Abstract
Background and Aims
Suaeda aralocaspica is a C4 summer annual halophyte without Kranz anatomy that is restricted to the deserts of central Asia. It produces two distinct types of seeds that differ in colour, shape and size. The primary aims of the present study were to compare the dormancy and germination characteristics of dimorphic seeds of S. aralocaspica and to develop a conceptual model of their dynamics.
Methods
Temperatures simulating those in the natural habitat of S. aralocaspica were used to test for primary dormancy and germination behaviour of fresh brown and black seeds. The effects of cold stratification, gibberellic acid, seed coat scarification, seed coat removal and dry storage on dormancy breaking were tested in black seeds. Germination percentage and recovery responses of brown seeds, non-treated black seeds and 8-week cold-stratified black seeds to salt stress were tested.
Key Results
Brown seeds were non-dormant, whereas black seeds had non-deep Type 2 physiological dormancy (PD). Germination percentage and rate of germination of brown seeds and of variously pretreated black seeds were significantly higher than those of non-pretreated black seeds. Exposure of seeds to various salinities had significant effects on germination, germination recovery and induction into secondary dormancy. A conceptual model is presented that ties these results together and puts them into an ecological context.
Conclusions
The two seed morphs of S. aralocaspica exhibit distinct differences in dormancy and germination characteristics. Suaeda aralocaspica is the first cold desert halophyte for which non-deep Type 2 PD has been documented.
Key words: Borszczowia, cold desert halophyte, physiological seed dormancy, seed germination, Suaeda
INTRODUCTION
Plant species have developed complex survival strategies that are evident in different stages of the life cycle, and this is particularly true for annual species (Gutterman, 2002). Adaptation to the desert environment via special seed dispersal and germination mechanisms is often the key to survival and development of these plants (Gutterman, 1993; Baskin and Baskin, 1998). Different species of desert plants may develop various dispersal strategies (i.e. escape or protection strategies) and germination strategies (i.e. opportunistic or cautious strategies) that are adaptations to the harsh environments (Gutterman, 1993, 2002). The heteromorphic (or dimorphic) seeds from one plant species may represent a combination of dispersal and germination strategies, some of which are unique combinations of the two opposing strategies.
Seed heteromorphism is a phenomenon in which a single individual produces different morphophysiological types of seeds, and it is common in Asteraceae, Chenopodiaceae and Poaceae (Harper, 1977; Imbert, 2002). Seed dimorphism can be considered as one type of seed heteromorphism. Heteromorphic seeds usually differ in colour, size and shape, as well as in dispersal, dormancy and germination (Baskin and Baskin, 1998; Wei et al., 2007). Worldwide, more than 200 species are reported to exhibit seed heteromorphism (Imbert, 2002).
Heteromorphic seeds generally have different germination responses that are thought to be a bet-hedging strategy advantageous in harsh and unpredictable environments (Venable, 1985; Khan and Gul, 1998; Wei et al., 2007). Seed germination responses have been tested in many heteromorphic species, e.g. Suaeda moquinii (Khan et al., 2001), Suaeda salsa (Li et al., 2005) and Salicornia europaea (Philipupillai and Ungar, 1984). However, a few questions still need to be answered about the germination biology of seeds of these species. First, no studies have confirmed seed dormancy class, level and type (sensu Baskin and Baskin, 2004), although seemingly it appears that one morph is non-dormant and the second morph has physiological dormancy (Imbert, 2002). Secondly, several authors who tested the effect of salinity on germination of heteromorphic seeds in halophytic species found that different seed types had different salt tolerance (Philipupillai and Ungar, 1984; Takeno and Yamaguchi, 1991; Khan and Gul, 1998; Khan et al., 2001). Nevertheless, few studies have tested the salt tolerance of the dormant morph after dormancy release (Ungar, 1979). For example, it is not suitable to use (fully) dormant or conditionally dormant seeds, rather than cold stratified (non-dormant) ones, to study the effects of salts on germination (Baskin et al., 2006).
Only a few heteromorphic species inhabiting inland cold desert habitats have been studied (Wei et al., 2007, 2008). In the inland, cold desert habitats of China, most of the heteromorphic species inhabit harsh environmental conditions, such as saline deserts. Seeds of a number of halophytic species from these regions (Atriplex micrantha, Suaeda aralocaspica and Salsola affinis) are heteromorphic (Commissione Redactorum Florae Xinjiangensis, 1994; Wei et al., 2007, 2008).
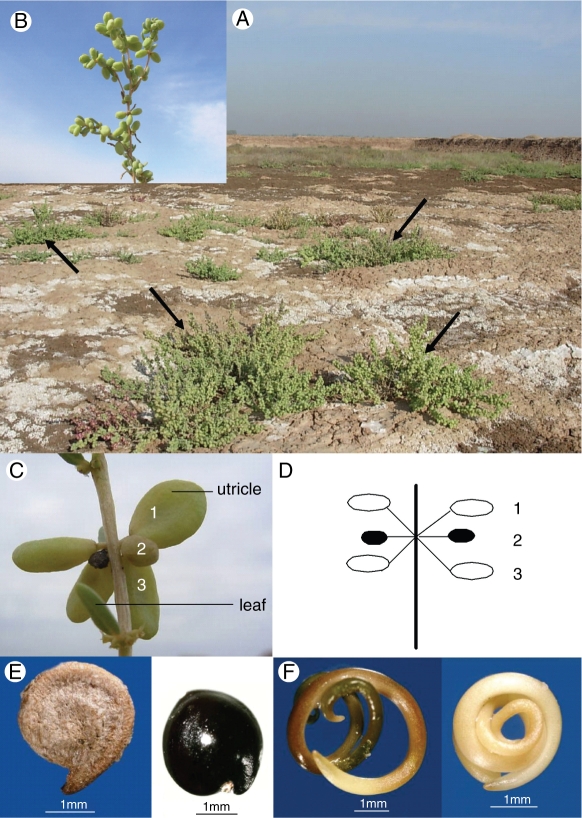
Suaeda aralocaspica (=Borszczowia aralocaspica) is the only member of section Borszczowia of the genus Suaeda (Schütze et al., 2003; Kapralov et al., 2006), and it is restricted to central Asia (Mabberley, 1997). In China, S. aralocaspica is found only in the inland cold desert of the Junggar Basin, Xinjiang. This plant species is a monoecious annual that grows up to 20–50 cm in height, has grey-green leaves and unisexual flowers, and is commonly found in the Gobi desert, where it grows in saline–alkaline sandy soils (Commissione Redactorum Florae Xinjiangensis, 1994) (Fig. 1A, B). It is a C4 plant without Kranz anatomy (Voznesenskaya et al., 2001; Schütze et al., 2003; Kapralov et al., 2006). Plants bloom in August and produce dimorphic fruits and seeds on the same plant in September (Fig. 1C–F). Two types of S. aralocaspica utricles and seeds are recorded in the Flora of China and in the Flora Xinjiangensis. According to our observations in the field, S. aralocaspica occurs in almost pure patches or co-occurs with other species such as Salicornia europaea, Karelinia caspica, Phragmites australis and Salsola subcrassa.
Fig. 1.
Suaeda aralocaspica. (A) Natural habitat (note white salt crust) of S. aralocaspica (arrows) with saline–alkaline sandy soil. (B) S. aralocaspica branch in fruiting stage. (C) Positions of utricles on a branch; numbers 1 and 3 each contain one brown seed and number 2 one black seed. (D) Schematic drawing showing the glomerules of utricles and distribution pattern of brown and black seeds. (E) Brown (left) and black (right) seeds. (F) Fully developed planospiral embryo of brown (left) and black (right) seeds.
Survival of annual plants under desert conditions is related mainly to mechanisms that ensure that germination occurs at the right time and in a suitable place for plant establishment (Gutterman, 1993). For the desert annual growing in stressful and unpredictable environments, the seed germination strategy may be the most significant factor determining survival as this is the only way their populations can be maintained from one year to the next. We hypothesized that S. aralocaspica has also developed a special strategy in the seed stage that is part of its suite of adaptations to the harsh cold, salty desert habitats. Thus, the following questions were asked: what are the differences, if any, in (1) dormancy-breaking and germination requirements of the dimorphic seeds of this species, and (2) the ability of the two seed morphs to recover from salt stress. The results were used to construct a conceptual model of its germination ecology.
MATERIALS AND METHODS
Seed collection and field site description
Fresh seeds of Suaeda aralocaspica (Bunge) Freitag & Schütze (=Borszczowia aralocaspica Bunge) were collected from dry inflorescences in natural populations growing in desert saline soils at the Hutubi Cattle Breeding Farm (44°19′N, 86°57′E; 429 m a.s.l.) near the Fukang Field Research Station of the Chinese Academy of Sciences, in Xinjiang, China, on 2 October, 2006. During the first 2 weeks, the fresh seeds were tested for germination behaviour or stored at −18 °C. One reason for testing seed germination immediately after harvest is that seed germination behaviour may change during storage (Baskin and Baskin, 1998; Baskin et al., 2006). Seeds used in the dry storage experiment were collected in October, 2005 at the same field site.
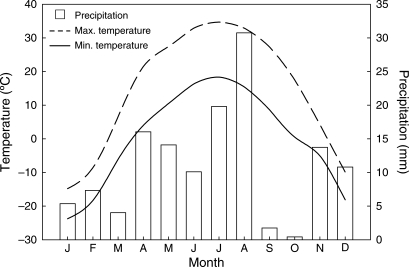
The research area is an inland cold desert with typical temperate desert climate. Meteorological data based on 3 years (2004–2006) indicated that the mean annual temperature was 6·4 °C, the highest summer temperature 40·9 °C, the lowest winter temperature −40·3 °C, mean temperature of the warmest month (July) 26·5 °C and the mean temperature of the coldest month (January) −19·3 °C. Annual precipitation (rain and snow) was 134·3 mm, annual potential evaporation 1965 mm and frost-free period 175 days. Mean monthly precipitation and mean monthly maximum and minimum temperatures at the Fukang Field Research Station during 2004–2006 are shown in Fig. 2.
Fig. 2.
Mean (2004–2006) precipitation and mean minimum and maximum temperatures at the Fukang Field Research Station.
Soil conductivity
Salinities of soil samples collected from ten randomly chosen areas within the study population of S. aralocaspica in October, 2007 were analysed by the residue drying quality measure (Bao, 2000). Mean total soil salinities in the 0–5-, 5–10- and 10–30-cm soil layers were 5·64 ± 0·54 % (965·09 ± 92·4 mmol L−1 NaCl), 5·29 ± 0·59 % (905·09 ± 100·96 mmol L−1 NaCl) and 2·29 ± 0·25 % (391·85 ± 42·78 mmol L−1 NaCl), respectively. Minimum and maximum soil salinities in the 0–5-cm soil layers were 1·93 % (330·25 mmol L−1 NaCl) and 7·85 % (1343·26 mmol L−1 NaCl), respectively. However, the maximum soil salinity in 0–1-cm soil layer can reach as high as 22·38 % (3829·57 mmol L−1 NaCl). Only NaCl was chosen to study the effect of salt on seed germination because it is the major component of salinity in some regions of the Junggar Basin of Xinjiang (Qian et al., 2003). In addition, the results of our germination experiments can be compared directly with most similar studies reported in the literature, which also have used NaCl to test the effect of salinity on seed germination.
Seed morphology and mass
Thirty individual plants were chosen randomly in the natural habitat of S. aralocaspica, and type and number of seeds on a total of 90 branches were determined.
Length, width and height of 20 of each of the two seed types (morphs) were measured, and five groups of 1000 seeds were weighed using an electronic analytical balance (Sartorius BP 221 S, Sartorius, Germany) for each seed type to determine average seed size and mass.
Imbibition tests
An imbibition test was conducted at room temperature (21–25 °C, 45 % relative humidity) using four replicates of 25 dry seeds of each morph. The dry mass of each group of 25 seeds was determined (time 0), and the seeds were then placed on moist (distilled water) Whatman No. 1 filter paper in 5-cm-diameter Petri dishes. After 1 h, the seeds were removed from the dishes, blotted dry with filter paper, reweighed and returned to the Petri dishes. Seed mass was measured again after 3, 6, 9, 12 and 22 h of water absorption. The imbibition test of brown seeds was terminated after 9 h because the seeds had begun to germinate. Relative increase in fresh weight (Wr) of seeds was calculated as Wr = [(Wf − Wi)/Wi] × 100, where Wi is the initial seed weight and Wf the weight after a certain time (Baskin et al., 2004). The tests were repeated four times, and the standard error was calculated.
Effect of light and temperature on germination of the two seed morphs
To investigate the germination behaviour of fresh seeds, four replicates of 25 fresh seeds of each morph were incubated on two layers of Whatman No.1 filter paper moistened with 2·5 mL of distilled water in 5-cm-diameter plastic Petri dishes. Petri dishes were sealed with parafilm and incubated at daily (12/12-h) temperature regimes of 5 : 15, 5 : 25, 10 : 25, 15 : 30 and 20 : 35 °C in light (12-h daily photoperiod) and in continuous dark (seeds in black bags) for 20 d. These thermoperiods represent the mean daily maximum and minimum monthly temperatures at the Fukang Field Research Station during the growing season: 5 : 15 °C (early April and October), 5 : 25 °C (late April), 10 : 25 °C (May and September), 15 : 30 °C (June and August) and 20 : 35 °C (July). A seed was considered to be germinated when the radicle had emerged. Germination in light was examined every 2 d for 20 d; germinated seeds were removed at each counting. Seeds incubated in dark were checked only after 20 d.
The rate of germination in light was estimated using a modified Timson's index of germination velocity: germination index = ΣG/t, where G is seed germination percentage at 2-d intervals and t the total germination period (Khan and Ungar, 1997). According to this equation, the highest value obtained is 50 (i.e. 1000/20), and a higher value indicates more rapid germination.
Breaking dormancy of black seeds
Germination of fresh black seeds of S. aralocaspica in light or dark in all temperature regimes was less than 40 %, indicating a proportion of black seeds are dormant. Therefore, effects of cold stratification, seed coat scarification, seed coat removal, gibberellic acid (GA3) and dry storage on dormancy-break were tested to explore the kind of dormancy in black seeds.
Cold stratification
Washed quartz sand moistened with distilled water (sand moisture content 11–13 %) was placed beneath two layers of filter paper in 10-cm-deep × 20-cm-diameter metal boxes. Fresh (2 weeks after collection) black seeds were placed on the filter paper, and the metal boxes were closed and placed in a refrigerator in the dark for 2, 4, 6 or 8 weeks at constant 5 °C.
After treatments, black seeds were transferred under green light to Petri dishes. Germination conditions were the same as those described in the experiment on the effects of light and temperature on germination of the two seed morphs. Black seeds collected at the same time that had not been stratified were used as control.
Testa treatments
This experiment aimed to determine if the testa mechanically restricts embryo growth and thus if the embryo lacks sufficient growth potential to break through it. The seed coats of four replications of 25 seeds were carefully scarified with a scalpel on the lenticular side. Testae of another four replications of 25 seeds were removed manually. Treated seeds and intact seeds (control) were incubated at a daily temperature regime of 10 : 25 °C in light for 20 d. Seeds were checked every 2 d for emergence of the radicle during this 20-d period.
Dry storage
After-ripening is one of the characteristics of seeds with non-deep physiological dormancy (PD). Thus, this experiment aimed to determine if seeds come out of dormancy (after-ripen) during dry storage. Black seeds were stored for 1 year in a closed cotton bag at room temperature (16–26 °C, 30–50 % relative humidity). After dry storage, four replications of 25 seeds were incubated in distilled water at a daily temperature regime of 10 : 25 °C in light for 20 d. Four replications of 25 fresh (2-week-old) black seeds collected at the same time were incubated as controls. Seeds were checked every 2 d for emergence of the radicle during this 20-d period.
GA3 treatments
GA3 is a plant growth regulator that can break dormancy in seeds with non-deep PD. To test the effects of GA3 on dormancy breaking, four replicates of 25 black seeds were incubated in 0 (distilled water control), 0·1, 1 and 10 mmol L−1 GA3 solutions at a daily temperature regime of 10 : 25 °C in light for 20 d. Seeds were checked every 2 d for emergence of the radicle during this 20-d period.
A daily temperature regime of 10 : 25 °C was chosen for testa, GA3 and dry storage experiments because from a previous experiment this was found to be optimal for germination of fresh black seeds in both light and dark (see Fig. 4). In addition, 10 : 25 °C represents the natural minimum and maximum temperature regime in May, when most seeds germinate in the natural habitat.
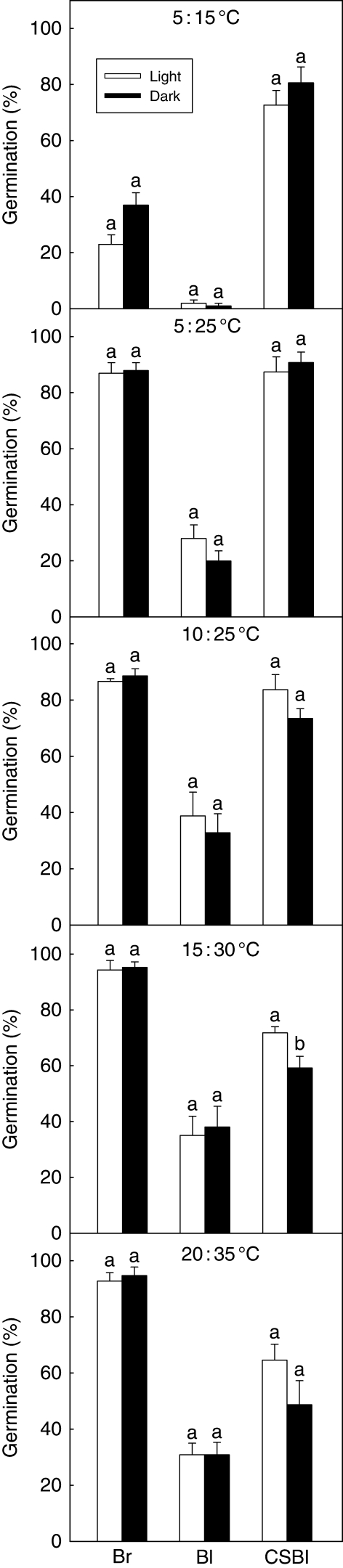
Fig. 4.
Effect of temperature and light on germination of non-treated brown seeds (Br), non-treated black seeds (Bl) and 8-week cold-stratified black seeds (CSBl) of Suaeda aralocaspica. Different lower-case letters indicate significant differences in germination percentages of the same seed type and same treatment between light and dark at a given temperature.
Effect of salinity on germination and recovery of germination
The effects of 0 (distilled water control), 200, 400, 600, 800, 1000, 1400, 2000 and 4000 mmol L−1 NaCl on germination of seeds (brown seeds, black seeds and black seeds after 8-week cold stratification) were tested at a daily temperature regime of 10 : 25 °C in light. Four replicates of 25 seeds in 5-cm-diameter plastic Petri dish were exposed to each treatment. Seed germination percentages were calculated after incubation for 20 d. Ungerminated seeds from 20-d NaCl pre-treatments were rinsed three times with distilled water and then incubated for 10 d in Petri dishes that contained 2·5 mL distilled water. Recovery percentage was calculated by the following formula: [(a – b)/(c – b)] × 100, where a is the total number of seeds that germinated in salt solution plus those that recovered to germinate in distilled water after the ungerminated seeds kept in NaCl solutions for 20 d were transferred to distilled water for 10 d, b is the number of seeds germinated in salt solution and c is the total number of seeds tested (Gul and Weber, 1999). Final germination was recorded as (a/c) × 100. Seed viability was expressed as [(a + d)/c] × 100, where d is the number of embryos that stained pink in the TTC solution following the seed germination test. Viability of seeds that did not germinate in the experiments was tested by the TTC method (embryos are placed in a 1 % solution of 2,3,5-triphenyl-2H-tetrazolium chloride) (Baskin and Baskin, 1998).
Data analysis
All data were expressed as mean ± s.e. Proportions were arcsine transformed before statistical analysis to ensure homogeneity of variance (non-transformed data appear in all figures). These data were analysed using SPSS Version 12·0 for Windows (SPSS Inc., 2003). General linear model (GLM) analysis of variance (ANOVA) was used to compare treatment effects. One-way ANOVA tested for differences among testa, GA3 and dry storage treatments. Two-way ANOVA was used to test the significance of main effects (seed type and salinity) and their interaction on germination in the ‘effect of salinity on germination and recovery of germination’ experiment. Three-way ANOVA was used to test the significance of main effects (seed type, temperature, light condition) and their interaction on germination in the ‘effect of light and temperature on germination of the two seed morphs’ experiment, and to test the significance of main effects (cold stratification, light condition, temperature) and their interaction on germination in the ‘cold stratification’ experiment. Tukey's HSD test and paired two-tailed tests were performed for multiple comparisons to determine significant (P < 0·05) differences between individual treatments (Sokal & Rohlf, 1995).
RESULTS
Seed morphology and mass
There were 2320 brown and 964 black seeds from a total of 90 branches of 30 individual plants, a ratio of 2·41 : 1; most commonly, the ratio is 2 : 1 (Fig. 1C, D). Numerous male and female flowers were usually mixed in glomerules, which frequently produced only three seeds. The two brown seeds usually were located at the two lateral sides of the glomerules and the black seeds in the middle (Fig. 1C, D).
Seed dispersal was observed in the natural habitat of S. aralocaspica. The two types of seeds matured nearly at the same time in autumn. Brown seeds were dispersed long distances by wind. However, black seeds were not easily dispersed by wind because their utricles were small, and some of them stuck to the branches tightly (Fig. 1C).
Field observations show that S. aralocaspica produces two types of utricles: large, 6–10 mm in diameter, obovate, pericarp fleshly and endocarp membranous; and small, about 3 mm in diameter and pyriform (Fig. 1C). Each of the two types of utricles produces a distinct type of seed (Fig. 1E). The oblate brown seed has a soft seed coat and the elliptical black seed has a rigid seed coat. The brown and black morphs also differ in length, width, height and weight (Table 1). For both seed types, the embryo is fully developed and planospiral (Fig. 1F).
Table 1.
Morphological characteristics and mass of brown and black seeds of Suaeda aralocaspica
| Type | Colour | Shape | Length (mm, mean ± s.e.) | Width (mm, mean ± s.e.) | Height (mm, mean ± s.e.) | Mass of 1000 seeds (g, mean ± s.e.) |
|---|---|---|---|---|---|---|
| A | Brown | Oblate | 3·17 ± 0·05 | 2·80 ± 0·03 | 0·70 ± 0·01 | 2·51 ± 0·06 |
| B | Black | Elliptical | 2·45 ± 0·01 | 2·31 ± 0·02 | 1·43 ± 0·02 | 2·33 ± 0·03 |
Imbibition tests
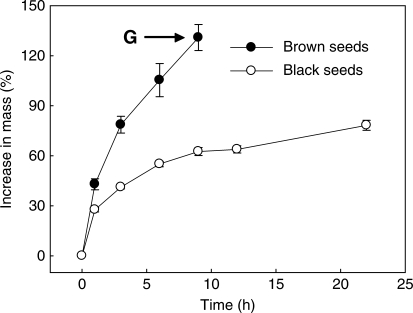
The dimorphic seeds had different water uptake characteristics. Brown seeds imbibed water readily and followed a typical pattern of rapid initial water uptake, with seed mass increasing by 42·9 ± 3·3 % after 1 h, 78·6 ± 5·0 % after 3 h and 130·9 ± 7·8 % after 9 h (Fig. 3). By contrast, black seeds imbibed water slowly, with seed mass increasing by 27·6 ± 1·3 % after 1 h, 41·2 ± 1·1 % after 3 h and only 78·2 ± 3·0 % after 22 h (Fig. 3).
Fig. 3.
Imbibition curves for brown and black seeds of Suaeda aralocaspica in distilled water. ‘G’, time germination begins.
Effect of light and temperature on germination of the two non-treated morphs
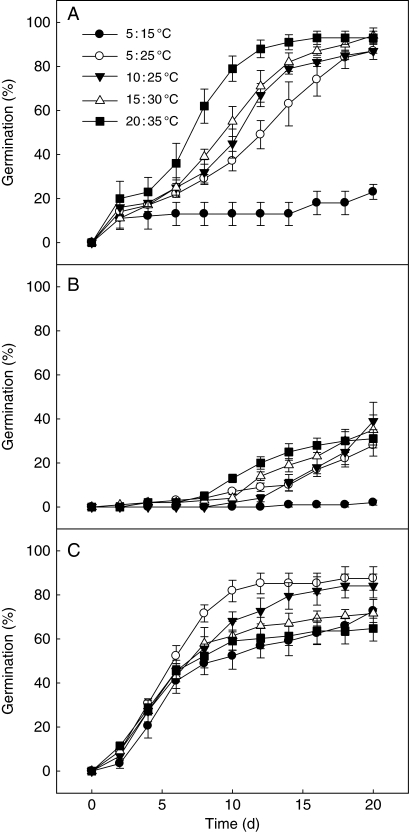
A three-way ANOVA showed that germination was significantly affected by seed type (P < 0·001), temperature (P < 0·001), their interaction (P ≤ 0·001) and the interaction between seed type and light condition (P ≤ 0·02) (Table 2). No significant difference (P = 0·771) was observed in germination percentage between incubation in light and incubation in dark within a seed type of S. aralocaspica at any temperature regime (Fig. 4; Table 2). Germination percentages of brown seeds in all treatments were >87 % in all temperature regimes in light except 5 : 15 °C, whereas those of non-treated black seeds were <39 % (Fig. 4).
Table 2.
Three-way ANOVA of effects of seed type, temperature, light condition and their interactions on seed germination of Suaeda aralocaspica
| Source | d.f. | SS | MS | F-value | P-value |
|---|---|---|---|---|---|
| Seed type (S) | 1 | 27206·338 | 27206·338 | 567·792 | <0·001 |
| Temperature (T) | 4 | 17449·708 | 4362·427 | 91·043 | <0·001 |
| Light (L) | 1 | 4·085 | 4·085 | 0·085 | 0·771 |
| S × T | 4 | 1018·920 | 254·730 | 5·316 | 0·001 |
| S × L | 1 | 271·766 | 271·766 | 5·672 | 0·020 |
| T × L | 4 | 222·206 | 55·552 | 1·159 | 0·338 |
| S × T × L | 4 | 192·350 | 48·087 | 1·004 | 0·413 |
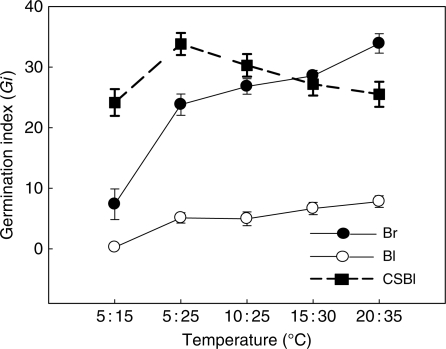
During 20 d of incubation, cumulative germination percentage and germination velocity of brown seeds were significantly higher than those of non-treated black seeds at the same temperature regime (Figs 5 and 6). For example, cumulative germination of brown seeds was 23 ± 3 % at 5 : 15 °C, whereas only 2 ± 1 % germination was recorded for black seeds at the same thermoperiod. Germination percentage of brown seeds reached 93 ± 3 % at the highest thermoperiod (20 : 35 °C), but the germination of black seeds was only 31 ± 4 % at this regime. At 5 : 15 °C and 20 : 35 °C, the germination index of brown seeds was 7·4 ± 2·5 and 34·0 ± 1·6, respectively, whereas for black seeds it was only 0·3 ± 0·2 and 7·8 ± 1·0, respectively (Fig. 6).
Fig. 5.
Cumulative germination percentages (mean ± s.e.) of (A) non-treated brown seeds, (B) non-treated black seeds and (C) 8-week cold-stratified black seeds of Suaeda aralocaspica incubated at different temperature regimes in light for 20 d.
Fig. 6.
Germination rates of non-treated brown seeds (Br), non-treated black seeds (Bl) and 8-week cold-stratified black seeds (CSBl) of Suaeda aralocaspica at different temperature regimes (night/day) in light.
Breaking dormancy of black seeds
Cold stratification
A three-way ANOVA showed that germination of black seeds was significantly affected by length of cold stratification period (P < 0·001), light condition (P ≤ 0·004), temperature (P < 0·001) and the interaction between the length of cold stratification period and temperature (P < 0·001; Table 3).
Table 3.
Three-way ANOVA of effects of length of cold stratification, light condition, temperature and their interactions on seed germination of Suaeda aralocaspica
| Source | d.f. | SS | MS | F-value | P-value |
|---|---|---|---|---|---|
| Cold stratification (CS) | 4 | 31048·174 | 7762·043 | 129·932 | <0·001 |
| Light (L) | 1 | 501·160 | 501·160 | 8·389 | 0·004 |
| Temperature (T) | 4 | 6307·740 | 1576·935 | 26·397 | <0·001 |
| CS × L | 4 | 47·338 | 11·834 | 0·198 | 0·939 |
| CS × T | 16 | 9487·339 | 592·959 | 9·926 | <0·001 |
| L × T | 4 | 270·407 | 67·602 | 1·132 | 0·344 |
| CS × L × T | 16 | 1090·054 | 68·128 | 1·140 | 0·323 |
Germination of black seeds was significantly increased by cold stratification (Figs 4 and 5; Table 4). As stratification time increased from 0 to 8 weeks, there was a decrease in the minimum temperature at which a moderate or high percentage of the seeds could germinate (Table 4). Thus, germination of seeds at 5 : 15 °C increased from about 2 % in 0-week-stratified (control) seeds to >70 % in 8-week-stratified seeds in light and from 1 to >80 % in darkness. The rate of germination also increased with cold stratification with an increase in the germination index of black seeds at 5 : 15 °C from 0·25 ± 0·19 in 0-week-stratified (control) seeds to 24·2 ± 2·2 in 8-week-stratified seeds (Fig. 6). Eight weeks of cold stratification increased the germination of freshly harvested black seeds from 1–41 to 49–91 % in both light and dark over the range of test temperature regimes. The germination percentage of black seeds at 5 : 15, 5 : 25, 10 : 25 and 20 : 35 °C reached the same level in light and in dark, but at 15 : 30 °C, the germination percentage in light was significantly higher than it was in dark [Fig. 4 (CSBl); Table 4].
Table 4.
Germination percentages (mean ± s.e.) of black seeds of Suaeda aralocaspica at five thermoperiods in light and dark after 0–8 weeks of cold stratification
| Cold stratification time (weeks) |
||||||
|---|---|---|---|---|---|---|
| 0 (control) | 2 | 4 | 6 | 8 | ||
| Light | 5 : 15 °C | 2 ± 1Cc | 14 ± 4Cb | 42 ± 5Ba | 77 ± 2Aa | 73 ± 5Aab |
| 5 : 25 °C | 29 ± 5Bb | 43 ± 3Ba | 48 ± 7Ba | 85 ± 2Aa | 88 ± 5 Aa | |
| 10 : 25 °C | 41 ± 9BCa | 38 ± 6Cab | 41 ± 4Ca | 76 ± 4ABa | 84 ± 5Aab | |
| 15 : 30 °C | 36 ± 7Bb | 33 ± 10Bab | 33 ± 4Ba | 51 ± 4ABb | 72 ± 2Aab | |
| 20 : 35 °C | 32 ± 4BCb | 16 ± 2Cb | 36 ± 6BCa | 51 ± 8ABb | 65 ± 6Ab | |
| Dark | 5 : 15 °C | 1 ± 1Cb | 21 ± 4BCb | 32 ± 9Ba | 66 ± 3Aab | 81 ± 6Aab |
| 5 : 25 °C | 21 ± 4Cab | 48 ± 2Ba | 34 ± 9BCa | 83 ± 6Aa | 91 ± 4Aa | |
| 10 : 25 °C | 34 ± 7Ca | 28 ± 4Cb | 45 ± 5BCa | 64 ± 4ABab | 74 ± 3Aab | |
| 15 : 30 °C | 40 ± 8ABa | 27 ± 7Bb | 33 ± 4ABa | 50 ± 9ABb | 59 ± 4Abc | |
| 20 : 35 °C | 32 ± 5ABa | 11 ± 4Bb | 35 ± 6ABa | 43 ± 11Ab | 49 ± 9Ac | |
Different upper-case letters indicate significant differences in germination percentages among different cold stratification times at the same temperature and different lower-case letters in each column with the same light condition indicate significant differences in germination percentages of seeds among different temperatures.
Testa treatments
Scarification and testa removal significantly increased final germination percentage from 30 ± 7 to 80 ± 4 % (P = 0·001) and 90 ± 5 % (P < 0·001), respectively. Germination index was also significantly increased from 5·2 ± 1·6 to 30·6 ± 1·7 (P < 0·001) and 40·8 ± 2·5 (P < 0·001), respectively.
GA3 treatments
After 20 d of incubation, germination percentage of black seeds at 0 (control), 0·1, 1 and 10 mmol L−1 GA3 was 18 ± 4, 24 ± 2, 21 ± 1 and 54 ± 8 %, respectively. GA3 treatment at 10 mmol L−1 significantly increased (P = 0·006) germination percentage of black seeds, but 0·1 and 1 mmol L−1 GA3 had no effect (P = 0·228 and P = 0·510, respectively) on germination.
Dry storage
One-year of dry storage at room temperature significantly increased (P = 0·004) germination percentage of black seeds, from 29 ± 3 to 57 ± 5 %.
Effect of salinity on germination and recovery of germination
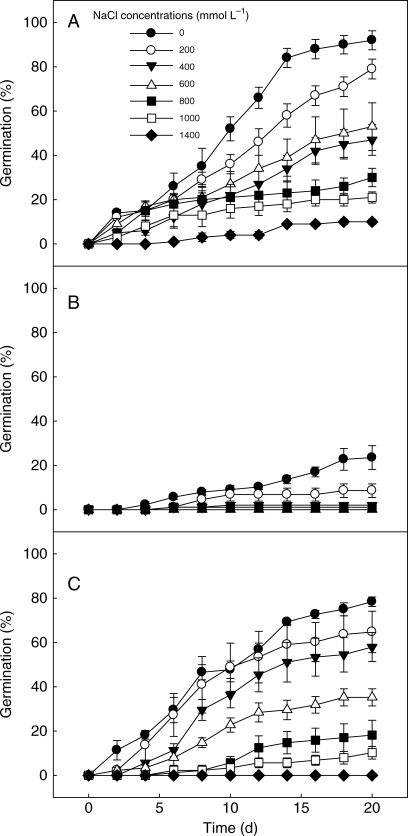
Germination of non-treated brown, non-treated black and 8-week cold-stratified black seeds was significantly affected by salinity (P < 0·001). Brown seeds germinated to ≥ 10 % at 0–1400 mmol L−1, while the untreated black seeds germinated to ≥ 8 % only at 0–200 mmol L−1 NaCl. Furthermore, brown seeds germinated to higher percentages than non-treated black seeds. For example, germination percentages of brown seeds at 200 mmol L−1 NaCl was 80 ± 5 %, whereas that of non-treated black seeds was only 9 ± 3 % (Table 5). As salinity increased from 0 to 600 mmol L−1 NaCl, both the germination (%) and germination velocity of brown seeds decreased, and no seeds germinated in a solution of greater than 1400 mmol L−1 NaCl (Fig. 7A; Table 5). For non-treated black seeds, the highest germination (24 ± 5 %) occurred in distilled water, and no seeds germinated in a solution of greater than 800 mmol L−1 NaCl (Fig. 7B; Table 5). At a given salt concentration, 8-week cold-stratified black seeds germinated to higher percentages, and over a wider range of salt solutions, than non-stratified black seeds (Fig. 7C; Table 5).
Table 5.
Effect of NaCl on germination and germination recovery of seeds of Suaeda aralocaspica
| Salinity (mmol L−1) | Initial germination (%, mean ± s.e.) | Recovery percentage (mean ± s.e.) | Final germination (%, mean ± s.e.) | Total viable seeds (%, mean ± s.e.) | Viable ungerminated seed (%, mean ± s.e.) | |
|---|---|---|---|---|---|---|
| Brown seeds | 0 | 94 ± 4a | 25 ± 25b | 95 ± 4a | 100 ± 0a | 5 ± 4c |
| 200 | 80 ± 5ab | 51 ± 17ab | 93 ± 1a | 100 ± 0a | 7 ± 1c | |
| 400 | 48 ± 7c | 79 ± 7ab | 88 ± 5a | 100 ± 0a | 12 ± 5c | |
| 600 | 53 ± 11bc | 86 ± 3a | 94 ± 2a | 100 ± 0a | 6 ± 2c | |
| 800 | 31 ± 4cd | 79 ± 4ab | 85 ± 4ab | 100 ± 0a | 15 ± 4bc | |
| 1000 | 22 ± 2d | 83 ± 7a | 87 ± 5a | 100 ± 0a | 13 ± 5c | |
| 1400 | 10 ± 1e | 76 ± 9ab | 78 ± 8ab | 100 ± 0a | 22 ± 8bc | |
| 2000 | 0 ± 0f | 51 ± 4ab | 51 ± 4c | 100 ± 0a | 49 ± 4a | |
| 4000 | 0 ± 0f | 61 ± 8ab | 61 ± 8bc | 100 ± 0a | 39 ± 8ab | |
| Black seeds, unstratified | 0 | 24 ± 5a | 23 ± 6a | 41 ± 6a | 100 ± 0a | 59 ± 6c |
| 200 | 9 ± 3ab | 12 ± 3abc | 19 ± 5b | 100 ± 0a | 81 ± 5b | |
| 400 | 2 ± 1b | 15 ± 3abc | 17 ± 3bc | 100 ± 0a | 83 ± 3ab | |
| 600 | 0 ± 0b | 7 ± 2abc | 7 ± 2bc | 100 ± 0a | 93 ± 2ab | |
| 800 | 1 ± 1b | 10 ± 4abc | 11 ± 3bc | 100 ± 0a | 89 ± 3ab | |
| 1000 | 0 ± 0b | 20 ± 5ab | 20 ± 5b | 100 ± 0a | 80 ± 5b | |
| 1400 | 0 ± 0b | 3 ± 1bc | 3 ± 1bc | 100 ± 0a | 97 ± 1ab | |
| 2000 | 0 ± 0b | 1 ± 1c | 1 ± 1c | 100 ± 0a | 99 ± 1a | |
| 4000 | 0 ± 0b | 3 ± 3bc | 3 ± 3bc | 100 ± 0a | 97 ± 3ab | |
| Black seeds after 8 weeks cold stratification | 0 | 78 ± 2a | 11 ± 7c | 81 ± 3a | 100 ± 0a | 19 ± 3c |
| 200 | 65 ± 9ab | 28 ± 14bc | 73 ± 8ab | 100 ± 0a | 27 ± 8bc | |
| 400 | 58 ± 7a | 50 ± 11ab | 77 ± 6ab | 100 ± 0a | 23 ± 6bc | |
| 600 | 35 ± 4bc | 54 ± 3ab | 70 ± 1ab | 100 ± 0a | 30 ± 1bc | |
| 800 | 18 ± 7cd | 43 ± 8abc | 53 ± 8bc | 100 ± 0a | 47 ± 8ab | |
| 1000 | 10 ± 3d | 65 ± 8a | 68 ± 8abc | 100 ± 0a | 32 ± 8abc | |
| 1400 | 0 ± 0e | 60 ± 3ab | 60 ± 3abc | 100 ± 0a | 40 ± 3abc | |
| 2000 | 0 ± 0e | 53 ± 3ab | 53 ± 3bc | 100 ± 0a | 47 ± 3ab | |
| 4000 | 0 ± 0e | 44 ± 4abc | 44 ± 4c | 100 ± 0a | 56 ± 4a |
Different lower-case letters in each column for the same seed type indicate significant differences across all concentrations of NaCl.
Fig. 7.
Effect of NaCl on germination of (A) non-treated brown seeds, (B) non-treated black seeds and (C) 8-week cold-stratified black seeds of Suaeda aralocaspica incubated at 10 : 25 °C in light.
After they were transferred from NaCl to distilled water, ungerminated brown and black seeds had the ability to recover. As the pretreatment salinity increased, the germination recovery percentage of brown seeds and of 8-week cold-stratified black seeds increased, and for all NaCl concentrations the recovery germinations were significantly higher than that of the control (P < 0·01). However, the highest recovery germination percentages of non-stratified black seeds were for seeds exposed to distilled water (Table 5).
Final germination percentages of brown seeds, non-stratified black seeds and 8-week cold-stratified black seeds were higher in distilled water than at any NaCl concentration (Table 5). However, seed viability was not affected by salt concentration, even for seeds pretreated with 4000 mmol L−1 NaCl (Table 5). Compared with brown seeds and 8-week cold-stratified black seeds, more non-treated black seeds remained ungerminated after incubation in various salt concentrations and in distilled water. In addition, the higher the pretreatment salinity, the higher the percentage of seeds that did not germinate, even after the recovery process (Table 5).
DISCUSSION
The evolution of mechanisms that increase survival and fitness of species in unpredictable and stressful environments has led to the development of a number of morphological and physiological adaptations in seeds that affect their dormancy and their dispersal distance (Harper et al., 1970). Suaeda aralocaspica is an inland cold desert annual halophyte that produces two distinct types of seeds (no intermediate types) that differ in morphology, dormancy and germination. The present study has demonstrated that both morphological and physiological polymorphism exist in seeds of this species. These differences presumably represent the combination of different complementary adaptive strategies in one plant and have ecological significance for its successful survival in inland cold salt deserts.
Fresh brown seeds of S. aralocaspica are highly permeable to water, have a fully developed embryo and are non-dormant. Thus, they can germinate rapidly to high percentages in water over a wide range of alternating temperature regimes in light and in dark. However, although fresh black seeds of S. aralocaspica are water-permeable, the amount and rate of water absorption by them is lower than it is in brown seeds. Furthermore, black seeds germinated slowly and to low percentages in all incubation conditions. Fresh black seeds exhibit non-deep PD (sensu Baskin & Baskin, 2004) that can be broken by a few weeks of cold stratification. Furthermore, promotion of germination by GA3, after-ripening in dry storage and disruption or removal of the testa also indicates that these seeds have non-deep PD. These results suggest that growth potential of the embryo in a high percentage of fresh intact dormant seeds is too low to overcome the mechanical resistance of the seed coat. Thus, GA3, dry storage and cold stratification treatments increase the growth potential of the embryo to the point at which resistance of the seed coat is overcome, and thus the seed germinates (radicle protrusion). On the other hand, damaging or removing the seed coat in fresh back seeds lowers (or removes) the mechanical resistance to embryo growth to the point at which the radicle can elongate, even though the embryo is at a relatively low growth potential, and thus the seed germinates.
Five types of non-deep PD are recognized by Baskin and Baskin (2004), based on the patterns of change in physiological responses to temperature during dormancy breaking. Germination tests of black seeds of S. aralocaspica after various periods of cold stratification indicated that during dormancy break (dormancy → non-dormancy) the minimum temperature at which seeds could germinate to a moderate to high percentage decreased. Thus, it is concluded that black seeds of S. aralocaspica have Type 2 non-deep PD, the type expected for a summer annual such as S. aralocaspica (Baskin and Baskin, 2004). Until now, salinity has not been included as a ‘physiological correlate’ (see table 4·3 in Baskin and Baskin, 1998) in the dormancy continuum as seeds cycle between dormancy and non-dormancy. However, the present study shows that the range of NaCl concentrations at which seeds of a halophyte can germinate increases during dormancy break by cold stratification. Thus, sensitivity to salinity should be added to the list of physiological correlates of the dormancy continuum.
The evolution of different seed types in halophytic species, which may vary in their level of dormancy, can extend the germination period and also produce a persistent seed bank that provides for the long-term recruitment of seedlings (Ungar 1987, 1991). Fresh brown seeds of S. aralocaspica are non-dormant. They can germinate quickly in a relatively wide range of environmental conditions and therefore would be expected to have an ‘opportunistic’ germination strategy (Gutterman, 1993). By contrast, fresh black seeds are in non-deep PD and would be expected to have a ‘cautious’ germination strategy, in which not all of the seeds germinate at one time (Gutterman, 1993).
A few studies have shown that dimorphic or polymorphic seeds are non-dormant or have PD. Large seeds of Atriplex prostrata and Salicornia europaea, two inland salt marsh plants, were non-dormant, and thus they germinated to >90 %. However, the small seeds had primary dormancy, a light requirement for germination and appeared to exhibit dormancy cycling (Carter and Ungar, 2003). Salsola affinis, an inland salt desert annual, produces three types of seeds. Type A and Type B seeds are non-dormant, whereas Type C seeds need a 4-week cold stratification period to break PD (Wei et al., 2007). The germination percentage of brown seeds of Arthrocnemum indicum was >80 % in distilled water at thermoperiods of 10 : 20 °C (Khan and Gul, 1998). Ninety-five per cent of the brown seeds of Halopyrum mucronatum germinated in the non-saline controls at all temperature regimes tested (Khan and Ungar, 2001). Small and/or black seeds of other species, for example Hedypnois cretica, Crepis aspera and S. salsa, also had non-deep PD (EI-Keblawy, 2003; Li et al., 2005). By contrast, small and/or black seeds of Arthrocnemum indicum, Salsola komarovii, Suaeda moquinii and Halopyrum mucronatum are non-dormant (Takeno and Yamaguchi, 1991; Khan et al., 1998, 2001; Khan and Ungar, 2001).
A light requirement for germination is frequently associated with small seeds, which are considered to contain rather small amounts of reserve materials. In this case, it might be advantageous for them to germinate under conditions where photosynthesis occurs very soon after germination (Mayer and Poljakoff-Mayer, 1989). The present results showed there was no difference between germination in light and dark in brown (large) and black (small) seeds of S. aralocaspica. Thus, this species may not be expected to form a persistent seed bank. However, even 8 weeks of cold stratification did not break dormancy in all of the black seeds, and salinity may induce secondary dormancy in both brown and black seeds. Thus, it seems that based on differences among seeds in this aspect of their requirements for dormancy break, the species does have the potential to form a seed bank.
Temperature is an important factor regulating germination of dimorphic seeds of S. aralocaspica. As temperature increased from 5 : 15 to 20 : 35 °C, germination percentage and germination velocity of fresh brown seeds increased, which means that temperature is not a limiting factor for germination from early spring until late autumn. Thus, germination of brown seeds mainly depends on moisture conditions in the field. However, germination of fresh black seeds was prevented by physiological dormancy. In the natural habitat, seeds mature in autumn, and they need 6–8 weeks of moist stratification in the cold early spring to break non-deep PD. In early spring, temperatures rise to about 5–10 °C, and the soil is wet due to melting snow and ice. This cold stratification effect of breaking dormancy has also been reported for many other plants inhabiting semi-deserts and deserts with cold winters (Huang et al., 2004a, b; Wei et al., 2007; Qu et al., 2008a). In such deserts, temperatures can be as low as −20 °C in winter, and the soil is frozen. If the time for moist stratification (about 0–10 °C) in spring is not long enough, some of the seeds may remain dormant and enter the soil seed bank.
Data from this investigation with S. aralocaspica indicated differences in the responses of brown and black seeds to salinity. Compared with fresh black seeds, brown seeds are much more salt-tolerant: they can germinate to high percentages in NaCl solutions and in relatively higher soil salt concentrations. Thus, 10 % of fresh brown seeds can germinate in 1400 mmol L−1 whereas <10 % of black seeds can germinate at 200 mmol L−1 NaCl (Table 5). Differences in germination of dimorphic seeds vary among halophytes. Germination of small seeds of Salicornia europaea was reduced from 52 % in distilled water to 1 % in a 3 % NaCl solution, and germination of large seeds was reduced from 94 % in distilled water to 35 % in a 3 % NaCl solution (Ungar, 1979). Khan et al. (2001) also found that brown seeds of S. moquinii germinated to 30 % in 1000 mmol L−1 NaCl, whereas only 8 % of black seeds did so in 600 mmol L−1 NaCl. Similar salinity tolerances for dimorphic seeds were reported for Atriplex triangularis (Khan and Ungar, 1984), Arthrocnemum indicum (Khan and Gul, 1998) and S. salsa (Li et al., 2005). No significant difference was observed in germination percentage between the two seed types of Salsola komarovii except at 500 mmol L−1 NaCl, where only the long-winged type germinated (Takeno and Yamaguchi, 1991).
Few previous studies have focused on the dynamic changes in germination response to salinity when dormancy conditions of halophyte seeds were altered. The present study showed that when non-deep PD was broken by 8 weeks of cold stratification, germination percentages and germination velocity of black S. aralocaspica seeds in distilled water and in saline solutions were significantly higher than those that had not been stratified. Furthermore, cold-stratified seeds germinated in higher soil salt concentrations (1000 mmol L−1 NaCl) than non-stratified seeds (800 mmol L−1 NaCl). Thus, cold stratification causes an increase in growth potential of the embryo, and as a result the radicle can break through the seed coat, i.e. seeds germinate.
As for other halophytes, when ungerminated S. aralocaspica seeds are transferred to distilled water, a percentage of them have the ability to recover to germinate. About 51–86 % of the ungerminated brown seeds recovered, whereas only 1–19 % of the ungerminated black seeds did so. However, germination recovery of cold-stratified black seeds reached 28–65 %. Thus, germination recovery of ungerminated brown seeds, non-stratified black seeds and cold-stratified black seeds was not complete, and it decreased with an increase in pretreatment concentration of NaCl solutions. Higher pretreatment concentrations of NaCl solutions appeared to prevent the recovery of germination. After recovery of germination, the TTC test demonstrated that most ungerminated seeds were still viable. This means that secondary dormancy was induced by salt pretreatment of the seeds. The higher the pretreatment salt concentration, the higher the percentage of seeds induced into dormancy. By contrast, the recovery of germination of black seeds of S. moquinii after they were transferred to distilled water following various salinity treatments for 20 d was nearly complete (82–100 %) at 5 : 15 °C (Khan et al., 2001). In the present study, 59–97 % of the brown seeds recovered. Like other halophytes in inland cold salt deserts (Qu et al., 2008a, b; Wei et al., 2008), S. aralocaspica seeds have the capacity to remain viable after exposure to hypersaline conditions. Thus, seeds induced into secondary dormancy would be expected to remain in the soil seed bank.
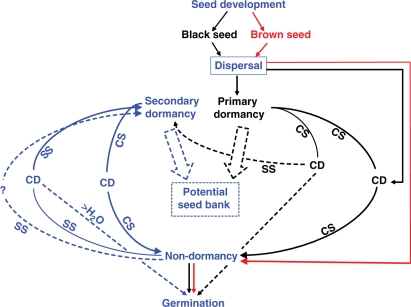
A model summarizing the dynamics of seed dormancy, germination and potential to form a seed bank for dimorphic seeds of S. aralocaspica is shown in Fig. 8. The dimorphic seeds are matured and dispersed in October. Fresh black seeds are in non-deep PD, and they need the spring temperatures to break dormancy [0 : 10 °C (March); 5 : 15 °C (April)]. It is too cold and dry in winter to satisfy the dormancy-breaking (cold stratification) requirement. After a short period of low temperatures (2–8 weeks), a proportion of the black seeds become conditionally dormant or non-dormant and germinate. However, a further proportion of the seeds need more than 8 weeks of cold to break dormancy, and they may remain in the soil until at least the next spring before they can germinate. Furthermore, some conditionally dormant or non-dormant black seeds that do not germinate in spring may enter secondary dormancy during the growing season via salt stress. The fresh brown seeds are non-dormant, and they can germinate in a wide range of temperatures.
Fig. 8.
Conceptual model of the dynamics of seed dormancy and germination of black (black lines), brown (red lines) and both brown and black (blue lines) seeds of Suaeda aralocaspica. CD, conditional dormancy; cs, cold stratification; ss, salt stress; > H2O, dilution by water.
Salinity may induce brown and non-dormant black seeds into conditional dormancy. For a percentage of these conditionally dormant seeds, which are quiescent due to high salt concentration, water from rain or snowmelt may dilute soil salinity and allow them to germinate. However, if salinity stress is not reduced in the germination season, the seeds would enter secondary dormancy and perhaps the seed soil bank. We presume that the secondarily dormant brown and black seeds need to be cold stratified the next spring to come out of dormancy. Further studies are needed to verify (or not) the presence of a persistent seed soil bank in S. aralocaspica.
Thompson (1981) suggested that both seed bank and seed polymorphism maintain the adaptation of the population to the average long-term conditions in its habitat, and this seems to be the case for S. aralocaspica. The present study clearly suggests an ecological significance of the dimorphic seeds in this species. The dimorphic seeds have different dispersal strategies: brown seeds have the ability to be dispersed further away from the mother plant than do black seeds (our personal observations). In this case, brown seeds may ‘explore’ new habitats and black seeds germinate in situ, near the mother plant. Furthermore, fresh brown seeds are non-dormant, and fresh black seeds are dormant. Finally, germination of non-dormant seeds of the two types have different optimum thermoperiods and different salinity tolerance limits. It is likely that as a strategy for sexual reproduction, seed dimorphism allows S. aralocaspica to gain competitive advantages in highly unstable environments and reduces the effect of spatial and temporal changes of surroundings on the success of its reproduction, thus permitting this species successfully to inhabit the harsh desert habitats.
ACKNOWLEDGMENTS
Our thanks are extended to Gehan K. M. G. Jayasuriya, Biology Department, University of Kentucky, USA, for valuable advice on data analysis. Z.H. thanks The Chinese Academy of Sciences for awarding him a fellowship for a 3-month visit to the Biology Department, University of Kentucky, Lexington, USA. This research work was supported financially by the Key Basic Research and Development Plan of China (2007CB106802), Key Project of CAS (KZCX2-YW-431) and National Natural Science Foundation of the People's Republic of China (30570281, 30570296).
LITERATURE CITED
- Bao SD. Soil chemistry and agriculture analysis. Beijing: China Agriculture Press (in Chinese); 2000. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin CC, Thompson K, Baskin JM. Mistakes in germination ecology and how to avoid them. Seed Science Research. 2006;16:165–168. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Davis BH, Baskin CC, Gleason SM, Cordell S. Physical dormancy in seeds of Dodonaea viscosa (Sapindales, Sapindaceae) from Hawaii. Seed Science Research. 2004;14:81–90. [Google Scholar]
- Carter CT, Ungar IA. Germination response of dimorphic seeds of two halophyte species to environmentally controlled and natural conditions. Canadian Journal of Botany. 2003;81:918–926. [Google Scholar]
- Commissione Redactorum Florae Xinjiangensis. Flora Xinjiangensis. Urumchi: Xinjiang Science & Technology & Hygiene Publishing House (in Chinese); 1994. [Google Scholar]
- EI-Keblawy A. Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae) Canadian Journal of Botany. 2003;81:550–559. [Google Scholar]
- Gul B, Weber DJ. Effect of salinity, light, and temperature on germination in Allenrolfea occidentalis. Canadian Journal of Botany. 1999;77:240–246. [Google Scholar]
- Gutterman Y. Seed germination of desert plants. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Gutterman Y. Survival strategies of annual desert plants. Berlin: Springer-Verlag; 2002. [Google Scholar]
- Harper JL. Population biology of plants. London: Academic Press; 1977. [Google Scholar]
- Harper JL, Lovell DH, Moore KG. The shapes and sizes of seeds. Annual Review of Ecology and Systematics. 1970;1:327–351. [Google Scholar]
- Huang ZY, Dong M, Gutterman Y. Factors influencing seed dormancy and germination in sand, and seedling survival under desiccation, of Psammochloa villosa (Poaceae), inhabiting the moving sand dunes of Ordos, China. Plant and Soil. 2004;a 259:231–241. [Google Scholar]
- Huang ZY, Dong M, Gutterman Y. Caryopses dormancy, germination and seedling emergence in sand, of Leymus racemosus (Poaceae), a perennial sand dune grass inhabiting the Junggar Basin of Xinjiang, China. Australian Journal of Botany. 2004;b 52:519–528. [Google Scholar]
- Imbert E. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology, Evolution and Systematics. 2002;5:13–36. [Google Scholar]
- Kapralov MV, Akhani H, Voznesenskaya EV, Edwards G, Franceschi V, Roalson EH. Phylogenetic relationships in the Salicornioideae/Suaedoideae/Salsoloideae s.l. (Chenopodiaceae) clade and a clarification of the phylogenetic position of Bienertia and Alexandra using multiple DNA sequence datasets. Systematic Botany. 2006;31:571–585. [Google Scholar]
- Khan MA, Gul B. High salt tolerance in germinating dimorphic seeds of Arthrocnemum indicum. International Journal of Plant Sciences. 1998;159:826–832. [Google Scholar]
- Khan MA, Ungar IA. The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. American Journal of Botany. 1984;71:481–489. [Google Scholar]
- Khan MA, Ungar IA. Effect of thermoperiod on recovery of seed germination of halophytes from saline conditions. American Journal of Botany. 1997;84:279–283. [PubMed] [Google Scholar]
- Khan MA, Ungar IA. Alleviation of salinity stress and the response to temperature in two seed morphs of Halopyrum mucronatum (Poaceae) Australian Journal of Botany. 2001;49:777–783. [Google Scholar]
- Khan MA, Ungar IA, Gul B. Action of compatible osmotica and growth regulators in alleviating the effect of salinity on the germination of dimorphic seeds of Arthrocnemum indicum L. International Journal of Plant Sciences. 1998;159:313–317. [Google Scholar]
- Khan MA, Gul B, Weber DJ. Germination of dimorphic seeds of Suaeda moquinii under high salinity stress. Australian Journal of Botany. 2001;49:185–192. [Google Scholar]
- Li WQ, Liu XJ, Khan MA, Yamaguchi S. The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. Journal of Plant Research. 2005;118:207–214. doi: 10.1007/s10265-005-0212-8. [DOI] [PubMed] [Google Scholar]
- Mabberley DJ. The plant-book. A portable dictionary of the vascular plants. 2nd edn. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Mayer AM, Poljakoff-Mayer A. The germination of seeds. 4th edn. Oxford: Pergamon Press; 1989. [Google Scholar]
- Philipupillai J, Ungar IA. The effect of seed dimorphism on the germination and survival of Salicornia europaea L. populations. American Journal of Botany. 1984;71:542–549. [Google Scholar]
- Qian YB, Zhang LY, Wu ZN. Characteristics of eco-environment in the margin regions of the Junggar Basin, Xinjiang. Arid Land Geography. 2003;26:30–36. (in Chinese with English abstract) [Google Scholar]
- Qu XX, Baskin JM, Wang L, Huang ZY. Effects of cold stratification, temperature, light and salinity on seed germination and radicle growth of the desert halophyte shrub, Kalidium caspicum (Chenopodiaceae) Plant Growth Regulation. 2008;a 54:241–248. [Google Scholar]
- Qu XX, Huang ZY, Baskin JM, Baskin CC. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically-widespread halophyte shrub Halocnemum strobilaceum. Annals of Botany. 2008;b 101:293–299. doi: 10.1093/aob/mcm047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze P, Freitag H, Weising K. An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae) Plant Systematics and Evolution. 2003;239:257–286. [Google Scholar]
- Sokal RR, Rohlf EJ. Biometry. 3rd edn. San Francisco: Freeman; 1995. [Google Scholar]
- Takeno K, Yamaguchi H. Diversity in seed germination behavior in relation to heterocarpy in Salsola komarovii. The Botanical Magazine, Tokyo. 1991;104:207–215. [Google Scholar]
- Thompson PA. Ecological aspects of seed germination. In: Thomson JR, editor. Advances in research and technology of seeds. Amsterdam: Centre for Agricultural Publishing and Documentation; 1981. pp. 9–42. [Google Scholar]
- Ungar IA. Seed dimorphism in Salicornia europaea L. Botanical Gazette. 1979;14:102–108. [Google Scholar]
- Ungar IA. Population ecology of halophyte seeds. The Botanical Review. 1987;53:301–334. [Google Scholar]
- Ungar IA. Ecophysiology of vascular halophytes. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Venable DL. The evolutionary ecology of seed heteromorphism. The American Naturalist. 1985;126:557–595. [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414:543–546. doi: 10.1038/35107073. [DOI] [PubMed] [Google Scholar]
- Wei Y, Dong M, Huang ZY. Seed polymorphism, dormancy, and germination of Salsola affinis (Chenopodiaceae), a dominant desert annual inhabiting the Junggar Basin of Xinjiang, China. Australian Journal of Botany. 2007;55:1–7. [Google Scholar]
- Wei Y, Dong M, Huang ZY, Tan DY. Factors influencing seed germination of Salsola affinis, a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora. 2008;203:134–140. [Google Scholar]