Abstract
Background and Aims
Studies on xylogenesis focus essentially on the stem, whereas there is basically no information about the intra-annual growth of other parts of the tree. As roots strongly influence carbon allocation and tree development, knowledge of the dynamics of xylem production and maturation in roots at a short time scale is required for a better understanding of the phenomenon of tree growth. This study compared cambial activity and xylem formation in stem and roots in two conifers of the boreal forest in Canada.
Methods
Wood microcores were collected weekly in stem and roots of ten Abies balsamea and ten Picea mariana during the 2004–2006 growing seasons. Cross-sections were cut using a rotary microtome, stained with cresyl violet acetate and observed under visible and polarized light. The number of cells in the cambial zone and in differentiation, plus the number of mature cells, was counted along the developing xylem.
Key Results
Xylem formation lasted from the end of May to the end of September, with no difference between stem and roots in 2004–2005. On the contrary, in 2006 a 1-week earlier beginning of cell differentiation was observed in the stem, with cell wall thickening and lignification in roots ending up to 22 d later than in the stem. Cell production in the stem was concentrated early in the season, in June, while most cell divisions in roots occurred 1 month later.
Conclusions
The intra-annual dynamics of growth observed in stem and roots could be related to the different amount of cells produced by the cambium and the patterns of air and soil temperature occurring in spring.
Key words: Abies balsamea, boreal forest, cambium, cell differentiation, cell wall thickening, lignification, Picea mariana, root, stem, xylem
INTRODUCTION
Several studies have recently investigated tree-ring growth in conifers (Deslauriers et al., 2003; Schmitt et al., 2004; Deslauriers and Morin, 2005; Dufour and Morin, 2007; Ko Heinrichs et al., 2007; Rossi et al., 2007). Cambial activity and xylem formation have been widely analysed, describing the dynamics of xylem development and cell differentiation over time (Rossi et al., 2006a; Deslauriers et al., 2008) and relating tree growth with endogenous (Ladefoged, 1952; Rossi et al., 2008a) or environmental factors (Rossi et al., 2006b; Rossi et al., 2007; Seo et al., 2008). However, all these studies essentially focused on stem growth. There is basically no information about the intra-annual growth of the other parts of the tree.
In trees, the root system is one of the main carbon sinks and constitutes a significant part of the plant biomass, monopolizing >50 % of the whole metabolism (Troeng and Linder, 1982). Although root growth can strongly influence carbon allocation and tree development (Fayle, 1968), little knowledge is available about root xylem formation at a short time scale. Some authors illustrated primary and secondary root tissues in vascular plants by anatomical observations without describing their development (Denne, 1972; Krause and Eckstein, 1993; Krause and Morin, 1999). Despite the well-known differences in anatomy and physiology between stem and roots (Panshin and de Zeeuw, 1970; Savidge, 2000), the mechanisms of xylogenesis and the factors involved in regulating cell formation are supposed to be similar in these two parts of a tree (Catesson, 1994; Larson, 1994). A longer period of cambial activity is assumed to occur in roots than in the stem, although this information is based mainly on 1-year-long monitoring of Pinus strobus that lacks details about sampling and methodology employed (Stevens, 1931).
At the moment, the available information about the onset of cambial activity and xylem production in the different parts of trees is contradictory and often based on hypotheses never confirmed or rejected. According to Zimmermann and Brown (1971) and Funada et al. (2002), cambial activity should reactivate first close to buds and propagate basipetally, so that the cambium in roots should be the last meristem to start. Other authors instead affirmed that the progression of cambial onset in conifers presents no clear pattern or direction (Savidge and Wareing, 1984; Riding and Little, 1986; Savidge, 2000). The progression of the ending of cambial activity is also still controversial. Some investigations found that cambial activity ceased later at the base than at the top of the tree (Riding and Little, 1986). However, other authors proposed a different behaviour; in autumn, the reverse process would begin when the base of the tree becomes dormant and the pattern would propagate acropetally up to the top of the crown (Zimmermann and Brown, 1971; Kozlowski and Pallardy, 1997; Vaganov et al., 2006). As far as is known, no definitive pattern of onset and ending of xylem formation has been demonstrated in the various parts of trees.
The aim of this study was to analyse and compare cambial activity and xylem formation in the stem and roots of two North American conifers in order to assess the specific pattern of xylem differentiation in the different parts of the tree. Repetitive micro-samplings and histological analyses of the developing xylem were used to describe the phenology of cambial activity and timing of intra-annual tree-ring growth over three growing seasons in the boreal forest.
MATERIALS AND METHODS
Study sites and tree selection
The study was conducted on Abies balsamea (L.) Mill. and Picea mariana (Mill.) B.S.P. growing in two stands located 400 m apart and established after a forest fire in the early 1920s (Gagnon, 1989). The study sites were at Lac Simoncouche (48°12′N, 71°14′W, 350 m a.s.l., Québec, Canada), in the southern part of the boreal zone, in the balsam fir–white birch ecological region. The mean annual temperature recorded at the sites in 2004–2006 was 2·2 °C, with a mean annual precipitation of 984 mm.
Every year, ten A. balsamea and ten P. mariana with homogeneous diameters were chosen (Table 1). Trees with polycormic stems, partially dead crowns, reaction wood or evident damage were avoided. Trees were chosen from 20 pre-selected trees after having assessed growth rate homogeneity by counting the number of tracheids in the tree rings of three preceding growing seasons on microcores (Antonova and Stasova, 1997; Deslauriers et al., 2003).
Table 1.
Average and standard deviation of tree heights (H) and diameters at breast height (DBH) for Abies balsamea and Picea mariana during 2004–2006
| Species | Year | H (m) | DBH (cm) |
|---|---|---|---|
| A. balsamea | 2005 | 14·4 ± 1·7 | 18·4 ± 1·3 |
| 2006 | 14·0 ± 1·3 | 18·4 ± 2·3 | |
| P. mariana | 2004 | 17·9 ± 1·6 | 21·0 ± 2·7 |
| 2005 | 17·7 ± 1·8 | 21·2 ± 2·5 | |
| 2006 | 15·3 ± 1·3 | 18·6 ± 2·5 |
Microcoring
Tree-ring formation was studied weekly from May to November for 3 years, from 2004 to 2006. Limited access to the study sites and important snow cover did not allow sampling before May. In each tree, wood microcores (2 mm in diameter and 15–20 mm in length) were collected on the stem and on two main roots using surgical bone marrow sampling needles (DBMNI-1501 inter-V médical). Microcores contained phloem, cambium, the developing annual xylem and at least three preceding tree rings. In the stem, samples were taken at breast height (1·3 m) following a spiral pattern (Deslauriers et al., 2003). Spacing between samples was at least 2–8 cm horizontally and 2–3 cm vertically in order to minimize formation of wounds or resin ducts (Forster et al., 2000). In the root system, samples were taken every 3 cm following a sinuous pattern, beginning about 25 cm from the stump and only in the upper parts because of the eccentric growth of the roots. Microcores were placed in a solution of ethanol and water (for P. mariana) or left to air dry (for A. balsamea) and stored at 5 °C in order to avoid tissue deterioration of the cambial zone.
Histological analyses
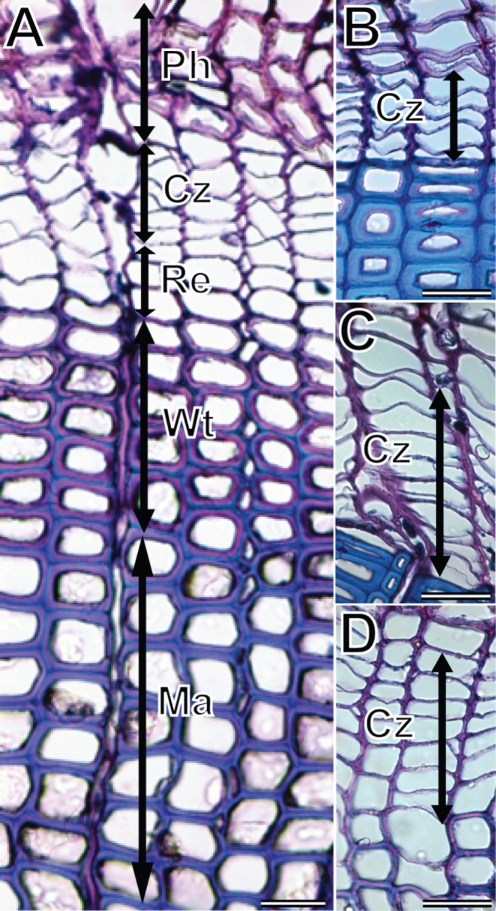
Microcores were dehydrated in successive immersions in ethanol and Histo-Clear™ and embedded in paraffin according to Rossi et al. (2006a). Transverse sections, 7 µm thick, were cut with a rotary microtome, stained with cresyl violet acetate (0·16 % in water), placed on slides and gently stretched with a fine needle. About 3500 sections were observed under visible and polarized light at ×400–500 (according to the quality of the sections) to distinguish the cells in the cambial zone and the differentiating xylem. The number of cells in the cambial zone, in radial enlargement, in cell wall thickening and the number of mature cells were counted along three radial files (Fig. 1A). In the cambial zone, cells had small radial diameters and thin walls (Skene, 1969; Antonova and Stasova, 1997; Fig. 1B–D). Cells in radial enlargement were larger than cambial cells with thin violet walls that were not birefringent under polarized light (Kutscha et al., 1975) (Fig. 1A). Cells in wall thickening were birefringent under polarized light and showed violet and blue walls (Abe et al., 1997). Walls of cells in secondary wall thickening changed from light violet, at the start of the process, to blue, when maturation was complete. Lignification was characterized by the appearance of a blue colour in the middle lamella that spread into secondary walls of the differentiating tracheids (Fig. 1A). Xylem tracheids were considered lignified and mature when walls were completely blue (Rossi et al., 2006b).
Fig. 1.
Cross-sections of Picea mariana at ×200: (A) developing xylem in mid-July, mature tracheids (Ma), wall thickening tracheids (Wt), radial enlargement tracheids (Re), cambial zone (Cz) and phloem (Ph); (B) cambial zone during dormancy at the beginning of May; (C) cambial zone during cell division at the end of May; (D) cambial zone at the end of cell division in mid-July. Scale bars = 30 µm.
The cell number in the three radial files was averaged for each tree and used to assess onset, duration and ending of the two phases of xylogenesis (radial enlargement and wall thickening). Each phase of xylogenesis was considered to occur when at least two radial files out of three showed cells in the given phase.
Standardization
The width of annual rings varied within the tree circumference and along stem and roots and, consequently, among the different samples (Schmitt et al., 2004). According to Rossi et al. (2003), the number of cells of the three previous years was counted on three radial files per sample and used to standardize the cell number around the circumference and along the roots. A ratio was obtained for each sample by dividing the mean cell number of the sample by the mean cell number of all samples per tree. The number of cells in each xylogenesis phase was then multiplied by the ratio to standardize the data according to the sample's relative position on the stem or roots (Deslauriers et al., 2003). According to the relative position of the sample, the standardized number of cells in each j-sample and i-phase (ncij) was calculated as:
with
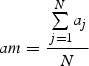 |
where nij was the number of cells counted, aj the mean cell number of the previous rings for each j-sample, N the number of j-samples and am the mean cell number of previous rings of all j-samples.
Statistical analyses
To compare the date of onset and ending of xylem tracheids differentiation between species and sampling location, a multifactor analysis of variance (MANOVA) was performed using the General Linear Models Procedure in SAS® (SAS Institute, Inc.). Before the analysis, data were examined to verify the normality of distribution and homogeneity of the variances (Quinn and Keough, 2002).
In order to assess dynamics of radial growth, the number of tracheids over time was fitted with the Gompertz function using JMP IN® 5·1 (SAS Institute, Inc.). For each factor (species, sampling location and year), the three parameters of the function were estimated by minimizing the sum of squared deviations between each observation and the predicted values by the fitted regression model. The Gompertz function was defined as:
where y is the weekly cumulative sum of tracheids, t the time of the year computed in Julian days, A the function asymptote and β and κ the x-axis placement and rate of change parameters, respectively. From each estimated function, the period of culmination of growth rate corresponding to the inflection point (tp) was calculated as the ratio between β and κ.
RESULTS
Cambial zone
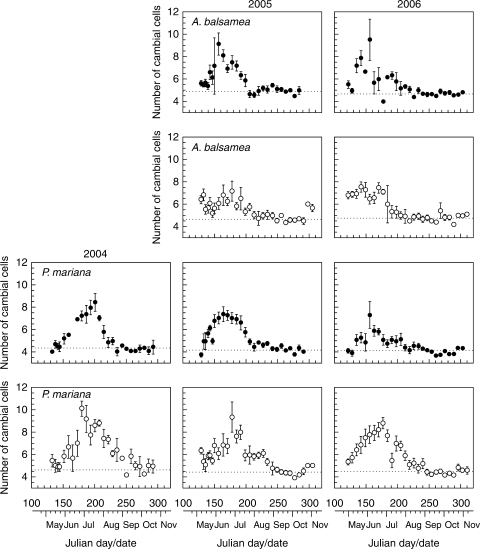
Similar annual dynamics were observed in the cambial zone of stem and roots of A. balsamea and P. mariana during 2004–2006 (Fig. 2). Without cell production, corresponding to the period from the end of August until the beginning of May, the vascular cambium consisted of for or five cells in both stem and roots (horizontal dotted line in Fig. 2). In May, the number of cambial cells increased to seven to nine, reaching a maximum between June and July. The number of cells in the cambial zone increased before the presence of tracheids in radial enlargement. In some cases, the number of cambial cells at the start of the sampling exceeded the minimum number of dormant cells. For example, the cambial zone in roots of A. balsamea in 2006 consisted of six or seven cells at the first sampling day (2 May or day of the year 122), whereas fewer than five cells defined the cambial zone during dormancy. After mid-July, the number of cambial cells gradually decreased until the end of August and, from September, was observed to remain constant.
Fig. 2.
Number of cells in the cambial zones observed in stem (closed circles) and roots (open circles) of Abies balsamea and Picea mariana during 2004–2006. Vertical bars represent s.e. Horizontal dotted lines represent the number of cells in the cambial zone during dormancy.
Xylem cell differentiation
In the three studied years, the onset of cell radial enlargement, corresponding to the beginning of xylem differentiation, varied from 19 May to 31 May and was synchronized between stem and roots in 2004 and 2005 (Tables 2 and 3). However, in 2006 cell enlargement in the stem started before that in roots (MANOVA, F = 11·10, P = 0·016, Table 3). In the stem, the highest values of enlarging cells (three or four) were observed between June and mid-July, while the greatest numbers of enlarging cells in roots were observed during July. The end of radial enlargement occurred in mid-August in both the stem and root.
Table 2.
Onset and ending of xylem differentiation in stem and roots of Abies balsamea and Picea mariana during 2004–2006
| Onset of differentiation |
Ending of differentiation |
||||
|---|---|---|---|---|---|
| Species | Year | Stem | Roots | Stem | Roots |
| A. balsamea | 2005 | 145 ± 7 | 144 ± 9 | 266 ± 7 | 263 ± 18 |
| 2006 | 139 ± 3 | 149 ± 11 | 271 ± 9 | 276 ± 21 | |
| P. mariana | 2004 | 152 ± 4 | 149 ± 3 | 278 ± 6 | 280 ± 8 |
| 2005 | 147 ± 4 | 150 ± 8 | 266 ± 9 | 270 ± 14 | |
| 2006 | 144 ± 4 | 147 ± 7 | 265 ± 7 | 287 ± 13 | |
Values are reported as average with standard deviation when timing was computed in Julian days.
Table 3.
F-statistic and resulting probability of the multifactor analysis of variance (MANOVA, P = 0·05) of onset and ending of xylem differentiation in stem and roots of Abies balsamea and Picea mariana during 2004–2006
| Onset of differentiation |
Ending of differentiation |
||||
|---|---|---|---|---|---|
| Year | Source | F | P | F | P |
| 2004 | Species | − | − | − | − |
| Location | 1·93 | 0·2019 | 0·15 | 0·7061 | |
| Species×location | − | − | − | − | |
| 2005 | Species | 3·10 | 0·0840 | 1·06 | 0·3088 |
| Location | 0·31 | 0·5774 | 0·02 | 0·8912 | |
| Species×location | 1·17 | 0·2840 | 0·70 | 0·4079 | |
| 2006 | Species | 0·56 | 0·4560 | 0·39 | 0·5337 |
| Location | 11·10 | 0·0016 | 10·35 | 0·0022 | |
| Species×location | 3·96 | 0·0520 | 4·36 | 0·0417 | |
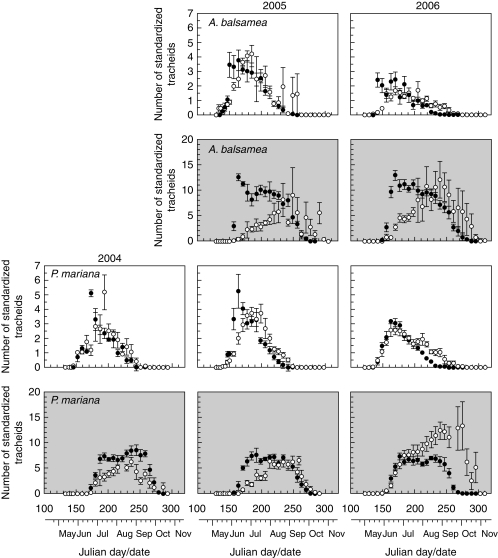
The onset of wall thickening occurred in June, earlier in the stem than in roots (Fig. 3). In the stem, the highest values of wall thickening cells were 10–12 for A. balsamea and 8–10 for P. mariana, observed at the end of July. In roots, the highest values of wall thickening were observed between August and September, with eight to ten and five or six tracheids in A. balsamea and P. mariana, respectively. The ending of cell wall thickening and lignification, corresponding to the end of xylem differentiation, occurred in the last days of September, in the same periods for stem and roots during 2004 and 2005 (Table 3). However, different endings of cell wall thickening were observed between the sample locations in 2006 (MANOVA, F = 10·35, P = 0·0022; Table 3) and statistically significant interactions with species (MANOVA, F = 4·36, P = 0·0417; Table 3).
Fig. 3.
Number of standardized tracheids in radial enlargement (white background) and wall thickening (grey background) in stem (closed circles) and root (open circles) for Abies balsamea and Picea mariana during 2004–2006. Vertical bars represent s.e.
Fitting the number of xylem tracheids
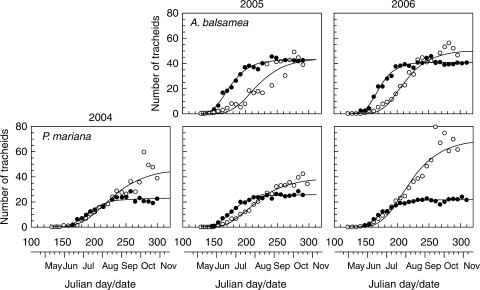
The patterns of tree-ring growth were examined in both stem and roots of A. balsamea and P. mariana (Fig. 4). The Gompertz function adequately fitted the observations, with R2 varying between 0·90 and 0·99 (Table 4), whereby roots showed slightly lower values than stems. Although the onset of xylem production was synchronous between the two sampling locations in 2004 and 2005, the patterns of growth clearly differed in stem and roots. Most tracheids were produced in June in the stem, while cell production occurred during July in roots; the date of the inflection point of the Gompertz equations was calculated at 20 June in the stem (day of the year 171), on average 30 d before that in roots (parameter tp; Table 4), indicating an early decline of the rate of cell production in the stem. Similarly, 50 % of the tracheids produced were delayed and achieved at the end of June in stems and at the beginning of August in roots.
Fig. 4.
Number of xylem cells over time and Gompertz equations for stem (closed circles) and roots (open circles) of Abies balsamea and Picea mariana during 2004–2006.
Table 4.
Parameters of the Gompertz function, R2 and date of the inflection point (tp) in stem and roots of Abies balsamea and Picea mariana during 2004–2006
|
A |
β |
κ (10–2) |
R2 |
tp |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Year | Stem | Roots | Stem | Roots | Stem | Roots | Stem | Roots | Stem | Roots |
| A. balsamea | 2005 | 42·68 | 44·49 | 8·21 | 6·86 | 4·88 | 3·28 | 0·99 | 0·91 | 168·24 | 209·14 |
| 2006 | 40·75 | 50·20 | 9·78 | 7·47 | 5·89 | 3·74 | 0·98 | 0·97 | 166·04 | 199·73 | |
| P. mariana | 2004 | 22·76 | 46·50 | 8·78 | 5·77 | 4·78 | 2·71 | 0·96 | 0·90 | 183·68 | 212·92 |
| 2005 | 25·59 | 39·01 | 8·74 | 6·13 | 5·12 | 3·01 | 0·99 | 0·98 | 170·70 | 203·65 | |
| 2006 | 21·65 | 69·64 | 8·46 | 6·22 | 5·03 | 3·00 | 0·98 | 0·94 | 168·19 | 207·33 | |
According to the asymptote of the fitted function (parameter A; Table 4), at the end of the growing season, the number of tracheids along a radial file in the stem of A. balsamea and P. mariana was 41·7 and 23·3, respectively. In roots, higher amounts of tracheids were observed (up to 75 % more than in the stem), except for A. balsamea in 2005, when a similar number of cells was counted in autumn. This observation was especially pronounced for cell production in roots of P. mariana in 2006.
DISCUSSION
In the last decade, many studies have been conducted on intra-annual wood formation in both conifer and broadleaf species, but none, as far as is known, concerned xylogenesis in roots. This investigation described and compared cell production and differentiation between stem and roots in two boreal forest species during three growing seasons. The results revealed similar xylem phenologies in 2004–2005, but delayed endings of cell differentiation in roots compared with the stem in 2006. The production of xylem was concentrated in the stem early in the season, while most cell divisions occurred 1 month later in roots.
Cambial reactivation is assumed to be promoted by auxin, produced in the younger shoots and exported basipetally along the stem to induce the production of xylem (Larson, 1969; Aloni, 2001) and regulate xylem development (Tuominen et al., 1997; Uggla et al., 1998). Following the basipetal movement of the auxin, periclinal divisions in the cambium should begin close to buds, spread downwards toward branches and stem and finally occur in the roots (Larson, 1969; Denne, 1979; Lachaud et al., 1999). However, in this study no pattern in the direction of the progression of the onset of cambial activity was observed between stem and roots in the two studied species, suggesting that the auxin required for cambial resumption could already be available in the dormant tissues before spring (Little and Wareing, 1981; Sundberg et al., 1991).
The hypotheses on the direction of cessation of cambial activity and xylem production through the different plant tissues are still contradictory (Zimmermann and Brown, 1971; Riding and Little, 1986; Vaganov et al., 2006). In the present investigation, the ending of xylem differentiation occurred in similar periods in 2004–2005, but in 2006 a longer period of xylem formation was observed in roots than in the stem. This longer differentiation period in the developing root tissues corresponded to a higher number of xylem cells produced, particularly in P. mariana. The ending of latewood differentiation is related to cell production in vascular cambium; the higher the number of cells produced along a radial row, the longer the overall period of tree ring formation becomes (Gričar et al., 2005, Rossi et al., 2008a). Incorporation of wall constituents in latewood cells is an energy-expensive process for a tree and wall thickening and lignification can require up to 40 d for a xylem tracheid (Rossi et al., 2006b). The greatest number of cells produced by roots in 2006 led to a higher accumulation of developing cells in differentiation and delayed the conclusion of xylem maturation.
The onset of xylem production occurred early in the season and terminated at the end of August, at temperatures still favourable for growth. This could indicate a different physiological mechanism involved in controlling xylem phenology at the beginning and ending of growth, with effects of both internal and external factors on cambial activity. Thermal sums are known to precisely estimate the beginning of growth (Schmitt et al., 2004; Seo et al., 2008) and Rossi et al. (2007) assessed a threshold temperature limiting spring carbon allocation in xylem of Alpine treeline trees, which suggests an important influence of temperature on cambial reactivation in these environments. On the contrary, the cessation of cell production is unrelated to the annual temperature trend (S. Rossi et al., 2008). Rossi et al. (2006b) observed that the growth rate in the stem culminated during the summer solstice, when the photoperiod was maximum. However, this work reports a different growth pattern in roots, with delays of up to 1 month for the culmination of growth in the soil. At the end of cell division, high amounts of abscisic acid were detected in stems and associated with the termination of cell production (Lachaud, 1989), but proof that abscisic acid directly induces cambial dormancy is still lacking.
In the boreal forest, temperature is the main factor driving xylogenesis during spring (Antonova and Stasova, 1997; Vaganov et al., 1999; Deslauriers and Morin, 2005). Vascular cambium is a sink for non-structural carbohydrates, and cambial activity requires a continuous supply of energy in the form of sucrose, extracted from the storage tissues for the first formed cells, or produced by photosynthesis (Hansen and Beck, 1994; Oribe et al., 2003). Temperature allows the metabolism of carbon allocation to be accomplished (Körner, 1998). Although cell differentiation was estimated to begin in the same period at both sample locations, stem and roots concentrated most xylem production in two different periods of the year, with earlier tracheid accumulation observed in the stem. In spring, air temperature increases sharply in May–June, although the soil temperature remains close to zero during snowmelt and is observed to increase at least 2 weeks later than the air (Rossi et al., 2007). This delayed temperature increase in the soil could explain the different patterns of xylem growth observed between stem and roots.
Further investigations should be interesting to follow the reactivation of the cambial zone during the spring. In the present study, sampling only started at the beginning of May and approximately within half of the observations, the number of cambial cells exceeded the minimum number counted at the end of the previous year. As reported in the literature, physiological processes occurred in the cambial zone before cell division is observed (Frankenstein et al., 2005). However, the present method did not allow the observation of structural modifications in the cambial zone in order to identify the onset of cell production according to Frankenstein et al. (2005).
In conclusion, the xylem of stem and roots in A. balsamea and P. mariana showed similar periods of cell differentiation during 2004–2006, but with different intra-annual growth dynamics. This behaviour could be related to internal and external factors such as the amount of cells produced by the vascular cambium and the different patterns of air and soil temperature in spring.
ACKNOWLEDGEMENTS
This work was funded by the Consortium de recherche sur la forêt boréale commerciale and the Natural Sciences and Engineering Research Council of Canada (NSERCC). We thank M. Boulianne, G. Savard, A. Castro Estupiñan, A. Turcotte, S. Pedneault, G. Dumont-Frenette, C. Tremblay and P. Émond for technical support, and give special thanks to D. Walsh, P.-Y. Plourde, A. Deslauriers and T. Anfodillo for their comments during the preparation of the paper.
LITERATURE CITED
- Abe H, Funada R, Ohtani J, Fukazawa K. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids. Trees – Structure and Function. 1997;11:328–332. [Google Scholar]
- Aloni R. Foliar and axial aspects of vascular differentiation: hypotheses and evidence. Journal of Plant Growth Regulation. 2001;20:22–34. [Google Scholar]
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in larch (Larch sibirica Ldb.) stems. Trees – Structure and Function. 1997;11:462–468. [Google Scholar]
- Catesson AM. Cambial ultrastructure and biochemistry – changes in relation to vascular tissue differentiation and the seasonal cycle. International Journal of Plant Sciences. 1994;155:251–261. [Google Scholar]
- Denne MP. A comparison of root- and shoot-wood development in conifer seedlings. Annals of Botany. 1972;36:579–587. [Google Scholar]
- Denne MP. Wood structure and production within the trunk and branches of Picea sitchensis in relation to canopy formation. Canadian Journal of Forest Research. 1979;9:406–427. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees – Structure and Function. 2005;19:402–408. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Canadian Journal of Forest Research. 2003;33:190–200. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in Southern Italy. Tree Physiology. 2008;28:863–871. doi: 10.1093/treephys/28.6.863. [DOI] [PubMed] [Google Scholar]
- Dufour B, Morin H. Focusing modelling on the tracheid development period – an alternative method for treatment of xylogenesis intra-annual data. Dendrochronologia. 2007;25:125–133. [Google Scholar]
- Fayle DCF. Radial growth in tree roots – distribution, timing, anatomy. Toronto: Faculty of Forestry; 1968. Technical report no. 9. [Google Scholar]
- Forster T, Schweingruber FH, Denneler B. Increment puncher: a tool for extracting small cores of wood and bark from living trees. IAWA Journal. 2000;21:169–180. [Google Scholar]
- Frankenstein C, Eckstein D, Schmitt U. The onset of cambium activity – a matter of agreement? Dendrochronologia. 2005;23:57–62. [Google Scholar]
- Funada R, Kubo T, Sugiyama T, Fushitani M. Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. Journal of Wood Science. 2002;48:75–80. [Google Scholar]
- Gagnon R. Maintien après feu de limites abruptes entre des peuplements d'épinettes noires (Picea mariana) et des formations de feuillus intolérants (Populus tremuloides et Betula papyrifera) dans la région du Saguenay-Lac Saint-Jean (Québec) Le Naturaliste Canadien. 1989;116:117–124. [Google Scholar]
- Gričar J, Čufar K, Oven P, Schmitt U. Differentiation of terminal latewood tracheids in silver fir during autumn. Annals of Botany. 2005;95:959–965. doi: 10.1093/aob/mci112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Beck E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees – Structure and Function. 1994;8:172–182. [Google Scholar]
- Ko Heinrichs D, Tardif J, Bergeron Y. Xylem production in six tree species growing on an island in the boreal forest region of western Quebec, Canada. Canadian Journal of Botany. 2007;85:518–525. [Google Scholar]
- Kozlowski TT, Pallardy SG. Growth control in woody plants. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Krause C, Eckstein D. Dendrochronology of roots. Dendrochronologia. 1993;11:9–23. [Google Scholar]
- Krause C, Morin H. Root growth and absent rings in mature black spruce and balsam fir, Quebec, Canada. Dendrochronologia. 1999;16–17:21–35. [Google Scholar]
- Kutscha NP, Hyland F, Schwarzmann JM. Certain seasonal changes in Balsam Fir cambium and its derivatives. Wood Science and Technology. 1975;9:175–188. [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Lachaud S. Participation of auxin and abscisic acid in the regulation of seasonal variations in cambial activity and xylogenesis. Trees – Structure and Function. 1989;3:125–137. [Google Scholar]
- Lachaud S, Catesson AM, Bonnemain JL. Structure and functions of the vascular cambium. Comptes Rendus de l'Académie des Sciences – Series III – Sciences de la Vie. 1999;322:633–650. doi: 10.1016/s0764-4469(99)80103-6. [DOI] [PubMed] [Google Scholar]
- Ladefoged K. The periodicity of wood formation. Det Kongelig Danske Vidensk Selsk Skrift Dan Biol. 1952;7:1–98. [Google Scholar]
- Larson PR. Wood formation and the concept of wood quality. New Haven, CT: Yale University; 1969. [Google Scholar]
- Larson PR. The vascular cambium: development and structure. Berlin: Springer-Verlag; 1994. [Google Scholar]
- Little CHA, Wareing PF. Control of cambial activity and dormancy in Picea sitchensis by indol-3-yl acetic and abscisic acids. Canadian Journal of Botany. 1981;59:1480–1493. [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees – Structure and Function. 2003;17:185–192. [Google Scholar]
- Panshin AJ, de Zeeuw C. Textbook of wood technology. New York, NY: McGraw-Hill Book Co; 1970. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Riding RT, Little CHA. Histochemistry of the dormant vascular cambium of Abies balsamea: changes associated with tree age and crown position. Canadian Journal of Botany. 1986;64:2082–2087. [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA Journal. 2006;a 27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytologist. 2006;b 170:301–310. doi: 10.1111/j.1469-8137.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152:1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carrer M. Age-dependent xylogenesis in timberline conifers. New Phytologist. 2008;a 177:199–208. doi: 10.1111/j.1469-8137.2007.02235.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Gričar J, Seo J-W, Rathgeber CBK, Anfodillo T, et al. Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography. 2008;b (in press) [Google Scholar]
- Savidge RA. Intrinsic regulation of cambial growth. Journal of Plant Growth Regulation. 2000;20:52–77. [Google Scholar]
- Savidge RA, Wareing PF. Seasonal cambial activity and xylem development in Pinus contorta in relation to endogenous indol-3-yl-acetic and (S)-abscisic acid levels. Canadian Journal of Forest Research. 1984;14:676–682. [Google Scholar]
- Schmitt U, Jalkanen R, Eckstein D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fennica. 2004;38:167–178. [Google Scholar]
- Seo J-W, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiology. 2008;28:105–112. doi: 10.1093/treephys/28.1.105. [DOI] [PubMed] [Google Scholar]
- Skene DS. The period of time taken by cambial derivatives to grow and differentiate into tracheids in Pinus radiata D. Don. Annals of Botany. 1969;33:253–262. [Google Scholar]
- Stevens CL. Root growth of white pine (Pinus strobus L.) Yale University School Forest Bulletin. 1931;32:1–32. [Google Scholar]
- Sundberg B, Little CHA, Cui K, Sandberg G. Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant, Cell & Environment. 1991;14:241–246. [Google Scholar]
- Troeng E, Linder S. Gas exchange in a 20 year old stand of Scots pine. Physiologia Plantarum. 1982;54:7–23. [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B. A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiology. 1997;115:577–585. doi: 10.1104/pp.115.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz E, Sundberg B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signalling. Plant Physiology. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature. 1999;400:149–151. [Google Scholar]
- Vaganov EA, Hughes MK, Shashkin AV. Growth dynamics of conifer tree rings – images of past and future environments. Berlin: Springer-Verlag; 2006. [Google Scholar]
- Zimmermann MH, Brown CL. Trees: structure and function. New York, NY: Springer-Verlag; 1971. [Google Scholar]