Abstract
Background and Aims
Although much is known about the vegetative traits associated with early monocot evolution, less is known about the reproductive features of early monocotyledonous lineages. A study was made of the embryology of Tofieldia glutinosa, a member of an early divergent monocot clade (Tofieldiaceae), and aspects of its development were compared with the development of other early divergent monocots in order to gain insight into defining reproductive features of early monocots.
Methods
Field-collected developing gynoecial tissues of Tofieldia glutinosa were prepared for histological examination. Over 600 ovules were sectioned and studied using brightfield, differential interference contrast, and fluorescence microscopy. High-resolution digital imaging was used to document important stages of megasporogenesis, megagametogenesis and early endosperm development.
Key Results
Development of the female gametophyte in T. glutinosa is of a modified Polygonum-type. At maturity the female gametophyte is seven-celled and 11-nucleate with a standard three-celled egg apparatus, a binucleate central cell (where ultimately, the two polar nuclei will fuse into a diploid secondary nucleus) and three binucleate antipodal cells. The antipodal nuclei persist past fertilization, and the process of double fertilization appears to yield a diploid zygote and triploid primary endosperm cell, as is characteristic of plants with Polygonum-type female gametophytes. Endosperm development is helobial, and free-nuclear growth initially proceeds at equal rates in both the micropylar and chalazal endosperm chambers.
Conclusions
The analysis suggests that the shared common ancestor of monocots possessed persistent and proliferating antipodals similar to those found in T. glutinosa and other early-divergent monocots (e.g. Acorus and members of the Araceae). Helobial endosperm among monocots evolved once in the common ancestor of all monocots excluding Acorus. Thus, the analysis further suggests that helobial endosperm in monocots is homoplasious with those helobial endosperms that are present in water lilies and eudicot angiosperms.
Key words: Tofieldia, Tofieldiaceae, Alismatales, monocots, embryology, female gametophyte, antipodals, development, endosperm
INTRODUCTION
Collectively, monocots are a remarkably diverse (Cronquist, 1988; Davis et al., 2004, 2006; Soltis et al., 2005, 2007) and ancient clade of flowering plants (Dahlgren et al., 1985, Cronquist, 1988; Friis et al., 2004; Soltis et al., 2005). From this perspective, reconstructing the biological features that defined the first or ancestral monocots might be viewed as a daunting task. Nevertheless, probable synapomorphies of monocots include a long list of vegetative features: the presence of a single cotyledon in the mature embryo, rhizomatous growth, shoot-borne roots, loss of a vascular cambium (and secondary growth), loss of vessel elements in stems and leaves, a dissected stele (atactostele) and parallel leaf venation (Arber, 1925; Dahlgren et al., 1985; Cronquist, 1988; Stevens, 2001; although see Stevens, 2006, for discussion of some disputed synapomorphic characters).
In contrast to the numerous vegetative synapomorphies of monocots, far less is known about the reproductive features that defined the first monocots. Helobial endosperm development is more common in monocots than in ‘dicots’ (Palser, 1975; Dahlgren et al., 1985) but does not occur in the shared common ancestor of all monocots (Floyd and Friedman, 2000; Stevens, 2001). Septal nectaries are restricted to the monocots, and appear early in the evolution of this clade (Igersheim et al., 2001). Successive wall formation during microsporogenesis can be traced back to the shared common ancestor of monocots (Furness et al., 2002), and early monocots (like the majority of angiosperms) inherited Polygonum-type female gametophyte development from their shared common ancestor with eudicots, magnoliids and Chloranthaceae (Dahlgren et al., 1985; Williams and Friedman, 2004).
To shed further light on the origin, early evolution and diversification of embryological characters within monocots, Tofieldia glutinosa (Tofieldiaceae) was chosen for the present examination. Recent phylogenetic analyses place Tofieldia in or closely allied with the Alismatales, an early-diverging clade within monocots (Soltis et al., 2000, 2007; APG II, 2003; Davis et al., 2004, 2006; Graham et al., 2006), with a fossil record extending back 110 to 120 million years (Friis et al., 2004). Within Alismatales, Graham et al. (2006) place Tofieldiaceae as sister to all remaining alismatids, and Soltis et al. (2007) place Tofieldiaceae as sister to all alismatids except the Araceae. Davis et al. (2004) provide the most detailed phylogeny of the Alismatales (sensu lato), placing Tofieldiaceae as sister to all alismatids except the Araceae, while Davis et al. (2006) present an unresolved polytomy involving Tofieldiaceae, Araceae, and a clade that includes Acorales and the remaining alismatids (see Graham et al., 2006, for discussion of conflicting phylogenetic signals in the rbcL and atpA data sets of Davis et al., 2004, 2006). These molecular phylogenetic accounts stand in contrast to previous (morphologically based) classifications that placed Tofieldia within the Melanthiaceae (now Liliales; Dahlgren et al., 1985) and Petrosaviaceae (now Petrosaviales; Tamura, 1998). The currently accepted phylogenetic position of Tofieldia makes it ideal for the examination of evolutionary patterns among early divergent monocots and the reconstruction of defining characteristics of the first monocots.
In this report, an account of female gametophyte development in Tofieldia glutinosa is provided, with special attention given to megasporogenesis, the behaviour of the antipodals, the fertilization process, and early endosperm development. Tofieldia had been studied previously by Seelieb (1924) and Sokoloska-Kulczycka (1980, 1985), but as will be demonstrated, some features of its embryology may have been mischaracterized. In addition to the embryological examination of Tofieldia, several reproductive features that are likely to have characterized the common ancestor of the Alismatales, as well as other early-divergent clades of monocots, are reconstructed. The overriding goal in this phylogenetically based comparative analysis of monocot female gametophyte and endosperm patterns of development is to identify the embryological features that defined and shaped the early evolution and diversification of this remarkable clade.
MATERIALS AND METHODS
Tofieldia glutinosa is a small, perennial herb native to North America from Alaska south to Oregon and Wyoming (Hitchcock, 1944a, b; Packer, 1993). It is synonymous with Narthecium glutinosum Michx. and Triantha glutinosa (Michx.) Baker. Tofieldia species have hermaphroditic flowers with three partially fused carpels (carpels are post-genitally united, and congentially united only at the base; Igersheim et al., 2001), each containing numerous ovules, surrounded by a whorl of anthers. Flowers and buds at various stages of development were collected in July 2006, June 2007 and July 2007 from South Prairie Bog (Gifford Pinchot National Forest) in Washington (US Department of Agriculture – Forest Service permit number 42-M03966-G).
Flowers were fixed for 24 h in 3 : 1 (95 % ethanol:acetic acid) and stored in 70 % ethanol or fixed in ‘triple fix’ (2 % formaldehyde, 1 % glutaraldehyde, 2 % acrolein in Pipes buffer), rinsed, and stored in Pipes buffer (pH 6·8, with 5 mm EGTA and 1 m MgSO4). Gynoecial tissues were dehydrated through a graded ethanol series, then infiltrated and embedded with glycol methacrylate (JB-4 embedding kit; Electron Microscopy Sciences, Hatfield, PA, USA). Embedded tissue was serially sectioned into 4-μm-thick ribbons and mounted onto slides. Slides were stained with either 0·1 % toluidine blue or 0·25 µg mL−1 of DAPI (4′,6-diamidino-2-phenylindole) in 0·05 m Tris (pH 7·2). A Zeiss Axiophot microscope (Carl Ziess, Oberkochen, Germany) equipped with a Zeiss Axiocam digital camera was used for digital imaging using brightfield, DIC and fluorescence optics. Fluorescence was visualized with an HBO 100-W burner with excitation filter (365 nm, band pass 12 nm), dichroic mirror (FT395; Zeiss) and barrier filter (LP397; Zeiss). Images were processed with Adobe Photoshop CS2 (Adobe Systems, San Jose, CA, USA). All Photoshop operations were applied to the entire image except as noted in figure captions.
Parsimony analyses were performed using the program MacClade (Sinauer, Sunderland, MA, USA). The phylogenies used in the present analyses are based on publications by Graham et al. (2006) and Soltis et al. (2007).
RESULTS
Megasporogenesis
Previous studies suggested that megasporogenesis in Tofieldia calyculata is variable, yielding either one or two functional megaspores (Sokolowska-Kulczycka, 1980, 1985). Consequently, the female gametophytes of T. calyculata were reported to be of the bisporic Allium-type, the Veratrum Lobelianum-type (a variant of the Allium-type), or the monosporic Polygonum-type (seven-celled, eight-nucleate). Bisporic types of female gametophytes were reported to develop approximately twice as frequently as the monosporic Polygonum-type (Sokolowska-Kulczycka, 1980, 1985). However, no evidence of bisporic female gametophyte development in the related species T. glutinosa was found after examining 110 ovules in various stages of megasporogenesis.
The megasporocyte of T. glutinosa contains a large, conspicuous nucleus (Fig. 1A). Meiosis I produces two dyad cells (Fig. 1B, C). In the majority of cases, the micropylar dyad cell is smaller from its inception (Fig. 1C). Meiosis II proceeds normally in the chalazal dyad cell, producing two unequally sized megaspores, of which the chalazal-most cell is larger (Fig. 1D). In the micropylar dyad, meiosis II frequently aborts during prophase II. In these instances, condensed chromosomes can be seen as long as the cell remains distinctly visible, up to and through the earliest stages of female gametophyte development (Fig. 1D). When meiosis II is completed in both the micropylar and chalazal dyads, a tetrad of megaspores is produced. Of these only the chalazal-most megaspore is functional (Fig. 1E). Thus, the development of the female gametophyte of T. glutinosa is strictly monosporic. The remaining three (or two, if meiosis II aborts in the micropylar dyad) nonfunctional megaspores (Fig. 1F) are only visible as distinct cells until the first free-nuclear mitotic division of the functional megaspore, at which point the degenerating megaspores become indistinguishable from one another and appear only as a dark-staining mass (Fig. 2A). The functional megaspore is approx. 35–50 µm long and 12–20 µm wide.
Fig. 1.
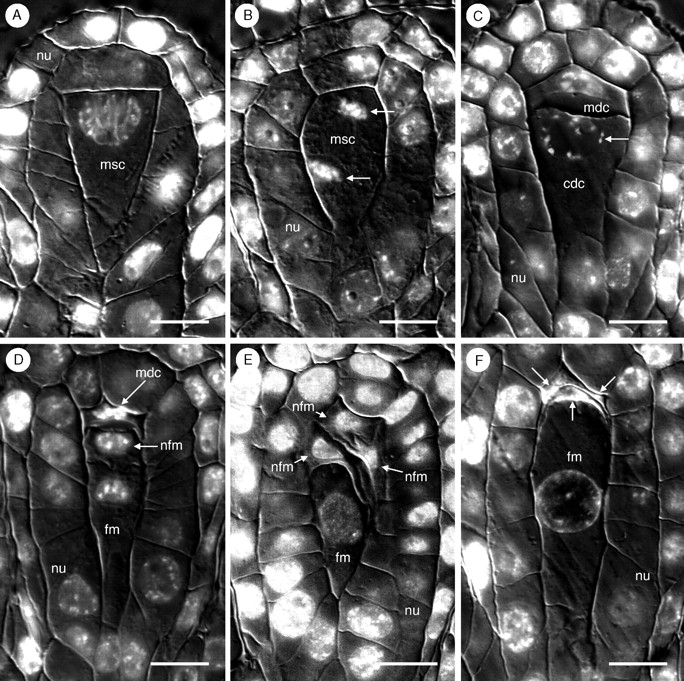
Megasporogenesis in Tofieldia glutinosa produces a single functional megaspore. (A) Megasporocyte surrounded by two cell layers of nucellus. (B) First meiotic division of megasporocyte. Arrows indicate nuclei derived from meiosis I. (C) Dyad stage. The chalazal dyad cell, with nucleus in prophase (arrow) is significantly larger than the micropylar dyad cell. (D) Completion of meiosis II in the chalazal dyad to produce a functional megaspore. The micropylar dyad cell is arrested in prophase II. (E) Tetrad stage in which meiosis II was completed in both the micropylar and chalazal dyad cells. The chalazal-most cell is the functional megaspore. (F) Functional megaspore with three crushed and degenerated megaspores (arrows). All figures are oriented such that the micropylar pole is at the top and the chalazal pole is at the bottom. All sections were stained with the DNA fluorochrome DAPI and visualized with differential interference contrast (DIC) and fluorescence optics. Images are composites of two micrographs of the same section (one with fluorescence and one with DIC). cdc, Chalazal dyad cell; fm, functional megaspore; mdc, micropylar dyad cell; msc, megasporocyte; nfm, nonfunctional megaspore; nu, nucellus. Scale bars = 10 µm.
Fig. 2.
Syncytial development of the female gametophyte in Tofieldia glutinosa. (A) Two-nucleate stage with nuclei (arrows) located at each pole of the female gametophyte and separated by a large central vacuole. Degenerated megaspores are still visible, but not discernable from one another. (B) Transition to a four-nucleate female gametophyte. The two nuclei (arrows) at the micropylar pole are emerging from telophase while the two sets of chromosomes (arrows) at the chalazal pole are still in telophase. Note that the mitotic divisions are perpendicular to one another. (C) Four-nucleate (arrows) female gametophyte. (D) Eight-nucleate female gametophyte prior to cellularization and migration of polar nuclei. All sections were stained with toluidine blue. The red box indicates digital superposition of the nucleus from the adjacent histological section. cv, Central vacuole; dm, degenerate megaspores; int, integument; nu, nucellus; pn, polar nucleus. Scale bars = 10 µm.
Female gametophyte development
Syncytial development of the female gametophyte of T. glutinosa is fundamentally similar to that of most angiosperms with a Polygonum-type female gametophyte. The female gametophyte becomes polarized following the first free nuclear mitotic division of the functional megaspore, with one nucleus situated at the micropylar pole of the female gametophyte, and the other at the chalazal pole (Fig. 2A). The two nuclei border a large central vacuole that fills virtually all of the space between them (Fig. 2A). At this stage, the female gametophyte is approx. 50–65 µm in length and 20–30 µm in width. A second wave of free nuclear mitosis (Fig. 2B) yields a pair of nuclei at each pole of the female gametophyte (Fig. 2C). These mitotic divisions occur perpendicular to each other (Fig. 2B). At the four-nucleate stage, the female gametophyte ranges from 65 µm to 85 µm in length and 25 to 35 µm in width. All four nuclei undergo a third mitotic division to produce four nuclei at each pole of the female gametophyte (Fig. 2D).
Upon cellularization, the egg apparatus, which is comprised of a single egg cell and a pair of synergids, is confined to a relatively small space between the central cell and the nucellus at the micropylar pole of the female gametophyte (Fig. 3A, B). All three cells of the egg apparatus are densely cytoplasmic and each has a wall in contact with the micropylar boundary of the female gametophyte (Fig. 3A, B), although in non-median sections, the egg cell often appears to be positioned below the synergids. The two polar nuclei, which are initially positioned at opposite poles, migrate toward the centre of the female gametophyte and meet along the periphery of the central cell, approximately one-half to two-thirds of the distance from the micropylar end of the female gametophyte (Fig. 3C). When the polar nuclei meet, the female gametophyte is approx. 80–100 µm long and 30–40 µm wide. As the female gametophyte grows, the egg apparatus also expands, protruding into the central cell (Fig. 3D). A rudimentary filiform apparatus is recognizable inside the synergids at this stage (Fig. 3D). The polar nuclei fuse prior to fertilization to form a large secondary nucleus with a single large nucleolus (Fig. 3E). This fusion occurs near the spot where the polar nuclei initially come in contact with each other, and is followed by the migration of the secondary nucleus towards the chalazal end of the central cell.
Fig. 3.
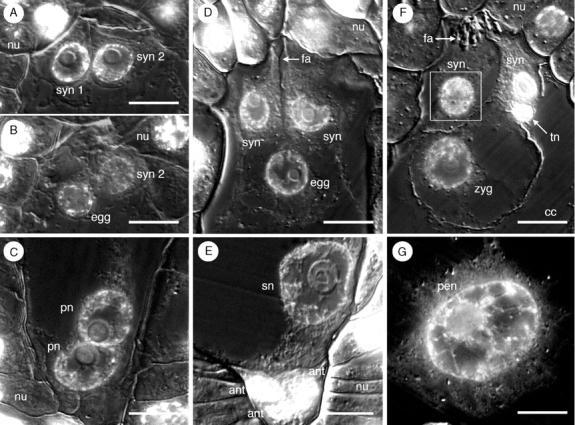
Development of egg apparatus and central cell in Tofieldia glutinosa. (A) and (B) Serial sections of synergid cells and egg in recently cellularized female gametophyte (prior to fusion of the polar nuclei). At this stage, cells of the egg apparatus are confined to a narrow region between the central cell and the micropylar pole of the female gametophyte. syn 2 refers to the same synergid in (A) and (B). (C) Polar nuclei just prior to their fusion. (D) Mature egg apparatus containing an egg cell, two synergids and a rudimentary filiform apparatus. Cells of the egg apparatus are much larger at this stage than in previous stages. (E) Mature central cell containing a secondary nucleus, seen in close proximity to the antipodals. (F) Post-fertilization egg apparatus containing a zygote, two synergids, a pronounced filiform apparatus, and a tube nucleus (from a pollen tube). (G) Post-fertilization central cell containing a large primary endosperm nucleus. All sections were stained with the DNA fluorochrome DAPI and visualized with differential interference contrast (DIC) and fluorescence optics. Images are composites of two micrographs of the same section (one with fluorescence and one with DIC). The white box indicates digital superposition of the nucleus from the adjacent histological section. ant, Antipodal cell; cc, central cell; egg, egg cell; fa, filiform apparatus; nu, nucellus; pen, primary endosperm nucleus; pn, polar nucleus; sn, secondary nucleus; syn, synergid cell; tn, tube nucleus; zyg, zygote. Scale bars = 10 μm.
At maturity (as denoted by a secondary nucleus in the central cell), the egg cell is highly vacuolate compared with the synergids, which remain densely cytoplasmic (Fig. 3D, F). The egg nucleus, which is initially positioned in the centre of the egg cell (Fig. 3D), occupies the periphery of the egg cell by the time fertilization occurs. The zygote nucleus is also seen in this position, close to the cell wall (Fig. 3F). Following fertilization (see below) the primary endosperm nucleus (the result of a fusion event involving the secondary nucleus and a second sperm nucleus; Fig. 3G) is located in close proximity to the antipodal cells at the extreme chalazal end of the central cell. At the time of fertilization, the female gametophyte measures as much as 205 µm long and 110 µm wide.
Double fertilization
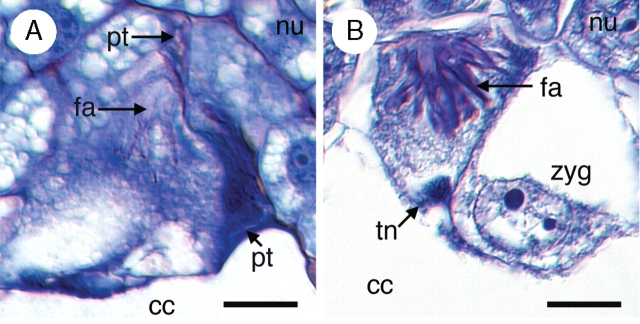
Following pollination, numerous pollen tubes can be seen penetrating the stigma (Fig. 4A) and growing through the stylar canal (Fig. 4B). Upon entering the ovary, pollen tubes radiate in all directions until they reach receptive ovules, at which point individual pollen tubes grow through the micropyle (Fig. 4C) and penetrate the nucellus of ovules (Fig. 4D). Pollen tube growth through the nucellus is intercellular (Fig. 4E). The pollen tube enters the female gametophyte through one of the two synergids, where it deposits two sperm. The remnants of the pollen tube, a single tube nucleus, and the filiform apparatus (which becomes much more elaborate in advance of fertilization) remain visible inside the degenerating synergid for some time after fertilization (Fig. 5A, B; also see Fig. 3F). The zygote nucleus typically contains two nucleoli (Fig. 5B) and remains undivided during the early stages of endosperm development (see below).
Fig. 4.
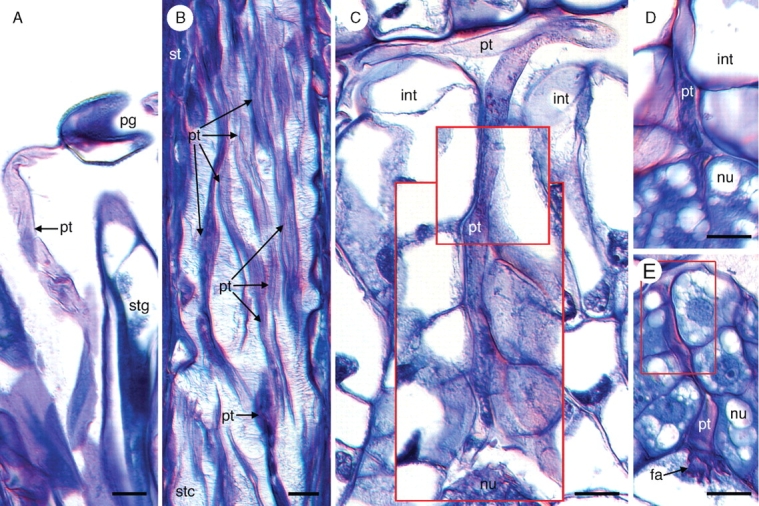
Pollen tube growth in Tofieldia glutinosa. (A) Pollen tube growing out of a pollen grain on the stigma. (B) Numerous pollen tubes grow through the stylar canal. (C) Pollen tube growth through the micropyle. This image is a composite of three serial histological sections of the same ovule. Red boxes indicate digital superposition of pollen tube and surrounding tissue from adjacent histological sections. (D) Penetration of the nucellus by a pollen tube. Pollen tube growth through the nucellus is intercellular. (E) Pollen tube growth through the nucellus and into the filiform apparatus of the female gametophyte. The red box indicates digital superposition of the pollen tube and surrounding tissue from the adjacent histological section. All sections were stained with toluidine blue. fa, Filiform apparatus; int, integument; nu, nucellus; pg, pollen grain; pt, pollen tube; st, style; stc, stylar canal; stg, stigma. Scale bars = 10 µm.
Fig. 5.
Post-fertilization egg apparatus in Tofieldia glutinosa. (A) Remnants of the pollen tube are visible at the micropylar pole of the female gametophyte. (B) A well-developed filiform apparatus inside the degenerated synergid cell. The tube nucleus is still visible at this stage. Sections were stained with toluidine blue and visualized with DIC optics. cc, Central cell; fa, filiform apparatus; nu, nucellus; syn, synergid cell; tn, pollen tube nucleus; zyg, zygote. Scale bars = 10 µm.
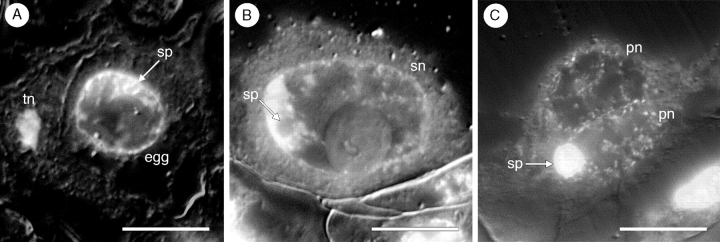
Three fertilization events and over 100 fertilized female gametophytes were observed. One sperm nucleus fuses with the egg nucleus (Fig. 6A) and the second sperm nucleus fuses with the secondary nucleus (Fig. 6B). Double fertilization appears to be synchronous, since in those three fertilization events neither the central cell nor the egg cell was found to be fertilized when the other was not. Although the polar nuclei usually fuse prior to fertilization, one instance was observed in which the second fertilization event involved the triple fusion of two unfused polar nuclei and a single sperm nucleus (Fig. 6C).
Fig. 6.
Double fertilization in Tofieldia glutinosa. (A) Fertilization of the egg nucleus. The sperm nucleus is still visible within the egg nucleus and the tube nucleus is present in the adjacent synergid. (B) Fertilization of the secondary nucleus by the second sperm nucleus. The sperm nucleus is still visible (with nucleolus) within the secondary nucleus. (C) Second fertilization event involving two unfused polar nuclei and a sperm nucleus. All sections were stained with the DNA fluorochrome DAPI and visualized with differential interference contrast (DIC) and fluorescence optics. Images are composites of two micrographs of the same section (one with fluorescence and one with DIC). egg, Egg cell; pn, polar nucleus; sn, secondary nucleus; sp, sperm nucleus; syn, synergid cell; tn, tube nucleus. Scale bars = 10 µm.
Early endosperm development
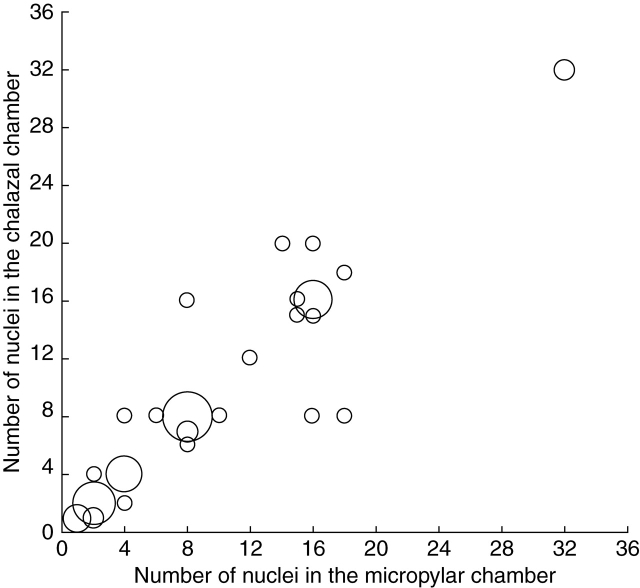
After the first mitotic division of the primary endosperm nucleus (Fig. 7A), a wall forms between the two daughter nuclei and partitions the former central cell into two chambers: a large micropylar chamber and a significantly smaller chalazal chamber (Fig. 7B, C). Free-nuclear mitosis is initiated at near-equal rates in each chamber (Fig. 8). This differs slightly from endosperm development in T. calyculata, where free-nuclear development in the chalazal chamber is limited from the onset in the vast majority of cases (Sokolowska-Kulczycka, 1980). Despite initially near-equal rates of free-nuclear development, the chalazal chamber, which is more densely cytoplasmic, remains the smaller of the two chambers. Endosperms containing up to 64 nuclei (32 in each chamber) were observed in this study, and at that stage, the zygote had not yet divided.
Fig. 7.
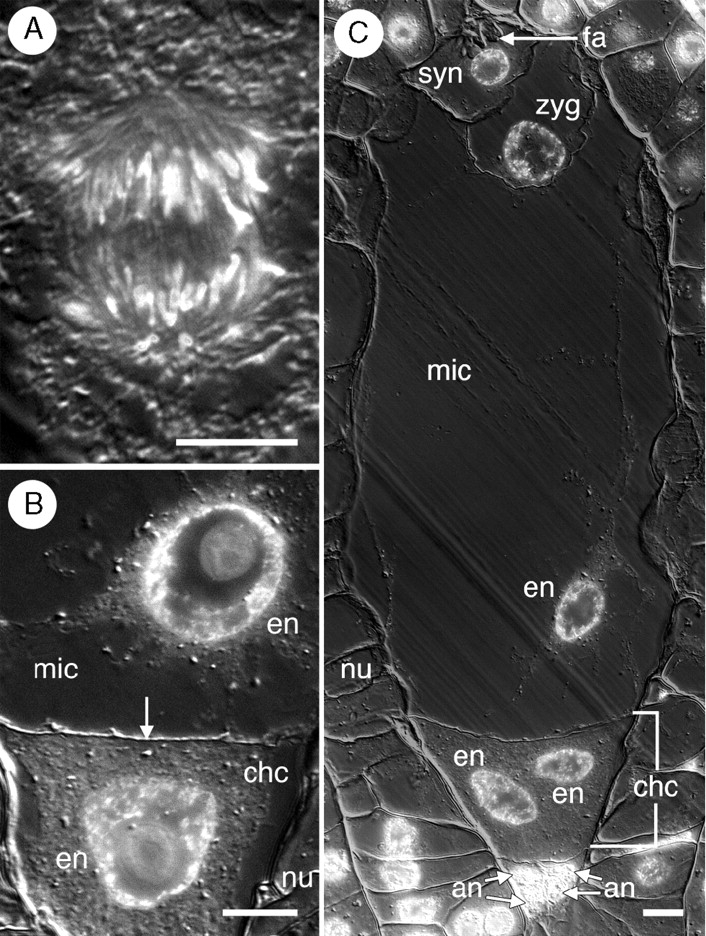
Helobial endosperm development in Tofieldia glutinosa. (A) The first mitotic division of the primary endosperm nucleus occurs in the bottom half of the central cell. (B) Two-celled, two-nucleate endosperm. A wall (arrow) forms between the two daughter nuclei of the primary endosperm nucleus partitioning the central cell into a large micropylar chamber and a smaller chalazal chamber. (C) Post-fertilization female gametophyte with binucleate chalazal endosperm chamber and binucleate (one nucleus is visible in this section) micropylar endosperm chamber. The zygote is visible at the micropylar pole. One synergid, the filiform apparatus, and four of six antipodal nuclei are also visible in this section. All sections were stained with DAPI and visualized with DIC and fluorescence optics. All images are composites of two micrographs of the same section (one utilizing fluorescence optics and one utilizing DIC optics). an, Antipodal nuclei; chc, chalazal chamber of endosperm; en, endosperm nucleus; fa, filiform apparatus; mic, micropylar chamber of endosperm; nu, nucellus; syn, synergid cell; zyg, zygote. Scale bars = 10 µm.
Fig. 8.
Relative rates of syncytial development in the micropylar and chalazal endosperm chambers in Tofieldia glutinosa. Endosperm nuclear proliferation occurs at roughly equal rates in each chamber. Endosperm beyond the 64-nucleate stage was not observed. The size of each data point is proportional to the number of times the specific endosperm configuration was observed.
Antipodal proliferation
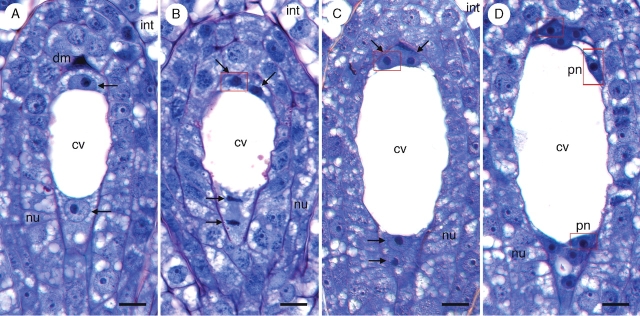
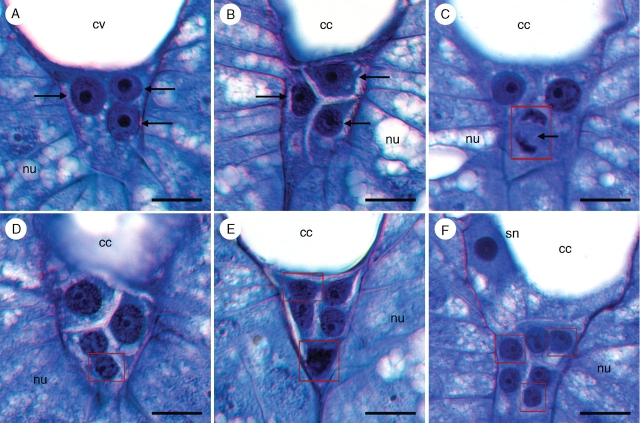
At the end of the syncytial phase of female gametophyte development, three antipodal nuclei occupy a narrow, densely cytoplasmic pocket at the chalazal end of the female gametophyte (Fig. 9A). Their nuclei stain darkly and each contains a pronounced nucleolus (Fig. 9). Cellularization of the chalazal region yields three uninucleate antipodal cells (Fig. 9B). As the female gametophyte matures, each of the three antipodal cells undergoes a single free-nuclear division (Fig. 9C–E). These nuclear divisions occur prior to fertilization and are usually asynchronous. The order in which antipodal nuclei divide seems to be irregular, and on only one occasion were all three antipodal nuclei seen dividing simultaneously. Additional cell divisions do not occur, so the number of antipodal cells remains fixed at three. Of the 396 mature prefertilization female gametophytes examined, 90 contained three antipodal nuclei, 39 contained four nuclei, 58 contained five nuclei, and 208 contained a full complement of six antipodal nuclei. Just prior to fertilization, the female gametophyte of T. glutinosa is seven-celled and ten-nucleate (the two polar nuclei in the central cell having fused to yield a single secondary nucleus). The binucleate antipodal cells (Fig. 9F) persist past fertilization and are still visible when endosperm begins to develop (Fig. 7). On one occasion, eight antipodal nuclei were observed, and, in this instance, there were two binucleate cells and a single cell containing four nuclei.
Fig. 9.
Proliferation of antipodal nuclei in Tofieldia glutinosa. (A) Three antipodal nuclei (arrows) prior to cellularization of the female gametophyte. (B) Three uninucleate antipodal cells (arrows) following cellularization of the female gametophyte. (C) Antipodal nucleus in mitosis (arrow). Mitotic divisions of the antipodals are usually asynchronous. (D) Single binucleate antipodal and two uninucleate antipodals. (E) Two binucleate antipodals and a single uninucleate antipodal. (F) Three binucleate antipodal cells. The secondary nucleus is also visible in this section. All sections were stained with toluidine blue. Red boxes indicate digital superposition of nuclei from adjacent histological sections. cc, Central cell; cv, central vacuole; nu, nucellus; sn, secondary nucleus. Scale bars = 10 µm.
After the endosperm proceeds through three or four rounds of mitosis, the antipodal nuclei become less discernable from one another, and are often small and irregularly shaped. Degenerate antipodals are visible in the female gametophyte for some time after fertilization. Very little is left of the antipodals by the time endosperm development reaches the two-celled, 64-nucleate stage.
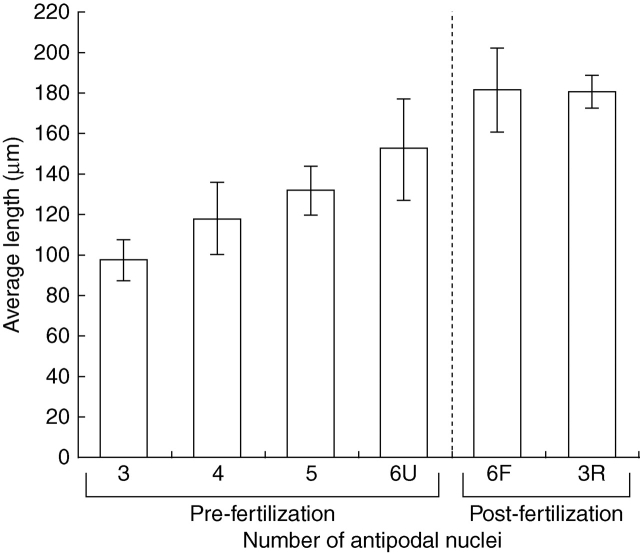
Female gametophyte length directly correlates with the number of antipodal nuclei found inside the gametophyte (r2 = 0·7381; Fig. 10). In addition, there is a burst of female gametophyte growth associated with the fertilization process (Fig. 10). In several instances however, large female gametophytes with only three antipodal nuclei were found (Fig. 10). It is unlikely, however, that antipodal nuclei in these female gametophytes did not proliferate since gametophytes with three antipodal nuclei occur in two significantly different size ranges (t-test; P = 4·6 × 10−22; Fig. 10). Thus, the presence of three antipodal nuclei in large female gametophytes is likely to be the result of either nuclear fusion within the antipodal cells or a complete degeneration of one nucleus in each cell.
Fig. 10.
Female gametophyte length relative to the number of antipodal nuclei present in Tofieldia glutinosa. 6U refers to unfertilized female gametophytes with six antipodal nuclei. 6F refers to fertilized female gametophytes with six antipodal nuclei measured before division of the primary endosperm nucleus. 3R refers to fertilized female gametophytes in which the number of antipodal nuclei has been reduced from six to three.
DISCUSSION
Female gametophyte development in Tofieldia
The mature female gametophyte is seven-celled and 11-nucleate, although after the two polar nuclei fuse to produce a diploid secondary nucleus, the gametophyte contains ten nuclei. Nevertheless, development of the female gametophyte of T. glutinosa is essentially Polygonum-type with subsequent amplification of the nuclear contents of the three antipodals. The present results are largely in agreement with Seelieb's embryological study of T. calyculata (Seelieb, 1924), but contrast with the studies of Sokolowska-Kulczycka (1980, 1985) who reported considerable variation in female gametophyte development of T. calyculata during megasporogenesis. According to Sokolowska-Kulczycka (1980, 1985), monosporic Polygonum-type female gametophytes occur in slightly less than one-third of all ovules, while the majority of female gametophytes are bisporic, and develop according to either the Allium-type or a variant referred to as the Veratrum Lobelianum-type (seven-celled, eight-nucleate, derived from the chalazal dyad in both types). However, no evidence of bisporic female gametophyte development was found in T. glutinosa.
Sokolowska-Kulczycka (1980) did not directly observe several key stages of megasporogenesis. Instead, the distinction between monospory and bispory was made on the basis of the number of degenerated megaspores identified adjacent to the developing female gametophyte (two or three). This approach can be problematic, since fully degenerated megaspores can be difficult to discern from one another, and often appear simply as a crushed necrotic mass of tissue (Fig. 2A). Furthermore, in many taxa with Polygonum-type female gametophytes, including T. glutinosa, the micropylar dyad cell may abort prior to or during the second meiotic division process, such that only two degenerated cells are observable upon initiation of the functional megaspore (Maheshwari, 1950; Bouman, 1984; Johri et al., 1992; Russell, 2001). The present data show that in T. glutinosa early degeneration of the micropylar dyad cell occurs in approx. 30 % of ovules. Thus, a monosporic female gametophyte could easily be mistaken for a bisporic female gametophyte if the distinction is made solely on the basis of the number of degenerated cells formed during megasporogenesis.
Reports of bisporic female gametophyte development are frequently inaccurate. Maheshwari (1955) reviewed 58 families in which bispory was reported to occur and concluded that, in at least 29 of these families, findings of bisporic female gametophyte development were not justified. Maheshwari (1955) attributed many of these inaccuracies to incomplete observations during megasporogenesis, and to misinterpretation of the triad stage (in which the chalazal-most cell – the functional megaspore – is mistaken for an undivided chalazal dyad cell). More recently, reports of bispory have been refuted in Illicium (Williams and Friedman, 2004), Kadsura (Battaglia, 1986; Friedman et al., 2003) and Epipactis (Fredrikson, 1992). While it is possible that interspecific (and intraspecific, in the case of T. calyculata) variation in female gametophyte development exists in Tofieldiaceae, this is unlikely to be the case. Far more probable is that there is variation within Tofieldia species with respect to the completion of meiosis II in the micropylar dyad cell.
Antipodal proliferation in Tofieldia and other monocots
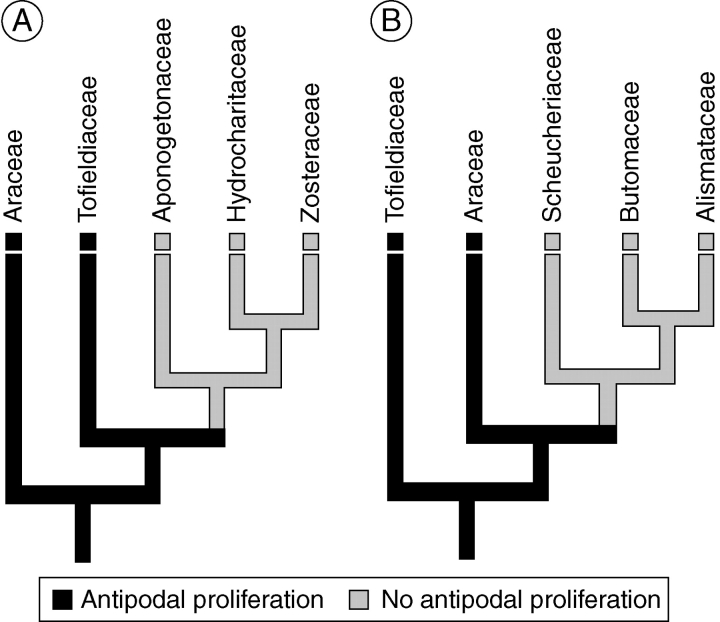
Proliferation of antipodal nuclei during female gametophyte maturation is the only deviation from the plesiomorphic Polygonum-type developmental pattern in T. glutinosa. It is unclear at this point whether the behaviour of the antipodals in T. glutinosa is anomalous or representative of Tofieldiaceae. Seelieb (1924) describes and illustrates a normal Polygonum-type female gametophyte in T. calyculata and does not suggest that the antipodals proliferate. Sokolowska-Kulczycka (1980) specifically reports that increased numbers of antipodal nuclei or cells were not found in T. calyculata, although no images of mature female gametophytes were published. Clearly, variation in antipodal behaviour in different species of Tofieldia is possible. However, the preponderance of antipodal proliferation within other members of the Alismatales as well as in Acorus (Table 1) suggests that antipodal proliferation is probably plesiomorphic in Tofieldiaceae based on current interpretations of angiosperm phylogeny (Fig. 11). Thus, the reported lack of antipodal proliferation in T. calyculata could represent a character reversion or simply have resulted from inadequate sampling of developmental stages.
Table 1.
Reports of antipodal proliferation in basal eudicots, magnoliids and monocots
‘Antipodal proliferation type’ refers to either nuclear proliferation (resulting in extra antipodal nuclei) or cellular proliferation (resulting in extra antipodal cells). All family/ordinal assignments conform to the recommendations of APG II (2003). NB In most cases, only a few taxa within a given lineage have been examined. Taxa not mentioned here either have not been studied, or are reported to lack proliferating antipodals.
* The extra antipodal reported by Wylie (1904) is more likely to be the chalazal endosperm chamber.
† Johri et al.'s (1992) report of no antipodal proliferation conflicts with other reports cited here.
Fig. 11.
Evolution of antipodal proliferation in Alismatales. (A) Phylogeny of Soltis et al. (2007). Antipodal proliferation has been reported in Hydrocharitaceae (Wylie, 1904), but more recent studies suggest that this is not actually the case (Johri et al., 1992). (B) Phylogeny of Graham et al. (2006). Parsimony analysis reveals that antipodal proliferation is the ancestral condition for Alismatales.
In many angiosperm lineages, especially within the monocots, antipodals, once formed, continue to develop (Table 1; see also Williams and Friedman, 2004) through a free nuclear proliferation (as is the case in T. glutinosa), a cellular proliferation, or both. Among monocots, antipodal proliferation has been reported in Acorus and members of the Alismatales, Arecales, Asparagales, Liliales, Pandanales, Poales and Zingiberales (for a complete list of references, see Table 1). In extreme cases (as in the Poaceae or Pandanaceae), several hundred antipodal cells may be formed (Cheah and Stone, 1975; Anton and Cocucci, 1984; Johri et al., 1992). In other lineages, such as Acorus calamus, only five antipodal cells are produced (Buell, 1938).
Williams and Friedman (2004) discussed variations in antipodal development in early divergent monocots, eudicots, magnoliids and Chloranthaceae. They were not, however, able to trace the evolutionary transition between non-proliferating and proliferating antipodals to a specific node on the angiosperm phylogeny (Williams and Friedman, 2004). Parsimony analysis reveals that antipodal proliferation is likely to be the ancestral condition for monocots (Fig. 12). Depending on the phylogenetic position of Chloranthaceae, which remains uncertain (Qiu et al., 1999; Graham and Olmstead, 2000; Graham et al., 2000; Soltis et al., 2000; APG II, 2003; Hilu et al., 2003; Davis et al., 2004; Graham et al., 2006; Qiu et al., 2006; Moore et al., 2007), antipodal proliferation may even be a synapomorphy of the common ancestor of monocots, magnoliids, eudicots and Chloranthaceae, although this would require a reversion in magnoliids and early eudicots (Fig. 12A). Alternatively, if Chloranthaceae is sister to the magnoliid clade as some recent studies suggest (Stevens, 2001; Hansen et al., 2007), antipodal proliferation in Chloranthaceae could be homoplasious with the similar phenomenon in monocots (Fig. 12B). The present analysis further suggests that modest proliferation of the sort seen in early-divergent monocots like Tofieldia, Acorus (Buell, 1938) and members of the Araceae (Campbell, 1903, 1905; Gow, 1908, 1913; Coulter, 1908), in which only a few supernumerary antipodals or antipodal nuclei are formed, is the ancestral condition among monocotyledonous plants.
Fig. 12.
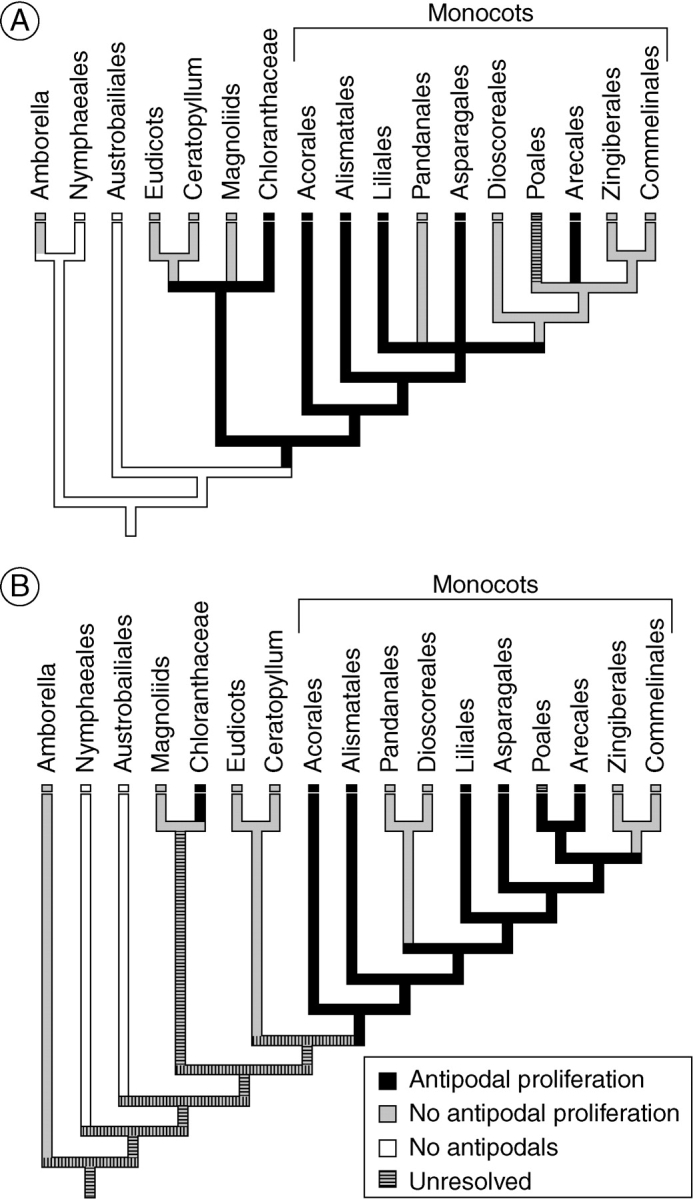
Evolution of antipodal proliferation in monocots. In all cases except for the Poales, the character state assigned to the group has been resolved as the ancestral condition for that group based on the present analysis. The ancestral condition within the Poales is unresolved, and is classified as polymorphic. (A) Phylogeny of Soltis et al. (2007). (B) Phylogeny of Graham et al. (2006). For both of these phylogenetic hypotheses of angiosperm and monocot relationships, parsimony analysis reveals that antipodal proliferation either evolved in or was present in the common ancestor of all monocots.
In angiosperms, the antipodals are perhaps the most variable component of the mature female gametophyte (Johri et al., 1992). They may be ephemeral, degenerating soon after their formation, as in Arabidopsis (Murgia et al., 1993; Rojek et al., 2005) and many other taxa, or may persist to and even well beyond fertilization (Johri et al., 1992; Williams and Friedman, 2004). Parsimony analyses have demonstrated that in those lineages diverging above the paraphyletic basal grade of Amborella, Nymphaeales and Austrobaileyales, persistence of the antipodal cells is the ancestral condition (Williams and Friedman, 2004). The present parsimony analysis shows this to be the case for monocots as well (Fig. 13). Ephemeral antipodals appear to be a derived condition most commonly found in higher eudicots (e.g. Violaceae, Cucurbitaceae, Solanaceae, Rutaceae, Malpighiaceae, etc.; Johri et al., 1992).
Fig. 13.
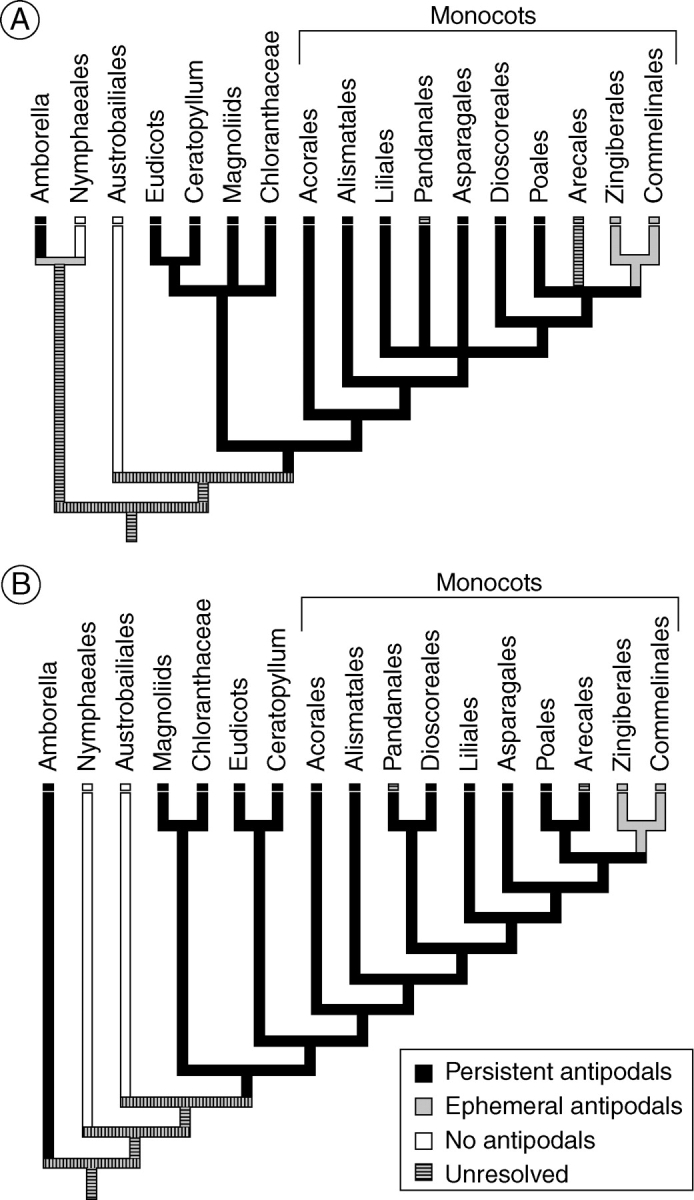
Evolution of persistence of antipodals in monocots. In all cases except for the Pandanales and the Arecales, the character state assigned to the group has been resolved as the ancestral condition for that group based on our analysis. The ancestral conditions within the Pandanales and the Arecales are unresolved, and are classified as polymorphic. (A) Phylogeny of Soltis et al. (2007). (B) Phylogeny of Graham et al. (2006). Parsimony analysis reveals that the common ancestor of all angiosperms exclusive of Amborella, Nymphaeales and Austrobaileyales had persistent antipodals. It is unclear whether the persistent antipodals in Amborella evolved independently or were inherited from the common ancestor of all angiosperms.
The physiological role (or roles) of antipodals has not been definitively demonstrated, but there is reasonable support for the idea that antipodals may be important in the transfer of nutrients from the sporophyte to the female gametophyte and endosperm (Westermaier, 1890; Riddle, 1898; Ikeda, 1902; Lloyd, 1902; Schnarf, 1929; Brink and Cooper, 1944; Coe, 1954; Kapil and Bhatnagar, 1981; Mogensen, 1981; Johri et al., 1992). The position of antipodals at the interface of the sporophytic tissue and the female gametophyte and endosperm is certainly congruent with this view, as is the presence of wall ingrowths in the antipodals of certain taxa (Westermaier, 1890; Mogensen, 1981). Nevertheless, for now it is unclear whether modest levels of antipodal proliferation (as in Tofieldia) are adaptive (e.g. represent a functional benefit to the seed) or are a neutral variation of female gametophyte structure.
Helobial endosperm
Helobial endosperm development is common in monocots (Table 2; see also Swamy and Parameswaran, 1963; Palser, 1975), but ab initio cellular endosperm is the ancestral condition of this clade, a feature most likely inherited from the shared common ancestor of all angiosperms (Floyd and Friedman, 2000). Endosperm development in Acorus, Amborella, most Nymphaeales and Austrobaileyales, and early-divergent eudicots is of the cellular type (Buell, 1938; Johri et al., 1992; Floyd and Friedman, 2000). However, parsimony analysis reveals that in monocots the transition from cellular to helobial endosperm occurred after the divergence of Acorus, assuming that Acorus is sister to all other monocots (Chase et al., 1993, 1995, 2000; Duvall et al., 1993; Davis et al., 1998; Doyle and Endress, 2000; Graham and Olmstead, 2000; Graham et al., 2000, 2006; Hilu et al., 2003; Soltis et al., 2007), and helobial endosperm should be considered the ancestral condition for all monocots excluding Acorus (Fig. 14).
Table 2.
Reports of helobial endosperm development in monocots
All family/ordinal assignments conform to the recommendations of APG II (2003).
* Nuclear endosperm has also been reported in these families
Fig. 14.
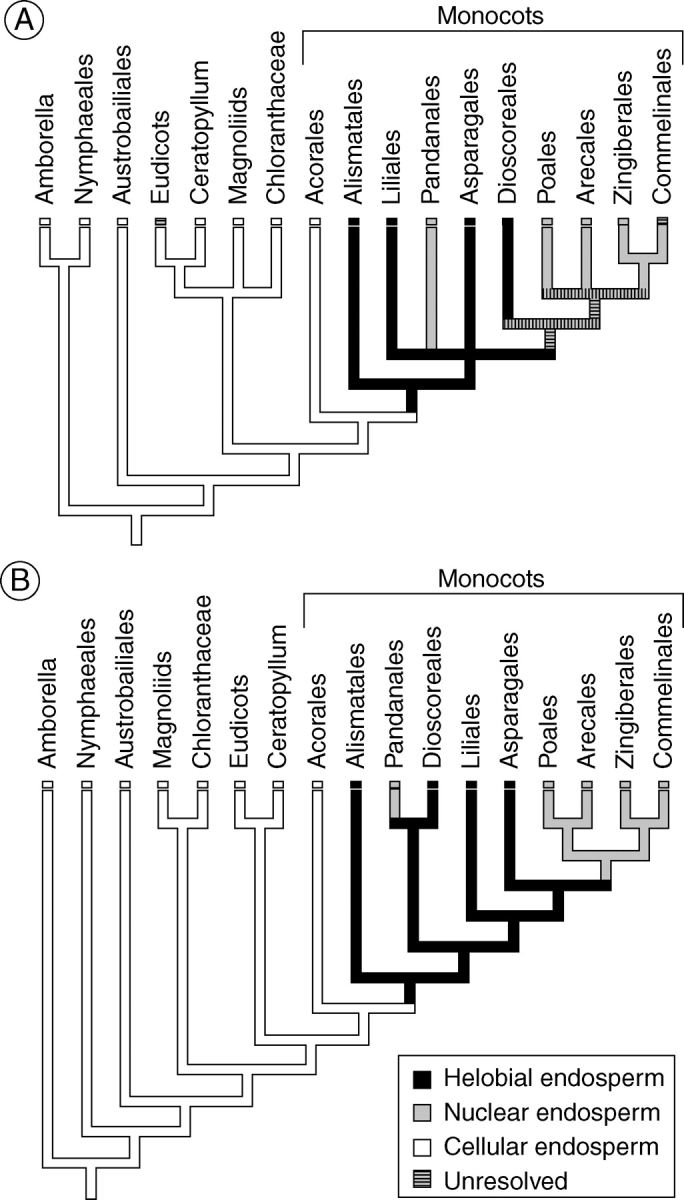
Evolution of helobial endosperm development in angiosperms. In all cases except for the Commelinales and eudicots, the character state assigned to the group has been resolved as the ancestral condition for that group based on the present analysis. The ancestral conditions within Commelinales and eudicots are unresolved, and are classified here as polymorphic. (A) Phylogeny of Soltis et al. (2007). (B) Phylogeny of Graham et al. (2006). Parsimony analysis reveals that helobial endosperm development evolved in the common ancestor of all monocots excluding the Acorales.
Floyd and Friedman (2000) recognized that the cellular endosperm of Acorus was unique among early-divergent monocots. However, the angiosperm phylogeny relied upon in their analysis (Qiu et al., 1999) has undergone revision, and they did not pinpoint the transition from cellular to helobial endosperm to such an early phase of monocot evolution. In monocots there is likely to be only a single evolutionary origin of helobial endosperm (Fig. 14), and occurrences of helobial endosperms in some eudicots and magnoliids, and also in Cabomba are certainly homoplasious with those of monocots (Floyd and Friedman, 2000).
CONCLUSIONS
A detailed account of female gametophyte development has been provided in Tofieldia glutinosa from the initiation of megasporogenesis through fertilization and the early stages of endosperm development. It can be said with certainty that the female gametophyte of T. glutinosa is monosporic (Fig. 1), and produces seven cells and 11 nuclei (one egg, two synergids, an initially binucleate central cell, and three binucleate antipodals) (Figs 2, 3 and 9). Tofieldia glutinosa produces a helobial endosperm (Fig. 7), whose early growth occurs at equal rates in both the micropylar and chalazal endosperm chambers (Fig. 8).
Based on the present study of T. glutinosa and the analysis of the embryological literature pertaining to early-divergent monocots, two important conclusions can be drawn about the reproductive characters of ancestral monocots. First, the ancestral monocot female gametophyte had persistent antipodal cells that underwent a modest proliferation (Figs 12 and 13). Whether this proliferation was nuclear or cellular in nature is unclear, but it was almost certainly simple, producing only a few supernumerary antipodal cells or nuclei in contrast with the elaborate proliferation found in some highly derived monocot lineages. Second, endosperm development in the first monocots was of the cellular type (Floyd and Friedman, 2000), but transitioned once to the helobial type after the divergence of Acorus from the remainder of the monocot clade (Fig. 14). Finally, we suggest that because of its prevalence and early appearance within this clade, helobial endosperm should be considered one of the more salient features of early monocots.
ACKNOWLEDGEMENTS
We thank Kirsten Ryerson for her assistance with field collections and histological preparations, Andrea Ruchty and the staff of the Mount Adams Ranger District (Gifford Pinchot National Forest, Trout Lake, WA, USA) for their assistance with collection permits and field collections, and the two anonymous reviewers for their helpful suggestions towards the improvement of the manuscript. We gratefully acknowledge funding from the National Science Foundation (IOB-0446191 to W.E.F.).
LITERATURE CITED
- Anton AM, Cocucci AE. The grass megagametophyte and its possible phylogenetic implications. Plant Sytematics and Evolution. 1984;146:117–121. [Google Scholar]
- APG II. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Arber A. Monocotyledons: a morphological study. Cambridge: Cambridge University Press; 1925. [Google Scholar]
- Asplund I. Embryological studies in the genus Typha. Svensk Botanisk Tidskrift. 1972;66:1–17. [Google Scholar]
- Asplund I. Embryological studies in the genus Sparganium. Svensk Botanisk Tidskrift. 1973;67:177–200. [Google Scholar]
- Aulbach-Smith CA, Herr JM., Jr Development of the ovule and female gametophyte in Eustachys petraea and E. glauca (Poaceae) American Journal of Botany. 1984;71:427–438. [Google Scholar]
- Bambacioni-Mezzetti V. Ricerche morfologiche sulle Lauracee, Lo sviluppo dell'ovulo e dei sacchi pollinici nel Laurus nobilis L. Annali di Botanica. 1935;21:1–19. [Google Scholar]
- Battaglia E. Embryological questions. 7. Do new types of embryo sac occur in Schisandra? Annals of Botany. 1986;44:69–82. [Google Scholar]
- Batygina TB, Shamrov , II, Kolesova GE. Embryology of the Nymphaeales and Nelumbonales. II. The development of the female embryonic structures. Botanicheskii Zhurnal. 1982;67:1179–1195. [Google Scholar]
- Berg RY. Megagametogenesis and seed development in Dendromecon rigida (Papaveraceae) Phytomorphology. 1967;17:223–233. [Google Scholar]
- Berg RY. Development of ovule, embryo sac, and endosperm in Dipterostemon and Dichelostemma (Alliaceae) relative to taxonomy. American Journal of Botany. 1996;83:790–801. doi: 10.3732/ajb.90.6.937. [DOI] [PubMed] [Google Scholar]
- Berg RY. Development of ovule, embryo sac, and endosperm in Triteleia (Themidaceae) relative to taxonomy. American Journal of Botany. 2003;90:937–948. doi: 10.3732/ajb.90.6.937. [DOI] [PubMed] [Google Scholar]
- Borwein B, Coetsee ML, Krupko S. Development of the embryo sac of Restio dodii and Elegia racemosa. Journal of South African Botany. 1949;15:1–11. [Google Scholar]
- Bouman F. The ovule. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer-Verlag; 1984. [Google Scholar]
- Brink RA, Cooper DC. The antipodals in relation to abnormal endosperm behavior in Hordeum jubatun × Secale cereale hybrid seeds. Genetics. 1944;29:391–406. doi: 10.1093/genetics/29.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne ET. Morphological studies in Aletris. I. Development of the ovule, megaspores and megagametophyte of A. aurea and their connection with the systematics of the genus. American Journal of Botany. 1961;48:143–147. [Google Scholar]
- Buell MF. Embryogeny of Acorus calamus. Botanical Gazette. 1938;99:556–568. [Google Scholar]
- Campbell DH. Studies on the Araceae: the embryo-sac and the embryo of Aglaonema and Spathicarpa. Annals of Botany. 1903;17:665–687. [Google Scholar]
- Campbell DH. Studies on Araceae III. Annals of Botany. 1905;19:329–349. [Google Scholar]
- Campbell DH. The embryo-sac of Pandanus. Bulletin of the Torrey Botanical Club. 1909;36:205–220. [Google Scholar]
- Campbell DH. The embryo-sac of Pandanus coronatus. Bulletin of the Torrey Botanical Club. 1910;37:293–295. [Google Scholar]
- Cass DD, Jensen WA. Fertilization in barley. American Journal of Botany. 1970;57:62–70. [Google Scholar]
- Cave MS. Sporogenesis and embryo sac development of Hesperocallis and Leucocrinum in relation to their systematic position. American Journal of Botany. 1948;35:343–349. [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, Morgan D, Les DH, Mishler BD, et al. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden. 1993;80:528–580. [Google Scholar]
- Chase MW, Stevenson DW, Wilkin P, Rudall PJ. Monocot systematics: a combined analysis. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. London: Royal Botanic Gardens, Kew; 1995. pp. 685–730. [Google Scholar]
- Chase MW, Soltis DE, Soltis PS, Rudall PJ, Fay MF, Hahn WH, et al. Higher-level systematics of the monocotyledons: an assessment of current knowledge and a new classification. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 3–16. [Google Scholar]
- Cheah CH, Stone BC. Embryo sac and microsporangium development in Pandanus (Pandanaceae) Phytomorphology. 1975;25:228–238. [Google Scholar]
- Coe GE. Cytology of reproduction in Cooperia pedunculata. American Journal of Botany. 1953;40:335–343. [Google Scholar]
- Coe GE. Distribution of Carbon 14 in ovules of Zephyranthes drummondii. Botanical Gazette. 1954;115:342–346. [Google Scholar]
- Coulter JM. Contribution to the life history of Ranunculus. Botanical Gazette. 1898;25:73–88. [Google Scholar]
- Coulter JM. Relation of megaspores to embryo sacs in angiosperms. Botanical Gazette. 1908;45:361–366. [Google Scholar]
- Crane CF, Carman JG. Mechanisms of apomixis in Elymus rectisetus from Eastern Australia and New Zealand. American Journal of Botany. 1987;74:477–496. [Google Scholar]
- Cronquist A. The evolution and classification of flowering plants. 2nd edn. New York, NY: New York Botanical Garden; 1988. [Google Scholar]
- Dahlgren KVO. Endosperm- und Embryobildung bei Zostera marina. Botaniska Notiser. 1939;1939:607–615. [Google Scholar]
- Dahlgren RMT, Clifford HT, Yeo PF. The families of the monocotyledons: structure evolution and taxonomy. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Davis GL. Systematic embryology of the angiosperms. New York, NY: John Wiley & Sons; 1966. [Google Scholar]
- Davis JI, Simmons MP, Stevenson DW, Wendel JF. Data decisiveness, data quality, and incongruence in phylogenetic analysis: an example from monocotyledons using mitochondrial atpA sequences. Systematic Biology. 1998;47:282–310. doi: 10.1080/106351598260923. [DOI] [PubMed] [Google Scholar]
- Davis JI, Stevenson DW, Petersen G, Seberg O, Campbell LM, Freudenstein JV, et al. Phylogeny of the monocots, as inferred from rbcL and atpA sequence variation, and a comparison of methods for calculating jackknife and bootstrap values. Systematic Botany. 2004;29:467–510. [Google Scholar]
- Davis JI, Petersen G, Seberg O, Stevenson DW, Hardy CR, Simmons MP, et al. Are mitochondrial genes useful for the analysis of monocot relationships? Taxon. 2006;55:857–870. [Google Scholar]
- Doyle JA, Endress PK. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences. 2000;161:S121–S153. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Duvall MR, Clegg MT, Chase MW, Clark WD, Kress WJ, Hills HG, et al. Phylogenetic hypotheses for the monocotyledons constructed from rbcL sequence data. Annals of the Missouri Botanical Garden. 1993;80:607–619. [Google Scholar]
- Eunus AM. Contributions to the embryology of the Liliaceae. 5. Life history of Amianthium muscae-toxicum Walt. Phytomorphology. 1951;1:73–79. [Google Scholar]
- Fernando DD, Cass DD. Development and structure of ovule embryo sac, embryo, and endosperm in Butomus umbellatus (Butomaceae) International Journal of Plant Sciences. 1996;157:269–279. [Google Scholar]
- Floyd SK, Friedman WE. Evolution of endosperm developmental patterns among basal flowering plants. International Journal of Plant Sciences. 2000;161:S57–S81. [Google Scholar]
- Fredrikson M. The development of the female gametophyte of Epipactis (Orchidaceae) and its inference for reproductive ecology. American Journal of Botany. 1992;79:63–68. [Google Scholar]
- Friedman WE. Embryological evidence for developmental lability during early angiosperm evolution. Nature. 2006;441:337–340. doi: 10.1038/nature04690. [DOI] [PubMed] [Google Scholar]
- Friedman WE, Gallup WN, Williams JH. Female gametophyte development in Kadsura: implications for Schisandraceae, Austrobaileyales, and the early evolution of flowering plants. International Journal of Plant Sciences. 2003;164:S293–S305. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. Araceae from the early Cretaceous of Portugal: evidence on the emergence of monocotyledons. Proceedings of the National Academy of Sciences of the USA. 2004;101:16565–16570. doi: 10.1073/pnas.0407174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness CA, Rudall PJ, Sampson FB. Evolution of microsporogenesis in angiosperms. International Journal of Plant Sciences. 2002;163:235–260. [Google Scholar]
- Geerinck D. Genera des Haemodoraceae et des Hypoxidaceae. Bulletin van National Plantentium van België. 1969;39:47–82. [Google Scholar]
- Goldblatt P. Phylogeny and classification of Iridaceae. Annals of the Missouri Botanical Garden. 1990;77:607–627. [Google Scholar]
- Gow JE. Studies in Araceae. Botanical Gazette. 1908;46:35–42. [Google Scholar]
- Gow JE. Observation on the morphology of the aroids. Botanical Gazette. 1913;56:127–142. [Google Scholar]
- Graham SW, Olmstead RG. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. American Journal of Botany. 2000;87:1712–1730. [PubMed] [Google Scholar]
- Graham SW, Reeves PA, Burns ACE, Olmstead RG. Microstructural changes in noncoding chloroplast DNA: interpretation, evaluation, and utility of indels and inversions in basal angiosperm phylogenetic inference. International Journal of Plant Sciences. 2000;161:S83–S96. [Google Scholar]
- Graham SW, Zgurski JM, McPherson MA, Cherniawsky DM, Saarela JM, Horne ESC, et al. Robust inference of monocot deep phylogeny using an expanded multigene plastid data set. Aliso. 2006;22:3–20. [Google Scholar]
- Grayum MH. A summary of evidence and arguments supporting the removal of Acorus from the Araceae. Taxon. 1987;36:723–729. [Google Scholar]
- Grayum MH. Systematic embryology of the Araceae. The Botanical Review. 1991;57:167–203. [Google Scholar]
- Haeckel I. Über Iridaceen. Flora. 1930;125:1–82. [Google Scholar]
- Halada L, Erdelska O. Reproductive biology of Ruscus hypoglossum L. in Slovakia. Acta Biologica Cracovienia, Série Botanique. 2005;47:213–217. [Google Scholar]
- Hamann U. Beitrag zur Embryologie der Centrolepidaceae mit Bemerkungen über den bau der Blüten und Blütenstände und die systematische Stellung der familie. Berichte der Deutschen Botanischen Gesellschaft. 1962;75:153–171. [Google Scholar]
- Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Jansen RK. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae) Molecular Phylogenetics and Evolution. 2007;45:547–563. doi: 10.1016/j.ympev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Hill TG. The structure and development of Triglochin maritimum. Annals of Botany. 1900;14:83–107. [Google Scholar]
- Hilu KW, Borsch T, Müller K, Soltis DE, Soltis PS, Savolainen V, et al. Angiosperm phylogeny based on matK sequence information. American Journal of Botany. 2003;90:1758–1776. doi: 10.3732/ajb.90.12.1758. [DOI] [PubMed] [Google Scholar]
- Hitchcock CL. The Tofieldia glutinosa complex of western North America. American Midland Naturalist. 1944;a 31:487–498. [Google Scholar]
- Hitchcock CL. Correction: the Tofieldia glutinosa complex of western North America. American Midland Naturalist. 1944;b 32:784. [Google Scholar]
- Igersheim A, Buzgo M, Endress PK. Gynoecium diversity and systematics in basal monocots. Botanical Journal of the Linnean Society. 2001;136:1–65. [Google Scholar]
- Ikeda T. Studies in the physiological functions of antipodals and the phenomena of fertilization in Liliaceae. I. Tricyrtis hirta. Bulletin of the College of Agriculture, Tokyo. 1902;5:41–72. [Google Scholar]
- Jane W.-N. Ultrastructure of embryo development in Arundo formosana Hack. (Poaceae) International Journal of Plant Sciences. 1999;160:46–63. [Google Scholar]
- Johri BM, Ambegaokar KB, Srivastava PS. Comparative embryology of angiosperms. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Joshi AC. Morphology of Tinospora cordifolia, with some observations on the origin of the single integument, nature of synergidae, and affinities of the Menispermaceae. American Journal of Botany. 1939;26:433–439. [Google Scholar]
- Kanta K. Morphology and embryology of Piper nigrum L. Phytomorphology. 1962;12:207–221. [Google Scholar]
- Kapil RN, Bhatnagar AK. Ultrastructure and biology of the female gametophyte in flowering plants. International Review of Cytology. 1981;70:291–337. [Google Scholar]
- Kircher P. Untersuchungen zur Blüten- und Infloreszenzmorphologie, Embryologie und Systemaik der Restionaceen im Vergleich mit Gramineen und verwandten Familien. Dissertationes Botanicae. 1986;94:1–219. [Google Scholar]
- Kress WJ. The phylogeny and classification of the Zingiberales. Annals of the Missouri Botanical Garden. 1990;77:698–721. [Google Scholar]
- Lakshmanan KK. Embryological studies in the Bromeliaceae. 1. Lindmania penduliflora (C.H. Wright) Stapf. Proceedings of the Indian Academy of Science B. 1967;65:49–55. [Google Scholar]
- Lazarte JE, Palser BF. Morphology, vascular anatomy and embryology of pistillate and staminate flowers of Asparagus officinalis. American Journal of Botany. 1979;66:753–764. [Google Scholar]
- Linder HP, Rudall PJ. The megagametophyte in Anarthria (Anarthriaceae, Poales) and its implications for the phylogeny of the Poales. American Journal of Botany. 1993;80:1455–1464. [Google Scholar]
- Lloyd FE. The comparative embryology of the Rubiaceae. Memoirs of the Torrey Botanical Club. 1902;8:27–112. [Google Scholar]
- McAllister F. The development of the embryo sac of Smilacina stellata. Botanical Gazette. 1909;48:200–215. [Google Scholar]
- Maddison WP, Maddison DR. MacClade. Sinauer, Sunderland, Massachusetts, USA: 1992. [Google Scholar]
- Maheshwari P. An introduction to the embryology of angiosperms. 1st edn. New York, NY: McGraw-Hill Book Co; 1950. [Google Scholar]
- Maheshwari SC. The occurrence of bisporic embryo sacs in angiosperms – a critical review. Phytomorphology. 1955;5:67–99. [Google Scholar]
- Mauritzon J. Samenbau und Embryologie einiger Scitamineen. Acta Universitatis Lundensis. 1936;30:1–31. [Google Scholar]
- Mogensen HL. Ultrastructural localization of adenosine triphosphate in the ovules of Saintpaulia ionantha (Gesneriaceae) and its relation to synergid function and embryo sac nutrition. American Journal of Botany. 1981;68:183–194. [Google Scholar]
- Moore MJ, Bell CD, Soltis PS, Soltis DE. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proceedings of the National Academy of Sciences of the USA. 2007;104:19363–19368. doi: 10.1073/pnas.0708072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier DM. Contributions to the embryology of Ranunculaceae. Botanical Gazette. 1895;20:241–248. 296–304. [Google Scholar]
- Munro SL, Linder HP. The embryology and systematic relationships of Prionium serratum (Juncaceae: Juncales) American Journal of Botany. 1997;84:850–860. [PubMed] [Google Scholar]
- Murgia M, Huang B.-Q, Tucker SC, Musgrave ME. Embryo sac lacking antipodal cells in Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 1993;80:824–838. [Google Scholar]
- Packer JG. Two new combinations in Triantha (Liliaceae) Novon. 1993;3:278–279. [Google Scholar]
- Palser BF. The bases of the angiosperm phylogeny: embryology. Annals of the Missouri Botanical Garden. 1975;62:621–646. [Google Scholar]
- Patterson TB, Givnish TJ. Phylogeny, concerted convergence, and phylogenetic niche conservatism in the core Liliales: insights from rbcL and ndhF sequence data. Evolution. 2002;56:233–252. doi: 10.1111/j.0014-3820.2002.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Proddubnaya-Arnoldi VA. Comparative embryology of the Orchidaceae. Phytomorphology. 1967;17:312–320. [Google Scholar]
- Qiu Y-L, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, et al. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature. 1999;402:404–407. doi: 10.1038/46536. [DOI] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Hendry TA, Li R, Taylor DW, Issa MJ, et al. Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes. Taxon. 2006;55:837–856. [Google Scholar]
- Rao AN, Wee YC. Embryology of the pineapple, Ananas comosus (L.) Merr. New Phytologist. 1979;83:485–497. [Google Scholar]
- Rao CV. Contributions to the embryology of Palmae. 2. Ceroxylinae. Journal of the Indian Botanical Society. 1959;38:46–75. [Google Scholar]
- Rao MK, Kumari KA, Grace JR. Cytology of antipodals cells with some observations on the male and female gametophyte development in pearl millet, Pennisetum americanum (L.) Leeke. Botanical Gazette. 1983;144:201–206. [Google Scholar]
- Riddle LC. The embryology of Alyssum. Botanical Gazette. 1898;26:314–324. [Google Scholar]
- Rojek J, Kuta E, Bohdanowicz J. In vitro culture promotes partial autonomous endosperm development in unfertilized ovules of wild-type Arabidopsis thaliana var. Columbia. Sexual Plant Reproduction. 2005;18:29–36. [Google Scholar]
- Rudall PJ, Linder HP. Megagametophyte and nucellus in Restionaceae and Flagellariaceae. American Journal of Botany. 1988;75:1777–1786. [Google Scholar]
- Rudall PJ, Owens SJ, Kenton AY. Embryology and breeding systems in Crocus (Iridaceae) – a study in causes of chromosome variation. Plant Systematics and Evolution. 1984;148:119–134. [Google Scholar]
- Russell SD. Female gametogenesis. In: Bhojwani SS, Soh WY, editors. Current trends in the embryology of angiosperms. Dordrecht: Kulwer Academic Publishers; 2001. pp. 67–88. [Google Scholar]
- Sachar RC, Arora U. Some embryological aspects of Amomum dealbatum and Hedychium acuminatum. Botanical Gazette. 1963;124:353–360. [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. Effects of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L.) Australian Journal of Plant Physiology. 1983;10:137–144. [Google Scholar]
- Sass JE. The development of endosperm and antipodal tissue in Argentine waxy maize. American Journal of Botany. 1946;33:791–795. [Google Scholar]
- Sastri RLN. Studies in the Lauraceae. VI. Comparative embryology and phylogeny. American Journal of Botany. 1963;27:425–433. [Google Scholar]
- Schnarf K. Embryologie der Angiospermen. Berlin: Bornträger; 1929. [Google Scholar]
- Schnarf K. Vergleichende Embryologie der Angiospermen. Berlin: Bornträger; 1931. [Google Scholar]
- Seelieb W. Beiträge zur Entwicklungsgeschichte von Tofieldia calyculata (L.) Wahlenb. Botaniska Notiser. 1924;77:172–178. [Google Scholar]
- Simpson MG. Embryological development of Lachnanthes caroliniana (Haemodoraceae) American Journal of Botany. 1988;75:1394–1408. [Google Scholar]
- Sokolowska-Kulczycka A. Development of bisporic embryo sacs in Veratrum lobelianum Bernh. ActaBiologica Cracoviensia, Série Botanique. 1973;14:85–98. [Google Scholar]
- Sokolowska-Kulczycka A. Embryological studies of Tofieldia calyculata (L.) Whlb. Acta Biologica Cracoviensia, Série Botanique. 1980;22:113–128. [Google Scholar]
- Sokolowska-Kulczycka A. The influence of temperature on the frequency of developmental types of ES in Tofieldia calyculata (L.) Whlb. Acta Biologica Cracoviensia, Série Botanique. 1985;27:1–7. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zannis M, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Soltis DE, Soltis PS, Endress PK, Chase MW. Phylogeny and evolution of angiosperms. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Soltis DE, Gitzendanner MA, Soltis PS. A 567-taxon data set for angiosperms: the challenges posed by Baysian analyses of large data sets. International Journal of Plant Sciences. 2007;168:137–157. [Google Scholar]
- Stevens PF. Angiosperm Phylogeny Website. 2001 onwards http://www.mobot.org/MOBOT/research/APweb/ . 24 April 2007. [Google Scholar]
- Stevens PF. Book review: Phylogeny and evolution of angiosperms. International Journal of Plant Sciences. 2006;167:607–611. [Google Scholar]
- Sulbha K. The embryology of Iphigenia indica Kunth. Phytomorphology. 1954;4:180–191. [Google Scholar]
- Swamy BGL. On the post-fertilization development of Trillium undulatum. Cellule. 1948;52:7–14. [PubMed] [Google Scholar]
- Swamy BGL, Parameswaran N. The helobial endosperm. Biological Review. 1963;38:1–50. [Google Scholar]
- Tamura MN. Nartheciaceae. In: Kubitski K, editor. The families and genera of vascular plants. Vol. III. Berlin: Springer; 1998. pp. 381–392. Liliinae. [Google Scholar]
- Tilton VR, Lersten NR. Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. III. Nucellus and megagametophyte. New Phytologist. 1981;88:477–504. [Google Scholar]
- Weatherwax P. Persistence of the antipodal tissue in the development of the seed of maize. Bulletin of the Torrey Botanical Club. 1926;53:381–384. [Google Scholar]
- Werker E, Fahn A. Seed anatomy of Pancratium species from three different habitats. Botanical Gazette. 1975;136:396–403. [Google Scholar]
- Westermaier M. Zur Embryologie der Phanerogamen, insbesondere über die sogenannten Antipoden. Acta Nova der Kaiserlich Leopoldinisch – Carolinisch Deutschen Akademie der Naturforscher. 1890;57:1–39. [Google Scholar]
- Wiger J. Embryological studies on the families Buxaceae, Meliaceae, Simaroubaceae and Burseraceae. Sweden: Dissertation, Lund University; 1935. [Google Scholar]
- Williams JH, Friedman WE. The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): implications for understanding the origin and early evolution of monocots, eumagnoliids, and eudicots. American Journal of Botany. 2004;91:332–351. doi: 10.3732/ajb.91.3.332. [DOI] [PubMed] [Google Scholar]
- Wylie RB. The morphology of Elodea canadensis: contributions from the Hull Botanical Laboratory LII. Botanical Gazette. 1904;37:1–22. [Google Scholar]
- Yamaura A. Karyologische und embryologische Studien über einige Bambusa-Arten. (Vorläufige Mitteilung) Botanical Magazine, Tokyo. 1933;47:551–555. [Google Scholar]
- Yoshida O. Embryologische Studien über die Ordnung Piperales. 1. Embryologie von Chloranthus japonicus Sieb. Journal of the College of Arts and Sciences, Chiba University (Natural Science) 1957;2:172–178. [Google Scholar]