Abstract
Background and Aims
Cadmium (Cd) causes Fe-deficiency-like symptoms in plants, and strongly inhibits photosynthesis. To clarify the importance of Cd-induced Fe deficiency in Cd effects on photosynthesis, the recovery processes were studied by supplying excess Fe after the Cd symptoms had developed.
Methods
Fe-citrate at 10 µm or 50 µm was given with or without 10 µm Cd(NO3)2 to hydroponically cultured poplars (Populus glauca ‘Kopeczkii’) with characteristic Cd symptoms. Ion, chlorophyll and pigment contents, amount of photosynthetic pigment–protein complexes, chlorophyll fluorescence and carbon assimilation were measured together with the mapping of healing processes by fluorescence imaging.
Key Results
In regenerated leaves, the iron content increased significantly, while the Cd content did not decrease. As a result, the structural (increase in the amount of photosynthetic pigments and pigment–protein complexes, decrease in the F690/F740 ratio) and functional (elevation of CO2 fixation activity and ΔF/Fm′) recovery of the photosynthetic machinery was detected. Cd-induced, light-stress-related changes in non-photochemical quenching, activity of the xanthophyll cycle, and the F440?/F520 ratio were also normalized. Imaging the changes in chlorophyll fluorescence, the recovery started from the parts adjacent to the veins and gradually extended to the interveinal parts. Kinetically, the rate of recovery depended greatly on the extent of the Fe supply, and chlorophyll a/b ratio and ΔF/Fm′ proved to be the most-rapidly reacting parameters.
Conclusions
Iron deficiency is a key factor in Cd-induced inhibition of photosynthesis.
Key words: Cadmium, chlorophyll–protein, iron deficiency, poplar, Populus glauca Haines 1906 var. Kopeczkii, fluorescence imaging, chlorophyll fluorescence induction
INTRODUCTION
Cadmium (Cd), a widespread pollutant, occurs in urban and industrial wastes (galvanic batteries, dyes), in chemical fertilizers, and is also a by-product of zinc mining. It has hardly any biological function (Lane et al., 2005) but it does induce many injuries in all organisms, including plants (Sanità di Toppi and Gabbrielli, 1999; Fodor, 2002). Cd has been shown to disturb both ion (Clemens, 2006) and water balance (Barceló and Poschenrieder, 1990). It has also been connected with retardation of plant growth and inhibition of diverse metabolic processes such as photosynthesis, respiration and assimilation of nitrate, and has an influence on the accumulation of different metabolites (van Assche and Clijsters, 1990; Sanità di Toppi and Gabbrielli, 1999, and references therein). With regard to photosynthesis, stomatal closure (Perfus-Barbeoch et al., 2002), inhibition of carbon assimilation and photosynthetic electron transport have been demonstrated, often correlated with premature senescence of chloroplasts (Krupa and Baszyński, 1995; Prasad, 1995; Myśliwa-Kurdziel et al., 2002). In common with the situation with stress factors, the activity of photosystem II (PSII) has proved to be the most sensitive, as shown by a decreased variable fluorescence (Sigfridsson et al., 2004; Küpper et al., 2007). Several sites of action have been suggested at both the donor and acceptor sides of PSII (Sigfridsson et al., 2004; Pagliano et al., 2006). In addition, Cd has strong effects on the development of the photosynthetic apparatus; it inhibited the synthesis of chlorophylls (Chl; Padmaja et al., 1990) and their stable binding to proteins (Horváth et al., 1996), thereby decreasing the accumulation of pigment–lipoprotein complexes, particularly photosystem I (PSI; Sárvári et al., 1999; Sárvári, 2005). Inhibition of photosynthesis induces oxidative stress (Romero-Puertas et al., 2004; Benavides et al., 2005), which can contribute to the degradation of photosynthetic structures and induction of senescence (McCarthy et al., 2001), and to stress acclimation of plants through signal-transduction processes (Maksymiec, 2007).
Although numerous effects of Cd on photosynthesis have been described, the exact molecular mechanism of action is not well understood. Existing theories have focused either on direct effects, such as Cd binding to important SH residues of enzymes and the replacement of essential ions at the active sites (van Assche and Clijsters, 1990), or on indirect effects including membrane damage, and disturbances in water and ion balance. With respect to whole plants, direct effects of Cd on photosynthesis have rarely been published, as Cd concentration is quite low in chloroplasts (Siedlecka and Krupa, 1999). Cd present at micromolar concentration could bind competitively to the essential Ca-binding sites in PSII during photoactivation of the water-splitting system (Faller et al., 2005) and direct inhibition of the oxygen evolution can not be excluded (Pagliano et al., 2006).
Indirect effects of Cd are more frequently mentioned under in vivo conditions. Cd-induced Fe deficiency in the shoots (Siedlecka and Krupa, 1996a, 1999; Fodor et al., 2005) may be of particular importance from the point of view of the development of photosynthetic apparatus, as Chl synthesis and stability of thylakoid complexes all depend on the availability of Fe. In fact, many effects of Cd on photosynthesis strongly resemble those of Fe deficiency; Fe-deficiency causes chlorosis, the amount of PSI decreases strongly, and the organization of the antennae changes (Abadía et al., 1989; Moseley et al., 2002; Timperio et al., 2007). Reduced activity of electron transport, and similar changes in Chl a fluorescence and thermal energy dissipation of excess light energy can be observed in Fe-deficient plants (Morales et al., 1998; Belkhodja et al., 1998), as in the case with Cd stress (Küpper et al., 2007). In addition, the severity of Cd symptoms was shown to depend strongly on the availability of Fe. In plants grown on Fe-deficient nutrient solution, Cd translocation on a larger scale into the shoot was observed, and both photosystems showed higher sensitivity to Cd (Siedlecka and Krupa, 1999). At the same time, elevated Fe supply applied together with Cd treatment warded off most Cd effects (Siedlecka and Krupa, 1996a, b; Siedlecka et al., 1997; Meda et al., 2006). Positive effects on growth, Rubisco and electron transport activity and Chl and carotenoid content were demonstrated. However, not only elevated Fe contents but, at the same time, lowered Cd uptake and translocation were observed in such studies in Phaseolus vulgaris (Siedlecka and Krupa, 1996a); thus these positive effects may well be attributed to the lowered Cd concentration in leaves.
In order to determine whether the beneficial effect of excess Fe was only connected with the recovery of Cd-induced Fe deficiency or was rather due to lowered Cd concentration, the aim of this study was to examine the effect of Fe, supplied at different concentrations in the presence or absence of Cd, on the recovery of well-developed Cd symptoms. The other goal was to elucidate the responsiveness of different photosynthetic parameters to Cd-induced Fe deficiency by studying the kinetics of their recovery under the above-mentioned conditions. To follow the spatial progress of regeneration, multicolour fluorescence imaging was used (Buschmann et al., 2000), which has proved to be a sensitive method for detecting stress (Lichtenthaler and Babani, 2000).
MATERIALS AND METHODS
Plant material and growth conditions
Experiments were performed on plants of poplar, Populus glauca Haines, 1906 ‘Kopeczkii’, grown in a growth chamber with 12/12 h light (100 µmol m−2 s−1)/dark periods, 20/18 °C and 70/75 % relative humidity. Plants were treated in hydroponics, in quarter-strength Hoagland solution [1·25 mm Ca(NO3)2, 1·25 mm KNO3, 0·5 mm MgSO4, 0·25 mm KH2PO4, 0·08 µm CuSO4, 4·6 µm MnCl2, 0·19 µm ZnSO4, 0·12 µm Na2MoO4, 11·56 µm H3BO3] with Fe-citrate at different concentrations as the Fe source to give the following treatments: control (Ctrl) = 10 µm Fe-citrate; Cad = 10 µm Cd, 10 µm Fe-citrate; Ctrl-50 = 50 µm Fe-citrate; Cad-50 = 10 µm Cd, 50 µm Fe-citrate. Plants were treated from their four-leaf stage, as shown in Table 1. Leaves that developed before/after the treatment were numbered down and up on the shoot (−3,–2,−1/+1,+2,+3).
Table 1.
Treatments applied on poplar plants after reaching their 4-leaf stage
| 1st week |
2nd and 3rd week |
|||||
|---|---|---|---|---|---|---|
| Treatment (1·w/2–3·w) | 10 µm Fe | 50 µm Fe | 10 µm Cd | 10 µm Fe | 50 µm Fe | 10 µm Cd |
| Ctrl/Ctrl | × | × | ||||
| Ctrl/Ctrl-50 | × | × | ||||
| Cad/Cad | × | × | × | × | ||
| Cad/Ctrl | × | × | × | |||
| Cad/Ctrl-50 | × | × | × | |||
| Cad/Cad-50 | × | × | × | × | ||
| Cad-50/Cad-50 | × | × | × | × | ||
Plants were treated with/without 10 µm Cd(NO3)2 for 1 week, and then allowed to recover or be treated under different circumstances. Ctrl (control) = 10 µm Fe-citrate; Cad = 10 µm Cd, 10 µm Fe-citrate; Ctrl-50 = 50 µm Fe-citrate; Cad-50 = 10 µm Cd, 50 µm Fe-citrate.
Determination of element concentrations
Dried (1 week, 60 °C) leaves were digested by HNO3 for 30 min at 60 °C and then in H2O2 for 90 min at 120 °C. After filtration by MN 640W paper, ion contents were measured by ICP-MS (inductively connected plasma mass spectrometer; ThermoFisher Scientific, USA) for micro-elements and by ICP-OES (inductively connected plasma optical emission spectrometer; Perkin-Elmer, USA) for macro-elements.
Pigment determination
Chlorophyll contents were determined in 80 % (v/v) acetone extracts by a UV-VIS spectrophotometer (Shimadzu, Japan) using the absorption coefficients of Porra et al. (1989).
For the determination of xanthophyll cycle components, leaf discs adapted to 100 µmol m−2 s−1 light or dark for 30 min were used. Leaves were powdered in liquid nitrogen and extracted with 80 % (v/v) acetone containing 0·1 % (v/v) NH4OH at 4 °C. Components were separated by HPLC method (Goodwin and Britton, 1988) using a Nucleosil C18 column in an HPLC-system equipped with an UV/VIS detector (JASCO Int. Co., Japan). The eluents were acetonitrile : water mixture (9 : 1, 0·01 % (v/v) triethylamine) and ethyl acetate. Zeaxanthin, as a standard, was used to identify the peaks and calculate the pigment concentrations (Tóth et al., 2002). The de-epoxidation state of xanthophyll cycle pigments (DEEPS) was calculated as (Z + 0·5A)/(V + A + Z), where Z, A and V are zeaxanthin, antheraxanthin and violaxanthin, respectively.
Chlorophyll–protein (CP) complexes
Isolation of thylakoids as well as the separation and identification of CP complexes was performed as described previously (Sárvári and Nyitrai, 1994). Briefly, washed thylakoids were solubilized with mainly glycosidic detergents (dodecanoyl sucrose : nonyl glycoside : lithium dodecyl sulphate = 4·5 : 4·5 : 1, w/w/w, purchased from Sigma and Calbiochem-Novabiochem), using a detergent to Chl ratio of 10 : 15 (w/w). CPs were separated by Deriphat (Cognis, Dusseldorf, Germany) gel electrophoresis (‘green gels’). The relative amounts of CP bands separated by electrophoresis were determined as the percentage of Chl applied on the gel by densitometry of the scanned gels using the Phoretix software (Phoretix International, Newcastle upon Tyne, UK). Thin slices of green gels were transferred to the top of denaturing gels for a second-dimension run according to the protocol of Laemmli (1970) but using 10–18 % acrylamide gradient. The amounts of bands identified by their polypeptide pattern as belonging to a given complex were summed up. The absolute amounts of specific complexes were calculated as μg Chl cm−2 leaf material by dividing the Chl content of 1-cm−2 leaf pieces among the complexes according to their percentage proportions.
Measurement of CO2 fixation
CO2 fixation was estimated by a radioisotope method (Láng et al., 1985). The radioactivity of leaf discs was measured in toluene containing 0·5 % (w/v) 2,5-diphenyl- oxazol and 0·005 % (w/v) 1,4-di[2-(5-phenyl)-oxazolyl]- benzene by a Beckmann LS-5000 TD liquid scintillation counter (Beckmann Instruments, Fullerton, CA, USA).
Fluorescence induction measurements
Fluorescence induction measurements of leaf samples were performed using a PAM 101–102–103 chlorophyll fluorometer (Walz, Effeltrich, Germany). Leaves were adapted to dark for 30 min. The F0 level of fluorescence was determined by switching on the measuring light [modulation frequency of 1·6 kHz and photosynthetic photon flux density (PPFD) <1 µmol m−2 s−1]. The maximum fluorescence yield (Fm) in the dark-adapted state and Fm′ in the light-adapted state were measured by applying a 0·7-s pulse of white light (PPFD of 3500 µmol m−2 s−1; light source, KL 1500 electronic; Schott, Mainz, Germany) which saturated PSII electron transport thus closing all PSII traps. For quenching analysis actinic white light (PPFD of 100 µmol m−2 s−1; KL 1500 electronic) was provided. Simultaneously with the onset of actinic light the modulation frequency was switched to 100 kHz. F0′ was measured by turning off the actinic light and applying 3 s of weak far-red light (102-FR, setting 11, emission peak at 730 nm; Walz, Effeltrich, Germany,) to ensure that all PSII traps were open.
The parameter Fv/Fm means the maximal efficiency, whereas ΔF/Fm′ is the actual efficiency (effective quantum yield of photochemical energy conversion in actinic light) of PSII. Non-photochemical quenching (NPQ) is related to the non-photochemical quenching of Chl fluorescence. SV0 shows the rate of NPQ of the light-harvesting antennae. Fluorescence induction parameters were determined by the equations according to Genty et al. (1989), Gilmore and Yamamoto (1991) and Baker and Rosenqvist (2004):
Fluorescence imaging
A compact flash-lamp fluorescence imaging system was used (Szigeti, 2008) for imaging the fluorescence at 440, 520, 690 and 740 nm wavelengths. Excitation was carried out by xenon-lamp flashes of 16·7 Hz filtered by DUG 11 (Schott, Mainz, Germany) to ensure the appropriate exciting wavelength (λexc = 360–370 nm). The detection of fluorescence was performed by CCD video camera (objective; Nikon-AF Nikkor, Japan, 1 : 1·4 D, 50 mm diameter) at the four different wavelengths using appropriate interference filters. Accumulation of 400 images was chosen as a suitable number of successive readout images. Images were taken from the adaxial side of light-adapted leaves at room temperature and corrected by the filter sensitivity parameters and the inhomogeneity of the exciting light. Corrections and arithmetical operations were performed by Camille 1·05 software (Photonetics, Kehl, Germany). The 8-bit images were transformed into pseudo-colour pictures by ImageJ software package (http://rsb.info.nih.gov/ij).
The changes in the F690/F740, F690–F740 and F440/F520 parameters were followed. The F690/F740 and F690–F740 parameters both refer to the Chl content but the F690–F740 parameter reports more sensitively about small changes – even at lower Chl contents (Solti et al., 2008b). F440/F520 ratio refers to the strength of stress effects (Lichtenthaler and Babani, 2000).
Statistical analysis
The results are mainly two parallel measurements in three replicate experiments under each condition. Unpaired t-tests and ANOVA were performed on the data. The term ‘significantly different’ means that the similarity of samples is P < 0·05.
RESULTS
Changes in physiological parameters during the treatment and regeneration period (Table 1) were followed in both leaves which emerged under Cd treatment (+2) and in those developed before the treatment (−2). Cd symptoms, which developed completely within 1 week, were similar in both leaf types but their strength depended on the developmental stage of leaves. Leaves that reached 50–60 % of their final size at the beginning of Cd treatment (−2) had their photosynthetic apparatus fully developed, and were less sensitive to the Cd treatment (data not shown). In young leaves the Cd symptoms were stronger (see below). Both leaf types behaved similarly under regeneration but ‘+2’ leaves showed more typical and vigorous recovery processes. Hence, the results presented are those obtained mostly with ‘+2’ leaves.
Ion contents
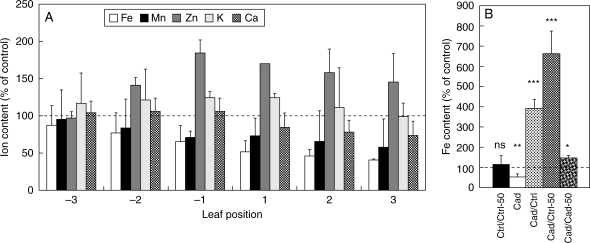
Concentrations of macro-elements did not differ significantly in the control leaves assayed [K/Ca contents were 35·6 ± 1·1/6·6 ± 0·4 and 37·1 ± 5·7/6·0 ± 1·3 mg g−1 dry mass in ‘–2’ and ‘+2’ leaves, respectively]. However, the concentrations of micro-elements, particularly Fe and Mn, decreased somewhat in the upper leaves (Fe/Mn/Zn contents were 125 ± 14/103 ± 10/31 ± 1 and 105 ± 20/89 ± 20/33 ± 1 µg g−1 d. wt in ‘−2’ and ‘+2’ leaves, respectively).
Cd content of ‘+2’ leaves was about 50 ± 18 µg g−1 d. wt after 7 d of Cd treatment. It did not vary significantly among leaves assayed. After 14 d, it rose to 286 ± 105 and 257 ± 56 µg Cd g−1 d. wt in Cad/Cad and Cad/Cad-50 type plants (Table 1), respectively. Cd treatment modified the concentrations of essential cations in leaves (Fig. 1A). K content hardly changed but the concentration of Ca diminished slightly in the upper leaves. Concerning the micro-elements, Zn concentration increased (by 20–60 %, depending on the leaf-type examined), while the concentration of Mn and Fe decreased, particularly in the upper leaves. At the end of the 1-week regeneration period with supplemental Fe, there was a 4- and 7-fold increase in Fe concentration in ‘+2’ leaves of Cad/Ctrl and Cad/Ctrl-50 plants, respectively (Fig. 1B) without any change in their Cd contents. This increase in Fe content was rapid (data not shown) in Cad/Ctrl-50 plants, reaching 146·3 ± 29·8 µg g−1 d. wt (higher than the Ctrl/Ctrl value of 116·6 ± 13·7 µg g−1 d. wt) after 1-d recovery (on the 8th day), and slower in Cad/Ctrl plants reaching 178·0 µg g−1 d. wt on the 10th day of treatment. The Fe content of Cad/Cad-50 plants also increased but it did not differ significantly from that of the control at the 14th day (Fig. 1B). At the same time, supplemental Fe nutrition did not cause any change in the Fe content of the leaves in Ctrl/Ctrl-50 plants.
Fig. 1.
Changes in ion contents of differently treated poplar plants. (A) Ion content of leaves of plants treated with Cd for 14 d. Values are given as the percentage of controls. (B) Fe content of ‘+2’ leaves of treated plants after 14 d of treatment. Control value: 105 ± 20 µg g−1 d. wt. Data are means of two replicate experiments. *, P < 0·05; **, P < 0·01, ***, P < 0·001 (one-way ANOVA). For treatments, see Table 1.
Leaf development
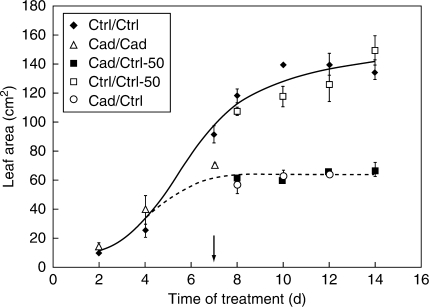
Leaf area growth of Cd-treated plants (+2 leaf: 65·4 ± 3·6 cm2 at the end of the treatment) significantly lagged behind the controls (138·8 ± 8·9 cm2). Cd not only decreased the intensity of area growth but the whole process was shorter, and Cd-treated plants reached the final leaf size sooner than controls (7 d versus 9 d). Therefore, leaf development could not be regenerated in any regeneration type by applying supplemental Fe (Fig. 2), and leaf sizes of regenerated and Cd-treated plants were essentially similar.
Fig. 2.
Leaf area growth of ‘+2’ leaves in differently treated poplar plants. The continuous line shows the control-type and the dashed line the Cd-treated-type growth curves. The arrow shows the start of the recovery period. For treatments, see Table 1.
Pigment contents
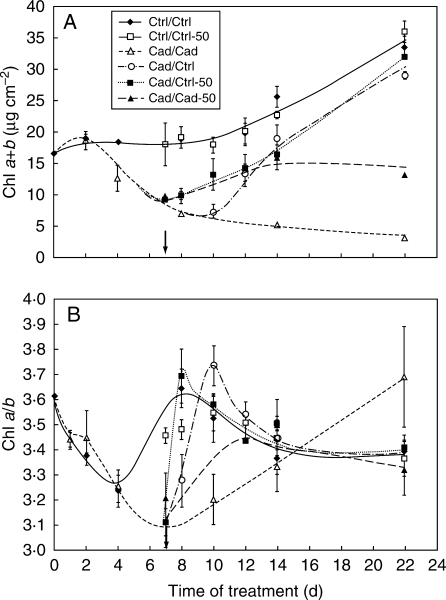
Chlorophyll content calculated on a leaf-area basis slightly but continuously increased in leaves of Ctrl/Ctrl and Ctrl/Ctrl-50 plants during treatment (Fig. 3A). The Chl accumulation was strongly reduced by Cd to the end of the first week. Chlorophyll content increased continuously in regenerated plants during the recovery but it did not reach the level of the controls in any case even after 2 weeks. Even the Cad/Cad-50 type plants showed regeneration. The rate of recovery depended greatly on the extent of the Fe supply.
Fig. 3.
Changes in (A) the Chl content and (B) Chl a/b ratio of ‘+2’ leaves for the different treatments. The Chl content of Cad/Ctrl and Cad/Ctrl-50 plants differed very significantly (P < 0·0001) from the Cad/Cad and Cad/Cad-50 types. The arrows show the start of the recovery period. For treatments, see Table 1.
The Chl a/b ratio, which relates to the ratio of core complexes to outer antennae, did show developmental changes in control leaves (from undeveloped to shaded). However, it dropped from the fully developed control level of 3·56 ± 0·08 to 3·15 ± 0·19 in Cd-treated plants. Interestingly, Chl a/b exceeded control values in the third week of Cd treatment. During the regeneration period, it began to increase very quickly and produced an ‘overshoot’ within 1–3 d depending on the type of treatment (Fig. 3B). After this ‘overshoot’, the Chl a/b ratio reached the level of controls in 1 week.
Carotenoid content increased by 10–15 % in Cd-treated leaves, the VAZ (violaxanthin + antheraxanthin + zeaxanthin) content being elevated the most (Table 2). It showed a tendency to decrease during the regeneration period but in spite of this it remained higher than in controls.
Table 2.
Carotenoid contents of poplar plants treated for 14 d. Carotenoid content of Ctrl/Ctrl is given in absolute amount (mmol carotenoid mol−1 Chl), and other treatment as percentage of the control values
| β-Carotene | Lutein | Neoxanthin | VAZ | Sum | |
|---|---|---|---|---|---|
| Ctrl/Ctrl | 17·20 ± 2·81 | 125·41 ± 7·71 | 20·36 ± 1·49 | 24·61 ± 2·50 | 187·80 ± 11·35 |
| Cad/Cad (%) | 151·6 ± 20·1 | 109·8 ± 4·9 | 107·3 ± 1·9 | 148·5 ± 17·4 | 117·3 ± 9·1 |
| Cad/Ctrl (%) | 146·0 ± 20·4 | 106·0 ± 7·6 | 106·0 ± 6·9 | 138·2 ± 13·6 | 113·8 ± 2·5 |
| Cad/Ctrl-50 (%) | 129·5 ± 25·2 | 106·1 ± 10·8 | 111·5 ± 8·0 | 126·5 ± 7·6 | 111·4 ± 10·3 |
For treatments, see Table 1.
VAZ, violaxanthin + antheraxanthin + zeaxanthin.
Fluorescence imaging
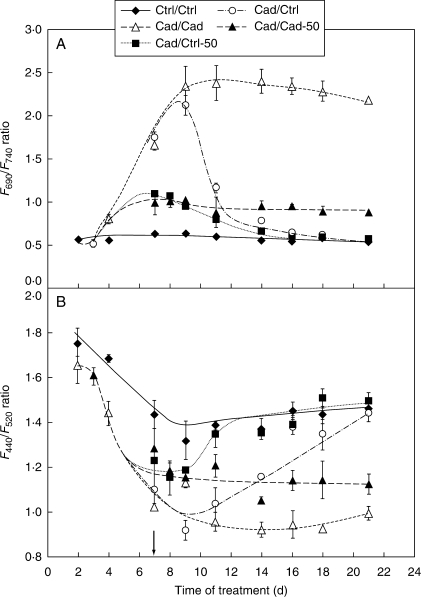
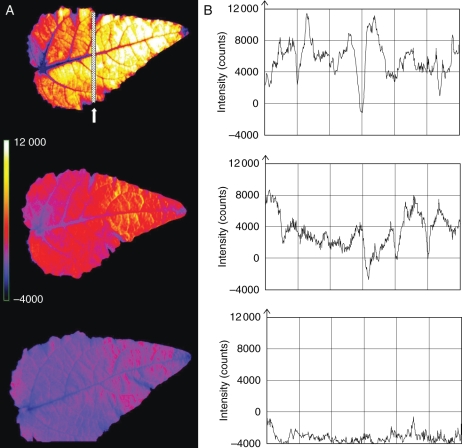
The fluorescence ratio F690/F740, which is closely associated with the Chl content and influenced by the organization of CPs in thylakoids, was strongly elevated due to Cd treatment (Fig. 4A). At the end of the 1-week regeneration period, this ratio had fully recovered except in the Cad/Cad-50 plants where it was stabilized at a slightly higher level than in the controls. The F690–F740 parameter, which is a more sensitive tool in mapping the differences in Chl content than F690/F740, showed that regeneration began at the leaf base and near to the major veins of recovering leaves, and later on expanded to the whole area of the leaf (Fig. 5). The leaf apex, similarly to ‘–2’ leaves (data not shown), did not show strong Cd effects, which manifested in a higher Chl content (Fig. 5A).
Fig. 4.
Changes in (A) the F690/F740 and (B) F440/F520 ratios of ‘+2’ leaves under different conditions. Only the Cad/Cad and Cad/Cad-50 plants showed strong significant differences (one-way ANOVA, P < 0·01) from the control at the end of the recovery period. The arrow shows the start of the recovery period. For treatments, see Table 1.
Fig. 5.
Distribution of fluorescence difference F690–F740 in ‘+2’ leaves of Cad/Ctrl-50 poplar plants during the recovery period (representative samples). (A) Pseudo-colour images taken on the 8th (top), 11th (middle) and 14th day (bottom). (B) The horizontal profiles of this parameter along the cross-section (see the dotted line) of corresponding leaves. The F690–F740 parameter shows similar but more sensitive distribution of Chl compared with the F690/F740 parameter (see Materials and Methods).
In Ctrl/Ctrl plants, the level of F440/F520 sharply decreased during the development of leaves and became stable after leaf area growth had finished (Fig. 4B). At the same time, however, it showed a stronger and further decrease in leaves of Cd-treated plants. This decrease was stopped by the addition of excess Fe together with Cd in Cad/Cad-50 plants, whereas the F440/F520 ratio was fully recovered in Cad/Ctrl and Cad/Ctrl-50 type plants during regeneration for 1 week. The recovery was also more rapid in Cad/Ctrl-50 than Cad/Ctrl samples.
Thylakoid composition
The CP composition of thylakoids was determined by separation of the solubilized complexes by native PAGE. The band pattern of green gels was similar to the ones published in Sárvári and Nyitrai (1994). The green bands were identified on the basis of their polypeptide patterns obtained by denaturing PAGE. The patterns were known from comparison with those of isolated PSI, PSII core and light-harvesting complex (LHC) II particles (E. Sarvari et al., unpubl. res.), and were basically similar to those obtained by, for example, Zhang and Scheller (2004).
In agreement with the Chl a/b ratio variations, thylakoid composition also showed distinct changes under Cd treatment. The most pronounced decrease was observed in the amount of PSI and LHCII complexes (Table 3). After 1 week, both complexes showed regeneration, although the accumulation of LHCII lagged behind the other complexes. Again, the order of effectiveness of conditions in regenerating power was as follows: Cad/Ctrl-50 > Cad/Ctrl > Cad/Cad-50 (Table 3).
Table 3.
Chlorophyll–protein complexes in differently treated poplar plants
| 7th day of treatment |
14th day of treatment |
||||||
|---|---|---|---|---|---|---|---|
| Complex | Ctrl (μg Chl cm−2 LA) | Cad (%) | Ctrl/Ctrl (μg Chl cm−2 LA) | Cad/Cad (%) | Cad/Ctrl (%) | Cad/Ctrl-50 (%) | Cad/Cad-50 (%) |
| PSI | 7·97 ± 0·23 | 32·7 ± 5·3 | 10·99 ± 0·49 | 26·4 ± 2·5 | 51·9 ± 1·4 | 67·6 ± 2·4 | 44·6 ± 1·5 |
| PSIICC | 1·54 ± 0·08 | 61·0 ± 2·6 | 2·58 ± 0·28 | 37·6 ± 11·2 | 58·5 ± 17·0 | 74·8 ± 13·9 | 50·0 ± 3·1 |
| PSIICA | 2·15 ± 0·55 | 83·7 ± 7·4 | 2·72 ± 0·48 | 62·1 ± 11·8 | 54·8 ± 8·8 | 81·2 ± 11·0 | 62·5 ± 10·3 |
| LHCII | 9·30 ± 1·00 | 39·0 ± 8·6 | 12·99 ± 0·65 | 23·6 ± 5·2 | 48,0 ± 1·9 | 57·8 ± 7·5 | 41·6 ± 6·2 |
Free pigment was 6–10 % of the total Chl applied on the gel.
CC, core complex; CA, connecting antenna; LA, leaf area; %, percentage of control.
For treatments, see Table 1.
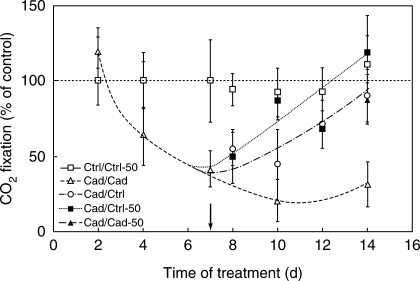
Photosynthetic activity
CO2 fixation (11·72 ± 0·73 µmol CO2 m−1 s−1 in fully developed leaves of Ctrl/Ctrl) was severely inhibited (by 75 %) under Cd treatment within 1 week, and no further decrease was observed (Fig. 6). It could be recovered to the Ctrl/Ctrl level by applying supplemental Fe in each recovery treatment in 1 week. The slopes of regeneration curves depended on the Fe concentration used for recovery.
Fig. 6.
Changes in 14CO2-fixation in ‘+2’ leaves under different treatments. The difference between controls and Cad/Cad values was strongly significant (one-way ANOVA, P < 0·0001) at the end of treatment, whereas regenerated plants did not differ from the controls (Tukey–Kramer comparison, P > 0·05). The arrow shows the start of the recovery period. For treatments, see Table 1.
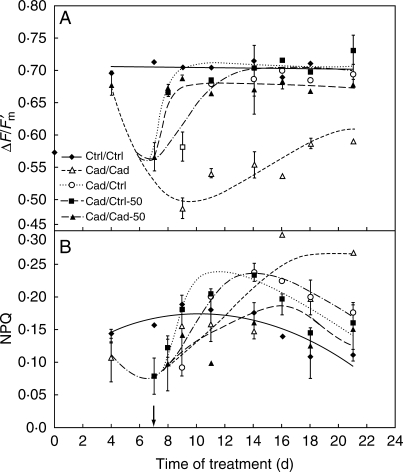
The maximal (Fv/Fm, data not shown) and actual efficiency of PSII (ΔF/Fm′) were nearly constant in leaves of controls during the treatment (Fig. 7A). Efficiency of PSII did not decrease in Cad-50/Cad-50 plants, showing the protective role of supplemental Fe applied together with the Cd treatment. Fv/Fm decreased to about 80 % (data not shown), and ΔF/Fm′ also dropped drastically to 71·0 ± 0·2 %, in Cd-treated plants after treatment for 1 week (Fig. 7A). During regeneration with different levels of Fe supply, both parameters started to rise up quickly, but with different kinetics. While the Cd-treated plants showed only a slight recovery, an increasing efficiency of PSII comparable to those without Cd could be observed under Cad/Cad-50 treatment.
Fig. 7.
Changes in (A) the actual efficiency of PSII, ΔF/Fm′ and (B) non-photochemical quenching, NPQ under different conditions. Only the Cad/Cad plant differed from the other groups (one-way ANOVA, P < 0·01) at the end of treatment. The arrow shows the start of the recovery period. For treatments, see Table 1.
NPQ
During the first week, NPQ of Cd-treated plants lagged behind the level of controls (Fig. 7B), while Cad-50/Cad-50 plants did not differ from controls (data not shown). During the recovery period, however, NPQ attained or exceeded the values of the controls. This ‘overshoot’ was similar to that of the Chl a/b ratio (Fig. 3B) but reached its maximum when the control-like Chl a/b level was approximated. The shapes of the NPQ curves were similar in the different recovery types but plants regenerated with 50 µm Fe showed the ‘overshoot’ faster than those supplied with a lower Fe concentration (10 µm Fe). Supplemental Fe applied together with Cd during regeneration was also effective but in this case the ‘overshoot’ was less pronounced. The level of NPQ also rose over the controls in leaves of Cd-treated plants, the recovery process, however, was much slower in this case. SV0, which is connected with antenna quenching, doubled under Cd treatment, and this increase could also be regenerated (data not shown).
During the measurement of fluorescence induction a phenomenon of longer adaptation time to actinic light could be observed due to Cd treatment. In addition, the NPQ in Cd-treated leaves was much higher than in controls (2·59 ± 0·50 vs. 0·790 ± 0·085) after exposure for 10 s to actinic light. The regeneration could be demonstrated by the shortening of the light adaptation time from about 30 min to nearly 15 min (approx. 10 min in controls).
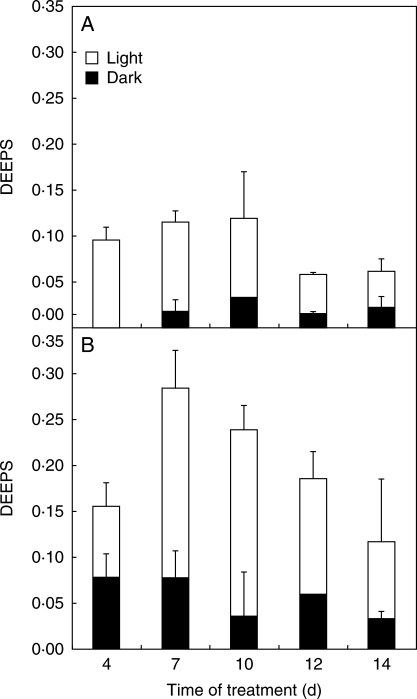
In fully developed ‘+2’ leaves of controls, the light- or dark-adapted de-epoxidation states of xanthophylls did not change during the treatment (Fig. 8). A decrease in the light-adapted de-epoxidation percentage could only be observed in shaded leaves. At the same time, Cd treatment significantly increased both the light and particularly the dark-adapted levels of de-epoxidated xanthophylls. During 1-week regeneration of Cad/Ctrl-50 plants, the level of de-epoxidation percentage tended to be similar to that of the young leaves of the controls.
Fig. 8.
Changes in the de-epoxidation state of xanthophyll cycle pigments (DEEPS) in ‘+2’ leaves of (A) Ctrl/Ctrl and (B) Cad/Ctrl-50 poplar plants during the time of treatment. Dark- (black) and light-adapted (white) sample pairs were collected side by side from the same leaves, and adapted to dark/100 µmol cm−2 s−1 light for 30 min.
DISCUSSION
Cd strongly disturbs the homeostasis of several essential metal ions, particularly that of Fe, and causes moderate-to-strong Fe deficiency in leaves (Siedlecka and Krupa, 1999; Meda et al., 2006). This Fe deficiency proved to be the most important factor among the effects of Cd on photosynthesis in poplar plants. The results show that not only did the excess Fe given together with Cd diminish the effects of Cd on photosynthesis, and at the same time reduce the Cd content of leaves (Siedlecka and Krupa, 1996a, b; Siedlecka et al., 1997) and provide protection against Cd effects on photosynthesis, but also by increasing the iron supply, and without a decrease of leaf Cd the plants recovered from the Cd symptoms that had developed. Leaves emerging during the treatment showed more characteristic symptoms and recovery. These changes will be discussed here.
Recovery of iron content
Cd-treated poplar plants experienced Fe deficiency that affected more heavily the younger parts of the shoot (Fig. 1A). In contrast to cucumber (Cucumis sativus), where both Fe uptake and translocation were inhibited competitively by Cd (Alcántara et al., 1994; Clemens, 2006), only Fe translocation from the roots to the shoot decreased markedly in Populus alba (Fodor et al., 2005). Long-term Fe deficiency induced by Cd treatment was shown to cause stable high expression of the components of the Fe uptake system (FRO, IDS2, IRT1) in root cells in Nicotiana tabacum (Yoshihara et al., 2006; Hodoshima et al., 2007). In addition to the activated Fe acquisition, the higher amount of available Fe for uptake and translocation (also up-regulated?) enabled regenerating plants to accumulate much more Fe in the shoots compared with controls (Fig. 1B). Even the Cad/Cad-50 treatment proved to be effective in increasing the Fe content of leaves. Fe was taken up in a very short period, as it was also found in recovering Fe-deficient Beta vulgaris plants (Larbi et al., 2004). In addition, the Fe concentration applied for regeneration did not influence the control leaves structurally or functionally. Therefore, excess Fe supply seems to be a promising method to recover the Cd-induced damage in developing leaves if it is connected with the Cd-induced Fe deficiency.
Restoration of thylakoids
To evaluate the effects of different treatments on photosynthetic parameters, leaf growth also has to be taken into consideration. Inhibition of leaf area growth was an early-expressed effect of Cd, which had completely developed before the recovery treatments (Fig. 2). Therefore, leaf growth did not influence the values of the different photosynthetic parameters during the regeneration process.
The development of the photosynthetic apparatus was particularly sensitive to iron deficiency due to the inhibition of Chl synthesis (Spiller et al., 1982) causing a parallel increase in the F690/F740 ratio (Fig. 4A). Retardation of the biogenesis of components with high Chl or Fe content by Cd led to differential reduction in the amount of complexes compared with the control values (PSI > LHCII > PSII), and consequently to a decreased Chl a/b ratio (Table 3 and Fig. 3B; Sárvári et al., 1999; Sárvári, 2005), as in the case of Fe-deficient plants (Timperio et al., 2007).
Each regeneration treatment promoted the accumulation of Chl (Fig. 3A). The increase in Chl content, which indicates de novo Chl biosynthesis, began right after the elevation of the Fe : Cd ratio, in contrast to the results of Larbi et al. (2004) where significant de novo Chl synthesis was not found on the first day of regeneration of Fe-deficient plants, probably due to the more severe damage in these plants. Elevation of Chl content was faster in Cad/Ctrl-50 than in Cad/Ctrl plants. The amount of accessible Fe or the higher Fe : Cd ratio seemed to be an important determinant of regeneration, as nearly the same effects were obtained in Cad/Ctrl and Cad/Cad-50 plants. Excess Fe applied together with 10 µm Cd also promoted the accumulation of Chl. These results demonstrate that Cd-induced Fe deficiency was the cause of the stagnation of the Chl content in developing leaves, and the recovery could be speeded up by the higher amount of accessible Fe. The Chl content was not able to recover totally, as in the case of regeneration of spinach (Timperio et al., 2007) or sugar beet (Larbi et al., 2004), after Fe deficiency, which further indicates the similarities between the Cd effect and Fe deficiency.
Variations in the Chl content and organization could also be visualized by fluorescence imaging using the F690/F740 ratio (Hák et al., 1990; Buschmann, 2007). With regard to the spatial dynamics of the process, however, the F690–F740 fluorescence difference turned out to be more informative. Due to its mathematical formula, it is more sensitive for lower – and also for increasing – Chl contents (Solti et al., 2008b). Using this parameter, an expanding regeneration from the major veins to the interveinal regions was demonstrated (Fig. 5). This also underlines the effect of Fe, which gets into the mesophyll cells through the leaf veins.
Parallel to Chl synthesis, a normalization of the Chl a/b ratio and an accumulation of pigment–lipoprotein complexes took place. While Chl and CPs accumulated continuously during the regeneration period, recovery of the Chl a/b ratio showed an ‘overshoot’ (Fig. 3B) compared with control values as the reaction centres recovered first followed by the antennae, similar to what happens during the process of greening (Kuttkat et al., 1997). This ‘overshoot’ coincided with the elevation of the Fe concentration to the control level. However, LHCII did not recover fully, probably due to the direct inhibiting effect of Cd on the transcription of apoLhc (Tziveleka et al., 1999).
Recovery of photosynthetic efficiency
The reduced photosynthetic activity of Cd-stressed plants (Figs 7 and 8; Küpper et al., 2007), showing similar changes in Chl a fluorescence and thermal energy dissipation of excess light energy to those of Fe-deficient plants (Morales et al., 1998; Belkhodja et al., 1998), was improved during regeneration. The amount of supplemental Fe had a strong influence on the speed of regeneration. Acclimation to Cd stress also resulted in a kind of recovery in some parameters (e.g. ΔF/Fm′ or NPQ) but these processes proved to be much less complete compared with regeneration in the presence of elevated Fe supply (Küpper et al., 2007; Solti et al., 2008a).
The maxima of Chl a/b ratio and the inflexion points of growing ΔF/Fm′ curves, irrespective of regeneration type, occurred at the same time (Figs 3B and 7A), both being in close correlation with thylakoid reorganization. In contrast, the decreased carbon-assimilating ability of Cd-treated leaves needed 1 week to reach the level of controls (Fig. 6). This could be connected with the observed strong reduction in the amount of Rubisco protein caused by Fe-deficiency (Timperio et al., 2007) but other effects of Cd on Rubisco cannot be excluded (Siedlecka and Krupa, 1996b).
Photoprotection
The reduced photosynthetic activity of Cd-treated leaves leads to light stress, and various protective mechanisms thus come into prominence. In the first week, NPQ was lower than in controls (Fig. 7B). Therefore, other protective mechanisms must have worked in this period. During the recovery, NPQ increased, as in Fe-deficient spinach leaves (Timperio et al., 2007), and showed an ‘overshoot’, the maximum of which coincided with the normalization of the Chl a/b ratio, i.e. accumulation of antennae (Figs 3B and 7). Elevated levels of de-epoxidated xantophylls (Fig. 8), as in Fe-deficient sugar beet (Morales et al., 1998; Larbi et al., 2004; Timperio et al., 2007), and expression of lil genes (Klimmek et al., 2006; Basa et al., 2007), together with alternative electron transport routes, may play a role in the enhanced NPQ. A higher amount of de-epoxidated xanthophylls may be attributed to the diminished zeaxanthin-epoxidase activity induced by Cd (Latowski et al., 2005). The regeneration of Cd symptoms led to normalization of the amount of de-epoxidated xanthophylls.
In addition to the different quenching mechanisms, synthesis of protective compounds may also have a role in defence against Cd stress. Parallel to leaf area growth, a decrease in F440/F520 took place independently of the treatment type (Fig. 4B). The main origin of blue-green fluorescence is the accumulation of cinnamoids, such as ferulic acid, in the cell walls (Meyer et al., 2003; Pineda et al., 2008), the concentration of which might have decreased due to rapid expansion of the leaf area. After this expansion had ended, blue and green fluorescence stabilized in control plants but began to rise under Cd treatments in accordance with the increase in phenylalanine ammonia lyase activity as shown by Kováčik and Bačkor (2007) and accumulation of phenolic compounds as shown by Zagoskina et al. (2007). However, the decrease in F440/F520 compared with the controls showed that the composition of these compounds changed under Cd treatment, with a green-fluorescing compound accumulating in the vacuoles (Solti et al., unpubl. res.). In regenerating plants the reduced F440/F520 ratio was restored in parallel with the increase in NPQ, showing a change in the mechanism of photoprotection. Therefore, these green-emitting compounds may have a role in the protection of chlorotic leaves. Their identity remains to be determined.
In conclusion, supplemental Fe had a strong positive influence on photosynthesis of Cd-treated poplar plants, proving the importance of Fe deficiency in Cd effects. The higher Fe concentration in solution (higher Fe : Cd ratio) caused faster regeneration. Elevated Fe concentration in leaves with unchanged or increased Cd content restored the Chl a/b ratio and ΔF/Fm′ as short-term responses, and normal amounts of Chl and CPs accumulated together with the recovery in the F690/F740 ratio and CO2 fixation activity as longer-term responses. The recovery started from the parts adjacent to the veins and gradually extended to the interveinal parts. The reactivation of the photosynthesis made the enhanced protective mechanisms (NPQ, violaxanthin cycle, blue-green fluorescence) unnecessary, which partially or totally declined during the recovery period.
ACKNOWLEDGEMENTS
We would like to thank Zsuzsa Ostorics for excellent technical assistance. This work was supported by grants of the Hungarian National Scientific Research Foundation (OTKA T-043646 and OTKA NN-74045).
LITERATURE CITED
- Abadía A, Lemoine Y, Trémolieres A, Ambard-Bretteville F, Rémy R. Iron deficiency in pea: effects on pigment, lipid and pigment–protein complex composition of thylakoids. Plant Physiology and Biochemistry. 1989;27:659–687. [Google Scholar]
- Alcántara E, Romera FJ, Cañete M, De la Guardia MD. Effects of heavy metals on both induction and function of root Fe(III) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. Journal of Experimental Botany. 1994;45:1893–1898. [Google Scholar]
- van Assche F, Clijsters H. Effects of metals on enzyme activity in plants. Plant, Cell and Environment. 1990;13:195–206. [Google Scholar]
- Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- Barceló J, Poschenrieder C. Plant water relations as affected by heavy metal stress: a review. Journal of Plant Nutrition. 1990;13:1–37. [Google Scholar]
- Basa B, Gárdonyi M, Tamás L, Sárvári É. Cd-induced changes in the expression of chlorophyll–protein complexes in Populus glauca. Budapest Meeting Abstracts 2007, Cell Stress and Chaperones online. 2007;12 4G_03_P. [Google Scholar]
- Belkhodja R, Morales F, Quílez R, López-Millán AF, Abadía A, Abadía J. Iron deficiency causes changes in chlorophyll fluorescence due to the reduction in the dark of the photosystem II acceptor side. Photosynthesis Research. 1998;56:265–276. [Google Scholar]
- Benavides MP, Gallego SM, Tomaro ML. Cadmium toxicity in plants. Brazilian Journal of Plant Physiology. 2005;17:21–34. [Google Scholar]
- Buschmann C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynthesis Research. 2007;92:261–271. doi: 10.1007/s11120-007-9187-8. [DOI] [PubMed] [Google Scholar]
- Buschmann C, Langsdorf G, Lichtenthaler HK. Imaging of blue, green and red fluorescence emission of plants: an overview. Photosynthetica. 2000;38:483–491. [Google Scholar]
- Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Faller P, Kienzler K, Krieger-Liszkay A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of photosystem II by competitive binding to the essential Ca2+ site. Biochimica et Biophysica Acta. 2005;1706:158–164. doi: 10.1016/j.bbabio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Fodor F. Physiological responses of vascular plants to heavy metals. In: Prasad MNV, Strzalka K, editors. Physiology and biochemistry of metal toxicity and tolerance in plants. Dordrecht: Kluwer Academic Publishers; 2002. pp. 149–177. [Google Scholar]
- Fodor F, Gáspár L, Morales F, Gogorcena Y, Lucena JJ, Cseh E, et al. The effect of two different iron sources on iron and cadmium allocation in cadmium exposed poplar plants (Populus alba L.) Tree Physiology. 2005;25:1173–1180. doi: 10.1093/treephys/25.9.1173. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gilmore AM, Yamamoto HM. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiology. 1991;96:635–643. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TW, Britton G. Distribution and analysis of carotenoids. In: Goodwin TW, editor. Plant pigments. London: Academic Press; 1988. pp. 62–132. [Google Scholar]
- Hák R, Lichtenthaler HK, Rinderle U. Decrease of the chlorophyll fluorescence ratio F690/F730 during greening and development of leaves. Radiation and Environmental Biophysics. 1990;29:329–336. doi: 10.1007/BF01210413. [DOI] [PubMed] [Google Scholar]
- Hodoshima H, Enomoto Y, Shoji K, Shimada H, Goto F, Yoshihara T. Differential regulation of cadmium-inducible expression of iron-deficiency-responsive genes in tobacco and barley. Physiologia Plantarum. 2007;129:622–634. [Google Scholar]
- Horváth G, Droppa M, Oravecz Á, Raskin VI, Marder JB. Formation of the photosynthetic apparatus during greening. Planta. 1996;199:238–243. [Google Scholar]
- Klimmek F, Sjödin A, Noutsos C, Leister D, Jansson S. Abundantly and rarely expressed Lhc protein genes exhibit distinct regulation patterns in plants. Plant Physiology. 2006;140:793–804. doi: 10.1104/pp.105.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik J, Bačkor M. Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water, Air, and Soil Pollution. 2007;185:185–193. [Google Scholar]
- Krupa Z, Baszyński T. Some aspects of heavy metals toxicity towards photosynthetic apparatus – direct and indirect effects on light and dark reactions. Acta Physiologiae Plantarum. 1995;17:177–190. [Google Scholar]
- Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Śetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytologist. 2007;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- Kuttkat A, Edhofer I, Eichacker LA, Paulsen H. Light-harvesting chlorophyll a/b-binding protein stably inserts into etioplast membranes supplemented with Zn-pheophytin a/b. The Journal of Biochemistry. 1997;272:20451–20455. doi: 10.1074/jbc.272.33.20451. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel MM. A cadmium enzyme from a marine diatom. Nature. 2005;435:42. doi: 10.1038/435042a. [DOI] [PubMed] [Google Scholar]
- Láng F, Sárvári É, Szigeti Z. Apparatus and method for rapid determination of photosynthetic CO2-fixation of leaves. Biochemie und Physiologie der Pflanzen. 1985;180:333–336. [Google Scholar]
- Larbi A, Abadía A, Morales F, Abadía J. Fe resupply to Fe-deficient sugar beet plants leads to rapid changes in the violaxanthin cycle and other photosynthetic characteristics without significant de novo chlorophyll synthesis. Photosynthesis Research. 2004;79:59–69. doi: 10.1023/B:PRES.0000011919.35309.5e. [DOI] [PubMed] [Google Scholar]
- Latowski D, Kruk J, Strzałka K. Inhibition of zeaxanthin epoxidase activity by cadmium ions in higher plants. Journal of Inorganic Biochemistry. 2005;99:2081–2087. doi: 10.1016/j.jinorgbio.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Babani F. Detection of photosynthetic activity and water stress by imaging the red chlorophyll fluorescence. Plant Physiology and Biochemistry. 2000;38:889–895. [Google Scholar]
- McCarthy I, Romero-Puertas MC, Palma JM, Sandalio LM, Corpas FJ, Gómez M, et al. Cadmium induces senescence symptoms in leaf peroxisomes of pea plants. Plant, Cell and Environment. 2001;24:1065–1073. [Google Scholar]
- Maksymiec W. Signaling responses in plants to heavy metal stress. Acta Physiologiae Plantarum. 2007;29:177–187. [Google Scholar]
- Meda AR, Scheuermann EB, Prechsl UE, Erenoglu B, Schaaf G, Hayen H, et al. Iron acquisition by phytosiderophores contributes to cadmium tolerance. Plant Physiology. 2006;143:1761–1773. doi: 10.1104/pp.106.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Cartelat A, Moya I, Cerovic ZG. UV-induced blue-green and far-red fluorescence along wheat leaves: a potential signature of leaf ageing. Journal of Experimental Botany. 2003;54:757–769. doi: 10.1093/jxb/erg063. [DOI] [PubMed] [Google Scholar]
- Morales F, Abadía A, Abadía J. Photosynthesis, quenching of chlorophyll fluorescence and thermal energy dissipation in iron-deficient sugar beet leaves. Australian Journal of Plant Physiology. 1998;25:403–412. [Google Scholar]
- Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, et al. Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO Journal. 2002;21:6709–6720. doi: 10.1093/emboj/cdf666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myśliwa-Kurdziel B, Prasad MNV, Strzalka K. Heavy metal influence on the light phase of photosynthesis. In: Prasad MNV, Strzalka K, editors. Physiology and biochemistry of metal toxicity and tolerance in plants. Dordrecht: Kluwer Academic Publishers; 2002. pp. 229–255. [Google Scholar]
- Padmaja K, Prasad DDK, Prasad ARK. Inhibition of chlorophyll synthesis in Phaseolus vulgaris L. seedlings by cadmium acetate. Photosynthetica. 1990;24:399–405. [Google Scholar]
- Pagliano C, Raviolo M, Dalla Vecchia F, Gabbrielli R, Gonnelli C, Rascio N, et al. Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.) Journal of Photochemistry and Photobiology B. Biology. 2006;84:70–78. doi: 10.1016/j.jphotobiol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. The Plant Journal. 2002;32:539–548. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Pineda M, Gáspár L, Morales F, Szigeti Z, Barón M. Multicolor fluorescence imaging of leaves – a useful tool for visualizing systemic viral infections in plants. Photochemistry and Photobiology. 2008 doi: 10.1111/j.1751-1097.2008.00357.x. DOI: 10.1111/j.1751–1097.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedman PE. Determination of accurate excitation coefficient and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Prasad MNV. Cadmium toxicity and tolerance in vascular plants. Environmental and Experimental Botany. 1995;35:525–545. [Google Scholar]
- Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río MA, Sandalio LM. Cadmium-induced subcellular accumulation of O2.− and H2O2 in pea leaves. Plant, Cell and Environment. 2004;27:1122–1134. [Google Scholar]
- Sanitàdi Toppi L, Gabbrielli R. Response to cadmium in higher plants. Environmental and Experimental Botany. 1999;41:105–130. [Google Scholar]
- Sárvári É. Effects of heavy metals on chlorophyll–protein complexes in higher plants: causes and consequences. In: Pessarakli M, editor. Handbook of photosynthesis. Boca Raton, FL: CRC Press; 2005. pp. 865–888. [Google Scholar]
- Sárvári É, Nyitrai P. Separation of chlorophyll–protein complexes by Deriphat polyacrylamide gradient gel electrophoresis. Electrophoresis. 1994;15:384–394. doi: 10.1002/elps.11501501159. [DOI] [PubMed] [Google Scholar]
- Sárvári É, Fodor F, Cseh E, Varga A, Záray Gy, Zolla L. Relationship between changes in ion content of leaves and chlorophyll–protein composition in cucumber under Cd and Pb stress. Zeitschrift für Naturforschung. 1999;54c:746–753. [Google Scholar]
- Solti Á, Szegi P, Basa B, Mészáros I, Sárvári É. Alleviation of Cd induced inhibition of photosynthesis under long-term Cd treatment in poplar. Cereal Research Communications. 2008;36(Suppl.):239–242. [Google Scholar]
- Solti Á, Gáspár L, Szigeti Z, Mészáros I, Sárvári É. F690–F740 is more suitable than F690/F740 for mapping the regeneration of Cd-induced chlorosis in poplar leaves by fluorescence imaging. Acta Biologica Szegediensis. 2008;52:191–194. [Google Scholar]
- Siedlecka A, Krupa Z. Interaction between cadmium and iron: accumulation and distribution of metals and changes in growth parameters of Phaseolus vulgaris L. seedlings. Acta Societatis Botanicorum Poloniae. 1996;65:277–282. [Google Scholar]
- Siedlecka A, Krupa Z. Interaction between cadmium and iron and its effects on photosynthetic capacity of primary leaves of Phaseolus vulgaris. Plant Physiology and Biochemistry. 1996;34:833–841. [Google Scholar]
- Siedlecka A, Krupa Z. Cd/Fe interaction in higher plants – its consequences for the photosynthethic apparatus. Photosynthetica. 1999;36:321–331. [Google Scholar]
- Siedlecka A, Krupa Z, Samuelsson G, Öquist G, Gardeström P. Primary metabolism in Phaseolus vulgaris plants under Cd/Fe interaction. Plant Physiology and Biochemistry. 1997;35:951–957. [Google Scholar]
- Sigfridsson KGV, Bernát G, Mamedov F, Styring S. Molecular interference of Cd2+ with photosystem II. Biochimica et Biophysica Acta. 2004;1659:19–31. doi: 10.1016/j.bbabio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Spiller SC, Castelfranco AM, Castelfranco PA. Effects of iron and oxygen on chlorophyll biosynthesis. I. In vivo observations on iron and oxygen-deficient plants. Plant Physiology. 1982;69:107–111. doi: 10.1104/pp.69.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigeti Z. Physiological status of cultivated plants characterised by multi-wavelength fluorescence imaging. Acta Agronomica Hungarica. 2008;56 DOI: 10.1556/Agr.56.2008.2. [Google Scholar]
- Timperio AM, D'Amici GM, Barta C, Loreto F, Zolla L. Proteomics, pigment composition, and organization of thylakoid membranes in iron-deficient spinach leaves. Journal of Experimental Botany. 2007;58:3695–3710. doi: 10.1093/jxb/erm219. [DOI] [PubMed] [Google Scholar]
- Tóth VR, Mészáros I, Veres Sz, Nagy J. Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field. Journal of Plant Physiology. 2002;159:627–634. [Google Scholar]
- Tziveleka L, Kaldis A, Hegedüs A, Kissimon J, Prombona A, Horváth G, et al. The effect of Cd on chlorophyll and light-harvesting complex II biosynthesis in greening plants. Zeitschrift für Naturforschung. 1999;54c:740–745. [Google Scholar]
- Yoshihara T, Hodoshima H, Miyano Y, Shoji K, Shimada H, Goto F. Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants. Plant Cell Reports. 2006;25:365–373. doi: 10.1007/s00299-005-0092-3. [DOI] [PubMed] [Google Scholar]
- Zagoskina NV, Goncharuk EA, Alyavina AK. Effect of cadmium on the phenolic compounds formation in the callus cultures derived from various organs of the tea plant. Russian Journal of Plant Physiology. 2007;54:237–243. [Google Scholar]
- Zhang S, Scheller HV. Light-harvesting complex II binds to several small subunits of photosystem I. The Journal of Biological Chemistry. 2004;279:3180–3187. doi: 10.1074/jbc.M311640200. [DOI] [PubMed] [Google Scholar]