially the CB1 receptor subtype, appears responsible for mediating the behavioral and physiological effects of cannabis and cannabinoid ligands such as Δ9-THC, there is evidence that non-CB neurotransmitter systems are also involved. A principal function of cannabinoid receptors is the modulation of non-CB neurotransmitter release via retrograde signaling (Hashimotodani et al., 2007; Szabo and Schlicker, 2005), so it stands to reason that these other neurotransmitter systems might play a role in the effects of cannabinoids. A considerable amount of preclinical research has focused on the involvement of GABA and opioid systems, and to a lesser degree, dopamine systems, in the effects of cannabinoids. There is substantial overlap in the effects produced by cannabinoids and drugs acting at central GABA and opioid systems, and neuroanatomical, neurochemical and behavioral studies support a functional link between the endogenous cannabinoids and these systems. CB1 receptors are co-localized with GABA receptors throughout the brain, CB1 receptor activity modulates the release of GABA, both GABA and CB ligands have been shown to impair memory and motor control, alleviate anxiety, induce hypothermia, increase feeding behavior, function as reinforcers, partially share discriminative-stimulus effects, and there are GABA-CB interactions on these outcomes (e.g., Barrett et al., 1995; DeSousa et al, 1994; Ferraro et al., 2001; Freund, 2003; Freund et al., 2003; Frosini et al., 2004; Griffiths and Weerts, 1997; Justinova et al., 2005; Kirkham, 2005; Mailleux and Vanderhaeghen, 1992; Ohno et al., 1992; Pertwee et al., 1988, 1991; Pertwee and Greentree, 1988; Rahminiwati and Nishimura, 1999; Rawls et al., 2004; Romero et al., 1996; van den Pol, 2003; Varvel et al., 2005; Wiley et al., 1995; Wilson and Nicoll, 2001). Likewise, CB1 receptors are co-localized with opioid receptors throughout the brain, CB1 receptor activity modulates the release of endogenous opioids, and there are shared effects of, and interactions between, opioids and cannabinoids on hypothermia, hypotension, intestinal motility, motor control, analgesia, reinforcement and reward, drug discrimination, and self reported effects (reviewed in Fattore et al., 2004; Maldonado and Valverde, 2003; Manzanares et al., 1999; Viganò et al., 2005; see also Haney, 2007; Justinova et al., 2004; Mendizábal et al., 2006; Solinas and Goldberg, 2005). Fewer studies have examined interactions between dopamine and cannabinoid systems, but notably, like all other drugs of abuse, cannabinoids elevate dopamine in the mesocorticolimbic dopamine system, which is thought to play a necessary role in its abuse potential (Chen et al., 1990; Leshner and Koob, 1999). Together, these studies indicate that GABA, opioid and dopamine systems are involved, to some degree, in the effects of cannabinoids; however, relatively few data have been collected in humans, so further research would be informative.
The aims of this study were to determine whether oral Δ9-tetrahydrocannabinol (Δ9-THC) could function as a discriminative stimulus in humans and to examine the substitution profile of triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-THC in an attempt to examine the potential involvement of GABAA, μ-opioid and dopamine receptors in the interoceptive effects produced by Δ9-THC. Drug-discrimination was chosen as the primary outcome variable because the data from this procedure are concordant with the actions of a drug at the receptor level (Holtzman and Locke, 1988). Substitution drug-discrimination procedures were used because drugs from several pharmacological classes could be tested in a single study. An extensive review of clinical studies that used drug-discrimination and subject report measures to examine the neuropharmacology of a range of drug classes (Kelly et al., 2003) indicated that drug-discrimination data conformed more closely to the results from in vitro and in vivo preclinical studies.
Methods
Subjects
Healthy, adult men and women with a history of cannabis use were recruited from the local community to participate in this experiment. All potential subjects completed demographic, drug-use history, medical history and personality questionnaires, as well as medical screens. In addition, because the commercially available preparation of Δ9-THC (Marinol®) is suspended in sesame oil, subjects were screened for an allergy to sesame seeds and sesame oil. Individuals with current or past histories of Axis I psychiatric disorder, including substance dependence disorders (except nicotine), were excluded from participating. All subjects were in good health with no contraindications to the drugs to be administered in the protocol. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. All subjects provided sober, written informed consent and the confidentiality of their personal information was maintained throughout.
Fifteen healthy adult subjects were enrolled. Two subjects withdrew because they did not like the drug effects and four subjects withdrew for reasons unrelated to the experimental protocol. Another subject completed the study, but this subject’s discrimination performance on sessions in which the control conditions were administered during the test phase (see below) was not different from chance, suggesting that the subject was unable to accurately discriminate Δ9-THC. Data from these subjects were not included in the analyses. Eight subjects (4 Caucasian males, 1 Caucasian/Middle-Eastern male, 1 Black male, 2 Caucasian females) completed this experiment and maintained accurate discrimination performance throughout the experiment.
Subjects ranged in age from 20 to 29 years (median = 22 years) and in education from 12 to 20 years (median = 16). Subjects ranged in weight from 65 to 120 kg (median = 73 kg). All subjects reported moderate cannabis use (range of 1–5 times/week; mean = 2.5). Subjects reported consuming 1 to 24 standard alcohol-containing beverages per week (mean = 11.0). Four subjects reported occasional tobacco cigarette use. Other lifetime drug use included amphetamine, cocaine, benzodiazepines, hallucinogens and opioids. Opioid use was primarily reported as therapeutic, but all other drug use was recreational. The reported frequency of other drug use was low, typically not in the month prior to screening. Consistent with the self-reported drug use, urine samples for seven of the eight subjects were positive for Δ9-THC prior to any experimental drug administration. In addition, one subject tested positive for cocaine during the medical screening and the first practice session; all subsequent urine samples were negative for cocaine.
General Procedures
Subjects were enrolled as outpatients at the General Clinical Research Center (GCRC) at the University of Kentucky Medical Center. Subjects completed two practice sessions to become familiarized with the behavioral measures and daily laboratory routine. Experimental drugs were not administered during these sessions. Subjects then completed between 31 and 36 (mean = 33.3) experimental sessions. Subjects were paid $40 per session to participate in this experiment and received additional performance-based payment as outlined below.
Subjects were informed that during their participation they would receive placebo, Δ9-THC, methylphenidate, hydromorphone and triazolam, administered orally, but were blind to the dose and order of administration. They were told that the purpose of the study was to see how different drugs affect mood and behavior and whether people are able to detect the presence of a drug. Subjects were asked to abstain from illicit drug use other than cannabis for the duration of the experiment, and to avoid any over-the-counter medication without prior approval, with the exception of non-steroidal anti-inflammatory analgesics. In addition, subjects were also asked not ingest food or caffeine for 4 hours prior to each experimental session, or alcohol for 12 hours prior to and following each experimental session.
Experimental sessions were conducted daily Monday through Friday, and subjects participated in 1–5 sessions per week. Subjects arrived at the GCRC between 8:00 to 9:00 AM on the day of scheduled sessions. Individual subjects were tested separately in a standard hospital room. At the beginning of each session, subjects completed field-sobriety, breath (Alcolyzer, AK Solutions USA, Palisades Park, NJ) and urine tests to assess drug use (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) and possible pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK). Subjects then consumed a low-fat snack. All female urine samples were negative for hCG.
Drug-Discrimination Procedure
This experiment used well-established drug-discrimination procedures in which subjects learn to discriminate between a Drug condition (i.e., 25 mg Δ9-THC) and a Not Drug condition (i.e., placebo) (e.g., Babalonis et al., 2008; Griffiths et al., 1990; Heishman and Henningfield, 1991; Lile et al., 2004, 2005a,b, 2006, 2007; Stoops et al., 2005, 2006; Rush et al., 2000, 2003). We chose to use a Drug versus Not Drug discrimination because instructing subjects to discriminate these conditions have yielded results that are more consistent with the pharmacology of drugs compared to studies that instruct subjects to discriminate between Drug A and Drug B (e.g., active versus placebo) as the training conditions. (e.g., Preston and Bigelow, 2000; Preston et al., 1992).
The experiment was conducted in three phases, presented in fixed order.
Sampling Phase
During two sampling sessions, subjects ingested three capsules that contained a total of 25 mg Δ9-THC. Δ9-THC was identified by letter code (e.g., Drug X; a unique letter code used for each subject), but the subjects were not explicitly informed of the contents of the capsules. Subjects were told that the capsules they were receiving were Drug X, and that they should pay attention to the effects of Drug X because in future sessions they can earn money by correctly identifying whether or not they have received Drug X.
Control Phase
The control phase was conducted to determine whether subjects could discriminate 25 mg Δ9-THC from placebo. During this phase, subjects ingested capsules under double-blind conditions. The subjects were instructed that they must decide whether they have received Drug X or Not Drug X and that they can change their mind throughout the experimental session based on what they think at the time. The subjects were also told that if they think they have received Drug X they can earn money by responding that they have received Drug X or if they think they did not receive Drug X or if they received a drug that has effects different from Drug X, they can earn money by responding that they received Not Drug X. Finally, the subjects were instructed that at the end of some experimental sessions (i.e., control sessions), a computer screen would inform them as to whether Drug X or Not Drug X was administered and that during these sessions earnings will be determined based on correct responding on the drug-discrimination task (see below), but that during other sessions the drug condition would not be disclosed (i.e., test sessions, see below) and that earnings during these sessions will be based on the average of their earnings from control sessions. These instructions were also used during the test phase described below. The criterion for having acquired the discrimination was ≥ 80% correct responding on the drug-discrimination task during the final 5-h assessment for four consecutive sessions. The order of drug administration was random except that each subject received each training condition, 25 mg Δ9-THC and placebo, at least twice every four sessions. If a subject did not meet the control criteria within 12 sessions, they were dismissed from the study.
Test Phase
Drug doses tested during the test phase included Δ9-THC (5, 7.5, 15 and 25 mg) methylphenidate (5, 10, 20 and 30 mg), hydromorphone (0.75, 1.5, 3 and 4.5 mg), triazolam (0.0625, 0.125, 0.25 and 0.375 mg) and placebo. For comparison, dose ranges for the clinical application of these drugs, as listed in the Physician’s Desk Reference (2004), are provided. The acute recommended dose range of Δ9-THC as an anti-emetic in adults is 2.5–10 mg, and for appetite stimulation is 5–15 mg/m2 (a means of dosing based on surface area instead of body weight; approximately 9–27 mg in a 70 kg person). The acute recommended dose range of methylphenidate for the treatment of attention deficit and hyperactivity disorder in adults is 20–30 mg. The acute recommended dose range of hydromorphone for the alleviation of pain in adults is 2–4 mg. The acute recommended dose range of triazolam for the treatment of insomnia is 0.125–0.5 mg. Each drug dose was administered once. The order of drug administration was random during this phase except that an active drug dose was never administered on more than three consecutive sessions.
Control sessions (i.e., 25 mg Δ9-THC or placebo) were also included in the test phase to monitor drug-discrimination performance, and comprised approximately 32% of sessions during the test phase. If a subject responded incorrectly on a control session (i.e., < 80% correct at the final, 5-h assessment), additional control sessions were scheduled. These additional control sessions continued until the subject accurately identified both of the training conditions once each on consecutive sessions. Placebo and 25 mg Δ9-THC were also included as test conditions (i.e., no feedback regarding drug-discrimination performance).
Outcome measures
In addition to drug-discrimination, several other measures were collected, including self-report questionnaires, performance tasks, physiological assessments, and a measure of drug reinforcement to more fully characterize the degree to which the behavioral and physiological effects of Δ9-THC overlap with drugs acting at these various neurotransmitter systems. Only a subset of the self-report questionnaire results will be presented to demonstrate that the dose range of each drug was biologically active and produced interoceptive effects.
The drug-discrimination task was completed 3, 4 and 5 h after drug administration; this abbreviated data collection schedule was used for this task based on pilot data from two subjects (data not shown) indicating that the onset of the discriminative-stimulus effects of Δ9-THC did not emerge for 3–4 h after drug administration. Self-reported drug-effect data were collected immediately prior to drug administration, and 1, 2, 3, 4 and 5 h after drug administration.
Drug-Discrimination Task
A point-distribution drug-discrimination task (e.g., Lile et al., 2007) was used to assess the discriminative-stimulus effects of the various drug conditions. Two circles labeled Drug X and Not Drug X were displayed on the computer screen, each associated with a training dose condition. Counters were displayed directly below the circles. Mouse button presses increased the counter associated with the circle where the cursor was located according to a fixed-interval 1-sec schedule. The cursor could be moved between the circles without any consequence for the fixed-interval schedule (i.e., no change-over-delay). Up to 60 points could be allocated across the two options. During control sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.24/point. Thus, subjects were able to earn a maximum of approximately $40.00/session on this task. The dependent variable for this task was the percent responding on the Drug X circle (i.e., drug-appropriate responding) at the final 5-h time point.
Subject-Rated Drug-Effect Questionnaires
Visual Analog Scale (VAS). Subjects rated 20 items (e.g., I feel: a good drug effect, high, shaky or jittery) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”.
Profile of Mood States (POMS). This 72-item adjective rating scale yields scores on eight mood clusters (e.g., Fatigue). Subjects rate each item by selecting one of five response options: “Not at all,” “A little bit”, “Moderately”, “Quite a bit” and “Extremely.” This version of the POMS consisted of the original 65-item experimental version of the POMS [i.e., described and validated by McNair et al. (1971)] plus an additional seven items (e.g., Fischman et al., 1990).
Drug Administration
All drug conditions were administered in a double-blind fashion. During each experimental session, subjects ingested three capsules with water. Methylphenidate (Mallinckrodt, St. Louis, MO), hydromorphone (Ethex Pharmaceuticals, St. Louis, MO) and triazolam (Roxane Labs, Columbus, OH) were prepared by encapsulating commercially available generic tablets in an opaque green size 00 capsule. Δ9-THC capsules contained Marinol® (Solvay Pharmaceuticals, Marietta, GA) capsules, which consist of dronabinol (i.e., synthetic Δ9-THC) in sesame oil. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch. Capsules were prepared by the University of Kentucky Medical Center Investigational Drug Service Pharmacy.
Data Analyses
Drug-discrimination data for each drug were analyzed separately as the percentage of drug-appropriate responding at the final, 5-h time point using one-factor, repeated-measure analysis of variance (ANOVA; JMP, SAS Institute Inc., Cary, NC) with Dose (Placebo, Dose 1, Dose 2, Dose 3 and Dose 4) as the factor. For the 25 mg Δ9-THC and placebo conditions, data were averaged across the sessions in which these conditions were presented during the test phase. Raw data from the self-reported drug-effect questionnaires were analyzed for each drug as the peak-effect (i.e., the mean of the maximum value observed for each subject 1–5 hr after drug administration) using one-factor, repeated-measure ANOVA. For all measures, effects were considered significant for p ≤ 0.05. If the main effect of Dose attained statistical significance, planned comparisons using contrast statements were used to compare active drug doses to placebo.
Results
Drug-discrimination task
The 8 subjects met the discrimination criterion in an average of 5.1 sessions (range = 4–8). During the final four sessions of the control phase, subjects reported an average of 0.0 (SEM = 0.0) percent Δ9-THC-appropriate responding on the drug-discrimination task during placebo sessions and 100.0 (SEM = 0.0) percent drug-appropriate responding during sessions when the training dose of Δ9-THC (i.e., 25 mg) was administered.
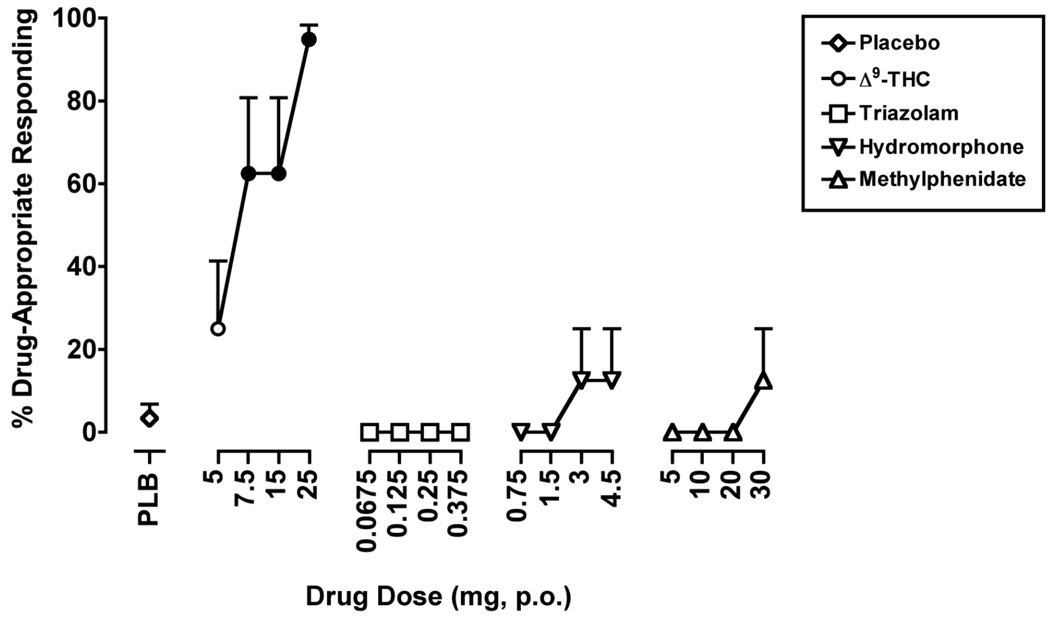
The one-factor, repeated-measure ANOVA that included placebo and the 4 active drug doses of Δ9-THC revealed a significant effect of Dose for percentage of Δ9-THC-appropriate responding (F4,28 = 8.3, p ≤ 0.001). When placebo and the training dose of Δ9-THC were administered during the test phase, they occasioned an average of 3.4 (SEM = 3.4) and 92.7 (SEM = 5.1) percent Δ9-THC-appropriate responding, respectively. The 7.5 and 15 mg doses of Δ9-THC each occasioned 62.5 (SEM = 18.3) percent Δ9-THC-appropriate responding, which, like the 25 mg dose of Δ9-THC, were significantly greater than placebo (Figure 1).
Figure 1.
The effects of Δ9-THC, triazolam, hydromorphone and methylphenidate on Δ9-THC-appropriate responding on the drug-discrimination task. Filled symbols indicate values that are significantly different from placebo. The x-axis represents the drug dose in mg; PLB denotes placebo. Data points show means of 8 subjects. Uni-directional brackets indicate 1 SEM.
The ANOVAs conducted on the point-distribution task data for the remaining drugs were not significant, indicating that no dose of any of the other drugs tested increased Δ9-THC-appropriate responding above placebo levels.
Self-Reported Drug-Effect Questionnaires
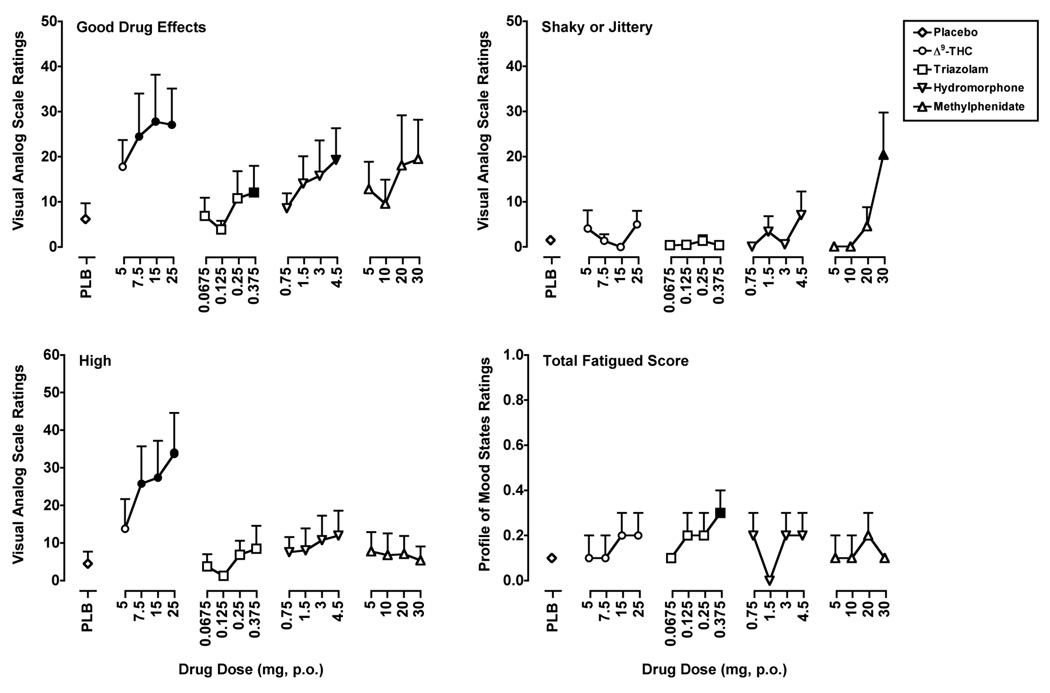
Results from selected items from the self-reported drug-effect questionnaires chosen to illustrate the varying profile of the subject-rated effects across drugs and to demonstrate that all of the drugs tested produced interoceptive effects are presented in Figure 2. Δ9-THC, triazolam, hydromorphone and methylphenidate significantly increased ratings on “positive” items from the self-reported drug-effect questionnaires. For example, the 7.5, 15 and 25 mg doses of Δ9-THC, the 0.375 mg dose of triazolam and the 4.5 mg dose of hydromorphone significantly increased peak ratings on the item Good Drug Effects (F’s4,28 = 3.1–3.3, p’s ≤ 0.05) from the VAS. Increased peak ratings on other questionnaire items were unique to a particular drug. For instance, only the 7.5, 15 and 25 mg doses of Δ9-THC only significantly increased ratings on the item High (F4,28 = 3.3, p ≤ 0.05) from the VAS. Triazolam alone increased ratings on the Total Fatigue scale from the POMS at the 0.375 mg dose (F4,28 = 2.6, p ≤ 0.05). Finally, only methylphenidate increased ratings of Shaky or Jittery from the VAS at the 30 mg dose (F4,28 = 3.6, p ≤ 0.01).
Figure 2.
Peak ratings for Δ9-THC, triazolam, hydromorphone and methylphenidate on the Visual Analog Scale items Good Drug Effects, Shaky/Jittery and High and on the Profile of Mood States Total Fatigue scale. All other details are as in Figure 1.
Discussion
One goal of the present study was to examine whether oral Δ9-THC would function as a discriminative-stimulus in humans. In one previous study, human subjects learned to discriminate smoked cannabis containing an active concentration of Δ9-THC (2.7%) from placebo cannabis (Chait et al., 1988). In that study, however, it was reported that some subjects based their discrimination responding, at least in part, on the taste or harshness of the cannabis cigarette. Oral Δ9-THC was used in the present study to eliminate the external cues and/or expectations associated with smoked cannabis. Although smoked cannabis is the route of administration typically used in the natural environment, the present results with oral Δ9-THC are directly applicable to smoked cannabis for at least two reasons. First, it is widely accepted that Δ9-THC is the primary active constituent of cannabis. Second, previous research that directly compared smoked cannabis with oral Δ9-THC demonstrated that although the time course of the drug effects differed, the drug effect profile on behavioral and physiological measures was similar (Chait and Zacny, 1992; Hart et al., 2002; Wachtel et al., 2002).
Δ9-THC functioned as a discriminative-stimulus and dose dependently increased drug-appropriate responding. Subjects learned to discriminate 25 mg Δ9-THC from placebo within 8 control sessions. In general, discriminative control of behavior was maintained throughout the test phase. Six of the eight subjects correctly identified placebo or 25 mg Δ9-THC every time a training condition was presented during the test phase. One subject inaccurately identified the active training condition (i.e., 25 mg Δ9-THC) during the test phase on two separate occasions. In both cases, each of the training conditions was administered once during the next two consecutive sessions, and the subject correctly identified the training conditions in these additional sessions. The other subject who inaccurately identified the training conditions during the test phase was unable to attend experimental sessions for approximately 2 weeks during this phase for personal reasons, and required five additional presentations of the training condition to re-acquire the discrimination upon return. When a 5-fold range of Δ9-THC doses were administered during the test phase, the lowest dose tested, 5 mg, engendered an average of 12.5% drug-appropriate responding, which consisted of 6 subjects reporting 0%, and 2 subjects reporting 100%, drug-appropriate responding. The same subjects also reported that the 7.5 and 15 mg doses of Δ9-THC shared discriminative-stimulus effects with the training dose. The 7.5 and 15 mg doses of Δ9-THC occasioned an average of 62.5% drug-appropriate responding, which was the result of 5 of 8 subjects allocating 100% of their responses to the drug-appropriate option and 3 of 8 allocating 0% of their responses to the drug-appropriate option. For 6 of the 8 subjects, the percent drug-appropriate responding was consistent across these two doses.
Because pilot data from two subjects (data not shown) indicated that the onset of the discriminative-stimulus effects of Δ9-THC did not emerge for 3–4 h after drug administration, the drug-discrimination task was only presented 3, 4 and 5 h after drug administration in an effort to minimize random responding or guessing during the early time points when subjects would be unable to discern the interoceptive cues of Δ9-THC. Those pilot data are consistent with the data shown here. When 25 mg Δ9-THC was administered, subjects occasionally reported 0–50% drug-appropriate responding at the 3-h presentation of the drug-discrimination task, but allocated responding to the drug-appropriate option at the 4- and 5-h assessments. Likewise, the self-reported drug effect and physiological data for Δ9-THC peaked at 3–4 h post-drug administration. It is possible that triazolam, hydromorphone or methylphenidate did not substitute for Δ9-THC because the peak effects for these other drugs typically occurred an hour earlier (i.e., 2–3 h; data not shown). However, despite observable self-reported effects for these drugs at the 3-h time point, less than 34% drug-appropriate responding was engendered by any of the doses of triazolam, hydromorphone or methylphenidate at the earlier time points. In addition, we have found in previous studies that once subjects decide that the drug they received could be characterized as Drug or Not Drug, they distribute 100% of their responses to the same option for the remainder of the session, even once the acute effects of the drug have dissipated (e.g., Lile et al., 2006).
The second goal of this study was to examine the potential involvement of GABAA, μ-opioid and dopamine receptors in the interoceptive effects produced by Δ9-THC. With the exception of Δ9-THC, none of the drugs tested shared discriminative-stimulus effects with the training dose of Δ9-THC, despite the fact that they all produced self-reported effects. In drug-discrimination studies in animals, partial substitution for the discriminative-stimulus effects of Δ9-THC was observed following administration of the GABAA positive modulators diazepam, phenobarbital and pentobarbital (Barrett et al., 1995; Browne and Weissman, 1981; Järbe and Hiltunen, 1988; Mokler et al., 1986; Wiley et al., 1995, Wiley and Martin, 1999). These data indicate that there is an overlap in the interoceptive effects produced by GABAA positive modulators and cannabinoids, particularly since drugs from other pharmacological classes generally fail to engender Δ9-THC-like discriminative-stimulus effects (Barrett et al., 1995; Browne and Weissman, 1981; Wiley et al., 1995). In the present study however, behaviorally active doses of triazolam did not occasion Δ9-THC-appropriate responding. Worth noting is that when multiple GABAA positive modulators were tested under the same experimental conditions, diazepam engendered more Δ9-THC-appropriate responding than chlordiazepoxide and midazolam (Barrett et al., 1995), suggesting that of the benzodiazepines tested, diazepam in particular produces an interoceptive cue similar to Δ9-THC. Worth noting is that Δ9-THC potentiated the discriminative-stimulus effects of pentobarbital when given in combination (Järbe and Ohlin, 1979; Järbe et al., 1975). Fewer research efforts have been aimed at evaluating μ-opioid agonists in animals trained to discriminate Δ9-THC, although the available data from substitution studies indicate that opioids engender only low levels of drug-appropriate responding (Browne and Weissman, 1981; Järbe et al., 1988,Järbe et al., 2006; McMahon, 2006; Wiley et al., 1995). For example, in rats trained to discriminate Δ9-THC, the μ-opioid agonists heroin or morphine did not engender drug-appropriate responding; however, when given in combination, these μ-opioid agonists shifted the Δ9-THC generalization curve leftward (Solinas et al., 2004; Solinas and Goldberg, 2005). In the initial study, it was also demonstrated that Δ9-THC elevated the endogenous μ-opioid agonist β-endorphin in the ventral tegmental area (VTA), and microinjections of β-endorphin into the VTA enhanced the discriminative-stimulus effects of non-discriminable doses of Δ9-THC, but had no effect when administered alone. Even fewer studies have tested dopaminergic drugs in animals discriminating Δ9-THC, but the available data have demonstrated that these drugs do not engender Δ9-THC-like discriminative-stimulus effects (Bueno et al., 1976; Järbe et al., 2006; McMahon, 2006). Taken together with the present findings, it appears that the discriminative-stimulus effects of Δ9-THC are not directly mediated by the GABAA, μ-opioid, or dopamine receptors acted upon by the drugs tested. However, it appears that ligands at the GABAA and μ-opioid receptor subtypes can modulate the discriminative-stimulus effects of Δ9-THC and that drug-discrimination procedures using drug combinations might be more sensitive to cannabinoid interactions with other neurotransmitter systems compared to substitution studies.
One of the limitations of the present study was a lack of a positive control. At the time this study was initiated, Δ9-THC was the only cannabinoid agonist approved by the US Food and Drug Administration. However, the cannabinoid agonist nabilone (Cesamet®) was recently approved for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional anti-emetic treatments and is now commercially available (Cesamet® product information, 2006). Preliminary data from an ongoing study indicate that nabilone shares discriminative-stimulus effects with Δ9-THC and would therefore be a useful positive control in future research (Lile et al., unpublished data). Worth noting is that interoceptive effects of nabilone were apparent at the 2–3 h following administration, demonstrating that a cannabinoid with a more rapid onset of action compared to Δ9-THC engendered drug-appropriate responding, which suggests that the lack of substitution by triazolam, hydromorphone and methylphenidate is not the result of the Δ9-THC discrimination being based on temporal cues.
The data generated by the present study established the procedures to investigate the discriminative-stimulus effects of Δ9-THC in human subjects, which should prove useful for future research. For example, data from drug-discrimination studies with Δ9-THC could provide important information about the neurobiological mechanisms underlying the effects of cannabinoids in humans. In addition to providing important basic science information, data from those studies could also reveal potential targets other than cannabinoid systems for medications development to manage cannabis-use disorders, and similarly, drug-discrimination studies with Δ9-THC could be used to screen potential medications. The results of this study also provide valuable groundwork for subsequent studies to examine the neuropharmacology of the effects of cannabinoids. Although the drug substitutions tested here did not reveal potential interactions with other neurotransmitter systems, the preclinical studies discussed above suggest that drug combination studies using these methods might be more sensitive, and support the use of these procedures for future research on the neuropharmacology of cannabinoids.
Acknowledgements
We are grateful to David Hudson, M.D., Rodrick Stuart, M.D. and Matthew Neltner, M.D. and the University of Kentucky General Clinical Research Center nursing staff for expert medical assistance. We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We also thank Drew Lally, Lisa Purdy, David Splichal for expert technical assistance. Finally, we thank Dr. Heather Bush for help with statistical analysis of the data.
These experiments comply with the current laws of the United States.
This research and the preparation of this manuscript were supported by grants from the National Institute on Drug Abuse (K01 DA018772) and the University of Kentucky Research Foundation awarded to Dr. Joshua A. Lile. Support was also provided by a Center for Biomedical Research Excellence (P20 RR015592) awarded to Dr. Thomas Curry and a General Clinical Research Center (M01 RR002602) awarded to Dr. Jay Perman by the National Center for Research Resources. The authors do not have any financial relationship with these funding sources.
Footnotes
There are no conflicts of interest with the sponsor.
References
- Babalonis S, Emurian CS, Martin CA, Lile JA, Kelly TH. Modulation of the discriminative-stimulus effects of triazolam across the menstrual cycle phase in healthy pre-menopausal women. Drug Alcohol Depend. 2008;94:276–280. doi: 10.1016/j.drugalcdep.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology. 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21:S227–S234. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Bueno OF, Carlini EA, Finkelfarb E, Suzuki JS. Delta 9-Tetrahydrocannabinol, ethanol and amphetamine as discriminative stimuli-generalization tests with other drugs. Psychopharmacologia. 1976;46:235–243. doi: 10.1007/BF00421108. [DOI] [PubMed] [Google Scholar]
- Cesamet Product Information. Valeant Pharmaceuticals International. 2006 [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology. 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, DeCosta BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- DeSousa NJ, Beninger RJ, Jhamandas K, Boegman RJ. Stimulation of GABAB receptors in the basal forebrain selectively impairs working memory of rats in the double Y-maze. Brain Res. 1994;641:29–38. doi: 10.1016/0006-8993(94)91811-2. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Spano MS, Deiana S, Fadda P, Scherma M, Fratta W. Cannabinoids and reward: interactions with the opioid system. Crit Rev Neurobiol. 2004;16:147–158. doi: 10.1615/critrevneurobiol.v16.i12.160. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Cassano T, Bebe BW, Siniscalchi A, O’Connor WT, Magee P, Tanganelli S, Cuomo V, Antonelli T. Cannabinoid receptor agonist WIN 55,212-2 inhibits rat cortical dialysate gamma-aminobutyric acid levels. J Neurosci Res. 2001;66:298–302. doi: 10.1002/jnr.1224. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration. J Pharmacol Exp Ther. 1990;253:160–170. [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trens Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Frosini M, Valoti M, Sgaragli G. Changes in rectal temperature and ECoG spectral power of sensorimotor cortex elicited in conscious rabbits by i.c.v. injection of GABA, GABA(A) and GABA(B) agonists and antagonists. Br J Pharmacol. 2004;141:152–162. doi: 10.1038/sj.bjp.0705593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals—implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Evans SM, Heishman SJ, Preston KL, Sannerud CA, Wolf B, Woodson PP. Low-dose caffeine discrimination in humans. J Pharmacol Exp Ther. 1990;252:970–978. [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J. 2006;151:754. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta 9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Discriminative-stimulus effects of d-amphetamine, methylphenidate, and diazepam in humans. Psychopharmacology. 1991;103:436–442. doi: 10.1007/BF02244241. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Locke KW. Neural mechanisms of drug stimuli: experimental approaches. Psychopharmacol Ser. 1988;4:138–153. [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology. 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Hiltunen AJ. Limited stimulus generalization between delta 9-THC and diazepam in pigeons and gerbils. Psychopharmacology. 1988;94:328–331. doi: 10.1007/BF00174684. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Johansson JO, Henriksson BG. Delta9-tetrahydrocannabinol and pentobarbital as discriminative cues in the Mongolian Gerbil (Meriones unguiculatus) Pharmacol Biochem Behav. 1975;3:403–410. doi: 10.1016/0091-3057(75)90048-9. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Ohlin GC. Discriminative effects of combinations of delta 9-tetrahydrocannabinol and pentobarbital in pigeons. Psychopharmacology. 1979;63:233–239. doi: 10.1007/BF00433555. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with Delta9-THC or (R)-methanandamide (AM-356) Psychopharmacology. 2006;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005;81:285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992;42:736–742. [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cogn Neurosci Rev. 2003;2:227–260. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Estradiol enhances the discriminative-stimulus and self-reported effects of d-amphetamine in healthy premenopausal women. Pharmacol Biochem Behav. 2007;87:289–296. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PEA, Rush CR. Discriminative-stimulus, self-reported, performance and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2006;142:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Hays LR, Rush CR. Tiagabine does not alter the discriminative-stimulus, reinforcing, subject-rated, performance or cardiovascular effects of oral cocaine in humans. Drug Alcohol Depend. 2004;76:81–91. doi: 10.1016/j.drugalcdep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel A, Glaser PEA, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus effects of d-amphetamine in humans. Neuropsychopharmacology. 2005a;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Wagner FP, Glaser PEA, Rush CR. Oxazepam does not modulate the behavioral effects of d-amphetamine in humans. Pharmacol Biochem Behavior. 2005b;82:270–279. doi: 10.1016/j.pbb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:6555–6568. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O. Participation of the opioid system in cannabinoid-induced antinociception and emotional-like responses. Eur Neuropsychopharmacol. 2003;13:401–410. doi: 10.1016/j.euroneuro.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernández-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. San Diego, CA: Educational and Industrial Testing Service; 1971. Profile of mood states [manual] [Google Scholar]
- Mendizábal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology. 2006;31:1957–1966. doi: 10.1038/sj.npp.1300957. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Nelson BD, Harris LS, Rosecrans JA. The role of benzodiazepine receptors in the discriminative stimulus properties of delta 9-tetrahydrocannabinol. Life Sci. 1986;38:1581–1589. doi: 10.1016/0024-3205(86)90497-2. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Wantanabe W. Intrahippocampal injections of benzozdiazepines and muscimol impair working memory but not reference memory of rats in the three-panel runway task. Eur J Pharmacol. 1992;219:245–251. doi: 10.1016/0014-2999(92)90302-k. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi AP, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Browne SE, Ross TM, Stretton CD. An investigation of the involvement of GABA in certain pharmacological effects of delta-9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1991;40:581–585. doi: 10.1016/0091-3057(91)90366-a. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Greentree SG. Delta-9-tetrahydrocannabinol-induced catalepsy in mice is enhanced by pretreatment with flurazepam or chlordiazepoxide. Neuropharmacology. 1988;27:485–491. doi: 10.1016/0028-3908(88)90130-x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Greentree SG, Swift PA. Drugs that stimulate or facilitate central GABAergic transmission interact synergistically with delta-9-tetrahydrocannabinol-induced to produce marked catalepsy in mice. Neuropharmacology. 1988;27:1265–1270. doi: 10.1016/0028-3908(88)90029-9. [DOI] [PubMed] [Google Scholar]
- Physician's Desk Reference. Oradell, NJ: Medical Economics Company, Inc; 2004. [Google Scholar]
- Preston KL, Bigelow GE. Effects of agonist-antagonist opioids in humans trained in a hydromorphone/not hydromorphone discrimination. J Pharmacol Exp Ther. 2000;295:114–124. [PubMed] [Google Scholar]
- Preston KL, Liebson IA, Bigelow GE. Discrimination of agonist-antagonist opioids in humans trained on a two choice saline-hydromorphone discrimination. J Pharmacol Exp Ther. 1992;261:62–71. [PubMed] [Google Scholar]
- Rahminiwati M, Nishimura M. Effects of delta 9-tetrahydrocannbinol and diazepam on feeding behevior in mice. J Vet Med Sci. 1999;61:351–355. doi: 10.1292/jvms.61.351. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Tallarida RJ, Kon DA, Geller EB, Adler MW. GABAA receptors modulate cannabinoid-evoked hypothermia. Pharmacol Biochem Behav. 2004;78:83–91. doi: 10.1016/j.pbb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman’s and Gilman’s: The Pharmacological Basis of Therapeutics. Ninth Edition. New York: McGraw-Hill; 1996. pp. 521–555. [Google Scholar]
- Romero J, García-Palomero E, Fernández-Ruiz JJ, Ramos JA. Involvement of GABA(B) receptors in the motor inhibition produced by agonists of brain cannabinoid receptors. Behav Pharmacol. 1996;7:299–302. doi: 10.1097/00008877-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW, Rowlett JK. Discriminative-stimulus effects of zolpidem, triazolam, pentobarbital and caffeine in zolpidem-trained humans. Exp Clin Psychopharmacology. 2000;8:22–36. doi: 10.1037//1064-1297.8.1.22. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3138–3192. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Involvement of mu-, delta-, and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology. 2005;179:804–812. doi: 10.1007/s00213-004-2118-x. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. A low dose of aripiprazole attenuates the subject-rated effects of d-amphetamine. Drug Alcohol Depend. 2006;84:206–209. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine and triazolam in humans. Experiment Clin Psychopharmacol. 2005;13:56–64. doi: 10.1037/1064-1297.13.1.56. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005;168:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Weighing the role of hypothalamic feeding of neurotransmitters. 2003;40:1059–1061. doi: 10.1016/s0896-6273(03)00809-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH. Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABAA antagonoist bicuculline. Psychopharmacology. 2005;178:317–327. doi: 10.1007/s00213-004-1988-2. [DOI] [PubMed] [Google Scholar]
- Viganò D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Effects of SR141716A on diazepam substitution for delta 9-tetrahydrocannabinol in rat drug discrimination. Pharmacol Biochem Behav. 1999;64:519–522. doi: 10.1016/s0091-3057(99)00130-6. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]