Abstract
Background
Transglutaminases have been studied in plants since 1987 in investigations aimed at interpreting some of the molecular mechanisms by which polyamines affect growth and differentiation. Transglutaminases are a widely distributed enzyme family catalysing a myriad of biological reactions in animals. In plants, the post-translational modification of proteins by polyamines forming inter- or intra-molecular cross-links has been the main transglutaminase reaction studied.
Characteristics of Plant Transglutaminases
The few plant transglutaminases sequenced so far have little sequence homology with the best-known animal enzymes, except for the catalytic triad; however, they share a possible structural homology. Proofs of their catalytic activity are: (a) their ability to produce glutamyl-polyamine derivatives; (b) their recognition by animal transglutaminase antibodies; and (c) biochemical features such as calcium-dependency, etc. However, many of their fundamental biochemical and physiological properties still remain elusive.
Transglutaminase Activity is Ubiquitous
It has been detected in algae and in angiosperms in different organs and sub-cellular compartments, chloroplasts being the best-studied organelles.
Possible Roles
Possible roles concern the structural modification of specific protein substrates. In chloroplasts, transglutaminases appear to stabilize the photosynthetic complexes and Rubisco, being regulated by light and other factors, and possibly exerting a positive effect on photosynthesis and photo-protection. In the cytosol, they modify cytoskeletal proteins. Preliminary reports suggest an involvement in the cell wall construction/organization. Other roles appear to be related to fertilization, abiotic and biotic stresses, senescence and programmed cell death, including the hypersensitive reaction.
Conclusions
The widespread occurrence of transglutaminases activity in all organs and cell compartments studied suggests a relevance for their still incompletely defined physiological roles. At present, it is not possible to classify this enzyme family in plants owing to the scarcity of information on genes encoding them.
Key words: Transglutaminases, polyamines, cross-links, chloroplast, light-dependence, cell wall, stress, programmed cell death, monocotyledons, dicotyledons, algae
INTRODUCTION
Why transglutaminases (TGases) have been studied in plants
The growth of plants is regulated by a cohort of environmental and internal factors, among which polyamines (PAs), essential juvenilation growth substances in all living organisms, regulate organogenesis and cell proliferation in higher plants and algae, apical growth of pollen, dormancy break as well as senescence and homeostatic adjustments in response to external stimuli and stresses. The first report on PA effects on plant growth was by Bertossi et al. (1965) in Helianthus tuberosus dormant tubers and further confirmed in several other plants. The molecular mechanism of action of PAs is only partially known. These amines are present in free and bound form. The increasing interest in the possible role of TGase in plants was due to its well-known ability to bind PAs covalently to some animal proteins (Beninati and Folk, 1988), thus modifying their structure and favouring the formation of nets resistant to mechanical stress and proteolysis (Martinet et al., 1990).
What are TGases?
Fifty years ago a transamidating activity was described in the liver and attributed to TGase (Fig. 1). Thereafter many reports showed that the enzyme family responsible for this activity is widespread in micro-organisms, plants, invertebrates and vertebrates (Folk, 1980; Lorand and Graham, 2003; Del Duca and Serafini-Fracassini, 2005). These enzymes catalyse the post-translational modification of proteins by forming stable intra- and inter-molecular bridges between a mid-chain glutamine residue and either a lysine residue or a free PA (Fig. 2); they have thus been called ‘biological glues’ (Griffin et al., 2002).
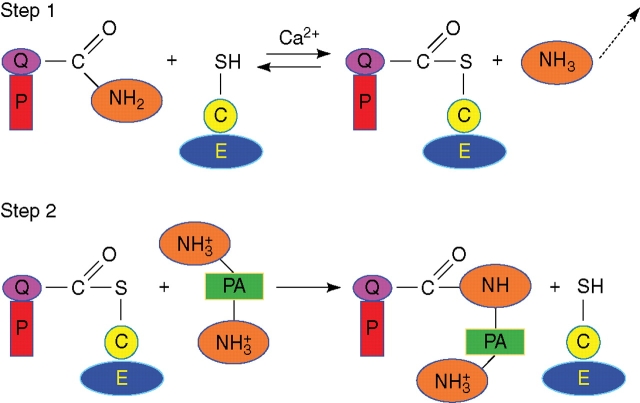
Fig. 1.
The two-step transamidase reaction of transglutaminase. All mammalian TGases belong to a superfamily of cysteine proteases, have structural homology and possess the catalytic triad of Cys-His-Asp/Asn; the reactivity of this Cys is activated by Ca2+, which causes a conformational change in the enzyme, allowing the access of the substrate to the binding site. Step 1: the active Ca2+-stabilized conformation of the enzyme forms a covalent intermediate between the active site thiol residue and a glutamyl residue in the protein substrate, releasing ammonia and activating the glutamine acyl moiety. Step 2: the active thioester undergoes an acyl transfer to a primary amine, in this case a polyamine, thus also introducing extra positive charges as PAs are protonated at physiological pH [or (not shown) to the lysyl residue of another protein]. A secondary cross-link might form between the free amine group of the bound polyamine and a glutamyl residue in another protein substrate thus forming bis-(γ-glutamyl)-PA derivatives (see Fig. 2). E, Transglutaminase; C, cysteine residue; PA, polyamine; P, protein substrate of transglutaminase; Q, glutamine residue.
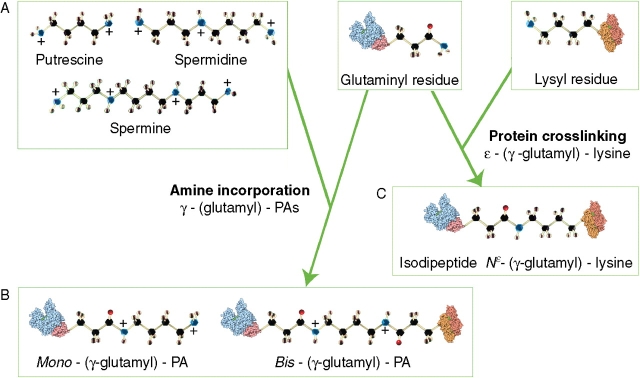
Fig. 2.
Transglutaminase products. The better recognized catalytic activity is the cross-linking activity which leads to N-(γ-glutamyl)-lysine bond formation or to the incorporation of any of various primary amines (e.g. aliphatic PAs) at a glutamyl (former Gln) residue of a substrate protein. (A) The three main aliphatic polyamines present in plants, putrescine (PU), spermidine (SD) and spermine (SM), have different backbone length and numbers of positive charges. (B) PAs (putrescine is the example illustrated) covalently bound to a single protein glutamyl residue by transglutaminase-action, forming mono-(γ-glutamyl)-PA derivatives as shown in Fig 1, or to two glutamyl residues, each located on the same or separate proteins, forming bis-(γ-glutamyl)-PA derivatives. The ‘bridges’ produced in the latter case have a different length according to the molecular length of the PA involved. The formation of mono-derivatives of PAs (the ‘cationization’ of a protein), although not relevant to the thermodynamic properties of the side-chain, causes a very relevant shift in the protein's solubility, stability and conformation, and affects its interaction with other molecules. (C) Glu (former Gln) and Lys residues covalently bound by transglutaminase, forming a cross-link shorter than that formed with PAs.
In animals, nine TGase isoenzymes (EC 2.3.2.13), have been identified at genomic level, but only six have been isolated and characterized at protein level. The best known are the ubiquitous type 2 tissue TGase (tTGase) and a plasma factor XIIIa (involved in the stabilization of fibrin clots) which becomes active only after proteolysis. Since its first description as an enzyme capable of calcium-dependent transamidation (Folk, 1980) (Fig. 1), tTGase has been attributed an increasing number of other biochemical capacities (Lorand and Graham, 2003). This enzyme is located both intra- (cytosol, mitochondria, nucleus) and extra-cellularly in the matrix where it appears to be involved in differentiation, transmembrane signalling, cell adhesion, organization of the extracellular matrix, and pro- and anti-apoptotic roles (Mukherjee et al., 1995; Lentini et al., 2004).
TRANSGLUTAMINASE DISTRIBUTION AND PROPERTIES IN PLANTS
TGases in plants
Twenty years ago the presence of a TGase activity was reported in higher plants. Despite the identification of γ-glutamyl-PA derivatives and the immuno-reactivity by TGase animal antibodies and other characteristics, research on plant TGases has been hampered by difficulties encountered in their purification and by the lack of significant amino acid sequence homologies between animal TGases and the polypeptides reported in the available plant databanks. A more recent computational analysis identified, in Arabidopsis thaliana, the presence of only one gene, AtPng1p, which encodes a putative N-glycanase containing the Cys-His-Asp triad of the TGase catalytic domain. AtPng1p is a single gene expressed ubiquitously, but at low levels, as shown by nested RT–PCR undertaken in different organs of Arabidopsis, at all growth stages and in all light conditions tested (Della Mea et al., 2004a). The recombinant protein was purified and an 86-kDa band was immuno-detected by use of three anti-(animal TGase) antibodies. Antibodies raised against this recombinant AtPng1p detected the same band in the Arabidopsis microsomal fraction and other bands of lower molecular mass in the cytosolic fraction, possibly proteolytic soluble fragments, a usual occurrence with TGases of animal origin. This finding is in keeping with the presence of TGase activities and different immuno-recognized bands in the extracts of several organs of the same plant. Analyses of γ-glutamyl derivatives revealed that the purified recombinant AtPng1p gene product acts as a TGase, having a Ca2+- and GTP-dependent transamidase activity. This was the first plant protein, isolated and characterized at the molecular level, displaying a TGase activity whose parameters agree with those typically exhibited by animal TGases. A structural comparison between the model of this protein and the crystal of factor XIII showed a considerable homology (Tasco et al., 2003). Despite having an amino acid sequence different from those of known animal TGases, with the exception of the active-site triad, AtPng1p shares with them immunological and biochemical properties and possibly an overall similar conformation, probably being a TGase with a different but convergent phylogenetic history. The presence of TGases in plants is further confirmed by the following data reported by several authors, except for point (e), in all plants assayed:
finding of typical products of its catalysis, e.g. glutamyl-PA derivatives;
observation that specific animal or synthetic TGase substrates undergo amine conjugation in the presence of plant extracts;
dose-dependent Ca2+ activation and inhibition by EGTA or EDTA of the activity;
immuno-recognition by antibodies raised against TGases of animal origin;
inhibition of the activity by active-site-specific inhibitors generated against animal TGases (Carvajal-Vallejos et al., 2007);
presence of a Cys in its active centre.
A putative TGase of 58 kDa was isolated from Helianthus thylakoids (Dondini, 1998). On this basis, two related cDNAs were cloned whose transcript is expressed mainly in young leaves and differentiated Zea mays callus under light exposure. This transcript has high homology with some Oryza expressed sequence tags and may be related to the AtPng1p, reported above, as well as to two Streptomyces TGases (Villalobos et al., 2004).
More recently, a pear TGase was sequenced, which showed a homology of 76 % with the Arabidopsis AtPng1p and of 99 % with an apple expressed sequence tag (Di Sandro et al., 2008).
TGases are located in various organs of plants
Meristems
Accomplishment of the cell-cycle, both in plants and animals, requires PAs, and a TGase activity was detected in dividing cells of apical meristems of stems and during the synchronous cell-cycle of parenchyma of Helianthus tuberosus tubers (Icekson and Apelbaum, 1987; Mossetti et al., 1987; Serafini-Fracassini et al., 1988) by immunodetection with animal TGase antibodies or by its transamidase activity, which was found to be low in the early G1 phase and gradually to increase later on until the S phase (Del Duca and Serafini-Fracassini, 2005); in parallel, PA conjugation to high-Mr protein increased (Del Duca et al., 2000a).
Mature vegetative organs
A comparative study of TGase activity and substrates in leaves, tubers, sprouts and flower buds of Helianthus revealed that this enzyme is widespread and that in the same organ multiple enzyme forms are present (Del Duca and Serafini-Fracassini, 2005). Lilley et al. (1998) detected a TGase activity in roots and shoots of dicotyledonous (pea and broad bean) and monocotyledonous (wheat and barley) plantlets. Roots exhibited a higher specific activity than leaves of the same age. In the root, TGase activity was present during early growth and development but afterwards decreased, while it was present in both developing and mature leaves.
Seeds
In seeds of Glycine the conjugation of PAs took place preferentially in the protein bodies during germination, where it was much higher than in the leaves (Kang and Cho, 1996).
Flower petals
TGase activity was also found in sterile (petals) and fertile (pollen) flower parts. A Ca2+-dependent transamidase activity was measured and deduced to be capable of TGase catalysis on the basis of recovery of glutamyl-PA derivatives in the corolla of Nicotiana tabacum flowers (Serafini-Fracassini et al., 2002). The high concentrations of bis-γ-glutamyl-putrescine (bis-PU) (at all stages) and of bis-γ-glutamyl-spermidine (bis-SD) (highest during development and when the corolla changes its shape to favour pollination) could be mainly related to the strengthening of the corolla cell walls, as discussed below.
Pollen
In apple pollen-tubes, a Ca2+-dependent TGase activity has been found both intracellularly and extracellularly. The intracellular TGase activity catalysed the incorporation of PAs into α-tubulin and actin monomers and relatively high-Mr complexes (Del Duca et al., 1997) (Fig. 3).
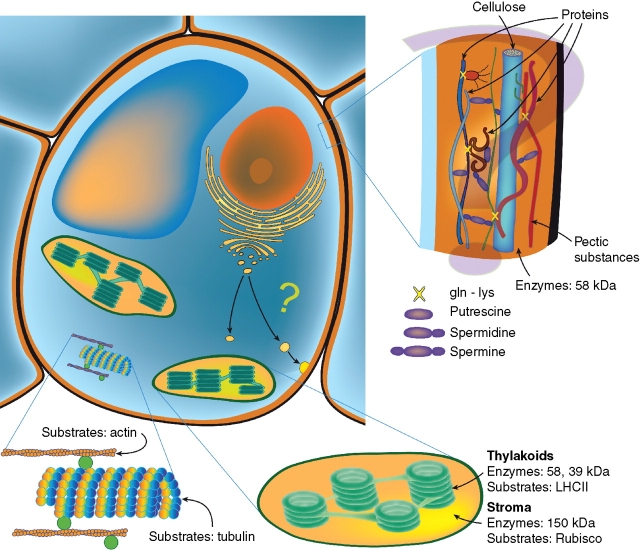
Fig. 3.
Location of transglutaminases in the plant cell. This class of enzyme has been found in cellular compartments of different plants as here schematically summarized. In chloroplasts, some transglutaminases of different molecular mass have been found both in the stroma, where the substrate is Rubisco, and in thylakoids, where the substrates are mainly the light-harvesting complexes (LHCII). The enzyme is also present in the cytosol, where tubulin and actin have been identified as substrates. The occurrence in the microsomal fraction and in the cell wall of TGases of the same molecular mass suggests the hypothesis that the enzyme was secreted through Golgi vesicles into the cell wall, where polyamines, known to be present, might be conjugated to various unidentified structural or enzymatic wall proteins.
The problem of incompatibility in reproduction
The success of reproduction is dependent on the compatibility between the male and female partners. The self-incompatibility (SI) response is a species-specific and genetically controlled mechanism. In the Rosaceae, glycoproteins, encoded by the stylar S locus, showing ribonuclease (S-RNase) activity regulate this molecular mechanism whereas the putative pollen determinants still need to be identified. The cytoskeleton involvement in SI phenomena is so far solely reported in incompatible Papaver tubes, where F-actin foci are formed by an uncharacterized cross-linking mechanism that blocks tube elongation, causing tube PCD. Nevertheless Di Sandro et al. (2008) reported that in the self-pollinated style (incompatible system) of the Rosaceae, the activity of TGase is higher than in the style pollinated with compatible pollen; moreover, high-mass aggregates of tubulin and punctuate aggregates of actin were also observed, suggesting a role for the cytoskeleton in SI. In vitro experiments with purified pollen TGase and purified actin and tubulin, have shown that the inhibition of tube growth in incompatible crosses might be mediated by an abnormal cytoskeletal reorganization caused by TGase-generated cross-linked protein networks.
Is TGase widely located in the cell wall?
The extracellular TGase observed either at the apical or the proximal parts of the pollen-tube, and on the surface of the grain, by laser confocal microscopy (Iorio et al., 2008) plays an essential role in apple pollen-tube growth, as suggested by the arrest of tube elongation by TGase inhibitors. Promising research suggests an involvement of this TGase in the interaction between pollen-tube and stylar cells in the extracellular matrix (D. Serafini-Fracassini et al., unpubl. res.).
It may be envisaged that TGases play a structural role in the organization of the cell wall of other cell types of higher plants. Indirect evidence of an interconnection between cell wall proteins and polysaccharides was obtained by the digestion of Helianthus parenchyma cells by cellulase and pectinase, which caused the disaggregation of high-mass PA-conjugated proteins (Del Duca and Serafini-Fracassini, 2005). A clear confirmation was obtained, in isolated cell walls of Nicotiana petals, by detection of TGase immuno-reactivity by western blotting and catalytic activity (Della Mea et al., 2007) and by the immunodetection of TGase by confocal microscopy when the corolla becomes more rigid (G. Cai, University of Siena, Italy; pers. comm.) (Fig. 3).
In lower plants, TGase activity affects the cell wall in the alga Chlamydomonas reinhardtii (Waffenschmidt et al., 1999). An early TGase activity catalyses the formation of a ‘soft envelope’, which organizes the self-assembly of glycoproteins and an oxidative cross-linking reaction renders the wall insoluble. Chlamydomonas secretes an extracellular 72-kDa TGase, the maximal activity of which precedes the insolubilization of the assembled hydroxyproline-rich glycoprotein. The addition of PAs at high concentrations disrupts the normal wall assembly, possibly by forming mono-derivatives.
TGases in chloroplasts and light effects
PAs are present in chloroplasts, where their biosynthesis is controlled by white light and their concentration is correlated to chlorophyll biosynthesis and photosynthetic rate. PAs are thought to affect regulatory mechanisms of the photosynthetic apparatus during photo-adaptation (Navakoudis et al., 2007); when added to osmotically stressed or senescent leaves, they preserve from degradation the thylakoid proteins and the large Rubisco subunit (Besford et al., 1993).
A highly active TGase was found widespread in green tissues of higher plants and algae. The covalent linkage of PAs to protein glutamyl residues was shown to occur in Beta vulgaris leaf extracts (Signorini et al., 1991) and in Helianthus isolated chloroplasts (Del Duca et al., 1994). In Medicago sativa a 39-kDa TGase subunit recognizes the large subunit of Rubisco as a substrate, causing its assembly (Kuehn et al., 1991).
A peculiar characteristic of chloroplast TGase is that its activity increases after exposure to white light during the assay. After the pioneering paper dealing with the light-stimulated occurrence of ‘fixed’ PAs in chloroplasts of Brassica pekinensis (Cohen et al., 1982), Margosiak et al. (1990) reported that PAs were actively incorporated by isolated chloroplasts of Medicago. A TGase-like activity is inducible in non-photosynthetically committed Helianthus explants grown in vitro under light condition and exposed to hormonal conditions that allow the differentiation of chloroplasts. The occurrence of a specific substrate was related to chloroplast differentiation (Del Duca et al., 1993) and identified later as the apoproteins of the chlorophyll a/b antenna complexes (LHCII, CP24, CP26 and CP29) of PSII (Del Duca et al., 1994). Indeed, PAs might be conjugated via a Ca2+- and light-stimulated TGase, as indicated by the identification of glutamyl-derivatives (Del Duca et al., 1995).
The first (Del Duca et al., 1994) and most frequently immuno-recognized protein in leaf extracts by antibodies raised against different animal TGases was a 58-kDa protein. Also in tuber parenchyma grown in vitro and exposed to light, a 58-kDa SDS–PAGE band immuno-recognized by TGase antibodies is rapidly synthesized and increases under light exposure (Del Duca et al., 2000a). Its purification proved very difficult and it is still unclear whether it represents an active form of the enzyme. A polyclonal antibody was raised against this 58-kDa form from thylakoids of Helianthus leaf chloroplasts (Dondini, 1998), which recognized mainly proteins in the granal thylakoids of Zea (Villalobos et al., 2001).
A TGase activity and bands of different molecular weight immuno-recognized by TGase antibodies were detected both in thylakoid- and stroma-enriched fractions of Helianthus chloroplasts, where the main forms were 58 and 150 kDa, respectively (Fig. 3); furthermore, a synergism, markedly affected by light, between the stromal TGase activity and the prevalent thylakoid TGase activity was observed during the TGase assay. One of the main substrates is LHCII in light conditions and after light → dark transition but not after the opposite transition (Dondini et al., 2003).
In the chloroplasts of Zea leaves, the TGase activity was also prevalent in thylakoids. When the LHCII was isolated and assayed for conjugation with PAs, spermine (the largest PA) was shown to be the most efficiently conjugated PA. The LHCII fraction also contained a 39-kDa band, which was identified as a calcium-dependent TGase localized closely to or in association with LHCII (Della Mea et al., 2004b). This suggested a role for this TGase in the photo-protection exerted by PAs against several factors such as UV-B, ozone, etc. as reported in several papers by the Kotzabasis group (Navakoudis et al., 2007). Thus, the similarities observed in monocots and dicots suggested that an LHCII-associated TGase is widespread.
Chloroplast TGase activity is regulated by the environment
The activity of TGase extracted from chloroplasts, but not those of animal origin, when detected in the test tube, is absolutely light-dependent. When radio-labelled PAs were incubated in the light with either purified thylakoid TGase (Thyl-TGase) or LHCII, mono- and bis-glutamyl-PAs were produced in similar amounts, as usually observed in vivo, while only traces were detectable in the dark (Della Mea et al., 2004b). The known light-induced conformational change of LHCII causes two specific glutamyl residues to modify their relative distance to a value close to the molecular dimensions of spermine which, by hypothesis, could then form a bis-glutamyl-SM bridge involving these residues, thus exerting a structural role in the stabilization of the complex. Two further LHCII glutamyl residues could be possible PA conjugation sites. However, in vivo they are positioned in a loop facing the granal lumen where the pH is lowered when photosynthesis is active, a condition that would not favour the activity of Thyl-TGase, which has a basic pH optimum (Del Duca et al., 2000b). The reported presence in LHCII of high-affinity sites for Ca2+, involved in membrane stacking occurring during photosynthesis, and of low-affinity sites, could also be relevant for the Ca2+-dependent action of Thyl-TGase in vivo.
Other factors, such as phytochrome and hormonal controls, are also involved in the regulation of the TGase activity. In the Zea chloroplasts isolated from meristematic calluses, the biosynthesis and conjugation of PAs show daily changes, possibly owing to an internal rhythm, also affected by external factors; in fact, both were increased during the subjective morning, when the light was on, decreased in the subjective afternoon (still in the light) and increased again during the subjective night; thus they were not strictly light-regulated. The effect was most marked in differentiating calluses, but less evident in growing ones, which were under a different hormonal treatment. Thus, it would appear that a significant role must also be played by the hormonal system that regulates tissue differentiation versus growth (Bernet et al., 1999).
A diurnal trend of free and bound putrescine and TGase activity was observed in forest plants, e.g. Quercus ilex, with the highest TGase value corresponding to the maximum light intensity (Pintó-Marijuan et al., 2007).
An additional mode of regulation by light in vivo is related to the availability of some plant TGase substrates: light regulates the expression of the genes for α- and β-tubulin and of several plastidial proteins such as Rubisco, LHCI, LHCII and others. This could occur concomitantly with the maturation of the thylakoids, a process in which TGase seems to participate (Del Duca et al., 1993), and during the light-induced greening of proplastids of Cucumis sativus endosperm when stimulated by cytokinin (Sobieszczuk-Nowicka et al., 2007). In cucumber etioplast membranes during their early greening stage, TGase antibodies could detect 30-kDa and 77-kDa bands; at the end of greening, the concentration of the latter dramatically decreased. The addition of kinetin increased spermidine conjugation in the early greening stage, but when chloroplast membranes were fully organized it decreased. PAs conjugated by Thyl-TGase could represent a component of the mechanism of etioplast-to-chloroplast transformation stimulated by kinetin.
TGase activity in algae is affected by light and salt stresses
TGase also appears to play a role in adaptation to saline stress. In Dunaliella salina, a green halophilic alga, subjected to abrupt hyper-saline stress under light, chloroplast TGases exhibit an immediate concentration change coincident with variations in enzymic activity; the photosynthetic complexes are severely affected with disappearance of many components on the two-dimensional gel, especially of the active trimeric form of the LHCII, but with the appearance of very high-Mr compounds, possibly owing to PA conjugation. Some TGase substrates, similar to those of higher plants (thylakoid photosynthetic complexes and Rubisco), were more intensely produced in the light and to a greater extent in algae acclimated to hyper-saline conditions (Del Duca and Serafini-Fracassini, 2005).
A Dunaliella PA-deficient variant strain (PA-vs) is characterized by a very low growth capacity and its TGase, chlorophylls and other chloroplast parameters are also deficient, but all recover upon the supply of putrescine. Concomitantly with an upturn in cell growth, chloroplast TGase activity increases 10-fold in the light (three times less in the dark). The PA-vs appears to be even more severely affected by salt stress than the wild-type and its TGase activity increases after stress, being always higher in the light than in the dark, showing an additive stress effect of salt and light. When Dunaliella is acclimated to high salinity, a considerable enhancement in content of chlorophyll a and b overlaps with the increase in TGase activity. This validates the view that in green algae TGase is involved in protection of the photosynthetic apparatus from stress (Del Duca and Serafini-Fracassini, 2005).
In the red alga Grateloupia doryphora, a moderate hyposaline shock caused an increase in free PAs, mainly due to a decrease in TGase activity, together with an apparent increase in their biosynthesis. This may account for the benefits in physiological performance during acclimation, since the photosynthetic rate increased in thalli when exposed to free PAs (García-Jiménez et al., 2007).
Programmed cell death
Developmental cell death (DCD)
During the complex, highly regulated senescence of petals controlled by growth factors and hormones, many events occur: nuclear blebbing, DNA laddering, cell wall modification, a decline in protein, water, chlorophyll and other pigment content, and a decrease in membrane integrity (Serafini-Fracassini et al., 2002; Rogers, 2006). It has been observed that spermine delays senescence and DCD in Nicotiana tabacum petals, retards DNA fragmentation and vacuole damage, and prolongs chloroplast viability with visible preservation of chlorophyll content by a molecular mechanism yet to be fully clarified (Serafini-Fracassini et al., 2002). To assess if these ‘juvenility’ factors might exert their role in bound form, Della Mea et al. (2007) searched for a Ca2+-dependent TGase activity. The contents of bis-PU and of bis-SD were highest during complete differentiation; thereafter they decreased with increasing age of the corolla, when mono-PU, on the contrary, significantly increased. The DCD exhibited an acropetal gradient, which was preceded by a maximum of TGase activity and shifted from the proximal to the distal part of the corolla. Some protein bands were immuno-recognized by three antibodies raised against mammalian, nematode and Arabidopsis TGases. The main immuno-recognized 58-kDa band, also the prevalent form in leaves, decreased during corolla life and was present in the soluble, microsomal, plastidial and cell wall fractions, whereas the peak location of a 38-kDa band, mainly a plastidial form, moved progressively from basal to distal parts of the corolla. TGase activities were detected in (a) the microsomes, where TGase activity is in general higher in the proximal part, peaking at the corolla opening; (b) the soluble fraction, where it is present only in the proximal part at senescence; (c) the plastids, where it shows an increasing trend; (d) the cell walls, prevailing in the distal part and progressively increasing (Della Mea et al., 2007). These data suggest a relationship between DCD and TGase; the latter, possibly released into the cell wall via Golgi vesicles (Fig. 3), could co-operate with cell wall strengthening, especially at the basal abscission zone and possibly during corolla shape change. The plastid TGase, stabilizing the photosystems, could favour the efficiency of photosynthesis and indeed sustain the energy requirements of senescence progression.
In animal systems, the role not only of TGase but also of free PAs in apoptosis is still controversial, depending on age of the cells, PA local concentration, etc.; however, contrarily to animal cells, plant cells can be less affected by excess PA, as plants can buffer this excess by binding PAs to TCA-soluble conjugates, such as cinnamic acids, or by storing them in the vacuole.
Programmed cell death induced by pathogens
PAs play a role also in plant defence against viral pathogens. Plants resisting pathogen attack, carrying the N resistance gene, frequently develop the hypersensitive response, a rapid cell death at the site of pathogen entry, with the effect of restricting pathogen multiplication and spread. Several lines of evidence suggest that hypersensitive response cell death is a form of localized PCD. In tobacco leaves, during the tobacco mosaic virus (TMV)-induced hypersensitive response, titres of free and conjugated PAs increased together with their biosynthetic enzymes. One day after TMV-inoculation of leaves, mono-PU and bis-SD were recovered and further increased in inoculated samples, but not in mock-inoculated ones. The amount of a 72-kDa protein immuno-recognized by AtPng1p polyclonal antibody increased after 3 d in TMV-inoculated leaves and in the lesion-enriched areas. TGase activity increased only in the intrinsic membrane protein fraction, and was more persistent in TMV-inoculated leaves than in the control, supporting the notion of its role in defence (Del Duca et al., 2007)
A role for TGase in defence against viruses is supported by findings in mammalian cells, where a growing number of viral proteins, as well as cellular proteins with which the latter interact, have been found to be modified by TGase, suggesting a novel function for tTGase in viral pathogenesis (Jeon and Kim, 2006).
CONCLUSIONS
Plant TGases share several common characteristics with those of animal TGases, despite having a different overall amino acid sequence except for the active site triad. TGases are present in low amount, but their widespread presence in all cell compartments and plant organs studied to date suggests their relevance as transamidating agents. Promising new fields of research appear to be related to relevant aspects of the life cycle, such as differentiation, PCD, fertilization, etc. The other catalytic features of the multifunctional animal TGases are still to be determined in plants.
ACKNOWLEDGEMENTS
We thank Prof. Elisabetta Verderio-Edwards of Nottingham Trent University (UK) for critical reading the manuscript and language suggestions, the technical assistance of Mr N. Mele for figure preparation, and for the original ideas and technical suggestions of the late Raoul Villanueva of the CNRS of Gif sur Yvette (France) and Arie Altman of the Hebrew University of Jerusalem, Rehovot (Israel). Funding is gratefully acknowledged from Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Fondo per gli Investimenti della Ricerca di Base, Project No. RBAU01KZ49 – Proteine modificate post-traduzionalmente da transglutaminasi durante la morte cellulare programata; Progetti di Rilevante Interesse Nazionale, Prot. 2005078830 – Interaction mechanisms for protein mediators of flower incompatibility in fertilization of fruit trees, to D.S.-F.).
LITERATURE CITED
- Beninati S, Folk JE. Covalent polyamine-protein conjugates: analysis and distribution. Advances in Experimental Medicine and Biology. 1988;250:411–422. doi: 10.1007/978-1-4684-5637-0_36. [DOI] [PubMed] [Google Scholar]
- Bernet E, Claparols I, Dondini L, Santos MA, Serafini-Fracassini D, Torné JM. Changes in polyamine content, arginine and ornithine decarboxylases and transglutaminase activities during light/dark phases of initial differentiation in maize calluses and their chloroplasts. Plant Physiology and Biochemistry. 1999;37:899–909. [Google Scholar]
- Bertossi F, Bagni N, Moruzzi G, Caldarera CM. Spermine as a new growth-promoting substance for Helianthus tuberosus (Jerusalem artichoke) in vitro. Experientia. 1965;21:81–82. [Google Scholar]
- Besford RT, Richardson CM, Campos JL, Tiburcio AF. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta. 1993;189:201–206. [Google Scholar]
- Carvajal-Vallejos PK, Campos A, Fuentes-Prior P, Villalobos E, Almeida AM, Barberà E, et al. Purification and in vitro refolding of maize chloroplast transglutaminase over expressed in Escherichia coli. Biotechnology Letters. 2007;29:1255–1262. doi: 10.1007/s10529-007-9377-7. [DOI] [PubMed] [Google Scholar]
- Cohen SS, Marcu DE, Balint RF. Light-dependent fixation of polyamines into chloroplasts of Chinese cabbage. FEBS Letters. 1982;141:93–97. [Google Scholar]
- Del Duca S, Serafini-Fracassini D. Transglutaminases of higher, lower plants and fungi. Progress in Experimental Tumor Research. 2005;38:223–247. doi: 10.1159/000084243. [DOI] [PubMed] [Google Scholar]
- Del Duca S, Favali A, Serafini-Fracassini D, Pedrazzini R. Transglutaminase-like activity during greening and growth of Helianthus tuberosus explants. Protoplasma. 1993;174:1–9. [Google Scholar]
- Del Duca S, Tidu V, Bassi R, Serafini-Fracassini D, Esposito C. Identification of transglutaminase activity and its substrates in isolated chloroplasts of Helianthus tuberosus. Planta. 1994;193:283–289. [Google Scholar]
- Del Duca S, Beninati S, Serafini-Fracassini D. Polyamines in chloroplasts: identification of their glutamyl- and acetyl-derivatives. Biochemical Journal. 1995;305:233–237. doi: 10.1042/bj3050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Duca S, Bregoli AM, Bergamini C, Serafini-Fracassini D. Transglutaminase-catalyzed modification of cytoskeletal proteins by polyamines during the germination of Malus domestica pollen. Sexual Plant Reproduction. 1997;10:89–95. [Google Scholar]
- Del Duca S, Allue Creus J, D'Orazi D, Dondini L, Bregoli AM, Serafini-Fracassini D. Tuber vegetative stages and cell cycle in Helianthus tuberosus: protein pattern and their modification by spermidine. Journal of Plant Physiology. 2000;a 156:17–25. [Google Scholar]
- Del Duca S, Dondini L, Della Mea M, Munoz de Rueda P, Serafini-Fracassini D. Factors affecting transglutaminase activity catalyzing polyamine conjugation to endogenous substrates in the entire chloroplast. Plant Physiology and Biochemistry. 2000;b 38:429–439. [Google Scholar]
- Del Duca S, Betti L, Trebbi G, Serafini-Fracassini D, Torrigiani P. Transglutaminase activity changes during the hypersensitive reaction (HR), a typical defence response of tobacco NN plants to TMV. Physiologia Plantarum. 2007;131:241–250. doi: 10.1111/j.1399-3054.2007.00950.x. [DOI] [PubMed] [Google Scholar]
- Della Mea M, Caparros-Ruiz D, Claparols I, Serafini-Fracassini D, Rigau J. AtPng1p: the first plant transglutaminase. Plant Physiology. 2004;a 135:1–9. doi: 10.1104/pp.104.042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Mea M, Di Sandro A, Dondini L, Del Duca S, Vantini F, Bergamini C, Bassi R, Serafini-Fracassini D. A Zea mays 39 kDa thylakoid transglutaminase catalyses the modification by polyamines of light harvesting complex II in a light-dependent way. Planta. 2004;b 219:754–764. doi: 10.1007/s00425-004-1278-6. [DOI] [PubMed] [Google Scholar]
- Della Mea M, De Filippis F, Genovesi V, Serafini-Fracassini D, Del Duca S. The acropetal wave of developmental cell death (DCD) of Nicotiana tabacum corolla is preceded by activation of transglutaminase in different cell compartments. Plant Physiology. 2007;144:1–13. doi: 10.1104/pp.106.092072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sandro A, Serafini-Fracassini D, Del Duca S, Della Mea M, De Franceschi P, Dondini L, et al. Pollen transglutaminase in pear self incompatibility and relationship with S-RNases and S-allele variability. Acta Horticulturae. 2008 (in press) [Google Scholar]
- Dondini L. Poliammine legate e transglutaminasi nelle piante. Italy: Università di Bologna; 1998. PhD Thesis. [Google Scholar]
- Dondini L, Del Duca S, Dall'Agata L, Bassi R, Gastaldelli M, Della Mea M, et al. Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates. Planta. 2003;217:84–95. doi: 10.1007/s00425-003-0998-3. [DOI] [PubMed] [Google Scholar]
- Folk JE. Transglutaminases. Annual Review of Biochemistry. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- García-Jiménez P, Just PM, Delgado AM, Robaina RR. Transglutaminase activity decrease during acclimation to hyposaline conditions in marine seaweed Grateloupia doryphora (Rhodophyta, Halymeniaceae) Journal of Plant Physiology. 2007;164:367–370. doi: 10.1016/j.jplph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochemical Journal. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icekson I, Apelbaum A. Evidence for transglutaminase activity in plant tissue. Plant Physiology. 1987;84:972–974. doi: 10.1104/pp.84.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio RA, Di Sandro A, Scarpellini A, Del Duca S, Serafini-Fracassini D, Verderio EAM. Visualisation of transglutaminase-mediated cross-linking activity in germinating pollen by laser confocal microscopy. Plant Biosystems. 2008;142 (in press) doi: 10.1080/11263500802150886. [Google Scholar]
- Jeon JH, Kim IG. Role of protein modifications mediated by transglutaminase 2 in human viral diseases. Frontiers in Bioscience. 2006;11:221–231. doi: 10.2741/1793. [DOI] [PubMed] [Google Scholar]
- Lentini A, Abbruzzese A, Caraglia M, Marra M, Beninati S. Protein-polyamine conjugation by transglutaminase in cancer cell differentiation. Amino Acids. 2004;26:331–337. doi: 10.1007/s00726-004-0079-3. [DOI] [PubMed] [Google Scholar]
- Lilley GR, Skill J, Griffin M, Bonner PL. Detection of Ca2+-dependent transglutaminase activity in root and leaf tissue of monocotyledonous and dicotyledonous plants. Plant Physiology. 1998;117:1115–1123. doi: 10.1104/pp.117.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature Reviews Molecular Cell Biology. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Kang H, Cho YD. Purification and properties of transglutaminase from soybean (Glycine max) leaves. Biochemical and Biophysical Research Communications. 1996;223:288–292. doi: 10.1006/bbrc.1996.0886. [DOI] [PubMed] [Google Scholar]
- Kuehn GD, Sotelo M, Morales T, Bruce-Carver MR, Guzman E, Margosiak SA. Purification and properties of transglutaminase from Medicago sativa L. (alfalfa) The FASEB Journal. 1991;5:A1510. [Google Scholar]
- Margosiak SA, Dharma A, Bruce-Carver MR, Gonzales AP, Louie D, Kuehn GD. Identification of the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase as a substrate for transglutaminase in Medicago sativa L. (alfalfa) Plant Physiology. 1990;92:88–96. doi: 10.1104/pp.92.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet N, Beninati S, Nigra TP, Folk JE. N1, N8-Bis(gamma-glutamyl)spermidine cross-linking in epidermal cell envelopes: comparison of cross-link levels in normal and psoriatic cell envelope. Biochemical Journal. 1990;271:305–308. doi: 10.1042/bj2710305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossetti U, Serafini-Fracassini D, Del Duca S. Conjugated polyamines during dormancy and activation of tuber of Jerusalem artichoke. In: Schreiber K, Schuette HR, Sembdner G, editors. Conjugated plant hormones structure metabolism and function. Berlin: Deutscher Verlag der Wissenschaften; 1987. pp. 369–375. [Google Scholar]
- Mukherjee BB, Nemir M, Beninati S, Cordella-Miele E, Singh K, Chackalaparampil I, et al. Interaction of osteopontin with fibronectin and other extracellular matrix molecules. Annals of the New York Academy of Sciences. 1995;760:201–212. doi: 10.1111/j.1749-6632.1995.tb44631.x. [DOI] [PubMed] [Google Scholar]
- Navakoudis E, Vrentzou K, Kotzabasis K. A polyamine- and LHCII protease activity-based mechanism regulates the plasticity and adaptation status of the photosynthetic apparatus. Biochimica et Biophysica Acta. 2007;129:706–716. doi: 10.1016/j.bbabio.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Pintó-Marijuan M, de Agazio M, Zacchini M, Santos MA, Torné JM, Fleck I. Response of transglutaminase activity and bound putrescine to changes in light intensity under natural or controlled conditions in Quercus ilex leaves. Physiologia Plantarum. 2007;131:159–169. doi: 10.1111/j.1399-3054.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: how and why do flowers die? Annals of Botany. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D, Del Duca S, D'Orazi D. First evidence for polyamine conjugation mediated by an enzymic activity in plants. Plant Physiology. 1988;87:757–761. doi: 10.1104/pp.87.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D, Del Duca S, Monti F, Poli F, Sacchetti G, Bregoli AM, et al. Transglutaminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotiana tabacum) flowers. Cell Death and Differentiation. 2002;9:309–321. doi: 10.1038/sj.cdd.4400954. [DOI] [PubMed] [Google Scholar]
- Signorini M, Beninati S, Bergamini CM. Identification of transglutaminase activity in the leaves of silver beet (Beta vulgaris L.) Journal of Plant Physiology. 1991;137:547–552. [Google Scholar]
- Sobieszczuk-Nowicka E, Di Sandro A, Del Duca S, Serafini-Fracassini D, Legocka J. Plastid-membrane-associated polyamines and thylakoid transglutaminases during etioplast-to-chloroplast transformation stimulated by kinetin. Physiologia Plantarum. 2007;130:590–600. [Google Scholar]
- Tasco G, Della Mea M, Serafini-Fracassini D, Casadio R. Building a low resolution model of a transglutaminase domain of an hypothetical N-glycanase from Arabidopsis thaliana. Amino Acids. 2003;25:197. [Google Scholar]
- Villalobos E, Torné JM, Rigau J, Ollés I, Claparols I, Santos M. Immunogold localization of a transglutaminase related to grana development in different maize cell types. Protoplasma. 2001;216:155–163. doi: 10.1007/BF02673867. [DOI] [PubMed] [Google Scholar]
- Villalobos E, Santos M, Talavera D, Rodríguez-Falcón M, Torné JM. Molecular cloning and characterization of a maize transglutaminase complementary DNA. Gene. 2004;336:93–104. doi: 10.1016/j.gene.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Waffenschmidt S, Kusch T, Woessner JP. A transglutaminase immunologically related to tissue transglutaminase catalyzes cross-linking of cell wall proteins in Chlamydomonas reinhardtii. Plant Physiology. 1999;121:1003–1015. doi: 10.1104/pp.121.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]