Abstract
Smokers often report an anxiolytic effect of cigarettes. In addition, stress-related disorders such as anxiety, post-traumatic stress syndrome, and depression are often associated with chronic nicotine use. To study the role of the α5 nicotinic acetylcholine receptor subunit in anxiety-related responses, control and α5 subunit null mice (α5 −/−) were subjected to the open field, light-dark box and elevated plus maze tests. In the open field and light-dark box, α5 −/− behaved like wild type controls. In the elevated plus, female α5 −/− mice displayed an anxiolytic-like phenotype while male α5 −/− mice were undistinguishable from littermate controls. We studied the hypothalamus-pituitary-adrenal axis by measuring plasma corticosterone and hypothalamic corticotropin releasing factor. Consistent with an anxiolytic-like phenotype, female α5 −/− mice displayed lower basal corticosterone levels. To test whether gonadal steroids regulate the expression of α5, we treated cultured NT2 cells with progesterone and found that α5 protein levels were up-regulated. In addition, brain levels of α5 mRNA increased upon progesterone injection into ovariectomized wild type females. Finally, we tested anxiety levels in the elevated plus maze during the estrous cycle. The estrus phase (when progesterone levels are low) is anxiolytic-like in wild type mice, but no cycle-dependent fluctuations in anxiety levels were found in α5 −/− females. Thus, α5-containing nAChRs may be mediators of anxiogenic responses, and progesterone-dependent modulation of α5 expression may contribute to fluctuations in anxiety levels during the ovarian cycle.
Keywords: nicotinic acetylcholine receptor, α5 subunit, sex differences, HPA axis, progesterone, anxiety, estrous cycle, qRT-PCR
Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) influence several central processes, including anxiety (Cordero-Erausquin et al. 2000; File et al. 2000; Labarca et al. 2001; Picciotto et al. 2002; Picciotto et al. 1995; Ross et al. 2000; Salas et al. 2003b). To date, the specific role of α5-containing nAChRs in anxiety-related manifestations is unknown.
nAChRs are pentamers formed by either α subunits (α7, α9, α10) or combinations of α and β subunits. In contrast to other α subunits, α5 cannot yield functional receptors when expressed alone or in combination with β subunits only (Ramirez-Latorre et al. 1996). The α5 subunit can form a receptor only when coexpressed with both another α and at least one β subunit. In this case, the presence of α5 modifies the pharmacology and the biophysical properties of nAChRs (Gerzanich et al. 1998; Groot-Kormelink et al. 2001; Wang et al. 1996). Interestingly, α5 null mice have been shown to be less sensitive to the effects of acute nicotine (Salas et al. 2003a), but a role for α5 in basal behavior in mice has not been described.
In humans, stressful events are often associated with anxiety and depression, symptoms that are more frequent in women than in men (Bracke 1998; Breslau et al. 1995; Olff et al. 2007). In animals, anxiety levels fluctuate during the ovarian cycle possibly due to the changes in endogenous steroid levels (Reddy & Kulkarni 1999). Stress-induced activation of the hypothalamus-pituitary-adrenal (HPA) axis leads to the release of corticotropin-releasing factor (CRF) from the paraventricular nucleus of the hypothalamus (Dallman et al. 1994), which ultimately results in the release of corticosterone (CORT) from the adrenal medulla (Caggiula et al. 1998; McEwen & Stellar 1993). Besides being an important regulator of the central nervous system during stress (Kim et al. 2006; Kosten & Ambrosio 2002; McEwen & Sapolsky 1995), CORT interacts with neurotransmitters involved in the reinforcing properties of various drugs, including nicotine (Koob & Nestler 1997; Piazza & Le Moal 1998). Interestingly, although both in males and females the levels of CORT are increased upon stress, the basal levels of CORT are much higher in females than in males (Critchlow et al. 1963). The nicotinic system is sexually dimorphic: in a recent report, it has been shown that chronic nicotine has an anxiolytic effect in the elevated plus maze in female, but not male, C57BL/6J mice (Caldarone et al. 2008). Since there are sexual differences in the risk for anxiety-related disorders, depression, and nicotine addiction (Evans et al. 2006; Kessler et al. 1994; Perkins 2001), the study of these differences is of obvious interest.
To address whether α5-containing nAChRs play a sex-specific role on anxiety-related behavior, we tested male and female α5 −/− mice in a battery of behavioral tests. Possible alterations of the HPA axis were also studied. In addition, we studied progesterone regulation of α5 levels in vivo and in vitro. Finally, we measured anxiety-like behavior during estrus and diestrus in wild type and α5 −/− female mice.
Materials and Methods
Animals
α5-deficient mice were generated as described previously (Salas et al. 2003a). Experiments were performed on littermate α5 null (−/−) and wild-type (+/+) mice backcrossed into a C57BL/6J background for up to nine generations. For figs 3D, E and 4A, C57BL/6J mice (The Jakson Laboratory, Bar harbor, Maine) were used. Mice were 2–6 months old and were housed two to five per cage in a room with a 12 hr light/dark cycle with food and water ad libitum. All testing procedures were approved by The Institutional Animal Protocol Review Committee at Baylor College of Medicine.
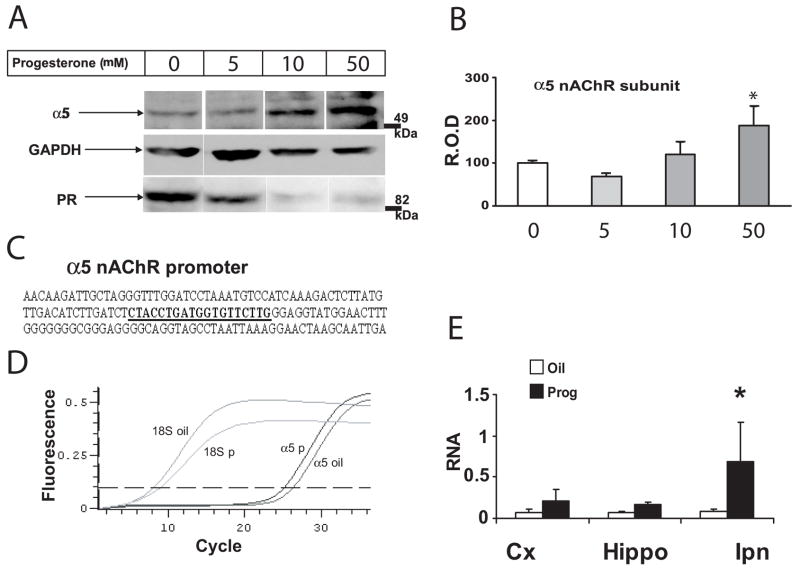
Figure 3. Progesterone-induced α5 subunit expression in NT2 cells and in vivo.
A) Representative immunoblots of total protein isolated from NT2 cells treated with progesterone for 48 h. Progesterone-dependent down-regulation of progesterone receptor (PR) was used as a positive control for the effect of progesterone. B) α5 protein levels are expressed as R.O.D. (relative optical density) normalized to GAPDH. A dose-dependent up-regulation of α5 was observed (F(3,20)=5.345, p<0.005). C) Partial α5 nAChR promoter sequence showing a putative progesterone binding site (bold and underlined). D) and E) Quantitative RT-PCR of α5 mRNA in mouse cortex, hippocampus and interpeduncular nucleus. D) Representative qPCR curves for α5 and 18S (normalization control) RNA in the cortex of female mice treated with oil (α5 oil and 18S oil) or progesterone (α5 p and 18S p, 1 μg/mouse for 24 h). Quantification was done at the level of the dotted line, which was manually set. E) Quantification of RNA signal (arbitrary units) from cortex, hippocampus and IPN of female mice treated with oil or progesterone. ANOVA effect of treatment (F(1, 18)=4.758, p<0.05. Posthoc LSD planned comparisons p(IPN)<0.05.
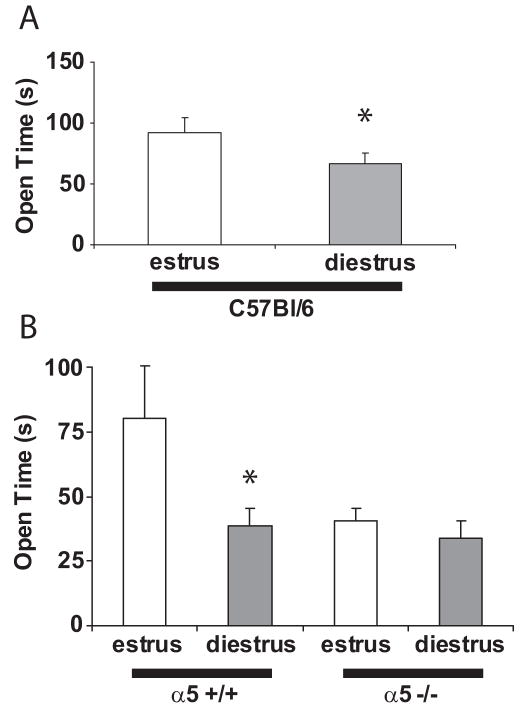
Figure 4. Effect of Estrous cycle on elevated plus maze behavior in C57BL/6J and α5 +/+ and α5−/− mutant mice.
A) Time in open arms, female C57BL/6J mice in estrus and diestrus (n=20 mice per group) *p<0.05. B) Time in open arms, female α5 +/+ and α5 −/− mice in estrus and diestrus (n=5 – 9 mice per group). *p<0.05 vs. same genotype, estrus vs. diestrus.
Behavioral tests
Behavioral tests were performed during the light phase, between 10 AM and 5 PM. The experimenter was blind to the genotypes of the mice. Once the experiments were concluded, mice were re-genotyped. For fig 1, mice were studied in the open field, light dark box, elevated plus and tail suspension test in that order, with at least a full day between experiments, but some mice were not tested on all experiments. Additional C57BL/6J and α5 mutant female mice were analyzed in behavioral studies that included ovarian cycle stage as one of the variables. In the behavioral experiments, a minimum of about 10 mice per genotype was used on each replication, and the experiments were done on two separate batches of mice. The data from different replications of the behavioral experiments were pooled after confirming that the groups showed the same results, unless specified. On the test day, mice were transferred from the colony room to the test room and were left undisturbed for at least 30 min prior to the start of the testing. The lighting conditions were kept stable at approximately 700 lux.
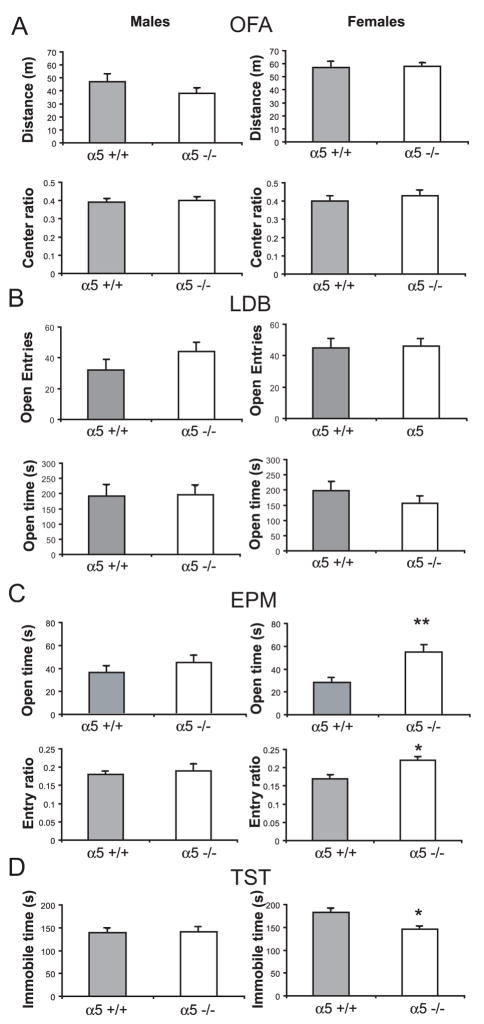
Figure 1. Locomotion, anxiety- and depression-related behaviors in the open field, light dark box, elevated plus maze and the tail suspension test in α5−/− mice.
Sex-specific averages (±S.E.M.) of A) Distance traveled and center/total distance ratio (open field activity, OFA). B) Entries to the open chamber and time in the open chamber (light/dark box, LDB). C) Time spent in open arms and open/total entries ratio (elevated plus maze, EPM). D) immobilization time (tail suspension test, TST) for wild-type (grey bars) and α5 −/− mice (white bars); n=15–33 mice per group. **p<0.01
Open field activity (OFA) test
Mice were placed in the center of an open field arena (40 × 40 cm) and movement was recorded for 30 min with a Versamax computer assisted tracking system (Accuscan Inc., Columbus, OH). The total distance traveled was used as a measure of locomotion. The ratio between the distance traveled in a 20 × 20 cm square in the center and the total distance traveled was calculated and used as a measure of anxiety-like behavior (Paylor et al. 1998). White noise (~55 dB) was present throughout the test.
Elevated plus maze (EPM)
Anxiety-like behavior was tested in the EPM test for 10 minutes. Briefly, mice were placed into a maze with two 25 × 7 cm corridors with black 15-cm high walls and two corridors with no walls, connected by a central square. The maze stood 50 cm above the floor. Time spent and percentages of entries into the open arms, which are measures of anxiety-like behavior (Pellow et al. 1985), were recorded with a computer-assisted system (The Observer, Noldus, The Netherlands). White noise (~55 dB) was present in the EPM room during adaptation to the room and test.
Light/dark box (LDB) test
Anxiety-like behavior was also tested in the LDB test for 20 minutes. Briefly, mice were placed into a maze (44 × 21 × 21 cm) with two chambers, one smaller (16 cm) and dark, one larger (28 cm) and lit, connected by an opening. Time spent and percentages of entries into the lit chamber were recorded with a computer-assisted system (The Observer, Noldus, The Netherlands). White noise (~55 dB) was present in the LDB room during adaptation to the room and test.
Tail suspension test (TST)
The tail suspension test was performed to study depression-like behavior (Trullas et al. 1989). Mice were taped by the tail to a metal bar connected to a transducer that transmitted movements to a computer. The time of immobility during a 6 min test was calculated using the software Tail suspension for Windows, Beta version 1.10 (Med Associates, St. Albans, VT).
Estrous cycle and anxiety-like behavioral tests
Determination of estrous cycle and behavior in EPM
The stages of estrous cycle were determined, before and after EPM, by analyzing cellular profiles of vaginal washes. All estrous cycle-related experiments were carried out in mice with well-established ovarian cycles. The estrus and diestrus phases (low and high progesterone levels, respectively) were chosen for comparison. Four hours after ovarian phase determination, mice were tested in the EPM as described above, and behavioral parameters were recorded and analyzed with the Anymaze tracking system (Stoelting Co., Wood Dale, IL).
Hormone analyses
Plasma corticosterone (CORT)
Experiments were performed between 4 PM and 6 PM at least two weeks after the behavioral tests. For the assessment of plasma CORT basal levels, mice were left undisturbed for 30 min, anesthetized and decapitated under anesthesia. Trunk blood was collected in ice-chilled heparinized tubes and stored at −20 °C. CORT was measured with an enzyme immunoassay kit (ALPCO, Windham, NH). The inter- and intra-assay coefficients of variance were 4.1% and 2.4% respectively, with a detection limit of 0.23 ng/ml.
Hypothalamic corticotropin releasing factor (CRF)
Brains were quickly removed and hypothalami were dissected under a microscope. Tissues were homogenized in 200 μl lysis buffer (10 mM Tris, pH 7.4, 1% phenylmethanesulfonyl fluoride) and after centrifugation (5000 g for 15 min at 4°C), the supernatant was treated following manufacturer’s instructions to extract and quantify CRF with a commercially available enzyme immunoassay kit (Phoenix Pharmaceuticals Inc., Belmont, CA). Samples were normalized to tissue protein concentration. Intra and inter-assay errors were less than 5% and 14%, respectively, and the detection limit was 0.1 ng/ml.
Cell culture
NT2 cell culture and gonadal steroid treatment
Human teratocarcinoma-derived NTera 2 (NT2) cells were kindly provided by Dr. Sadhan Majumder (University of Texas M. D. Anderson Cancer Center, Houston, Texas). These cells express the α5 nAChR subunit (Newman et al. 2002) and the progesterone (PR) and estrogen (ER) receptors (Pierson et al. 2005). NT2 cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, 1:1) medium, with 10% (v/v) fetal calf serum (HyClone, Logan, UT), 2 mM L-glutamine, penicillin and streptomycin (Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Media were changed every 2 days and cells were passaged when 60–80% confluent. Cells were maintained in culture for 5 days, followed by exposure to either progesterone (P4; 5, 10 or 50 nM), D-estradiol (E2; 0.05, 0.1 or 1 nM), or vehicle (0.1 % DMSO) in DMEM/F12 medium for 48 h.
Western-blot analysis
Cells were harvested with buffer A (in mM, Na2HPO4, 50; NaH2PO4, 50; pH 7.5; NaCl, 50; EDTA, 50; EGTA, 5; benzamidine, 5; iodoacetamide, 15; phenylmethylsulfonyl fluoride, 2) and pelleted by centrifugation. The pellets were rinsed three times with buffer A before buffer A with the addition of 3% Triton X-100 was used to solubilize nicotinic, progesterone (PR) and estrogen (ER) receptors. Protein concentration was determined using a BCA protein Assay (Pierce, Rockford, IL), and 100 μg of protein were loaded on 10% SDS-polyacrylamide gels, subjected to electrophoresis and transferred to a nitrocellulose membrane (BioRad, Hercules, CA). The membranes were blocked for 1 h with 5% nonfat dry milk and 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBS-T) at RT. The membranes were incubated with specific primary antibodies against either nAChR α5 subunit [AChRα5 (D-19) antibody, 1:500 dilution, Santa Cruz Biotechnologies, Santa Cruz, CA] or PR (1:500 dilution, DAKO, Carpinteria, CA) for 2 h at RT, followed by visualization with horseradish peroxidase-conjugated specific secondary antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA). Immunoreactive bands were detected with the SuperSignal substrate kit (Pierce, Rockford, IL). Western blots were quantified by measuring the relative optical densities (ROD) of the bands using the Scion image program (Scion Corp., Frederick, Maryland). Samples were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Quantitative RT-PCR
Female wild type mice were ovariectomized and 10 days later they were subcutaneously injected with 1 mg progesterone in sesame oil (5 mice) or sesame oil only (5 mice). Twenty four hours later their brains were rapidly dissected for tissue collection (hippocampus, cortex and midbrain including the interpeduncular nucleus). Using the RNAeasy mini kit (Qiagen, Valencia, CA), RNA was extracted and frozen until used. The RT reactions were prepared using the Superscript III kit (Invitrogen, Carlrsbad, CA) and PCR reactions were prepared with pfu DNA polymerase (Stratagene, La Jolla, CA), following manufacturer’s instructions. The PCR reactions (with primers specific for α5 nAChR subunit and for 18S RNA as a loading control) were run on a DNA Engine Opticon2 (MJ Research, Minneapolis, Mn) and the data was analyzed with the software package Opticon Monitor 3 (BioRad, Hercules, CA). Primers used were α5 Forward: 5′ CGTGTTCCTTGAGACTCTCTG; α5 Reverse: 5′ TAGTTTGCTGGCTGCGTCCAA; 18S Forward: 5′ ACCGCAGCTAGGAATAATGGA; 18S Reverse:5′ GCCTCAGTTCCGAAAACCA.
Statistical analysis and software
Data were tested for statistical significance by ANOVA with Neumann-Keuls post-hoc comparisons, or student t-test when appropriate. For RT-PCR data, LSD planned comparisons were used. P<0.05 was accepted as the level of significance. The putative α5 nAChR promoter sequence (~5 Kb upstream of the α5 cDNA in Chromosome 9) was screened for progesterone binding elements using the MatInspector software [Genomatix; (Cartharius et al. 2005)].
Results
Decreased anxiety- and depression-related behavior in α5 −/− female mice
In the OFA and LDB, no significant differences in behavior were found between α5 +/+ and α5 −/− mice, either males or females (Fig. 1A, B). In OFA, we performed an ANOVA with two independent variables (sex and genotype) and repeated measures (blocks of 2 min each). There was a significant effect of time (F(14, 224) = 1.9, p<0.05) and an interaction between time and sex (F(14,224) = 2.0, p<0.05). Since no effect of genotype (F(1, 16) = 2.0, p>0.1) or genotype x sex or genotype x time interaction were observed, the data was not further analyzed. In the DLB, there was no effect of genotype on transitions (F(1, 48) = 2.84, p>0.05) or time in light (F(1, 48) = 0.11, p>0.5), no effect of sex on transitions (F(1, 48) = 3.32, p> 0.05) or time in light (F(1, 48) = 0.021, p>0.5) and no sex x genotype interaction in transitions (F(1, 48) = 0.57, p>0.05) or time in light (F(1, 48) = 0.06, p>0.5). In contrast, in the EPM there was a significant effect of genotype on both time in open arm (F(1, 89) = 12.9, p<0.001) and entry ratio (F(1,89) = 4.08, p<0.05). In addition, there was a sex x genotype interaction on both time in open arm (F(1, 89) = 4.65, p<0.05 and entry ratio (F(1,89) = 4.56, p<0.05). On Newman-Keuls post hoc comparisons, mutant females were significantly different to both wild type females and to males, while no other comparison reached statistical significance. When we repeated the EPM experiment in a separate cohort of α5 +/+ and α5 −/− mice, we found a result consistent with the previous data. In these mice, the time in open arms were α5 +/+ female = 16 + 5; α5 −/− female = 43 ± 7 (p<0.01, LSD planned comparisons); α5 +/+ male = 34 ± 8; α5 −/− male = 29 ± 5 (p>0.05). The open/total entries ratios were α5 +/+ female = 0.17 + 0.04; α5 −/− female = 0.30 ± 0.04 (p<0.05); α5 +/+ male = 0.21 ± 0.04; α5 −/− male = 0.20 + 0.03 (p>0.05).
As shown before (Pelloux et al. 2005), immobilization time in the TST was greater in female than male wild type mice (Fig. 1D). In ANOVA, there was a significant effect of sex on mobility time (F(1, 106) = 5.02, p<0.05) and a significant sex x genotype interaction (F(1, 106) = 4.2, p<0.05). In Newman-Keuls post-hoc analysis, α5 +/+ female mice had significant less mobility than α5 −/− female, α5 −/− male, and α5 +/+ male mice (Fig 1D). No other comparison was significant.
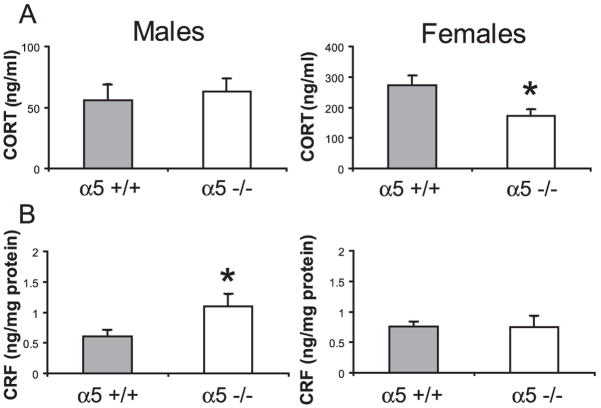
Lower basal plasma corticosterone levels in α5 mutant female mice and higher basal hypothalamic CRF levels in α5 −/− male mice
Figure 2A shows plasma CORT levels in basal conditions in control and α5 −/−mice. As already reported in the literature, female mice displayed higher CORT levels than males (Finn et al. 2004). In ANOVA, there was and effect of genotype (F(1, 68) = 7.88, p<0.01); an effect of sex (F(1, 68) = 49.4, p<0.0001); and an interaction (F(1, 68) = 14.7, p<0.001). On Newman-Keuls post hoc analysis, basal CORT levels for female α5 −/− mice were significantly lower than those for female controls (p< 0.001). No differences were found in CORT levels between control and α5 −/− male mice (Fig. 2A). IN CRF, ANOVA did not revealed significant effects of genotype (F(1, 49) = 2.89, p=0.1); sex (F(1, 49) = 0.53, p=0.5); or genotype x sex interaction (F(1, 68) = 3.50, p=0.07). Since there was a strong trend for a genotype x sex interaction and based on the CORT data, we performed an a priori LSD comparison. Baseline hypothalamic CRF levels were significantly higher in α5 −/− male mice compared to their littermates (LSD a priori comparison, p< 0.05) but no differences in CRF levels were found between control and α5 −/− females (Fig. 2B).
Figure 2. HPA hormone analysis in α5 −/− mice.
Sex-specific basal levels of A) plasma corticosterone. B) hypothalamic CRF in wild-type (grey bars) and α5 −/− (white bars) mice. n= 13–23 mice per group. *p<0.05
Progesterone-dependent up-regulation of α5 nAChR subunit expression in NT2 cells and in vivo
Upon binding of their ligand, steroid hormone receptors bind to specific hormone responsive elements on target genes and act as enhancers for regulation of transcription (Beato 1989). Because our results suggested that anxiety-like behaviors might be influenced by the levels of α5 protein in a sex-specific fashion, we tested the hypothesis that gonadal hormones affect the expression levels of the α5 subunit. These experiments were motivated by the presence of a putative progesterone responsive element in the promoter region of α5 (Fig. 3C). NT2 cells express nACh receptor subunits, including α5, as well as progesterone and estrogen receptors (Newman et al. 2002; Pierson et al. 2005). Exposure of NT2 cells to increasing doses of progesterone (5, 10, or 50 nM) for 48 h resulted in a significant increase in α5 nAChR subunit levels in a dose-dependent manner (F (3,20)= 5.3450, p< 0.01; Fig. 3A and 3B). Progesterone receptor levels, used as positive control for progesterone treatment, decreased in a dose-dependent manner [F (3,8)= 8.4670, p< 0.01], as expected (Wei et al. 1988) and Fig. 3A). NT2 cells were also exposed to different doses of estradiol (0.05, 0.1 and 1 nM) for 48 h and no changes were found compared to DMSO-treated cells (data not shown). These results demonstrate positive modulation of α5 nAChR subunit levels by progesterone but not estradiol. Extrapolation of our results to in vivo suggests dynamic alterations of α5 nAChR subunit levels over the estrous cycle. To further investigate the influence of progesterone on α5 expression levels we conducted quantitative RT-PCR experiments and found that ovariectomized female mice that received 1 μg progesterone 24 h before tissue harvesting had higher brain levels of α5 mRNA than animals receiving vehicle (F(1,28) = 4.728, p<0.05, LSD planned comparisons posthoc, IPN p<0.05, Fig. 3D, E).
Altered levels of anxiety-like behavior during the estrous cycle
In light of specific anxiogenic-like effects of the α5 nAChR subunit and its regulation by progesterone, we wanted to examine the levels of anxiety in female mice at different stages of the estrous cycle. We used the EPM test to measure anxiety-like behavior levels in female C57BL/6J mice with well-established ovarian cycles. Mice in diestrus, when progesterone is high (and therefore the α5 subunit levels should be high, according to Fig. 3), showed increased anxiety-like behavior. In fact, mice spent more time in the open arms of the maze during estrus (when progesterone is low) than during diestrus (F (1,31)= 4.3442, p< 0.05; Fig. 4A). No significant difference was found in the open entries/total entries ratio (not shown). These findings suggest the presence of a “partial” anxiolytic-like phenotype during the estrus phase, when progesterone and, in consequence α5 nAChRs levels, are low.
To further verify that changes in anxiety-related behavior during the estrous cycle are related to the α5 nAChR subunit we repeated the EPM experiment at estrus and diestrus, in α5 +/+ and α5 −/− mice. As seen in figure 4B, α5 +/+ mice behaved as more anxious during diestrus, like C57BL/6J mice did. In contrast, in α5 −/− mice no differences were found in EPM behavior between estrus and diestrus. In ANOVA, there was an effect of genotype (F(1, 22) = 5.32, p<0.05), no effect of cycle stage (F(1, 22) = 2.79, p>0.1), and a genotype x cycle stage interaction (F(1, 22) = 4.50, p<0.05). On LSD planned comparisons, there was a significant difference between estrus and diestrus for wild type (p<0.05) but not for mutant (p>0.1) mice. The data in Fig. 4B would suggest that the a5 −/− mice are actually more anxious than their wild-type littermates and this contradicts the results shown in Fig. 1C. However, these two figures should not be directly compared as Fig. 1 reflects the average behavior over four hormonal states (proestrus, estrus, metestrus, and diestrus) with unknown distribution among the various states. When animals are examined specifically during estrous and diestrus, diestrus is anxiogenic in wild type mice only. An alternative explanation is that the a5−/− mice might be more sensitive to the stress produced by daily vaginal swabs.
Finally, it should be noted that α5 mutant mice seem to breed at normal rates and that their estrous cycle is indistinguishable from that of wild type mice. If there is any reproductive abnormality in these mice it was undetectable during our observations.
Discussion
The deletion of the α5 nAChR subunit results in an anxiolytic-like effect in the EPM. No differences were observed between wild type and a5 −/− mice in OFA and LDB. However, it should be noted that in the OFA, the center ratio was higher than chance (0.25), possibly implying that under the experimental conditions used the center might not have been anxiogenic enough. We previously reported reduced anxiety-related behaviors in mice lacking the β4 nAChR subunit (Salas et al. 2003b), while other groups have shown changes in anxiety-like behavior in β3 (Booker et al. 2007) and α4 (Ross et al. 2000) null mice. Interestingly, these null mutations affect anxiety-like behaviors in a test-specific fashion, with no significant effects on the mildly anxiogenic OFA and LDB tests but a robust effect on the more stressful EPM test (Salas et al. 2003b). Another feature shared by α5 and β4 is that deletion of each subunit gene makes mice less sensitive to some of the behavioral effects of nicotine (Salas et al. 2004a;Salas et al. 2003a). Although the phenotype is not as strong as in β4 −/− mice, α5 −/− mice display diminished sensitivity to both nicotine-induced hypolocomotion and nicotine-induced seizures (Salas et al. 2003a). In addition, α5 and β4, together with α3, form a genomic cluster (Boulter et al. 1990), have overlapping promoter regulatory elements (McDonough et al. 2000) and assemble to form functional receptors (Wang et al. 1996). Therefore, it is tempting to speculate that the effects on anxiety are derived from channels composed of β4, β3, α4 and α5 subunits, or combinations. Given the diminished effects of nicotine on α3, α5 and β4 mutant mice (Salas et al. 2004a; Salas et al. 2003a; Salas et al. 2004b), it is possible that there is a link between nicotine’s effect and basal anxiety, which could partially explain the relationship between anxiety and tobacco use. In fact, very recent genome-wide association scan experiments have shown that variants in the region containing the α5, α3 and β4 subunits of nAChR are linked to increased tobacco smoke and lung cancer (Amos et al. 2008; Berrettini et al. 2008; Hung et al. 2008; Thorgeirsson et al. 2008). In addition, chronic nicotine treatment has been shown to be anxiolytic-like in female but not male C57BL/6J mice (Caldarone et al. 2008). Since chronic treatment is likely to desensitize receptors, and our data shows that a null mutation has a similar effect, it is reasonable to speculate that at least some of the effects of nicotine on anxiety are mediated by α5-containing nAChR desensitization.
Interestingly, the α5 null mutation leads to behavioral phenotypes in females only. Although sex differences have not been extensively studied in mice, sex-specific effects have been examined in the EPM paradigm. When hormone levels are not considered, C57BL/6J mice do not display sex differences in EPM behavior (Frick et al. 2000); and Fig. 1). However, fluctuations in behavior in the EPM occur in female mice as a function of the ovarian cycle (Galeeva & Tuohimaa 2001). These effects are attributable to cyclic changes in progesterone plasma levels. Plasma progesterone levels in mice are maximal during diestrus and lowest during estrus (Bastida et al. 2005; Maguire et al. 2005) while estrogen levels do not change in estrus vs. diestrus (Bastida et al. 2005). Similarly to Galeeva and colleagues (Galeeva & Tuohimaa 2001) we found that wild type female mice spent significantly more time in the open arms of the EPM during estrus than during diestrus.
Progesterone has two types of cellular effects. First, the classical effect of progesterone, termed “genomic effect”, is mediated by intracellular progesterone receptors that upon binding progesterone migrate to the nucleus and activate the promoter of several genes. This requires a considerable amount of time to take effect because gene transcription is a relatively slow process. The second type of effects of progesterone, termed “non-genomic”, is much faster as no gene transcription is required. The effects of progesterone on anxiety-like behavior are complex. When behavioral responses are measured shortly after injection of high doses of progesterone there is an anxiolytic-like effect (Reddy et al. 2005). This effect does not require the presence of progesterone receptors and is likely due to non-genomic actions of progesterone metabolites (Frye et al. 2006). However, when behavioral responses are tested few hours after progesterone treatment, the hormone has an anxiogenic rather than anxiolytic effect (Galeeva et al. 2003; Gulinello & Smith 2003), suggesting that the physiological effects of progesterone on anxiety likely reflect both genomic and non genomic mechanisms. Our results in the EPM indicate that the ability of α5-containing receptors to modulate anxiety might be a function of ovarian hormone levels. The presence of a putative progesterone responsive element in the promoter of the α5 nAChR subunit led to the testing of potential genomic effects of progesterone on α5 protein levels. We found that progesterone, at concentrations mimicking hormone levels found in estrus and diestrus (Bastida et al. 2005; Maguire et al. 2005), modulates the levels of α5. It has already been shown that GABAA receptor subunit expression and composition is modulated by progesterone both in vitro and in vivo (Biggio et al. 2001; Griffiths & Lovick 2005; Lovick 2006; Pierson et al. 2005; Weiland & Orchinik 1995) but this is the first report showing an effect of physiological concentrations of progesterone on nAChR subunit expression levels. Additional studies will be needed to address whether progesterone receptors actually bind and activate the α5 promoter or whether their influence occurs through other mechanisms. It should be noted, however, that mRNA levels are increased in vivo upon progesterone injection, suggesting a transcriptional effect. It has been previously reported that progesterone can block nAChRs expressed in Xenopus oocytes via allosteric mechanisms (Ke & Lukas 1996; Valera et al. 1992). However, the IC50 for such effect (~ 10 μM) is much higher than the values of plasma progesterone reported in the mouse (8 nM in estrus and 35 nM in diestrus (Maguire et al. 2005). Therefore, nAChR blockade by progesterone might not be relevant for the phenotypes observed in the α5 nAChR mutants. It is interesting that we found no estrogen effects on α5 nAChR subunit expression. Estrogen has been shown to affect anxiety-like behavior by different mechanisms (Walf & Frye 2006), but the effects of the estrous cycle on anxiety-related behavior seems to depend solely on the progesterone signal.
As previously described for CD1 mice (Pelloux et al. 2005), we found that wild type females have higher immobility time in the TST than wild type males. In addition, the α5 null mutation reduced immobility time in female mice but not in males. A similar result has been described for the deletion of the 5-HT1B receptor, a receptor thought to be involved in the mechanisms of anxiety. Female 5-HT1B null mice displayed ntidepressant-like responses in the TST whereas male null mice had no phenotype (Jones & Lucki 2005). An alternative interpretation is that immobility time in the tail suspension test may also reflect anxiety, as this is a procedure based on stress. The TST has been validated as a test sensitive to the effects of antidepressants (Trullas et al. 1989), but whether it truly reflects depression-like behavior is unknown.
Female α5 −/− mice have lower CORT levels than wild type females. Glucocorticoids increase depression-like behavior in rats in a dose-dependent manner (Johnson et al. 2006), and in humans, hypercortisolism is frequently observed in patients with major depression (Murphy 1991). Therefore, the sex-dependent differences in CORT levels between wild type and mutant mice might also explain the reduced depression-like behavior in α5 −/− females. It is intriguing that we found differences in CORT levels in female α5 −/− mice, which are concordant with the anxiolytic phenotype, but an opposite change in CRF levels in male α5 −/− mice. In general, females have much higher CORT levels than males, but in both there is an increase of CORT upon stress (Consoli et al. 2005). Considering the high expression levels of α5 in the adrenal gland (data not shown), a possible explanation might be that absence of α5 in the adrenals contributes to the decrease in plasma CORT levels. We hypothesize that in male mutants, a compensatory effect is present that is responsible for the increase in CRF, which in turn, would account for normal levels of CORT and normal anxiety behavior. Why this compensatory mechanism is absent in female α5 −/− mice, is just matter of speculation at this time.
Our results are important for the understanding of the influence of the nicotinic cholinergic system on anxiety and depression, especially in light of the relationship between stress-related disorders and nicotine addiction. Stress-related disorders such as anxiety, post-traumatic stress syndrome, and depression are often associated with chronic nicotine use (Laje et al. 2001; Markou et al. 1998; Picciotto et al. 2002). Depression and anxiety disorders are more common in women (Kessler et al. 1994), and women smokers with a history of depression are more likely to experience smoking relapse (Bock et al. 1996; Pomerleau et al. 2005). Furthermore, women may respond to nicotine differently than men (Perkins 2001), and might be less sensitive to pharmacological interventions aimed at smoking cessation (Evans et al. 2006; Perkins 2001). Because we found that lack of α5 reduces anxiety- and depression-like responses, and reduces the sensitivity to certain behavioral effects of nicotine (Salas et al. 2003a), antagonists selective for α5-containing nAChRs might prove beneficial for smoking cessation interventions, especially in female smokers.
Acknowledgments
This work was supported by the National Institute of Drug Abuse (NIDA) grant DA17173 to MDB. We thank Dr. Gad Shaulsky, Dr. Adam Kuspa and Rocío benabentos for technical help with the RT-PCR experiments.
References
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Tejada F, Penafiel R. Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization. Endocrinology. 2005;146:666–674. doi: 10.1210/en.2004-1004. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABAA-receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–241. doi: 10.1016/s0074-7742(01)46064-8. [DOI] [PubMed] [Google Scholar]
- Bock BC, Goldstein MG, Marcus BH. Depression following smoking cessation in women. J Subst Abuse. 1996;8:137–144. doi: 10.1016/s0899-3289(96)90151-0. [DOI] [PubMed] [Google Scholar]
- Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacology Biochemistry and Behavior. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, et al. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Bracke P. Sex differences in the course of depression: evidence from a longitudinal study of a representative sample of the Belgian population. Social Psychiatry & Psychiatric Epidemiology. 1998;33:420–429. doi: 10.1007/s001270050075. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Research. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, McAllister CG, Antelman SM, Perkins KA. The role of corticosteroids in nicotine’s physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience Letters. 2008;439:187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test) European journal of pharmacology. 2005;520:100–107. doi: 10.1016/j.ejphar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. discussion 31–22, 64–27. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers’ gender. Exp Clin Psychopharmacol. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. Eur J Pharmacol. 2000;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, Pfaff DW, Rhodes ME. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- Galeeva AY, Tuohimaa P, Shalyapina VG. The role of sex steroids in forming anxiety states in female mice. Neurosci Behav Physiol. 2003;33:415–420. doi: 10.1023/a:1022864011385. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, Sivilotti LG. Formation of functional alpha3beta4alpha5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br J Pharmacol. 2001;134:789–796. doi: 10.1038/sj.bjp.0704313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30:1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- Ke L, Lukas RJ. Effects of steroid exposure on ligand binding and functional activities of diverse nicotinic acetylcholine receptor subtypes. J Neurochem. 1996;67:1100–1112. doi: 10.1046/j.1471-4159.1996.67031100.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje RP, Berman JA, Glassman AH. Depression and nicotine: preclinical and clinical evidence for common mechanisms. Curr Psychiatry Rep. 2001;3:470–474. doi: 10.1007/s11920-001-0040-z. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle - implications for premenstrual syndrome in women. Exp Physiol. 2006 doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McDonough J, Francis N, Miller T, Deneris ES. Regulation of transcription in the neuronal nicotinic receptor subunit gene cluster by a neuron-selective enhancer and ETS domain factors. J Biol Chem. 2000;275:28962–28970. doi: 10.1074/jbc.M004181200. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol. 1991;38:537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- Newman MB, Kuo YP, Lukas RJ, Sanberg PR, Douglas Shytle R, McGrogan MP, Zigova T. Nicotinic acetylcholine receptors on NT2 precursor cells and hNT (NT2-N) neurons. Brain Res Dev Brain Res. 2002;139:73–86. doi: 10.1016/s0165-3806(02)00513-8. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensimotor gating: a behavioral characterization of Acra7-deficient mice. Learning and Memory. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Hagues G, Costentin J, Duterte-Boucher D. Helplessness in the tail suspension test is associated with an increase in ethanol intake and its rewarding effect in female mice. Alcohol Clin Exp Res. 2005;29:378–388. doi: 10.1097/01.alc.0000156123.10298.fa. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, LeNovere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Pierson RC, Lyons AM, Greenfield LJ., Jr Gonadal steroids regulate GABAA receptor subunit mRNA expression in NT2-N neurons. Brain Res Mol Brain Res. 2005;138:105–115. doi: 10.1016/j.molbrainres.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol Biochem Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide R, Beaudet AL, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates acute effects of nicotine in vivo. Molecular Pharmacology. 2003a;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004b;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered Anxiety-Related Responses in Mutant Mice Lacking the β4 Subunit of the Nicotinic Receptor. J Neurosci. 2003b;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Jackson B, Skolnick P. Genetic differences in a tail suspension test for evaluating antidepressant activity. Psychopharmacology (Berl) 1989;99:287–288. doi: 10.1007/BF00442824. [DOI] [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Wei LL, Krett NL, Francis MD, Gordon DF, Wood WM, O’Malley BW, Horwitz KB. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Molecular Endocrinology. 1988;2:62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res. 1995;32:271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]