Abstract
Background and Aims
Until recently, there was no consensus regarding the phylogenetic relationships of the Neotropical orchid genera Scuticaria Lindl. and Dichaea Lindl. However, recent evidence derived from both gross morphological and molecular studies supports the inclusion of Scuticaria and Dichaea in sub-tribes Maxillariinae and Zygopetalinae, respectively. The present paper describes the labellar micromorphology of both genera and seeks to establish whether labellar characters support the assignment of Scuticaria and Dichaea to these sub-tribes.
Methods
The labella of four species of Scuticaria and 14 species of Dichaea were examined using light microscopy and scanning electron microscopy, and their micromorphology was compared with that of representative species of Maxillariinae sensu lato and Zygopetalinae (Huntleya clade).
Key Results and Conclusions
In most specimens of Scuticaria examined, the papillose labella bear uniseriate, multicellular, unbranched trichomes. However, in S. steelii (Lindl.) Lindl., branched hairs may also be present and some trichomes may fragment and form pseudopollen. Multicellular, leaf-like scales were also present in one species of Scuticaria. Similar, unbranched hairs are present in certain species of Maxillaria Ruiz & Pav. (Maxillariinae sensu stricto) and Chaubardia Rchb.f. (Huntleya clade). As yet, moniliform, pseudopollen-forming hairs have not been observed for Zygopetalinae, but their presence in Scuticaria steelii, Maxillaria and Heterotaxis Lindl. supports the placing of Scuticaria in Maxillariinae. As other genera are sampled, the presence of branched hairs, hitherto unknown for Maxillariinae sensu lato, may prove to be a useful character in taxonomy and phylogenetic studies. Euglossophily occurs in Dichaea, as well as Chondrorhyncha Lindl. and Pescatorea Rchb.f. (Huntleya clade), and all three genera tend to lack distinctive labellar features. Instead, lip micromorphology is relatively simple and glabrous or papillose. However, two of the Dichaea species examined bear unicellular, labellar trichomes very similar to those found in Bifrenaria Lindl. (pollinated by both euglossine bees and Bombus spp.), and this feature may have arisen by convergence in response to similar pollination pressures.
Key words: Bifrenaria, Bifrenaria clade, Chaubardia, Chondrorhyncha, Dichaea, Dichaeinae, Heterotaxis, Huntleya clade, Huntleyinae, labellum, Maxillaria, Maxillariinae, papillae, Pescatorea, scales, Scuticaria, trichomes, Zygopetalinae
INTRODUCTION
The Neotropical orchid genera Scuticaria Lindl. and Dichaea Lindl. are assigned to the tribe Maxillarieae Pfitzer. This tribe, as recognized by Dressler (1990, 1993), contains, with the exception of Vandeae Lindl. and Polystachyeae Pfitzer, all the vandoid orchids with four pollinia. These, he assigned, on morphological grounds, to eight sub-tribes, namely Corallorhizinae Camus, Bergan & Camus, Zygopetalinae Schltr., Bifrenariinae Dressler, Lycastinae Schltr., Maxillariinae Benth., Dichaeinae Schltr., Telipogoninae Schltr. and Ornithocephalinae Schltr. Thus, Maxillarieae, as here circumscribed, is primarily American, although Corallorhizinae, considered to be the least advanced sub-tribe, also occurs in Eurasia. Plesiomorphic representatives of Maxillarieae are cormous with plicate leaves, whereas the most derived exhibit great vegetative diversity. Consequently, some taxa have corms with several internodes and plicate leaves, others possess pseudobulbs of one internode and conduplicate leaves, and still others display monopodial growth and cylindrical or laterally flattened leaves. Inflorescences are one- to many-flowered, but floral structure is more conservative than vegetative morphology, with some representatives having floral spurs.
Unfortunately, the exact position of Scuticaria and Dichaea within the tribe proved problematical and, until recently, despite numerous taxonomic revisions based solely on gross morphology, their phylogenetic relationships remained unresolved (Dressler, 1990, 1993; Brieger et al., 1994, 1995, 1996, 2000; Szlachetko, 1995).
Scuticaria is a small epiphytic genus of some nine species (Bennett and Christenson, 2002) found mainly in Brazil but also occurring in Peru, Ecuador, Colombia, Guyana and Venezuela. It is characterized by a very short, unifoliate stem with sulcate, contiguous, sub-terete to terete leaves. The inflorescence is lateral and one-flowered, and the flowers are relatively large and showy. The tepals are free, sub-similar, erect-spreading and the lateral sepals adnate to the column-foot, forming a prominent mentum. The labellum is sessile and attached to the column-foot, broad, concave, articulate and deeply three-lobed, the lateral lobes large and erect, whereas the mid-lobe is smaller. The column is erect, fleshy and semi-terete, lacks wings and extends basally forming a foot. The pollinarium consists of four pollinia, a tegular stipe and a viscidium; features it shares with both Zygopetalinae and Maxillariinae including Bifrenaria Lindl., Mormolyca Fenzl, Xylobium Lindl., certain species of Maxillaria Ruiz & Pav., as well as Trigonidium acuminatum Batem. ex Lindl. (Singer and Koehler, 2004). In common with Bifrenaria and Rudolfiella aurantiaca (Lindl.) Hoehne, the tegula of Scuticaria hadwenii Hort. ex. Hook is short and bifurcate whereas, in contrast to other species having this type of pollinarium, the viscidium is arcuate (Singer and Koehler, 2004). On account of its morphology, Dressler (1990), and later Brieger et al. (1994) and Szlachetko (1995), assigned Scuticaria to Maxillariinae sensu stricto – a sub-tribe characterized by pseudobulbs of a single internode with slender, short or elongate stem, distichous or secondarily spiral, duplicate and articulate leaves and lateral, one-flowered inflorescences. The small to large flowers usually have a hinged labellum and this may form a saccate mentum or distinct spur with the column-foot. The column is slender or short and the stigma entire. The operculate, terminal anther has reduced partitions, whereas the four pollinia are superposed with a viscidium and a more or less well developed stipe. However, by 1993, Dressler had reconsidered his earlier placing of Scuticaria and transferred it to Zygopetalinae.
Dichaea is a larger genus comprising approx. 111 epiphytic or lithophytic species found at high elevations in tropical and sub-tropical America, from Mexico to Brazil, where they often form large clumps or mats (Whitten et al., 2005). It has the following features: stems leafy, elongate, erect or pendulous, lacking pseudobulbs and concealed by imbricate leaf-sheaths. Leaves distichous, ascending to spreading or reflexed and coriaceous to membranous. Inflorescence axillary, leaf-opposed, one-flowered. Small, fleshy flowers subtended by an orbicular bract and a smaller, linear bracteole. Sepals free, sub-equal, spreading or connivent, the lateral sepals oblique and often forming an obscure mentum with the short column-foot. Petals are similar to sepals but often smaller and narrower. Labellum very fleshy, claw present or not, simple or lobed and continuous with base of column. Column stout, semi-terete, wingless or with a fleshy keel on each side at the base, and often with an obsolete foot with a glabrous or pubescent stigmatic ligule present on the ventral surface. Anther terminal, operculate, incumbent with reduced partitions and four waxy pollinia with a distinct viscidium and stipe. Capsule ellipsoid to obovoid, smooth or densely muricate (Ames and Correll, 1985). On the basis of its unique combination of characters, Dressler (1990), who had initially placed this genus in a sub-tribe of its own, Dichaeinae, within Maxillarieae, now assigned it to an informal clade within a more broadly circumscribed Zygopetalinae (Dressler, 1993), and was soon followed by Szlachetko (1995), who, likewise, assigned Dichaeinae to Zygopetalinae sensu lato. Dressler (1990), remarking on similarities between Dichaea and the Chondrorhyncha Lindl. complex, stated that Dichaea may be derived from Zygopetalinae-like ancestors and that the seemingly monopodial habit may in fact be due to extreme sympodial branching with each leaf-opposed inflorescence terminal on a stem of one internode. Indeed, despite its unusually long, pseudobulb-less, monopodial stems and warty or spiny capsules, flower and pollinarium structure in Dichaea is consistent with that of other Zygopetalinae (Whitten et al., 2000). Zygopetalinae sensu lato thus became divided on morphological grounds into several groups recognized either as separate sub-tribes (Huntleyinae, Zygopetalinae, Warreinae, Dichaeinae; e.g. Szlachetko, 1995) or as informal clades (e.g. Dressler, 1993).
Recently, however, molecular techniques have provided a powerful tool for investigating the phylogenetic relationships of orchids (e.g. Cameron et al., 1999; Whitten et al., 2000, 2005, 2007; Gravendeel et al., 2001; Pridgeon et al., 2001; Williams et al., 2001; Koehler et al., 2002; van den Berg et al., 2005; Dathe and Dietrich, 2006) and results, on occasion, have necessitated extensive taxonomic revision. Unfortunately, cladistic parsimony analyses of rbcL nucleotide sequence data alone were not sufficient to determine the status of Zygopetalinae (Cameron et al., 1999). Nevertheless, parsimony analyses of combined nuclear, ribosomal and plastid DNA sequence data of ITS (internal transcribed spacers) 1 and 2, matK, the trnL intron and the trnL–F intergene spacer (Whitten et al., 2000) strongly supported Zygopetalinae (including Dichaea and Cryptarrhena R. Br.). Parsimony analyses of combined DNA sequence data (Whitten et al., 2005) also produced highly resolved cladograms and supported Zygopetalinae as monophyletic, comprising a Zygopetalum clade (prominent pseudobulbs with leaves usually plicate, revolute); a Huntleya clade (pseudobulbs reduced or lacking, leaves conduplicate), including Dichaea, Huntleya Batem. ex. Lindl., Chaubardia Rchb.f. and the Chondrorhyncha complex, plus Cryptarrhena; and a Warrea clade. Whitten and co-workers (2000, 2005) thus favour the recognition of a more broadly defined Zygopetalinae. Vegetative and floral diversity, however, exacerbate the difficulties associated with the identification of synapomorphies that define Zygopetalinae sensu lato, although perhaps the most obvious features are the combination of four, flattened, superposed pollinia, a transversely narrow and slit-like stigma and an infrastigmatic keel to the column (Chondrorhyncha spp.), a tooth (Kefersteinia Rchb.f.) often basal (Cryptarrhena, Pescatorea Rchb.f., Warscewiczella Rchb.f.) or a ligule (Dichaea). Dichaea, however, differs in that it has a rounded stigma and variable pollinia (Whitten et al., 2000, 2005). Other features possibly characteristic of Zygopetalinae sensu lato are violet (not purple) floral pigmentation and the tendency to occupy shady, sub-optimal niches in forest canopies, often resulting in the formation of epidermal transformations (Whitten et al., 2005). Molecular analysis also demonstrated that Dichaea is sister to Huntleyinae, including Chaubardia, Chondrorhyncha and Pescatorea (Whitten et al., 2000), whereas Whitten et al. (2005) showed that Dichaea, Huntleya and Chaubardia are ‘highly supported as monophyletic on long branches’ and ‘are successively basal with strong support to the remaining taxa of the Huntleya clade comprising the Chondrorhyncha complex’. Characteristics of the Huntleya clade are the lack of pseudobulbs or the presence of very small pseudobulbs with conduplicate leaves. Furthermore, all members except Cryptarrhena are united in their possession of two apical bracts on the peduncle, the basal, adaxial bract enveloping the pedicel, ovary and inner bract while the smaller, apical, ligulate bract projects beneath the flower abaxial to the lip (Whitten et al., 2005).
Similarly, several authors (Whitten et al., 2000; Chase et al., 2003; Chase, 2005) favour a more broadly circumscribed Maxillariinae containing a number of taxa formerly recognized as sub-tribes by Dressler. These include Lycastinae, Maxillariinae sensu stricto and Bifrenariinae (including Scuticaria), as well as Xylobium, and this consensus is largely strongly supported by SW bootstrap analysis. Thus, whereas Dressler (1993), on morphological grounds, included Scuticaria in Zygopetalinae, and Brieger et al. (1994) and Szlachetko (1995) transferred it to Maxillariinae sensu stricto, molecular data (Whitten et al., 2000) support its inclusion in the Bifrenaria clade of Maxillariinae sensu lato (100 % bootstrap support). The placing of the Bifrenaria alliance in a more broadly circumscribed Maxillariinae is also supported by a number of other studies (e.g. Koehler et al., 2002; Chase et al., 2003; Chase, 2005; Whitten et al., 2007). Despite the anomalous terete, whip-like leaves of Scuticaria, its transfer to the Bifrenaria clade of Maxillariinae sensu lato is further supported in that flowers and pollinaria of some Scuticaria spp. are similar to those of Rudolfiella Hoehne. Furthermore, an inflorescence bearing few flowers is a synapomorphy shared by Scuticaria and Bifrenaria (Whitten et al., 2000). However, Koehler et al. (2002), who investigated the monophyly and phylogenetic relationships of the Bifrenaria complex by means of morphology and sequence data from nuclear and rDNA ITS and the chloroplast trnL–trnF region, also tested two species of Scuticaria and concluded that morphological and molecular studies involving more species of Scuticaria would be necessary for a complete understanding of phylogenetic relationships and character evolution within the Bifrenaria alliance.
There is no doubt that modern molecular techniques used in conjunction with more traditional methods such as morphology have resulted in a better understanding of orchid phylogeny and enabled the assignment of individual taxa to particular sub-tribes with a greater degree of confidence than was previously possible. As a result of these complementary approaches, the current view is that Scuticaria and Dichaea belong in Maxillariinae sensu lato and Zygopetalinae sensu lato, respectively.
Pollination of Scuticaria and Dichaea has rarely been studied. It is said that Scuticaria steelii is fragrant by morning, and Braga (1977) reports that it is pollinated by euglossine bees. Indeed, when presented with the fragrance of S. steelii, Euglossa stilbonata, bearing a pollinarium of this species, was attracted to the sample. However, this insect was considered too small to bring about effective pollination. Likewise, Dressler reported the pollination of Dichaea by male euglossine bees and, on a number of occasions, observed the pollinaria of D. panamensis on the clypeus of these insects. Pollinaria of D. panamensis have also been recorded on Euglossa allosticta, E. cyanaspis, E. despecta, E. dissimula, E. dressleri, E. heterosticta, E. mixta, E. tridentata, E. variabilis and Eufriesia pulchra. Moreover, pollinia of unidentified species of Dichaea have been reported for Euglossa allosticta, E. deceptrix and E. hansonii (Ackerman, 1983; Roubik and Ackerman, 1987). Folsom (1985, 1994), who studied pollination of Dichaea in Costa Rica and Colombia, also reported the pollination of this genus by Eulaema meriana. By staining the pollinia of D. potamophila Folsom, a species with large viscidia, and placing the plants at set points along the branches of a main tree in a cluster of chiparo trees (Pithecellobium Mart. sp.), he was able to record pollinator visits and the transfer of pollinia from flower to flower. Synchronized flowering occurs in this orchid and it emits a musty-sweet fragrance by day, yet closes late in the afternoon. Even after emasculation, flowers continued to emit fragrance and were pollinated in the morning by bees. These frequently arrived with several pollinia attached to the frons and, after flying in large circles (>5 m diameter) and hovering at flower height, 1–2 m downwind, they landed on the apical half of the labellum, climbed over it and scratched the base of the lip. Continuing to face the flower, the bees then hovered a few centimetres from it while transferring the collected fragrant compound to the hind tibia. Further landings often followed and, after 2–5 min, the pollinaria had collapsed forward and were ideally positioned to effect pollination. On occasion, self-pollination was observed, and in Puerto Rico D. hystricina is reportedly autogamous.
Since both Scuticaria (Braga, 1977; van der Cingel, 2001) and Dichaea (Folsom, 1985; Roubik and Ackerman, 1987; van der Cingel, 2001) are said to be pollinated by euglossine bees, it is reasonable to suppose that they are likely to have similar labellar micromorphology. Here, the labellar micromorphology of Scuticaria (currently assigned to Maxillariinae sensu lato and said to be euglossophilous) is compared with that of certain species assigned to the Bifrenaria clade (pollinated by euglossine bees and bumble-bees), Xylobium (pollinated by stingless bees – Meliponini) and Maxillariinae sensu stricto (largely pollinated by Meliponini), whereas the labellar micromorphology of Dichaea (currently assigned to Zygopetalinae and euglossophilous) is compared with that of euglossine-pollinated representatives of the Huntleya clade, in an attempt to determine the extent to which labellar structure is influenced by pollinator and/or phylogeny.
MATERIALS AND METHODS
Nine spirit-preserved specimens, representing four species of Scuticaria Lindl., and 38 spirit-preserved specimens, representing 14 species of Dichaea Lindl., were obtained from the herbarium of the Royal Botanic Gardens, Kew (Table 1). Their accession numbers are prefixed ‘K’. Further taxa for comparison were obtained from the same source. These included species of Chaubardia Rchb.f., Chondrorhyncha Lindl. and Pescatorea Rchb.f., all members of the Huntleya clade (Table 2). The names by which the specimens were originally collected are retained, but recent changes in nomenclature are noted. The authorities for plant names follow Brummit and Powell (1992). Specimens, originally stored in Kew mix [53 % ethanol (industrial methylated spirit), 37 % water, 5 % formaldehyde solution, 5 % glycerol], were transferred to and kept in Copenhagen mix [70 % ethanol (industrial methylated spirit), 28 % water, 2 % glycerol] for the duration of the study.
Table 1.
Specimens of Scuticaria and Dichaea species studied and their provenance
| Taxon | Accession no. | Collector | Provenance | Date of collection or donation | Notes |
|---|---|---|---|---|---|
| Scuticaria adverna | K57975 | Harley, R. M. et. al. (H50616) | Brazil, Bahia, Mun. Abaira. | 1992 | S. bahiensis (Davies and Stpiczyńska, 2008) |
| S. hadwenii (Lindl.) Hoehne | K12635 | 1931 | Purchased from Armstrong and Brown, Tunbridge Wells. | ||
| S. hadwenii (Lindl.) Hoehne | K73914 | 2005 | |||
| S. mooreana C. Schweinf. | K26282 | 1964 | Donated, Oddy, J. R; syn S. salesiana Dressler | ||
| S. steelii (Lindl.) Lindl. | K6903 | Guyana | Donated, Thompson, A., Mason, L. M. No. 1091 | ||
| S. steelii (Lindl.) Lindl. | K13811 | Brazil, Amazon region | 1948 | Donated, Garnett, C.S. | |
| S. steelii (Lindl.) Lindl. | K13812 | British Guiana | 1957 | Donated, Thompson, A. D. | |
| S. steelii (Lindl.) Lindl. | K14398 | 1950 | Donated, Garnett, C. S. | ||
| S. steelii (Lindl.) Lindl. | K37526 | Leppard, M. J. | Brazil | ||
| Dichaea brachypoda Rchb.f. | K8267 | Costa Rica | 1937 | Donated, Lankester, C. H. | |
| D. brachypoda Rchb.f. | K8268 | Costa Rica | 1930 | Donated, Lankester, C. H. | |
| D. brachypoda Rchb.f. | K8269 | Costa Rica | 1939 | Donated, Lankester, C. H. | |
| D. brachypoda Rchb.f. | K10257 | 1942 | Donated, Colman, J. | ||
| D. ciliolata Rolfe | K32353 | Mason, L. M. (2316) | Costa Rica | ||
| D. glauca (Sw.) Lindl. | K8270 | Costa Rica | 1939 | Donated, Lankester, C. H. | |
| D. glauca (Sw.) Lindl. | K42098 | Adams, B. R. (246) | Belize, Cayo District | 1980 | |
| D. hystricina Rchb.f. | K43905 | Adams, B. R. (263) | Belize, Toledo District | 1980 | |
| D. mosenii Cogn. | K63872 | Warren, R. | Brazil, Rio State, Serra do Mar | ||
| D. muricata (Sw.) Lindl. | K8271 | Costa Rica | 1955 | Donated, Lankester, C. H. | |
| D. muricata (Sw.) Lindl. | K14315 | Costa Rica | 1950 | Donated, Lankester, C. H. | |
| D. muricata (Sw.) Lindl. | K26896 | 1965 | Donated, Heidelberg Botanical Gardens | ||
| D. muricata (Sw.) Lindl. | K40917 | Brenan, J. P. M. | Mexico, Chiapas State | ||
| D. muricata (Sw.) Lindl. | K42372 | Adams, B.R. (259) | Belize, Toledo District | 1980 | |
| D. muricata (Sw.) Lindl. | K48237 | Storr, R. (081) | Brazil, Bahia | ||
| D. muricata (Sw.) Lindl. | K50169 | Hodgson (35) | Ecuador | ||
| D. muricata (Sw.) Lindl. var. maculata (Poepp. & Endl.) C. Schweinf. | K6802 | Peru | 1965 | Donated, Mason, L. M. | |
| D. neglecta Schltr. | K40914 | Brenan, J. P. M. | Mexico, Chiapas State | ||
| D. neglecta Schltr. | K45852 | Brenan, J. P. M. | Mexico | ||
| D. panamensis Lindl. | K37288 | Erskine, C. M. (E.238) | Brazil, Bahia | ||
| D. panamensis Lindl. | K42970 | Edwards, P. J. (1247) | Guyana, Kako River Expedition | 1979 | |
| D. pendula (Aubl.) Cogn. | K8272 | Sandwith, N. Y. (1384) | Guyana, Potaro River | 1937 | |
| D. pendula (Aubl.) Cogn. | K57878 | Hermans, J. | Brazil | 1994 | No. 2306 |
| D. picta Rchb.f. | K8273 | Trinidad | 1955 | Donated, Gillette, A. | |
| D. picta Rchb.f. | K10256 | Mason, L. M. | Guyana | 1960 | |
| D. picta Rchb.f. | K10258 | 1959 | Donated, Chelsea Physic Garden | ||
| D. picta Rchb.f. | K34246 | Dunsterville, G. C. K. (144) | Venezuela | ||
| D. pumila Rodr. | K634 | Lankester, C. H. (2) | Costa Rica | 1961 | |
| D. pumila Rodr. | K24906 | Brazil | 1964 | Donated, Mason, L. M. | |
| D. pumila Rodr. | K26918 | Brazil | 1963 | Donated, Mason, L. M. | |
| D. rendlei Gleason | K3128 | Surinam | 1961 | Donated, Kramer | |
| D. rendlei Gleason | K12542 | British Guiana | 1957 | Donated, Thompson, A. D. | |
| D. rendlei Gleason | K12543 | Guyana | 1957 | Donated, Mason, L. M. | |
| D. trulla Rchb.f. | K26008 | Brazil | 1963 | Donated, Mason, L. M. | |
| D. trulla Rchb.f. | K28325 | Mason, L. M. (1690) | Guyana | ||
| D. trulla Rchb.f. | K34993 | Honduras, Yojoa, San Pedro Sula. | Donated, Paul, J. A. | ||
| D. verrucosa Ames & Schweinf. | K8274 | Costa Rica | 1935 | Donated, Lankester, C. H. | |
| D. verrucosa Ames & Schweinf. | K13602 | Mexico | 1937 | Donated, Hinton, G. B. |
Table 2.
Specimens of the Huntleya clade studied and their provenance
| Taxon | Accession no. | Collector | Provenance | Date of collection or donation | Notes |
|---|---|---|---|---|---|
| Chaubardia heteroclita (Poepp. & Endl.) Dodson & D.E. Benn. | K72180 | Chase (18053) | |||
| C. klugii (C. Schweinf.) Garay | K57081 | da Silva, J. B. F | Brazil, Carajas Para | ||
| C. surinamensis Rchb.f. | K621 | Ecuador | det. G. Gerlach, 7·2·90 | ||
| C. surinamensis Rchb.f. | K27056 | det. G. Gerlach, 7·2·90 | |||
| Chondrorhyncha albicans Rolfe | K10246 | Costa Rica | |||
| C. albicans Rolfe | K12530 | ||||
| C. albicans Rolfe | K12531 | Costa Rica | |||
| C. andreae P. Ortíz | K64671 | ||||
| C. andreae P. Ortíz | K70116 | Hermans (4239) | 1998 | Obtained from Orquideas del Valle | |
| C. caloglossa (Schltr.) P.H. Allen | K13582 | Costa Rica | 1959 | syn. C. picta (Rchb.f.) Senghas | |
| C. maculata Garay | K44136 | Jenny, R. (16/82) | Colombia or Ecuador | ||
| Pescatorea cerina (Lindl. & Paxton) Rchb.f. | K14368 | Costa Rica | 1953 | Donated, Mason, L. M. | |
| P. cerina (Lindl. & Paxton) Rchb.f. | K14369 | Costa Rica | 1948 | Donated, Lankester, C. H. | |
| P. dayana Rchb.f. | K33285 | ||||
| P. wallisii Linden & Rchb.f. | K50035 | Ecuador | ex cult. Mrs L. Severin |
Following preliminary examination at light microscopy (LM) level, small samples of labellum were excised and prepared for scanning electron microscopy (SEM), as previously described (Davies and Turner, 2004b; Stpiczyńska et al., 2004). These were subsequently examined by means of a TESLA BS-300 scanning electron microscope at an accelerating voltage of 20–25 kV.
RESULTS
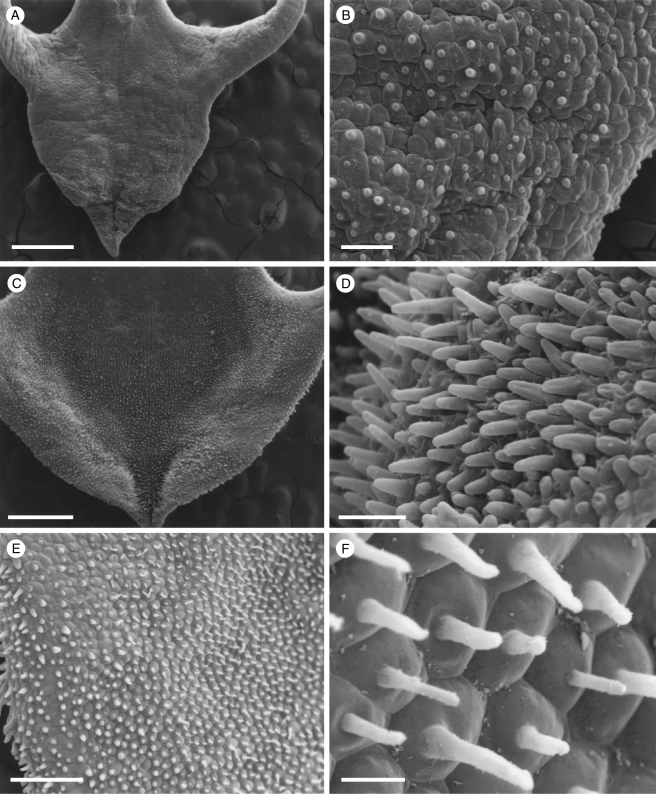
All species of Scuticaria examined possess papillose labella (Figs 1A, E, 2A and 3A). In S. hadwenii and a specimen labelled S. adverna (S. bahiensis K. L. Davies & M. Stpiczyńska; Davies and Stpiczyńska, 2008), short, unicellular, conical papillae occur both adaxially and abaxially (Figs 1B, and 2B, C), whereas in S. mooreana (syn. S. salesiana Dressler) they are most obvious on the mid-lobe. In S. steelii, however, unicellular, obpyriform papillae occur on the mid-lobe and the region distal to the callus, whereas unicellular, conical papillae occur alongside the callus, on the margins of the lateral lobes and abaxially. Conical to villiform papillae also occur along the veins of this species and these are often more obvious and pigmented than their counterparts elsewhere (Fig. 3B). Similarly, in S. mooreana, the veins are densely clothed with pronounced villiform papillae and many of these too contain pigment (Fig. 1E, F). In all cases, papillae found peripherally on the labellum tend to arise from angular, more or less isodiametric cells, whereas centrally these cells are often more than twice as long as wide (Figs 1B, D, 2B and 3B).
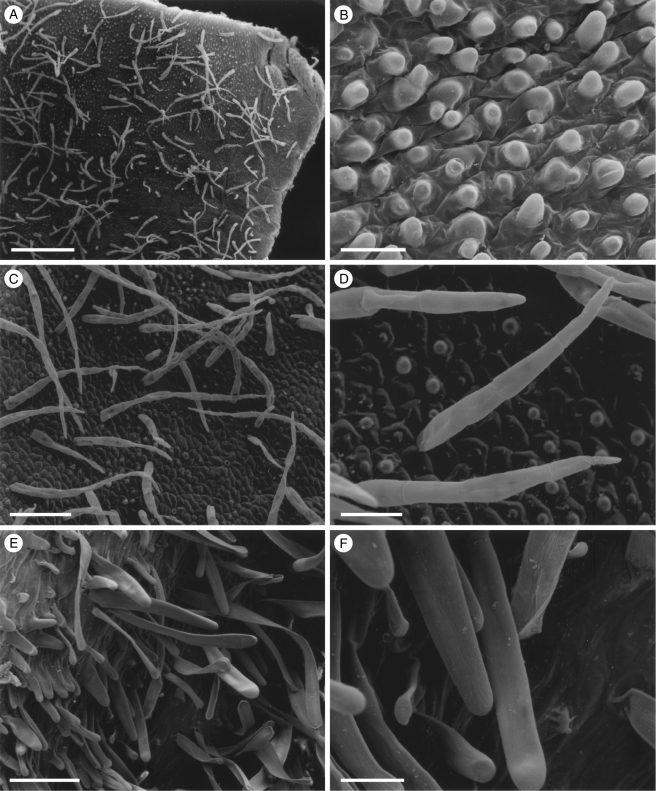
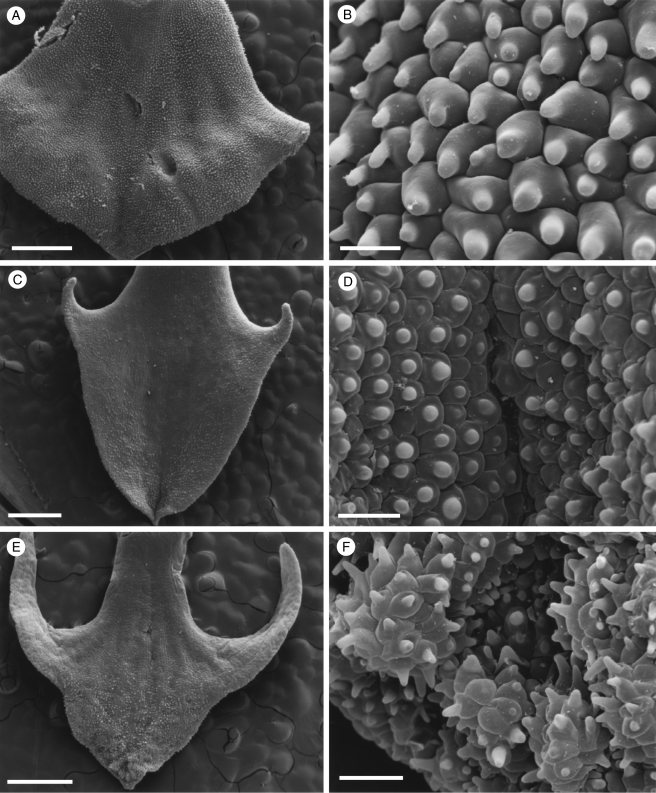
Fig. 1.
Labellar micromorphology of Scuticaria (A–F). Adaxial labellar surface (A–D) of S. hadwenii (accession no. K73914) showing uniseriate, unbranched, multicellular trichomes (A, C, D) and short, conical papillae (B). Unicellular, trichome-like papillae (E and F) of S. mooreana (syn. S. salesiana Dressler; accession no. K26282). Scale bars = 800, 80, 300, 90, 300 and 80 µm, respectively.
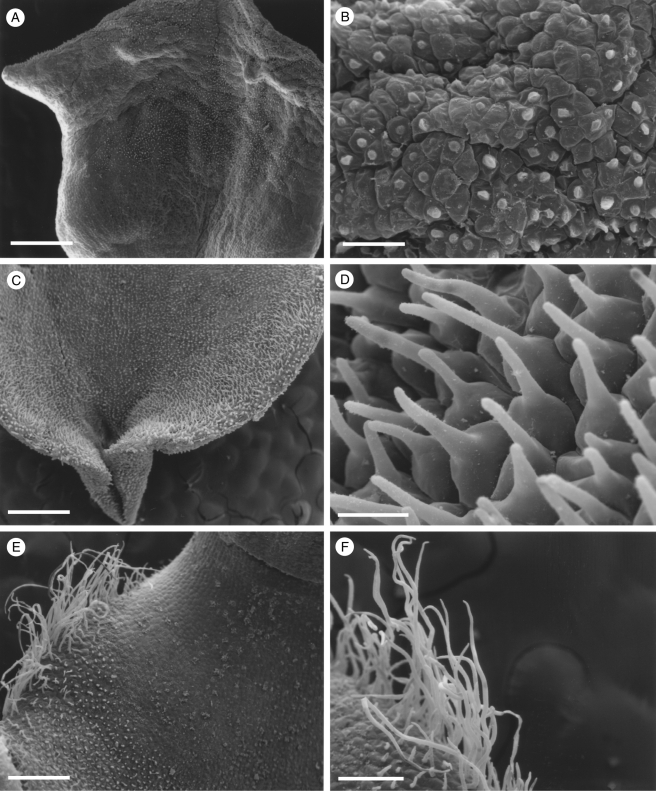
Fig. 2.
Labellar micromorphology of Scuticaria. Detail of labellar surface (A–F) of S. adverna (S. bahiensis K.L. Davies & M. Stpiczyńska; accession no. K57975) showing uniseriate, multicellular trichomes (A, D–F), conical papillae (B, C) and multicellular, scale-like structures (F). Scale bars = 900, 80, 90, 200, 100 and 100 µm, respectively.
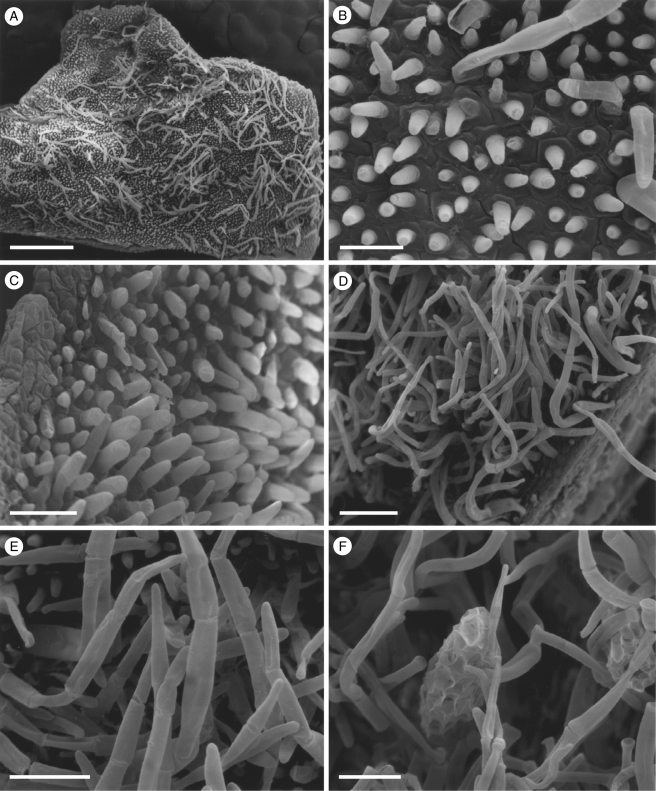
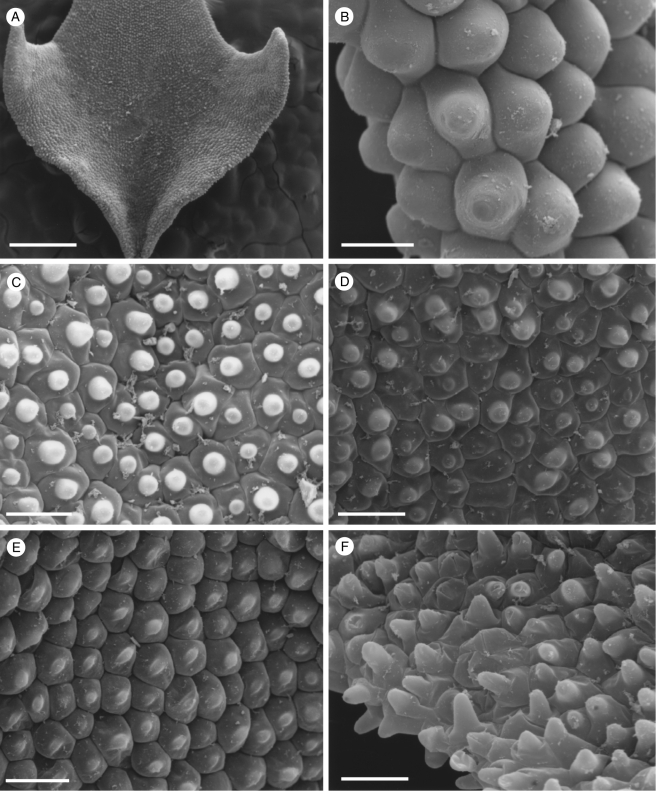
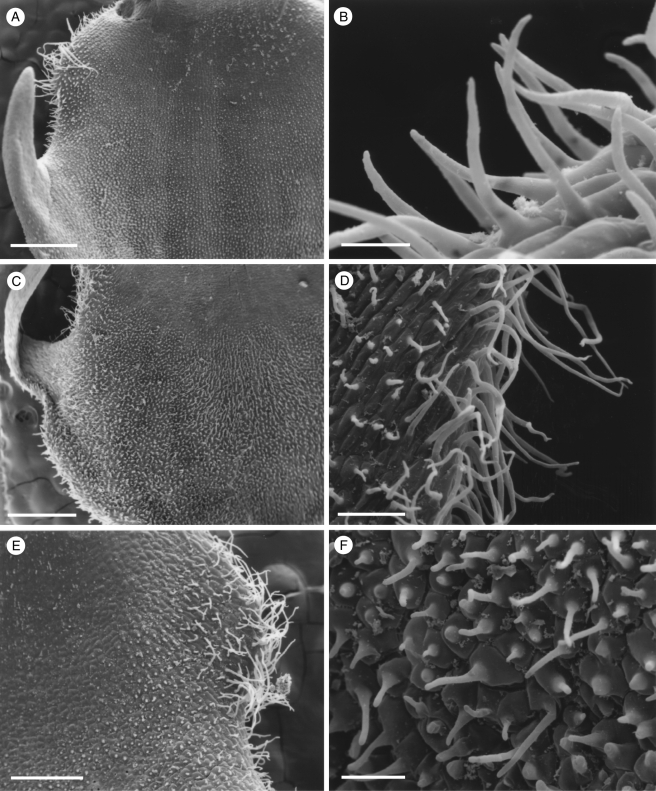
Fig. 3.
Labellar micromorphology of Scuticaria. Detail of labellar surface (A–F) of S. steelii (accession no. K13812) showing conical papillae (B) and uniseriate, multicellular, branched and unbranched trichomes (A, C–F), whose terminal cells may have verrucose walls (F). Scale bars = 700, 80, 100, 100, 70 and 50 µm, respectively.
Although villiform papillae can resemble trichomes (Fig. 1E, F), no hairs were found on the labellum of S. mooreana. Proximally, the lateral sepals of this species form a mentum, and here the labellum is glabrous. In S. hadwenii, uniseriate, unbranched, multicellular (2- to 17-celled, but mainly 4- to 8-celled) trichomes occur upon and alongside the labellar callus as well as proximally, close to the mentum (Fig. 1A, C, D). Hairs within the mentum may comprise ≥20 cells. Each labellar trichome consists of nucleated cells, 2–5 times as long as wide, a tapering terminal cell and a slightly bulbous basal cell (Fig. 1C, D). Similar hairs (6- to 9-celled) occur in S. adverna (Fig. 2A, D, E), but here they are found all over the adaxial surface of the labellum. Remarkably, in this species, multiseriate, epidermal, leaf-shaped scales, some five cells across, were present at the extreme proximal part of the lip (Fig. 2F). Unbranched (2- to 7-celled) and branched (11+ cells), multicellular trichomes with tapering tips and bulbous bases also occur on, alongside and distal to the labellar callus of S. steelii (Fig. 3A, C–F). These also occur inside the mentum. Some hairs have clavate tips (Figs 3F, and 4A, B). In one specimen of S. steelii (K13812), the terminal cells of the trichomes were verrucose (Figs 3F, and 4A, B). In this species, the callus is densely hirsute and, again, the individual trichomal cells are nucleated. Although the labellum of S. adverna lacked branched hairs, both branched and unbranched trichomes, identical to those of S. steelii, were found on a detached floral bract at the bottom of the specimen jar. It is assumed that this was once associated with the flower of S. adverna (Davies and Stpiczyńska, 2008).
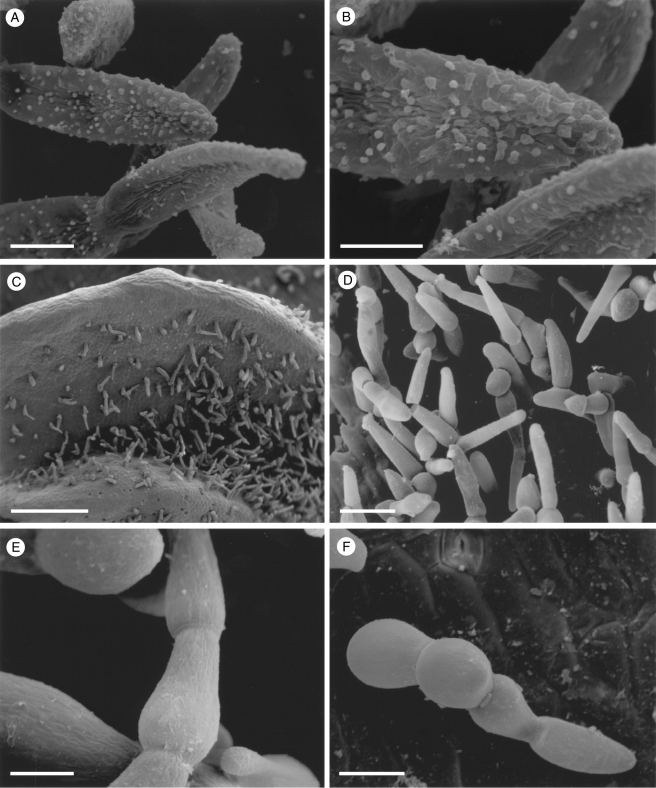
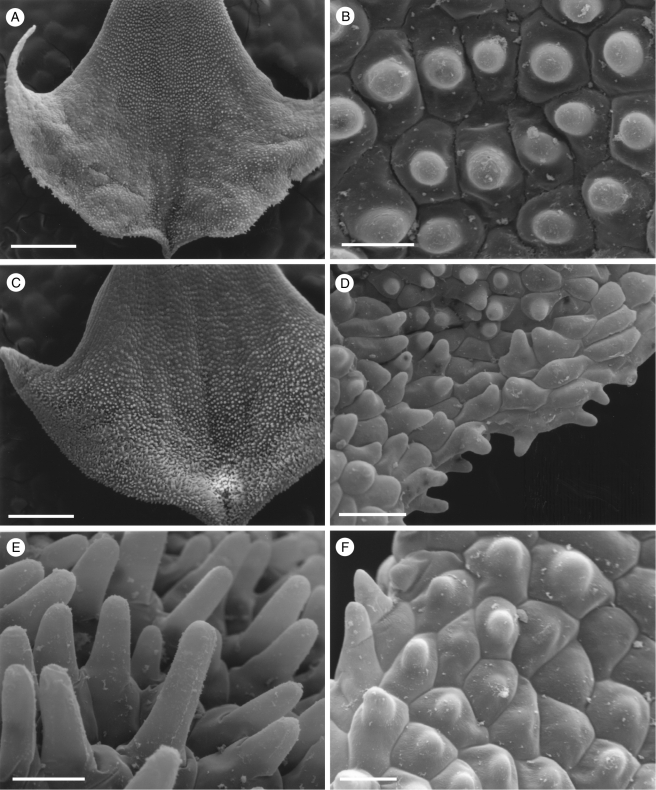
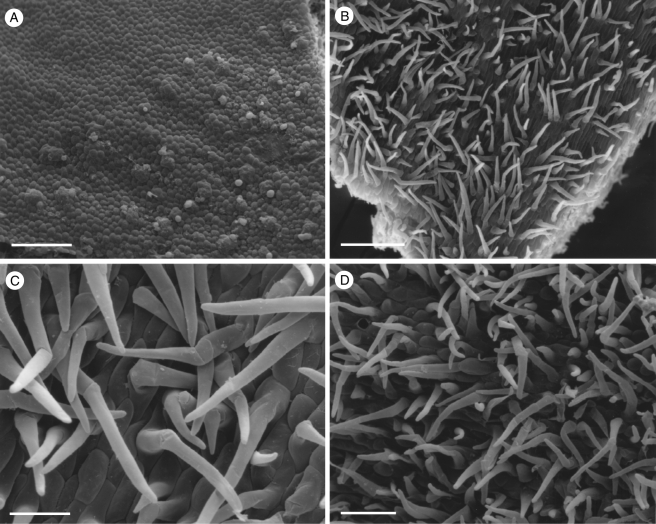
Fig. 4.
Labellar micromorphology of Scuticaria. Detail of verrucose walls (A, B) of terminal cells of labellar trichomes of S. steelii (accession no. K13812). Labellar surface (C) of S. steelii (accession no. K14398) with multicellular trichomes (D–F) showing stages in pseudopollen formation. Scale bars = 40, 20, 700, 100, 30 and 60 µm, respectively.
With one exception, staining of labellar trichomes of Scuticaria spp. with 0·25 % (w/v) toluidine blue/0·25 % (w/v) sodium tetraborate solution and IKI solution, respectively, did not reveal the presence of protein bodies or starch. However, the branched, multicellular trichomes of a single specimen of S. steelii (K14398), on preparing for LM, fragmented easily (Fig. 4C–F) and, unlike comparable, non-fragmented trichomes from other specimens of S. steelii, these contained numerous amyloplasts. Such hairs, thus share a number of features with the unbranched, moniliform, pseudopollen-forming trichomes of certain Maxillaria spp. (Davies and Winters, 1998; Davies et al., 2000, 2003; Davies and Turner, 2004a), in particular, members of the M. grandiflora (Humb., Bonpl. & Kunth) Lindl. alliance and some of those currently assigned to Heterotaxis Lindl. (Blanco et al., 2007; Whitten et al., 2007).
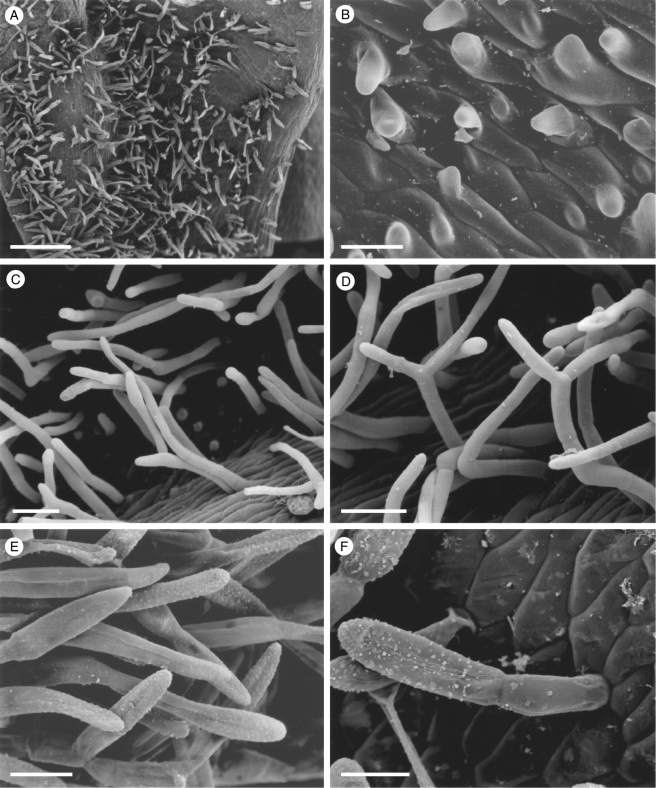
Despite differences in shape, the labella of all species of Dichaea examined showed considerable uniformity (Figs 5–10). In each case, the labellum is, to a greater or lesser degree, papillose and consists largely of more or less isodiametric cells with walls of varying thickness. Those cells comprising the lateral lobules, however, are elongate and up to five times as long as wide. The unicellular papillae are conical with pointed or rounded tips (Figs 5B, D, F, 6B–F; 7B, D–F; 8B, D, F, 9F, and 10B, D), although obpyriform papillae may occur distally in D. brachypoda (K10257). Conical papillae occur both adaxially and abaxially, the abaxial papillae and those comprising the labellar margin being most pronounced. Whereas conical papillae occur along the margins of the apical labellar lamina and distal margins of the lateral lobules (Figs 5F, 6B, F, 7D, F, and 9F), they are frequently absent from the proximal margins of the latter (e.g. Fig. 7A). Such papillae, however, occur on the proximal margin of the lobule in D. neglecta (K40914) and D. trulla (K26008, K28325). The margins of the proximal part of the labellum are usually glabrous, although in certain specimens (e.g. D. picta, K10258; D. pumila, K24906; D. rendlei, K12543), they are papillose. These papillae too are conical, with rounded and blunt or pointed tips. In some species (e.g. D. pendula, K8272; D. trulla K26008), there is a tendency for the papillose labellum to become minutely ciliolate proximally. In D. verrucosa (K13602, K 8274) and certain specimens of D. muricata (K14315, K26896, K42372, K50169 and K8271), small, densely hirsute auricles (shoulders) occur proximal to the lateral lobules (Figs 9A, C, E, and 10E) and shorter hairs extend from the auricles in both directions along the labellar margin. These auricular hairs are unicellular and flexuose with rounded or pointed tips (Figs 9B, D, and 10F). Trichomes were absent from all other species examined.
Fig. 5.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae of (A, B) D. glauca (accession no. K8270), (C, D) D. brachypoda (accession no. K8268) and (E, F) D. hystricina (accession no. K43905). Scale bars = 1 mm, 50 µm, 1 mm, 90 µm, 1 mm and 80 µm, respectively.
Fig. 10.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae of (A, B) D. muricata var. maculata (accession no. K6802) and (C, D) D. verrucosa (accession no. K8274). Unicellular trichomes (E, F) borne upon the labellar auricles of D. verrucosa (accession no. K8274). Scale bars = 1 mm, 900, 800, 50, 400 and 200 µm, respectively.
Fig. 6.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae of (A) D. pumila (accession no. K26918), (B) D. pumila (accession no. K24906), (C) D. panamensis (accession no. K42970), (D) D. neglecta (accession no. K40914), (E) D. trulla (accession no. K26008) and (F) D. picta (accession no. K10258). Scale bars = 900, 40, 70, 80, 80 and 70 µm, respectively.
Fig. 7.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae of (A, B) D. rendlei (accession no. K12543), (C–E) D. rendlei (accession no. K12542) and (F) D. mosenii (accession no. K63872). Scale bars = 900 µm, 40 µm, 1 mm, 100, 50 and 30 µm, respectively.
Fig. 8.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae of (A, B) D. ciliolata (accession no. K32353), (C, D) D. pendula (accession no. K8272) and (E, F) D. pendula (accession no. K57878). Scale bars = 900, 100, 900, 90, 300 and 30 µm, respectively.
Fig. 9.
Labellar micromorphology of Dichaea. Labellum and detail of conical labellar papillae and unicellular trichomes of (A, B) D. muricata (accession no. K42372), (C, D) D. muricata (accession no. K8271) and (E, F) D. muricata (accession no. K14315). Scale bars = 900, 40, 900, 100, 400 and 90 µm, respectively.
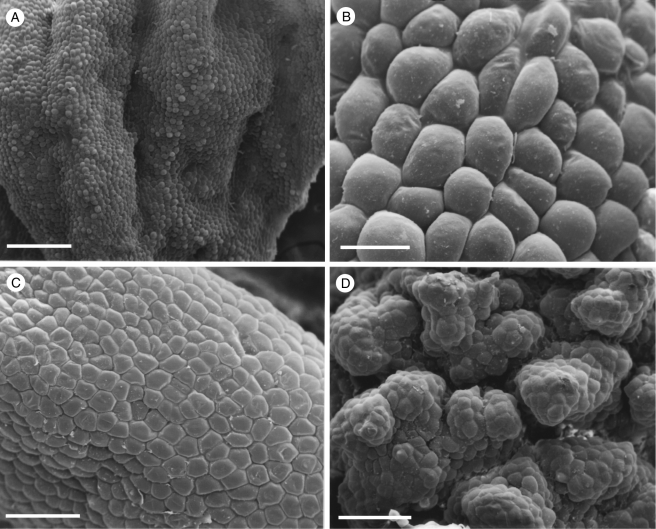
Specimens of the Huntleya clade exhibited diverse labellar micromorphology. The labellum of Chaubardia heteroclita (K72180) bears obpyriform papillae (Fig. 11A). Conical to obpyriform papillae are also present in C. klugii (K57081) and C. surinamensis (K621). However, much of the adaxial labellar surface of both these species also bears uniseriate, multicellular trichomes (Fig. 11B–D). Likewise, the labella of many of the Chondrorhyncha species examined are glabrous or papillose (Fig. 12A–C). In C. maculata [K44136; Daiotyla maculata (Garay) Dressler], the labellum is glabrous (Fig. 12C). In C. andreae [K70116; Euryblema andreae (Ortíz) Dressler], it is largely glabrous except for scattered conical papillae, whereas in specimen K64671 of the same species the labellum is papillose (Fig. 12A). These papillae are mostly obpyriform (Fig. 12B), with relatively few conical papillae present. Scattered, 2- to 3-celled, uniseriate trichomes were occasionally observed. Papillose labella are also present in C. albicans [Daiotyla albicans (Rolfe) Dressler] and C. caloglossa. In the latter species, most of the papillae are conical. However, a few villiform papillae were also observed. Labella of species of Pescatorea such as P. wallisii and P. cerina were usually rugose to verrucose (Fig. 12D). Unicellular, obpyriform, labellar papillae predominated in all Pescatorea spp. examined, but in P. cerina conical papillae were also present along the proximal edge of the labellum. No labellar trichomes were observed for this genus.
Fig. 11.
Labellar micromorphology of Chaubardia (A–D). Papillose labellum (A) of C. heteroclita (accession no. K72180) with obpyriform papillae. Hirsute labella (B, C) of C. klugii (accession no. K57081) and (D) C. surinamensis (accession no. K621), showing multicellular, uniseriate trichomes. Scale bars = 500, 200, 50 and 100 µm, respectively.
Fig. 12.
Labellar micromorphology of Chondrorhyncha (A–C) and Pescatorea (D). Papillose labellum (A, B) of C. andreae (accession no. K64671) with obpyriform papillae. Glabrous labellum (C) of C. maculata (accession no. K44136). Detail of rugose to verrucose labellum (D) of P. cerina (accession no. K14368). Scale bars = 700, 80, 200 and 200 µm, respectively.
DISCUSSION
Since the genera Scuticaria and Dichaea are both thought to be pollinated exclusively by euglossine bees, it is reasonable to assume that their labella would have evolved in like manner in response to pollinator pressure and, thus, exhibit similar micromorphology (Benzing, 1986; Stern et al., 2004; Dathe and Dietrich, 2006). However, this is not the case. Whereas the labella of most Scuticaria spp. are hirsute, those of the majority of Dichaea spp. studied lack hairs. However, labellar hairs are present in some species of Dichaea, but these are unicellular and unbranched, whereas those of Scuticaria are multicellular, uniseriate and unbranched or branched with, in some species, a tendency towards fragmentation and pseudopollen formation. Of the taxa currently assigned to Maxillariinae sensu lato hitherto studied, the labellar hairs of Scuticaria and Dichaea most closely resemble those of Maxillaria and Bifrenaria, respectively (Davies and Winters, 1998; Davies et al., 2000, 2003; Davies and Turner, 2004a; Davies and Stpiczyńska, 2006). Labellar hairs of Scuticaria showed no resemblance to those of Bifrenaria since the latter possesses unicellular, unbranched trichomes, and moniliform hairs are lacking (Davies and Stpiczyńska, 2006). Pollinaria of Bifrenaria harrisoniae (Hook.) Rchb.f. have been found on male Eufriesea violacea (Euglossini) as well as queens of Bombus brasiliensis (Bombini; Singer and Koehler, 2004). The resemblance of labellar hairs of Dichaea to those of Bifrenaria is thus perhaps best explained in terms of shared pollinators (euglossine bees in Dichaea; euglossine bees and bumble-bees in Bifrenaria), especially since in presumed ornithophilous species such as Bifrenaria aureo-fulva (Hook.) Lindl., labellar hairs are shorter and much more sparsely distributed (Davies and Stpiczyńska, 2006). However, an explanation for the similarity of the labellar trichomes of Scuticaria to those of Maxillaria is more difficult since, whereas Scuticaria is said to be pollinated by euglossine bees, Maxillaria is largely pollinated by stingless bees (Meliponini). This raises two possibilities; either Scuticaria is not exclusively pollinated by euglossine bees (Braga, 1977), or similar trichomes do not in this instance necessarily indicate a common pollinator but, rather, shared phylogeny.
In Maxillariinae sensu lato and its sister group Zygopetalinae (Whitten et al., 2000), multicellular hairs may be lacking (e.g. Bifrenaria, Chondrorhyncha, Pescatorea) or present (e.g. Rudolfiella, Teuscheria Garay, Xylobium, Maxillaria, Heterotaxis). However, moniliform, pseudopollen-producing trichomes are known only from certain species of Maxillaria, Heterotaxis, Xylobium and Teuscheria (Maxillariinae sensu lato; Davies and Winters, 1998; Davies et al., 2000, 2003; Davies and Turner, 2004a; Davies and Stpiczyńska, 2006). The pseudopollen-forming hairs of Scuticaria most closely resemble those of Maxillaria and Heterotaxis. Moreover, to date, pseudopollen has not been recorded for Zygopetalinae and thus, despite earlier claims, it would appear that Scuticaria is more closely related to Maxillariinae than Zygopetalinae. This is supported by both morphological (Dressler, 1990; Brieger et al., 1994; Szlachetko, 1995; Singer and Koehler, 2004) and molecular (Whitten et al., 2000; Koehler et al., 2002; Chase et al., 2003; Chase, 2005) evidence. Branched trichomes, present in S. steelii and on the floral bract of S. adverna, and multicellular, scale-like structures, as found in the latter species, have not been described for any other members of Maxillariinae sensu lato. It is claimed that S. steelii is euglossine-pollinated (Braga, 1977). However, the presence of pseudopollen in this species would tend not to support this assertion, since this feature is more characteristic of Meliponini-pollinated taxa (Davies et al., 2003; Davies and Turner, 2004a; Davies and Stpiczyńska, 2006).
Dichaea spp. lack distinctive labellar features, thus frustrating further analysis. In all cases, the labellum of Dichaea is papillose and, since conical papillae are considered the most common amongst angiosperms (Kay et al., 1981) and occur both in Maxillariinae (Davies and Turner, 2004a) and in representatives of the Huntleya clade, they provide little information about the phylogenetic relationships of this genus. Multicellular hairs, as found in Scuticaria and many species of Maxillaria and Heterotaxis, are absent from Dichaea. Two species, however, namely D. verrucosa and D. muricata, bear unicellular hairs. These hairs arise from auricles, and the latter, termed ‘shoulders’ by Folsom (1994), are said to be important to the pollination of many Dichaea spp. since they affect the positioning of visiting insects. Such hairs are absent from members of the Huntleya clade and from Maxillariinae sensu stricto (although unicellular hairs of a much stiffer appearance occur in the pseudocopulatory genus Mormolyca). However, they occur in Bifrenaria (Davies and Stpiczyńska, 2006), which, although known also to be pollinated by Bombus spp. (Singer and Koehler, 2004), like Dichaea, is largely euglossophilous. Conversely, whereas specimens of both Chondrorhyncha and Pescatorea lack multicellular hairs, Chaubardia, also a member of the Huntleya clade, has multicellular labellar trichomes reminiscent of those found in Maxillaria. Unfortunately, no pollination data for this genus are currently available and, consequently, investigations relating to whether this feature is due to shared phylogeny or convergence must wait until such information is forthcoming.
According to van der Cingel (2001), all members of Zygopetalinae hitherto studied, including members of the Huntleya clade, are pollinated by euglossine bees, mostly fragrance-gathering males. Of these, an unidentified species of Chondrorhyncha from Panama and Pescatorea wallisii were variously observed to be pollinated by Eulaema speciosa or Euglossa viridissima and Eulaema polychroma or Eulaema cingulata, respectively, whereas Euglossa hemichlora was found by Dodson and Frymire with a pollinarium from one or other of these orchid genera on its abdomen (all cited in van der Cingel, 2001). Thus, the absence of hirsute labella in these two orchid genera, as well as Dichaea, also appears to be consistent with certain euglossophilous pollination strategies.
For well over a century and a half, the gross morphology of the orchid labellum has been used to identify taxa. Yet, despite the importance of the labellum in taxonomy and pollination, until relatively recently, labellar micromorphology has been largely neglected. Notwithstanding difficulties in distinguishing between homoplasies relating to a common pollinator and synapomorphies that reflect a common ancestry, the ongoing collating of information relating to micromorphological characters remains worthwhile as a step towards a more complete data set for cladistic analyses. In the case of Dichaea, phylogenetic analysis based on labellar characters alone proved impossible owing to homoplasy and, therefore, a lack of sufficiently distinctive features. Nevertheless, branched floral trichomes, as found in S. steelii and S. adverna, and multicellular scale-like structures, as found in the latter species, are, to date, unique for Maxillariinae sensu lato and may prove to be sufficiently distinct for use both in taxonomy and phylogenetic studies.
ACKNOWLEDGEMENTS
K.L.D. is grateful to the Stanley Smith (UK) Horticultural Trust for their generous grant. The authors also acknowledge the help of the staff of the Royal Botanic Gardens Kew, UK and Alan Gregg, Swansea Botanical Complex, Swansea, UK.
LITERATURE CITED
- Ackerman JD. Specificity and mutual dependency of the orchid–euglossine interaction. Biological Journal of the Linnean Society. 1983;20:301–314. [Google Scholar]
- Ames O, Correll DS. Orchids of Guatemala and Belize. London: Constable and Company Ltd; 1985. [Google Scholar]
- Bennett DE, Jr, Christenson EA. A new species of Scuticaria from Peru. Orchid Digest. 2002;66(2):64–69. [Google Scholar]
- Benzing DH. The genesis of orchid diversity: emphasis on floral biology leads to misconceptions. Lindleyana. 1986;1:73–89. [Google Scholar]
- van den Berg C, Goldman DH, Freudenstein JV, Pridgeon AM, Cameron KM, Chase MW. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae) American Journal of Botany. 2005;92:613–624. doi: 10.3732/ajb.92.4.613. [DOI] [PubMed] [Google Scholar]
- Blanco MA, Carnevali G, Whitten WM, Singer RB, Koehler S, Williams NH, et al. Generic realignments in Maxillariinae (Orchidaceae) Lankesteriana. 2007;7:515–537. [Google Scholar]
- Braga P. Aspectos biologicos das Orchidaceae da Amazônica Central. Acta Amazonica, Manaus. 1977;7:1–89. [Google Scholar]
- Brieger FG, Maatsch R, Senghas K. Schlechter Die Orchideen 1713–2056. Berlin: Blackwell Wissenschafts-Verlag; pp. 2309–2436. 1994, 1995, 1996, 2000. [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew: Royal Botanic Gardens; 1992. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, et al. A phylogenetic analysis of the Orchidaceae: evidence from RBCL nucleotide sequences. American Journal of Botany. 1999;86:208–224. [PubMed] [Google Scholar]
- Chase MW. Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine. 2005;22(1):2–7. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, editor. Orchid conservation. Kota Kinabalu, Sabah, Malaysia: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination – America, Africa, Asia and Australia. Rotterdam, The Netherlands: A. A. Balkema; 2001. [Google Scholar]
- Dathe S, Dietrich H. Comparative molecular and morphological studies of selected Maxillariinae orchids. Wildenowia. 2006;36:89–102. [Google Scholar]
- Davies KL, Stpiczyńska M. Labellar micromorphology of Bifrenariinae Dressler (Orchidaceae) Annals of Botany. 2006;98:1215–1231. doi: 10.1093/aob/mcl204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. A new species of Scuticaria Lindl. (Orchidaceae) from Bahia, Brazil. Orchid Review. 2008 (in press) [Google Scholar]
- Davies KL, Turner MP. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2004;a 93:75–86. doi: 10.1093/aob/mch007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP. Pseudopollen in Eria Lindl. Section Mycaranthes Rchb.f. (Orchidaceae) Annals of Botany. 2004;b 94:707–715. doi: 10.1093/aob/mch195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP, Gregg A. Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae) Botanical Journal of the Linnean Society. 2003;143:151–158. [Google Scholar]
- Davies KL, Winters C. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae) Botanical Journal of the Linnean Society. 1998;126:349–361. [Google Scholar]
- Davies KL, Winters C, Turner MP. Pseudopollen: its structure and development in Maxillaria (Orchidaceae) Annals of Botany. 2000;85:887–895. [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Folsom JP. Pollination floral strategy and pollen flow in Dichaea sp. (Orchidaceae) American Journal of Botany. 1985;72:953–954. [Google Scholar]
- Folsom JP. Pollination of a fragrant orchid. Orchid Digest. 1994;58:83–99. [Google Scholar]
- Gravendeel B, Chase MW, de Vogel EF, Roos MC, Mes THM, Bachmann K. Molecular phylogeny of Coelogyne (Epidendroideae; Orchidaceae) based on plastid RFLPS, MATK, and nuclear ribosomal ITS sequences: evidence for polyphyly. American Journal of Botany. 2001;88:1915–1927. [PubMed] [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society. 1981;83:57–84. [Google Scholar]
- Koehler S, Williams NH, Whitten WM, Amaral MCE. Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spaces (ITS) and chloroplast trnL–trnF region. International Journal of Plant Sciences. 2002;163:1055–1066. [Google Scholar]
- Pridgeon AM, Solano R, Chase MW. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany. 2001;88:2286–2308. [PubMed] [Google Scholar]
- Roubik DW, Ackerman JD. Long-term ecology of euglossine orchid-bees (Apidae: Euglossini) in Panama. Oecologia (Berlin) 1987;73:321–333. doi: 10.1007/BF00385247. [DOI] [PubMed] [Google Scholar]
- Singer RB, Koehler S. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae) Annals of Botany. 2004;93:755–762. doi: 10.1093/aob/mch009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern WL, Judd WS, Carlsward BS. Systematic and comparative anatomy of Maxillarieae (Orchidaceae), sans Oncidiinae. Botanical Journal of the Linnean Society. 2004;144:251–274. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae) Annals of Botany. 2004;93:87–95. doi: 10.1093/aob/mch008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlachetko DL. Systema Orchidalium. Fragmenta Floristica et Geobotanica Supplementum. 1995;3:1–152. [Google Scholar]
- Whitten WM, Williams NH, Chase MW. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany. 2000;87:1842–1856. [PubMed] [Google Scholar]
- Whitten MW, Williams NH, Dressler RL, Gerlach G, Pupulin F. Generic relationships of Zygopetalinae (Orchidaceae: Cymbidieae): combined molecular evidence. Lankesteriana. 2005;5:87–107. [Google Scholar]
- Whitten WM, Blanco MA, Williams NH, Koehler S, Carneval G, Singer RB, et al. Molecular phylogenetics of Maxillaria and related genera (Orchidaceae: Cymbideae) based on combined molecular data sets. American Journal of Botany. 2007;94:1860–1889. doi: 10.3732/ajb.94.11.1860. [DOI] [PubMed] [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Orchidaceae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum and Trichocentrum and a new genus (Orchidaceae) Lindleyana. 2001;16:113–139. [Google Scholar]