Abstract
Objective
To investigate systematically the role of systemic corticosteroid therapy in non-arteritic anterior ischemic optic neuropathy (NA-AION).
Methods
The study consists of a cohort of 613 consecutive patients (696 eyes), first seen in our clinic from 1973 to 2000. Of this cohort, 312 patients (364 eyes) voluntarily opted for systemic steroid therapy, and 301 (332 eyes) for no treatment. At first visit, all patients in both groups had a detailed ophthalmic and medical history, and comprehensive ophthalmic evaluation. Visual evaluation was done by recording Snellen visual acuity, and visual fields with a Goldmann perimeter. The same ophthalmic evaluation was performed at each follow-up visit. Patients in the steroid-treated group were initially given 80 mg Prednisone daily for 2 weeks, and then tapered down to 70 mg for 5 days, 60 mg for 5 days, and then cutting down by 5 mg every 5 days. Visual outcome in the two groups was compared
Results
Median follow-up was 3.8 years. At 6 months from onset of NA-AION, of the eyes with initial visual acuity 20/70 or worse and seen within 2 weeks of onset, there was visual acuity improvement in 69.8% (95% confidence interval (CI): 57.3%, 79.9%) in the treated group, compared to 40.5% (95% CI: 29.2%, 52.9%) in the untreated group (odds ratio of improvement: 3.39; 95% CI:1.62, 7.11; p=0.001). Comparison of visual field defect at 6 months from onset of NA-AION, among those seen within 2 weeks of NA-AION onset with moderate to severe initial visual field defect, there was improvement in 40.1% (95% CI: 33.1%, 47.5%) of the treated group, and 24.5% (95% CI: 17.7%, 32.9%) of the untreated group (odds ratio: 2.06, 95% CI: 1.24, 3.40; p= 0.005). In both treated and untreated groups, the visual acuity and visual fields kept improving up to about 6 months from onset of NA-AION, and very little thereafter.
Conclusion
This study suggested that NA-AION eyes treated during the acute phase with systemic corticosteroids resulted in a significantly higher probability of improvement in visual acuity (p=0.001) and visual field (p=0.005) than in the untreated group. Both visual acuity and visual fields improved up to 6 months after onset of NA-AION.
Keywords: Anterior ischemic optic neuropathy, Optic disc edema, Optic nerve, Optic nerve head ischemia, Visual loss
So far, no treatment for non-arteritic anterior ischemic optic neuropathy (NA-AION) has been shown to be effective in an adequate clinical trial in recovering visual loss, despite various claims. Miller and Smith [38] in 1966, who first described “ischemic optic neuropathy” in 11 patients, treated six of them with corticosteroids. They stated: “Steroids and anti-coagulants have been used in these patients but the final evaluation of their efficacy awaits further study”. Foulds [6] in 1969 treated 13 of 24 patients with NA-AION with systemic corticosteroids; on comparing the treated with the untreated patients, he reported significant visual improvement in 85% (11 of 13) of the treated cases, compared to 45% (five of 11) of the untreated patients. Hayreh [11], based on studies of 14 patients with NA-AION, seen from 1970 to 1972 at the Edinburgh Royal Infirmary, Scotland, reported visual acuity improvement in 75% of the eight patients with NA-AION and in 17% of the six untreated patients.
In 1973, we decided to conduct a systematic study on the role of corticosteroid therapy in NA-AION, at the Ocular Vascular Clinic of the University of Iowa Hospitals and Clinics, for the following reasons:
Studies on small numbers of patients by Foulds [6] and Hayreh [11] showed encouraging results of beneficial effect of systemic corticosteroid therapy in NA-AION.
Miller and Smith [38], who used corticosteroid therapy in 6 of their 11 patients, commented that “the final evaluation of their efficacy awaits further study”.
One of us (SSH) in discussions with a large number of ophthalmologists during professional meetings in North and South America, Europe and Asia found that many ophthalmologists were treating these patients with systemic corticosteroid therapy empirically, for lack of any other alternative treatment.
The whole subject of the role of corticosteroid therapy in NA-AION was and is highly controversial, with some having strong opinions about the lack of any rationale for corticosteroid therapy in NA-AION. We felt that a comprehensive study in a large cohort of patients with NA-AION was essential, to determine whether this therapy was beneficial or not.
Thus, the primary objective of the study was to evaluate in a large cohort of NA-AION patients the role of systemic corticosteroid therapy during its acute phase.
Patients and methods
We have investigated prospectively, using a cohort study design, various aspects of NA-AION systematically in the Ocular Vascular Clinic at the Tertiary Care University of Iowa Hospitals and Clinics since 1973. The prospective study on NA-AION funded by the National Institute of Health (RO1 grant), including this study to investigate the role of corticosteroid therapy on visual outcome were approved by the Institutional Review Board. In our study, we investigated the effect of systemic corticosteroid therapy, given during the acute phase of NA-AION (i.e. when optic disc edema is present), on visual outcome; it included patients who were first seen in our clinic from 1973 to 2000, and thereafter followed serially for many months or years. The study consists of a cohort of 613 consecutive patients, first seen in our clinic from 1973 to 2000, and who fulfilled our inclusion and exclusion criteria for this study. Of this cohort, 312 patients (364 eyes) voluntarily opted for systemic steroid therapy, and 301 (332 eyes) for no treatment. The data on visual outcome were compared between the steroid-treated and untreated groups.
Criteria required for diagnosis of NA-AION and inclusion in the present study
These included: (1) a history of sudden visual loss, usually discovered in the morning, and an absence of other ocular, systemic or neurological diseases that might influence or explain the patient’s visual symptoms, (2) optic disc edema (ODE) at onset must have been documented in the Ocular Vascular Clinic and must still be present for inclusion in this study, (3) spontaneous resolution of ODE was observed, (4) the eye had optic discrelated visual field defects, (5) there was no neurologic, systemic or ocular disorder, which could be responsible for ODE and visual impairment, (6) the patient must not have had any treatment for NA-AION prior to our evaluation, and (7) there must be a follow-up of 2 months at least to provide enough length of follow-up to obtain valid information in the treated and control untreated groups.
Exclusion criteria
Patients who had any retinal or optic nerve lesion or any other factor (e.g., cataract) which could have influenced the visual status, were excluded. NA-AION patients with only background diabetic retinopathy were included, but those who had active neovascularization, vitreous hemorrhages, traction detachment or other complications influencing the visual acuity or fields were excluded. Patients who had a diagnosis of glaucoma and visual field loss were excluded; however, those with history of elevated intraocular pressure and on ocular hypotensive therapy, with a documented normal field prior to the onset of NA-AION, were included. Eyes with unreliable visual fields were excluded. Patients with a follow-up of less than 2 months were excluded.
Studies performed
The intention was to document the visual outcome by recording best-corrected visual acuity using the Snellen visual acuity chart, and visual field defects on manual kinetic perimetry with a Goldmann perimeter. The data were collected prospectively and systematically. A detailed ophthalmic and medical history was obtained at the patient’s first visit to our clinic (by S.S.H.); in the medical history, we elicited from the patient a detailed history of all previous or current systemic diseases, particularly of arterial hypertension, diabetes mellitus, ischemic heart disease, strokes, transient ischemic attacks, carotid artery disease and hyperlipidemia (also by systemic evaluation - see below). A comprehensive ophthalmic evaluation was performed at that time (by SSH). This included: recording of best corrected visual acuity, visual fields with a Goldmann perimeter (using I-2e, I-4e and V-4e targets regularly), relative afferent pupillary defect, intraocular pressure, slit-lamp examination of the anterior segment, lens and vitreous, direct and indirect ophthalmoscopy, stereoscopic color fundus photography, and, in acute cases, stereoscopic fluorescein fundus angiography. When giant cell arteritis was suspected, based on systemic symptoms, elevated erythrocyte sedimentation rate and/or C-reactive protein or suspicion of arteritic AION, a temporal artery biopsy was performed to rule out giant cell arteritis [14, 22, 25]. At each follow-up visit, the same ophthalmic evaluation and stereoscopic color fundus photography were done, except that fluorescein fundus angiography was not performed. At the initial visit, a detailed systemic evaluation was performed by a cardiologist, internist or the patient’s local physician. Where indicated, other systemic or neurologic investigations were done to rule out any systemic or neurologic cause of visual loss.
Corticosteroid therapy
Initially, as a part of our National Institute of Health funded prospective studies on various aspects of NA-AION, we started to study the role of systemic corticosteroid therapy for NA-AION, based on previous encouraging reports by Foulds [6] and Hayreh [11]. Since the number of cases seen in our clinic alone was small, we planned a large multicenter randomized clinical trial in the early 1970s to have a large cohort in a short period; that clinical trial was not funded by the National Eye Institute, because of a firm belief (based on no scientific data) among the reviewers of the study that corticosteroid therapy had no role at all in NA-AION. Therefore, we decided to continue with our study, as part of our ongoing prospective NA-AION studies. In this study, for logistic and financial reasons, the treatment decision was based on a “patient choice” scheme, instead of the conventional randomization.
All patients with NA-AION seen in the Ocular Vascular Clinic at Iowa City were given the same information that was in the proposed multicenter randomized study protocol; however, the choice to have corticosteroid therapy or not was entirely voluntary. It was made abundantly clear to the patients that we had no definite information as to whether corticosteroid therapy would help to improve their vision or not. All their questions were answered in an unbiased way, and they were informed about the various possible side effects of corticosteroid therapy. Patients were encouraged to consult their local physician or ophthalmologist to get more information about various aspects of corticosteroid therapy and to help them decide, but all of us in the clinic carefully refrained from biasing their choice. No one in the clinic had any input into his or her choice. Throughout the study period, the authors had no information at all about the number of patients who opted for one or the other mode of management and the outcome. Thus, all possible safeguards were placed against any potential bias in choice by the patient.
The patients were treated, evaluated, and followed according to the original protocol in the proposed controlled randomized study, except that the follow-up schedule was varied slightly, tailored to the convenience of the individual patient and Iowa’s geography and severe winter conditions.
Corticosteroid therapy protocol
This was the same as in our preliminary study [11]. The patients who opted to have corticosteroid therapy were started on 80 mg Prednisone daily (irrespective of their weight). After 2 weeks, tapering down of therapy was started in steps of 5 days each: to 70 mg, 60 mg, and then cutting down by 5 mg every 5 days to 40 mg until the ODE was no longer present. After that, it was rapidly tapered off. Thus, most patients were on the treatment for approximately 2–3 months only. Throughout the duration of corticosteroid therapy, the patients were closely monitored for any side effects and compliance in our clinic. The patients were also strongly advised to be followed by their local physicians while on the therapy.
Initially we discussed in detail with the Endocrinology Department of our University of Iowa Hospitals and Clinics the risks of treating diabetic patients with corticosteroid therapy, because of its side effects; they gave us the go-ahead, so long as the patients were closely monitored by their internists for diabetes mellitus. Thus, when diabetics opted for the corticosteroid therapy, before starting the therapy we had a thorough discussion with their internists about monitoring the patient closely while on corticosteroid therapy, and the therapy was given only if the internists consented to do so (most did consent). If the internist did not feel comfortable with the treatment, we did not give corticosteroid therapy to that patient. Therefore, when diabetic patients decided to have corticosteroid therapy, they were managed jointly with their internists so long as they were on the therapy. Thus, contrary to the highly prevalent impression, we have had no problems treating diabetics with corticosteroid therapy with these precautions.
The steroid therapy regimen was not altered, whether there was worsening, improvement or no change of visual function during the course of the treatment, because the whole object of the study was to determine the visual outcome following a standard steroid therapy regimen when the eye still had optic disc edema. Any alteration of treatment regimen would have introduced confounding factors in the results, i.e. different treatment regimens, at different time intervals after the onset of NA-AION. Moreover, as the data analysis showed, the number of eyes that worsened was too small to have provided statistically reliable information.
Follow-up protocol for all patients
The patients were followed about every 2 weeks as long as they were taking up to 40 mg Prednisone daily, and at 3–4 weekly intervals after that until they finished the therapy. When necessary, they were seen more often, in addition to this protocol. After that, they were followed at 3 months, 6 months and then yearly after that—a rare patient has been followed for as long as 33 years in our clinic. Patients who opted not to be treated with steroid were initially followed every 3–4 weeks for the first 3 months or till ODE resolved, and after that they were followed at 3 months, 6 months and then yearly after that.
Visual status evaluation
Visual acuity
This was tested using the Snellen visual acuity chart and under identical testing conditions, almost invariably by the same person (SSH), encouraging the patient to look around and take his/her own time in responding, to ensure that the testing provided the most reliable information about the visual acuity. The following steps of visual acuity were checked: 20/15, 20/20, 20/25, 20/30, 20/40, 20/50, 20/60, 20/70, 20/80, 20/100, 20/200, 20/400, counting fingers (CF), hand motion (HM), perception of light (PL), and no perception of light (NPL).
Visual fields
Throughout this study, we used kinetic perimetry. Automated perimetry did not exist when we started the study in 1973; moreover, the changing face of automated perimetry would make such long-term studies difficult—in fact, automated perimetry is still evolving. Both types of perimetry have their advantages and disadvantages, which are discussed elsewhere [18]. Visual field plotting was attempted in all patients with a visual acuity of hand motion or better at all visits, with a Goldmann perimeter using I-2e, I-4e and V-4e targets always, although occasionally other targets (including I-1e or those in between I-4e and V-4e) were used if it was felt that that would provide additional information for evaluation of the visual status. The method of testing visual fields used by us in eyes with NA-AION is described in detail elsewhere [26].
Central visual field was also tested by using the Amsler grid chart, which sometimes provided more reliable information than the visual fields.
Steps taken to reduce potential bias in visual evaluation
This was considered an extremely important issue to have reliable and unbiased information. Therefore, we took the following steps.
For visual acuity
This was achieved by mixing these patients with all other patients seen in the Ocular Vascular Clinic - many of them were in several other long-term ocular vascular studies being conducted in the Ocular Vascular Clinic simultaneously. Therefore, when evaluating the visual acuity, the tester was not aware of the diagnosis or treatment in any of the patients, in this or any other studies. Thus, every precaution was taken to avoid possible bias.
For visual fields
This was achieved by taking the following two steps:
While recording the visual fields: Perimetry for our entire department of Ophthalmology is centralized. During visual field recording, these patients were again mixed not only with all the patients seen in our clinic but also with those seen in other clinics in our department. Therefore, the perimetrists were totally unaware of the diagnosis or treatment.
While evaluating the visual field loss: Visual field evaluation for all NA-AION eyes in the entire cohort (i.e. the steroid therapy group as well as the untreated control group) was done jointly by mixing the two groups. At a time, the visual fields of each patient were arranged in chronological order by the research assistant. Then the visual fields were graded jointly and simultaneously, by the method described below, by three graders (SSH and two neuro-ophthalmologists who had never seen the patients). [Incidentally, SSH, being a dyslexic, cannot remember the names or faces of persons he meets; thus, all his life he has had socially the most embarrassing disability! However, that disability helped prevent any bias in this study for testing visual status.]. Thus, at the time of grading, none of the graders knew whether the patient was on treatment or not, and no attention was paid to the names. The visual fields were graded randomly, irrespective of whether the patient was on treatment or not, and without any knowledge of the identity of the patient. The method of visual field evaluation is described in detail below.
Evaluations of visual acuity, visual field defects and optic disc edema
Each was evaluated separately in a masked fashion, i.e. changes in visual acuity, visual fields and ODE were evaluated independently of each other, so that the severity of one did not influence evaluation of the other. In addition, in eyes that developed recurrence of NA-AION, only the data on visual evaluation collected up to the last follow-up visit of the first episode were used, i.e. before the onset of recurrence.
Optic disc evaluation
According to our follow-up protocol, the patients were followed about every 2 weeks as long as they were taking up to 40 mg Prednisone daily, and at 3–4 weekly intervals after that until they finished the therapy. Therefore, we recorded the date when ODE was last seen and the date when it had completely resolved. For visual assessment when ODE had resolved, visual acuity and visual field were evaluated on the visit when ODE had just resolved completely - this date would be within 2–4 weeks of the actual resolution of ODE.
Visual acuity evaluation
A change of at least three lines in the Snellen visual acuity chart was considered a significant change, which is equivalent to a logMAR (logarithm of the minimum angle of resolution) change of at least 0.30. We divided visual acuity into two categories for evaluation purposes:
Normal visual acuity was defined as 20/30 or better, because that category cannot show an improvement of three lines to achieve 20/20.
For data analysis, we chose to use those with 20/70 or worse visual acuity for determining improvement or deterioration for the following reasons: (a) poor visual acuity was defined as 20/70 or worse because patients with 20/70 or worse visual acuity are much more disabled visually compared to those with 20/60 or better, (b) also, we wanted to compare our data on visual outcome in the untreated cohort (representing the natural history) with the corticosteroid-treated cohort, with the “IONDT” (Ischemic Optic Neuropathy Decompression Trial) study [40, 31] (considered as the “gold standard” by most neuro-ophthalmologists) which used 20/64 as their inclusion criterion, and (c) moreover, like the IONDT study, corticosteroid therapy is an interventional study, and on a risk/benefit ratio in an interventional study with possible likelihood of some side effects, treatment of patients with 20/70 or worse visual acuity is justified, whereas with 20/60 or better visual acuity it may or may not be considered desirable. However, in the present study, it would have been unscientific and misleading not to include patients with visual acuity of better than 20/70 to determine the effect of corticosteroid therapy on visual outcome in those eyes, particularly to find out if corticosteroid therapy had any deleterious effect on the visual outcome, since eyes with good visual acuity cannot get much better. The data were originally collected irrespective of the level of visual acuity at initial visit.
Visual field evaluation
We wanted to evaluate quantitative and qualitative changes in visual fields plotted with the Goldmann perimeter during follow-up period. We tried three different strategies to find out which one of those would provide reliably the extent of visual loss, the amount of visual functional disability caused by the visual field loss, and the change during follow-up. The strategies were: (i) ranking the visual fields in their order from best to worst, (ii) the “counting dots” method used for visual field scoring originally described by Esterman [5], and (iii) an overall subjective grading of the visual fields; this was done because, unlike automated perimetry, it is not possible to have a quantitative measurement of visual field loss with kinetic perimetry. We found that the last method gave the best information, so we used it in this study. This method has proved reliable in our previous studies. [17, 20, 24, 30]
Overall subjective grading of the visual fields
All the visual fields plotted during the entire follow-up period were laid out in chronological order, and three clinicians experienced in the interpretation of visual fields done with a Goldmann perimeter (SSH and two neuro-ophthalmologists who had never seen the patients) simultaneously scrutinized them, and independently subjectively graded the severity of visual loss, taking into consideration all the parameters one considers while clinically evaluating a change in visual fields plotted with manual kinetic perimetry (because of the complexity of the Goldmann visual field defects, it is unfortunately difficult to define the exact parameters). Two types of evaluation of visual fields were performed using this method: (i) the entire visual field, and (ii) central and peripheral fields evaluated separately, to determine whether each one improved, deteriorated, or remained stable. In general, deterioration was defined as development of a new scotoma, a deepening or expanding scotoma, a generalized constriction not accounted for by any other ocular parameter, or overall deterioration. Improvement was the reverse of the above. Subtle changes were confirmed on more than one examination.
The entire visual field was graded into four levels - from zero (normal) to four (severe loss) in steps of 0.5 (and occasionally 0.25 when the differences were subtle), and the dates when each change was noted during the entire follow-up. The grade was judged by qualitatively assessing clinical computation of the amount of visual field loss, factoring in the functional disability produced by that defect; for example, inferior and/or central visual field defect, producing far more functional disability, was assigned a much higher grade than a corresponding loss in the upper field or elsewhere. The grading was started from the first visual field. A change from one grade to another was noted, and the date it occurred. Then the three graders compared their grades immediately, and if there was a disagreement, this was resolved by discussion at that time to reach a unanimous agreement. The findings were then condensed for descriptive purposes into minimal (grade 0.5), mild (grades >0.5–1.0), moderate (1.5 to 2.0), marked (2.5 to 3.0) and severe (3.5 to 4.0) loss. Examples of the various grades of visual fields are given elsewhere [17].
Statistical methods
Descriptive statistics (means, standard deviation, and percentages) were computed for the demographic, clinical variables, visual acuity and visual field defect at initial visit. Changes in visual acuity and visual field defect were assessed from initial visit to ODE resolution, from ODE resolution to 3 months, 6 months, and 9 months after resolution of ODE, and for the overall follow-up at 3 months, 6 months, and 1 year from initial visit. Since patient visits did not exactly fall at the specified time period for various logistic, seasonal or geographic reasons, a±6 week interval was used for the 3, 6, and 9 month follow-up and ±12 weeks for the 1 year follow-up. A logMAR difference in visual acuity of at least 0.30, in either direction, was considered a significant change (i.e. improved or deteriorated). At these same intervals, change in visual field loss was also examined, with a difference in grade of at least 0.5, in either direction being defined as improvement or deterioration. The percentages of improved and worse visual outcomes were calculated at each of these intervals. These were reported separately for those first seen and treated with corticosteroid within 2 weeks of onset of visual loss and those with corticosteroid therapy started longer than 2 weeks after visual loss. The patients who chose to have corticosteroid therapy were compared with our cohort of patients (301 patients, 332 eyes) in our natural history study of visual outcome in NA-AION [28] that did not opt for corticosteroid therapy with respect to their gender, systemic comorbidities, and smoking status using the Pearson Chi-square test, age at initial visit, using two- sample t-test, and their initial visual acuity and visual field defect using the Wilcoxon rank-sum test. The improvement in visual acuity (or visual field) at ODE resolution, and at 3 months, 6 months, and 1 year from onset of NA-AION were also compared between these two groups of patients that had an initial visual acuity of 20/70 or worse (or moderate to severe initial visual field defect) and were first seen (or treated) within 2 weeks of NA-AION onset. This was done using repeated measures logistic regression analysis fitted by the generalized estimating equations (GEE) method to account for the correlation of visual outcomes from the same eye over time, as well as between eyes from the same patient. The effect of comorbidities [such as arterial hypertension, ischemic heart disease, diabetes mellitus, transient ischemic attack (TIA)/cerebro-vascular accident (CVA), peripheral vascular disease] and smoking on improvement in visual outcome was also examined by including these factors as independent variables in the logistic model. Estimates (with 95% CI) of the probability of improvement in visual acuity (or visual field) and the odds ratio (with 95% CI) for improvement with corticosteroid relative to no corticosteroid were obtained from the fitted logistic regression model. The same analysis was performed for comparing worsening of visual outcome.
Results
The demographic characteristics of the 312 corticosteroid steroid-treated patients (364 eyes) in the study are summarized in Table 1. These are shown for all patients and for patients who started corticosteroid therapy within 2 weeks of NA-AION onset and those that started corticosteroid treatment more than 2 weeks after onset. In this study, almost all patients were Caucasian, which is in keeping with the overall population in this part of the country. At the initial visit, 53% of eyes that were started on corticosteroid within 2 weeks after onset of symptoms had visual acuity of 20/30 or better (Table 2). Visual acuity of 20/200–20/400 was present in 5%, and counting fingers or worse in 13% of eyes. Twenty-nine percent had minimal to mild visual field defect and 47% had marked to severe visual field defect.
Table 1.
Demographic and Clinical characteristics of NA-AION patients that meet inclusion criteria
| Demographic/ clinical variable |
With steroid therapy |
No steroid therapy: n=301 patients, 332 eyes |
With vs without steroid p value |
||
|---|---|---|---|---|---|
| All steroid-treated: n=312* patients, 364 eyes |
By start of steroid therapy |
||||
| ≤2 weeks of onset: n=236* patients, 263 eyes |
>2 weeks of onset: n=94* patients, 101 eyes |
||||
| Gender (male) | 188 (60%) | 129 (59%) | 59 (63%) | 175 (58%) | 0.594 |
| Age (mean±SD) | 59.2±12.6 | 59.2±12.6 | 59.3±12.0 | 62.0 ± 12.2 | 0.006 |
| Involved eye | |||||
| Right eye | 122 (39%) | 98 (42%) | 42 (45%) | 141 (47%) | |
| Left eye | 138 (44%) | 111 (47%) | 45 (48%) | 129 (43%) | |
| Both eyes | 52* (17%) | 27* (11%) | 7* (7%) | 31 (10%) | |
| Duration of steroid therapy | |||||
| Median (25th-75th percentile) |
9.3 (7.1–11.7) | 9.4 (7.3–11.7) | 9.1 (7.0–11.7) | — | |
| Follow-up (of eyes) | |||||
| Median | 3.8 years | 4.3 years | 3.0 years | 3.4 years | |
| (25th-75th percentile) | (1.4–9.1) | (1.7–9.2) | (1.3–9.0) | (1.3–7.4) | |
| Minimum—maximum | 2 mos–31 yrs | 2 mos–26 yrs | 2 mos–31 yrs | 2 mos–25 yrs | |
| Systemic conditions | |||||
| Hypertension | 107 (34%) | 72 (33%) | 35 (37%) | 128 (43%) | 0.036 |
| Ischemic heart disease | 57 (18%) | 36 (17%) | 21 (22%) | 66 (22%) | 0.258 |
| Diabetes mellitus | 83 (27%) | 47 (22%) | 36 (38%) | 97 (32%) | 0.126 |
| TIA/CVA | 18 (6%) | 13 (6%) | 5 (5%) | 28 (9%) | 0.097 |
| Peripheral vascular disease |
15 (5%) | 7 (3%) | 8 (9%) | 15 (5%) | 0.920 |
| Elevated cholesterol (>200) |
143 (of n=208) (69%) | 106 (of n=150) (71%) | 37 (of n=58) (64%) | 137 (of n=198) (69%) | 0.923 |
| Smoked current/past | (n=303 patients) 142 (47%) |
(n=213 patients) 98 (46%) |
(n=90 patients) 44 (49%) |
(n=296 patients) 145 (49%) |
0.603 |
| Diabetic retinopathy | 33 (11%) | 18 (8%) | 15 (16%) | 29 (10%) | 0.699 |
| Initial IOP (mean±SD) | (n=357 eyes) 16.2±3.5 |
(n=260 eyes) 16.2±3.4 |
(n=101 eyes) 16.2±3.8 |
(n=326 eyes) 16.3±4.6 |
0.699 |
There were 18 steroid-treated patients with both eyes involved with one eye treated ≤2 weeks of onset and the other eye treated > 2 weeks after onset
CVA = Cerebrovascular disorder; NA-AION = Non-arteritic anterior ischemic optic neuropathy; IOP = Intraocular pressure; SD = Standard deviation; TIA = Transient ischemic attack
Table 2.
Visual acuity and visual field at initial visit
| Steroid therapy started ≤2 weeks of onset |
Steroid therapy started >2 weeks of onset |
All eyes | |
|---|---|---|---|
| Visual acuity | (n=263 eyes) | (n=101 eyes) | (n=364 eyes) |
| 20/15–20/20 | 91 (35%) | 27 (27%) | 118 (32%) |
| 20–25–20/30 | 48 (18%) | 20 (20%) | 68 (19%) |
| 20/40–20/60 | 53 (20%) | 27 (27%) | 80 (22%) |
| 20/70–20/100 | 26 (10%) | 8 (8%) | 34 (9%) |
| 20/200–20/400 | 12 (5%) | 4 (4%) | 16 (4%) |
| Counting fingers or worse | 33 (13%) | 15 (15%) | 48 (13%) |
| Visual field defect | (n=262 eyes)# | (n=101 eyes) | (n=363 eyes) # |
| Minimal | 11 (4%) | 7 (7%) | 18 (5%) |
| Mild | 66 (25%) | 23 (23%) | 89 (25%) |
| Moderate | 62 (24%) | 21 (21%) | 83 (23%) |
| Marked | 86 (33%) | 35 (35%) | 121 (33%) |
| Severe | 37 (14%) | 15 (15%) | 52 (14%) |
Missing visual field defect data in one eye No significant difference in visual acuity (p=0.45) and visual field defect (p=0.96) between those that started steroid within 2 weeks of onset and those that started greater than 2 weeks of onset
Assessment of change in visual acuity
This was divided into three phases: (i) from initial visit to ODE resolution, (ii) from the time when ODE resolved to 3 months, 6 months, and 9 months after resolution, and (iii) overall change at 3 months, 6 months, and 1 year from the initial visit. Changes in visual acuity are shown in Tables 3, 4, and 5.
Table 3.
Visual acuity change from initial visit to optic disc edema (ODE) resolution
| Visual acuity at initial visit | Steroid started ≥2 weeks from onset (n=261 eyes) |
Steroid started >2 weeks from onset (n=101 eyes) |
||||
|---|---|---|---|---|---|---|
| n | Number (%) of eyes |
n | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
| 20/15–20/30 | 140 | — | 21 (15%) | 47 | — | 3 (6%) |
| 20/40 | 22 | 1 (5%) | 3 (14%) | 11 | 0 (0%) | 1 (9%) |
| 20/50–20/60 | 29 | 6 (21%) | 0 (0%) | 15 | 1 (7%) | 0 (0%) |
| 20/70–20/100 | 26 | 9 (35%) | 1 (4%) | 8 | 2 (25%) | 0 (0%) |
| 20/200–20/400 | 11 | 7 (64%) | 0 (0%) | 4 | 1 (25%) | 0 (0%) |
| Counting fingers or worse | 33 | 15 (45%) | 0 (0%) | 16 | 5 (31%) | 0 (0%) |
| VA 20/70 or worse | 70 | 31 (44%) | 1 (1%) | 28 | 8 (29%) | 0 (0%) |
Table 4.
Visual acuity change from optic disc edema (ODE) resolution at 3 months, 6 months, and 9 months after ODE resolution
| Visual acuity at ODE resolution | 3 months* after ODE resolution (n=344** eyes) |
6 months* after ODE resolution (n=316** eyes) |
9 months* after ODE resolution (n=304** eyes) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n** | Number (%) of eyes |
n** | Number (%) of eyes |
n** | Number (%) of eyes |
||||
| Improved | Worsened | Improved | Worsened | Improved | Worsened | ||||
| 20/15–20/30 | 182 | — | 10 (5%) | 165 | — | 10 (6%) | 161 | — | 12 (7%) |
| 20/40 | 31 | 1 (3%) | 0 (0%) | 27 | 3 (11%) | 3 (11%) | 27 | 3 (11%) | 3 (11%) |
| 20/50–20/60 | 48 | 4 (8%) | 2 (4%) | 46 | 8 (17%) | 3 (7%) | 43 | 8 (19%) | 3 (7%) |
| 20/70–20/100 | 28 | 3 (11%) | 0 (0%) | 26 | 7 (27%) | 0 (0%) | 25 | 9 (36%) | 0 (0%) |
| 20/200–20/400 | 24 | 5 (21%) | 0 (0%) | 24 | 14 (58%) | 0 (0%) | 24 | 15 (62%) | 0 (0%) |
| Counting fingers or worse | 31 | 12 (39%) | 0 (0%) | 28 | 16 (57%) | 1 (4%) | 24 | 13 (54%) | 1 (4%) |
| VA 20/70 or worse | 83 | 20 (24%) | 0 (0%) | 78 | 37 (47%) | 1 (1%) | 73 | 37 (51%) | 1 (1%) |
±6 weeks for 3 months and 9 months.
n includes the eyes that have a post-ODE resolution follow-up for VA of at least the lower limit specified
ODE = Optic disc edema; VA = Visual acuity
Table 5.
Visual acuity change from visual acuity at initial visit to 3 months, 6 months, and 1 year from first visit
| Time from first visit /Initial Visual Acuity | Steroid started ≤2 weeks from onset (n=261 eyes) |
Steroid started >2 weeks from onset (n=101 eyes) |
||||
|---|---|---|---|---|---|---|
| n** | Number (%) of eyes |
n** | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
| 3 months* | (n=261) | (n=101) | ||||
| 20/15–20/30 | 140 | — | 20 (14%) | 47 | — | 4 (9%) |
| 20/40 | 22 | 1 (5%) | 2 (9%) | 11 | 0 (0%) | 0 (0%) |
| 20/50–20/60 | 29 | 5 (17%) | 0 (0%) | 15 | 0 (0%) | 0 (0%) |
| 20/70–20/100 | 26 | 9 (35%) | 1 (4%) | 8 | 2 (25%) | 0 (0%) |
| 20/200–20/400 | 11 | 7 (64%) | 0 (0%) | 4 | 0 (0%) | 0 (0%) |
| Counting fingers or worse | 33 | 17 (52%) | 0 (0%) | 16 | 4 (25%) | 0 (0%) |
| 20/70 or worse | 70 | 33 (47%) | 1 (1%) | 28 | 6 (21%) | 0 (0%) |
| 6 months* | (n=244) | (n=94) | ||||
| 20/15–20/30 | 129 | — | 21 (16%) | 43 | — | 6 (14%) |
| 20/40 | 19 | 3 (16%) | 2 (11%) | 11 | 2 (18%) | 0 (0%) |
| 20/50–20/60 | 29 | 10 (34%) | 1 (3%) | 14 | 4 (29%) | 1 (7%) |
| 20/70–20/100 | 25 | 12 (48%) | 1 (4%) | 7 | 4 (57%) | 1 (14%) |
| 20/200–20/400 | 11 | 11 (100%) | 0 (0%) | 4 | 1 (25%) | 0 (0%) |
| Counting fingers or worse | 31 | 23 (74%) | 1 (3%) | 14 | 10 (71%) | 0 (0%) |
| 20/70 or worse | 67 | 46 (69%) | 2 (3%) | 25 | 15 (60%) | 1 (4%) |
| 1 year* | (n=222) | (n=83) | ||||
| 20/15–20/30 | 120 | — | 22 (18%) | 40 | — | 6 (15%) |
| 20/40 | 15 | 3 (20%) | 2 (13%) | 9 | 3 (30%) | 0 (0%) |
| 20/50–20/60 | 26 | 10 (38%) | 1 (4%) | 13 | 5 (38%) | 1 (8%) |
| 20/70–20/100 | 22 | 12 (55%) | 1 (5%) | 6 | 4 (67%) | 1 (17%) |
| 20/200–20/400 | 11 | 10 (91%) | 1 (9%) | 4 | 2 (50%) | 0 (0%) |
| Counting fingers or worse | 28 | 20 (71%) | 0 (0%) | 11 | 8 (73%) | 0 (0%) |
| 20/70 or worse | 61 | 42 (69%) | 2 (3%) | 21 | 14 (67%) | 1 (5%) |
±6 weeks for 3 and 6 months; ±12 weeks for 1 year
n includes the eyes that have a follow-up for visual acuity of at least the lower limit specified
From initial visit to ODE resolution: Of the eyes that started corticosteroid therapy within 2 weeks of onset of symptoms and had initial visual acuity of 20/70 or worse, 44% showed improvement from first visit to the time when ODE resolved, while 1% got worse (Table 3); however, in those with initial visual acuity of 20/60 or better, 13% (24 of 190 eyes) got worse. For patients who started corticosteroid therapy more than 2 weeks after onset of visual loss, change in visual status could have occurred at varying lengths of time before they were first seen or treated with corticosteroid in our clinic, and improvement and/or deterioration may have already occurred. That would have an effect on the percentage of deterioration or improvement that was observed for this group, which was smaller for both improvement and deterioration than those first started within 2 weeks of onset.
From the time when ODE resolved to 3 months, 9 months, and 1 year after the resolution: Improvement in visual acuity in the eyes with 20/70 or worse at the time of ODE resolution was observed in 24% at 3 months, 49% at 6 months, and 52% at 9 months after ODE had resolved (Table 4). Of the eyes with 20/60 or better visual acuity at ODE resolution, worsening visual acuity at 9 months after ODE resolution was seen in 8% (18 of 231), and in 1% of the eyes with visual acuity of 20/70 or worse at ODE resolution.
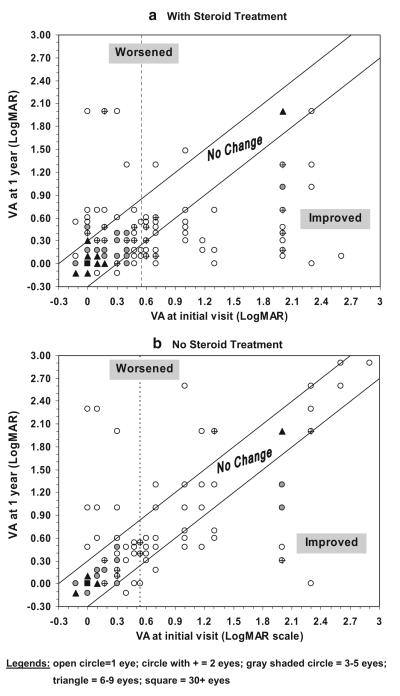
Overall change at 3 months, 6 months, and 1 year after the initial visit: In those that had corticosteroid therapy within 2 weeks of onset with visual acuity 20/70 or worse, there was an improvement in visual acuity in 47% at 3 months, 70% at 6 months, and 70% at 1 year after the initial visit (Table 5). Worsening of visual acuity at 1 year after the initial visit was seen in 16% (25 of 161) of those with initial visual acuity of 20/60 or better and in 3% of those with initial visual acuity of 20/70 or worse. The distribution of visual acuity change at 1 year in all eyes of patients treated with corticosteroid therapy is shown in Fig. 1a.
Fig. 1.
Of the eyes that were first seen within 2 weeks of onset of NA-AION, plot of visual acuity at 1 year versus at initial visit: steroid-treated (a) (top) and natural history (b) (bottom) groups. Vertical dotted line in both figures is at the level of visual acuity of 20/70; data to the left of that line represents visual acuity of better than 20/70, and to the right of that for worse than 20/70. The points to the right of the vertical line and in the improved region represent eyes that improved at 1 year: in the steroid group 69% (42/61—Table 5) and in the natural history group 42% (23/55)
Assessment of change in visual fields
Like the visual acuity, this assessment was also divided into three phases. Changes in visual field defect of the corticosteroid-treated eyes are shown in Tables 6, 7 and 8.
Table 6.
Visual field defect change from initial visit to optic disc edema (ODE) resolution
| Visual field defect at initial visit | Steroid started ≤2 weeks from onset (n=258 eyes) |
Steroid started >2 weeks from onset (n=101 eyes) |
||||
|---|---|---|---|---|---|---|
| n | Number (%) of eyes |
n | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
| Minimal | 10 | — | 6 (60%) | 6 | — | 2 (33%) |
| Mild | 66 | 19 (29%) | 18 (27%) | 23 | 4 (17%) | 4 (17%) |
| Moderate | 60 | 15 (25%) | 12 (20%) | 21 | 1 (5%) | 6 (29%) |
| Marked | 85 | 28 (33%) | 17 (20%) | 35 | 13 (37%) | 5 (14%) |
| Severe | 37 | 23 (62%) | — | 16 | 9 (56%) | — |
| Moderate to severe | 182 | 66 (36%) | 29 (16%) | 72 | 23 (32%) | 11 (15%) |
Table 7.
Visual field defect change from optic disc edema (ODE) resolution to 3 months, 6 months, and 9 months after ODE resolution
| Visual field defect at ODE resolution | 3 months* after ODE resolution (n=338** eyes) |
6 months* after ODE resolution (n=311** eyes) |
9 months* after ODE resolution (n=299** eyes) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n** | Number (%) of eyes |
n** | Number (%) of eyes |
n** | Number (%) of eyes |
||||
| Improved | Worsened | Improved | Worsened | Improved | Worsened | ||||
| Minimal | 26 | — | 1 (4%) | 25 | — | 1 (4%) | 24 | — | 1 (4%) |
| Mild | 85 | 5 (6%) | 2 (2%) | 76 | 4 (5%) | 1 (1%) | 75 | 5 (6%) | 1 (1%) |
| Moderate | 77 | 1 (1%) | 1 (1%) | 74 | 1 (1%) | 1 (1%) | 69 | 0 (0%) | 1 (1%) |
| Marked | 113 | 7 (6%) | 0 (0%) | 104 | 6 (6%) | 0 (0%) | 102 | 8 (8%) | 0 (0%) |
| Severe | 37 | 7 (19%) | — | 32 | 7 (22%) | — | 29 | 7 (24%) | — |
| Moderate to severe | 225 | 15 (7%) | 1 (0.4%) | 208 | 14 (7%) | 1 (0.5%) | 198 | 15 (8%) | 1 (0.5%) |
±6 weeks for 3 months and 9 months; ±12 weeks for 2 years
n includes the eyes that have a post-ODE resolution follow-up for visual field of at least the lower limit specified.
ODE = Optic disc edema
Table 8.
Visual field change from visual field at initial visit to 3 months, 6 months, and 1 year from first visit
| Time from first visit /Initial Visual Field | Steroid started ≤2 weeks from onset (n=261 eyes) |
Steroid started >2 weeks from onset (n=101 eyes) |
||||
|---|---|---|---|---|---|---|
| n** | Number (%) of eyes |
n** | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
| 3 months* | (n=256) | (n=99) | ||||
| Minimal | 10 | — | 7 (70%) | 6 | — | 1 (17%) |
| Mild | 66 | 20 (30%) | 18 (27%) | 23 | 7 (30%) | 5 (22%) |
| Moderate | 59 | 15 (25%) | 12 (20%) | 21 | 1 (5%) | 5 (24%) |
| Marked | 85 | 28 (32%) | 15 (18%) | 33 | 13 (39%) | 6 (18%) |
| Severe | 35 | 25 (71%) | — | 16 | 9 (56%) | — |
| Moderate to severe | 179 | 68 (38%) | 27 (15%) | 70 | 23 (33%) | 11 (16%) |
| 6 months* | (n=240) | (n=92) | ||||
| Minimal | 9 | — | 6 (67%) | 6 | — | 2 (33%) |
| Mild | 62 | 20 (32%) | 17 (27%) | 20 | 6 (30%) | 4 (20%) |
| Moderate | 56 | 14 (25%) | 12 (21%) | 21 | 1 (5%) | 5 (24%) |
| Marked | 82 | 28 (34%) | 15 (18%) | 30 | 12 (40%) | 5 (17%) |
| Severe | 31 | 25 (81%) | — | 15 | 9 (60%) | — |
| Moderate to severe | 169 | 67 (40%) | 27 (16%) | 66 | 22 (33%) | 10 (15%) |
| 1 year* | (n=215) | (n=79) | ||||
| Minimal | 8 | — | 5 (62%) | 6 | — | 2 (33%) |
| Mild | 52 | 18 (35%) | 14 (27%) | 17 | 4 (24%) | 3 (18%) |
| Moderate | 52 | 13 (25%) | 13 (25%) | 19 | 1 (5%) | 5 (26%) |
| Marked | 77 | 28 (36%) | 15 (19%) | 25 | 10 (40%) | 5 (20%) |
| Severe | 26 | 21 (81%) | — | 12 | 7 (58%) | — |
| Moderate to severe | 155 | 62 (40%) | 28 (18%) | 56 | 18 (32%) | 10 (18%) |
±6 weeks for 3 and 6 months; ±12 weeks for 1 year
n includes the eyes that have a follow-up for VF of at least the lower limit specified
From initial visit to ODE resolution: of the eyes that started corticosteroid therapy within 2 weeks of onset of symptoms and had moderate to severe initial visual field loss, 36% showed improvement from first visit to the time when ODE resolved, while 16% got worse (Table 6); however, when the initial visual field loss was minimal to mild, 32% (24 of 76) got worse.
From the time when ODE resolved to 3 months, 6 months, and 9 months after the resolution: improvement in visual fields in the eyes with moderate to severe loss at the time of ODE resolution was observed in 7% at 3 months, 7% at 6 months, and 8% at 9 months after ODE had resolved (Table 7). Worsening visual field 9 months after ODE had resolved was observed in 2% (two of 99) of the eyes with minimal to mild visual field loss at ODE resolution, and in 0.5% of the eyes with moderate to severe visual field loss at ODE resolution.
Overall change at 3 months, 6 months, and 1 year from the initial visit: of those who were treated with corticosteroid within 2 weeks of onset with moderate to severe visual field defect, there was improvement in 38% at 3 months, 40% at 6 months, and 40% at 1 year from initial visit (Table 8). In eyes with minimal to mild field defects initially, there was worsening in 33% (25 of 76) at 3 months, and similarly at 1 year (32%; 19 of 60) from first visit.
We also evaluated overall changes in the central 30° and the peripheral visual fields separately during follow-up. The central visual field was stable during the follow-up period in 47% of the eyes, improved in 35%, and worsened in 19%. There was improvement in peripheral visual field in 28% of the eyes, and worsening in 21%.
The recorded improvement in visual acuity may not always reflect genuine improvement in the optic nerve function, but could be simply due to the patient having learned by experience to read the test chart better by looking around and fixating eccentrically. This applies particularly to an eye that has a visual field defect passing through or just involving the central fixation spot, so that in such cases, by eccentric fixation, the patient may finally test much better without any actual improvement in the retinal or optic nerve function. Among the corticosteroid-treated eyes with improvement in visual acuity at 1 year from initial visit, improvement was due to eccentric fixation in 10% (eight of 77) of the eyes. If those with visual improvement due to eccentric fixation were considered as having no change in visual acuity, then in those treated with steroid within 2 weeks of NA-AION onset with initial visual acuity of 20/70 or worse, the genuine improvement in visual acuity is about 56%.
Comparison of steroid-treated (ST) group with natural history (NH) cohort
Overall, in the entire cohort of 613 consecutive patients, 51% opted for ST and 49% for NH. Breakdown of the number of patients opting for ST and NH, according to different decades of the study duration, showed that during the first decade of the study 58% opted for ST, and 42% for NH. During the second decade of the study, 39% opted for ST, and 61% opted for NH. During the last, remaining period of the study, 51% opted for ST, and 49% for NH.
The demographic and clinical characteristics of the cohort that volunteered to take steroid therapy (ST) and the cohort that decided not to take any treatment were compared (see Table 1). There was no significant difference in gender distribution (p=0.594), smoking status (p= 0.603), prevalence of ischemic heart disease (p=0.258), and peripheral vascular disease (p=0.920). Of the eyes seen within 2 weeks of onset in our NH cohort, the initial visual acuity and visual field defect did not differ significantly from those of the ST group (p=0.201 for visual acuity; p= 0.304 for visual field defect). However, the patients who opted for corticosteroid were found to be somewhat younger (59.2 vs 62.0; p= 0.006) and had a lower prevalence of arterial hypertension (34% vs 43%; p= 0.036). The non-steroid cohort also had a lower prevalence of TIA/CVA (6% vs 9%; p=0.097) and diabetes mellitus (27% vs 32%; p=0.126), but this was not significant at the 0.05 significance level. To account for these differences between the two groups, these variables were used as covariates in the logistic regression analyses that examined the effect of steroid therapy on improvement of visual outcomes.
Comparing visual acuity improvement in the eyes with initial visual acuity of 20/70 or worse that were first seen or treated with corticosteroid within 2 weeks of onset of NA-AION, a significantly higher probability of improvement was observed in the ST group (n=70 eyes) than in the NH cohort (n=71 eyes) at ODE resolution (p=0.004), and at 3 months (p=0.0002), 6 months (p=0.001), and 1 year (p= 0.0002) of NA-AION onset. The odds ratio for improvement in visual acuity from initial visit in the steroid-treated group relative to that in the natural history group was 4.45 (95% CI: 2.03, 9.75) at 3 months, 3.39 (95% CI: 1.62, 7.11) at 6 months, and 4.06 (95% CI: 1.92, 8.57) at 1 year (Table 9). The same results were observed after including age at onset, arterial hypertension, TIA/CVA, and diabetes mellitus as covariates in the logistic regression model, with these variables not showing a significant association with visual acuity improvement (age at onset p=0.817; hypertension p=0.589; TIA/CVA p=0.929; diabetes p=0.516). Fig. 1a (ST) and b (NH) gives the distribution of visual acuity change at 1 year in all eyes.
Table 9.
Comparison of percent improvement in visual acuity* among those with initial visual acuity of 20/70 of worse that were seen within 2 weeks on NA-AION onset who received steroid therapy within two weeks of onset and those that did not receive any steroid
| Follow-up period | Percent with improved visual acuity* (95% Confidence Interval) |
Odds ratio (95% CI) for VA improvement (steroid/no steroid) |
p value | |
|---|---|---|---|---|
| Steroid | No steroid** | |||
| At ODE resolution | 44.2% (33.1%, 56.0%) | 21.2% (13.2%, 32.2%) | 2.95 (1.42, 6.17) | 0.004 |
| 3 months from first visit | 47.1% (35.8%, 58.7%) | 16.7% (9.6%, 27.3%) | 4.45 (2.03, 9.75) | 0.0002 |
| 6 months from first visit | 69.8% (57.3%, 79.9%) | 40.5% (29.2%, 52.9%) | 3.39 (1.62, 7.11) | 0.001 |
| 1 year from first visit | 72.2% (60.2%, 81.6%) | 39.0% (27.6%, 51.7%) | 4.06 (1.92, 8.57) | 0.0002 |
Estimates obtained from the repeated measures logistic regression model fitted using the generalized estimating equations (GEE) method
From cohort of patients in our natural history study of NA-AION [28].
CI = Confidence interval; NA-AION = Non-arteritic anterior ischemic optic neuropathy; ODE = Optic disc edema; VA = Visual acuity
Since this study included patients that were seen over a period of 27 years, we also determined if the findings on visual acuity improvement differed across study periods. Comparing outcomes between patients seen in the first and second half of the study showed that differences between steroid-treated and the natural history group did not significantly vary between the study periods (p=0.29). There was also no overall significant difference in visual acuity improvement between patients seen in the two periods (p=0.41).
Comparing visual field improvement in the eyes with initial moderate to severe defects that were first seen or treated with corticosteroid within 2 week of onset of NA-AION showed a significantly higher probability of improvement in the corticosteroid group than in the untreated group at ODE resolution (p=0.001), and at 3 months (p= 0.0006), 6 months (p=0.005), and 1 year (p=0.006) from initial visit (Table 10). At 1 year from initial visit, the odds of improvement in visual field in those that had corticosteroid therapy was 2.03 (95% CI: 1.23, 3.36) times greater than those that had no treatment. The same results were observed after including age at onset, hypertension, and diabetes as covariates in the logistic regression model, with these variables not showing a significant association with visual field improvement (age at onset p=0.746; hypertension p=0.271; TIA/CVA 0.829; diabetes p=0.972).
Table 10.
Comparison of percent improvement in visual field defect* between those with initial moderate to severe visual field defect and were seen within 2 weeks of NA-AION onset who received steroid therapy within 2 weeks of onset and those that did not receive any steroid
| Follow-up period | Percent with improved visual field* (95% confidence interval) |
Odds ratio (95% CI) for VF improvement (steroid/no steroid) |
p value | |
|---|---|---|---|---|
| Steroid | No steroid** | |||
| At ODE resolution | 36.6% (29.7%, 43.9%) | 19.6% (13.8%, 27.0%) | 2.36 (1.41, 3.96) | 0.001 |
| 3 months from first visit | 37.7% (30.8%, 45.0%) | 19.7% (13.9%, 27.2%) | 2.47 (1.48, 4.12) | 0.0006 |
| 6 months from first visit | 40.1% (33.1%, 47.5%) | 24.5% (17.7%, 32.9%) | 2.06 (1.24, 3.40) | 0.005 |
| 1 year from first visit | 40.0% (33.0%, 47.3%) | 24.7% (17.8%, 33.1%) | 2.03 (1.23, 3.36) | 0.006 |
Estimates obtained from the repeated measures logistic regression model fitted using the generalized estimating equations (GEE) method
From cohort of patients in our natural history study of NA-AION [28]
CI = Confidence interval; NA-AION = Non-arteritic anterior ischemic optic neuropathy; ODE = Optic disc edema; VF = Visual field
Visual improvement was compared between the ST group and the NH cohort that were seen within 2 weeks of onset of NA-AION that had 20/40 to 20/60 initial visual acuity, and also in those with mild visual field loss. At 6 months from initial visit, there was improvement in visual acuity in 27% of the ST group and 17% in the NH cohort (p=0.897), and in the visual field in 32% of the ST group and 14% of the NH group (p=0.027).
Of the eyes with visual acuity improvement at 1 year from initial visit that were seen within 2 weeks on onset of NA-AION, it was found that improvement was due to eccentric fixation in 27% of the eyes in the NH cohort, and in 15% of the eyes in the ST group (p=0.172). If the comparison of visual acuity improvement between the ST group and the NH group was reassessed, with improvement due to eccentric fixation considered as no change, of those who presented with visual acuity of 20/70 or worse within 2 weeks of NA-AION onset, genuine improvement at 1 year from initial visit is 55.4% (95% CI: 42.8%, 67.2%) for the ST group and 27.7% (95% CI: 17.6%, 40.7%) in the NH group.
With respect to worsening of visual acuity in eyes with initial visual acuity of 20/60 or better and seen within 2 weeks of NA-AION onset, the ST group (n=190) and the NH group (n=130) were compared. There was no significant difference in the worsening of visual acuity at ODE resolution (p=0.384), with the estimated probability of worsening of 12.5% (95% CI: 9.0%, 18.1%) in the ST group and 10.2% (95% CI: 6.3%, 16.1%) in the NH cohort. This was also observed at 3 months (p=0.420), 6 months (p=0.150), and 1 year (p=0.100) from initial visit. The estimated probability of worsening at 1 year from initial visit was 16.5% (95% CI: 11.9%, 22.4%) in the ST group, and 11.1% (95% CI: 7.1%, 16.9%) in the NH cohort. Including arterial hypertension, TIA/CVA, diabetes mellitus, and age at onset as covariates showed no significant association of these factors with visual acuity deterioration (p>0.43).
For those with minimal to mild initial visual field defect, comparison of worsening of visual field among those seen within 2 weeks of NA-AION onset showed no significant difference at ODE resolution, with the estimated probability of worsening of 31.5% (95% CI: 22.4%, 42.4%) in the ST group, and 23.0% (95% CI: 14.6%, 34.3%) in the NH cohort (p=0.228). This was also observed at 3 months (p= 0.380), 6 months (p=0.317), and 1 year (p=0.524) from initial visit. The estimated probability of worsening of visual field at 1 year from initial visit was 31.7% (95% CI: 21.5%, 43.9%) in the ST group and 26.6% (95% CI: 16.8%, 39.5%) in the NH cohort. Including arterial hypertension, diabetes mellitus, TIA/CVA, and age at onset as covariates showed no significant association of these factors with visual field deterioration (p>0.16).
Discussion
NA-AION is a common, visually disabling disease, with a potential of involvement of both eyes [1, 3, 39]. So far, no treatment has proved effective in improving its visual outcome, in spite of various claims. Thus, the primary objective of the present study was to evaluate prospectively in a large cohort of NA-AION patients whether systemic corticosteroid therapy given during its acute phase (i.e. when optic disc edema is still present) has any beneficial effect. From a cohort of 696 consecutive eyes with NA-AION (who fulfilled our inclusion and exclusion criteria for the studies), seen in the Ocular Vascular Clinic at the University of Iowa Hospitals & Clinics from 1973 to 2000, we evaluated the natural history of visual outcome (in 332 eyes) [28] and compared that with those who opted voluntarily to have corticosteroid therapy (in 364 eyes). Percent improvement in visual acuity among those with initial visual acuity of 20/70 or worse and seen within 2 weeks of NA-AION onset, who opted to have corticosteroid therapy, was compared with those who opted not to [28]. This showed that 6 months after their initial visit, visual acuity improvement was 69.8% (95% CI: 57.3%, 79.9%) in the treated group, compared to 37.1% (95% CI: 28.4%, 46.7%) in the untreated group, with odds ratio of visual acuity improvement in the treated group of 3.39 (95% CI: 1.62, 7.11) (p=0.001) (Table 9). The findings were almost similar at 12 months from onset (Table 9). A similar comparison of visual field defects improvement at 6 months from initial visit among those with initial moderate to severe visual field defect, and seen or treated within 2 weeks of NA-AION onset, showed visual field improvement in 40.1% (95% CI: 33.1%, 47.5%) of those who had the corticosteroid therapy, compared to 24.5% (95% CI: 17.7%, 32.9%) of those without corticosteroid therapy, with odds ratio of visual field improvement in the treated group of 2.06 (95% CI: 1.24, 3.40) (p=0.005) (Table 10). The findings were almost similar at 1 year from initial visit (Table 10). This indicates that systemic corticosteroid therapy significantly improves both visual acuity and visual fields compared to the natural history of visual outcome [28].
The study also showed that, among eyes with mild visual loss (i.e. visual acuity of 20/40 to 20/60; mild visual field loss) seen within 2 weeks of onset of NA-AION, there was improvement in visual acuity in 27% of the treated group, and in 17% of the untreated group (p=0.897) [28], 6 months after the initial visit, and in the visual field in 32% of the treated group, and 14% of the untreated group (p=0.027) [28]. In this group of eyes with mild visual loss, at 6 months from initial visit, there was no significant difference in the worsening of visual acuity (p=0.150) and visual fields (p=0.317) between the treated and the untreated eyes. Table 5 shows that at 1 year of follow-up, of the 161 eyes with visual acuity of 20/15 to 20/60, visual acuity remained stable in 123 (76%) eyes; of the 161 eyes, 120 eyes had a visual acuity of 20/30 or better, which is normal and stayed normal in 82%. This shows that corticosteroid therapy had no deleterious effect on visual acuity or visual fields in these eyes; it showed a significant improvement in the visual fields.
Thus, the study showed that in eyes seen within 2 weeks of NA-AION onset and with marked visual loss (i.e. initial visual acuity of 20/70 or worse and initial moderate to severe visual field defect), both visual acuity and visual fields improved significantly for 6 months after the onset of NA-AION and very little thereafter. However, in eyes with mild visual loss (i.e. visual acuity of 20/40 to 20/60; mild visual field loss), although the visual field improved significantly in the treated group, visual acuity showed no significant difference between the two groups. This suggests that eyes with marked visual loss benefit much more from systemic corticosteroid therapy than those with mild visual loss, although in the latter group the visual fields did show significant improvement. The primary concern in treating patients with systemic corticosteroid therapy is invariably the possibility of systemic side effects. However, in NA-AION, corticosteroid therapy is required only for the duration of optic disc edema (i.e. 2–3 months at the maximum). In our study, we did not have to stop the therapy in any case, including the diabetics, because of any serious systemic side effects of corticosteroids.
Rationale for visual improvement with corticosteroid therapy in NA-AION
Naturally, the question arises, why did corticosteroid therapy help to improve the visual acuity and visual fields of NA-AION patients? To comprehend that, one has to consider some of the relevant basic aspects of NA-AION.
NA-AION is due to ischemia of the optic nerve head, which is primarily supplied by the posterior ciliary artery circulation [8, 15].
Ischemia of axons in NA-AION results in axoplasmic flow stasis, which in turn causes axoplasmic accumulation and consequent axonal swelling in the optic nerve head; that manifests as ODE [12, 13, 37].
It has been shown that, in the majority of NA-AION eyes, the optic disc has a small cup or none at all [2, 10]. Thus, there is crowding of the nerve fibers as they pass through a restricted space in the rigid opening in Bruch’s membrane and the small scleral canal. The importance of this factor is that the swollen axons in the restricted and unyielding space within the optic nerve head have to expand at the cost of other tissues in that restricted space. The only thing that they can compress to expand are the fine capillaries lying among them; that results in secondary vascular changes [12, 21]. A vicious circle may, therefore, be set up, in which compression of capillaries may further aggravate ischemia, particularly when perfusion pressure in them falls for any reason (as for example, during nocturnal arterial hypotension [23, 24, 30]). This is supported by the fact that in at least 73.3% of episodes of NA-AION, visual loss was discovered first upon awakening or a first opportunity to use vision critically after sleeping, because of fall of blood pressure during sleep [23].
On fluorescein fundus angiography, the optic disc with ODE in NA-AION always shows dye leaking from the capillaries in the optic nerve head and late staining. Fluorescein leakage may be due to two factors: (i) ischemic insult to the capillaries in the optic nerve head, and (ii) venous stasis produced by the capillary compression [12]. Foulds [6] also pointed out that increased capillary permeability due to anoxic capillary damage was an important factor in development of ODE in NA-AION.
Therefore, there are primary and secondary changes in the optic nerve head to produce ODE in NA-AION—the primary change being ischemic axoplasmic flow stasis in the axons and the secondary vascular changes and fluid leakage.
So far, the effect of systemic corticosteroids on axoplasmic flow stasis has not been studied in the optic nerve head. In one postmortem, in vitro study of the effect of cortisol on axoplasmic flow in prefrontal and temporal cortical neurons of four aged human brains, a “bell-shaped” effect was found: a stimulating effect at low concentrations, and a depressing effect at high concentrations [4]. Whether that has any relevance to in vivo axoplasmic flow stasis in the optic nerve head seen in NA-AION remains unknown.
Foulds [6] postulated that corticosteroid therapy in acute NA-AION reduces ODE by reducing the capillary permeability. There is ample evidence that corticosteroids work in many non-inflammatory diseases. For example, oral [9, 16] or intravitreal [7, 32, 33, 36] corticosteroid therapy reduces macular edema due to various causes. Although the exact mechanism by which steroids act in all these conditions is not known, it seems most probable that they alter capillary permeability and reduce fluid leakage. As discussed above, fluorescein angiography shows leakage of fluorescein in the optic nerve head when the disc is edematous in NA-AION but not in normal or atrophic discs—a proof of increased capillary permeability in ODE. We studied the effect of corticosteroid therapy on the resolution of ODE in this cohort of treated versus untreated NA-AION eyes, and that showed that those treated with corticosteroid therapy within 2 weeks after onset of NA-AION had significantly (p= 0.0006) faster ODE resolution than the untreated cases [27]. This would suggest reduction in capillary leakage, similar to that seen in macular edema with corticosteroid therapy.
Thus, from the above discussion, the most likely scenario that emerges to explain the beneficial effect of corticosteroid therapy on visual outcome in NA-AION seems to be as follows. The faster resolution of ODE with corticosteroid therapy compared to the untreated patients [27] → progressive decrease of compression of the capillaries in the optic nerve head → better blood flow in the capillaries → improved circulation in the optic nerve head → improved function of the surviving but not functioning hypoxic axons. There is a possibility that corticosteroids may have beneficial effects from some other unknown mechanisms; one of those mentioned has been inhibition of damage by free radicals. At the time of resolution of ODE (in eyes seen within 2 weeks of onset of NA-AION, with initial visual acuity of 20/70 of worse), the visual acuity improvement was 44.2% (95% CI: 33.1%, 56.0%) in the treated group compared to 21.2% (95% CI: 13.2%, 32.2%) in the untreated group [28], with odds ratio of visual acuity improvement of 2.95 (95% CI: 1.42, 6.17 CL; p=0.004) (Table 9). A similar comparison of visual field defect improvement (among those seen within 2 weeks of onset of NA-AION, with initial moderate to severe visual field defect) at the time of resolution of ODE, was 36.6% (95% CI: 29.7%, 43.9%) in the treated group, compared to 19.6% (13.8%, 27.0%) in the untreated group [28], with an odds ratio of visual field improvement in the treated group of 2.36 (95% CI: 1.41, 3.96) (p=0.001) (Table 10). Our natural history study of visual outcome in NA-AION [28] showed that visual acuity and visual fields in NA-AION keep improving for some time even after the resolution of ODE (i.e. up to about 6 months from onset), as was also seen in the corticosteroid group in this study (Tables 5 and 8).
Use of intravitreal triamcinolone acetonide for treatment of NA-AION
There have recently been two contradictory studies on this topic[34, 35]. Jonas et al. [34], in three patients, found that it had no beneficial effect on visual acuity. Kaderli et al. [35], in four eyes, reported visual acuity improvement, but without any improvement in visual fields. However, the study of Kaderli et al. [35] has some notable flaws which are discussed in detail elsewhere [19]. For example: (a) their study is based on only four eyes, (b) large natural history studies [28, 40] have shown spontaneous visual acuity improvement in 41% of eyes with NA-AION, and (c) more importantly, all the eyes in the study by Kaderli et al. [35] showed no improvement in visual fields and all had altitudinal visual field defects. We have found in studies on NA-AION and arteritic AION [28, 29] that apparent visual acuity improvement without visual field improvement is due to patient learning to fixate eccentrically and that does not represent a genuine visual improvement. In the study by Kaderli et al [35], eccentric fixation may explain why the visual acuity of the patients apparently improved, while the visual fields did not.
Most importantly, intravitreal triamcinolone injection in NA-AION eyes, which already have precarious circulation in the optic nerve head, can be harmful. Optic nerve head circulation depends upon the perfusion pressure (mean blood pressure minus IOP). Intravitreal injection increases the volume in the eyeball, thereby resulting in a transient rise of IOP. In addition, there are many reports showing a rise in IOP a few days or weeks after intravitreal triamcinolone. In NA-AION, with already precarious optic nerve head circulation, even a small rise in IOP for any reason can further compromise the circulation and result in further visual loss. Oral steroid therapy, by contrast, did not have that effect on IOP on a short-term treatment given in our study. Thus, one cannot equate oral and intravitreal steroid therapy in NA-AION.
Limitations and strengths of the study
As discussed above, from the strictly scientific point of view, the main limitation of this study is that corticosteroid therapy was not randomly assigned to patients. As discussed above, given that a multicenter clinical trial was not funded, we decided to conduct a “patient choice” study (i.e. the patients decided whether to take corticosteroid therapy or not) as the next best choice. As discussed above in detail, we took every possible step (a) to prevent leading patients towards selecting corticosteroid therapy over no treatment or vice versa, and (b), importantly, to reduce potential bias in visual evaluation. All those were observed very strictly throughout the study. Most importantly, the final data were subjected to careful statistical analysis, which showed among other things that the number of persons who fulfilled our inclusion and exclusion criteria and voluntarily opted for steroid therapy (51%) and those who wanted no treatment (49%) were similar in this entire cohort of 613 consecutive patients with NA-AION, first seen from 1973 to 2000 in the Ocular Vascular Clinic - this would not have occurred if there was any bias in selection. Moreover, to deal with the issue whether there was bias, we compared the baseline patient characteristics, including visual acuity, visual fields, and systemic diseases between the treated and untreated groups. The demographic and clinical characteristics of the cohort that volunteered to take steroid therapy and the cohort that decided not to take any treatment were compared (see Table 1). There was no significant difference in gender distribution (p =0.594), smoking status (p =0.603), prevalence of ischemic heart disease (p =0.258), and peripheral vascular disease (p = 0.920). Of the eyes seen within 2 weeks of onset in our natural history cohort, the initial visual acuity and visual field defect did not differ significantly from those of steroid treated group (p=0.201 for visual acuity; p=0.304 for visual field defect). There was no significant difference between the treated and untreated groups in prevalence of TIA/CVA (6% vs 9%; p=0.097) and diabetes mellitus (27% vs 32%; p=0.126).However, the patients who opted for corticosteroid were found to be somewhat younger (59.2 vs 62.0; p=0.006) and had a lower prevalence of arterial hypertension (34% vs 43%; p=0.036). To determine if differences in age, arterial hypertension, TIA/CVA, diabetes mellitus influenced the visual outcome in this study, they were accounted for in the statistical analysis by including them as covariates in the logistic regression model. This is similar to the analysis performed for epidemiological studies, which do not involve randomization to treatment groups. From the statistical analysis, we found that differences in age, arterial hypertension, TIA/CVA and diabetes mellitus showed no significant association with the primary outcome of visual acuity (age at onset p=0.817; hypertension p=0.589; TIA/CVA p=0.929; diabetes p=0.516) or visual field improvement (age at onset p=0.746; hypertension p=0.271; TIA/CVA p=0.829; diabetes p=0.972); nor did they alter the finding of a significantly greater likelihood of improvement in visual outcome with steroid therapy. Thus, the differences in age and in prevalence of arterial hypertension did not make any significant difference in the visual outcome. In addition, since the study included patients with both eyes having NA-AION, the statistical analysis also accounted for the correlation of eyes by using the generalized estimating equation (GEE) method in fitting the logistic regression model. Thus, we believe that concerns with respect to differences between the groups that may be due to the study not being randomized have been adequately addressed by the statistical analysis. Therefore, when all these facts are put together, one can conclude that in spite of the lack of conventional randomization, this study provides scientifically valid information about the role of corticosteroid therapy in NA-AION.
Moreover, the odds ratio for improvement in visual acuity from initial visit in the steroid-treated group relative to that in the natural history group was 4.45 (95% CI: 2.03, 9.75; p=0.0002) at 3 months, 3.39 (95% CI: 1.62, 7.11; p= 0.001) at 6 months and 4.06 (95% CI: 1.92, 8.57) at 1 year (Table 9). This is not at all a minor difference, given that the two groups were basically similar. Furthermore, the visual acuity outcome in the natural history [28] component of this overall study was identical to that in the randomized IONDT study [40].
The strength of our study is that it is based on the largest cohort of NA-AION patients ever followed so closely, for so long, by a single observer (SSH). Thus, there was consistency in the quality of evaluation and data throughout the entire duration of the study. Most importantly, we believe that in this blinding disease, this study provides some hope to desperate patients without any hope so far.
Conclusions
This study suggested that systemic corticosteroid therapy in NA-AION eyes given during the acute phase resulted in a significantly higher probability of improvement in visual acuity (p=0.001) and visual field (p=0.005) compared to an untreated group [28]. It also showed that both visual acuity and visual fields improved for up to 6 months after onset of NA-AION, and very little thereafter. Based on the mechanism of visual improvement with corticosteroid therapy discussed above, it would seem that the sooner the treatment is started, the better the chance of improvement, and that may be due to the fact that the longer the axonal ischemia persists, the more axons are likely to be damaged permanently. Most importantly, so far, no treatment for NA-AION has proved effective in visual improvement; significant visual benefits shown by this study provide some hope for these desperate patients suffering from this blinding disease.
Acknowledgements
We are extremely grateful to Drs. Randy H. Kardon, H. Stanley Thompson and Michael Wall, and Mrs. Patricia Podhajsky for their invaluable help with the visual field evaluation.
Supported by grant EY-1151 from the National Institutes of Health, Bethesda, Maryland, and in part by unrestricted grant from Research to Prevent Blindness, Inc., New York.
References
- 1.Beck RW, Hayreh SS, Podhajsky PA, Tan E-S, Moke PS. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:212–217. doi: 10.1016/s0002-9394(14)71038-4. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Servais GE, Hayreh SS. Anterior ischemic optic neuropathy IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94:1503–1508. [PubMed] [Google Scholar]
- 3.Beri M, Klugman MR, Kohler JA, Hayreh SS. Anterior ischemic optic neuropathy VII. Incidence of bilaterality and various influencing factors. Ophthalmology. 1987;94:1020–1028. doi: 10.1016/s0161-6420(87)33350-0. [DOI] [PubMed] [Google Scholar]
- 4.Dai J, Buijs R, Swaab D. Glucocorticoid hormone (cortisol) affects axonal transport in human cortex neurons but shows resistance in Alzheimer’s disease. Br J Pharmacol. 2004;143:606–610. doi: 10.1038/sj.bjp.0705995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esterman B. Grid for scoring visual fields. II. Perimeter. Arch Ophthalmol. 1968;79:400–406. doi: 10.1001/archopht.1968.03850040402007. [DOI] [PubMed] [Google Scholar]
- 6.Foulds WS. Visual disturbances in systemic disorders: optic neuropathy and systemic disease. Trans Ophthalmol Soc UK. 1969;89:125–146. [PubMed] [Google Scholar]
- 7.Greenberg PB, Martidis A, Rogers AH, Duker JS, Reichel E. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002;86:247–248. doi: 10.1136/bjo.86.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayreh SS. Optic disc vasculitis. Br J Ophthalmol. 1972;56:652–670. doi: 10.1136/bjo.56.9.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayreh SS. Pathogenesis of cupping of the optic disc. Br J Ophthalmol. 1974;58:863–876. doi: 10.1136/bjo.58.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayreh SS. Anterior ischaemic optic neuropathy III. Treatment, prophylaxis, and differential diagnosis. Br J Ophthalmol. 1974;58:98l–989. doi: 10.1136/bjo.58.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh SS. Optic disc edema in raised intracranial pressure V. Pathogenesis. Arch Ophthalmol. 1977;95:1553–1565. doi: 10.1001/archopht.1977.04450090075006. [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS. Fluids in the anterior part of the optic nerve in health and disease. Surv Ophthalmol. 1978;23:1–25. doi: 10.1016/0039-6257(78)90194-7. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS. Anterior ischaemic optic neuropathy: Differentiation of arteritic from non-arteritic type and its management. Eye. 1990;4:25–41. doi: 10.1038/eye.1990.4. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it -Myth and reality. Prog Retin Eye Res. 2001;20:563–593. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica. 2003;217:167–188. doi: 10.1159/000068980. [DOI] [PubMed] [Google Scholar]
- 17.Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye. 2004;18:1188–1206. doi: 10.1038/sj.eye.6701562. [DOI] [PubMed] [Google Scholar]
- 18.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Hayreh SS. Intravitreal triamcinolone for non-arteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2008;28:77–78. doi: 10.1097/WNO.0b013e31816781f3. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion: a 10-year prospective study. Graefes Arch Clin Exp Ophthalmol. 1990;228:281–296. doi: 10.1007/BF00920049. [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS, Massanari RM, Yamada T, Hayreh SMS. Experimental allergic encephalomyelitis I. Optic nerve and central nervous system manifestations. Invest Ophthalmol Vis Sci. 1981;21:256–269. [PubMed] [Google Scholar]
- 22.Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: Validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- 23.Hayreh SS, Podhajsky PA, Zimmerman B. Non-arteritic anterior ischemic optic neuropathy - Time of onset of visual loss. Am J Ophthalmol. 1997;124:641–647. doi: 10.1016/s0002-9394(14)70902-x. [DOI] [PubMed] [Google Scholar]
- 24.Hayreh SS, Podhajsky PA, Zimmerman B. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96. doi: 10.1159/000027399. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS, Zimmerman B. Management of giant cell arteritis. Ophthalmologica. 2003;217:239–259. doi: 10.1159/000070631. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: Their pattern and prevalence at the initial presentation. Arch Ophthalmol. 2005;123:1554–1562. doi: 10.1001/archopht.123.11.1554. [DOI] [PubMed] [Google Scholar]
- 27.Hayreh SS, Zimmerman MB. Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:1107–1121. doi: 10.1007/s00417-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 28.Hayreh SS, Zimmerman B. Non-arteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis: Report of a large study and review of literature. Acta Ophthalmol Scand. 2002;80:355–367. doi: 10.1034/j.1600-0420.2002.800403.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–624. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 31.Ischemic Optic Neuropathy Decompression Trial Research Group Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch Ophthalmol. 2000;118:793–798. [PubMed] [Google Scholar]
- 32.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:782–783. doi: 10.1007/s00417-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, Söfker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132:425–427. doi: 10.1016/s0002-9394(01)01010-8. [DOI] [PubMed] [Google Scholar]
- 34.Jonas JB, Spandau UH, Harder B, Saunder G. Intravitreal triamcinolone acetonide for treatment of acute nonarteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:749–750. doi: 10.1007/s00417-006-0332-4. [DOI] [PubMed] [Google Scholar]
- 35.Kaderli B, Avci R, Yucel A, Guler K, Gelisken O. Intravitreal triamcinolone improves recovery of visual acuity in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2007;27:164–168. doi: 10.1097/WNO.0b013e31814a5a9a. [DOI] [PubMed] [Google Scholar]
- 36.Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, Baumal C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 37.McLeod D, Marshall J, Kohner EM. Role of axoplasmic transport in the pathophysiology of ischaemic disc swelling. Br J Ophthalmol. 1980;64:247–261. doi: 10.1136/bjo.64.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller GR, Smith JL. Ischemic optic neuropathy. Am J Ophthalmol. 1966;62:103–115. doi: 10.1016/0002-9394(66)91685-0. [DOI] [PubMed] [Google Scholar]
- 39.Newman NJ, Scherer R, Langenberg P, Kelman S, Feldon S, Kaufman D, Dickersin K, Ischemic Optic Neuropathy Decompression Trial Research Group The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002;134:317–328. doi: 10.1016/s0002-9394(02)01639-2. [DOI] [PubMed] [Google Scholar]
- 40.The Ischemic Optic Neuropathy Decompression Trial Research Group Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625–632. [PubMed] [Google Scholar]