Abstract
Background and Aims
The source of nitrogen plays an important role in salt tolerance of plants. In this study, the effects of NaCl on net uptake, accumulation and transport of ions were investigated in Nerium oleander with ammonium or nitrate as the nitrogen source in order to analyse differences in uptake and cycling of ions within plants.
Methods
Plants were grown in a greenhouse in hydroponics under different salt treatments (control vs. 100 mm NaCl) with ammonium or nitrate as the nitrogen source, and changes in ion concentration in plants, xylem sap exuded from roots and stems, and phloem sap were determined.
Key Results
Plant weight, leaf area and photosynthetic rate showed a higher salt tolerance of nitrate-fed plants compared with that of ammonium-fed plants. The total amount of Na+ transported in the xylem in roots, accumulated in the shoot and retranslocated in the phloem of ammonium-fed plants under salt treatment was 1·8, 1·9 and 2·7 times more, respectively, than that of nitrate-treated plants. However, the amount of Na+ accumulated in roots in nitrate-fed plants was about 1·5 times higher than that in ammonium-fed plants. Similarly, Cl− transport via the xylem to the shoot and its retranslocation via the phloem (Cl− cycling) were far greater with ammonium treatment than with nitrate treatment under conditions of salinity. The uptake and accumulation of K+ in shoots decreased more due to salinity in ammonium-fed plants compared with nitrate-fed plants. In contrast, K+ cycling in shoots increased due to salinity, with higher rates in the ammonium-treated plants.
Conclusions
The faster growth of nitrate-fed plants under conditions of salinity was associated with a lower transport and accumulation of Na+ and Cl− in the shoot, whereas in ammonium-fed plants accumulation and cycling of Na+ and Cl− in shoots probably caused harmful effects and reduced growth of plants.
Key words: Mineral cycling, Nerium oleander, nitrogen source, salinity, xylem and phloem transport
INTRODUCTION
Nerium oleander is an evergreen sclerophyllous C3 shrub, native to arid regions in a broad area from Morocco to southern China, and planted widely as an ornamental plant in many warm parts of the world, including hot desert areas (Huxley, 1992). It is of interest for revegetation and landscaping, because of its ornamental value and its capacity to acclimate to adverse environmental conditions. Several investigators have reported remarkable drought tolerance of N. oleander (Björkman and Powles, 1984; Demmig et al., 1988). Salinity can inhibit plant growth by a range of mechanisms, including low external water potential, ion toxicity and interference with the uptake of nutrients, particularly K+ (Munns, 1993; Tester and Davenport, 2003). Previous studies have indicated that the salt tolerance of N. oleander is achieved by inhibition of uptake of Na+ and Cl− and their accumulation in the shoot, as well as maintenance of a high K+/Na+ ratio in plants (Hajji, 1979; Abdolzadeh et al., 1998).
Plant metabolism of nitrogen is influenced by the inorganic form of nitrogen supplied. Since salinity affects the uptake and assimilation of nitrogen in various plant species, the nitrogen source plays an important role in salt tolerance of plants. In most studied species, including wheat (Leidi et al., 1991), maize (Lewis et al., 1989), peanuts (Silberbush and Lips, 1988), pea (Speer et al., 1994; Frechilla et al., 2001), muskmelon (Adler and Wilcox, 1995) and sunflower (Ashraf, 1999), plants grown with ammonium as the nitrogen source show a higher level of salt sensitivity than those grown with nitrate. Similarly, a study of salt tolerance of N. oleander with different nitrogen sources indicates that ammonium-fed plants show a greater reduction in dry weight under saline conditions than those grown with nitrate. One of the main reasons for such a difference might be related to a different distribution and accumulation of Na+ and Cl− in roots and shoots (Abdolzadeh et al., 1998). These different patterns were suggested to be the result of the relative contribution of xylem and phloem import and of export via the phloem. It is generally accepted that an increased K+/Na+ ratio and reduced Na+ translocation from the root to the shoot contribute to the overall salt tolerance in glycophytes (Tester and Davenport, 2003; Parida and Das, 2005). It is of interest, therefore, to evaluate the rate of phloem and xylem transport between roots and shoots to quantify the effects of NaCl on uptake and transport of ions within intact plants using different nitrogen sources. In Ricinus communis, Lupinus albus and Banksia prionotes, small amounts of phloem exudate can be collected after a small incision is made in the aerial part of plants. Most of our knowledge about transport of ions comes from these species (Marschner, 1995; Jeschke and Pate, 1995). The same property was found in N. oleander; it spontaneously exudes phloem sap that can be collected by making shallow incisions in the stem (A. Abdolzadeh, pers. obs.).
Armstrong and Kirkby (1979) used a model that assumes Ca2+ is not translocated in the phloem. Such an assumption may not be completely valid, as illustrated by the finding of 45Ca in a part of the root system that was not supplied with labelled Ca2+ in a spilt-root experiment (Lambers et al., 1982); however, the Ca2+ concentration in phloem sap in comparison with other macronutrients is, indeed, negligible. Thus, the xylem flux of Ca2+ can be estimated from the accumulation of Ca2+ in shoots. The xylem fluxes of other ions are then calculated from the Ca2+ flux and the ion/Ca2+ ratios in the xylem sap. Finally, the phloem fluxes are calculated from the difference between accumulation rates and xylem fluxes (Armstrong and Kirkby, 1979; Touraine et al., 1988; Gouia et al., 1994, Lu et al., 2005; Niu et al., 2007).
In the present study, growth and changes in ion concentrations in whole plants, xylem sap exuded from roots and stems, and phloem sap were determined in N. oleander exposed to saline conditions and grown with either ammonium or nitrate. Based on the above model, the uptake and flows of major ions in shoots and roots were estimated in order to elucidate the relationship between some mineral cycling and salinity tolerance as influenced by different nitrogen sources.
MATERIALS AND METHODS
Plant material and growth conditions
Plants were grown in a greenhouse from shoot cuttings of a single tree of N. oleander L. (Rosebay). Plants of a similar size were transferred to a hydroponics culture with ammonium or nitrate as the nitrogen source. The nutrient solution was made with tap water and contained 0·5 mm MgSO4, 0·5 mm KH2PO4 and micronutrients (Gibson, 1987) in all treatments. Other nutrients included 2·5 mm KNO3 and 1·5 mm Ca(NO3)2 for the nitrate treatment, and 1·5 mm CaCl2, 2·75 mm (NH4)2SO4 and 2·5 mm KCl for the ammonium treatment. The pH of the nutrient solution was adjusted daily to 6·3 ± 0·2 with 0·1 m KOH or H2SO4, and the nutrient solution was changed every week. Salinity treatments (100 mm NaCl) were started 3 weeks after transfer to nutrient solution. Salinity treatments were started with 25 mm NaCl and increased in three steps to 100 mm to avoid osmotic shock. The average daily maximum and minimum temperature in the greenhouse during the growing period were 32 and 18°C, respectively. The relative humidity was between 42 and 75%. Four plants in each treatment were harvested at days 1, 3, 7, 15 and 30 after starting the salt treatment for growth assessment and chemical analyses. The phloem and xylem sap collections were carried out on the same days (days 1, 3, 7, 15 and 30 after starting the salt treatment). Leaf area was determined by an automatic leaf area meter (Model AAM-7 Hyashi Denkoh Co. Ltd, Tokyo, Japan).
Gas exchange measurements
CO2 assimilation (net photosynthesis) and transpiration rates of attached leaves were measured using a portable gas exchange system (ADC Infrared Gas Analyzer type LCA4 with PLC4 chamber, Hertfordshire, UK) at various time intervals during the study. Measurements were taken from 1000 h to 1500 h, at a CO2 concentration of approx. 360 µmol mol−1, vapour pressure deficit of 1·2 kPa and a photosynthetuic photon flux density of 1000 µmol m−2 s−1. Each measurement took approx. 7 min, and the parameters were recorded every 30 s. The mean of the values obtained during the stable part of the experiment (lasting 4 min) was used as one value. Water-use efficiency (μmol CO2 fixed per mmol water lost) was calculated by dividing net photosynthesis by transpiration rates.
Xylem and phloem sap collection
Phloem exudate was collected from 0900 h to 1100 h by making shallow incisions in the middle of the stem using a sharp razor blade. Phloem sap exuded from the incisions almost immediately in small droplets. The first drop of sap may contain cell sap and was discarded (Jeschke and Pate, 1995). Approximately 50–100 µL of phloem sap was collected by a Hamilton syringe over a 2–3 min period, and pooled into small plastic vials. Total carbon, nitrogen and carbohydrate concentrations were measured in the exuded sap. The total C and N concentration was determined in freeze-dried phloem sap after the dried powder was mixed with 4 g of cobalt oxide using a C-N Corder (Yanaco Co. Type TNC-600, Kyoto, Japan). Hippuric acid was use as a standard. The freeze-dried samples were dissolved in 7 mL of distilled water and passed through an ion exchange column (Amberlite CG-120 cation exchange resin and Amberlite CG-400 anion exchange resin, Sigma, Tokyo, Japan) before quantification of soluble sugars by high-performance liquid chromatography (HPLC; Shimazu Co., Kyoto, Japan). The mobile phase was water, and separation was carried out at 80°C at a flow rate of 0·5 mL min−1. Xylem sap was collected from stems (from 1000 h to 1200 h) and roots separately. In stems, after collection of phloem sap the external tissue of the vascular cambium, including the phloem, was peeled off, and then the stem was cut and the exuded sap was collected. After approx. 1–2 min, about 100 µL of xylem sap was collected. In the case of small volumes for plants under a prolonged period of salinity, the stem segments were gently pressurized with air. For roots, one or two of the main cut roots were sealed in a pressure chamber and mildly pressurized with air, and the exuded sap was collected. For evaluation of the purity of the xylem sap, the concentration of carbohydrates was measured in xylem sap as described above. All sap samples were stored at –20°C until inorganic ion analysis.
Chemical analyses
At each harvest, about 0·8 g of plant material was ground in liquid nitrogen, extracted with distilled water, mixed with a homogenizer, boiled for 15 min in a water bath and the supernatant was separated by centrifugation. Extractions were performed three times and the resulting supernatants were pooled. The supernatants were analysed for the concentration of ions by ion chromatography (Abdolzadeh et al., 1998).
Empirical model for uptake, transport and accumulation of ions
The uptake of ions between day 15 and day 30 was calculated from the increment of the ion content in plant organs and divided by 15 (μmol per organ per day) from the following equation
| 1 |
where ΔK is the increment of K+ from day 15 to 30 during the experimental period, and the subscripts ‘l’, ‘r’ and ‘st’ indicate leaves, roots and stems, respectively.
Based on the model introduced by Armstrong and Kirkby (1979), flows of ions were calculated. This method is based on the following assumptions: (a) transport between organs occurs exclusively through the xylem and phloem; (b) transport occurs by mass flow and any two compounds are translocated in proportion to their molar ratio; and (c) phloem transport of Ca2+ is assumed to be negligible. In shoots, the sum of the increment of any ion, e.g. ΔKs (μmol per organ per day), during a defined period equals the difference between the flows of the xylem (Jion, x) and phloem (Jion, p). For example, for K+
| 2 |
Assuming that Ca2+ was not transported in the phloem, its xylem transport was estimated from the absolute Ca2+ increase in shoots, and this equation simplifies to
| 3 |
Assuming mass flow in the xylem, the ratio of the flows of K+ and Ca2+ are equal to their molar ratio in the xylem
| 4 |
Using eqn (3) substituting ΔCa2+s for JCa, x in eqn (4), the flow of K+ in the xylem is then
| 5 |
Xylem transport of K+ in roots and shoots was then calculated by multiplying the Ca2+ increment in the shoot by the K+/Ca2+ molar ratios in the xylem sap exuded from excised roots and intact shoots. The recirculation of ions in the phloem was the difference between xylem transport and accumulation in shoots. Therefore, the flow of K+ in the phloem (separately in the root and shoot) can be estimated using eqn (2)
| 6 |
Flows of other ions could be estimated correspondingly. The mean of the ion/Ca2+ molar ratio between days 15 and 30 as determined by analysis of xylem sap exuded from excised roots and from the middle part of stems was used for calculation of flows in root and shoot. The higher flow of an ion in the shoot xylem compared with that in the root xylem indicates release of this ion into the xylem in the shoot from the phloem (cycling). The release was calculated from the difference of the ion flow in the xylem in the shoot and root. Similarly, a lower flow of an ion in the shoot xylem than in the root xylem indicates resorption of this ion from the xylem sap during ascent (observed only for Na+). The resorption of ions was calculated from the difference of the ion flow in the xylem in the root and shoot. All values obtained for the uptake and accumulation rates were divided by the mean dry weight over days 15–30 assuming linear growth during these days, and all numbers in the models are presented as μmol g−1 d. wt d−1 (Armstrong and Kirkby, 1979; Touraine et al., 1988; Gouia et al., 1994; Lu et al., 2005; Niu et al., 2007). This allows comparison of flows in plants of different sizes and the opportunity to highlight the specific effects of NaCl and nitrogen source on these flows that cannot be accounted for by differences in biomass.
Statistical analysis
The experiments were carried out using a completely randomized factorial design. Statistical analyses of data were carried out using SAS statistical software (SAS Institute Inc., 2001). All data subjected to analysis of variance, and comparisons of means were performed using the l.s.d. test.
RESULTS
Growth and gas exchange
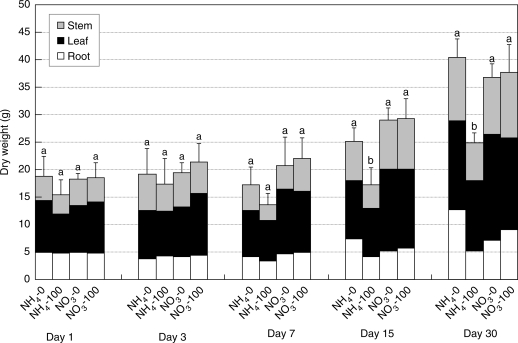
The total dry weight of plants increased similarly over time in both nitrogen treatments under non-saline conditions (Fig. 1). However, the response of plants to salinity depended on the nitrogen source. There was no significant difference in total dry weight of NO3-fed plants due to salinity, but salinity markedly reduced the total dry weight of NH4-fed plants. The effect of salinity was more severe in roots of NH4-fed plants. At the end of the experimental period, root dry weight of the NH4-fed plants was 43·8% lower than that of NO3-fed plants under conditions of salinity.
Fig. 1.
Effect of salinity on dry weight of leaves, roots and stems of Nerium oleander plants grown for 30 d at 100 mm NaCl as affected by ammonium or nitrate as the nitrogen source. Vertical bars represent s.e. (n = 4) for the total dry weight of plants. Columns not sharing the same letter within each group indicate significant differences between treatments for total dry weight according to the l.s.d. test (P < 0·05).
The decrease in total leaf area due to salinity was more severe in NH4-fed plants than in NO3-fed plants (Table 1). At day 30, total leaf area of the NH4-fed plants under treatment with saline was 44% lower than that of control plants, whereas in the NO3-treated plants it was only 29% lower. The decline in photosynthetic rate was also more severe under saline treatment in NH4-fed plants than in NO3-fed plants. Transpiration was slightly higher in NH4-fed plants than in NO3-fed plants under control conditions, but there was no change due to salinity in either nitrogen treatment. Water-use efficiency was lower in NH4-fed plants in control conditions, but declined similarly with both nitrogen sources with saline treatment.
Table 1.
Effect of salinity on rate of photosynthesis and transpiration, water-use efficiency and total leaf area in Nerium oleander plants after about 1 month of exposure to 100 mm NaCl as affected by ammonium or nitrate as the nitrogen source
| NH4+-N |
NO3−-N |
|||
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | |
| Photosynthetic rate (μmol CO2 m−2 s−1) | 8·0 ± 0·44a | 4·7 ± 0·54b | 7·6 ± 0·29a | 5·3 ± 0·46b |
| Transpiration rate (mmol H2O m−2 s−1) | 5·4 ± 0·27a | 5·4 ± 0·41a | 4·7 ± 0·26a | 4·7 ± 0·32a |
| Water-use efficiency (μmol CO2 fixed per mmol water lost) | 1·48 ± 0·13ab | 0·87 ± 0·14c | 1·62 ± 0·12a | 1·13 ± 0·15bc |
| Leaf area (cm2) | 1740 ± 106a | 981 ± 74b | 1644 ± 73a | 1178 ± 93b |
Values are means ± s.e. of four replicate plants. Rows not sharing the same letter indicate significant differences between treatments according to the l.s.d. test (P < 0·05).
Ion concentration in plants
The concentrations of Na+ and Cl− were very low in control plants, but increased significantly with increasing time of exposure to salinity (Table 2). The concentrations of Na+ and Cl− in roots were much higher than those in leaves. The concentrations of Na+ and Cl− in leaves and stems were significantly higher in NH4-fed plants than in NO3-fed plants after 15 d of exposure to salinity; however, after 30 d of the salt treatment, a significant difference remained only in stems. The concentration of both Na+ and Cl− in roots was significantly higher in NO3-fed plants than in NH4-fed plants, and increased with time of exposure to salinity.
Table 2.
Na+, K+, Ca2+ and Cl− concentration (mg g−1 d wt) and the K+: Na+ ratio in Nerium oleander plants at days 15 and 30 of salt treatments (control vs. 100 mm NaCl) with ammonium or nitrate as the nitrogen source
| NH4+-N |
NO3−-N |
||||
|---|---|---|---|---|---|
| Harvest day | 0 | 100 | 0 | 100 | |
| Leaf | |||||
| Na+ | 15 | 0·70 ± 0·20 (321) | 1·74 ± 0·08 (584) | 0·87 ± 0·05 (561) | 1·33 ± 0·44 (832) |
| 30 | 0·46 ± 0·11 (327) | 2·34 ± 0·23 (1310) | 0·69 ± 0·05 (583) | 2·43 ± 0·15 (1766) | |
| K+ | 15 | 11·3 ± 0·50 (3040) | 8·7 ± 0·49 (1725) | 14·4 ± 0·59 (5477) | 10·7 ± 1·77 (3653) |
| 30 | 10·4 ± 0·28 (4287) | 7·9 ± 1·29 (2589) | 14·7 ± 0·75 (7275) | 10·9 ± 0·79 (4795) | |
| Ca2+ | 15 | 1·97 ± 0·04 (518) | 1·16 ± 0·36 (223) | 1·51 ± 0·17 (608) | 0·91 ± 0·20 (325) |
| 30 | 1·67 ± 0·11 (672) | 0·96 ± 0·15 (310) | 1·78 ± 0·45 (860) | 1·03 ± 0·08 (429) | |
| Cl− | 15 | 1·94 ± 0·12 (576) | 6·41 ± 0·52 (1397) | 1·48 ± 0·40 620) | 3·37 ± 0·38 (1366) |
| 30 | 1·27 ± 0·37 (581) | 5·83 ± 0·20 (2117) | 1·28 ± 0·19 (697) | 5·78 ± 0·12 (2730) | |
| K+/Na+ | 15 | 9·47 | 2·95 | 9·76 | 4·39 |
| 30 | 13·11 | 1·98 | 12·48 | 2·72 | |
| Root | |||||
| Na+ | 15 | 0·64 ± 0·05 (209) | 7·88 ± 0·40 (1094) | 0·73 ± 0·50 (163) | 10·43 ± 0·60 (2584) |
| 30 | 0·58 ± 0·17 (322) | 12·43 ± 0·51 (2769) | 0·60 ± 0·05 (184) | 16·00 ± 0·10 (6268) | |
| K+ | 15 | 9·4 ± 2·32 (1792) | 4·9 ± 1·09 (402) | 18·4 ± 1·17 (2538) | 5·1 ± 0·34 (737) |
| 30 | 8·6 ± 0·80 (2785) | 4·7 ± 0·11 (618) | 20·0 ± 2·56 (3612) | 4·3 ± 0·21(1452) | |
| Ca2+ | 15 | 0·35 ± 0·10 (65) | 0·40 ± 0·07 (32) | 0·52 ± 0·12 (66) | 0·74 ± 0·36 (106) |
| 30 | 0·57 ± 0·15 (182) | 0·67 ± 0·08 (85) | 0·81 ± 0·09 (143) | 0·72 ± 0·02 (161) | |
| Cl− | 15 | 2·00 ± 0·27 (420) | 10·65 ± 0·60 (959) | 1·31 ± 0·68 (189) | 16·04 ± 1·24 (2579) |
| 30 | 1·71 ± 0·36 (611) | 10·53 ± 0·96 (1521) | 1·39 ± 0·15 (277) | 13·70 ± 1·20 (3484) | |
| K+/Na+ | 15 | 8·59 | 0·37 | 15·57 | 0·29 |
| 30 | 8·65 | 0·22 | 19·63 | 0·23 | |
| Stem | |||||
| Na+ | 15 | 0·44 ± 0·06 (137) | 2·50 ± 0·08 (471) | 0·74 ± 0·45 (288) | 1·54 ± 0·43 (619) |
| 30 | 0·49 ± 0·88 (246) | 3·49 ± 0·87 (1039) | 0·85 ± 0·57 (383) | 2·35 ± 0·56 (1221) | |
| K+ | 15 | 9·3 ± 0·61 (1697) | 10·0 ± 1·72 (1109) | 15·6 ± 1·61 (3561) | 11·7 ± 1·45 (2764) |
| 30 | 10·4 ± 0·96 (3053) | 7·9 ± 2·19 (1378) | 15·7 ± 1·86 (4181) | 10·6 ± 0·03 (3228) | |
| Ca2+ | 15 | 0·73 ± 0·03 (130) | 0·81 ± 0·18 (88) | 0·87 ± 0·07 (193) | 0·59 ± 0·06 (136) |
| 30 | 1·17 ± 0·10 (336) | 0·96 ± 0·20 (163) | 1·16 ± 0·02 (300) | 0·90 ± 0·09 (269) | |
| Cl− | 15 | 1·74 ± 0·15 (351) | 4·65 ± 1·22 (569) | 1·60 ± 0·42 (404) | 2·71 ± 0·43 (970) |
| 30 | 1·44 ± 0·12 (469) | 5·17 ± 0·77 (999) | 1·51 ± 0·10 (442) | 4·09 ± 0·04 (1378) | |
| K+/Na+ | 15 | 12·39 | 2·35 | 12·36 | 4·47 |
| 30 | 12·41 | 1·33 | 10·92 | 2·64 | |
Values are means ± s.e. of four replicate plants.
Values in parentheses represent the ion content (μmol organ−1).
The concentration of K+ was significantly higher in NO3-fed plants than in NH4-fed plants in all organs under control conditions (Table 2). Salinity induced a significant reduction in the K+ concentration in both nitrogen treatments, and this pattern was not affected by time of exposure. The decrease in K+ concentration in leaves due to salinity was similar in both nitrogen treatments. In contrast, the decrease in concentration of K+ in roots was more severe in NO3-fed plants than in NH4-fed plants. The K+/Na+ ratio with saline treatment was higher in leaves and stems and lower in roots of NO3-fed plants compared with thoise fed NH4. At day 30, the K+/Na+ ratio was 4·5 in leaves of NO3-fed plants under saline conditions compared with 3·4 in NH4-treated plants
The concentration of Ca2+ decreased similarly in leaves and stems due to salinity in both nitrogen treatments, but did not change markedly in roots (Table 2).
The concentration of ions in xylem and phloem sap
The concentration of sugars in xylem sap exuded from roots and shoots was negligible, as expected (Table 3). The equivalent sum of anions and cations was lower in xylem sap exuded from roots than in xylem sap exuded from the stem. Salinity induced a marked increase in the equivalent sum of anions and cations in xylem sap of both roots and stems, especially in NH4-fed plants. The phloem exudate had a high dry matter content (>100 g L−1), a high carbon (99–130 g L−1) and nitrogen (3–7 g L−1) concentration, with a C/N ratio of around 20, a high sugar concentration (72–83 g L−1) and a high concentration of anions and cations, especially potassium, phosphate and chloride. Salinity caused a marked reduction in the phloem sap carbon and nitrogen concentrations in NH4-fed plants, compared with those in NO3-fed plants.
Table 3.
Effect of salinity (control vs. 100 mm NaCl) on total C, N, amino acids, soluble sugars and the sum of equivalent anion (Cl−, SO42−, NO3− and PO43−) and equivalent cation (Na+, K+, Ca2+, Mg2+ and NH4+) concentrations in phloem sap and xylem sap exuded from excised roots and from the middle part of stems of Nerium oleander plant after 30 d exposure to 100 mm NaCl as affected by ammonium or nitrate as nitrogen source
| NH4+-N |
NO3−-N |
|||
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | |
| Phloem sap | ||||
| Total C (mg mL−1) | 130·8 ± 6·44 | 99·6 ± 8·28 | 121·2 ± 4·76 | 113·4 ± 4·93 |
| Total N (mg mL−1) | 7·06 ± 1·11 | 3·95 ± 1·37 | 4·44 ± 0·68 | 5·22 ± 1·32 |
| C/N ratio | 18·52 | 25·23 | 27·30 | 21·73 |
| Sugar (mg mL−1) | 83·2 ± 5·33 | 75·4 ± 6·12 | 78·6 ± 9·62 | 72·8 ± 12·02 |
| Σ Anion (meq L−1) | 111·1 | 183·1 | 83·2 | 156·4 |
| Σ Cation (meq L−1) | 103·9 | 144·3 | 114·8 | 171·4 |
| Xylem sap root | ||||
| Sugar (mg mL−1) | 1·34 ± 0·77 | 2·92 ± 1·34 | 1·32 ± 0·68 | 2·14 ± 1·35 |
| Σ Anion (meq L−1) | 13·0 | 67·8 | 13·3 | 56·6 |
| Σ Cation (meq L−1) | 10·55 | 78·8 | 19·5 | 67·1 |
| Xylem sap shoot | ||||
| Sugar (mg mL−1) | 1·23 ± 0·97 | 3·04 ± 1·82 | 1·40 ± 1·11 | 2·03 ± 1·58 |
| ΣAnion (meq L−1) | 72·4 | 131·6 | 59·6 | 82·5 |
| Σ Cation (meq L−1) | 70·6 | 169·1 | 90·7 | 114·4 |
Values are means ± s.e. of four replicate plants.
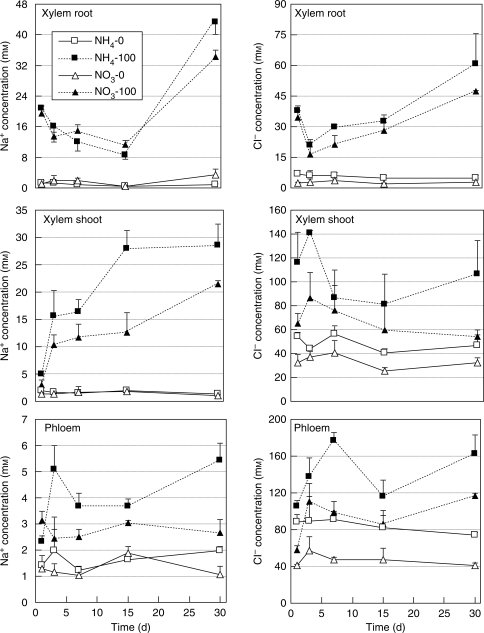
The concentration of Na+ and Cl− in xylem sap exuded from roots or stems, and that in phloem sap was negligible in control conditions, but increased drastically due to salinity. However, the increase in Na+ concentration in sap due to salinity was much lower in xylem sap exuded from stems in comparison with that exuded from roots (Fig. 2). In contrast to Na+, with salinity treatment the Cl− concentration was less in xylem sap exuded from roots than in that exuded from stems. The smallest increase in concentration of Na+ was observed in phloem sap in both nitrogen treatments under conditions of salinity. The increase in Na+ concentration due to salinity was markedly lower in xylem sap exuded from stems and phloem sap of NO3-fed plants than in that from NH4-fed plants. No significant difference was observed in Na+ concentration in xylem sap exuded from roots between nitrogen treatments due to salinity, except at day 30 when the Na+ concentration was markedly higher in NH4-fed plants. Similarly to Na+, with saline treatment the concentration of Cl− in NO3-fed plants was lower than that in NH4-fed plants in both xylem and phloem sap.
Fig. 2.
Changes in Na+ and Cl− concentrations in xylem sap exuded from roots and stems, and phloem sap of Nerium oleander plants grown for 30 d at 100 mm NaCl as affected by ammonium or nitrate as the nitrogen source. Values are the means ± s.e. of four replicate plants.
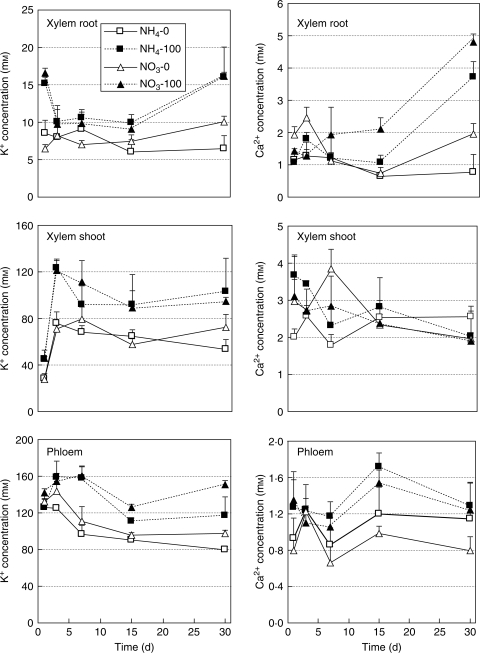
The concentration of K+ was very low in xylem sap exuded from roots, increased markedly in xylem sap exuded from stems, and exhibited the highest concentration in phloem sap in both nitrogen treatments (Fig. 3). In control plants, the concentration of K+ was markedly higher in NO3-fed plants than in NH4-fed plants at day 30 in both xylem and phloem sap. Salinity increased the K+ concentration in both xylem and phloem sap. There was no significant difference in the K+ concentration between NH4- and NO3-fed plants in xylem sap exuded from roots and stems under saline conditions. However, under saline conditions, the K+ concentration in phloem sap was significantly higher in NO3-fed plants.
Fig. 3.
Changes in K+ and Ca2+ concentrations in xylem sap exuded from root and stem, and phloem sap of Nerium oleander plants grown for 30 d at 100 mm NaCl as affected by ammonium or nitrate as the nitrogen source. Values are the means ± s.e. of four replicate plants.
The Ca2+ concentration increased in xylem sap exuded from roots which had undergone saline treatment, with higher concentrations in NO3-fed plants than in NH4-fed plants (Fig. 3). The Ca2+ concentration did not show any significant changes in xylem sap exuded from stems and phloem sap with both nitrogen sources under salinity treatment.
Modelling of flow
The total increments of ions during 15 d per plant organ were determined (Table 2), and the sum of the increments in different organs (μmol per plant from day 15 to 30) was considered the total net uptake of ions (Table 4). Means of the ion/Ca2+ molar ratio on day 15 and 30 from analysis of xylem sap exuded from roots and stem are presented in Table 5. Both the Na+/Ca2+ and the Cl−/Ca2+ molar ratio increased under saline conditions in both phloem and xylem sap. However, the increase due to salinity was much lower in NO3-fed plants than in NH4-fed plants. The K+/Ca2+ ratio did not change markedly in xylem sap exuded from roots due to either salinity or nitrogen treatment, but it did increase due to salinity in xylem sap exuded from the stem.
Table 4.
Increase in Na+, K+, Ca2+ and Cl− (μmol g−1 d. wt d−1) in Nerium oleander plants from day 15 until day 30 (ion uptake) after the start of salt treatment (control vs. 100 mm NaCl) as affected by ammonium or nitrate as nitrogen source
| NH4+-N |
NO3−-N |
|||
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | |
| Ca2+ | ||||
| Shoot | 2·24 | 1·47 | 1·72 | 1·28 |
| Root | 0·77 | 0·86 | 0·84 | 0·50 |
| Total | 3·01 | 2·32 | 2·56 | 1·78 |
| Na+ | ||||
| Shoot | 0·81 | 11·47 | 0·75 | 7·79 |
| Root | 0·75 | 26·86 | 0·23 | 33·38 |
| Total | 1·56 | 38·33 | 0·98 | 41·17 |
| K+ | ||||
| Shoot | 15·91 | 8·80 | 11·28 | 7·97 |
| Root | 6·58 | 3·46 | 11·75 | 6·48 |
| Total | 22·48 | 12·26 | 24·47 | 14·45 |
| Cl− | ||||
| Shoot | 0·87 | 9·79 | 0·56 | 8·41 |
| Root | 1·26 | 9·01 | 0·96 | 8·20 |
| Total | 2·13 | 18·81 | 1·52 | 16·61 |
Ion uptake was calculated by subtracting the ion content of 30-d-old plants from that of 15-d-old plants (Table 2). All values were divided by the mean dry weight between days 15 and 30.
Table 5.
Change in the ion/Ca2+ molar ratio (mean of days 15 and 30) of phloem sap and xylem sap exuded from excised roots and intact shoots of Nerium oleander plants due to 100 mm NaCl as affected by ammonium or nitrate as nitrogen source
| NH4+-N |
NO3−-N |
|||
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | |
| Phloem sap | ||||
| Na+/Ca2+ | 1·55 | 3·15 | 1·61 | 2·05 |
| K+/Ca2+ | 72·99 | 77·34 | 110·05 | 101·37 |
| Cl−/Ca2+ | 66·99 | 96·47 | 49·47 | 74·77 |
| Xylem root | ||||
| Na+/Ca2+ | 0·90 | 9·79 | 1·17 | 6·17 |
| K+/Ca2+ | 8·83 | 6·75 | 7·57 | 6·36 |
| Cl−/Ca2+ | 6·67 | 23·17 | 1·99 | 11·51 |
| Xylem stem | ||||
| Na+/Ca2+ | 0·65 | 11·94 | 0·65 | 8·27 |
| K+/Ca2+ | 23·35 | 41·68 | 30·92 | 43·60 |
| Cl−/Ca2+ | 17·12 | 40·30 | 13·67 | 26·63 |
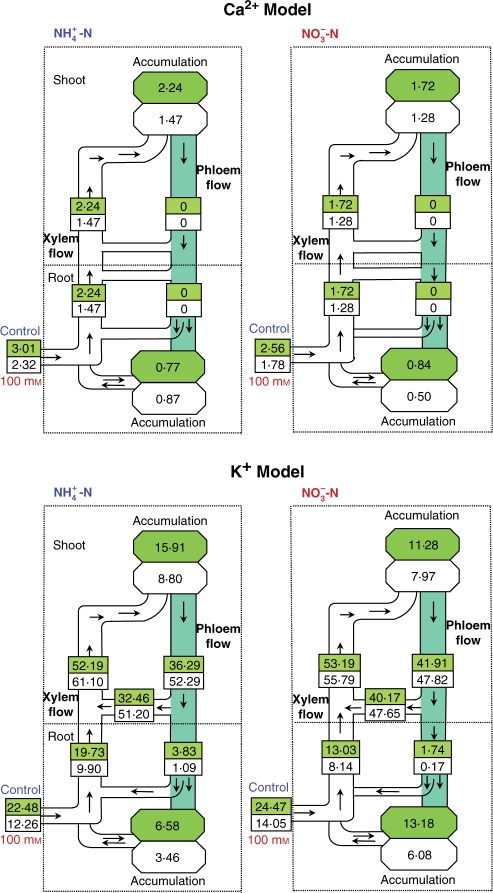
Total uptake of Ca2+ and its flow in the xylem was reduced by salinity in both nitrogen treatments (Fig. 4).
Fig. 4.
Effect of salinity (control vs. 100 mm NaCl) on the flow profile of net uptake, transport and accumulation of Ca2+ and K+ in Nerium oleander plants. Net uptake and accumulation were calculated from the difference in ion content at days 15 and day 30. Transport was calculated from the ion/Ca ratio obtained from analysis of xylem sap exuded from roots and stems. All numbers denote μmol g−1 d. wt d−1.
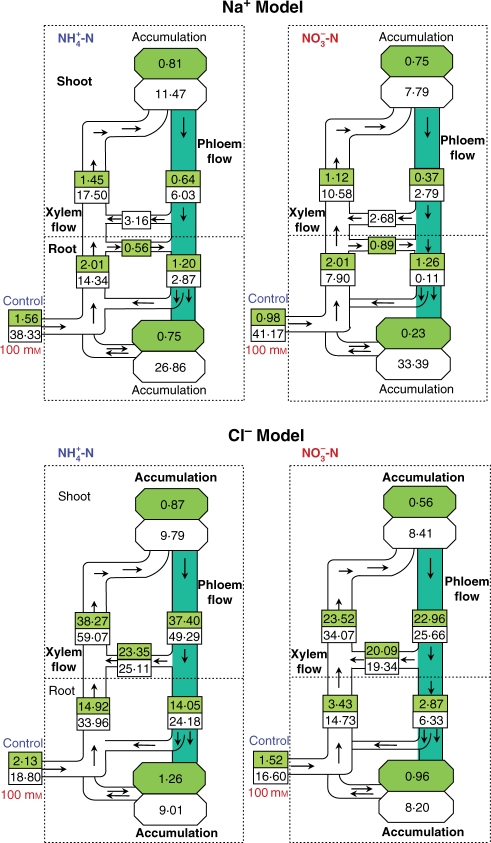
The flow of K+ in the root xylem (based on the K+/Ca2+ ratio in xylem sap exuded from the root) was much lower than the flow in shoot xylem (based on the K+/Ca2+ ratio in the xylem sap exuded from the shoot; Fig. 5). The only possible way to explain these differences in flows between roots and shoots would be release of this ion from phloem to xylem and its retranslocation back to the shoot (cycling). The cycling of K+ in shoots, i.e. the amount of K+ loading into the xylem from the phloem in the shoot before entering the root, was calculated from the difference in ion flow in the xylem in the shoot and that in the root. There was not a great difference in the uptake of K+ between the two sources of nitrogen, but uptake of K+ in the shoot was reduced by >54% due to salinity in NH4-fed plants compared with 47% in NO3-fed plants. The xylem root transport of K+ under saline conditions decreased markedly under both nitrogen treatments. The transport of K+ via xylem and phloem in shoots was more than three times higher than that in roots under both nitrogen treatments, and was increased by salinity.
Fig. 5.
Effect of salinity (control vs. 100 mm NaCl) on the flow profile of net uptake, transport and accumulation of Na+ and Cl− in Nerium oleander plants.. Net uptake and accumulation were calculated from the difference in ion content at days 15 and day 30. Transport was calculated from the ion/Ca ratio obtained from analysis of xylem sap exuded from roots and stems. All numbers denote μmol g−1 d. wt d−1.
The lower flow of Na+ in shoot xylem compared with that in roots under control conditions indicates resorption of Na+ from the xylem during the ascent of sap (Fig. 6). The resorption was calculated by the difference in ion flow in root xylem and shoot xylem. However, the greater flow of Na+ in shoot xylem compared with that in the roots under saline conditions indicates a greater release of Na+ from phloem to xylem during sap ascent. The Na+ uptake due to salinity was higher when plants received NO3 rather than when they were supplied with NH4, but most Na+ accumulated in the roots. In the NO3 treatment under saline conditions, about 81% of absorbed Na+ accumulated in roots, as compared with 70% in NH4-fed plants. Also, with saline treatment, transport of Na+ in both the xylem stream and the phloem in NH4-fed plants was almost twice that in NO3-fed plants. Under saline conditions, cycling of Na+ was markedly higher in NH4-fed plants than in NO3-fed plants.
Cl− uptake was higher in NH4-fed plants than in those fed NO3, and increased further due to salinity. The Cl− flow in xylem and phloem was much higher in both shoot and root in NH4-fed plants than in NO3-fed plants under control conditions, and increased much more under saline conditions in the NH4 treatment than in NO3-fed plants (Fig. 7). Cycling of Cl− in shoots was more than twice that in roots in both nitrogen treatments.
DISCUSSION
The objective of this study was to evaluate the effect of salinity on uptake, transport and accumulation of ions in N. oleander grown with either ammonium or nitrate. The higher salt tolerance of NO3-fed plants confirmed the results of a previous study (Abdolzadeh et al., 1998). The photosynthetic rate decreased more severely with saline treatment in NH4-fed plants. A higher transpiration rate and lower water-use efficiency were observed in NH4-fed plants compared with NO3-fed plants, even under control conditions. It has been reported that NH4-N increases stomatal conductance in white clover (Høgh-Jensen and Schjoerring, 1997), and enhances the transpiration rate in alfalfa (Khan et al., 1994) and sunflower (Ashraf, 1999). In both alfalfa and sunflower, leaf water and osmotic potential were lower than those of plants grown with NO3 under saline conditions, as was described previously in N. oleander (Abdolzadeh et al., 1998).
Under conditions of salinity, the concentration of Na+ in xylem sap exuded from roots was lower than that in the root medium, suggesting high K+ selectivity and/or K+/Na+ exchange across the plasmalemma of the root epidermis and cortex in both nitrogen treatments (Adler and Wilcox, 1995). The Na+ concentration in xylem sap exuded from the roots was higher than that in xylem sap exuded from stems, with a stronger decrease in NO3-fed plants than in those fed NH4. The prevention of Na+ accumulation in shoots by the maintenance of a low Na+ concentration in the ascending xylem sap can be realized by minimizing Na+ entry into the xylem from the root symplast, or by maximizing retrieval from the xylem before reaching sensitive tissues in the shoot. In this way, a H+/Na+ antiporter or Na+ uniporter could be responsible for the control of Na+ translocation to the shoot (Fortmeier and Schubert, 1995; Tester and Davenport, 2003). As a result, Na+ accumulated mostly in roots in NO3-fed plants and was transported less to shoots. In contrast, a higher amount of Na+ was transported to and accumulated in shoots in NH4-fed plants, probably causing toxicity.
The phloem exudate, which was collected by making shallow incisions in the stem of N. oleander, had a high carbon and nitrogen concentration, with a C/N ratio of around 20, a high sugar concentration and a high concentration of anions and cations (Table 3). These features are similar to those of phloem sap from a number of other species, including R. communis (Hall and Baker, 1972), L. albus (Pate and Sharkey, 1974), Brassica oleracea (Shelp, 1987), Nicotiana glauca (Hocking, 1980), Lactuca sativa (Van Helden et al., 1994) and Eucalyptus globulus (Pate et al., 1998), confirming that the collected fluid was, indeed, phloem sap.
Using a modelling technique (Armstrong and Kirkby, 1979; Touraine et al., 1988; Gouia et al., 1994), it has been possible to determine flows and accumulation of cations and Cl− via phloem and xylem in whole plants of N. oleander. Using this approach a minor error is inevitable when assuming zero Ca2+ transport in the phloem. Several investigators reported that calcium is taken up by roots from soil solution and delivered to the shoot via xylem and cannot be redistributed in the plant via the phloem (White and Broadley, 2003). Lambers et al. (1982) reported the finding of 45Ca in a part of the root system that was not supplied with labelled Ca in a split-root experiment. However, this error must be small considering the vastly different concentrations of Ca2+ and other ions in phloem and xylem sap. A similar assumption was made for calculation of nutrient flows in bean and cotton (Gouia et al., 1994), tobacco (Lu et al., 2005), wheat (Peng and Li, 2005) and maize (Niu et al., 2007). There was a large difference between flows of ions in roots and shoots via xylem and phloem. Cycling of ions from xylem to phloem and vice versa was the only way to balance transport. Three different cycling pathways were observed. First, the ions cycled only in shoots and did not enter the roots. In this way, translocated ions (mostly K+ and Cl−) in the phloem were again released into the xylem in the shoot. A change in concentration and composition of the phloem sap along the pathway between source and sink has been reported in several plants (Marschner, 1995). Secondly, ions translocated in the phloem towards the root via the phloem were transferred to the xylem (part of K+, Na+ and Cl−). In this pathway, cycling occurred in the whole plant. Comprehensive studies on mineral nutrient cycling in whole plants have been carried out in white lupin and castor bean (Jeschke and Pate, 1991; Jeschke et al., 1992; Peuke et al., 1996). Thirdly, cycling occurred only in roots; transported ions (Na+) in root xylem transferred to the phloem in roots (resorption) did not enter the shoot xylem stream. The short-term cycling of recently absorbed nutrients and long-term cycling by retranslocation of mobilized K+ have been reported in L. albus (Jeschke et al., 1992). Resorption (unloading) of Na+ from the xylem to living cells along the pathway of the xylem sap ascending from the roots to shoots has been reported by several investigators (Fortmeier and Schubert, 1995; Tester and Davenport, 2003).
In control plants, Na+ cycling occurred mostly in roots in both nitrogen treatments. However, under salinity, Na+ cycling in whole plants increased in both nitrogen treatments. In NO3-fed plants, a large amount of Na+ accumulated in roots and did not cycle in plants. Consequently, cycling of Na+ was higher in NH4-fed plants than in NO3-fed plants. It is generally accepted that reduced Na+ translocation from the root to the shoot contributes to the overall salt tolerance in glycophytes (Tester and Davenport, 2003). Jeschke and Pate (1991) reported marked retention of Na+ in the root of R. communis, leading to low intake of this ion by young leaves. The difference in cycling pattern of Na+ via xylem and phloem in NH4- and NO3-fed plants that caused the different accumulation of Na+ in roots and shoots plays an important role in salt tolerance of N. oleander. Probably, higher cycling and accumulation of Na+ in shoots led to higher toxicity, and this would be partly responsible for growth reduction in NH4-fed plants.
The K+ and Cl− concentrations indicated a similar pattern of cycling, presumably because these ions have a similarly high mobility in xylem and phloem (Marschner, 1995). Most cycling of these ions between phloem and xylem occurred in shoots, and only a small amount was translocated to roots and then recycled via the xylem. For K+ under NH4 nutrition, cycling plays an important role in maintenance of the charge balance in shoots and roots for NH4 uptake (Speer et al., 1994). The value of K+ uptake and accumulation in the shoot decreased most in NH4-fed plants compared with control plants with the salt treatment, which indicates a more harmful effect of salinity in these plants. However, cycling via xylem and phloem increased in shoots and decreased in roots due to salinity with both nitrogen treatments. Cycling of nutrients is a process that smoothes out fluctuations in external supply (Cooper et al., 1989).
The Cl− concentrations indicated a high mobility, and its flow via both xylem and phloem was high even in treatments without salinity, especially in NH4-fed plants. It seems that a high rate of transport of Cl− was not related to restriction of a high concentration of this ion from active leaves. However, Cl− might play a role in cation–anion balance during transport or in pH regulation (Jeschke et al., 1995). In NO3-fed plants, cycling of Cl− was not increased to the same extent by salinity as it was in NH4-fed plants. Jeschke et al. (1992) reported a marked increase in Na+ and Cl− flows and uptake in the presence of NaCl in white lupin. Also, increases in Na+ and Cl− concentrations in phloem sap have been reported in maize under saline conditions (Lohaus et al., 2000). These authors estimated that 13–36% of Na+ and Cl− imported into the leaves through the xylem was exported again via the phloem. High cycling and accumulation of Cl− might also increase toxicity and inhibition of growth in NH4-fed plants.
Modelling of flows provides new information on possible causes of salt damage under different nitrogen sources. Better acclimation of NO3-fed plants was probably related to a lower transport to and accumulation of Na+ and Cl− in the shoot, and a better balance of K+ accumulation in both shoots and roots. On the other hand, in NH4-fed plants, increased accumulation and cycling of Na+ and Cl− and decreased accumulation of K+ in shoots due to salinity probably led to harmful effects.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of the Ministry of Education, Science and Culture of Japan. Also, we are indebted to students of the Laboratory of Applied Plant Ecology in Okayama University for technical assistance.
LITERATURE CITED
- Abdolzadeh A, Shima K, Chiba K. Role of ammonium and nitrate as nitrogen source on salt tolerance in Nerium oleander L. Journal of the Japanese Society of Revegetation Technology. 1998;23:237–248. [Google Scholar]
- Adler PR, Wilcox GE. Ammonium increases the net rate of sodium influx and partitioning to the leaf of muskmelon. Journal of Plant Nutrition. 1995;18:1951–1962. [Google Scholar]
- Armstrong MJ, Kirkby EA. Estimation of potassium recirculation in tomato plants by comparison of the rates of potassium and calcium accumulation in the tops with their fluxes in the xylem stream. Plant Physiology. 1979;63:1143–1148. doi: 10.1104/pp.63.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. Interactive effect of salt (NaCl) and nitrogen source on growth, water relations and photosynthetic capacity of sunflower (Helianthus annuus L.) Annals of Applied Biology. 1999;135:509–513. [Google Scholar]
- Björkman O, Powles SB. Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta. 1984;161:490–504. doi: 10.1007/BF00407081. [DOI] [PubMed] [Google Scholar]
- Cooper HD, Clarkson DT. Cycling of amino-nitrogen and other nutrients between shoots and roots in cereals – a possible mechanism integrating shoot and root in the regulation of nutrient uptake. Journal of Experimental Botany. 1989;40:753–762. [Google Scholar]
- Demmig B, Winter K, Kruger A, Czygan F. Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiology. 1988;87:17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortmeier R, Schubert S. Salt tolerance of maize (Zea mays L.): the role of sodium exclusion. Plant, Cell and Environment. 1995;18:1041–1047. [Google Scholar]
- Frechilla S, Lasa B, Ibarretxe L, Lamsfus C, Aparicio-Tejo P. Pea responses to saline stress is affected by the source of nitrogen nutrition (ammonium or nitrate) Plant Growth Regulation. 2001;35:171–179. [Google Scholar]
- Gibson AH. Evaluation of nitrogen fixation by legumes in the greenhouse and growth chamber. In: Elkan GH, editor. Symbiotic nitrogen fixation technology. New York: Marcel Dekker; 1987. pp. 321–369. [Google Scholar]
- Gouia H, Ghorbal MH, Touraine B. Effects of NaCl on flows of N and mineral ions and on NO3− reduction rate within whole plants of salt-sensitive bean and salt-tolerant cotton. Plant Physiology. 1994;105:1409–1418. doi: 10.1104/pp.105.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajji M. Effect of salt on the growth and mineral nutrition of oleander. Physiologie Végétale. 1979;17:517–524. [Google Scholar]
- Hall SM, Baker DA. The chemical composition of Ricinus phloem exudate. Planta. 1972;106:131–140. doi: 10.1007/BF00383992. [DOI] [PubMed] [Google Scholar]
- Hocking PJ. Redistribution of nutrient elements from cotyledons of two species of annual legumes during germination and seedling growth. Annals of Botany. 1980;45:383–396. [Google Scholar]
- Høgh-Jensen H, Schjoerring JK. Effects of drought and inorganic N source on nitrogen fixation and carbon isotope discrimination in Trifolium repens. Plant Physiology and Biochemistry. 1997;35:55–62. [Google Scholar]
- Huxley A, Griffiths M, Levy M. The new Royal Horticultural Society dictionary of gardening. London: Macmillan Press; 1992. [Google Scholar]
- Jeschke WD, Pate JS. Cation and chloride partitioning through xylem and phloem within the whole plant of Ricinus communis L. under conditions of salt stress. Journal of Experimental Botany. 1991;42:1105–1116. [Google Scholar]
- Jeschke DW, Pate JS. Mineral nutrition and transport in xylem and phloem of Banksia prionotes (Proteaceae), a tree with dimorphic root morphology. Journal of Experimental Botany. 1995;46:895–905. [Google Scholar]
- Jeschke WD, Wolf W, Hartung W. Effect of NaCl salinity on flows and partitioning of C, N, and mineral ions in whole plants of white lupin, Lupinus albus. Journal of Experimental Botany. 1992;43:777–788. [Google Scholar]
- Jeschke WD, Klagges S, Hilpert A, Bhatti AS, Sarwar G. Partitioning and flow of ions and nutrients in salt-treated plants of Leptochloa fusca L. Kunth. New Phytologist. 1995;130:23–35. [Google Scholar]
- Khan MG, Silberbush M, Lips SH. Physiological studies on salinity and nitrogen interaction in alfalfa. II. Photosynthesis and transpiration. Journal of Plant Nutrition. 1994;17:669–682. [Google Scholar]
- Lambers H, Simpson RJ, Beilharz VC, Dalling MJ. Growth and translocation of C and N in wheat (Triticum aestivum) grown with a split root system. Physiologia Plantarum. 1982;56:421–429. [Google Scholar]
- Leidi EO, Silberbush M, Lips SH. Wheat growth as affected by nitrogen type, pH and salinity. I. Biomass production and mineral composition. Journal of Plant Nutrition. 1991;14:235–24. [Google Scholar]
- Lewis OAM, Leidi EO, Lips SH. Effect of nitrogen source on growth response to salinity stress in maize and wheat. New Phytologist. 1989;111:155–160. doi: 10.1111/j.1469-8137.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Lohaus G, Hussmann M, Pennewiss K, Schneider H, Zhu JJ, Sattelmacher B. Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. Journal of Experimental Botany. 2000;51:1721–1732. doi: 10.1093/jexbot/51.351.1721. [DOI] [PubMed] [Google Scholar]
- Lu YX, Li CJ, Zhang FS. Transpiration, potassium uptake and flow in tobacco as affected by nitrogen forms and nutrient levels. Annals of Botany. 2005;99:153–160. doi: 10.1093/aob/mci104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Munns R. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell and Environment. 1993;16:15–24. [Google Scholar]
- Niu J, Chen F, Mi G, Li C, Zhang F. Transpiration, and nitrogen uptake and flow in two maize (Zea mays L.) inbred lines as affected by nitrogen supply. Annals of Botany. 2007;99:153–160. doi: 10.1093/aob/mcl237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida AK, Dasa AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Pate JS, Sharkey PJ. Phloem bleeding from legume fruits – a technique for study of fruit nutrition. Planta. 1974;120:229–243. doi: 10.1007/BF00390291. [DOI] [PubMed] [Google Scholar]
- Pate J, Shedley E, Arthur D, Adams M. Spatial and temporal variations in phloem sap composition of plantation-grown Eucalyptus globules. Oecologia. 1998;117:312–322. doi: 10.1007/s004420050664. [DOI] [PubMed] [Google Scholar]
- Peng Z, Li C. Transport and partitioning of phosphorus in wheat as affected by P withdrawal during flag-leaf expansion. Plant and Soil. 2005;268:1–11. [Google Scholar]
- Peuke AD, Glaab J, Kaiser WM, Jeschke DW. The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. IV. Flow and metabolism of inorganic nitrogen and malate depending on nitrogen and salt treatment. Journal of Experimental Botany. 1996;47:377–385. [Google Scholar]
- Shelp BJ. The composition of phloem exudate and xylem sap from broccoli (Brassica oleracea var italica) supplied with NH4+, NO3− or NH4NO3. Journal of Experimental Botany. 1987;38:1603–1618. [Google Scholar]
- Silberbush M, Lips SH. Nitrogen concentration, ammonium/nitrate ratio and NaCl interaction in vegetative and reproductive growth of peanuts. Physiologia Plantarum. 1988;74:493–498. [Google Scholar]
- Speer M, Brune A, Kaiser WM. Replacement of nitrate by ammonium as the nitrogen source increases the salt sensitivity of pea plants. I. Ion concentrations in roots and leaves. Plant, Cell and Environment. 1994;17:1215–1221. [Google Scholar]
- Tester M, Davenport R. Na+ tolerance Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine B, Grignon N, Grignon C. Charge balance in NO3-fed soybean, estimation of K+ and carboxylate recirculation. Plant Physiology. 1988;88:605–612. doi: 10.1104/pp.88.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden M, Freddy W, Van Bee TA. Phloem sap collection from lettuce (Lactuca sativa L.): chemical comparison among collection methods. Journal of Chemical Ecology. 1994;20:1573–1561. doi: 10.1007/BF02033720. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Annals of Botany. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]