Abstract
Background and Aims
Relationships between autumn flowering, precipitation and temperature of plant species of Mediterranean coastal shrublands have been described, but not analysed experimentally. These relationships were analysed for two species of co-occurring, dominant, autumn-flowering shrubs, Globularia alypum and Erica multiflora, over 4 years and in experimentally generated drought and warming conditions. The aim was to improve predictions about the responses and adaptations of flowering of Mediterranean vegetation to climate change.
Methods
Beginning of anthesis and date of maximum flowering intensity (‘peak date’) were monitored over 4 years (2001–2004) on a garrigue land type in the noth-east of the Iberian Peninsula. Two experimental treatments were applied, increased temperature (+0·73°C) and reduced soil moisture (–17%) relative to untreated plots.
Key Results
Flowering of Globularia alypum and Erica multiflora differed greatly between years depending on the precipitation of the previous months and the date of the last substantial rainfall (>10 mm). Globularia alypum flowered once or twice (unimodal or bimodal) as the result of differences in the distribution and magnitude of precipitation in late-spring and summer (when floral buds develop). The drought treatment delayed and decreased flowering of Globularia alypum in 2001 and delayed flowering in 2002. Warming extended the period between the beginning of flowering and the end of the second peak for autumn flowering in 2001 and also increased peak intensity in 2002. Flowering of Erica multiflora was unaffected by either treatment.
Conclusions
Autumn flowering of Globularia alypum and Erica multiflora is more dependent on water availability than on temperature. Considerable inter-annual plasticity in the beginning of anthesis and peak date and on unimodal or bimodal flowering constitutes a ‘safe strategy’ for both species in relation to varying precipitation and temperature. However, severe changes in precipitation in spring and summer may severely affect flowering of Globularia alypum but not Erica multiflora, thus affecting development/structure of the ecosystem if such conditions persist.
Key words: Globularia alypum, Erica multiflora, autumn flowering, drought, global warming, Mediterranean
INTRODUCTION
The study of plant phenology (the timing of life cycle stages) is important in understanding responses of vegetation to climate change; improved knowledge of the geographical distribution of the timing of phenological phases and their relationship to climate will improve predictions of the effects (Schwartz, 1999; Kramer et al., 2000; Peñuelas and Filella, 2001; Hollister et al., 2005; Menzel et al., 2006). Studies based on long phenological series, supported by field-based surveys or satellite remote sensing, have shown that phenophases (budding, leafing, flowering) are particularly sensitive to temperature and that they have been occurring progressively earlier in Europe in the last century (Menzel, 2000; Osborne et al., 2000; Rötzer and Chmielwski, 2001; Ahas et al., 2002; Walther et al., 2002; Parmesan and Yohe, 2003; Gordo and Sanz, 2005; Piao et al., 2006; Schwartz et al., 2006). It has been reported that an increase of 1·4°C in the last five decades has advanced leaf unfolding by up to 16 d at a Mediterranean site in Catalonia (Peñuelas et al., 2002). Recently, Menzel et al. (2006) reported an average advancement of spring/summer events (bud-bursting, flowering and fruiting) of 2·5 d per decade for the whole of Europe. Fewer studies have focused on plant processes occurring in autumn, so the relationships between environmental conditions and changes in phenophases occurring then are not as clear nor as well known as those in spring. There is some evidence of later onset of autumn phenological phases but there is no general agreement about the patterns (Menzel and Fabian, 1999; Menzel, 2000; Peñuelas et al., 2002; Walther et al., 2002; Chen et al., 2005; Gordo and Sanz, 2005).
The climate of the Western Mediterranean region is characterized by a pronounced seasonality: hot, dry summers, cool winters and mild, rainy springs and autumns. Species grow and flower mainly in spring, thus avoiding winter cold and summer drought. Rather exceptionally for Mediterranean shrubland communities, Globularia alypum and Erica multiflora, which are co-occurring dominant species in some coastal Mediterranean shrublands, grow (Llorens et al., 2004) and flower (Llorens and Peñuelas, 2005) in the autumn (Orshan, 1989; Castro-Díez and Montserrat-Martí, 1998; Picó and Retana, 2001; Tèbar et al., 2004). Observational and experimental studies of Mediterranean plants have shown that environmental control of non-spring flowering is variable, with temperature being one of the most important factors (Nilsen and Muller, 1981; Picó and Retana, 2001, Peñuelas et al., 2002; Jato et al., 2004; Ogaya and Peñuelas, 2004). Restricted water availability is another important factor associated with delayed flowering and decreased flower and fruit production (Gordon et al., 1999; Ogaya and Peñuelas, 2004; Peñuelas et al., 2004a, b; Llorens and Peñuelas, 2005; Giménez-Benavides et al., 2007).
Understanding what controls the phenology of vegetation is essential in trying to understand possible future changes in relation to changing weather and climate. General conclusions of most of the general circulation models (GCMs) are that, over this century, mean global surface temperature will increase by 1·1–6·4°C, depending on the socio-economic scenarios and the resulting emissions of greenhouse gases (IPCC, 2007). Models also predict decreased rainfall and increased variability in its distribution (De Luís et al., 2001). Although there is no general agreement regarding the future amounts and timing of precipitation in the Mediterranean area, plant communities are likely to be greatly affected by the increased potential evapotranspiration linked to warming (Le Houérou, 1996; Piñol et al., 1998). These expected climatic changes may pose particular problems for plants with growth (e.g. leaf appearance) and reproduction (e.g. flowering) triggered by environmental conditions such as temperature or water availability. Such conditions act as cues that regulate plant growth and development, and specifically may critically affect plant reproduction and flowering (Peñuelas and Filella; 2001; Peñuelas et al., 2004a; Llorens and Peñuelas, 2005; Sherry et al., 2007).
Flowering responses to climate change seem to be species-specific since co-existing species may have different environmental constraints (Peñuelas et al., 2002; Ogaya and Peñuelas, 2004; Llorens and Peñuelas, 2005). The consequences of a change (advance or delay) of flowering phenology might be a decoupling of species interactions, such as between plants and their pollinators, altered competitive relationships and different abundance ranges of species, for example by changed seedling recruitment (Bond, 1995; Gordo and Sanz, 2005). Together, all these readjustments may lead to important changes in the structure, composition and functioning of the ecosystem (Peñuelas and Filella, 2001; Hollister et al., 2005) and therefore changes in phenological processes may have huge socio-economic consequences around the world.
The aims of this study were (1) to quantify the flowering of two common Mediterranean species that flower in autumn, Globularia alypum and Erica multiflora, in relation to environmental conditions, (2) to explain the relationships between flowering phenology and environmental conditions that vary between years (mostly precipitation), and (3) to study the responses to experimental field warming and drought that simulate future climate conditions forecasted by GCMs and ecophysiological models (IPCC, 2001, 2007; Peñuelas et al., 2005). We hypothesized: (a) that the beginning of anthesis and date of maximum flowering intensity (peak date) will vary between years according to the precipitation, since these phenophases occur after the summer drought and therefore under water deficit conditions; and (b) that flowering would be delayed and/or the intensity of the flowering peak would be reduced in response to projected future drier conditions, especially in Globularia alypum, which begins to flower in late-summer, and warming would enhance flowering, especially in Erica multiflora, which flowers later than Globularia alypum, in wet and colder periods.
MATERIALS AND METHODS
Study site and plant species
The study was carried out in a dry calcareous shrubland (Rosmarino–Ericion) in the Garraf Natural Park in North-East Spain (41°18′N, 1°49′E), at 210 m asl. Precipitation during the 4 years of the study (2001–2004) showed great inter-annual variability, from 458 mm in 2001 to 956 mm in 2002, but the distribution throughout the year was typically Mediterranean, with spring, and especially autumn, rains (Fig 1). The driest periods were June, July and August, which together had an average of 80 mm precipitation, and January, with 20 mm. Temperature variation through the year was typically Mediterranean, with moderately cold winters and hot summers. The mean average annual air temperature was 15·2°C and the monthly mean maximum was 27°C in August 2003 and the minimum was 5°C in January 2005.
Fig. 1.
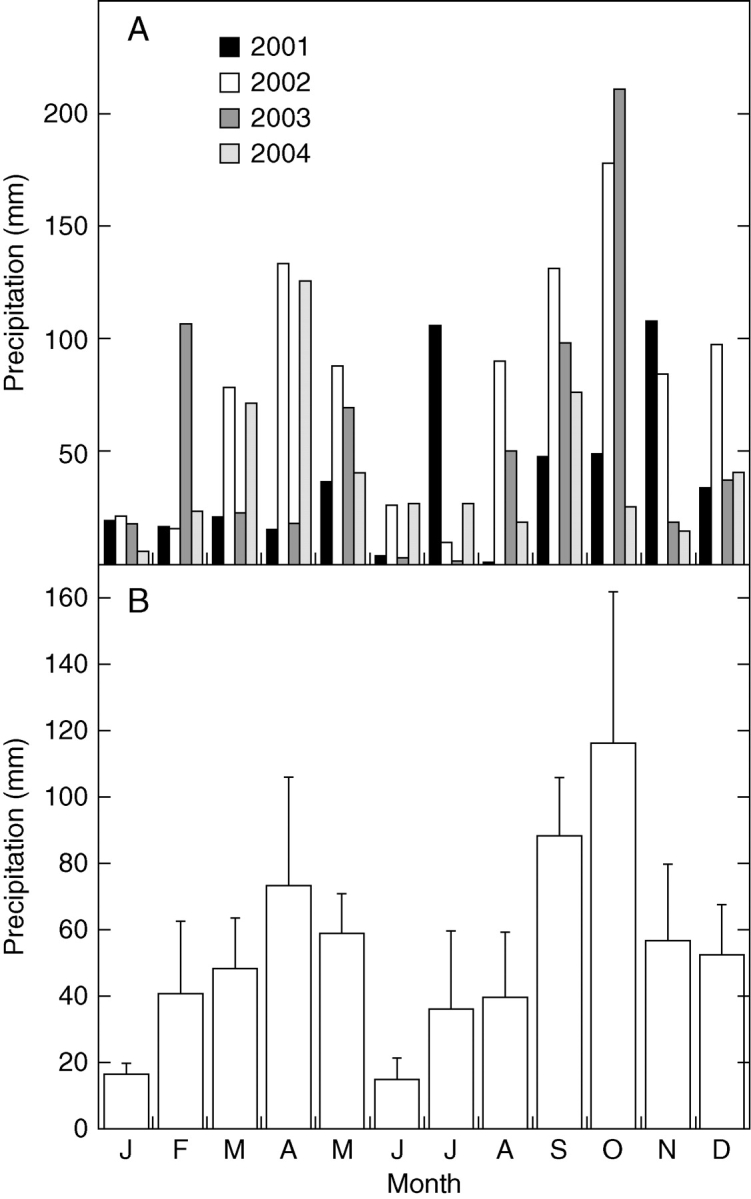
(A) Monthly precipitation and (b) monthly average precipitation based on the 4 years of study (2001–2004). Bars indicate the s.e.m. (n = 4 year means).
The site is a south-facing slope in abandoned old field terraces. The substrate consists of limestone and marls, with a many rock outcrops. In summer 1982 and spring 1994 the area suffered severe fires that destroyed Pinus halepensis forest and converted it into a garrigue (uncultivated land of a calcareous soil overgrown with low scrub). The dominant species are Erica multiflora L. and Globularia alypum L. Both are re-sprouter shrubs, distributed in dry calcareous and rocky places in the Mediterranean Basin (Bolòs and Vigo, 1995). Pinus halepensis was reintroduced by seeding after the last fire and is gaining dominance. Other common species are Dorycnium pentaphyllum, Pistacia lentiscus, Rosmarinus officinalis, Fumana ericoides, Fumana thymifolia and Helianthemum syriacum. The plant community has an average annual above-ground net primary productivity of 160 g m−2 (Peñuelas et al., 2007) and the plant cover in 2005 reached 75%.
Experimental treatments
Three separated experimental treatments were imposed, field-scale night-time warming, drought and control, and the response to warming and drought were compared separately (no interaction plots) to the response of control plots. Plots were 4 × 5 m2, allowing for a buffer strip of 0·5 m at the perimeter. Each treatment was replicated three times, giving a total of nine plots (three warming, three drought and three controls; see Appendix). The experimental treatments were applied continuously from 1999 to 2005, although this paper presents only results from 2001–2004.
Drought treatment
The drought treatment was applied for 2–3 month periods during the spring and autumn growing seasons by covering the vegetation with waterproof, transparent covers. For the rest of the year the plots were not protected from rain. The drought plots were set up similarly to the warming plots (see below) except that the curtain material was a transparent plastic and that the movement of the curtains was governed only by rain and wind. When the drought treatment was in operation, rain sensors activated the curtains to cover the plots whenever it rained and removed the curtains when the rain stopped. The curtains were removed automatically when the wind speed exceeded 10 m s−1.
Warming treatment
The warming treatment consisted of covering the vegetation during the night time with reflective aluminium foil curtains (ILS ALU, AB Ludvig Svensson, Sweden; Beier et al., 2004). Solar energy warms plots during the day and a fraction of the energy is re-radiated back to the atmosphere at night as longwave infrared (IR) radiation; covering reduced the loss of IR radiation. The curtains reflected 97% of the direct and 96% of the diffuse radiation from the vegetation to the night sky. Light scaffolding carrying the reflective aluminium curtain was arranged around the plots in the warming treatment. The plots were covered automatically when solar radiation was less than 0·4 Wm−2. The curtains were removed automatically if wind speed exceeded 10 m s−1 (Beier et al., 2004). In order to avoid influencing the hydrological cycle, rain sensors trigger the automatic removal of the covers when it rained at night.
This method had the advantage that unintended edge effects and artifacts were minimized. Measurements of curtain movements, temperature, precipitation, water input into the plots, radiation balance during the treatment periods, relative humidity and wind speed showed that edge effects on the temperature increase, as well as unintended effects on wind and moisture conditions, were minimal. Since night-time warming involves leaving the plots open during the daytime, the effect on radiation incident on the plots was negligible (Beier et al., 2004).
Untreated controls
Three untreated control plots were set up for comparison, with similar light scaffolding as for the warming and drought treatments, but without any curtains.
Environmental conditions were monitored in all plots. Precipitation within the plots was measured using three water collectors. Soil moisture was also measured weekly by means of three time domain reflectometry (TDR) probes installed per plot. Air (+20 cm) and soil temperatures (at 5 and 10 cm depth) and the functioning of the treatments (curtain closure and removal detected by magnetic sensors) were recorded (Campbell Scientific Datalogger, Logan, UT, USA).
Data collection and analysis
The timing of the autumn flowering of Erica multiflora and Globularia alypum was monitored from observations made biweekly during the less active flowering period and weekly during the most active flowering period, over four consecutive years (2001–2004). From four to 12 Erica multiflora plants and 11–15 Globularia alypum plants were monitored in each plot. For each plant the current flowering percentage was estimated as the amount of functional flowers (live flowers with at least a petal and stamen) relative to the maximum flowering potential, defined as the number of new shoots where flower heads can develop. Four flowering categories were categorized depending on the percentage of shoots with functional flowers: 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). The percentage of branches with functional flowers per plot for each census occasion was computed as the mean of the values of all plants in the plot. This procedure was followed until no new flowers opened. Three traits were used to characterize the flowering process: beginning date (date at which flowering was greater than 1%), peak date (date when flowering per plot reached its maximum intensity), and the intensity of the peak.
Statistical analyses
All the statistical analyses were performed using one value per plot, obtained from averaging the plants measured per plot. Drought and warming treatments were always compared separately with controls. Data values expressed as percentage were arcsine-square-root transformed to homogenize variances. Repeated measures (RM-) ANOVAs were conducted to test inter-annual differences in the beginning and peak dates and intensities in control plots and also to check the global effects of the warming or drought treatments on flowering. ANOVAs were used to test potential effects of the treatments on the peak intensity. Post-hoc (Fisher PLSD) tests were performed for comparisons between years for each species. Survival analyses were used to test differences in the beginning and peak dates between treatments and controls. Survival time was defined as the number of days from January 1 until the beginning of flowering or the peak day occurred. The Kaplan–Meier non-parametric method was used for the computation of survival curves and log-rank (Mantel–Cox) statistics to test for differences between treatments and controls. Drought and warming curves were always compared separately with controls. Correlation analyses were conducted in order to examine the relationship of the accumulated precipitation and the average temperature (from different periods prior to flowering) with the flowering traits. In addition, the relationship was tested with the date when the last important rain event (>10 mm in less than 36 h) just before the peak date took place, since this threshold has been shown to be the most significant. In the exploratory analyses, correlations with other environmental variables such as soil moisture and minimum, maximum and average temperatures were tested. The strongest correlations were between flowering and accumulated precipitation, which seemed to integrate water availability better than weekly soil moisture measurements at this semi-arid site with high evaporative demand. Because of this, only correlations of flowering with precipitation are given. The analyses were conducted with treatment mean values, and therefore with values having known associated variance. Thus a model II regression by means of the reduced major axis method was used (Sokal and Rohlf, 1995). For Globularia alypum, in cases of bimodal flowering pattern, we always used the most prolific peak (the second one in 2001 and the first one in 2003). Survival analyses, ANOVAs, Fisher PLSD tests, RM-ANOVAs, and correlation and regression analyses were performed using the Statview software package (Abacus Concepts Inc., Cary, NC, USA).
RESULTS
Environmental conditions
The drought treatment reduced mean annual soil moisture by an average of 17% with respect to control plots (2001–2004 period). But this effect varied between periods and years (Table 1). During the period of flower development (spring and summer), the soil moisture in drought plots was reduced by an average of 19% compared to control plots, although in 2001 the reduction was 32% (Table 1). The average soil moisture both in summer and the whole spring–summer periods varied significantly between years (P < 0·001). The experimental drought reduced (but not significantly; P = 0·11) the average soil moisture in the spring–summer period (Table 1). The warming treatment did not affect soil moisture.
Table 1.
Mean (± s.e., n = 3) soil moisture (%) in summer and in spring–summer periods in control and droughted plots
| Summer (1 June to 31 August) |
Spring–Summer (1 April to 31 August) |
|||
|---|---|---|---|---|
| Year | Control | Drought | Control | Drought |
| 2001 | 9 (1·2)ab | 5 (0·4)a | 11 (1·2)a | 8 (0·4)a |
| 2002 | 10 (1·3)a | 10 (0·8)b | 16 (1·4)b | 14 (0·6)b |
| 2003 | 6·5 (0·7)b | 7 (0·7)c | 14 (1)ab | 12 (0·1)c |
| 2004 | 11 (0·9)a | 9 (0·6)bc | 16 (1·2)b | 14 (0·9)b |
Significant differences (Fisher post-hoc test) among years are indicated by different letters. Bold type indicates a significant effect of drought treatment on soil moisture (ANOVA).
Covering at night warmed the plots, increasing mean air temperature through the year by an average of 0·73°C compared to control plots. The increase was greater for soil mean temperature (0·9°C), and especially for the minimum temperature (1·5°C). The drought treatment did not affect the mean or minimum air temperature, or the soil temperature.
Characterization of the flowering and treatment effects
Globularia alypum presented two flowering patterns depending on the year (Figs 2 and 3). In 2001 and 2003 two peaks occurred, whereas in 2002 and 2004 only one peak was noted. Flowering in this species varied significantly between years (RM-ANOVA: beginning and first peak date, P < 0·0001; first peak intensity, P = 0·01; second peak date, P = 0·0002; Table 2) and correlated with precipitation, as described below.
Fig. 2.
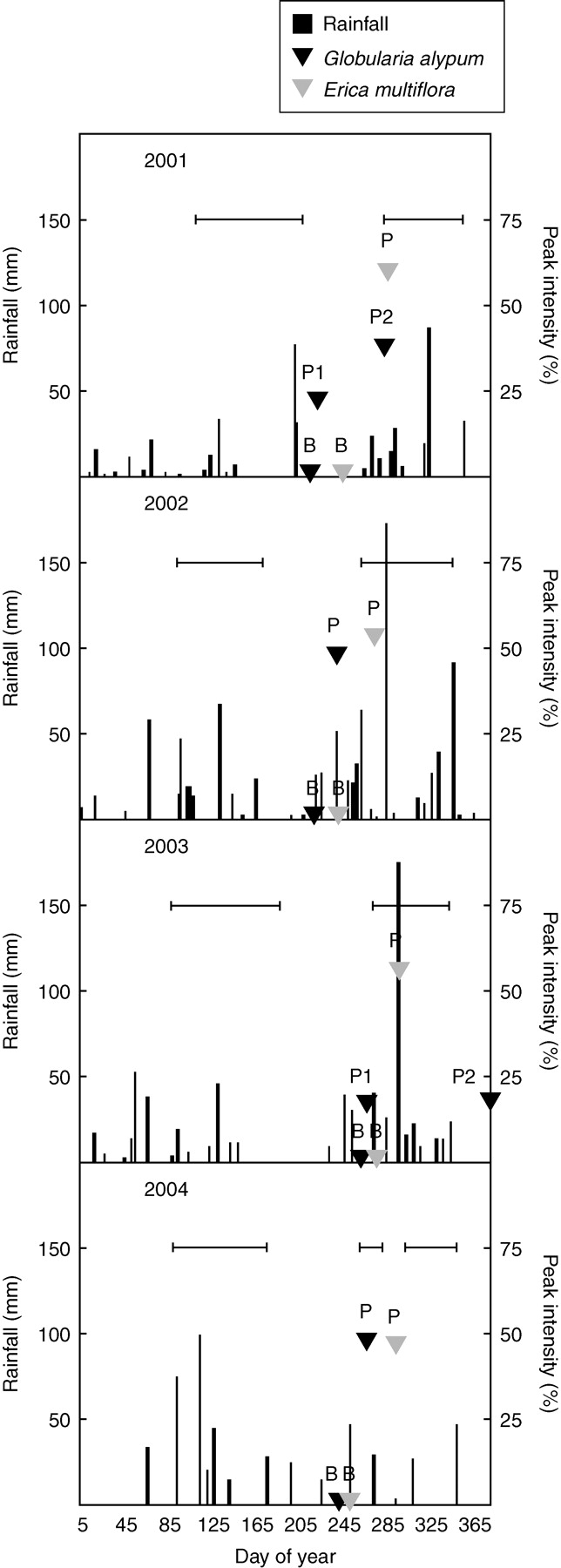
Distribution and magnitude of rainfall and beginning of anthesis (‘B’) and peak date (‘P’, date of maximum flowering intensity) for each year of the study for Globularia alypum and Erica multiflora in control plots. For Globularia alypum ‘P1’ indicates the first peak and ‘P2’ the second peak. Horizontal bars show the duration of the drought treatment.
Fig. 3.
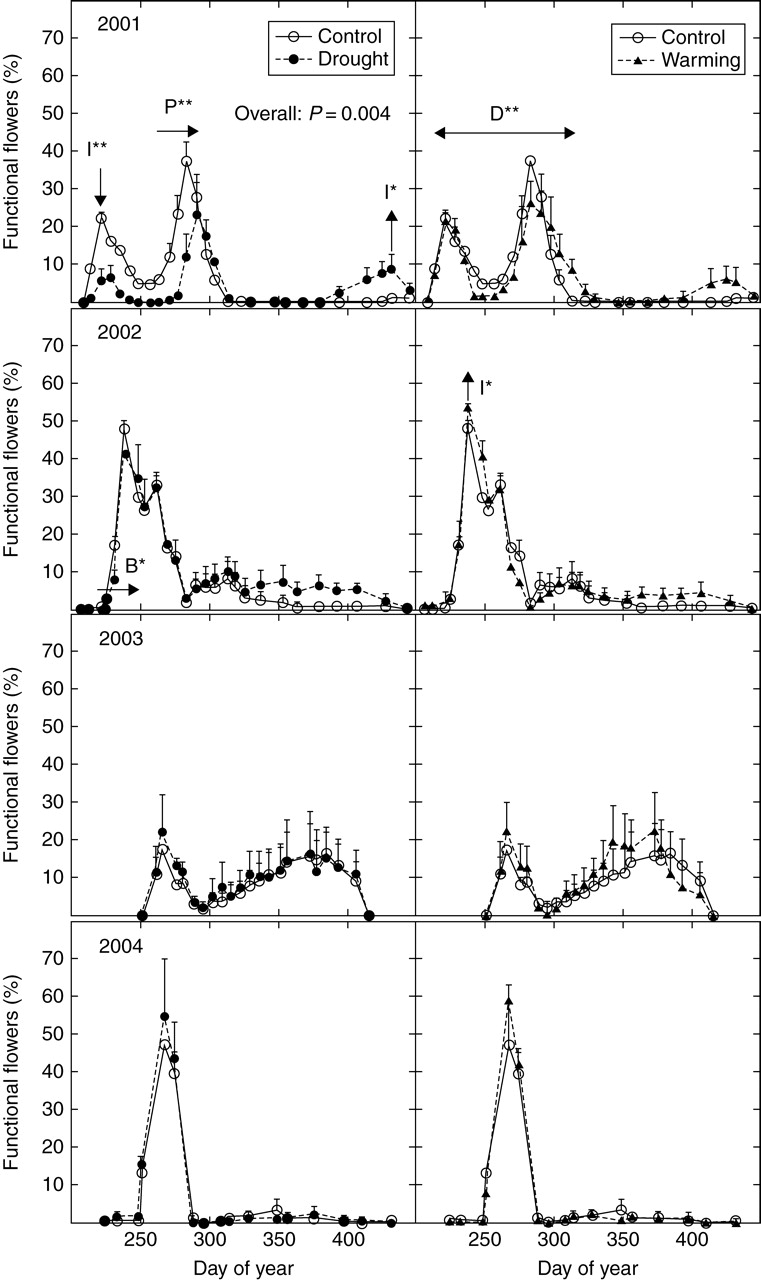
Annual flowering patterns for Globularia alypum in control, drought and warming treatments. Letters indicate significant treatment effects on the beginning date (B), peak date (P), peak intensity (I) and flowering duration (D): **, P < 0·05; *, P < 0·1. Arrows indicate the direction of the change.
Table 2.
Annual mean dates of the beginning of flowering and peaks (expressed in days of the year, DOY), and peak intensity (%) for Globularia alypum and Erica multiflora. Each value is the mean of the variable in control and treated plots if the treatment did not affect it
| First bloom |
Second bloom |
|||||
|---|---|---|---|---|---|---|
| Species | Year | Beginning DOY | DOY | Intensity (%) | DOY | Intensity (%) |
| Globularia alypum | 2001 | 212a | 223a | 22a | 285a | 32 |
| 2002 | 219a | 238b | 48b | |||
| 2003 | 261b | 268bc | 17a | 372b | 21 | |
| 2004 | 241bc | 267bc | 48b | |||
| Erica multiflora | 2001 | 248a | 293a | 57 | ||
| 2002 | 241a | 271b | 56 | |||
| 2003 | 276b | 299a | 56 | |||
| 2004 | 251a | 296a | 53 | |||
Different letters indicate significantly different means (Fisher post-hoc test of the ANOVA, P < 0·05). Bold type indicates significant inter-annual variability (RM-ANOVA, P < 0·001).
Drought treatment affected the flowering of Globularia alypum in 2001 (RM-ANOVA: P = 0·004). The intensity of the first peak was reduced by 13% (ANOVA: P = 0·03) and drier conditions retarded the second peak by almost 10 d (Log-rank: P = 0·02; Fig. 3). Imposed drought also tended to increase the percentage of open flowers at the end of the flowering period (ANOVA: P = 0·09; Fig. 3). In the next 3 years that were monitored, appearance of the earlier open flowers of Globularia alypum was delayed by 11 d in 2002 (Log-rank: P = 0·06; Fig. 3). In the warming plots, the period between the beginning of flowering and the end of the second peak was longer (ANOVA: P = 0·04) in 2001: that included reduced flowering at the end of the late-summer peak and enhanced flowering at the end of the autumn peak. In 2002, an increased peak intensity of 6% was marginally significant (ANOVA: P = 0·06; Fig. 3).
Erica multiflora flowered with a single peak only once each year throughout the 4 years; Figs 2 and 4). The mean dates of the onset of flowering and the peak differed significantly between years (RM-ANOVA: P < 0·0001; Table 2) and correlated with precipitation. Flowering started later in 2003, and the peak date in 2002 was earlier than in other years. Peak intensity was about 56% and did not vary between years (Table 2). For Erica multiflora neither drought nor warming treatments affected flowering (Fig. 4).
Fig. 4.
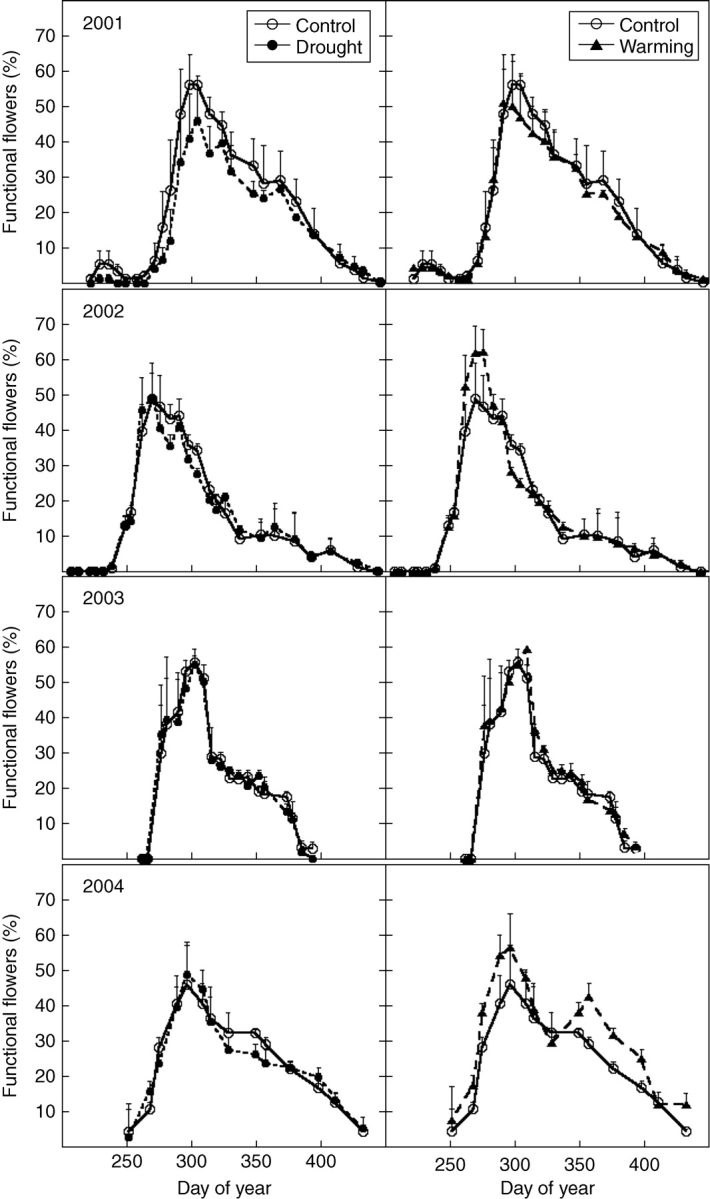
Annual flowering patterns for Erica multiflora in control, drought and warming treatments. There were no significant effects of any treatment.
Rainfall and flowering
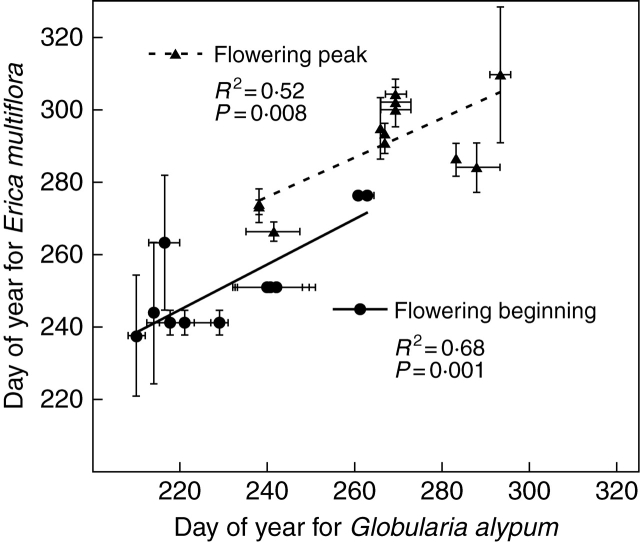
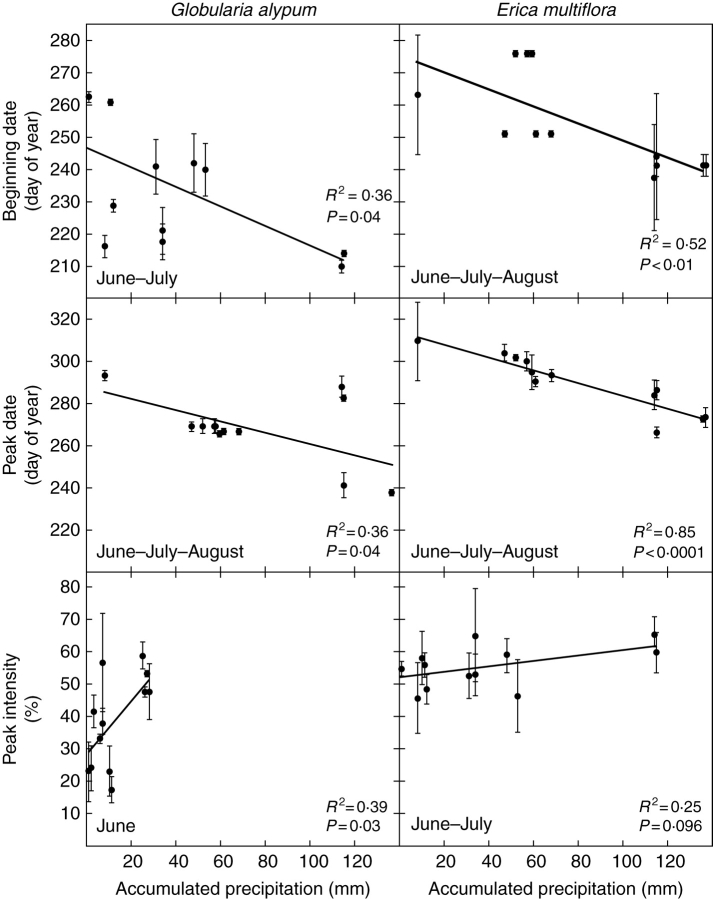
The beginning of flowering and peak date for both species varied synchronously within years (beginning: R2 = 0·68, P = 0·001; peak: R2 = 0·52, P = 0·008; Fig 5), indicating that flowering in both is similarly affected by environmental variables. Precipitation was more closely correlated with flowering than was temperature. There was a significant, negative relationship between the beginning date of Globularia alypum and Erica multiflora and the accumulated precipitation in June–July (R2 = 0·36, P < 0·04) and June–July–August (R2 = 0·52, P = 0·008), respectively (Fig. 6).
Fig. 5.
Synchronicity between the beginning of flowering and peak dates for Globularia alypum and Erica multiflora. Each symbol corresponds to a mean value per treatment (n = 12). Bars indicate the s.e.m. (n = 3 plots).
Fig. 6.
Relationships between the beginning of the flowering, the peak date and peak intensity for Globularia alypum and Erica multiflora and the accumulated precipitation in preceding months (period indicated in each panel). Each symbol corresponds to a mean value per treatment (n = 12). Bars indicate the s.e.m. (n = 3 plots).
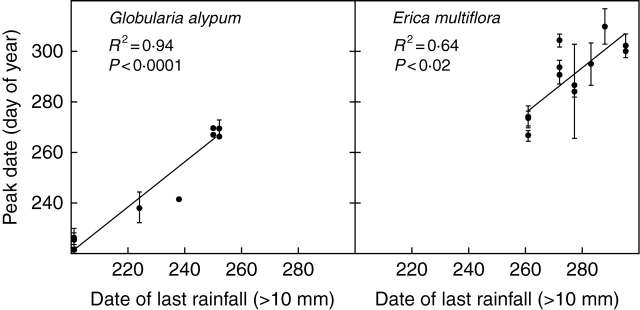
There were also negative correlations between the peak dates for Globularia alypum and Erica multiflora and the accumulated precipitation in June–July–August (R2 = 0·36, P = 0·04 and R2 = 0·85, P < 0·0001, respectively; Fig. 6). A significant, positive relationship was found between the peak intensity of Globularia alypum and the accumulated precipitation in June (R2 = 0·39, P = 0·03; Fig. 6). The peak intensity for Erica multiflora correlated with the accumulated precipitation in June and July (R2 = 0·25, P = 0·096; Fig. 6). Control of the peak date for both species by precipitation was also manifested in the significant relationship between the peak date and the date of the last rain (>10 mm) before peak date (Globularia alypum, R2 =0·94, P < 0·0001; Erica multiflora, R2=0·64, P < 0·02; Fig. 7).
Fig. 7.
Relationships between the peak date for Globularia alypum and Erica multiflora and the date of the last rainfall exceeding 10 mm before the peak date. Each symbol corresponds to a mean value per treatment (n = 12). Bars indicate the s.e.m. (n = 3 plots).
DISCUSSION
Synchronization of flowering for Globularia alypum and Erica multiflora was similar between years, suggesting that they were influenced in the same way by the environment (Fig. 5). As described for other species in the Iberian Peninsula (Peñuelas et al., 2004a), advanced and increased flowering was found in response to increased precipitation during the preceding months (Fig. 6). The results show that the date of maximum flowering intensity of both species responded to rainfall, in particular for Globularia alypum, which uses water less conservatively than Erica multiflora (Llorens et al., 2003). Our findings agree with those of Llorens and Peñuelas (2005) regarding the important role of water availability in determining year-to-year shifts in flowering for these species. Precipitation was found to trigger anthesis and synchronization of flowering of trees and shrubs in a tropical forest in Costa Rica that undergoes periods of drought (Opler et al., 1976). An opportunistic response to water availability has been considered the simplest explanation for patterns at sites where water is seasonally limiting and it is considered a ‘safe strategy’ for the control of flowering (Friedel et al., 1993; Castro-Díez and Montserrat-Martí, 1998; Corlett and Lafrankie, 1998).
Besides the inter-annual variability in the beginning and peak date, Globularia alypum flowered once or twice, depending on the year (Figs 2 and 3). Multiple flowering patterns have also been described for other species (Opler et al., 1976; Picó and Retana, 2001) and it has been considered an adaptive response to the unpredictability of drought intensity and duration (Llorens and Peñuelas, 2005). The majority of the flower buds of Globularia alypum develop in late-spring and early summer, in the apex of new shoots. In years such 2002 and 2004, with relatively wet springs and summers (Figs 1 and 2), flower buds opened in a single and large peak in late-summer or autumn (Fig. 3). In 2001 and 2003 the flowering pattern in Globularia alypum was bimodal. In both years there were longer dry periods than in 2002 and 2004; 70 d from mid-spring to early summer in 2001 and 98 d from late-spring to mid-summer in 2003, whereas the dry period lasted only 55 d in 2002 and 63 d in 2004 (Fig. 2). Moreover, high temperatures in the summer of 2003 increased the loss of water by evapotranspiration (the mean temperature during June–August in 2003 was 26·4°C compared with the average of 24·3°C). The bimodal flowering patterns observed in 2001 and 2003 exhibited differences, probably associated with the variable distribution and magnitude of rains in summer–autumn. In 2001, after the long dry period, rain in early summer released only a small fraction of the floral head buds, which corresponded with the late-summer peak. This increase in water availability was probably insufficient and later rains (24 mm collected on day-of-year 265) were needed to stimulate a second peak in autumn, which was bigger than the first peak (Fig. 2). In 2003, extended drought periods caused high mortality of new shoots and consequently of flower buds (pers. obs.), which resulted in the diminution of the first peak in late summer. In this year, other flower-head buds were formed on top of shoots grown in autumn and these opened in a second peak in winter. Conversely, flower buds of Erica multiflora appeared in August–September and flowers opened gradually in autumn or even in winter in a single and lengthened peak, independent of the meteorological conditions (Fig. 4). The unimodal flowering pattern and the lack of inter-annual variability in the peak intensity in Erica multiflora may be linked with its conservative use of water (Llorens et al., 2003), a strategy that allows this species to maintain higher water potentials, and thus more continuous activity.
Some significant changes were detected in the flowering process in droughted plots, which were manifested as delays in the beginning of flowering and peak date, and reductions in the peak intensity. But, as observed in other studies with Mediterranean species, the effects were clearly species-specific and depended on the conditions in each year (Ogaya and Peñuelas, 2004; Peñuelas et al., 2004a; Llorens and Peñuelas, 2005). In spite of the clear relationship between the timing of flowering and water availability, drought treatment did not have global effects throughout the 4 years. Erica multiflora was unaffected by drought, which significantly affected flowering of Globularia alypum in 2001. In that year, the reduced peak intensity in flowering of Globularia alypum with drought was followed by an increased percentage of functional flowers at the end of the flowering period (when the drought treatment had ceased). This suggests a great ability of Globularia alypum to flower in favourable periods (mostly linked to water availability). The effects of drought in our study agreed with those of a study in 1999 and 2000 (Peñuelas et al., 2004a, Llorens and Peñuelas, 2005). More marked changes in flowering for this species were found during the first 3 years of experimentation (1999–2001), probably related to a combination of the particular environmental conditions of each year and the higher effect of the drought treatment on soil moisture during the first 3 years (29% soil moisture decrease vs. 14% decrease in 2002–2004). The apparent paradox involving the significant relationships between flowering and water availability and the absence of a significant effect of our experimental drought treatment on flowering of Erica multiflora may be explained by the natural inter-annual variability of soil moisture in the summer and spring–summer periods being greater than the reduction of soil moisture in droughted plots (Table 1). Moreover, in most years the autumn drought treatment started after the late-summer rains.
Complementary studies at a community level showed that after 7 years of experimental drought, neither biomass accumulation nor the relative abundances of Globularia alypum and Erica multiflora were negatively affected (Prieto, 2007). In fact, Globularia alypum seems to have a competitive advantage over co-existing species. Drier conditions may not reduce the capacity of established plants to re-sprout but they may affect the recruitment of young plants. In 2001, when flowering of Globularia alypum was delayed by the drought treatment, there was also a reduction in its seedling recruitment, probably due to a reduction in pollinators, injuries caused by late frost, and indirect effects on germination and survival (Peñuelas et al., 2004a). Longer-term studies are required in order to better understand the potential effects of drought on the features Mediterranean shrublands, and the future distribution of these species.
Many observational studies have reported advancements in flowering phenology as a consequence of increased temperatures (Farnsworth et al., 1995; Suzuki and Kudo, 1997; Menzel and Fabian, 1999; Sandvik and Totland, 2000; Abu-Asab et al., 2001; Peñuelas and Filella, 2001; Fitter and Fitter, 2002; Walther et al., 2002; Sherry et al., 2007). Peñuelas et al. (2002) estimated that plants in the Mediterranean region flowered on average 6 d earlier than in 1952. However, the warming effect on flowering seems to be species-specific (Peñuelas and Filella, 2001; Peñuelas et al., 2002), and moreover depends on the local and regional variability of climatic conditions (Kudo and Suzuki, 2003), the life form (Farnsworth et al., 1995), the time when it occurs and the specificities of the environmental conditions occurring at each moment of the year (Fitter and Fitter, 2002; Gordo and Sanz, 2005; Sherry et al., 2007). In the present study, the flowering of neither species was very sensitive to increased temperatures (only slight responses of Globularia alypum); this can be considered as a reasonable result because the flower-head buds of Globularia alypum appear in late-spring/summer and the flower buds of Erica multiflora in late summer, in both cases when temperatures are high, and because the warming treatment only increased the temperature by 0·73°C, which was lower than other experimental studies (see the TERACC website for all warming experiments: http://www.umaine.edu/teracc/). Previous studies have shown that the timing of vegetative growth of Erica multiflora is very responsive to increased temperature (Peñuelas et al., 2004b, 2007; Prieto, 2007). However, this process takes place in late-winter and spring when temperatures are lower and thus the warming treatment alleviates them. In addition, we did find any negative effects of the warming treatment due to accentuated summer temperatures and drought in either of the studied species.
In summary, our results show that the beginning of anthesis and the peak date (date of maximum flowering intensity) vary in these two species depending on the precipitation during the preceding months and the date of the last rainfall (>10 mm), especially in Globularia alypum. The plasticity in flowering of this species in response to water availability was also reflected in its multiple flowering patterns. The results suggest that precipitation-dependent autumn flowering is a safe strategy for both species when faced with unpredictable water availability in the Mediterranean area. However, extreme changes in rainfall patterns in spring and summer may seriously affect the phenology of flowering of Globularia alypum, whereas the phenology of Erica multiflora seems much less sensitive.
ACKNOWLEDGEMENTS
We owe thanks to Paula Bruna for facilities at the start of this work and for providing the phenological data in 2001, and to Vanesa Cruz for her help in the collection of the data. This research was funded by the EU under the projects CLIMOOR (Contract ENV4-CT97-0694) and VULCAN (Contract EVK2-CT-2000–00094), and we also received financial help from the Spanish Government (grants CGL2004–1402/BOS and CGL2006–04025/BOS), the Catalan Government (SGR2005–00312), the European project ALARM (Contract 506675), and the Fundación Banco Bilbao Vizcaya 2004 grant.
APPENDIX
Distribution of the experimental plots (4 × 5 m) with night-time warming (W) or drought (D) treatments and controls (C).

LITERATURE CITED
- Abu-Asab MS, Peterson PM, Shetler SG, Orli SS. Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodiversity and Conservation. 2001;10:597–612. [Google Scholar]
- Ahas R, Aasa A, Menzel A, Fedotova VG, Scheifinger H. Changes in European spring phenology. International Journal of Climatology. 2002;22:1727–1738. [Google Scholar]
- Beier C, Emmett B, Gundersen P, Tietema A, Peñuelas J, Estiarte M, et al. Novel approaches to study climate change effects on terrestrial ecosystems in the field: drought and passive night-time warming. Ecosystems. 2004;7:583–597. [Google Scholar]
- Bolòs O, Vigo J. Flora dels Països Catalans. Barcelona: Editorial Barcino; 1995. [Google Scholar]
- Bond WJ. Effects of global change on plant–animal synchrony: implications for pollination and seed dispersal in Mediterranean habitats. In: Moreno JM, Oechel WC, editors. Global change and Mediterranean-type ecosystems. New York: Springer-Verlag; 1995. pp. 35–57. [Google Scholar]
- Castro-Díez P, Montserrat-Martí G. Phenological pattern of fifteen Mediterranean phanaerophytes from Quercus ilex communities of NE-Spain. Plant Ecology. 1998;139:103–112. [Google Scholar]
- Chen X, Hu B, Yu R. Spatial and temporal variation of phenological growing season and climate change impacts in temperate eastern China. Global Change Biology. 2005;11:1118–1130. [Google Scholar]
- Corlett RT, Lafrankie JV. Potential impacts of climate change on tropical Asian forest through an influence on phenology. Climatic Change. 1998;39:439–453. [Google Scholar]
- De Luís M, García-Cano MF, Cortina J, Raventós J, González-Hidalgo JC, Sánchez JR. Climatic trends, disturbances and short-term vegetation dynamics in a Mediterranean shrubland. Forest Ecology and Management. 2001;147:25–37. [Google Scholar]
- Farnsworth EJ, Nuñez-Farfán J, Careaga SA, Bazzaz FA. Phenology and growth of three temperate forest life forms in response to artificial soil warming. Journal of Ecology. 1995;83:967–977. [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Friedel MH, Nelson DJ, Sparrow AD, Kinloch JE, Maconochie JR. What induces central Australian arid zone trees and shrubs to flower and fruit? Australian Journal of Botany. 1993;41:307–319. [Google Scholar]
- Giménez-Benavides L, Escudero A, Iriondo JM. Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytologist. 2007;173:367–382. doi: 10.1111/j.1469-8137.2006.01932.x. [DOI] [PubMed] [Google Scholar]
- Gordo O, Sanz JJ. Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia. 2005;146:484–495. doi: 10.1007/s00442-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Gordon C, Woodin SJ, Alexander IJ, Mullins CE. Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytologist. 1999;142:243–258. [Google Scholar]
- Hollister RD, Webber PJ, Tweedie CE. The response of Alaskan arctic tundra to experimental warming: differences between short- and long-term responses. Global Change Biology. 2005;11:525–536. [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, et al., editors. IPCC. Climate change 2001: the scientific basis. Cambridge: Cambridge University Press; 2001. Contribution of Working Group I in the Third Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Solomon S, Qin D, Manning M, et al., editors. IPCC. Climate change 2007: the physical science basis. Cambridge, UK and New York: Cambridge University Press; 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Jato V, Rodríguez-Rajo J, Dacosta N, Aira M. Heat and chill requirements of Fraxinus flowering in Galicia (NW Spain) Grana. 2004;43:217–223. [Google Scholar]
- Kramer K, Leinonen I, Loustau D. The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: an overview. International Journal of Biometeorology. 2000;44:67–75. doi: 10.1007/s004840000066. [DOI] [PubMed] [Google Scholar]
- Kudo G, Suzuki S. Warming effects on growth, production, and vegetation structure of alpine shrubs: a five-year experiment in northern Japan. Oecologia. 2003;135:280–287. doi: 10.1007/s00442-003-1179-6. [DOI] [PubMed] [Google Scholar]
- Le Houérou HN. Climate change, drought and desertification. Journal of Arid Environments. 1996;34:133–185. [Google Scholar]
- Llorens L, Peñuelas J. Experimental evidence of future drier and warmer conditions affecting flowering of two co-occurring Mediterrnean shrubs. International Journal of Plant Science. 2005;166:235–245. [Google Scholar]
- Llorens L, Peñuelas J, Filella I. Diurnal and seasonal variations in the photosynthetic performance and water relation of two co-occurring Mediterranean shrubs, Erica multiflora and Globularia alypum. Physiologia Plantarum. 2003;118:84–95. doi: 10.1034/j.1399-3054.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Llorens L, Peñuelas J, Estiarte M, Bruna P. Contrasting growth changes in two dominant species of a Mediterranean shrubland submitted to experimental drought and warming. Annals of Botany. 2004;94:843–853. doi: 10.1093/aob/mch211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel A. Trends in phenological phases in Europe between 1951 and 1996. International Journal of Biometeorology. 2000;44:76–81. doi: 10.1007/s004840000054. [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, et al. European phenological response to climate change matches the warming pattern. Global Change Biology. 2006;12:1969–1976. [Google Scholar]
- Nilsen ET, Muller WH. Phenology of the drought-deciduous shrub Lotus scoparius: climatic controls and adaptive significance. Ecological Monographs. 1981;51:323–341. [Google Scholar]
- Ogaya R, Peñuelas J. Phenological patterns of Quercus ilex, Phillyrea latifolia, and Arbutus unedo growing under a field experimental drought. Ecoscience. 2004;11:263–270. [Google Scholar]
- Opler PA, Frankie GW, Baker HG. Rainfall as a factor in the release, timing, and synchronization of anthesis by tropical trees and shrubs. Journal of Biogeography. 1976;3:231–236. [Google Scholar]
- Orshan G. Plant pheno-morphological studies in Mediterranean type ecosystems. Dordrecht: Kluwer Academic Publishers; 1989. [Google Scholar]
- Osborne CP, Chuine I, Viner D, Woodward FI. Olive phenology as a sensitive indicator of future climatic warming in the Mediterranean. Plant, Cell and Environment. 2000;23:701–710. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I. Responses to a warming world. Science. 2001;294:793–795. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Comas P. Changed plant and animal life cycles from 1952 to 2000 in Mediterranean region. Global Change Biology. 2002;8:531–544. [Google Scholar]
- Peñuelas J, Filella I, Zhang X, Llorens L, Ogaya R, Lloret F, et al. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytologist. 2004;a 161:837–846. doi: 10.1111/j.1469-8137.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Gordon C, Llorens L, Nielsen T, Tietema A, Beier C, et al. Non-intrusive field experiments show different plant responses to warming and drought among sites, seasons and species in a North–South European gradient. Ecosystems. 2004;b 7:598–612. [Google Scholar]
- Peñuelas J, Filella I, Sabaté S, Gracia C. Natural systems: terrestrial ecosystems. In: Llebot JE, editor. Climate change in Catalonia. Barcelona: Institut d'Estudis Catalans; 2005. pp. 517–553. [Google Scholar]
- Peñuelas J, Prieto P, Beier C, Cesaraccio C, De Angelis P, de Dato G, et al. Response of plant species richness and primary productivity in shrublands along a north–south gradient in Europe to seven years of experimental warming and drought. Reductions in primary productivity in the heat and drought year of 2003. Global Change Biology. 2007;13:2563–2581. [Google Scholar]
- Piao S, Fang J, Zhou L, Ciais P, Zhu B. Variations in satellite-derived phenology in China's temperate vegetation. Global Change Biology. 2006;12:672–685. [Google Scholar]
- Picó FX, Retana J. The flowering pattern of the perennial herb Lobularia maritima: an unusual case in the Mediterranean basin. Acta Oecologica. 2001;22:209–217. [Google Scholar]
- Piñol J, Terradas J, Lloret F. Climate warming, wildfire hazard, and wildfire occurrence in coastal eastern Spain. Climatic Change. 1998;38:345–357. [Google Scholar]
- Prieto P. Phenology, biomass and community composition changes in European shrublands submitted to experimental warming and drought. Spain: Universitat Autònoma de Barcelona; 2007. PhD Thesis. [Google Scholar]
- Rötzer T, Chmielewski FM. Phenological maps of Europe. Climate Research. 2001;18:249–257. [Google Scholar]
- Sandvik S, Totland Ø. Short-term effects of simulated environmental changes on phenology, reproduction, and growth in the late-flowering snow herb Saxifraga stellaris L. Ecoscience. 2000;7:201–213. [Google Scholar]
- Schwartz MD. Advancing to full bloom: planning phenological research for the 21st century. International Journal of Biometeorology. 1999;42:113–118. [Google Scholar]
- Schwartz MD, Ahas R, Aasa A. Onset of spring starting earlier across the Northern Hemisphere. Global Change Biology. 2006;12:343–351. [Google Scholar]
- Sherry RA, Zhou X, Gu S, Arnone JA, III, Schimel DS, Verburg PS, Wallace LL, Luo Y. Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences of the USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: WH Freeman; 1995. [Google Scholar]
- Suzuki S, Kudo G. Short-term effects of simulated environmental change on phenology, leaf traits and shoot growth of alpine plants on a temperate mountain, northern Japan. Global Change Biology. 1997;3:108–115. [Google Scholar]
- Tèbar FJ, Gil L, Llorens L. Flowering and fruiting phenology of a xerochamaephytic shrub community from the mountain of Mallorca (Balearic islands, Spain) Plant Ecology. 2004;174:293–303. [Google Scholar]
- Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]