Abstract
Background and Aims
Few studies have examined the dynamics of specialist plant–pollinator interactions at a geographical scale. This knowledge is crucial for a more general evolutionary and ecological understanding of specialized plant–pollinator systems. In the present study, variations in pollinator activity, assemblage composition and pollen limitation were explored in the oil-producing species Nierembergia linariifolia (Solanaceae).
Methods
Pollen limitation in fruit and seed production was analysed by supplementary hand pollination in five wild populations. Pollinator activity and identity were recorded while carrying out supplementary pollination to assess the effect of pollinators on the degree of pollen limitation. In two populations, pollen limitation was discriminated into quantitative and qualitative components by comparing supplementation and hand cross-pollination in fruit set and seed set. The effect of flower number per plant on the number of flowers pollinated per visitor per visit to a plant was examined in one of these populations as a possible cause of low-quality pollination by increasing geitonogamy.
Results and Conclusions
Although pollen limitation was evident along time and space, differences in magnitude were detected among populations and years that were greatly explained by pollinator activity, which was significantly different across populations. Floral display size had a significant effect on the visitation rate per flower. Limitation by quality clearly affected one population presumably due to a high proportion of geitonogamous pollen. The great inter-population variation in plant–pollinator interaction (both in pollinator assemblages composition and pollinator activity) and fitness consequences, suggests that this system should be viewed as a mosaic of locally selective processes and locally specialized interactions.
Key words: Nierembergia linariifolia, Centris, Chalepogenus, pollen limitation, pollen quality, oil-producing flowers, specialized pollination, floral display, assemblage composition, geographic variation, Solanaceae, tests of equivalence
INTRODUCTION
In recent years, the specialization of plant–pollinator interactions has been the focus of much discussion and debate (e.g. Waser et al., 1996; Johnson and Steiner, 2000; Fenster et al., 2004). Traditionally (e.g. Stebbins, 1970) it was believed that the evolutionary trend should be towards specialization, considering it more advantageous than generalization (Fenster et al., 2004). However, this view was challenged based on the increasing evidence that most flowering plant species are pollinated by many species of diverse animal groups, and on the idea that stochastic variation in the composition of pollinator assemblages in space and time would favour generalization in pollination systems (Schemske and Horvitz, 1984; Herrera, 1988, 1996; Waser et al., 1996; Gómez and Zamora, 1999; Herrera, 2005).
Two current actively discussed topics may favour our understanding of the evolution of plant–pollinator relationships, namely pollen limitation and geographic variation in plant–pollinator interactions. On one hand, pollen limitation studies (i.e. studies examining if there is a reduction in fruit or seed set as a result of quantitatively or qualitatively insufficient pollen deposition) provide a way of evaluating the reproductive consequences of pollinator services (Ashman et al., 2004; Knight et al., 2005; Aizen and Harder, 2007). Thus, an intraspecific study on the magnitude of pollen limitation allows evaluating the ecological consequences of different local interactions.
Pollen limitation comprises two components: quantity and quality limitation. However, the importance of the latter has seldom been recognized and its magnitude is rarely quantified (Aizen and Harder, 2007, and references therein).The quantity of pollen deposited on the stigma can decrease as a result of pollinators visiting fewer flowers and/or delivering less pollen per visit (Ashman et al., 2004). Thus, the quantitative component of pollen limitation is related to both pollinator frequency (i.e. number of visits) and pollinator assemblage composition (i.e. efficiency of visits).
In addition, quality can also become a limiting factor if pollinators deposit incompatible pollen on stigmas (Totland et al., 1998; Ramsey and Vaughton, 2000; Aizen and Harder, 2007). Therefore, self-incompatible species with multiple flowers per plant may be particularly prone to pollen limitation by quality if geitonogamous incompatible pollen is deposited on their stigmas (Snow et al., 1996; Totland et al., 1998; Ramsey and Vaughton, 2000). Hence, to shed light on the ecological significance of pollen limitation it is necessary to assess the relative importance of both the quantity and quality of pollen in limiting reproductive output (Ramsey and Vaughton, 2000; Aizen and Harder, 2007).
On the other hand, a geographic perspective on plant–pollinator interaction should allow understanding macroevolutionary processes such as specialization or generalization by examining patterns at a microgeographic scale that are the consequence of local adaptations to different ecological contexts (Gould and Johnston, 1972; Thompson, 1994; Herrera et al., 2006; Alonso et al., 2007). This perspective would allow determining whether specialization is an inherent property of a species or an attribute of local populations, thus establishing the limits of conceptual generalizations in plant–pollinator systems (Fox and Morrow, 1981; Thompson, 1994; Herrerra, 2005; Alonso et al., 2007). Studies on geographic variation in pollinator assemblages have generally been performed on moderately generalized to generalized pollination systems (Herrera et al., 2006), with very few studies focusing on the effects of geographical and temporal variations of pollinators on fitness in specialized systems (but see Baker et al., 2000; Moody-Weis and Heywood, 2001). The general consensus is that more empirical studies are needed to characterize specialized pollination systems at both a spatial and temporal scale (Waser et al., 1996; Fenster et al., 2004; Kay and Schemske, 2004; Herrera, 2005).
Oil-secreting flowers and oil-collecting bees are examples of highly specialized plant-pollinator systems (Steiner and Whitehead, 1991; Johnson and Steiner, 1997, 2000). Flowers offering fatty oils are only found in a number of genera from ten families of flowering plants (Buchmann, 1987; Neff and Simpson, 2005). These flowers are visited by highly specialized bees comprising only 1·4 % of the bee species in the world (Buchmann, 1987; Cocucci et al., 2000). There is scarce information on the pollination ecology of most plant species with oil-producing flowers (but see Vogel, 1990; Cocucci, 1991; Vogel and Machado, 1991; Cocucci and Vogel, 2001; Steiner and Whitehead, 2002; Pauw, 2005), let alone the consequence of pollinators on fitness and cross-pollination (Gomes Teixeira and Machado, 2000; Machado, 2004).
In the present study, variations in pollinator activity, assemblage composition and pollen limitation are explored in the oil-producing species Nierembergia linariifolia (Solanaceae). The main aims of this study were to determine if (a) there is spatial and temporal variation in assemblage composition and pollinator activity; (b) the magnitude of pollen limitation varies among populations and years; and (c) there is an effect of pollinator assemblage, activity and diversity on the magnitude of pollen limitation among populations and years.
MATERIALS AND METHODS
Study species
Nierembergia linariifolia (Solanaceae) is a perennial subshrub endemic to central Argentina, South America. It has hermaphroditic flowers with a fertile column emerging from the centre of the almost flat limb of the salverform corolla. This column is formed by the apical end of the style surrounded by five stamens (Cocucci, 1991). The stigma is receptive and pollen is viable throughout the flower life, and neither age nor pollen grain deposition cause it to decline (Cocucci, 1984; Cocucci, 1991).
Nierembergia linariifolia is a completely self-incompatible species except in one of the populations studied (see below) where artificial selfing and bagging flowers produced fruits with a few seeds (Cocucci, 1991; Cosacov, 2004). No significant differences in the mean number of ovules per flower (grand mean 55·8 ± 16·69 s.d.) were observed among five populations of this species in the same geographical range (J. Nattero, unpubl. res.; e.g. Pampa de Achala, 57·62 ± 19·19, n = 165; Capilla del Monte, 55·95 ± 16·17, n = 108; Villa Animí = 53·86 ± 14·718, n = 101). Plants bloom for 3–4 weeks with each individual flower lasting 2–3 d.
Previous studies have reported oil-collecting bee species of the genera Chalepogenus, Tapinotaspis (Tribe Tapinotaspidini) and Centris (Tribe Centridini) as pollinators of this plant species (Simpson and Neff, 1983; Cocucci, 1984, 1991; Cocucci et al., 2000). In subtropical and temperate zones, Centris bee species are mainly found in plains and dry habitats, whereas Chalepogenus species are found in montane and more humid areas (Cocucci, 1984; Cocucci, 1991; Roig-Alsina, 1999, 2000). Oil-collecting behaviour and pollinator mechanisms vary greatly among bee species (Cocucci, 1991). Preliminary observations suggest that T. chalybaea and C. tricolor visit an average of 7·5 flowers per plant per visit, Ch. nigripes and Ch. brevipilli 2·5 flowers per visit and Ch. parvus 4 flowers per plant per visit (J. Nattero, unpubl. res.).
Study area
The climate, topography and biogeographical background of central Argentina are very heterogeneous, with a considerably diversity of environmental conditions and plant communities (Cabrera, 1971). Five wild populations were deliberately chosen to represent different ecological contexts where differences among populations were expected to be found in pollinator activity, assemblage composition and/or relative abundance of bee species.
Pampa de Achala (ACH) is a granitic upland plateau (1800–2300 m) located in the Sierras Grandes mountain range in the province of Córdoba. The mean temperature in January and July is 14·4 °C and 6·3 °C, respectively, with a mean annual rainfall of 725 mm. The vegetation consists of a mosaic of grasslands and Polylepis australis woodlands. Capilla Del Monte (CAP) and Charbonier (CHAR) are located in the Chaco Serrano Forest, a montane variant of the Chaco Forest which is the most extensive dry forest area in South America (Cabrera, 1971). Altitude ranges between 400 m and 1300 m, mean January and July temperatures are 23 °C and 11 °C, respectively, and the mean annual rainfall is 550 mm. The vegetation consists of low and open woodland dominated by Prosopis species and Aspidosperma quebracho-blanco (Cabrera, 1971). Deán Funes (DF) is located in the Chaco Occidental Forest (200–700 m), a semi-arid lowland variant of the above forest. Mean January and July temperatures are 23·4 °C and 10·8 °C, respectively, with a mean annual rainfall of 560 mm. The dominant vegetation consists of xerophitic species such as Aspidosperma quebracho-blanco, Schinopsis lorentzii, Acacia caven and Opuntia quimilo (Cabrera, 1971). The University Campus (CU) is located in the Espinal area (200–450 m), humid savanna-like woodland dominated by Prosopis forests with psammophyle or halophyle grasslands and bush (Cabrera, 1971). Mean January and July temperatures are 24 and 10·5 °C, respectively, and the mean annual rainfall is 677 mm.
The above climatic data were obtained as deviations from agro-climatic potentials covering a 30-year time span (De Fina, 1992). These variables were summarized as an environmental factor, the first axis of the principal component analysis (PCA), which accounted for 87 % of the total climatic variation and was mainly explained by temperature. Variations in plant density among sites were also included in the analysis. Mean plant density was measured as the number of N. linariifolia flowering plants within a 2-m2 plot. This was measured six times at randomly selected points in every study population. ACH presented the highest plant density, while CAP and CU had the lowest attributable to a moderate level of grazing. Sampling sites including information on altitude, flowering periods, vegetation type, environmental conditions and mean plant density of each population are presented in Table 1. Distance among populations ranged between 4 km and 80 km (mean = 47·84 km).
Table 1.
Location, geographic characteristics, type of vegetation and plant density of the Nierembergia linariifolia populations studied for pollen limitation in fruit and seed set in central Argentina
| Pampa de Achala (ACH) | Capilla del Monte (CAP) | Charbonier (CHAR) | University (CU) | Deán Funes (DF) | |
|---|---|---|---|---|---|
| Longitude | 64°46′00″W | 64°32′00″W | 64°31′60″W | 64°10′60″W | 64°20′60″W |
| Latitude | 31°36′00″S | 30°52′00″S | 30°46′00″S | 31°23′60″S | 30°25′60″S |
| Altitude (m a.s.l.) | 2193 | 972 | 850 | 383 | 679 |
| Vegetation type | Montane grassland | Chaco serrano forest | Chaco serrano forest | Espinal | Chaco occidental forest |
| Peak flowering | Mid-December | Mid-October | Mid-October | Mid-November | Mid-October |
| Annual mean rainfall (mm) | 725 | 608 | 537 | 677 | 560 |
| January mean temperature (°C) | 14·4 | 22·6 | 23·4 | 24·3 | 23·4 |
| Mean plant density (plants m−2) | 5·85 | 2·3 | 2·85 | 1·8 | 4·20 |
Pollinator assemblage composition and pollinator activity
Pollinator visits were recorded on the same days the pollen limitation experiments were carried out (see below). This way the fruit and seed set of each population can be related to the activity of the pollinators observed. On each visit, six to eight plots (approx. 1 m2) including approx. 100 flowers were observed for 15–30 min in each population. All flower visitors entering a plot were identified and the number of flowers visited recorded. The mean number of visits per flower per hour was then calculated as: Vf = V/F/T, where V is the total number of visits to flowers, F the total number of flowers in the plot, and T the observation time in hours. Vf was also calculated for each bee species, which provides a relative index of abundance for the pollinator species in each site.
To assess if there was a geographic pattern in assemblage composition, a Mantel test was performed using dissimilarity matrices of assemblage composition (1-Jaccard) and geographical distances (in km) between all population pairs. The assemblage composition of the populations studied for 2 years was a combination of the data recorded during both seasons. To determine whether environmental variables and mean plant density were affecting pollen limitation by influencing pollinator activity, a multiple linear regression was performed using Vf as response variable. Since ecological specialization (or generalization) might affect the magnitude of pollen limitation (see Knight et al., 2005), the level of specialization was characterized in each population and year studied using Simpson's diversity index (Simpson, 1949) which includes richness and evenness and has been proposed as a good generalization estimator in plant–pollinator systems (Sahli and Conner, 2006). A simple linear regression between Simpson's diversity index and Vf was carried out among populations and years.
Pollen limitation by quantity
The spatial and temporal components of pollen limitation of the fruit and seed set were studied in three populations (ACH, CAP and CU). CHAR and DF were only studied during the second year.
To obtain virgin flowers before manipulation, 11–32 plants were chosen in each population, their open flowers were removed (around three to seven flowers per plant) and the buds were covered with a mesh to exclude pollinators. The experiments were carried out the following day on newly opened flowers. Available flowers were identified by painting the persistent calyx and classified into two sub-sets: open-pollination and supplemented hand-pollination. For the latter, flowers were exposed to natural pollination between 09·00 and 15·00 h, when pollinators are most active, and then supplemented with cross-pollen (i.e. mixed loads of pollen from three to five individuals located >3 m away from the recipient) until saturating the stigma. This was evident because the stigmas were wet and glossy when virgin and became opaque when saturated with pollen. A total of 20–70 flowers was used per plant per treatment, which on average represented 70 % of the total flower production. Treatments were carried out 3–5 times during the flowering season on successive new flowers. Fruits were collected 3–4 weeks later. This procedure was repeated the following year on a different subset of flowering plants.
To control the possible influence of limitations by resource allocation on fitness (Zimmerman and Pike, 1988), naturally pollinated flowers from plants receiving no type of treatment were used as controls for pollen-supplemented plants (10–31 plants per site). In order to consider small-scale environmental heterogeneity (nutrients, humidity, temperature and radiation) that might account for differences in reproductive success by affecting pollinator activity or resource availability (Haig and Westoby, 1988; Herrera, 1995), control and treated plants were alternately selected along a single transect that crossed the extent of each population (50–100 m long).
To test whether pollen supplementation affected the seed set of non-supplemented flowers on the same plant, the seed sets of open-pollinated flowers in control and treated plants of each population were compared with a one-way ANOVA. A two one-sided t-test (TOST) for equivalence assessment was also performed (Schuirmann, 1987). This analysis is particularly appropriate when rigorous evidence of equivalence between two treatments is needed, as the failure to reject the null hypothesis (i.e. statistical differences between means) is not always enough evidence of similarity (see Westlake, 1976; Garrett, 1997; Dixon and Pechmann, 2005). For this analysis, the definition of the magnitude of the similarity region (−ε, +ε) was based on results obtained in different pollination experiments, which showed that an effect of a treatment is significant when the difference of means is equal or higher than 80 % of the standard deviation of the control.
Additionally, as indirect evidence of a potential compensatory mechanism (i.e. more seeds of smaller size), the slope of the relationship between the mean number of seeds per fruit and fruit length was studied to find out if it differed between non-supplemented flowers of control plants and supplemented flowers of treated plants (Zimmerman and Aide, 1989). A compensatory mechanism should be evident when fruits from supplemented flowers had as steep a slope in the relation seeds/fruits size – i.e. a stronger increase of seed number in relation to fruit size increase – than fruits from control flowers. Since a covariance test (ANCOVA) is the standard method for testing differences in regression coefficients between groups (Sokal and Rohlf, 1995), an ANCOVA model was constructed including a categorical term coding for plant treatment (i.e. control vs. treated plants), a continuous term for fruit length, and a treatment × fruit length term. The interaction term, if significant, indicates that the relationship between seed number and fruit length varied between treatments.
As there was no evidence of resource allocation nor of a compensatory mechanism (see Results), all the subsequent analyses were performed on open and supplemented flowers of treated plants.
The effects of flower treatment (i.e. open vs. supplemented pollination) on fruit set (fruits produced/flowers treated per plant), seed set (mean number of seeds per fruit per plant), and seed production per plant (seed set × fruit set × number of treated flowers) were examined separately for each site and year. Since seed set and seed production per plant were strongly and significantly correlated (Pearson's r = 0·85, P = 0·01) both fitness measures are reported whenever different patterns were obtained (see Results). As the distribution of the fruit set data was not normal, a non-parametric Wilcoxon's matched paired test that uses median values instead of mean values was used to examine differences between groups (Sokal and Rohlf, 1995). Differences in seed set and seed production per plant were analysed using a paired t-test (Sokal and Rohlf, 1995). The effects of pollination treatment, year and population on seed set and seed production were examined with a three-way ANOVA including only the three populations studied both years. To examine the effects of pollination treatments and population on these measures of fitness at a broader spatial scale, a two-way ANOVA was performed using the experiments carried out during the second year (2003) in the five populations studied. The sampling site was treated as a fixed-effects factor given that the five populations were deliberately chosen to represent different environmental conditions along the distribution range of N. linariifolia.
A pollen limitation index (L) was calculated for each plant according to Larson and Barrett (2000) as: L = 1 – (Po/Ps), where Po and Ps are seed set under open and supplemented pollination, respectively. Values range from 0 to 1, with L = 0 indicating no pollen limitation. To visualize the spatial and temporal contributions that explained variance in pollen limitation, the effects of year and population on mean pollen limitation index were assessed by a two-way ANOVA including only the three populations studied both years.
Pollen limitation by quality
As previous observations indicated that pollinators visit many flowers per plant favouring geitonogamous pollination, it was of interest to evaluate whether N. linariifolia experienced pollen limitation by quality. Two populations that differed in the level of self-compatibility were selected, expecting pollen limitation by quality to be higher in the fully self-incompatible population. In the CU population, N. linariifolia presented a moderate level of self-compatibility (i.e. hand self-pollination set an average of five seeds per fruit) while in the ACH population plants were completely self-incompatible (i.e. hand self-pollination did not set seeds; Cosacov, 2004). Thus, the ACH population was expected to be more suceptible to pollen limitation by pollen quality than the CU population.
To examine the occurrence of pollen limitation due to pollen quality, cross-pollination treatments were simultaneously carried out with pollen supplementation and open pollination on the same individuals in CU and in ACH populations during 2003. The stigmas of open-pollinated and pollen-supplemented flowers were exposed to receive autogamous and geitonogamous self-pollen in addition to cross-pollen from distant plants. On the other hand, bagged, emasculated and cross-pollinated flowers only received cross-pollen from three to five plants located at least 3 m away from the recipient. Fruit sets and seed sets were compared among cross-pollinated, pollen-supplemented and naturally pollinated flowers. A non-parametric Kruskal–Wallis test was used to examine differences in fruit set since it did not distribute normally, whereas a one-way ANOVA was performed to examine differences in seed set (Sokal and Rohlf, 1995).
Factors affecting pollen limitation
In order to determine whether the frequency of visitations (Vf) and floral density explain pollen limitation patterns, a multiple linear regression using mean pollen limitation index (L) as a response variable was performed among the populations studied during 2003. The effect of Vf on L and natural seed set (i.e. open pollinated flowers of treated plants) was also examined by a simple linear regression among populations and years.
To examine whether differences in the magnitude of pollen limitation between populations (i.e. between population differences in L) could be explained by differences in assemblage composition (i.e 1-Jaccard), a Mantel test was performed including only the data obtained during the second sampling year. A simple linear regression was performed between Simpson's diversity index and mean pollen limitation index among populations and years to determine if variations in the level of specialization affected the magnitude of pollen limitation.
Floral display and pollinator visitations
To determine if floral display size (i.e. number of flowers per plant) influences pollinator attraction (in turn affecting the quantity of pollen deposited) and whether it is related to the probability of geitonogamous pollination (in turn affecting the quality of pollen deposited), the number of visits to each plant (visitation rate per plant) and the number of flowers pollinated per visitor per approach to a plant (Gp) were measured in 16 plants in ACH during 2003. At the flowering peak, 17 censuses of 15 min were carried out on each plant between 0900 h and 1500 h. Simple linear regressions were performed between the number of flowers per plant and both the variables measured.
Most statistical analyses were carried out using the InfoStat statistical software (Di Rienzo et al., 2000). The equivalence test was performed using the R statistical software (R Development Core Team, 2006) and the Mantel test was performed with the Mantel software for Windows version 1·18 (Calvanti, 1988).
RESULTS
Pollinator assemblage composition and pollinator activity
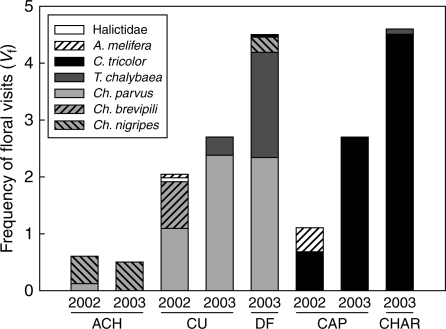
Seven bee species were observed visiting N. linariifolia flowers (Fig. 1). The main pollinators were solitary oil-collecting bees of the Apidae family accounting for 96·45 % of the floral visits (Figs 1 and 2). Apis mellifera, observed in the CU and CAP populations, and an unidentified species of Halictidae, observed exclusively in the CU population, were responsible for the remaining 4·55 %.
Fig. 1.
Mean number of visits per flower per hour (Vf), pollinator assemblage composition, and relative contribution of the pollinator species to the total number of visits recorded in five populations of N. linariifolia during two years. Populations are ordered by geographic proximity. Key: white for pollen-collecting bees; light grey and hatched grey for small- and medium-sized oil-collecting bees, respectively; dark grey and black for large-sized oil-collecting bees.
Fig. 2.
Oil-collecting bee species visiting the flowers of N. linariifolia: (A) the large-sized Tapinotaspis chalybaea sweeping the entire oil-producing area with the oil-collecting brushes on its long middle legs, with the anthers and stigma in contact with the ventral part of the thorax; (B) the small-sized Chalepogenus parvus collecting oil with its forelegs on a sector of the corolla without pollinating; (C) the medium-sized Ch. nigripes collecting oil with its forelegs, with the anthers and stigma in contact with its forehead; (D) the large-sized Centris tricolor seizing the stamens with its mandibles and sweeping a large area of the corolla with the foreleg oil-collectors; similarly to Ch. nigripes with the anthers and stigma in contact with the forehead.
A significant variation in the composition and relative abundance of pollinators was observed between populations. The number of pollinator species also varied between years (Fig. 1). Only one or two species of visitors accounted for >90 % of the total visits recorded in each population. The most frequent pollinator of each population did not vary between years (Fig. 1). These were Ch. parvus, C. tricolor and Ch. nigripes for CU, CAP and ACH, respectively (Figs 1 and 2).
The Mantel test revealed a geographic pattern of pollinator assemblage composition by associating assemblage dissimilarity and geographic distances between populations (Z = 0·695, P = 0·025). The populations located in the most arid region of the distribution range of N. linariifolia (CAP and CHAR) had the most similar pollinator assemblages, sharing C. tricolor bees as the most frequent pollinator. The most different pollinator assemblage was that of CU, where two non-oil-collecting bee species were present (Fig. 1).
The number of visits per flower per hour also varied significantly between populations (Fig. 1). The highest values of Vf were recorded in CHAR (2003) and DF (2003) and the lowest in the upland population of ACH (2002 and 2003). The multiple regression model carried out with the environmental factor and plant density explained a high proportion of the total variance of Vf (R2 = 0·97, P = 0·01, n = 5). The environmental factor was positive and significantly correlated with Vf (P = 0·0092), while mean plant density was only marginally correlated with this variable (P = 0·07). No relationship was recorded between Simpson's diversity index and Vf across populations and years (P > 0·05).
Pollen limitation by quantity
Test of assumptions
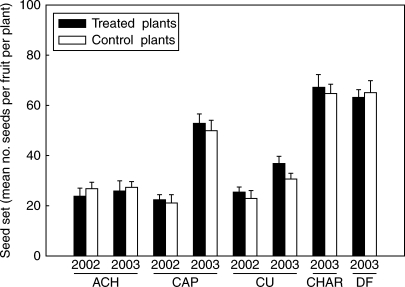
No significant differences were observed in the seed set of naturally pollinated flowers between control and treated plants (F1,20–60 = 0·09 – 2·83 n.s.; Fig. 3). In the equivalence test, the null hypothesis of dissimilarity was rejected in all cases except for CU population for the second year (Table 2). Since in this population, contrarily to the expectation of resource limitation, open-pollinated flowers of treated plants produced more seeds per fruit than open pollinated ones of control plants, a possible resource reallocation mechanism affecting open-pollinated flowers of treated plants could be disregarded.
Fig. 3.
Mean (± s.e) seed set following open-pollination in treated and control plants in N. linariifolia populations studied in 2002 and/or 2003. No significant differences were observed (Tuckey's test: P > 0·05 in all cases). Abbreviations as in Table 1.
Table 2.
Statistical summary of the equivalence tests for seed set following open-pollination in treated and control plants in N. linariifolia populations studied in 2002 and/or 2003
| Year | Population | Mdiff | s.d.diff | CI (diff) | CI (P) | P |
|---|---|---|---|---|---|---|
| 2002 | ACH | 3·16 | 3·90 | −3·43–9·75 | −8·67–15·00 | 0·002 |
| 2002 | CAP | –1·22 | 3·91 | −8·00–5·56 | −10·18–7·75 | 0·017 |
| 2002 | CU | –2·58 | 3·37 | −8·66–3·50 | −9·40–4·23 | 0·034 |
| 2003 | ACH | 1·33 | 3·79 | −5·02–7·60 | −3·02–6·50 | 0·001 |
| 2003 | CAP | –2·83 | 5·57 | −12·24–6·57 | −15·71–10·04 | 0·013 |
| 2003 | CU | –6·20 | 3·70 | −12·55–0·14 | −9·89–( −2·51) | 0·160 |
| 2003 | DF | 1·99 | 5·70 | −7·00–11·00 | −14·00–18·00 | 0·004 |
| 2003 | CHAR | –2·70 | 6·24 | −13·32–7·9 | −17·11–11·78 | 0·001 |
Mdiff = mean of the difference; s.d.diff = standard error of the difference; CI (diff) = the 1 – alpha confidence interval for the difference; CI (P) = the 1 – alpha confidence interval for the difference.
The slope of the relationship between number of seeds and fruit length did not differ between treatments (interaction term in ANCOVA, F1,254 =1·17, P = 0·31), indicating the absence of a compensatory mechanism, while the categorical term indicated a strong overall differences in seed set between treatments (F1,254 = 21·43, P < 0·0001).
Pollen limitation tests
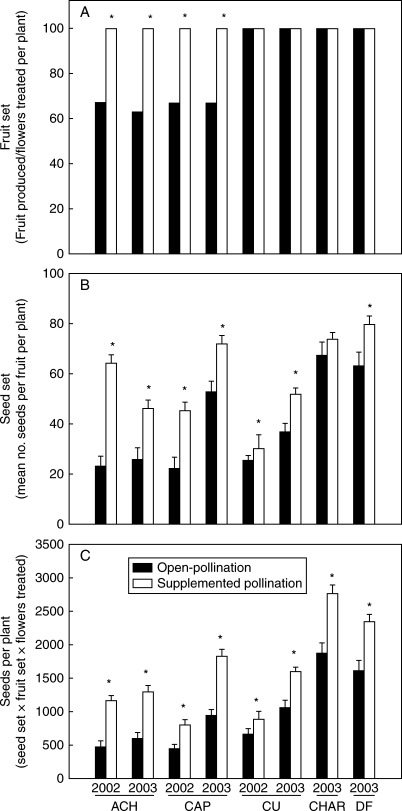
The supplemented pollination tests showed pollen limitation in fruit set in ACH and CAP (both years), but no pollen limitation was detected by this fitness measure in CU (both years), CHAR and DF (Fig. 4A). Pollen limitation was more acute in the seed set per fruit. Supplemented pollination produced a significantly higher number of seeds per fruit than open-pollinated flowers in all but one population (CHAR; Fig. 4B). The increase in seed set following supplemented pollination was variable, ranging from 19 % to >100 % (Fig. 4B). Seed production per plant was significantly higher after supplementation in all the populations studied (Fig. 4C).
Fig. 4.
Pollen limitation in N. linariifolia populations studied in 2002 and/or 2003. (A) Median fruit set, (B) mean (± s.e.) seed set and (C) mean (± s.e.) seed produced per plant. Asterisks indicate a significant increase in fruit, seed set or seed production with supplemented pollination (P < 0·05). Abbreviations as in Table 1.
Seed set and seed production per plant were significantly dependent on pollination treatment, population and year as shown by the results of a three-way ANOVA of the populations studied in both years (Table 3). Considering seed set per fruit, differences between pollination treatments varied between populations and years, as indicated by the marginally significant three-way interaction term of ANOVA (Table 3). On the contrary, the effects of the pollination treatments on seed production per plant only varied between years (as indicated by a significant F-value for the population × year interaction; Table 3). The results of the two-way ANOVA including all the populations showed significant effects of pollination treatment (F1,239 = 30·52, P < 0·0001; F1,175 = 105·72, P < 0·0001 ) and population (F4,239 = 27·82, P < 0·0001; F4,175 = 48·38, P < 0·0001) on both seed set and number of seeds per plant. Spatial and temporal components of variance accounted for 48 % and 19 % of the variance explained in the magnitude of pollen limitation, respectively. Intra-plant variation (i.e. coefficient of variation of the seed set) was highly variable among populations (grand mean 47·93 ± 9·36 s.d.). ACH and CAP were the populations with the highest values (mean CV = 60·56 ± 12·14 and 56·8 ± 10·81 in 2002 and 2003, respectively).
Table 3.
Three-way ANOVA of the effects of treatment (open vs. supplemented pollination), population and year (2002 vs. 2003) on mean seed set and seeds produced per plant
| Source of variation | d.f. | MS | F | P |
|---|---|---|---|---|
| Seed set | ||||
| Treatment | 1 | 10201·92 | 39·35 | <0·0001 |
| Population | 2 | 1269·37 | 4·90 | 0·0086 |
| Year | 1 | 3718·59 | 14·34 | 0·0002 |
| Treatment × population | 2 | 1045·43 | 4·03 | 0·0195 |
| Treatment × year | 1 | 246·88 | 0·95 | 0·3306 |
| Population × year | 2 | 5587·00 | 21·55 | <0·0001 |
| Treatment × population × year | 2 | 711·82 | 2·75 | 0·0672 |
| Error | 228 | 259·25 | ||
| Seeds produced per plant | ||||
| Treatment | 1 | 13721603·3 | 92·3 | <0·0001 |
| Population | 2 | 464111·23 | 3·12 | 0·0464 |
| Year | 1 | 10055979 | 67·64 | <0·0001 |
| Treatment × population | 2 | 319613·37 | 2·15 | 0·1193 |
| Treatment × year | 1 | 842435·91 | 5·67 | 0·0183 |
| Population × year | 2 | 1965146·22 | 13·22 | <0·0001 |
| Treatment × population × year | 2 | 342907·43 | 2·31 | 0·1024 |
| Error | 199 | 148661·31 |
The analysis included the three populations of N. linariifolia studied for 2 years.
Quantity vs. quality in pollen limitation
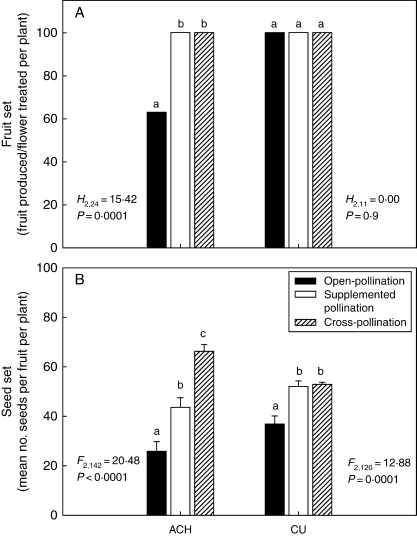
No differences in fruit set or seed set were observed between cross- and supplemented pollination in CU (Fig. 5A, B). In the ACH population, cross-pollination only provided a significantly higher yield than pollen supplementation in seed set (Fig. 5A, B). Thus, the quality of pollen limited seed set but not fruit set in ACH. This was as expected because only a small proportion of compatible pollen is required to initiate fruit formation.
Fig. 5.
Effect of pollen quantity and quality in female fitness of N. linariifolia. (A) Median fruit set and (B) mean (± s.e.) seed set, following open-pollination, pollen supplementation and cross-pollination in ACH and CU populations. Columns with different letters indicate significant intra-population differences (P < 0·05). Abbreviations as in Table 1.
Factors affecting pollen limitation
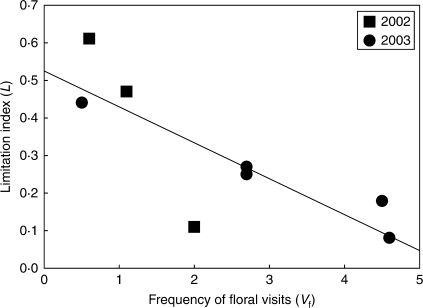
The multiple regression model obtained with Vf and mean plant density for the mean pollen limitation index explained a high proportion of the total variance (R2 = 0·90, P = 0·04, n = 5). The mean pollen limitation index was negatively and significantly correlated with Vf (P = 0·036) but not with mean plant density (P = 0·49). Natural seed set, which varied strongly among populations (F7,214 = 17·86, P = 0·001), was significantly explained by Vf (R2 = 0·87, P = 0·0008, n = 8) as was the magnitude of pollen limitation in seed set (Fig. 6). Differences in the magnitude of pollen limitation between populations were not associated with differences in assemblage composition among populations (Z = 24·363, P = 0·755, n = 5), nor Simpson's diversity index between populations and years (P = 0·582, n = 8).
Fig. 6.
Regression between mean number of visits per flower per hour (Vf) and pollen limitation index (L). L = 1 – (Po/Ps) where Po is the seed set of open-pollinated flowers and Ps is the seed set of supplemented flowers; a value of L = 0 indicates no pollen limitation. Regression equation y = 0·52 – 0·1x; R2 = 0·67; P < 0·001.
Floral display and pollinator visitations
The number of visits per plant was significantly explained by the number of flowers per plant (R2 = 0·91, P = 0·0001, n = 32), suggesting that floral display influenced pollinator attraction. Display size also had a significant effect on the number of flowers visited per plant per approach (i.e. geitonogamous pollination; R2 = 0·24, P = 0·038, n = 32).
DISCUSSION
Variation in pollinator assemblage composition
Variations in pollinator composition between populations of the same plant species have been reported previously (e.g. Herrera, 1988; Gómez and Zamora, 1999; Herrera, 2005; Nattero and Cocucci, 2007). However, these studies were mostly performed on generalized plant–pollinator systems. In the present study, significant variations in pollinator assemblage composition between populations were documented in a system regarded as one of the most specialized within angiosperms (Johnson and Steiner, 2000).
Considering the number of species visiting this plant at a geographical scale, the pollination system of Nierembergia linariifolia could be considered generalized (Waser et al., 1996; Knight et al., 2005). However, from a functional point of view (Fenster et al., 2004) this species is considered highly specialized, since different oil-collecting bee species have been recorded as the only or main pollinators in all populations. Therefore, at the geographical scale it is ecologically generalized and functionally specialized (Ollerton et al., 2007). Locally, the number of pollinator species interacting with N. linariifolia ranged between two and five, suggesting different levels of species-to-species specialization among populations. However, the facts that in all the populations studied only one species (or two, as in the DF population) accounted for >90 % of the floral visits, and that the most frequent pollinator varied among populations but not between years, suggest that N. linariifolia interacts with a local-specific pollinator species and that population differences may remain consistent across years. Therefore, this plant–pollinator system could be considered an evolutionary mosaic of locally specialized interactions. These results are in line with the idea that plant specialization is a local phenomenon rather than a trait of the species along its geographic range (Fox and Morrow, 1981; Thompson, 1994, 1999; Herrera, 2005).
Magnitude and extent of pollen limitation
As far as is known, this is the first study examining pollen limitation in a particularly highly specialized oil-rewarding species and among the few on pollen limitation in specialized pollination systems (but see Ackerman and Montalvo, 1990; Johnson and Bond, 1997; Baker et al., 2000). Two hypotheses have been proposed regarding pollen limitation in specialized pollination systems. On one hand, a corollary of Stebbins (1970) ‘most effective pollinator’ principle suggests that specialization may provide more reliable pollination with lower levels of pollen limitation. Alternatively, plants relying on specialized pollinators may experience greater variation in their pollination success because the abundance of these pollinators may fluctuate greatly in space or time and hence are more prone to pollen limitation (Neal et al., 1998; Knight et al., 2005). The present results support the second hypotheses as pollen limitation was detected in practically all the populations and years. Considering the populations studied in both seasons, 66 % (two out of three) of the populations experienced pollen limitation in fruit set whereas seed set and seed production per plant were consistently limited by pollen in all the populations in both years. On the other hand, considering the five populations studied during the second year, 40 % (two out of five) of the populations showed limitation in fruit set, 80 % (four out of five) experienced limitation in seed set and all the populations experienced pollen limitation regarding the number of seeds per plant. The fact that pollen limitation was more acute in seed set and seed number per plant than in fruit set suggests that in some populations enough pollen was transferred to the stigmas to enable fruit maturation, but not to ensure a full seed set.
Factors affecting pollen limitation
Quantifying the effect of pollinator abundance on pollen limitation has been a problem in specialized animal-pollinated plant species (Baker et al., 2000; Larson and Barrett, 2000; Moody-Weis et al., 2001). However, the present results suggest that the frequency of visitation to a great extent explains variations in natural seed set and the magnitude of pollen limitation. In CHAR, where the highest frequency of floral visits was recorded, neither fruit set nor seed set were pollen limited and pollen limitation was only detected when considering total seed production. Accordingly, in the ACH population that had the lowest frequency of visits, pollen limitation was detected in the three fitness measures considered. The present results also indicate that temperature was the main factor influencing pollinator abundance, with lower temperatures associated with a lower pollinator activity in agreement with previous studies (Roubik, 1989; Herrera, 1995; Buide, 2006). Thus, pollination success in ACH was noticeably inferior probably due to the low temperatures characteristic of this upland population.
As low plant density can reduce the number of compatible mates (Lamont et al., 1993; Ǻgren, 1996; Knight, 2003; Ashman et al. 2004) and decrease pollinator attraction (Jennersten, 1988; Kunin, 1997; Moody-Weis and Heywood, 2001; Waites and Ǻgren, 2004), to find some sort of relationship between plant density, magnitude of pollen limitation and frequency of floral visits was expected. However, although plant density slightly explained pollinator activity, it was not related to the mean pollen limitation index. In addition, it has been reported that pollen limitation between plant species decreased as the number of pollinating taxa increased, suggesting that higher levels of specialization are associated with higher levels of pollen limitation (Knight et al., 2005). However, in this study neither pollinator assemblage composition, nor the diversity of pollinating taxa, had any effect on the frequency of floral visits or on the pollen limitation index.
Relative magnitudes of quantity and quality limitation
Different results were obtained in the experiments performed to study pollen limitation by quality in two selected populations. Although low density would make the CU population more susceptible to insufficient pollen quality (Ramsey and Vaughton, 2000), no evidence was found that the quality of pollen limited reproduction. These results could be explained by the fact that this population showed a certain level of self-compatibility (Cosacov, 2004). In contrast, pollen quality limited seed set in the ACH population: 44 % of the total amount of pollen limitation was due to the quantity of pollen deposited on the stigmas, while 56 % was related to the quality of pollen. Thus, the magnitude of pollen limitation by quality was even more important than pollen limitation by quantity. This population presented the highest plant flowering density among the populations studied but is completely self-incompatible (i.e. hand self-pollination produced no seeds; Cosacov, 2004). Thus, some floral traits such as multiple flowers per plant and pollinator behaviour (i.e. flight distances and geitonogamous pollination) might be factors affecting the deposition of incompatible pollen. In fact, it was observed that the number of flowers visited on a plant per visit increases with floral display (i.e. number of flowers per plant), thus increasing self-pollination by geitonogamy. In addition, observations made in this population suggest that flight distances range between 0·5 m and 1·5 m, favouring cross-pollination between very close plants. As N. linariifolia has limited seed dispersal, a proportion of outcrossed matings may be accounted by movement among related individuals, hence causing the deposition of incompatible pollen (Loveless and Hamrick, 1984).
Evolutionary implications of pollen limitation
In addition to the among-population variation in pollen limitation, the fact that pollen limitation was evident in most of the populations studied and consistent between years, even in sites with a relatively high frequency of floral visits, may suggest that pollen limitation is a permanent condition for the studied species. Burd's model of ovule packaging (Burd, 1995) provides an adaptive explanation to flower and ovule overproduction. The author predicted that species experiencing unpredictably fluctuating pollinator environments might evolve overproduction of flowers and/or ovules as a bet-hedging mechanism to allow them to take advantage of highly successful individual flowers when the among-flower variance in pollination is high or during years with high pollinator activity. Thus, a higher response to experimental pollen supplementation should occur in species experiencing greater variance in pollen acquisition (Ashman et al., 2004). Since N. linariifolia plants rely on a reduced group of pollinators and pollinator activity varies between sites and years, it is bound to experience unpredictable fluctuating pollinator environments as other plants with specialized pollination systems do (Johnson and Steiner, 2000). In fact, the variance in seed production was high at intra-plant, inter-plant and inter-population levels in N. linariifolia, and a strong response to supplementation was detected. In addition, it was observed that populations with highly fluctuating fertilization success (higher intra-plant coefficient of variation of seed set) also presented a higher response to experimental pollen supplementation. Furthermore, under the bet-hedging hypothesis the degree of pollen limitation would be expected to vary in relation to pollinator activity. Hence, the high explanatory power of the frequency of floral visits on natural seed set and pollen limitation index further supports this hypothesis. Additional evidence in this line is the fact that N. linariifolia fruits with very few seeds (i.e. three or four seed per fruit) do mature despite their potential for an average of 63·2 seeds per fruit. Thus, the absence of selective abortion of few-seeded fruits could be an adaptation to low levels of pollinator activity, suggesting a history of unpredictable pollinator environments as previously proposed in other plant–pollinator systems (Moody-Weis and Heywood, 2001).
Larger floral display would be adaptive under the bet-hedging hypotheses, as it was also demonstrated that plants with larger floral display were visited more frequently. However, a larger floral display also increases geitonogamous pollination and therefore increases the quality component of pollen limitation.
These results suggest that the quandary between limitation by quantity and quality could actually comprise a selective force to achieve the optimum number of flowers per plant that maximizes the attraction to pollinators and minimizes geitonogamous visits. An alternative way to solve this dilemma could be an increase in flower size which would promote pollinator attraction (increasing the individual's display) but would have no cost to reproduction as it does not favour geitonogamy. This hypothesis must be tested in studies combining phenotypic selection with both components of pollen limitation, the latter being essential to an appropriate understanding of the ecological significance and evolutionary implications of selection patterns on floral traits.
Concluding remarks
The great inter-population variation observed in pollinator identity and activity, and in the magnitude of pollen limitation suggests that these different scenarios may entail different evolutionary outcomes (e.g. Thompson, 1994, 1999). In this regard, spatial variation in pollen limitation was greater than temporal variation within populations, indicating that different ecological contexts produced greater variations in the reproductive consequences than stochastic interannual intra-population fluctuations. Given that pollen limitation is a prerequisite for the occurrence of phenotypic selection, pollinator activity affects plant fitness, and the biotic and abiotic contexts differed among populations, the present results reinforce the idea that a mosaic of different selective processes on floral evolution and plant–pollinator interaction may be occurring along the geographic range of N. linariifolia.
ACKNOWLEDGEMENTS
We thank Alicia N. Sérsic, Santiago Benitez-Vieyra and Marcela Moré for their useful comments in previous versions of this manuscript, Francisco Córdoba for his field assistance and Catriona Kirkwood for her linguistic advice. We especially thank the anonymous reviewers for very helpful comments on this paper. We thank Doctorate in Biology, University of Cordoba. This study was funded by the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 5174), the Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (197/05) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 01-33755).
LITERATURE CITED
- Ackerman JD, Montalvo AM. Short-and long-termed limitations to fruit production in a tropical orchid. Ecology. 1990;71:263–272. [Google Scholar]
- Ǻgren J. Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria. Ecology. 1996;77:1779–1790. [Google Scholar]
- Aizen M, Harder LD. Expanding the limits of the pollen-limitation concept: efects of pollen quantity and quality. Ecology. 2007;88:271–281. doi: 10.1890/06-1017. [DOI] [PubMed] [Google Scholar]
- Alonso C, Mutikainen P, Herrera CM. Ecological context of breeding system variation: sex, size and pollination in a (predominantly) gynodioecious shrub. Annals of Botany. 2007;100:1547–1556. doi: 10.1093/aob/mcm254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell D, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Baker AM, Barrett SCH, Thompson JD. Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae) Oecologia. 2000;124:529–535. doi: 10.1007/s004420000417. [DOI] [PubMed] [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–369. [Google Scholar]
- Buide ML. Pollination ecology of Silene acutifolia (Caryophyllaceae): floral traits variation and pollinator attraction. Annals of Botany. 2006;97:289–297. doi: 10.1093/aob/mcj032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M. Ovule packaging in stochastic pollination and fertilization environments. Evolution. 1995;49:100–109. doi: 10.1111/j.1558-5646.1995.tb05962.x. [DOI] [PubMed] [Google Scholar]
- Cabrera AL. Fitogeografía de la República Argentina. Buenos Aires: Sociedad Argentina de Botánica; 1971. [Google Scholar]
- Calvanti MJ. Mantel for Windows version 1·18. Test for Association between Two Symmetric Distance Matrices with Permutation Iterations. 1988. http://life.bio.sunysb.edu/morph/soft-mult.html. (4 July 2008)
- Cocucci AA. Polinización en Nierembergia hippomanica (Solanaceae) Kurtziana. 1984;17:31–47. [Google Scholar]
- Cocucci AA. Pollination biology of Nierembergia (Solanaceae) Plant Systematics and Evolution. 1991;174:17–35. [Google Scholar]
- Cocucci AA, Vogel S. Oil producing species of Sisyrinchium (Iridaceae) and their pollination by oil-collecting bees in subtropical South America. Flora. 2001;196:26–46. [Google Scholar]
- Cocucci AA, Sérsic AN, Roig-Alsina A. Oil-collecting structures in Tapinotaspidini: their diversity, function and probable origin (Hymenoptera: Apidae) Mitteilungen der Münchner Entomologischen Gesellschaft. 2000;90:51–74. [Google Scholar]
- Cosacov A. Sistema reproductivo y limitación de polen en Nierembergia linariaefolia. Argentina: University of Cordoba; 2004. Graduation Thesis. [Google Scholar]
- De Fina AL. Aptitud agroclimática de la República Argentina. Buenos Aires: Academia Nacional de Agronomía y Veterinaria; 1992. [Google Scholar]
- Di Rienzo J, Robledo W, Casanoves F, Balzarini M. InfoStat, Software estadístico. Córdoba: Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba; 2000. [Google Scholar]
- Dixon MP, Pechmann JHK. A statistical test to show negligible trend. Ecology. 2005;86:1751–1756. [Google Scholar]
- Fenster C, Armbruster W, Willson P, Dudash M, Thomson J. Pollination syndromes and floral specialization. Annual Review of Ecology and Systematics. 2004;35:375–403. [Google Scholar]
- Fox LR, Morrow PA. Specialization: species property or local phenomenon? Science. 1981;211:887–893. doi: 10.1126/science.211.4485.887. [DOI] [PubMed] [Google Scholar]
- Garrett KA. Use of statistical tests of equivalence (bioequivalence tests) in plant pathology. Phytopathology. 1997;87:372–374. doi: 10.1094/PHYTO.1997.87.4.372. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Zamora R. Generalization vs. specialization in the pollination system of Hormathophyla spinosa (Cruciferae) Ecology. 1999;80:796–805. [Google Scholar]
- Gomes Teixeira LA, Machado IC. Sistema de polinização e reprodução de Byrsonima sericea Dc (Malpighiaceae) Acta Botanica Brasílica. 2000;14:347–357. [Google Scholar]
- Gould SJ, Johnston RF. Geographic variation. Annual Review of Ecology and Systematics. 1972;3:115–151. [Google Scholar]
- Haig D, Westoby M. On limits to seed production. The American Naturalist. 1988;131:757–759. [Google Scholar]
- Herrera CM. Variation in mutualism: the spatiotemporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society. 1988;35:95–125. [Google Scholar]
- Herrera CM. Microclimate and individual variation in pollinators: flowering plants are more than their flowers. Ecology. 1995;76:1516–1524. [Google Scholar]
- Herrera CM. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman and Hall; 1996. pp. 65–87. [Google Scholar]
- Herrera CM. Plant generalization on pollinators: species property or local phenomenon? American Journal of Botany. 2005;92:13–20. doi: 10.3732/ajb.92.1.13. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 278–294. [Google Scholar]
- Jennersten O. Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conservation Biology. 1988;2:359–366. [Google Scholar]
- Johnson SD, Bond WJ. Evidence for widespread pollen limitation of fruiting success in Cape wildflowers. Oecologia. 1997;109:530–534. doi: 10.1007/s004420050113. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution. 2000;15:140–143. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- Kay KM, Schemske DW. Geographic patterns in plant-pollinator mutualistic networks: comment. Ecology. 2004;85:875–878. [Google Scholar]
- Knight TM. Floral density, pollen limitation, and reproductive success in Trillium gradiflorum. Oecologia. 2003;137:557–563. doi: 10.1007/s00442-003-1371-8. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution, and Systematics. 2005;36:467–497. [Google Scholar]
- Kunin WE. Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. Journal of Ecology. 1997;85:225–234. [Google Scholar]
- Lamont BB, Klinkhamer PGL, Witkowski TF. Population fragmentation may reduce fertiliy to zero in Banksia goodii – a demonstration of the allee effect. Oecologia. 1993;94:446–450. doi: 10.1007/BF00317122. [DOI] [PubMed] [Google Scholar]
- Larson BMH, Barrett SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- Loveless MD, Hamrick JL. Ecological determinants of genetic structure in plant populations. Annual Review of Ecology and Systematics. 1984;15:65–95. [Google Scholar]
- Machado IC. Oil-collecting bees and related plants: a review of the studies in the last twenty years and case histories of plants occurring in NE Brazil. In: Freitas M, Pereira JOP, editors. Solitary bees, conservation, rearing and management for pollination: a contribution to the International Workshop on Solitary Bees and their Role in Pollination. Fortaleza: Imprensa Universitaria; 2004. pp. 252–282. [Google Scholar]
- Moody-Weis MJ, Heywood JS. Pollination limitation to reproductive success in the Missouri evening primrose Oenothera macrocarpa (Onagraceae) American Journal of Botany. 2001;88:1615–1622. [PubMed] [Google Scholar]
- Nattero J, Cocucci AA. Geographic variation in floral traits of the tree tobacco in relation to its hummingbird pollinator fauna. Biological Journal of the Linnean Society. 2007;90:657–667. [Google Scholar]
- Neal PR, Dafni A, Giurfa M. Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annual Review in Ecology and Systematics. 1998;29:345–373. [Google Scholar]
- Neff JL, Simpson BB. Other rewards: oils, resins, and gums. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Ontario: Enviroquest; 2005. pp. 314–328. [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon. 2007;56:717–728. [Google Scholar]
- Pauw A. Inversostyly: a new stylar polymorphism in an oil-secreting plant, Hemimeris racemosa (Scrophulariaceae) American Journal of Botany. 2005;92:1878–1886. doi: 10.3732/ajb.92.11.1878. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. Vienna: R Foundation for Statistical Computing; 2006. R: a language and environment for statistical computing. http://www.R-project.org. (accessed 4 July 2008) [Google Scholar]
- Ramsey M, Vaughton G. Pollen quality limits seed set in Burchardia umbellata (Colchicaceae) American Journal of Botany. 2000;87:845–852. [PubMed] [Google Scholar]
- Roubik DW. Ecology and natural history of tropical bees. New York, NY: Cambridge University press; 1989. [DOI] [PubMed] [Google Scholar]
- Roig-Alsina A. Revisión de las abejas colectoras de aceites del género Chalepogenus Holmberg (Hymenoptera, Apidae, Tapinotaspidini) Revista del Museo Argentino de Ciencias Naturales. 1999;1:67–101. [Google Scholar]
- Roig-Alsina A. Claves para las especies argentinas de Centris (Hymenoptera, Apidae) con descripción de nuevas especies y notas sobre su distribución. Revista del Museo Argentino de Ciencias Naturales. 2000;2:171–193. [Google Scholar]
- Sahli HF, Conner JK. Characterizing ecological generalization in plant-pollination systems. Oecologia. 2006;148:365–372. doi: 10.1007/s00442-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Horvitz CC. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science. 1984;225:519–521. doi: 10.1126/science.225.4661.519. [DOI] [PubMed] [Google Scholar]
- Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Journal of Pharmacokinetics Biopharmaceutics. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Simpson EH. Measurements of diversity. Nature. 1949;163:688. [Google Scholar]
- Simpson BB, Neff JL. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York, NY: Nostrand Reinhold; 1983. pp. 142–159. [Google Scholar]
- Snow AA, Spira TP, Simpson R, Klips RA. The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall; 1996. pp. 191–216. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York, NY: Freeman; 1995. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characters in Angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Steiner KE, Whitehead VB. Oil flowers and oil bees: further evidence for pollinator adaptation. Evolution. 1991;45:1493–1501. doi: 10.1111/j.1558-5646.1991.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Steiner KE, Whitehead VB. Oil secretion and the pollination of Colpias mollis (Scrophulariaceae) Plant Systematics and Evolution. 2002;235:53–66. [Google Scholar]
- Thompson JN. The geographic mosaic of evolving interactions. In: Leather SR, Watt AD, Mill NJ, Walters KFA, editors. Individuals, populations and patterns in ecology. Andover, UK: Intercept Press; 1994. pp. 419–431. [Google Scholar]
- Thompson JN. The evolution of species interactions. Science. 1999;284:2116–2118. doi: 10.1126/science.284.5423.2116. [DOI] [PubMed] [Google Scholar]
- Totland Ø, Andersen HL, Bjelland T, Dahl V, Eide W, Houge S, et al. Variation in pollen limitation among plants and phenotypic selection on floral traits in an early-spring flowering herb. Oikos. 1998;82:491–501. [Google Scholar]
- Vogel S. History of the Malpighiaceae in the light of pollination ecology. Memoirs of the New York Botanical Garden. 1990;55:130–142. [Google Scholar]
- Vogel S, Machado IC. Pollination of four sympatric species of Angelonia (Scrophulariaceae) by oil-collecting bees in Ne Brazil. Plant Systematics and Evolution. 1991;178:153–178. [Google Scholar]
- Waites AR, Ǻgren J. Pollinator visitation, stigmatic pollen loads and among-population variation in seed set in Lythrum salicaria. Journal of Ecology. 2004;92:512–526. [Google Scholar]
- Waser NM, Chitka L, Price MV, Williams NM, Ollerson J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Westlake WJ. Symmetrical confidence intervals for bioequivalence trials. Biometrics. 1976;32:741–744. [PubMed] [Google Scholar]
- Zimmerman JK, Aide TM. Patterns of fruit production in a Neotropical orchid: pollinator vs. resource limitation. American Journal of Botany. 1989;76:67–73. [Google Scholar]
- Zimmerman M, Pyke GH. Reproduction in Polemonium: assessing the factors limiting seed set. The American Naturalist. 1988;131:723–738. [Google Scholar]