Abstract
Background and Aims
Plants adapted for pollination by rodents tend to exhibit a distinct floral syndrome that includes dull coloured and geoflorous inflorescences and nocturnal anthesis and nectar production. On the basis of their floral traits, it was predicted that two African Colchicum species (C. scabromarginatum and C. coloratum) are rodent-pollinated.
Methods
Field studies were carried out in the semi-arid Succulent Karoo region of South Africa. Live trapping of rodents was conducted and pollen loads on the rodents were quantified. The daily periodicity of nectar production was determined. Selective exclusion and controlled pollination experiments were also conducted.
Key Results
Live-trapped rodents were found to carry large amounts of Colchicum pollen on the fur of their snouts, and in their faeces. Birds were occasional pollinators of flowers of C. coloratum. During the evening, nectar volume and concentration increased for both species. When vertebrates were excluded from C. scabromarginatum and C. coloratum plants, there was a significant decrease in seed set compared with open control plants. By contrast, vertebrate exclusion did not significantly affect seed production of a congener, C. hantamense, which has floral traits associated with insect pollination. Breeding system experiments revealed that both C. scabromarginatum and C. coloratum require pollinators for seed production. Colchicum scabromarginatum is strictly self-incompatible, whereas C. coloratum is partially self-compatible.
Conclusions
Pollination by rodents occurs in two African Colchicum species. C. scabromarginatum appears to depend exclusively on rodents for seed production, while birds and autonomous selfing may contribute to seed production in C. coloratum. These are the first records of rodent pollination in the Colchicaceae.
Key words: Convergent evolution, floral syndrome, pollination, rodents, birds, insects, Colchicum scabromarginatum, Colchicum coloratum, Succulent Karoo, southern Africa
INTRODUCTION
The semi-arid Succulent Karoo region of South Africa is considered to be a biodiversity hotspot (Mucina et al., 2006). Reasons for this diversity are varied and may include pollinator-driven speciation (Johnson, 2006; van der Niet et al., 2006). The evolution of a rodent pollination system in a succulent Karoo geophyte, Massonia depressa (Hyacinthaceae), was reported by Johnson et al. (2001). These authors predicted that other geophytes in the same area are also rodent-pollinated. This prediction was based on apparent convergent floral morphology between flowers of these geophytes and rodent-pollinated Cape proteas (cf. Rourke and Wiens, 1977; Wiens and Rourke, 1978; Wiens et al., 1983). In the present study, two Colchicum species (C. scabromarginatum and C. coloratum) from the succulent Karoo region were identified as likely candidates for a rodent pollination system on the basis of their ‘therophilous’ floral traits.
Plants adapted for pollination by non-flying mammals (including rodents, marsupials and primates) tend to have robust flowers that are dull in colour, cup-shaped and situated at ground level (geoflorous) (Wiens and Rourke, 1978). They also produce copious amounts of nectar and a have a stigma–nectar distance of about 10 mm (Wiens et al., 1983). The time of flowering of non-flying mammal-pollinated plants tends to be late winter, which has been suggested to reflect the willingness of mammals to supplement their diet with nectar at this time of low food availability (Rourke and Wiens, 1977).
Many authors have remarked on the ‘yeasty’ odour of the flowers of such plants. This is assumed to be the primary cue for long-distance attraction as mammals, such as rodents, have a well-developed sense of smell and the well-hidden flowers are usually pollinated at night when visual cues would not be effective (Rourke and Wiens, 1977; Rebelo and Breytenbach, 1987). Scent emission, nectar secretion and floral anthesis tend to be nocturnal, coinciding with the activity patterns of many mammals, such as most rodents (Wiens et al., 1983; Johnson et al., 2001).
Verification of non-flying mammal pollination is difficult because the animals' nocturnal habits largely preclude direct field observations. Evidence must be obtained from other lines of investigation, which may include examination of pollen loads on the fur and in the faeces of trapped animals (Carthew and Goldingay, 1997) and selective exclusion of vertebrates from flowers. If the latter treatment results in reduced fecundity, and birds and bats can be excluded as flower visitors using other evidence, then pollination by rodents can be inferred.
To test the hypothesis that C. scabromarginatum and C. coloratum subsp. coloratum are pollinated by rodents, the following research questions were addressed. (1) Do plants have a breeding system that makes them dependent on pollinator visits for seed production? (2) Are floral morphology, nectar properties and nectar secretion patterns consistent with rodent pollination? (3) Do rodents visit flowers and act as pollen vectors for the study species? (4) Does experimental exclusion of rodents result in diminished seed production?
MATERIALS AND METHODS
Study species and study sites
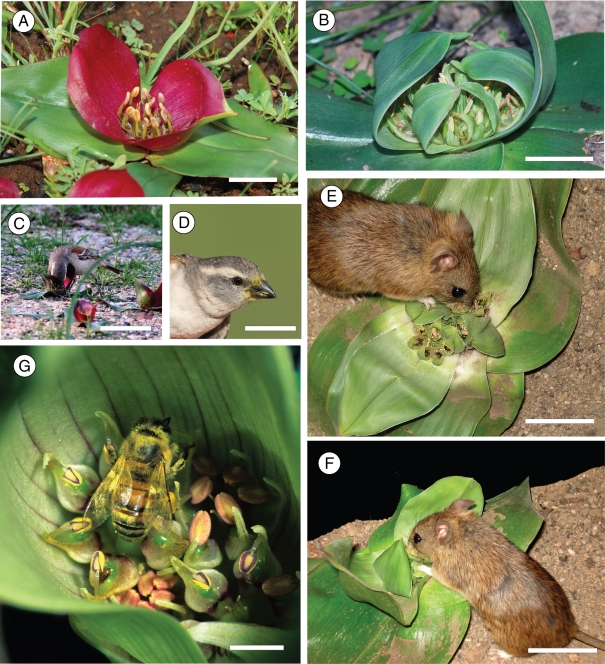
All species of the genus Androcymbium (±60 species) have been included within an expanded circumscription of the genus Colchicum (±90 species; Manning et al., 2007). The expanded genus, Colchicum, family Colchicaceae, is defined by its reduced or absent stem, androecial nectaries and 2–4-porate pollen (Manning et al., 2007; Vinnersten and Manning, 2007). The genus Colchicum is widely distributed through Africa and the Mediterranean, with marked centres of diversity in winter-rainfall regions of both hemispheres (Vinnersten and Manning, 2007). The flowers of both Colchicum scabromarginatum (Schltr. and K.Krause) J.C.Manning and Vinn (= Androcymbium scabromarginatum) and C. coloratum J. C. (Manning and Vinn) (= Androcymbium pulchrum) are geoflorous and the inflorescences are robust and cup-shaped. Like rodent-pollinated proteas (Wiens et al., 1983), these two Colchicum species also flower in winter. Colchicum scabromarginatum has the dull-coloured floral bracts that are typical of rodent-pollinated plants (Fig. 1B), whereas in C. coloratum the bracts are a reddish colour (Fig. 1A) (Membrives et al., 2002). The third species included in this study is Colchicum hantamense (Engl.) J.C.Manning and Vinn. This species displays insect pollination characteristics, such as white-coloured floral bracts (Membrives et al., 2002) and a sweet spicy scent. Honeybees frequently visit flowers of this species (Fig. 1G; C. Kleizen and S. D. Johnson, pers. obs.). Therefore, Colchicum hantamense was used for comparisons with an insect-pollinated congener. Voucher specimens of the three study species are deposited in the Bews Herbarium, Pietermaritzburg.
Fig. 1.
(A) Inflorescence of Colchicum coloratum. Scale bar = 5 cm. (B) Inflorescence of Colchicum scabromarginatum. Scale bar = 5 cm. (C) Cape sparrow visiting a C. coloratum inflorescence at site 1. Scale bar = 10 cm. (D) C. coloratum pollen deposited on the feathers surrounding the beak of the bird. Scale bar = 5 cm. (E, F) Aethomys pushes its head among C. scabromarginatum anthers to reach the nectar, with pollen visibly dusted on the snout of the rodent. Scale bar = 5 cm. (G) A honeybee visiting a C. hantamense inflorescence. Scale bar = 5 mm.
This study was carried out in the semi-arid Succulent Karoo region of South Africa during August, 2006 and July–September, 2007. Two large populations of C. scabromarginatum (>1000 plants) were located in sparse vegetation on Naries farm 30 km north of Springbok. One population was on the top of a ridge (29°41′S, 17°39′E, elevation 794 m) and the other occurred alongside a dam (29°41′S, 17°40′E, elevation 703 m). Several populations of C. coloratum were located in the vicinity of Niewoudtville. These populations were situated at the following sites: an empty plot in the centre of town (31°22′S, 019°06′E, elevation 713 m), a dolerite ridge on the farm Glen Lyon (31°25′S, 019°09′E, elevation 774 m), a slope on Glen Lyon farm (31°23′S, 019°09′E, elevation 742 m), alongside a river on Glen Lyon farm (31°22′S, 019°15′E, elevation 703 m), the Nieuwoudtville Flower Reserve (31°21′S, 019°08′E, elevation 747 m), Matjiesfontein farm (31°28′S, 019°04′E, elevation 700 m) and Hotbergfontein farm (31°22′S, 019°12′E, elevation 758 m). The population of Colchicum hantamense that we studied was located in the Nieuwoudtville Flower Reserve(31°21′S, 019°08′E, elevation 761 m).
Breeding system
To establish whether the study species depend on pollinator visits for seed production and whether or not they possess a genetic self-incompatible system, a breeding system experiment was conducted. Twenty inflorescences of both C. scabromarginatum and C. coloratum were covered by pollinator-excluded cages while the plants were in the budding phase. Once flowers had opened, three treatments were applied to each inflorescence: (1) pollinated by hand with pollen from a different plant (after which the flower was emasculated), (2) pollinated by hand with self-pollen to determine whether plants are self-compatible and (3) unmanipulated to test for autonomous self-fertilization. At the end of the flowering season, the number of seeds in one locule per flower was counted and multiplied by three to estimate the number of seeds per flower (there are three locules per flower).
Floral and nectar characteristics
In order to determine daily variation in nectar, the standing crop of nectar was measured from all six nectaries of ten different randomly selected flowers every 3 h for approximately 24 h for both C. scabromarginatum and C. coloratum. Nectar volume was measured using 100-μL capillary tubes (Drummond Scientific Company, Broomall, PA, USA) and the nectar concentration was quantified using a 0–50 % field refractometer (Bellingham and Stanley, Tunbridge Wells, UK). The pattern of floral anthesis in C. coloratum was obtained by recording the number of open flowers on 20 inflorescences approximately every 4 h for 22 h. For C. scabromarginatum, C. coloratum and C. hantamense, the floral dimensions were measured from ten flowers, each sampled from different plants, and rounded off to the nearest 0·5 mm.
Rodent trapping and pollen loads
On the nights of the 17, 18, 19 and 20 July, 2007, 60 Sherman traps were laid out in the C. scabromarginatum population. On the nights of the 8, 9 and 10 August, 2006, 84 ‘gutter-pipe’ traps were laid out amongst the C. coloratum inflorescences on the dolerite ridge on Glen Lyon farm. Between 6 August and 12 September, 2007, 90 traps were set every evening for three consecutive nights at each of the six C. coloratum sites. Traps were set at dusk and laid out in lines of 15 traps, with 4 m between each trap; all traps were baited with rolled peanut butter and oats. Traps were checked in the early morning (between 0600 and 0700 h) and once a rodent had been captured, that trap was not re-used. Captured rodents were identified and temporarily placed in a plastic bag with a hole in one corner through which the snout of the rodent protruded. The fur just around the nose of each trapped rodent was swabbed for 10 s with a small block of fuschin gelatine (Beattie, 1971). Each of the fuschin gelatine samples was then melted onto a slide and the number of pollen grains was counted over three scans of the length of the coverslip. Rodent faeces were collected from the traps and stored in 70 % alcohol. Liquid fuschin gelatine was added to this solution, and this mixture was mounted on a slide. These samples were also examined microscopically for the presence of pollen. This would include pollen ingested directly, through feeding and indirectly through grooming (Fleming and Nicolson, 2002).
As it is virtually impossible to observe rodents in the field at night (Wiens et al., 1983), we recorded the foraging behaviour of captured rodents. An individual Aethomys namaquensis (Namaqua rock mouse) was released into a glass tank (100 cm long by 100 cm wide) with a 10-cm-deep layer of sand containing four fresh C. scabromarginatum inflorescences. Fresh Oxalis flowers from the same area were also placed in the tank in order to determine if the rodents visit flowers selectively. In another experiment, an Aethomys namaquensis individual and Gerbillurus paeba (Hairy-footed gerbil) individual were released separately into a tank with four fresh C. coloratum inflorescences. Later, inflorescences of the apparently insect-pollinated species C. hantamense were placed in the same tank. The foraging behaviour of the rodents was observed from 1800 until 0100 h.
Observations during daylight were conducted for a total of 10 h in the C. scabromarginatum populations and a total of 20 h in the C. coloratum populations. The C. hantamense population was observed for 4 h. During the field observations of C. coloratum at site 1, birds (sparrows, weaverbirds and starlings only) were observed to visit the flowers and feed on nectar (Fig. 1C), with pollen visibly dusted on the feathers surrounding the beaks of the birds (Fig. 1D). Subsequently, 4 h of mist-netting was conducted at site 1 in order to catch birds after a feeding bout to verify if the pollen originated from Colchicum flowers. Captured birds were identified and the feathers surrounding the beak of the bird were swabbed with a small block of fuschin gelatine (Beattie, 1971). Microscopic slides were created and analysed using the same procedure as from captured rodents. No birds were observed visiting C. coloratum inflorescences at any of the other sites.
Selective exclusion experiments
In order to investigate the importance of rodents for seed production, ten pairs of C. scabromarginatum plants from the ridge population at Naries and ten pairs of C. coloratum plants at Glen Lyon were selected. One plant per pair was enclosed in a wire cage with a mesh diameter of 15 × 20 mm. This enclosure excluded rodents but allowed insects free access to the flowers. The other plant was left unmanipulated as a control. At the end of the flowering season, the number of seeds per flower was counted. This experiment was also conducted on C. hantamense. Given observations of bee visitation to flowers of C. hantamense, selective exclusion of rodents was expected to have no effect on seed set of this species.
RESULTS
Breeding system
Cross-pollination of both C. scabromarginatum and C. coloratum plants resulted in significantly higher seed set than when the plants were self-pollinated or unmanipulated (Table 1). Colchicum scabromarginatum plants only set seed when cross-pollinated. Colchicum coloratum plants set only one-third as many seeds when self-pollinated as when cross-pollinated and even fewer seeds when left unmanipulated (Table 1). All Colchicum species are hypogynous (Meyer, 2000), and the three study species appeared to be protogynous.
Table 1.
Results of controlled pollination experiments to determine the breeding system of Colchicum scabromarginatum and C. coloratum
| Seeds per flower |
||||||
|---|---|---|---|---|---|---|
| Species | n (plants) | Unmanipulated | Self-pollinated | Cross-pollinated | χ2 | P |
| C. scabromarginatum | 20 | 0 (0–6) | 0 (0–12) | 114 (15–228) | 27·9 | <0·0001 |
| C. coloratum | 20 | 3 (0–24) | 18 (0–72) | 52·5 (6–150) | 36·8 | <0·0001 |
Values are medians (range) and were analysed using Friedman's test with plant treated as a blocking factor.
Floral traits
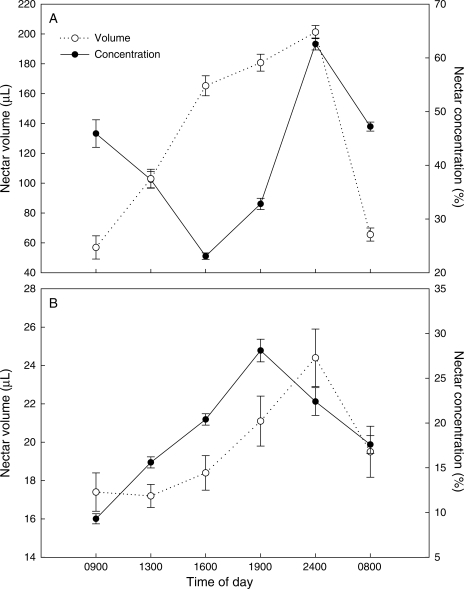
Large volumes of nectar (up to 210 µL) were found in individual C. scabromarginatum flowers during the night. In C. scabromarginatum the maximum average volume of nectar (193·3 µL) was secreted at midnight, after which the average volumes declined until after midday when they started increasing again (Fig. 2A). The average concentration of C. scabromarginatum nectar increased steadily throughout the day, reaching a maximum of 64·8 % sucrose at midnight (Fig. 2A). In C. coloratum the average nectar volume and concentration increased slowly throughout the day, reaching a maximum volume of 24·4 µL at midnight and maximum concentration of 28·1 % sucrose at 1900 h (Fig. 2B). The sticky nectar secreted by C. scabromarginatum and C. coloratum is readily apparent, whereas the nectar secreted by C. hantamense is difficult is see with the naked eye.
Fig. 2.
Daily variation in the average nectar volume and concentration per flower for (A) Colchicum scabromarginatum and (B) C. coloratum. Bars represent ± 1 s.e.
In the genus Colchicum, the nectar is secreted from androecial nectaries, which are situated at the base of the filaments (Manning et al., 2007). The nectar wells up into a chamber which is an average of 5·25 mm wide by 3·7 mm deep in C. scabromarginatum and 3·65 mm wide by 2·65 mm deep in C. coloratum (Table 2). The nectar chambers of C. scabromarginatum and C. colchicum were noticeably larger, with a more sculptured structure, in contrast to the significantly smaller chamber size of C. hantamense (2·4 mm wide by 1·55 mm deep, Table 2). The results from the floral measurements (Table 2) show that both C. scabromarginatum and C. coloratum follow the 10-mm rule of the therophilous syndrome and that the average stigma–nectar distances of both species are significantly greater than that of C. hantamense (F2,27 = 83·002, P = 0·000001). The floral measurements show that the mean inflorescence diameter and the mean number of flowers per inflorescence were greater in C. scabromarginatum than in C. coloratum and C. hantamense (Table 2). The uppermost bracts of C. scabromarginatum curve over the flowers and were also thicker and appeared less likely to tear or rip than the bracts of either C. coloratum or C. hantamense.
Table 2.
Floral characteristics measured for each of the Colchicum species
| Characteristic | C. scabromarginatum | C. coloratum | C. hantamense |
|---|---|---|---|
| Nectar chamber width (mm) | 5·25 ± 0·23a | 3·65 ± 0·18b | 2·4 ± 0·21c |
| Nectar chamber depth (mm) | 3·7 ± 0·2a | 2·65 ± 0·15b | 1·55 ± 0·12c |
| Anther height (mm) | 10·25 ± 0·29a | 11·7 ± 0·4a | 4·65 ± 0·24b |
| Stigma height (mm) | 8·95 ± 0·68a | 14·8 ± 1·22b | 7·3 ± 0·47a |
| Stigma–nectar distance (mm) | 10·2 ± 0·47a | 13 ± 0·61b | 4·6 ± 0·23c |
| Diameter of inflorescence (cm) | 14·36 ± 1·63a | 5·1 ± 0·38b | 3·46 ± 0·9b |
| Flowers per inflorescence | 11·8 ± 1·17a | 3·1 ± 0·23b | 3·3 ± 0·6b |
Values are means ± s.e. and dissimilar letters indicate significant (P < 0·05) differences between the species, as determined by one-way ANOVA followed by post-hoc Tukey LSD tests (n = 10).
At the start of the floral anthesis experiment (0800 h), there were 14 open flowers (0·7 per inflorescence) on the 20 marked C. coloratum inflorescences. After approximately 24 h, there were 42 open flowers (2·1 per inflorescence) on these inflorescences. Floral anthesis took place mainly in the afternoon. In the 4-h period between 1200 and 1600 h, 11 flowers (39 %) opened. The number of flowers that opened per time period differed significantly from values expected if opening was random (χ2 = 12·5, P < 0·05).
Rodent trapping and pollen loads
Trapping in the C. scabromarginatum population resulted in the capture of eight individual rodents, all Aethomys namaquensis, commonly known as the namaqua rock mouse (family Muridae, subfamily Murinae; Table 3). A total of 28 individuals representing four rodent species were captured when trapping was conducted in the C. coloratum populations (Table 3). Nocturnally active rodents included two murid species (family Muridae, subfamily Murinae), Aethomys namaquensis and Mus minutoides (the pygmy mouse), and one gerbil species (family Muridae, subfamily Gerbillinae), Gerbillurus paeba (the hairy-footed gerbil). Rhabdomys pumilio (family Muridae, subfamily Murinae; the Cape striped field mouse) was the only diurnal species captured. Rodents were captured amongst C. coloratum inflorescences at all of the six sites except for site 1. Microscopic examination of the fuschin gelatin blocks showed that C. scabromarginatum pollen was the only pollen present and was abundant on the snouts (mean of 154 ± 19 grains per slide) and in the faeces (mean of 21·3 ± 4·8 grains per slide) of all of the eight captured rodents from the C. scabromarginatum population (Table 3). Colchicum coloratum pollen was found on the snouts of 24 of the 28 captured rodents from the C. coloratum populations (mean of 29 ± 5·8 grains per slide). Faeces from all of the 28 rodents contained C. coloratum pollen (mean of 214 ± 34·5 grains per slide) (Table 3). In the rodents captured amongst C. coloratum plants, there was only one other type of pollen (an unidentified Asteraceae) and this was very sparse.
Table 3.
Pollen loads of the rodents captured at the different sites
| Year | Site | Rodent species | No. of animals captured | No. of animals with pollen on snout (mean pollen count per slide ± s.e.) | No. of animals with pollen in faeces (mean pollen count per slide ± s.e.) |
|---|---|---|---|---|---|
| C. scabromarginatum | |||||
| 2007 | Naries | Aethomys namaquensis | 8 | 8 (154 ± 19) | 8 (21·3 ± 4·8) |
| C. coloratum | |||||
| 2006 | Glen Lyon | Aethomys namaquensis | 9 | 6 (5·5 ± 2·6) | 9 (212 ± 155·6) |
| 2007 | Site 1 | 0 | – | – | |
| Site 2 | Gerbillurus paeba | 1 | 1 (26) | 1 (155) | |
| Site 3 | Mus minutoides | 2 | 2 (14 ± 0·5) | 2 (125 ± 0) | |
| Rhabdomys pumilio | 1 | 1 (20) | 1 (185) | ||
| Site 4 | Aethomys namaquensis | 3 | 3 (63 ± 16·7) | 3 (405 ± 159·4) | |
| Mus minutoides | 1 | 1 (22) | 1 (94) | ||
| Site 5 | Aethomys namaquensis | 3 | 3 (54 ± 8·4) | 3 (411 ± 129·2) | |
| Rhabdomys pumilio | 2 | 2 (43 ± 12·5) | 2 (232 ± 43·5) | ||
| Site 6 | Rhabdomys pumilio | 2 | 2 (28 ± 11) | 2 (158 ± 80) | |
| Gerbillurus paeba | 4 | 3 (17 ± 7·2) | 4 (166 ± 14·5) | ||
The captive rodents all visited the respective Colchicum inflorescences at approximately midnight (Fig. 1E, F). The animals moved between all the inflorescences and had pollen dusted on their snouts (Fig. 1E, F). The pollen-covered snouts of the rodents made contact with the stigmas while they lapped nectar. As the rodents lapped up the nectar, their snouts moved very rapidly and appeared to push down firmly into the flower. However, the flowers were not damaged in any way after each feeding bout. All the rodents ignored the other flowers placed in the tanks (Oxalis and C. hantamense, respectively). After visiting all the flowers in the tank, the rodents spent several minutes grooming pollen from their fur.
During the field observations, no insects were observed to visit either C. scabromarginatum or C. coloratum, but bees were frequently observed to visit C. hantamense inflorescences. From 4 h of mist-netting for birds at C. coloratum site 1, a total of 19 birds were captured. Microscopic analysis of the fuschin gelatine samples showed that the Cape sparrow, Passer melanurus (Family Passeridae), the Cape weaver, Ploceus capensis (Family Ploceidae) and the masked weaver, Ploceus intermedius (Family Ploceidae) carried large amounts of C. coloratum pollen on their feathers (an average of 614 pollen grains, s.e. = 112, n = 18). The Cape glossy starling, Lamprotornis nitens (Rafinesque) (family Sturnidae), however, was also captured in the mist-net after feeding on C. coloratum nectar, yet there was no pollen found on the feathers of this bird.
Selective exclusion experiment
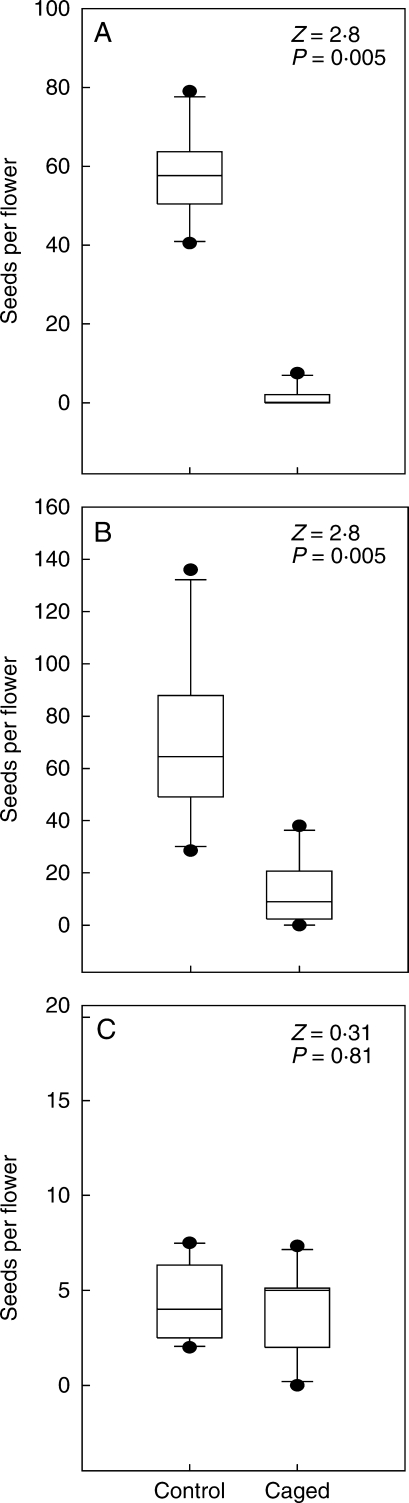
The exclusion of rodents from C. scabromarginatum and C. coloratum plants resulted in a significant decline in seed set relative to the unmanipulated controls, but this was not evident in the apparently insect-pollinated species C. hantamense (Fig. 3). Excluding rodents from the C. scabromarginatum, C. coloratum and C. hantamense flowers reduced seed set by approximately 97, 82 and 8 %, respectively (Fig. 3).
Fig. 3.
The effect of exclusion of vertebrates on the median number of seeds produced per flower in plants of (A) Colchicum scabromarginatum, (B) C. coloratum and (C) C. hantamense. Z values were obtained from the Wilcoxon test for paired samples. Samples sizes were 13 plants per treatment group for all species.
DISCUSSION
The results of the present study show that both C. scabromarginatum and C. coloratum are rodent-pollinated species. Captive rodents willingly and non-destructively foraged on inflorescences of these species, but not on those of the bee-pollinated congener C. hantamense, and are clearly effective pollen vectors. Copious amounts of Colchicum pollen was deposited on the snouts of field-caught rodents as well as those in the tank experiments (Fig. 1E, F). The cup-like shape of the inflorescence and the distance between the nectar and stigma in C. scabromarginatum and C. coloratum (greater than 10 mm) ensures that a rodent's snout fits neatly into the flower and brushes against the stigmas and anthers while the animal laps nectar from the nectary chambers (Fig. 1E, F). The position of the uppermost bracts curved over the flowers of C. scabromarginatum would most likely make it more difficult for insects or birds to reach the flowers than rodents, which are able to push the bracts aside to reach the nectar.
The breeding experiment showed that both of the study species are dependent on pollinator visits for seed production – C. scabromarginatum is a strongly self-incompatible species, while C. coloratum is partially self-incompatible. The latter result is consistent with the findings of Membrives et al. (2002), who performed breeding system experiments on plants of C. coloratum that had been grown from seed and concluded that the species was ‘preferentially self-incompatible’.
Experimental exclusion of vertebrates resulted in sharp and significant decreases in seed set in both C. scabromarginatum and C. coloratum (Fig. 3). This was consistent with the apparent complete absence of insect visitors to inflorescences of these species. Although birds and bats would also have been excluded with this technique, the effect of the treatment was almost certainly due to rodent exclusion as birds were not observed as flower visitors in the populations in which this experiment was conducted and flower-feeding bats are not known from the Succulent Karoo region. These results contrast with those obtained for the insect-visited species C. hantamense in which vertebrate exclusion had no effect on seed set (Fig. 3). As C. hantamense is not capable of autogamy (Membrives et al., 2002), this lack of an effect of caging indicates that the cages did not hinder access by insect pollinators.
By flowering in winter and early spring, C. scabromarginatum and C. coloratum, like therophilous Protea species, may provide an important energy source for rodents such as Aethomys namaquensis that breed in late July (Fleming and Nicolson, 2002). The primarily nocturnal anthesis and nectar secretion patterns also correlate with the nocturnal activity of rodent pollinators. The large mean standing crop of nectar in C. scabromarginatum and C. coloratum (Fig. 2) is also consistent with vertebrate pollination. By contrast, flowers of the insect-pollinated species C. hantamense contain very small volumes of nectar and this is secreted mainly during daylight hours (Membrives et al., 2002). During the scope of this study, it was not possible to investigate the measures of phenology for the three species involved. However, Membrives et al. (2002) investigated the reproductive biology of C. coloratum and C. hantamense, but not of C. scabromarginatum. They found that there were 185 ± 20 d between emergence and senescence for C. colchicum and 191 ± 13 d for C. hantamense, and that both species flower for approximately 2 months.
How ecologically important are rodents to C. scabromarginatum and C. coloratum?
The observation of short-billed birds visiting C. coloratum inflorescences and carrying Colchicum pollen at one of our study sites indicates that this species may not have a strict dependence on rodents for pollination. The site where this was observed was the only one situated in an urban area and sparrows and other birds may be more plentiful and opportunistic at this site. However, the nocturnal nectar secretion and capture of rodents carrying Colchicum pollen at five out of the six sites where C. coloratum was studied make a strong case for rodents being its primary pollinators. Nevertheless, the possibility that birds are secondary pollinators of this species cannot be excluded. Indeed, the reddish floral bracts of this species could be interpreted as a trait for attraction of birds. Weaverbirds are known to be legitimate pollinators of flowers (Botes et al., 2008). However, as far as we are aware, there are no studies that show sparrows pollinating flowers.
The ecological dependence of C. scabromarginatum on rodents is undoubtedly much higher than in C. coloratum. The former has extremely cryptic green inflorescences with bracts that have to be folded open to gain access to the flowers (Fig. 1B). The plants are often hidden in the vegetation or among rocks and were not seen to be visited by birds or insects. It is also strictly self-incompatible and incapable of autogamy (Table 1).
The evolution of rodent pollination in geophytes
Rodent pollination has now been found in two African geophytic lineages – Hyacinthaceae (Johnson et al., 2001) and Colchicaeae (present study). There are many studies recording flower visitation and pollination by arboreal mammals (Lumer, 1980; Kress et al., 1994); however, the Succulent Karoo region has a rich representation of geophytes in which insect-pollinated flowers are situated close to the ground and thus pre-adapted for pollination by terrestrial rodents. Such flowers probably often receive exploratory visits by hungry rodents, and if these have mutations for traits that make rodents more effective than insects at transferring pollen, then it is not hard to imagine selection shaping flowers along the lines of a rodent pollination floral syndrome. This study, like that of Johnson et al. (2001), has shown that floral syndromes can be useful for generating testable hypotheses about the existence of particular pollination systems. The clade containing C. scabromarginatum and C. coloratum has two other species. Of these, C. circinatum (Manning et al., 2007) is possibly rodent-pollinated given its dull green colour and viscous nectar.
ACKNOWLEDGEMENTS
We thank the Northern Cape Department of Nature and Environmental Conservation for providing us with the appropriate permits. We thank Neil McGregor for his friendly co-operation and discussions in Nieuwoudtville, as well as Dennis Hansen, Timo van der Niet and Christo Botes for their help with mist-netting birds; and Dennis Hansen for photographing birds visiting C. coloratum. We also thank the staff at Naries for their help during this study. Funding by the South African National Research Foundation is gratefully acknowledged.
LITERATURE CITED
- Beattie AJ. A technique for the study of insect-borne pollen. Pan-Pacific Entomologist. 1971;47:82. [Google Scholar]
- Botes C, Johnson SD, Cowling RM. Coexistence of succulent tree aloes: partitioning of bird pollinators by floral traits and flowering phenology. Oikos. 2008;117:875–882. [Google Scholar]
- Carthew SM, Goldingay RL. Non-flying mammals as pollinators. Trends Ecol. Evol. 1997;12:104–108. doi: 10.1016/s0169-5347(96)10067-7. [DOI] [PubMed] [Google Scholar]
- Fleming PA, Nicolson SW. How important is the relationship between Protea humiflora (Proteaceae) and its non-flying mammal pollinators? Oecologia. 2002;132:361–368. doi: 10.1007/s00442-002-0921-9. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 296–306. [Google Scholar]
- Johnson SD, Pauw A, Midgley J. Rodent pollination in the African lily Massonia depressa (Hyacinthaceae) American Journal of Botany. 2001;88:1768–1773. [PubMed] [Google Scholar]
- Kress JW, Schatz GE, Andrianifahanana M, Morland HS. Pollination of Ravenala madagascariensis (Strelitziaceae) by lemurs in Madagascar: evidence for an archaic coevolutionary system? American Journal of Botany. 1994;81:542–551. [Google Scholar]
- Lumer C. Rodent pollination of Blakea (Melastomataceae) in a Costa Rican cloud forest. Brittonia. 1980;32:512–517. [Google Scholar]
- Manning J, Forest F, Vinnersten A. The genus Colchicum L. redefined to include Androcymbium Willd. based on molecular evidence. Taxon. 2007;56:872–882. [Google Scholar]
- Membrives N, Caujape-Castells J, Pedrola-Monfort J. Reproductive biology of the genus Androcymbium (Colchicaceae) in western southern Africa. Orsis. 2002;17:37–59. [Google Scholar]
- Meyer N L. Colchicaceae. Strelitzia. 2000;10:587. [Google Scholar]
- Mucina L, Jürgens N, le Roux A, Rutherford MC, Schmiedel U, Esler KJ, et al. Succulent Karoo Biome. In: Mucina L, Rutherford MC, editors. The vegetation of South Africa, Swaziland and Lesotho. Pretoria: South African National Biodiversity Institute; 2006. pp. 221–299. Strelitzia 19. [Google Scholar]
- van der Niet T, Johnson SD, Linder HP. Macro-evolutionary data suggest a role for reinforcement in pollination system shifts. Evolution. 2006;60:1596–1601. doi: 10.1554/05-705.1. [DOI] [PubMed] [Google Scholar]
- Rebelo AG, Breytenbach GJ. Mammal pollination in the Cape Flora. In: Rebelo AG, editor. A preliminary synthesis of pollination biology in the Cape Flora 141. Pretoria: South African National Scientific Programmes Report, CSIR; 1987. 109–125. [Google Scholar]
- Rourke JP, Wiens D. Convergent floral evolution in South African and Australian Proteaceae and its possible bearing on pollination by nonflying mammals. Annals of the Missouri Botanical Garden. 1977;64:1–17. [Google Scholar]
- Vinnersten A, Manning J. A new classification of Colchicaceae. Taxon. 2007;56:171–178. [Google Scholar]
- Wiens D, Rourke JP. Rodent Pollination in southern African Protea species. Nature. 1978;276:71–73. [Google Scholar]
- Wiens D, Rourke JP, Casper BB, Rickart EA, LaPine TR, Peterson CJ, Channing A. Nonflying mammal pollination of southern African proteas: a non-coevolved system. Annals of the Missouri Botanical Garden. 1983;70:1–31. [Google Scholar]