Abstract
Background and Aims
This study examined level of causal relationships amongst functional traits in leaves and conjoint pitcher cups of the carnivorous Nepenthes species.
Methods
Physico-chemical properties, especially lignin content, construction costs, and longevity of the assimilatory organs (leaf and pitcher) of a guild of lowland Nepenthes species inhabiting heath and/or peat swamp forests of Brunei, northern Borneo were determined.
Key Results
Longevity of these assimilatory organs was linked significantly to construction cost, lignin content and structural trait of tissue density, but these effects are non-additive. Nitrogen and phosphorus contents (indicators of Rubisco and other photosynthetic proteins), were poor predictors of organ longevity and construction cost, suggesting that a substantial allocation of biomass of the assimilatory organs in Nepenethes is to structural material optimized for prey capture, rigidity and escape from biotic and abiotic stresses rather than to light interception. Leaf payback time – a measure of net carbon revenue – was estimated to be 48–60 d. This is in line with the onset of substantial mortality by 2–3 months of tagged leaves in many of the Nepenthes species examined. However, this is a high ratio (i.e. a longer minimum payback time) compared with what is known for terrestrial, non-carnivorous plants in general (5–30 d).
Conclusions
It is concluded that the leaf trait bivariate relationships within the Nepenthes genus, as in other carnivorous species (e.g. Sarraceniaceae), is substantially different from the global relationship documented in the Global Plant Trait Network.
Key words: Botanical carnivory, carbon gain, functional traits, leaf chemistry, leaf lifespan, leaf mass per unit area, Nepenthes, pitcher, payback time
INTRODUCTION
A large body of studies exists on factors influencing lifespan of plant assimilatory organs (Kikuzawa, 1991) and the link of these factors with the physiological and growth/structural traits of the organs (Reich et al., 1997, 2003). For example, leaf longevity (LL) is related to various leaf traits including nitrogen (N) and phosphorus (P) contents, leaf mass per unit area (LMA, or its derivatives of thickness and tissue density) and maximum photosynthetic capacity (Amax). It is also entrenched in the literature that LL increases with increasing leaf toughness/sclerophylly and defence due to the presence of complex, non-palatable, structural carbohydrate compounds including tannins, lignin, wax and cuticles (Kikuzawa, 1991; Turner, 1994; Wright et al., 2004); these are products requiring high cost to acquire, and hence tissue construction cost (CC) often varies positively with LL. It is now increasingly being widely accepted that variation amongst these traits is captured by a single axis in multidimensional space (Reich et al., 1997; Westoby et al., 2002; Wright et al., 2004). What is not yet clear is how applicable these correlated traits are across and within life-form categories. For example, the carnivorous plants typically grow in high light and high moisture conditions, but are extremely constrained in terms of nutrient availability and uptake (Clarke and Leen, 2004; Ellison, 2006). Does such a constraint affect their leaf physico-chemical properties, the interrelationship of these traits, and consequently the positions of carnivorous plants on the global leaf economic spectrum axis? Studies by Knight (1992), Chapin and Pastor (1995), Ellison and Gotelli (2002), Mendez and Karlsson (2005), Wakefield et al. (2005), Ellison (2006), Osunkoya et al. (2007) and Farnsworth and Ellison (2008) suggest that the some scaling relationships among leaf traits in carnivorous species (mainly members of Sarraceniaceae, Nepenthaceae and Urticulariaceae) diverge from the worldwide leaf economics spectrum. However, in the above studies on carnivorous plants not all the links of leaf functional traits, especially those of photosynthetic physiology, CC, defense and lifespan, were examined.
In an earlier paper (Osunkoya et al., 2007), it was reported that tissue CC of members of the genus Nepenthes, whose abundance and centre of origin is in South-east Asia, varied appreciably across species, but by far the greatest difference was detected between the pitcher and the leaf. That study also detected a weak link between CC and N or P, but a strong one with potassium (K), although N serves as one of the direct input variables to the derivation of CC. It was then hypothesized that for Nepenthes species, factors other than the production of photosynthetic machinery (which calls for high N and P inputs), including accumulation of compounds to provide greater resistance against herbivore damage, tearing and bending and hence organ deformation (e.g. lignin) or to reduce water loss/increase prey-capture (e.g. wax/lipids), may better explain CC values. Indeed work by Pavlovic et al (2007) has shown a much lower photosynthetic rate in Nepenthes leaves compared with the global value – further proof that machineries of photosynthesis may not necessarily predict CC in this group of plants. Hence here (a) the lignin content and lifespan of the assimilatory organs of eight Nepenthes species of Brunei, northern Borneo, that were the focus of the investigation in Osunkoya et al. (2007), are reported, and (b) a search was conducted to find links of these two functional traits with those of earlier reported physico-chemical properties, especially with CC, nutrient composition (N and P) and mechanical/structural traits (LMA and its derivatives). In addition, because of the cup-like nature and liquid retention (reservoir) capacity of the pitcher, it was hypothesized that this organ should contain more structural materials for minimizing organ deformation/deterioration, such as higher lignin (fibre) content, than those of the conjoint flat photosynthetic lamina.
MATERIALS AND METHODS
Filed sites and study species
Data for this comparative study come from five species (Nepenthes albomarginata Lobb, N. ampullaria Jack, N. bicalcarata Hook,f, N. gracilis Korth and N. mirabilis Druce) and three distinct varieties of N. rafflesiana Jack (var. typical, var. elongata and var. gigantia); the latter, because their gross differences are regarded as ‘species’ (see Phillipps and Lamb, 1996; Clarke and Leen, 2004; Osunkoya et al., 2007). These species occur sympatrically in heath/Kerangas forests of Brunei, northern Borneo, South-east Asia. Nepenthes produces two organs of assimilation: (1) a main photosynthetic flat base (henceforth called the leaf), and (2) a modified non- or limited-photosynthetic, jug-like leaf blade at the end of the leaf tendril/petiole (henceforth called the pitcher) – a passive trap that can catch/digest prey and absorb the breakdown products. The pitcher is a modified epiascidiate leaf blade, in which the adaxial surface curls around and fuses to form the inner wall of the pitcher tube (Owen and Lennon, 1999). Fuller description, including morphology, growth habit and extent of distribution of these species can be found in Phillipps and Lamb (1996) and Clarke and Leen (2004).
Detailed description of the collection sites of the leaf and pitcher samples of these Nepenthes species, the subsequent morphometric measurements taken [i.e. organ area, dry mass and thickness for estimation of tissue mass per unit area (TMA) and density], as well as sample initial preparation for the chemical analyses are fully reported in Osunkoya et al. (2007). In summary, plant materials were collected in sunny heath and peat swamp habitats from four to six localities in Brunei, northern Borneo, in South-east Asia. The materials were washed in distilled water, dried at 40 °C and subsequently milled to fine powder. Samples for individual sites were then stored over silica gel in desiccators to maintain dryness prior to nutrient analyses.
Foliar and pitcher chemistry and longevity
The following analyses were carried out on samples collected per site, per species. Total nitrogen (N) was determined in triplicate on 1·0-g samples per site (replicate) by the micro-Kjeldahl method (Helrich, 1990). Ash content (g g−1) was determined in triplicate by incinerating 1 g samples of the dried leaves in a muffle furnace at 500 °C until a white-grey residue remained (3–4 h). Heat of combustion was measured in triplicate per site using 1 g, with a Gallenkamp bomb calorimeter (model CRA-305, UK) calibrated against benzoic acid pellets of known energy value. Ash-free heat of combustion was calculated by converting the heat of combustion on a total dry weight to the corresponding ash free weight. Leaf construction cost (mass), defined as grams of glucose (+ minerals) required to synthesize 1 g of carbon skeleton (leaf tissue) (Poorter et al., 2006), was calculated using a formula based on the growth efficiency of leaf tissue, heat of combustion, ash and nitrogen content according to Williams et al. (1987; for the formula, see Osunkoya et al., 2007).
Lignin content was assayed by the Klason procedure (Rowland and Roberts, 1994; Hatfield and Fukushima, 2005) involving 0·5 g of the dried and milled sample refluxed for 4 h with 72 % H2SO4 followed by vacuum filtration of the insoluble lignin residue in a washed pre-weighed sintered crucible. It must be noted that this procedure does not measure the exact amount of lignin in a plant tissues as the residues obtained contain other non-hydrolysable complex compounds including wax/cutins and suberin (Jin et al., 2003; Hatfield and Fukushima, 2005) However, because these products are all associated with leaf scelerotization under the physiologically stressing conditions such as in heath/Kerangas soils of low inherent fertility in which the Nepenthes species inhabit, the Klason lignin assay should give an indication of strength and stiffness (sometimes called modulus of elasticity) of the test material.
Organ longevity was determined in the field where materials were collected for leaf trait measurements. In November 2005 through to July 2006, for each species, except N. rafflesiana var. typical, organ longevity was estimated at each of four to six sites by tagging and following the fates of four young, healthy and fully developed leaves and pitchers with the lid still closed in each of five individual plants. Thus 4 leaves and 4 pitchers × 5 individual plants × (4–6 sites) = 80–120 leaves or pitchers were tagged per species. The tagged organs were observed every month for a period of 8 months (until July 2006). At each census period, the organs were recorded as alive or dead, based on colour and visual appearance. A leaf was considered dead when it had turned completely brown or had fallen off. A pitcher was considered dead when a significant proportion or the whole cup became dry, turned brown and/or had fallen off. The census data collected is an indication of the operational lifespan (in terms of photosynthesis/and or nutrient capture) as the organs were not tagged at their primordial stages. Census data collected were used to estimate the organ mortality rate (λ) and operational half lifetime (t0·5) for each species according to Lieberman (1985) and Suarez (2003). The mortality rate (λ) was estimated for each species through the non-linear adjustment of the survival probability as a function of time, λ = (loge No – loge Nf)/t, where λ is considered constant through time, and No and Nf are counts at the beginning and at the end of the census interval t. The half lifetime [t0·5 = (loge 2)/loge λ] is used as a surrogate of lifespan, and is defined as the time it would take a leaf or pitcher population to loose 50 % of its members.
Statistical analysis
The data were analysed using SPSS software (ver. 16·0). The data were confirmed for homogeneity of variances and hence there was no need for transformation prior to parametric analysis. Differences between sites (replicates), species and assimilatory organs for all measured variables were tested using multi-factorial ANOVA. A preliminary analysis indicated that within species, site differences were not significant for the majority of the traits examined. Hence data for all sites were pooled. Using species means, correlations were carried out between all measured variables. Multiple linear regressions were also carried out to explore: (a) to what extent the physical and chemical traits can predict longevity of the organs, and (b) to see if the effects of these explanatory traits are additive on longevity.
RESULTS
Figure 1 shows the patterns of organ survival with time. At 8 months, organ longevity varied appreciably across species (F6,457 = 2·05; P < 0·001) and between the two organs (F1,481 = 13·89; P < 0·001; Fig. 1). As expected, longevity was significantly lower in the pitcher cup relative to the leaf (Table 1). In most species close to 100 % of the tagged leaves and cups were still alive and functional by 2 months; thereafter they all experienced near linear loss with time (a type I survivorship pattern), with the exception of leaves of N. gracilis and N rafflesiana var. gigantia which did not start senescence until 3 and 4 months, respectively. It is noteworthy that pitchers of N. mirabilis experienced total loss by 2 months. It appeared that N. bilcalacarta pitchers lasted the longest with an estimated half lifetime of about 6 months (Fig. 1 and Table 1), while those of the remaining species were much lower ranging between 1 and 3·5 months. In leaves, half lifetime ranged from 1·5 months (N. mirabilis) to between 4–8 months (N. rafflesiana var. gigantea, N. rafflesiana var. elongata and N. albomarginata) to >10 months (N. gracilis, N. bilcalcarata and N. ampullaria).
Fig. 1.
Patterns of survival of leaves (closed symbols) and pitchers (opened symbols) of Nepenthes species over an 8-month period in heath and peat swamp forests of Brunei, northern Borneo. For each species, data have been pooled over all (four to six) sites surveyed. Note that the two varieties of N. rafflesiana (var. elongata and var. gigantia) are plotted on the same graph in (F).
Table 1.
Mean (± s.e.) estimated half lifetime and lignin content of the assimilatory organs of Nepenthes species
| Longevity, t0·5 (months) |
Lignin (%) |
|||
|---|---|---|---|---|
| Nepenthes species | Leaf | Pitcher | Leaf | Pitcher |
| N. albomarginata | 7·69 ± 1·37 | 3·46 ± 0·98 | 26·57 ± 0·53 | 23·45 ± 0·80 |
| N. ampullaria | 14·10 ± 3·90 | 2·40± 0·99 | 32·57 ± 2·09 | 24·39 ± 1·45 |
| N. bicalcarata | 9·71 ± 1·18 | 6·01 ± 1·85 | 30·09 ± 2·67 | 26·25± 0·67 |
| N. gracilis | 13·97 ± 2·34 | 2·96 ± 0·99 | 36·80± 0·55 | 32·90 ± 0·81 |
| N. mirabilis | 1·54 ± 0·43 | 0·93± 0·00 | 32·89 ± 0·57 | 31·23± 0·31 |
| N. rafflesiana var. typical | NA | NA | 31·87 ± 1·34 | 22·02 ± 0·12 |
| N. rafflesiana var. elongata | 5·59 ± 1·88 | 0·95 ± 0·31 | 31·09 ± 1·04 | 20·57 ± 0·29 |
| N. rafflesiana var. gigantia | 4·21 ± 0·78 | 1·10± 0·01 | 29·28 ± 0·39 | 17·92 ± 0·94 |
| Mean | 7·95 ± 1·25 | 2·77 ± 0·40 | 31·30 ± 0·76 | 24·81 ± 1·03 |
| F-ratio amongst species within organs | 6·58** | 2·41* | 4·42** | 31·74*** |
| F-ratio between organs | 9·78*** | 109·18*** | ||
For longevity, at the initiation of the survey, sample size (n) was ≥80 leaves or pitchers per species. For lignin content, data are based on leaves and pitchers from six sites per species with at least three readings per site.
* P < 0·05; ** P < 0·02, *** P < 0·01; NA, data not available.
In all cases lignin content was significantly higher in the leaf relative to the cup (F1,89 = 109·18; P < 0·001; Table 1), with N. gracilis exhibiting the highest value both for the leaf and the cup (36·80 % vs. 32·90 %, respectively). In leaves the lowest lignin is in N. albomarginata (26·57 %); the remaining six species had a lignin content of 30–33 % in their leaves. In the pitcher cup, the lowest lignin contents were recorded in the three varieties of N. rafflesiana (19–22 %).
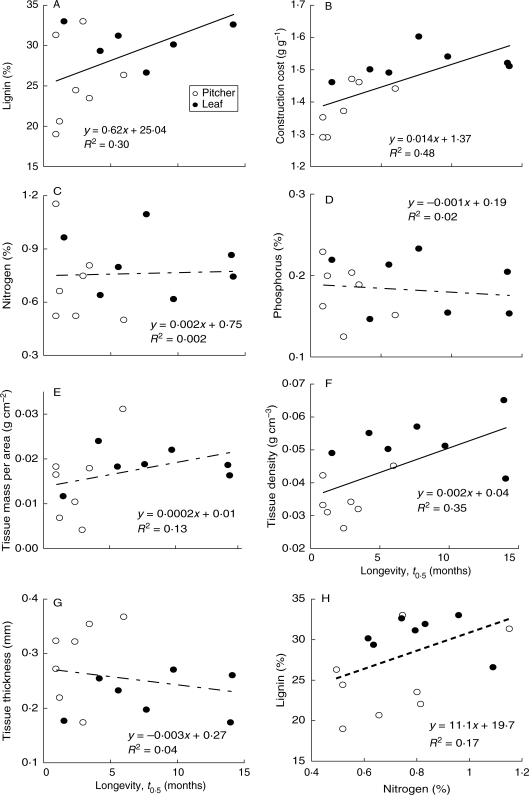
Trait differences between organs and amongst species for CC, N, P, TMA, thickness and tissue density have been fully reported elsewhere (Osunkoya et al., 2007). Cross-species relationships between organ longevity, lignin content, N, P, CC and physical traits of TMA, density and thickness are given in Table 2. As in previous studies (Osunkoya et al., 2007), a greater number of the bivariate relationships were significant (P < 0·05) when the leaf and pitcher data are used as two independent data points (nine out of 28 with additional three pair-wise comparisons being marginally significant at P < 0·10; Table 2, right upper section) than when using either the leaf (one out of 28 with an additional three significant at P < 0·10) or the pitcher (four out of 28 at P < 0·05; Table 2, left lower section). The well-known frequent exchange of biochemical materials between the leaf and its conjoint pitcher (see Schulze et al., 1999; Osunkoya et al., 2007) may have been responsible for the fewer numbers of significant links between traits at the individual organ levels. Overall, trends can be summarized as follows: across species and organs, longevity and lignin content are positively correlated (r = 0·54, n =14; P = 0·04; Fig. 2A); organ longevity also increased significantly with CC mass (r = 0·69, n = 14; P = 0·008; Fig. 2B) but not with leaf nutritional value (e.g. N and P) or with leaf economic spectrum trait of TMA (Table 2 and Fig. 2C–E). Out of the three physical/structural traits examined, variation in tissue concentration (density) was the most influential on organ longevity and chemical properties (Fig. 2E–G and Table 2; for details, see Osunkoya et al., 2007); tissue density was positively related to CC mass (r = 0·64, n = 16; P = 0·01), lignin content (r = 0·51, n = 16; P = 0·05) and organ longevity (r = 0·59, n =14; P =0·03). Interestingly, N and lignin content appeared positively related, though the trend is only marginally significant (r = 0·44, n = 16; P = 0·09; Fig. 2H). Stepwise multiple regression analyses showed that the effects of CC, lignin, N content and tissue density, though influential individually, are non-additive on organ longevity (Table 3), i.e. once any of these four traits, especially CC, is incorporated into the model, the influence of the others is dampened. Thus it appears that there is a high level of collinearity amongst these traits and consequently the inclusion of other traits in the model does not explain any additional variability in organ longevity beyond that which is explained by CC. Most of the changes in variance explained (i.e. change in R2) are <2 % and are non-significant, except N inclusion with CC where approx. 10 % change in R2 was observed, though still only marginally significant (P = 0·06) (Table 3).
Table 2.
Matrix of correlation coefficients (r) between pairs of physico-chemical properties, construction costs and longevity of the assimilatory organs of eight Nepenthes species
| Longevity (t0·5) | Lignin | Nitrogen | Phosphorus | Tissue mass per area | Tissue density | Tissue thickness | Construction cost | |
|---|---|---|---|---|---|---|---|---|
| Longevity (t0·5) | – | 0·54* | 0·04 | −0·13 | 0·35 | 0·59* | −0·20 | 0·69** |
| Lignin | 0·41, 0·21 | – | 0·44† | 0·12 | 0·05 | 0·51* | −0·42† | 0·59** |
| Nitrogen | −0·17, –0·38 | −0·09, 0·50 | – | 0·72*** | −0·16 | 0·19 | −0·30 | 0·18 |
| Phosphorus | −0·28, –0·39 | 0·04, 0·14 | 0·76*, 0·73 | – | −0·17 | 0·03 | −0·22 | −0·30 |
| Tissue mass per area | 0·15, 0·59 | −0·35, –0·16 | −0·56, –0·18 | −0·26, –0·20 | – | 0·50* | 0·48† | 0·25 |
| Tissue density | 0·09, 0·42 | 0·12, –0·11 | 0·22, –0·29 | −0·02, –0·06 | 0·14, 0·72* | – | −0·49* | 0·64** |
| Tissue thickness | 0·15, 0·43 | −0·26, –0·14 | −0·62†, 0·02 | −0·18, –0·24 | 0·62†, 0·80* | −0·68†, 0·18 | – | −0·37 |
| Construction cost | 0·38, 0·75* | −0·36, 0·59 | 0·15, –0·08 | −0·41, –0·49 | −0·03, 0·10 | 0·56, 0·02 | −0·49, 0·11 | – |
The right upper section of the matrix are the correlations across species and organs using mean values of the leaf and pitcher of each species as two independent data points for the bivariate relationships; n = 16, except for correlation with longevity where data are not available for N. rafflesiana var. typical and hence n = 14. The left lower section of the matrix are the correlations across species within the leaf and pitcher, respectively; n = 8 for each case, except for correlation with longevity where n = 7.
Significant trends are highlighted in bold: † P < 0·10; * P < 0·05; ** P < 0·02, *** P < 0·01.
Fig. 2.
Relationships between longevity as determined by half lifetime (t0·5) vs. physico-chemical properties (A–G) and between nitrogen vs. lignin content (H) in eight Nepenthes species from Brunei, Borneo. Each point is the mean values from four to six sites; leaf (closed circles) and pitcher (open circles). Continuous lines indicate significant correlation across species and assimilatory organs at P < 0·05; faint dashed lines indicate non-significant trends at P > 0·10; the bold dashed line for nitrogen–lignin relationship indicates a marginally significant (P = 0·08) trend. For significance of trends within organs, see Table 2.
Table 3.
Summary statistics of stepwise multiple regression analyses of likely factors influencing organ longevity in eight Nepenthes species in Brunei, northern Borneo
| Model fit | Intercept | Slope | Variance explained (R2) | Prob | Change in R2 | Significance of R2 change |
|---|---|---|---|---|---|---|
| Construction cost (CC) | −1·369 | 12·066 | 0·696 | 0·001 | – | – |
| TMA* | 3·384 | 1·419 (1·62, 1·82) | 0·179 | 0·132 | – | |
| Tissue density (D) | 3·316 | 2·000 | 0·332 | 0·031 | – | – |
| Lignin (L) | −4·958 | 3·496 | 0·275 | 0·05 | – | – |
| Nitrogen (N) | 0·573 | 0·051 (− 2·39, –2·14) | 0·001 | 0·961 | – | – |
| CC + D | −1·094 | 11·651, 0·15 | 0·697 | 0·001 | 0·001 | 0·849 |
| CC + L | −1·078 | 12·364, –0·21 | 0·697 | 0·001 | 0·001 | 0·886 |
| CC + N | −1·781 | 13·690, –1·126 | 0·785 | 0·001 | 0·089 | 0·056 |
| CC + D + L | −0·762 | 11·95, 0·159, –0·234 | 0·698 | 0·006 | 0·002 | 0·971 |
| CC + D + L + N | −2·878 | 12·38, 0·098, 0·899, –1·271 | 0·795 | 0·004 | 0·099 | 0·293 |
All traits were log transformed prior to regression analysis in order to compare allometry exponents with the global data set of Wright et al. (2004). In the multiple regressions, each trait was inserted in order of magnitude of its explanatory power (R2 value) when only the term is in the model. CC-mass had the highest R2 value and was inserted first, followed by the term with the second highest R2 value and so on. The 95 % confidence interval values for the global data are given in parentheses in the Slope column for organ longevity vs. nitrogen content and organ longevity vs. tissue mass per unit area.
* TMA, Tissue mass per unit area.
DISCUSSION
Concentration of lignin in green leaves has received less attention in the literature (see also Poorter et al., 1997; Diehl et al., 2008) and it appears that there are no studies reporting this trait for carnivorous plants. Overall, the average leaf lignin in the Nepenthes species studied (mean = 31·30 ± 0·76 %) though higher, is not significantly different from those detected in the leaves of non-carnivorous plants cohabiting with them (e.g. species of Alphitonia, Commersonia, Ploarium, Symplocos and Syzygium; mean lignin content: 27·32 ± 4·56 %; F1,12 = 1·31; P = 0·28, O. O. Osunkoya, unpubl. res.). However, within the time interval of the survey, Nepenthes species did experience a significantly lower leaf survival (overall mean survival: 33·04 %; 95 % confidence interval: 25·55–40·53 %) than those of non-carnivorous, small–medium-size plant species listed above (overall mean survival: 43·13 %, 95 % confidence interval: 20·50–80·25 %; Omar-Ali, 2006). Nepenthes growth habit (being a herb/small-climbing shrub) could have been a contributing factor to the observed lower survival as they are less likely to escape trampling by ground animals and other forms of biotic attack compared with their non-carnivorous counterparts.
TMA, a product of thickness and tissue density, has been identified as a crucial trait of the leaf economic spectrum (Wright et al., 2002, 2004). It was expected that a positive relationship would be detected between lignin and the structural trait of TMA (or its components) because a high lignin concentration, a major constituent of cell walls and vascular bundles, will lead to more robust, sturdy leaves (Westoby et al., 2002). Only tissue density upheld this hypothesis, suggesting that the thicker leaf in Nepenthes does not necessarily translate to a tougher organ, perhaps due, in part, to the presence of scattered palisade layers, large intercellular spaces (especially in the leaves; Muntassir, 2007; Pavlovic et al., 2007) and/or storage of non-structural carbohydrate in the protoplasm of their cells. In fact, in some studies (e.g. Wilson et al., 1999; Westoby et al., 2002), components of TMA (i.e. lamina depth and tissue density or measures closely related to them, e.g. cell volume) have been advocated as better indices of plant strategies than TMA.
The significant interspecific positive relationship between lignin and CC in the Nepenthes studied has validated that lignin – an expensive, protective compound – is a better predictor of investment cost than any of the nutritional traits (especially N and P) in this carnivorous guild (see also Osunkoya et al., 2007). Lignin also has a positive correlation with N, albeit marginally significant (Fig. 2H). This suggests that the lignin relationship with N (a nutritive trait) may be more of a co-ordination rather than of a physically enforced trade-off in contrast to what prevails in other plant species (Turner, 1994; Westoby et al., 2002). A lignin–N trade-off would have implied a causal effect in which a greater concentration of fibres, cell walls and hence lignin, etc. will leave less room for N-rich mesophyll, but this trend was never observed in the present study. Thus in Nepenthes, it is likely that the higher lignin content serves as a protective function in organs with higher N concentration so as to confer (on the organ) a prolonged lifespan (for further explanation, see below). Indeed Westoby et al. (2002), Reich et al. (2003) and Wright et al. (2005) argued that some functional ecological traits are substantially independent of each other (as seen here for N vs. lignin and N vs. CC), expressing different aspects of a plant's ecology, and may thus not have strong causal, unidirectional relationships. Rather such traits may exhibit a co-ordination (reciprocal) effect in which trait values function more successfully as a combination, rather than because one trait drives another mechanically or physiologically. It remains to be seen how consistent and widespread the lignin–N positive relationship is in other carnivorous species.
In all species, lignin content, tissue density, tissue mass per unit area, CC, and longevity were higher in the leaf relative to the pitcher (Table 1; see also Osunkoya et al., 2007). Lower longevity in the pitcher is probably related to its high physiological function, especially its frequent production of digestive enzymes via secretory glands (Schulze et al., 1999). This activity inevitably makes the pitcher more liable to a higher risk of self damage compared with the leaf. Interestingly, because of the intricate and fibrous appearance of the pitcher, it had been hypothesized (see Introduction) that the pitcher would have a much higher lignin content than the leaf, but the opposite trend was detected. This is probably due to the need for longer retention of the leaf as it contains more expensive compounds, like N and P, than the pitcher cup. The positive and significant correlation between longevity and lignin content reflects reduced palatability of the leaves and pitchers to herbivores with increasing structural materials. Indeed, it was observed that the level of herbivory on leaves of N. gracilis is minimal, in line with its relatively higher lignin content and higher longevity. In contrast, pitchers of N. mirabilis and N. rafflesiana var. typical were observed to be riddled with insect holes in view of their relatively higher N content (see table 2 of Osunkoya et al., 2007) and/or low lignin value (Table 1).
Westoby et al. (2002) and Wright et al. (2004) contend that increased leaf longevity is of paramount importance for plants in a resource-limited environment (such as the heath/Kerangas forest) as it means longer residence time of nutrients in the plant and allows payback of the initial cost of production of the organ itself. A corollary to this is that if organ longevity is to increase, the structure needs to be protected over time to minimize all forms of damage (herbivores, pathogens and physical forces), thus requiring extra structural materials (see Kikuzawa, 1991; Turner, 1994; Reich et al., 1997; Shiodera et al., 2008), and hence the reason why longevity also correlates positively with lignin and TMA (or for Nepenthes with its component of tissue density). Consequently longevity should increase with CC, as found in the Nepenthes species studied, and in other non-carnivorous species as well (e.g. Eamus and Prichard, 1998; Suarez, 2003). Others (e.g. Villar and Merino, 2001; Poorter et al., 2006) have argued that often it is not CC that varies with longevity, but the ratio of CC to carbon gain (i.e. CC: maximum photosynthetic rate, Amax) because this currency (often called payback time) measures net carbon revenue. Currently, there are no data on the photosynthetic performance of the species studied, but it is possible to draw on the limited data in the literature to evaluate this interesting hypothesis. Pavlovic et al. (2007) measured Amax of one of the species in this study, N. mirabilis, and obtained a value close to 0 and 0·0244 µmol CO2 g−1 d. wt s−1 for the pitcher and the leaf, respectively. A leaf CC value 1·46 g g−1 was obtained for the same species (Osunkoya et al., 2007). This suggests, for the leaf, a ratio of CC: Amax of 1·46/0·0244 = 60 d. Similarly, using all available Amax and CC data in the literature for Nepenthes leaf (Osunkoya et al., 2007; Pavlovic et al., 2007; A. M. Ellison and J. D. Karagatzides, Harvard Forest, Harvard University, Petersham, MA, USA, unpubl. res.), a ratio of 1·52/0·0313 = 48 d is obtained. This minimum payback time of 48–60 d is in line with the onset of substantial mortality by 2–3 months of tagged leaves of many of the Nepenthes species in this study (see Fig. 1). However, this is a high ratio (i.e. a longer minimum payback time) when compared with what is known for terrestrial non-carnivorous plants in general (5–30 d; Saeki and Nomoto, 1958; Jurik and Chabot, 1986; Williams et al., 1989; Villar and Merino, 2001; Poorter et al., 2006). The payback time is even higher for the pitcher cup (because of its low Amax and if contribution from trapping is excluded), and one can only wonder if the minimum critical point (i.e. marginal dividend) is ever attained for the pitcher before being discarded. Alternatively, it could be argued that the trapping capability of the pitcher contributes indirectly to the plant carbon and nitrogen gain potential (Schulze et al., 1997) thus helping to lower critical payback time, and may account for earlier pitcher senescence compared with the leaf as observed in this study. Nepenthes gracilis leaves experienced the highest survival, with 65 % of the leaves still present at 8 months (i.e. 240 d; Fig. 1D) implying a lifespan that is thrice beyond the mean critical payback time. It is not inconceivable that prior to this time (i.e. 240 d), the leaf net dry mass return per time per leaf area (i.e. net carbon revenue) is already optimized, but the organ is still being retained: (a) because of nutrient withdrawal to newly formed assimilatory organs, and (b) because net revenue, even though decelerating, had not deteriorated to zero (Westoby et al., 2002). This strategy of retention beyond payback time may help explain the widespread nature and high density of N gracilis compared with the other species in this study (Clarke and Leen, 2004; O. O. Osunkoya, pers. obs.). A shoot carbon budget model favoured keeping leaves this long to maximize extension growth (Ackerly, 1999).
CONCLUSIONS
The links between CC, tissue density, lignin and longevity as seen here exemplify the direct and indirect causal relationships in leaf economic spectrum traits (Wright et al., 2002, 2004; Reich et al., 2003). In Nepenthes, as in other carnivorous species (e.g. Sarraceniaceae; see Farnsworth and Ellison, 2008), causal relationships amongst leaf traits are apparent, but the scales or extent of the relationships definitely differ significantly from that described by the universal spectrum of leaf traits found in non-carnivorous plants. For example, in Nepenthes species a weak link and hence an insignificant scaling relationship exists between CC and nutrient contents, especially N (a measure of Rubisco and other photosynthetic proteins), probably due to the dual function of their assimilatory organs and a substantial allocation of their biomass to structural leaves optimized for prey capture rather than light interception. There appears to be a positive link rather than a trade-off between lignin and N which may reflect an evolutionary co-ordination and/or adaptation to protect acquired but limited nitrogenous compounds that are chronically lacking in the soils of Nepenthes habitats. Organ lifespan is definitely a function of CC and structural materials (e.g. lignin and tissue density), but the effects of theses explanatory traits are non-additive on the response variable (i.e. longevity). Minimum payback time is longer than that estimated for non-carnivorous plants, but may be in line with Nepenthes low photosynthetic rate, long leaf lifespan and consequently longer revenue amortization.
ACKNOWLEDGEMENTS
We thank the Chemistry Department, Universiti Brunei Darussalam, Brunei for providing facilities for lignin analyses.
LITERATURE CITED
- Ackerly D. Self shading, carbon gain and leaf dynamics: a test of alternative primary models. Oecologia. 1999;119:300–310. doi: 10.1007/s004420050790. [DOI] [PubMed] [Google Scholar]
- Chapin CT, Pastor J. Nutrient limitation in the northern pitcher plant. Sarracenia purpurea. Canadian Journal of Botany. 1995;73:728–734. [Google Scholar]
- Clarke C, Leen C. Pitcher plants of Sarawak. Sabah, Malaysia: Natural History Publications Borneo Sdn. Bhd; 2004. [Google Scholar]
- Diehl P, Mazzarino JM, Fontenla S. Plant limiting nutrients in Andean-Patagonianwoody species: effects of inter annual rainfall variation, soil fertility and mycorrhizal infection. Forest Ecology and Management. 2008;255:2973–2980. [Google Scholar]
- Eamus D, Prichard H. A cost-benefit analysis of leaves of four Australian savannah species. Tree Physiology. 1998;18:537–545. doi: 10.1093/treephys/18.8-9.537. [DOI] [PubMed] [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous Plants. Plant Biology. 2006;8:1–8. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proceedings of the National Academy of Sciences of the USA. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsowth EJ, Ellison AM. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous species. Journal of Ecology. 2008;96:213–221. [Google Scholar]
- Hatfield R, Fukushima R. Can lignin be accurately measured? Crop Science. 2005;45:832–839. [Google Scholar]
- Helrich K. Official methods of analysis. 15th edn. Vol. 1. Arlington, VA: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Jin Z, Akiyama T, Chung BY, Matsumoto Y, Iiyama K, Watanabe S. Changes in lignin content of leaf litter during mulching. Phytochemistry. 2003;64:1023–1031. doi: 10.1016/s0031-9422(03)00423-0. [DOI] [PubMed] [Google Scholar]
- Jurik TW, Chabot BF. Leaf dynamics and profitability in wild strawberries. Oecologia. 1986;69:296–304. doi: 10.1007/BF00377637. [DOI] [PubMed] [Google Scholar]
- Kikuzawa K. A cost-benefit analysis of leaf habitats and leaf longevity of trees and their geographical patterns. American Naturalist. 1991;138:1250–1263. [Google Scholar]
- Knight SE. Cost of carnivory in the common bladderwort. Urtcularia macrohiza. Oecologia. 1992;89:348–355. doi: 10.1007/BF00317412. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Lieberman M, Peralta R, Hartshorn GS. Mortality patterns and stand turnover rates in a wet tropical forest in Costa Rica. Journal of Ecology. 1985;73:915–924. [Google Scholar]
- Mendez M, Karlsson PS. Nutrient stoichiometry in Pinguicula vulgaris: nutrient availability, plant size, and reproductive status. Ecology. 2005;86:982–991. [Google Scholar]
- Muntassir NAA. Comparative anatomy of the assimilatory organs of Nepenthes species. Brunei: Universiti Brunei Darussalam; 2007. MSc Thesis. [Google Scholar]
- Omar-Ali K. Leaf attributes in the tropics: a comparison of habitats in Brunei. Brunei: Universiti Brunei Darussalam; 2006. BSc Thesis. [Google Scholar]
- Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM. Construction costs and physico-chemical properties of Nepenthes species in Northern Borneo. Annals of Botany. 2007;99:895–906. doi: 10.1093/aob/mcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen TPJ, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Pavlovic A, Masarovicova E, Hudak J. Carnivorous syndrome in Asia pitcher plants of the genus Nepenthes. Annals of Botany. 2007;100:527–536. doi: 10.1093/aob/mcm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipps A, Lamb A. Pitcher-plants of Borneo. Sabah, Malaysia: Natural History Publications Borneo Sdn. Bhd; 1996. [Google Scholar]
- Poorter H, Berkel YV, Den-Hertog JD, Dijkstra P, Gifford RM, Griffin KL, et al. The effects of elevated CO2 on the chemical composition, construction costs of 27 C3 species. Plant, Cell & Environment. 1997;20:472–482. [Google Scholar]
- Poorter H, Pepin S, Rijkers T, de Jong Y, Evans JR, Kőrner C. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. Journal of Experimental Botany. 2006;57:355–371. doi: 10.1093/jxb/erj002. [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. Proceedings of National Academy of Science of the USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craines JM, Oleksyn J, Westoby M, et al. The evolution of plant functional variation: traits, spectra and strategies. International Journal of Plant Science. 2003;164:S143–164. [Google Scholar]
- Rowland AP, Roberts JD. Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre method. Communications in Soil Science and Plant Analysis. 1994;25:269–277. [Google Scholar]
- Saeki T, Nomoto N. On the seasonal change of the photosynthetic activity of some deciduous and evergreen broadleaf trees. Botancal Magazine Tokyo. 1958;71:235–241. [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Schulze W, Frommer WB, Ward JM. Transporters of ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. The Plant Journal. 1999;17:637–646. doi: 10.1046/j.1365-313x.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Shiodera S, Rahajoe JS, Kohyama T. Variation in longevity of leaves of co-occurring understorey plants in a tropical montane rainforest. Journal of Tropical Ecology. 2008;24:121–133. [Google Scholar]
- Suarez N. Leaf longevity, construction, and maintenance costs of three mangrove species under field conditions. Photosynthetica. 2003;41:373–381. [Google Scholar]
- Turner IM. Sclereophylly: primarily protective? Functional Ecology. 1994;8:669–705. [Google Scholar]
- Villar R, Merino J. Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytologist. 2001;151:213–226. doi: 10.1046/j.1469-8137.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- Wakefield AE, Gotelli NJ, Wittman SE, Ellison AM. Prey addition alters nutrient stoichiometry of the carnivorous plant Sarracenia purpurea. Ecology. 2005;86:1737–1743. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Williams K, Percival F, Merino J, Mooney HA. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant, Cell & Environment. 1987;10:725–734. [Google Scholar]
- Williams K, Field CB, Mooney HA. Relationships amongst leaf construction cost, leaf longevity and light environment in rainforest plants of the genus Piper. American Naturalist. 1989;133:198–211. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson J. Specific leaf area and leaf dry matter content as alternative predictor of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
- Wright IJ, Westoby M, Reich PB. Convergence towards higher mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology. 2002;90:534–543. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackley DD, Baruch Z, Bongers F, et al. The worldwide leaf economic spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Cornelissen JHC, Falster DS, Garnier E, et al. Assessing the generality of global leaf trait relationships. New Phytologist. 2005;166:485–496. doi: 10.1111/j.1469-8137.2005.01349.x. [DOI] [PubMed] [Google Scholar]