Abstract
Background and Aims
The premature production of alpha-amylase without visible germination has been observed in developing grain of many cereals. The phenomenon is associated with cool temperatures in the late stages of grain growth but the mechanisms behind it are largely unknown. The aim of this study was to replicate the phenomenon under controlled conditions and investigate the possibility of a mechanistic link with grain size or endosperm cavity size.
Methods
Five wheat (Triticum aestivum) genotypes differing in their susceptibility to premature alpha-amylase were subjected to a range of temperature shocks in controlled environments. A comparison was then made with plants grown under ambient conditions but with grain size altered by using degraining to increase the assimilate supply. At maturity, alpha-amylase, grain area and endosperm cavity area were measured in individual grains.
Key Results
Both cold and heat shocks were successful in inducing premature alpha-amylase in susceptible genotypes, with cold shocks the most effective. Cold shocks also increased grain area. Degraining resulted in increased grain area overall, but the larger grain did not have higher alpha-amylase. Analysis of individual grain found that instances of high alpha-amylase were not associated with differences in grain area or endosperm cavity area.
Conclusions
Pre-maturity alpha-amylase is associated with temperature shocks during grain filling. In some cases this coincides with an increase in grain area, but there is no evidence of a mechanistic link between high alpha-amylase and grain or endosperm cavity area.
Key words: Alpha-amylase, pre-maturity alpha-amylase, late maturity alpha amylase, temperature, grain size, endosperm cavity, wheat, Triticum aestivum
INTRODUCTION
The role of alpha-amylase in germination is well studied; less well characterized is the phenomenon of premature alpha-amylase. Pre-maturity alpha-amylase (PMA, also called late maturity amylase) refers to the sporadic production of alpha-amylase during the later stages of grain filling. This genetic defect is frequently detected in commercially grown wheat and other cereals (Lunn et al., 2001). Although it was initially associated with pre-harvest sprouting, PMA is not necessarily accompanied by sprouting and is now thought to be under independent genetic control (Mares and Mrva, 2008).
In some genotypes PMA appears to be constitutively expressed while in others the occurrence is sporadic (Mares and Mrva, 2008). In the field, PMA is often associated with cool, wet conditions during grain development (Gale et al., 1987; Mrva and Mares, 1994). PMA has also been detected in controlled environment experiments when temperature shocks are applied during grain filling and it has been proposed that there is a subset of genotypes that require a temperature shock to express PMA (Mares and Mrva, 2008). Cold shocks have been effective in inducing PMA in Australian genotypes, but similar experiments with UK genotypes have proved less reliable (Gale et al., 1987; Major, 1999; Tjin-Wong-Joe, 2004). Heat shocks have also been found to stimulate PMA in some cases (Randall and Moss, 1990; Major, 1999). The variability in results is partially explained by the observation of Mares and Mrva (2008) that a temperature shock is only effective if applied during a ‘window of sensitivity’, which extends from 25–30 d after anthesis under typical Australian conditions.
One limitation in relating such results to field-grown plants is the lack of a mechanistic model linking variation in temperature to alpha-amylase production. A central question is whether temperature shocks act directly on alpha-amylase-producing cells or indirectly through their effect on grain morphology. Although both temperature and grain morphology have been proposed as PMA-regulating factors the link between the two has not been investigated.
Evers et al. (1995) and Evers (2000) reviewed the evidence for a link between large grain size and high alpha-amylase activity. The evidence presented follows two lines: firstly, of the varieties commonly grown in the UK those classed as large-grained were all in the high alpha-amylase (low Hagberg falling number) category; secondly, some studies have found a gradient in alpha-amylase activity within ears and within spikelets that correlates with the typical weight distribution of individual grains. Although not conclusive, these studies suggest a fundamental mechanistic link between grain size and alpha-amylase that is expressed both within and between genotypes. The studies also suggest that it is external grain dimensions (grain area) rather than grain weight that is most associated with PMA. Kindred et al. (2005) also found a correlation between grain area and alpha-amylase. They suggested that the underlying relationship is between alpha-amylase and the size of the endosperm cavity, with grain area acting as an imperfect proxy. As also seen by Evers (2000), the correlation occurred both between genotypes and within genotypes grown at different nitrogen levels. The proposal that PMA might be associated with the size and disruption of the endosperm cavity relates to observations that alpha-amylase produced during PMA tends to be localized around the grain crease, possibly being produced in the aleurone layer surrounding the endosperm cavity (Cornford et al., 1987; Evers et al., 1995; Greenwell et al., 2001; Tjin-Wong-Joe, 2004; Kindred et al., 2005). Again, there is some disagreement between UK studies and those carried out in Australia where PMA was found to have a random distribution throughout the aleurone (Mrva et al., 2006; Mares and Mrva, 2008). The relationship between cavity size and temperature shocks has not been investigated.
Here, we apply a range of temperature shocks and look at the effect on alpha-amylase and grain morphology in several UK winter wheat genotypes. A comparison is made with plants grown under ambient temperatures but with grain morphology altered by using degraining to increase the assimilate supply.
MATERIALS AND METHODS
Plant material
Seeds of the UK winter wheat (Triticum aestivum L.) varieties ‘Maris Huntsman’, ‘Rialto’, ‘Spark’, ‘Option’ and ‘Potent’ were obtained from J. Flintham (John Innes Centre, Norwich, UK). Based on results from the field, ‘Maris Huntsman’, ‘Rialto’ and ‘Spark’ represent high, medium and low susceptibility to PMA respectively (J. Flintham, pers. comm.), while the remaining genotypes are less well characterized. ‘Maris Huntsman’ and ‘Spark’ were chosen to provide within-treatment controls for the complex temperature regimes where it was not feasible to include other control treatments.
Temperature shock
Plants were vernalized at 6 °C for 8 weeks then transferred to 1·3-L pots in a glasshouse (pots contained John Innes No. 2 Compost: Keith Singleton's Seaview Nurseries, Cumbria, UK). Secondary shoots (tillers) were removed periodically to leave the main shoot plus four secondary shoots. Plants were reared in the glasshouse until anthesis then transferred to controlled-environment cabinets (Fitotron, Sanyo Gallenkamp, Loughborough, UK; or Conviron, Controlled Environments, Winnipeg, Canada). The glasshouse was heated to provide a minimum of 15/5 °C day/night temperature, water was provided by capillary matting wetted automatically three times a day, and supplementary light (400 W high pressure sodium) was used to maintain a minimum 16-h day length. For practical reasons experiments were carried out in two stages at different times of year (see Table 1 for details).
Table 1.
Outline of day/night temperatures (°C) and timing of treatments in the temperature-shock experiments
| Treatment stage |
||||
|---|---|---|---|---|
| Treatment | Pre-anthesis | Pre-treatment | Induction* | Ripening |
| Mid → Low | Glasshouse | 22/22 | 12/12 | 22/22 |
| Mid → High | Glasshouse† | 20/10 | 30/20 | 30/15 |
| High → Mid | Glasshouse | 30/15 | 18/12 | 30/15 |
*Induction started at 550 degree-days after anthesis and lasted 8 calendar days.
†Mid → High planted in a glasshouse on 25 May 2006 and transferred during June 2006. Other treatments were planted in a glasshouse on 2 Dec. 2005 and transferred during Feb. 2006.
Plants were checked regularly and tagged at anthesis. When a sufficient number of plants reached anthesis on the same day, this subset of plants was transferred to one of three cabinets. This ensured that all plants used were at a comparable developmental stage. Each cabinet temperature was initially set to be similar to that of the glasshouse. Over the next 12 d, temperatures were gradually adjusted until a defined pre-treatment temperature was reached. Three cabinets were used to apply three temperature shocks: Mid → Low, Mid → High, and High → Mid, as shown in Table 1. The third temperature shock, ‘High → Mid’, was designed to replicate previous experiments carried out under Australian conditions (Mrva and Mares, 2001). In each case the temperature shock started approximately 550 degree-days after anthesis (degree-DAA, base temperature 0 °C) and lasted for 8 d. The start time ranged from 25 to 30 DAA depending on the pre-treatment regime. Plants remained in the cabinets until 60 DAA when the grain was harvested. The cabinets were maintained on a 14/10 h light/dark cycle with a relative humidity of 70 %, photosynthetic photon flux density was 250–300 µmol m−2 s−1 throughout and pots were kept near field capacity using an automatic drip-irrigation system attached to each pot.
Degraining
Plants were grown from seed in a polytunnel where they underwent vernalization at ambient temperatures over winter. Secondary shoots were removed periodically to leave the main shoot plus four secondary shoots.
At anthesis half of the plants were left intact while half were subjected to 50 % spikelet removal (degrained) in order to stimulate compensatory grain growth in the remaining grains. Degraining was achieved by excising an entire row of spikelets from one side of the main ear and removing the whole ear from all secondary shoots.
For three genotypes, ‘Maris Huntsman’, ‘Spark’ and ‘Rialto’, the degraining treatment was combined with a cold shock applied in controlled-environment cabinets (as above). Plants were transferred to the cabinets at anthesis and underwent either an ‘ambient’ treatment (similar to the temperatures in the polytunnel) or a Mid → Low (B) treatment, as described in Table 2. At 28 DAA both control and Mid → Low (B) plants were returned to the polytunnel. The cabinets were maintained on a 16/8 h light/dark cycle with a humidity of 70 % and a photosynthetic photon flux density of 500 µmol m−2 s−1 throughout.
Table 2.
Outline of day/night temperatures (°C) and timing of treatments in the degraining experiments
| Treatment stage |
||||
|---|---|---|---|---|
| Treatment | Pre-anthesis | Pre-treatment | Induction* | Ripening |
| Ambient† | Polytunnel | 25/15 | 25/15 | Polytunnel |
| Mid → Low (B) | Polytunnel | 25/15 | 13/11 | Polytunnel |
*Induction started at 330 degree-days after anthesis and lasted 15 calendar days.
†‘Option’ and ‘Potent’ remained in the polytunnel from planting (26 Dec. 2005) until maturity and are also classed as ambient.
Grain alpha-amylase assay
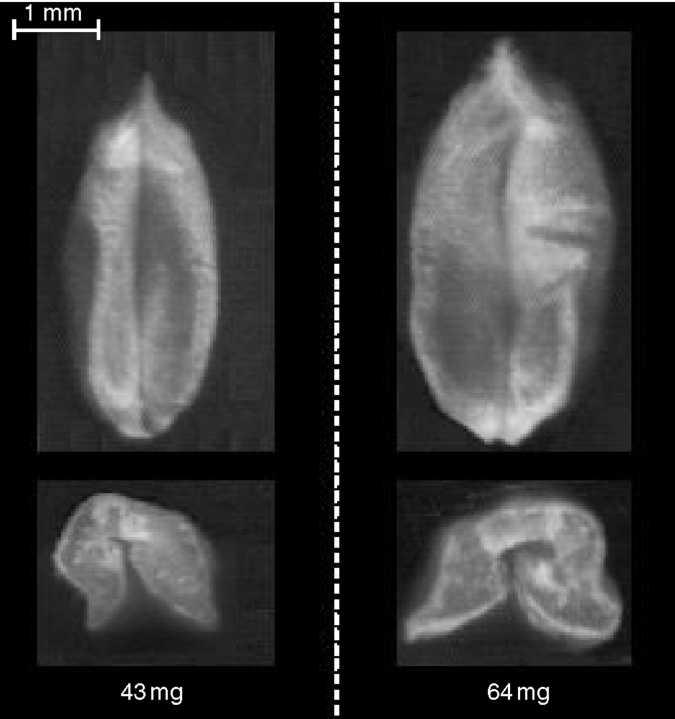
All grain was sampled at random from within a target region consisting of florets 1 and 2 from spikelets 7 to 11 (counting acropetally) of the main shoot. For each plant one grain was removed, weighed and an image taken of the grain area (plan view of ventral side) and transverse section (following hand-dissection with a scalpel). Images were acquired with a flat-bed scanner (ScanExpress, Mustek, Taiwan) and analysed using ImagePro Plus (Media Cybernetics, Marlow, UK). Transverse sections were used to measure the area of the endosperm cavity (Fig. 1).
Fig. 1.
Typical grain images for ‘Rialto’, showing the range in grain weight, grain area and endosperm cavity area.
High pI alpha-amylase protein content was assayed using a high pI-specific ELISA (Value Added Wheat CRC, North Ryde, NSW, Australia; Mrva and Mares, 2001). The alpha-amylase assay was carried out using a single half-grain from each plant. The distal half of each grain was reduced to 20 ± 2 mg before being homogenized for use in the assay. Grain halves were homogenized in microcentrifuge tubes using a rotating ball grinder (RotoMix, 3M ESPE AG, Seefeld, Germany) and 0·5 mL of extraction buffer (85 mmol NaCl) was added to each tube. Tubes were incubated for 1 h at room temperature on a shaker, after which 100 µL of grain extract was taken for use in the assay. Absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Benchmark, Watford, UK) and results zeroed against extraction buffer blanks.
Statistical analysis
The data presented are means of eight grains per treatment with each grain taken from one of eight replicate plants. Statistical analysis was carried out using two-way ANOVA with multiple comparisons (l.s.d., P < 0·05) using Genstat 8 (VSN International Ltd., Hemel Hempstead, UK). For alpha-amylase, log-transformed data was also tested but was found to show similar output to untransformed data.
RESULTS
Temperature shock
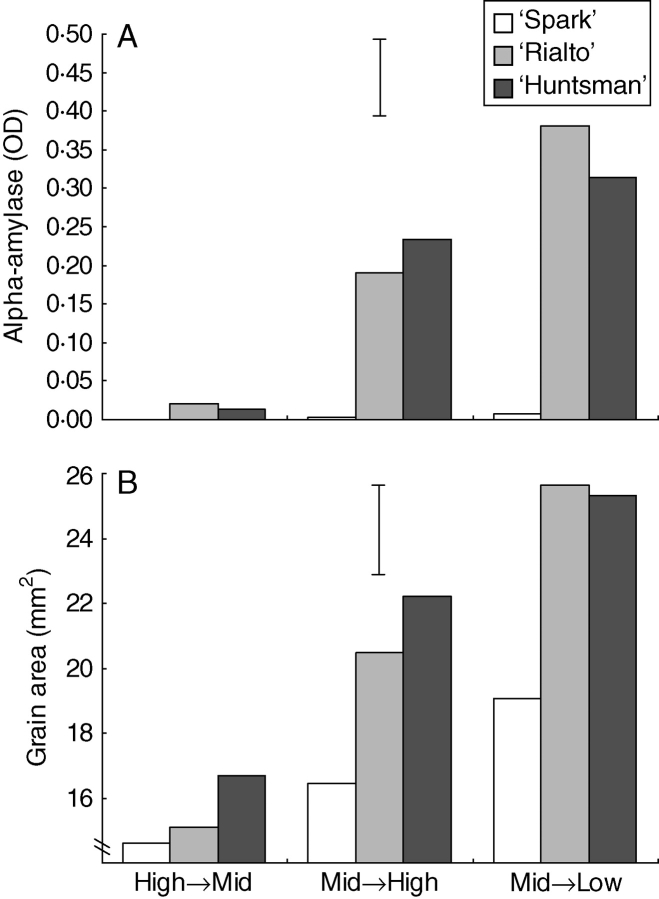
Environment, genotype and environment × genotype all produced significant differences in alpha-amylase. Grain area showed a similar response, although the environment × genotype effect was not significant. ‘Spark’, a PMA-resistant genotype, produced negligible levels of alpha-amylase in all treatments (Fig. 2). The Mid → Low and the Mid → High temperature shock stimulated alpha-amylase production in ‘Maris Huntsman’ and ‘Rialto’. The High → Mid temperature shock, which was designed to replicate previous experiments carried out under Australian conditions (Mrva and Mares, 2001), resulted in negligible alpha-amylase levels in all three genotypes.
Fig. 2.
(A) Grain alpha-amylase content and (B) grain area of mature winter wheat subjected to different temperature regimes during grain filling (temperature-shock experiments; see details in Table 1). Data are means of eight replicate plants. Vertical bars indicate l.s.d.0·05 for genotype × environment.
Across the three treatments there was a clear correlation between alpha-amylase content and grain area, with cooler temperatures over the grain-filling period resulting in larger grains with more alpha-amylase (Fig. 2). A similar correlation was also present within treatments.
Degraining
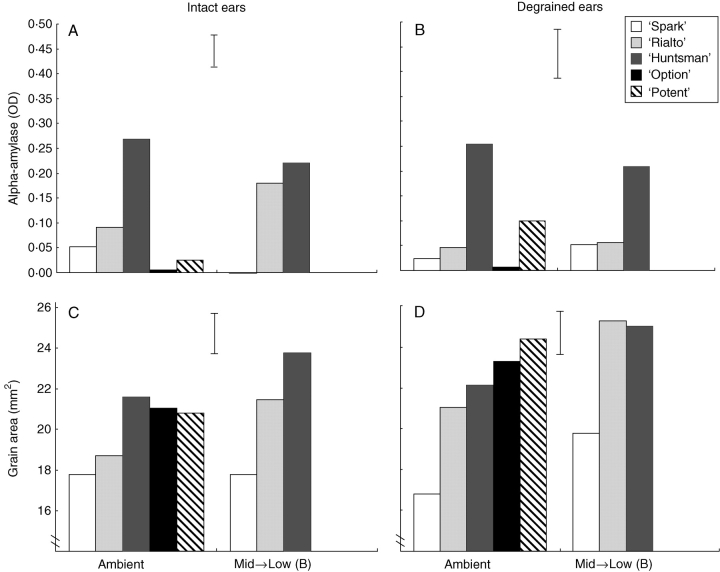
In the degraining experiment, Mid → Low (B) successfully stimulated alpha-amylase in intact ‘Rialto’ plants (Fig. 3). ‘Maris Huntsman’ produced high alpha-amylase levels in both ambient and Mid → Low (B) treatments. As in Fig. 2, a positive relationship was found between grain area and alpha-amylase for ‘Maris Huntsman’, ‘Rialto’ and ‘Spark’; however, this correlation was not seen in the two additional genotypes ‘Option’ and ‘Potent’ (Fig. 2).
Fig. 3.
(A, B) Grain alpha-amylase content and (C, D) grain area of intact and degrained winter wheat subjected to different temperature regimes during grain filling (degraining experiments; see details in Table 2). Data are means of eight replicate plants. Vertical bars indicate l.s.d.0·05 for genotype × environment.
In the intact plants, environment, genotype and environment × genotype all produced significant differences in alpha-amylase. Grain area showed a similar response, although the environment × genotype effect was not significant. In the degrained plants, only genotype had a significant effect on alpha-amylase. Grain area again showed significant differences due to environment and genotype but not environment × genotype. Degraining resulted in significantly larger grains (Fig. 3), when averaged over all five genotypes grown under ambient conditions. The increased grain area did not, however, produce significant changes in alpha-amylase content.
Combining degraining and Mid → Low (B) treatments had an additive effect on grain area, producing larger grains than either treatment on its own (Fig. 3). Again, the increased grain area was not associated with an increase in alpha-amylase. In fact, the expected increase in alpha-amylase seen in ‘Rialto’ following the Mid → Low (B) treatment was absent in the degrained plants (Fig. 3). The Mid → Low (B) treatment did stimulate PMA in intact ‘Rialto’ plants, but the lower levels of alpha-amylase overall suggest this treatment was less effective than the previous Mid → Low treatment that was applied at a later stage of grain filling.
Grain and cavity size
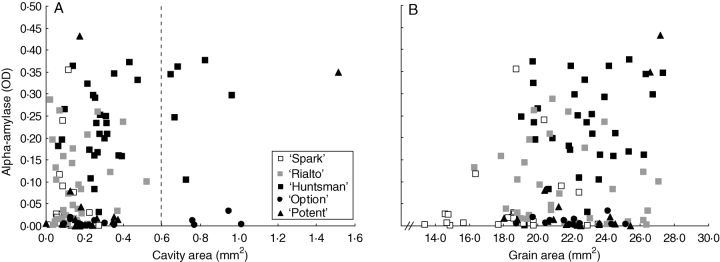
Figure 4 shows grain area, cavity area and alpha-amylase content for individual grains, with data combined from all of the treatments used in the degraining experiments. Alpha-amylase appears to show a weak positive correlation with grain area when genotypes are ignored. However, a regression analysis with genotypes as groups shows that the association is due to differences between genotypes. Within genotypes there is no association between alpha-amylase and grain or cavity area.
Fig. 4.
The relationship between alpha-amylase content and (A) endosperm cavity area and (B) grain area. Grain to the right of the line in (A) are designated as having a ‘large cavity’. Data are for single grains grown under a range of conditions as indicated in Fig. 3.
Any cavity with an area more than 1·5 s.d. from the mean (i.e. >0·6 mm2) is classed as a ‘large cavity’. ‘Maris Huntsman’ and ‘Option’ account for the majority of instances of large cavities; these instances were not associated with the degraining or Mid → Low (B) treatments (data not shown). Neither is there a higher incidence of high alpha-amylase content among those grains with ‘large cavities’ (Fig. 4A).
DISCUSSION
The understanding of pre-maturity alpha-amylase (PMA) has often been hindered by the sporadic way in which it occurs, with levels of alpha-amylases varying widely between plants, between ears and between grain. The experiments here rely on a relatively small sample size (eight grain, each from a replicate plant), with grain taken from a target region thought to have the highest occurrence of PMA (Gale et al., 1987). Adopting this method and analysing the results on a single-grain basis appears to provide a good system for probing the mechanisms behind PMA.
Temperature shock
These results confirm that ‘Maris Huntsman’ is highly susceptible to PMA while ‘Spark’ appears resistant. ‘Rialto’ falls into an intermediate category, with PMA only expressed under particular growth conditions. As such, ‘Rialto’ provides a suitable model to test the possible link between alpha-amylase and grain or endosperm cavity area. It is this intermediate category that is of most interest here, as it gives insight into the physiological processes underlying PMA.
Of the three temperature regimes applied here, Mid → Low was the most effective in inducing PMA. The Mid → Low (B) treatment applied in the degraining experiments (Fig. 3) confirmed the effectiveness of a cold shock in stimulating the production of alpha-amylase. This is consistent with results of similar cold-shock experiments using Australian wheat genotypes (Mares and Mrva, 2008). The Mid → High regime was also effective in inducing PMA, suggesting that a heat shock has a similar effect. The negligible levels of alpha-amylase in all three genotypes after the third temperature shock ‘High → Mid’ was surprising given that this represented a similar temperature change to the other treatments and was applied at a similar developmental stage (550 degree-DAA). Nevertheless this attempt to replicate Australian conditions involved applying a wide range of temperatures pre-anthesis and pre-treatment, which can reduce the effectiveness of the cool shock (Mares and Mrva, 2008).
Temperature influences many aspects of grain development, not least the rate and duration of grain filling. In general, wheat has an optimum temperature for grain filling of 15 °C, with each 1 °C rise above this resulting in a 3–5 % reduction in single grain weight (Wardlaw et al., 1989). In the temperature-shock experiment the temperatures varied over the grain-filling period, but overall the average temperature applied in each regime (Table 1) showed a negative association with grain area (Fig. 2). The tendency for cool temperatures during grain filling to produce both larger grain and increase PMA may explain the association found between the two traits in field-grown plants.
As lower temperatures increase both grain area and PMA the possibility of a mechanistic link between the two emerges. It is possible that cold shocks and cool periods in the field induce PMA by altering grain filling, e.g. by decoupling the growth of the aleurone layer from the expansion of the endosperm or endosperm cavity. This possibility is supported by the positive association found between grain size and PMA in other circumstances (Evers, 2000; Kindred et al., 2005). Taken in isolation the results of the temperature-shock experiment are consistent with this proposition, although the results from the degraining experiment appear to counter this. In the degraining experiment the association between grain area and PMA was lost. Given this, and the fact that both cold and heat shocks can be effective, it is more likely that temperature affects PMA through other means.
Grain area and PMA
The degraining experiment was designed to test if there was a causal link between grain area and PMA as suggested by other studies (Evers et al., 1995; Evers, 2000; Greenwell et al., 2001). Degraining generally increases grain size by reducing the number of grains competing for photoassimilates and stimulating compensatory grain growth in the remaining grain (Borras et al., 2004). For the four genotypes that showed a positive response to degraining there was an average increase in grain area of 11 %. This is lower than has been seen in some studies but comparable to the 11 % expected for wheat in general (Borras et al., 2004). Despite the increase in grain area there was no general increase in alpha-amylase. Only ‘Potent’ showed an increase in both traits with the alpha-amylase level remaining relatively low throughout. Similarly, the between-genotype association of grain area and PMA seen in the temperature-shock experiment and by Evers (2000) was not seen when all five genotypes were tested. Similar results were seen when grain weight rather than area was used (data not shown). Even though this comparison of five genotypes is far from comprehensive, the fact that ‘Potent’ and ‘Option’ have grain areas equal to or greater than ‘Maris Huntsman’, yet have consistently lower levels of alpha-amylase, indicates that grain area is not a reliable predictor of PMA. This is confirmed by the analysis of individual grains, with many instances of PMA detected in grain of average area (Fig. 4B). Although it remains possible that particular cases of enlarged grain or particular genetic determinants of grain size also influence PMA, our results do not support the suggestion of a mechanistic relationship between the two traits.
Endosperm cavity area and PMA
Kindred et al. (2005) found that reducing the amount of applied N resulted in increased alpha-amylase and endosperm cavity size across several genotypes. In addition to this indirect association, when comparing ‘large-cavity grains’ to ‘small-cavity grains’ they found a similar positive relationship between cavity size and alpha-amylase within genotypes grown under the same N level. In the present work, there was a weak association between grain area and endosperm cavity area (data not shown) and grain area showed a weak association with alpha-amylase content (Fig. 4). However, despite the range of alpha-amylase levels detected, no clear relationship with cavity area was seen under any of the treatments (Fig. 4). A similar lack of association was seen whether cavity size was expressed as area or maximum diameter (data not shown). The different results may reflect differences in the methods used to measure alpha-amylase; Kindred et al. (2005) measured alpha-amylase levels by placing half-grains transverse-side down on agar and measuring the extent of starch degradation. It may be that differences in cavity size alter the amount of alpha-amylase reaching the agar without altering the overall alpha-amylase level in the homogenized grain (as measured here).
Thus, neither total grain area nor endosperm cavity area explained the variation in PMA seen here. Observations of PMA occurrence in tall and semi-dwarf genotypes have also revealed no obvious link with grain morphology, indicating that significant changes in the GA signal transduction pathway are not involved. Nonetheless, production of alpha-amylase in the aleurone is GA dependent and GA-insensitive genotypes are generally less prone to PMA than their tall parents (Mares and Mrva, 2008). It is likely that GA and GA-sensitivity are key factors in stimulating PMA here. The most effective treatment (Mid → Low) was applied in the final stages of grain filling as the grain was approaching physiological maturity. In vitro experiments have shown that in the presence of GA, aleurone tissue from such immature grain can be induced to synthesise alpha-amylase when subjected to a suitable stimulus. Successful stimuli have included low temperature, high temperature, drying and incubation in a buffer (Bewley and Black, 1994). Given the range of treatments that can induce alpha-amylase in detached grain, it has been suggested that a regulatory mechanism exists in vivo to inactivate the GA signal, or make the aleurone layer insensitive to GA (Cornford and Black, 1985). One candidate for this regulatory mechanism is the GA/ABA ratio. Peak ABA levels also occur in the later stages of grain filling as maturation begins (King, 1976; Finkelstein, 2004). Applying a temperature shock during the window of sensitivity could stimulate PMA by disrupting this regulatory mechanism, e.g. by altering the GA/ABA ratio itself or the sensitivity of the aleurone to the two hormones.
ACKNOWLEDGEMENTS
We thank Tony Evers for instructive comments on the manuscript, Daryl Mares and Kolumbina Mrva for assistance in developing methods, Janice Haycox for technical assistance, and James Simmonds and John Flintham for providing plant material. This work was sponsored by the Department of Environment, Food and Rural Affairs, and the Biotechnology and Biological Sciences Research Council through the Sustainable Arable LINK Programme, and supported by Home Grown Cereals Authority.
LITERATURE CITED
- Bewley J, Black M. Seeds; physiology of development and germination. New York: Plenum Press; 1994. [Google Scholar]
- Borras L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research. 2004;86:131–146. [Google Scholar]
- Cornford C, Black M. Alpha-amylase content of pre-mature unsprouted wheat grains. Journal of Cereal Science. 1985;3:295–304. [Google Scholar]
- Cornford C, Black M, Chapman J. Sensitivity of developing wheat grains to gibberellin and production of alpha-amylase during grain development and maturation. In: Mares D, editor. Fourth international symposium on preharvest sprouting in cereals. Boulder, CO: Westview Press; 1987. pp. 283–292. [Google Scholar]
- Evers A. Grain size and morphology: implications for quality. In: Schofield J, editor. Wheat structure, biochemistry and functionality. Cambridge, UK: The Royal Society of Chemistry; 2000. pp. 19–24. [Google Scholar]
- Evers A, Flintham J, Kotecha K. Alpha-amylase and grain size in wheat. Journal of Cereal Science. 1995;21:1–3. [Google Scholar]
- Finkelstein R. The role of hormones during seed development and germination. In: Davies PJ, editor. Plant hormones. Biosynthesis, signal transduction, action! Vol. 3. Berlin: Springer; 2004. pp. 513–537. [Google Scholar]
- Gale M, Salter A, Lenton J. The induction of germination alpha-amylase during grain development in unfavourable weather conditions. In: Mares D, editor. Fourth international symposium on preharvest sprouting in cereals. Boulder, CO: Westview Press; 1987. pp. 273–281. [Google Scholar]
- Greenwell P, Cauvain S, Bhandari D, Flintham J, Gale M, Briarty M, Evers A. Prediction and prevention of excessive enzyme activity in cereals through investigation and manipulation of causal factors. London: Home-Grown Cereals Authority; 2001. HGCA Project report no. 247. [Google Scholar]
- Kindred DR, Gooding MJ, Ellis RH. Nitrogen fertilizer and seed rate effects on Hagberg falling number of hybrid wheats and their parents are associated with alpha-amylase activity, grain cavity size and dormancy. Journal of the Science of Food and Agriculture. 2005;85:727–742. [Google Scholar]
- King RW. Abscisic acid in developing wheat grains and its relationship to grain growth and maturation. Planta. 1976;132:43–51. doi: 10.1007/BF00390329. [DOI] [PubMed] [Google Scholar]
- Lunn G, Major B, Kettlewell P, Scott R. Mechanisms leading to excess alpha -amylase activity in wheat (Triticum aestivum L.) grain in the U.K. Journal of Cereal Science. 2001;33:313–329. [Google Scholar]
- Major B. Environmental factors affecting pre-maturity alpha-amylase activity in winter wheat (Triticum aestivum. Milton Keynes, UK: Open University; 1999. [Google Scholar]
- Mares D, Mrva K. Late-maturity alpha-amylase: low falling number in wheat in the absence of preharvest sprouting. Journal of Cereal Science. 2008;47:6–17. [Google Scholar]
- Mrva K, Mares D. Environmental and genetic factors which control late maturity alpha amylase in wheat. In: Panozzo J, Downie P, editors. Proceedings of the 44th Australian Cereal Chemistry Conference; Melbourne: Royal Australian Chemical Institute; 1994. pp. 78–79. [Google Scholar]
- Mrva K, Mares DJ. Induction of late maturity alpha-amylase in wheat by cool temperature. Australian Journal of Agricultural Research. 2001;52:477–484. [Google Scholar]
- Mrva K, Wallwork M, Mares D. Alpha-amylase and programmed cell death in aleurone of ripening wheat grains. Journal of Experimental Botany. 2006;57:877–885. doi: 10.1093/jxb/erj072. [DOI] [PubMed] [Google Scholar]
- Randall P, Moss H. Some effects of temperature regime during grain filling on wheat quality. Australian Journal of Agricultural Research. 1990;41:603–617. [Google Scholar]
- Tjin-Wong-Joe A. Alpha-amylase in wheat genotypes. Milton Keynes, UK: Open University; 2004. [Google Scholar]
- Wardlaw I, Dawson I, Munibi P. The tolerance of wheat to high temperatures during reproductive growth. 2. Grain development. Australian Journal of Agricultural Research. 1989;40:15–24. [Google Scholar]