Abstract
Background and Aims
The optimal period for seedling emergence depends on factors such as habitat preference, life cycle and geographical distribution. This research was performed to clarify the role of temperature in regulating processes leading to seedling emergence of the European continental Scilla bifolia and the Atlantic Narcissus pseudonarcissus and Hyacinthoides non-scripta.
Methods
Experiments in natural conditions were performed to examine the phenology of embryo growth, seed germination in the soil and seedling emergence. Effects of temperature conditions on embryo growth, seed germination, seedling growth and leaf formation were studied in temperature-controlled incubators.
Key Results
In nature, embryo growth of all three species was initiated from the moment the seeds were dispersed in spring and continued during summer. A sequence of high temperature followed by a lower temperature was required to complete embryo growth and initiate germination. Seeds of H. non-scripta and N. pseudonarcissus germinated in autumn once they attained the critical E:S ratio, while seeds of S. bifolia started germinating when temperatures were low in winter. Seedlings developed normally, but slowly, only when placed in low temperature conditions (5 or 10 °C), resulting in a time lag between the moment of radicle protrusion and seedling emergence in the field.
Conclusions
A continuous development of the embryo and seedlings of the three species was observed from the moment the seeds were dispersed until seedlings emerged. A sequence of high summer temperatures followed by decreasing autumn and winter temperatures was required for all developmental processes to be completed. Although a time lag occurs between radicle protrusion and seedling emergence, the term ‘epicotyl dormancy’ does not apply here, due to the absence of a period of developmental arrest. Timing of first seedling emergence differed between the three species and could be related to differences in geographical distribution.
Key words: Hyacinthoides non-scripta, Scilla bifolia, Narcissus pseudonarcissus, germination, E:S ratio, epicotyl dormancy, seedling development, monocotyledon, geophytes
INTRODUCTION
An embryo that does not entirely fill the seed is a common feature in ripe seeds of several Angiosperm taxa (Martin, 1946). A large number of these species have an underdeveloped embryo at the moment of dispersal, meaning that it has to grow within the seed before germination can occur (Grushvitzky, 1967). These seeds are termed morphologically dormant (MD) or morphophysiologically dormant (MPD), where there is an additional physiological block preventing germination (Nikolaeva, 1977). So far, eight types of MPD have been defined, based on temperature requirements for embryo growth and physiological dormancy break, and on the ability of gibberellic acid to overcome dormancy (Baskin and Baskin, 2004). According to Martin (1946), the level of embryo development in ripe monocotyledon seeds varies from underdeveloped rudimentary embryos to fully developed linear embryos. Genera that include species with underdeveloped embryos are especially common in Liliales and certain Asparagales families. An overview of dormancy types in Liliaceae s. str. shows that five out of eight types of MPD are known to occur in this group (Kondo et al., 2006). Although dormancy has been studied in other monocotyledon taxa, data on embryo growth requirements are particularly scarce.
Timing of seedling emergence is a crucial event in a plant's life cycle, affecting the plant's chances of becoming established and reaching the reproductive phase (Harper, 1977). Seedlings of most species emerge shortly after the seed has germinated in the soil. Thus, timing of seedling emergence is mainly regulated by dormancy breaking and germination requirements of the seed. In some other species, a considerable time lag exists between the moment of radicle protrusion in the field and emergence of the seedling (e.g. Baskin and Baskin, 1985). In most cases these seeds show epicotyl dormancy, meaning that a physiological block prevents further growth of the epicotyl (Barton, 1933). A time lag between germination and seedling emergence can, however, also result from a reduced seedling growth rate (Karlsson et al., 2005).
In general, dormancy is considered a mechanism to avoid periods that are favourable for germination but unfavourable for subsequent seedling establishment (Vleeshouwers et al., 1995). The most favourable period for seedling establishment can vary according to the geographical distribution and climatic conditions. Differing climatic conditions are, therefore, often reflected in dormancy breaking requirements of seeds (Skordilis and Thanos, 1995). Habitat preference and life cycle of the species are other factors determining the optimal period for seedling emergence and thus affecting seed behaviour (Nikolaeva, 1999). In the present study temperature requirements for germination and seedling emergence of Hyacinthoides non-scripta, Scilla bifolia (Hyacinthaceae) and Narcissus pseudonarcissus (Amaryllidaceae) were compared. These three species have a very similar ecology, being long-lived spring geophytes growing in temperate deciduous forests (Blackman and Rutter, 1954; Barkham, 1980; Keller, 2004). Although vegetative reproduction through formation of daughter bulbs occurs regularly, formation of seeds is also an important means of propagation (Knight, 1964; Barkham, 1992). The main difference in ecology between the studied species is found in their geographical distribution. Hyacinthoides non-scripta and N. pseudonarcissus both have a European Atlantic distribution, while S. bifolia has a European continental distribution. Hyacinthoides non-scripta grows abundantly on the British Isles and the western parts of Belgium and France, while scattered populations occur up to North-western Germany and the Alps (Thompson and Cox, 1978). Native populations of N. pseudonarcissus occur from England up to Western Germany, Switzerland and Northern Italy (Caldwell and Wallace, 1955). Scilla bifolia, on the other hand, is distributed throughout Central and Southern Europe, its range extending westwards to France and Belgium and eastwards up to the Caucasus and Turkey (Hegi, 1975).
Seed morphological traits such as embryo size and seed coat impermeability undoubtedly affect seed dormancy. Amongst related taxa these traits can be considered fairly conservative (e.g. Corner, 1976). As a consequence, when comparing seed germination syndromes of different species, the phylogenetic relatedness of these species should not be neglected (Nikolaeva, 1999). Studies based on molecular data strongly support inclusion of Hyacinthaceae and Amaryllidaceae in the Asparagales group (Chase et al., 2000; Tamura et al., 2004). Hyacinthoides and Scilla are both included in the Hyacintheae tribe and can thus be regarded as closely related genera (Pfosser and Speta, 1999).
The aim of this study was to elucidate and compare timing of embryo growth, seed germination in the soil and seedling emergence in natural conditions of the three spring geophytes H. non-scripta, S. bifolia and N. pseudonarcissus. A series of experiments in controlled conditions was performed to test how temperature regulates: (a) embryo growth; (b) germination; and (c) seedling development. Since these three species have a very similar ecology, an attempt was made to explain how biogeographical differences result in interspecific variation in timing of germination and emergence.
MATERIALS AND METHODS
Studied species
Seeds of Hyacinthoides non-scripta (L.) Chouard ex Rothm. and Narcissus pseudonarcissus L. were collected from populations growing in a deciduous forest patch near Gembloux, Belgium (50°36'N 4°44'E). Scilla bifolia L. seeds were collected from a single population growing in a forest on a steep slope in Couvin, Belgium (50°04'N 4°30'E). Experiments were performed on seeds sampled on three different occasions from 2005 to 2007 (Table 1). Ripe fruits were harvested and spread open at the laboratory, so seeds could fall out. The seeds of the studied species are rather intolerant to dry storage, therefore all experiments were started within 2 weeks after collecting the seeds. Visibly deficient and immature seeds were excluded from the experiments.
Table 1.
Sampling dates of seeds used in the experiments, and initial and critical E:S ratio: mean ± s.e.m.; n = 20
| Date of collection |
E:S ratio |
||||
|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | Initial | Critical | |
| Hyacinthoides non-scripta | 28 June | 30 June | 6 June | 0·58 ± 0·03 | 0·71 ± 0·02 |
| Narcissus pseudonarcissus | 26 May | 1 June | 15 May | 0·42 ± 0·02 | 0·79 ± 0·01 |
| Scilla bifolia | 11 May | 11 May | 4 May | 0·24 ± 0·01 | 0·87 ± 0·01 |
Phenology of embryo growth, germination and seedling emergence
Two separate phenology studies were started in 2005 and 2007, both lasting for 1 year. In 2005 the phenology of embryo growth and seedling emergence was investigated in natural conditions. To study the phenology of embryo growth, 20 nylon bags were filled with 30 seeds each and 10 g of white sand. These bags were buried at a depth of 5 cm in an experimental garden near Leuven, Belgium. Every 2 weeks a nylon bag was exhumed and the E:S ratio (embryo length/seed length) of 20 randomly selected seeds was determined. The seeds were cut in half under a dissecting microscope, and embryo length and seed length were measured using an ocular micrometer. The E:S ratio of seeds that had germinated was not determined; instead, for these seeds the critical E:S ratio was used. The critical E:S ratio is defined as the average E:S ratio of 20 randomly selected seeds with split seed coat, but no radicle protrusion.
Timing of seedling emergence was determined by sowing three replicates of 50 seeds in plastic flower pots filled with potting soil. Seeds were sown at a depth of 1 cm and pots were buried at soil level in the garden. A nearby building prevented direct sunlight from reaching these pots. These shady conditions are similar to those the seeds experience from a closed canopy in their natural habitat. The pots were covered with a net to prevent disturbance by birds, and a molluscicide was applied regularly. Emerged seedlings were counted and removed every week.
The experiment on phenology of seedling emergence was repeated using seeds collected in 2007. Additionally, in 2007 the phenology of germination in the soil was investigated. For each species, three nylon bags were filled with 100 seeds each and buried 5 cm deep in plastic flower pots filled with garden soil. These pots were buried at soil level in the garden near Leuven. Every fortnight these nylon bags were exhumed, and germinated seeds were counted. Seeds that had not germinated were reburied in the garden. Seeds were regarded as germinated when the length of the radicle exceeded 1 mm. Soil temperature at a depth of 1 cm was measured at an open place in the garden. Maximum and minimum temperature were recorded daily before 1000 h and averaged over a 1 week period.
Temperature requirement for embryo growth
The effects of four different temperature conditions on embryo growth were investigated using temperature-controlled incubators. At the start of the experiment, seeds of all three species, collected in 2005, were placed in 10 cm Petri dishes on a filter paper (MN 440) and moistened with approx. 10 mL of distilled water. During the course of the experiment, filter papers were watered regularly to prevent the seeds from drying. Two incubators were set at constant 5 and 23 °C. In these incubators, a 12 h photoperiod was established, whereby light was provided by fluorescent tubes (Philips TLD 80) with a photosynthetic photon flux density (PPFD) of 52 µmol m−2 s−1. Approximately 350 seeds of each species were placed at both 5 and 23 °C for 32 weeks. A second batch of approx. 250 seeds was incubated at 23 °C for 8 weeks and thereafter transferred to 5 °C for another 24 weeks. A third batch of approx. 200 seeds was placed first at 23 °C for 16 weeks and then moved to 5 °C for 16 weeks. In each condition, 20 seeds were selected randomly every fortnight and the E:S ratio was determined as described above. In seeds that were placed at 23 °C prior to 5 °C, the E:S ratio was determined every fortnight from the moment they were transferred to 5 °C.
Temperature requirement for germination
Three experiments were performed to determine temperature requirements for dormancy break and germination. The experiments were performed with seeds of H. non-scripta and S. bifolia collected in 2006 and with seeds of N. pseudonarcissus collected in 2007. In each condition, three replicates of 50 seeds were placed on a filter paper in a Petri dish and moistened with distilled water. Germination was investigated using temperature-controlled incubators set at constant temperatures of 5, 10, 23 and 30 °C or at daily fluctuating temperatures of 15/6, 20/10 and 30/20 °C (12 h/12 h). All seeds received light during a 12 h photoperiod. In the case of daily fluctuating temperatures, the photoperiod coincided with the 12 h high temperature part of the cycle. Filter papers were remoistened regularly, and germinated seeds were counted and removed every week.
In a prolonged germination experiment, seeds were incubated at 5, 10, 23, 15/6 and 20/10 °C for 40 weeks, without any pre-treatment. The effect of a high temperature pre-treatment on germination was tested by moist incubating seeds at 23 °C for 0, 4, 8, 12 and 16 weeks. After this high temperature pre-treatment, seeds were transferred to incubators at 5, 10, 15/6 and 20/10 °C. A third experiment was performed to determine the effect of pre-treatment at different temperatures on germination at 10 and 5 °C. Therefore, seeds were initially placed at 30, 23, 30/20, 20/10 and 15/6 °C for 16 weeks and subsequently transferred to 10 and 5 °C. Since seeds of all three species are dispersed before summer, these temperatures simulate the summer (high) followed by autumn and winter (lower) temperature sequence. Once the seeds were transferred to lower temperature conditions, the experiment was continued for another 16 weeks for seeds of H. non-scripta and N. pseudonarcissus, and for 24 weeks in the case of S. bifolia.
Temperature requirement for seedling development
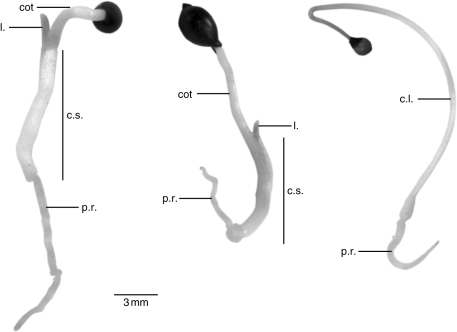
Germination of H. non-scripta and N. pseudonarcissus is hypogeal. Only one leaf, which develops in the cotyledonary sheath region, appears above ground during the first growing season (Fig. 1). In S. bifolia the cotyledon is pushed above soil level, through extension of the basal part of the cotyledon. Therefore, the first leaf is termed a seed leaf or cotyledonary leaf, and germination of S. bifolia can be regarded as epigeal.
Fig. 1.
Seedlings of Hyacinthoides non-scripta (left), Narcissus pseudonarcissus (centre) and Scilla bifolia (right). l., leaf; cot: cotyledon; c.s., cotyledonary sheath; p.r., primary root; c.l., cotyledonary leaf.
In this experiment, the effect of post-germination temperature on seedling growth was investigated, as was the effect of temperature on leaf formation. Seedling development was tested in five different temperature conditions. For each temperature condition, three replicates of 50 seeds collected in 2007 were moist incubated at 23 °C for 12 weeks. After this high temperature pre-treatment, seeds of N. pseudonarcissus and H. non-scripta were transferred to 10 °C to induce germination, while seeds of S. bifolia were transferred to 5 °C. At these lower temperature conditions, checks were made for germinated seeds every week. Seeds that had germinated were transferred to another Petri dish and moved to 5, 10, 20/10 or 23 °C. In a fifth temperature condition, germinated seeds were moved to 10 °C for 6 weeks, before being transferred to 23 °C. In every replicate Petri dish, the first ten seeds that had germinated were set aside and used for measurement of seedling length. Seedling length (from seed to root tip) of N. pseudonarcissus and H. non-scripta was measured every week for 20 weeks following germination or until a leaf was formed that had clearly separated from the central axis (Fig. 1). In the case of S. bifolia, total seedling length (from seed to root tip) was measured every week for 20 weeks, or until total length exceeded 50 mm. The arbitrary size was set as 50 mm, since seedlings with this length resembled seedlings with a curved seed-leaf at the moment of emergence in natural conditions. Hereafter, seedlings of S. bifolia that have grown to 50 mm are referred to as seedlings with leaf formation. From the moment the seeds of H. non-scripta and N. pseudonarcissus had germinated, seedlings that had formed a leaf were counted weekly for 20 weeks. Scilla bifolia seedlings were counted and discarded if total seedling length exceeded 50 mm. For S. bifolia the weekly increase in seedling length was also calculated as: (length week n + 1) – (length week n).
A move-along experiment (sensu Baskin and Baskin, 2003) was performed to check whether germination and seedling emergence under simulated laboratory conditions resembled those in the field. Three replicates of 50 seeds were moved through a temperature regime resembling the natural summer–autumn–winter–spring sequence: 16 weeks at 23 °C 8 weeks at 10 °C 16 weeks at 5 °C 8 weeks at 10 °C. During the course of the experiment, germinated seeds and seedlings with leaf/seed-leaf formation (in the same way as mentioned above) were counted every week.
Data analysis
The effect of duration/temperature of pre-treatment and the effect of germination temperature on final germination percentage was statistically analysed using a two-way analysis of variance (ANOVA). All data were arcsine transformed prior to analysis to stabilize variances.
In the experiment on seedling growth and leaf formation, the effect of temperature condition on the percentage leaf formation after 20 weeks was tested using a one-way ANOVA, followed by a Tukey multiple comparisons test. The same statistics was applied to analyse effects of post-germination temperature on the time required for leaf development and the length of H. non-scripta and N. pseudonarcissus seedlings at the moment of leaf development. In the latter, conditions with <10 germinated seeds were excluded from the analysis.
RESULTS
Phenology of embryo growth, germination and seedling emergence
A considerable difference in embryo elongation required for germination was observed between the studied species (Table 1). In seeds of H. non-scripta a 13 % increase in the E:S ratio occurred, while a 63 % increase had occurred in seeds of S. bifolia. The limited embryo growth in seeds of H. non-scripta can be attributed to both a larger initial E:S ratio and a lower critical E:S ratio. The initial E:S ratio and the increase of E:S in seeds of N. pseudonarcissus is intermediate between seeds of H. non-scripta and S. bifolia.
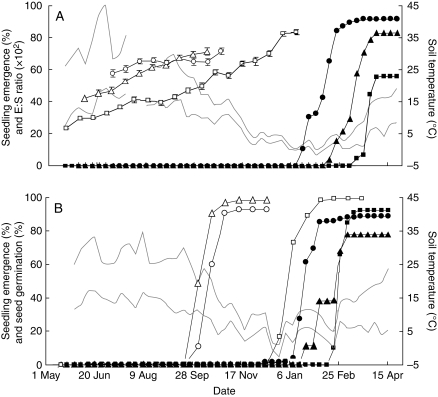
In all three species the E:S ratio increased gradually from the moment they were sown in spring or early summer 2005 until germination (Fig. 2A). In seeds of H. non-scripta, the E:S ratio mainly increased in the first 4 weeks after burial and in the last 2 weeks of observation. In the last bag exhumed, about 86 % of H. non-scripta seeds had germinated. The embryo in seeds of N. pseudonarcissus and S. bifolia grew considerably during high temperature conditions in summer. In about half of the N. pseudonarcissus seeds exhumed on 11 October 2005, embryo elongation was completed and seeds had germinated. In S. bifolia, however, embryo elongation was completed during conditions of reduced temperature in autumn and winter. At the moment the last bag with S. bifolia seeds was exhumed, on 12 January 2006, about 65 % of the seeds had germinated.
Fig. 2.
Phenology of embryo growth, seed germination and seedling emergence of Narcissus pseudonarcissus (triangles), Hyacinthoides non-scripta (circles) and Scilla bifolia (squares). (A) Average E:S ratio (open symbols; n = 20) and cumulative percentage seedling emergence (filled symbols; n = 3) of seeds sown in 2005. (B) Cumulative percentage seed germination (open symbols; n = 3) and seedling emergence of seeds sown in 2007. Grey lines indicate the mean weekly maximum and minimum soil temperature. Vertical bars denote the s.e.m. for the E:S ratio.
Although seedlings started to emerge later in 2005 as compared with 2007, the order of first seedling emergence was the same in both years. Seedlings of H. non-scripta always emerged first, followed by seedlings of N. pseudonarcissus and S. bifolia (Fig. 2A, B). Seedlings of H. non-scripta started to emerge in winter, when temperatures dropped below 10 °C. Emergence continued until the beginning of spring, resulting in a 91·3 ± 4·2 % (mean ± s.d.) and 88·7 ± 3·1 % emergence of seeds buried in 2005 and 2007, respectively. Narcissus pseudonarcissus seedlings were observed to emerge between 15 February and 5 April 2006 and between 23 January and 5 March 2008. In 2006, seedlings of N. pseudonarcissus emerged fairly continuously, resulting in a final emergence rate of 82·7 ± 0·6%. An erratic emergence of seedlings was recorded in 2008, very probably due to intermittent frost periods. All S. bifolia seedlings emerged in a 4 week time interval when temperatures started rising in spring. In 2006, only 56·0 ± 14·4 % of the seeds had emerged as seedlings in this 4 week period, contrasting with a 92·0 ± 4·0 % emergence in 2008.
Germination percentages recorded in the nylon bags buried in 2007 were always higher than the percentage of emerged seedlings (Fig. 2B). Germinated seeds of N. pseudonarcissus and H. non-scripta were recorded first in early autumn on 4 October 2007. The last germinated seeds of these species were recorded on 15 November 2007. By this time, seeds of H. non-scripta and N. pseudonarcissus had germinated to 92·9 ± 1·7 and 97·9 ± 2·7 %, respectively. Seeds of S. bifolia had germinated to 99·0 ± 1·0 % between 27 December 2007 and 21 February 2008.
Temperature requirement for embryo growth
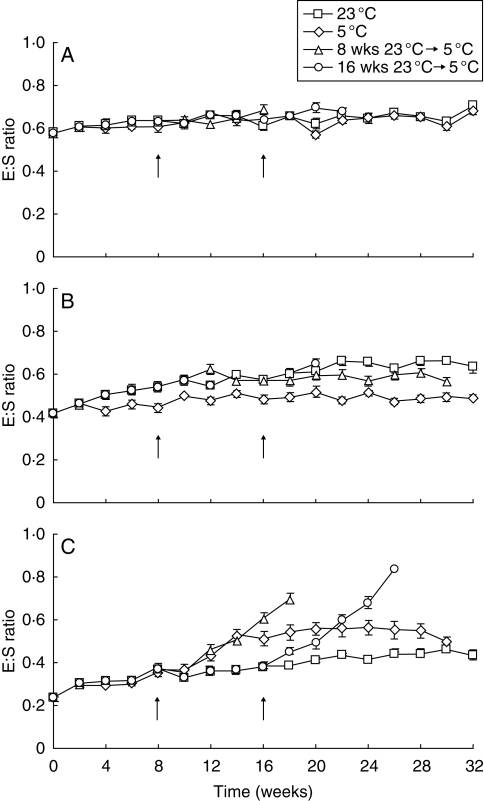
Embryo growth in seeds of H. non-scripta was low in all conditions tested (Fig. 3A). The critical E:S ratio for germination did not differ significantly from the average E:S ratio after 32 weeks of incubation at 5 or 23 °C, while none of the seeds had germinated in these conditions. In seeds of N. pseudonarcissus incubated at 5 °C for 32 weeks, only an approx. 5 % increase of the E:S ratio was observed (Fig. 3B). Embryo growth was, however, initiated in seeds incubated at 23 °C, but was not completed when they remained at this temperature condition for 32 weeks. When seeds were transferred to 5 °C after 8 weeks at 23 °C, approx. 5 % of the seeds attained the critical E:S ratio and had germinated after 22 weeks at 5 °C. The E:S ratio of most N. pseudonarcissus seeds that were moved to 5 °C after 16 weeks at 23 °C continued to increase after transfer. This resulted in about 45 % germination after 6 weeks of incubation at 5 °C.
Fig. 3.
Average E:S ratio of seeds of (A) Hyacinthoides non-scripta, (B) Narcissus pseudonarcissus and (C) Scilla bifolia during incubation at 5, 23 and 23 °C prior to transfer to 5 °C. For the latter treatment, the arrows indicate the moment of transfer to 5 °C after 8 or 16 weeks at 23 °C. Vertical bars denote the s.e.m.; n = 20.
In seeds of S. bifolia, the E:S ratio increased from 0·24 ± 0·01 (mean ± s.e.m.) to 0·44 ± 0·02 during 32 weeks at 23 °C (Fig. 3C). This was still well below the critical E:S ratio required for germination. Within 12 weeks after transfer to 5 °C, following 8 and 16 weeks at 23 °C, the E:S ratio increased to the critical E:S ratio in approx. 82 and 97 % of the seeds, respectively. At this time the experiment was terminated. The E:S ratio in seeds incubated at 5 °C for 32 weeks had increased to an average of 0·50 ± 0·02. However, in approx. 27 % of the seeds the embryo had reached the critical E:S ratio during incubation.
Temperature requirement for germination
In the prolonged germination experiment, up to 29·3 ± 1·3 % (mean ± s.e.m.) and 16·7 ± 1·8 % germination was recorded for S. bifolia seeds incubated for 40 weeks at 5 and 10 °C, respectively. Hyacinthoides non-scripta seeds incubated at 10 °C had germinated to 14·7 ± 2·9 % during 40 weeks. In all other conditions tested, germination of these two species was negligible (<6 %). Seeds of N. pseudonarcissus incubated at the same temperature condition for 40 weeks never germinated to >6 %.
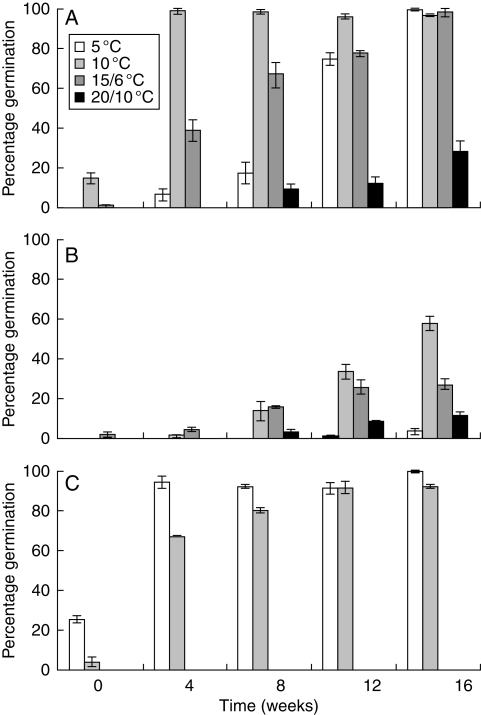
Pre-treating seeds of the studied species at 23 °C had a significant (P < 0·05) stimulating effect on subsequent germination at lower temperatures (Fig. 4). The temperature at which seeds were incubated after pre-treatment also significantly affected final germination percentages. A 4 week pre-treatment of H. non-scripta seeds at 23 °C was already sufficient to attain 98·7 ± 1·3 % germination during 16 weeks at 10 °C, which can be considered the optimal temperature for germination (Fig. 4A). Extending the pre-treatment period resulted in higher germination percentages at sub-optimal temperature conditions. A similar pattern was found for seeds of N. pseudonarcissus, although final germination percentages were considerably lower (Fig. 4B). A maximum germination percentage of 57·3 ± 3·5 % was attained for seeds incubated at 10 °C, following 16 weeks of pre-treatment at 23 °C. Scilla bifolia seeds, however, germinated best at 5 °C and to a lesser extent at 10 °C (Fig. 4C). No germination was recorded for S. bifolia seeds incubated at 15/6 and 20/10 °C following a pre-treatment at 23 °C.
Fig. 4.
Final germination percentage of (A) Hyacinthoides non-scripta, (B) Narcissus pseudonarcissus and (C) Scilla bifolia seeds incubated at 5, 10, 15/6 and 20/10 °C, following pre-treatment at 23 °C for 0, 4, 8, 12 or 16 weeks. Vertical bars denote the s.e.m.; n = 3.
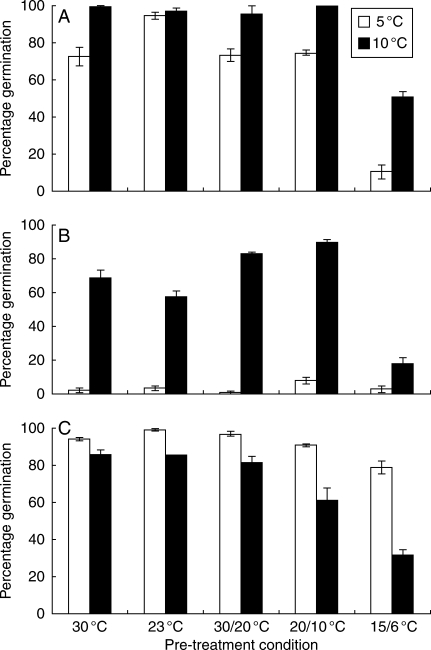
Similar to the previous experiment, the pre-treatment temperature and the germination test temperature significantly affected the final germination percentages of the three species studied (P < 0·05). In general, germination was lowest when seeds were pre-treated at 15/6 °C (Fig. 5). Final germination percentages were fairly similar for all other pre-treatment temperatures applied. Here again, seeds of H. non-scripta and N. pseudonarcissus germinated to the highest percentage when transferred to 10 °C after pre-treatment (Fig. 5A, B). Moreover, almost no seeds of N. pseudonarcissus germinated when incubated at 5 °C. The opposite was true for seeds of S. bifolia, the seeds of which germinated to a higher percentage at 5 °C (Fig. 5C).
Fig. 5.
Final percentage germination of (A) Hyacinthodes non-scripta, (B) Narcissus pseudonarcissus and (C) Scilla bifolia seeds incubated at 5 and 10 °C following pre-treatment at 23, 30, 15/6, 20/10 and 30/20 °C for 16 weeks. Vertical bars denote the s.e.m.; n = 3.
Temperature requirements for seedling development
The temperature to which seeds of H. non-scripta, N. pseudonarcissus and S. bifolia are subjected after germination significantly affects leaf formation (Table 2). In all three species a leaf is developed in >85 % of the germinated seeds, when placed at 5 and 10 °C. For seeds that remained at 5 °C, however, it took on average 2–3 weeks more before a leaf had developed, as compared with seeds that remained at 10 °C. Note also that, at the moment the leaf was discernible, seedlings of H. non-scripta and N. pseudonarcissus incubated continuously at 10 °C, after transfer from 23 °C, were significantly longer than seedlings at 5 °C. Although more than half of the H. non-scripta seedlings at 20/10 °C had formed a leaf after 20 weeks, it took considerably longer for the leaf to develop, and seedlings were significantly smaller at the moment the leaf became discernible. Leafs were not formed in seedlings of N. pseudonarcissus and H. non-scripta placed at 23 °C. After 20 weeks at 23 °C, seedlings of these species had grown to 13·1 ± 0·7 cm (mean ± s.e.m.) and 8·7 ± 0·7 cm, respectively. Seedlings of H. non-scripta transferred to 23 °C after 6 weeks at 10 °C grew longer than in any other temperature condition, but nonetheless a leaf was formed in only 62·2 % of the seedlings.
Table 2.
The average percentage of seedlings with a leaf after 20 weeks of post-germination incubation at 23 °C, 20/10 °C, 10 °C, 5 °C and 6 weeks at 10 °C followed by transfer to 23 °C. The average time (weeks), after transfer from 23 °C, required before the leaf becomes discernible and the average seedling length at the moment of leaf formation. For each species, means followed by the same letter are not significantly different at the P < 0·05 level (Tukey multiple comparisons test), n = 3
| Leafs formed (%) | Weeks to leaf development | Seedling length (mm) | |
|---|---|---|---|
| Hyacinthoides non-scripta | |||
| 23 °C | 0·0 a | – | – |
| 20/10 °C | 54·7 b | 14·3 a | 14·9 a |
| 10 °C | 99·3 c | 8·8 b | 24·8 b |
| 5 °C | 99·2 c | 11·7 b | 19·7 c |
| 6 weeks 10 °C → 23 °C | 62·2 b | 9·1 a | 28·6 b |
| Narcissus pseudonarcissus | |||
| 23 °C | 0·0 a | – | – |
| 20/10 °C | 11·6 a | – | – |
| 10 °C | 93·3 b | 11·7 a | 40·5 a |
| 5 °C | 91·4 b | 14·2 b | 26·9 b |
| 6 weeks 10 °C → 23 °C | 16·2 a | – | – |
| Scilla bifolia | |||
| 23 °C | 23·7 a | – | |
| 20/10 °C | 0·0 a | – | |
| 10 °C | 89·9 b | 6·3 a | |
| 5 °C | 86·6 b | 8·2 b | |
| 6 weeks 10 °C → 23 °C | 75·6 b | 7·9 c |
–, data too limited.
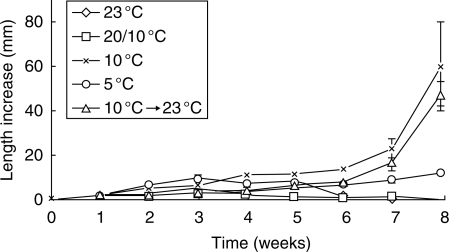
More than 75 % of S. bifolia seedlings moved to 23 °C after 6 weeks at 10 °C had grown to >50 mm and had formed a seed leaf (Table 2). The length of S. bifolia seedlings increased rapidly after transfer to 23 °C following 6 weeks at 10 °C (Fig. 6). A similar pattern was found for seedlings of S. bifolia, that were kept continuously at 10 °C. The opposite was true for seedlings stored continuously at 23 and 20/10 °C. In these seedlings, the length increase dropped to almost zero after week 6.
Fig. 6.
Average weekly length increase of seedlings of Scilla bifolia placed at different temperatures following germination at 5 °C after a 12 week pre-treatment at 23 °C. Vertical bars denote the s.e.m.; 5 < n < 30.
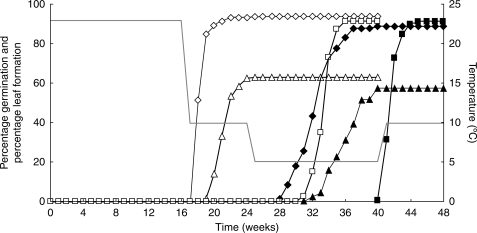
Seeds of H. non-scripta and N. pseudonarcissus that were moved through a sequence of temperatures resembling natural conditions germinated at 10 °C following 16 weeks at 23 °C (Fig. 7). When these seedlings were moved further along the temperature sequence, leaves became discernible during a 16 week period at 5 °C. Seeds of H. non-scripta germinated faster and formed leaves earlier than seeds of N. pseudonarcissus. Scilla bifolia seeds did not germinate until they were moved to 5 °C. Seed leaf formation started at the end of the 16 week period at 5 °C and continued in seedlings that were moved to 10 °C.
Fig. 7.
Cumulative average germination percentage (open symbols) and percentage of seedlings with a discernible leaf (filled symbols) of Hyacinthoides non-scripta (diamonds), Narcissus pseudonarcissus (triangles) and Scilla bifolia (squares) during a move-along experiment. The grey line indicates the incubation temperature. n = 3.
DISCUSSION
Amongst the species studied, variation existed in the degree of embryo elongation required for germination. In S. bifolia, there was a 63 % increase of the E:S ratio preceding germination. In seeds of H. non-scripta, on the other hand, the embryo grew very little (Fig. 2). Moreover, for H. non-scripta seeds, the small increase in embryo length could mainly be attributed to growth of the embryo during the final stages before radicle protrusion. The level of embryo growth required before germination in seeds of N. pseudonarcissus is somewhat intermediate in comparison with S. bifolia and H. non-scripta seeds. In terms of dormancy, this means that a gradient can be observed in the extent of morphological dormancy present in the studied species. In the case of H. non-scripta, one could hardly refer to them as having MD. Although MD has been frequently observed in monocotyledons (Kondo et al., 2006), data of Martin (1946) suggest that in numerous species of Hyacinthaceae, Amaryllidaceae and related families, the embryos are already fully developed in ripe seeds. In some species, e.g. Allium ursinum, the embryo occupies only a small amount of the total ripe seed volume, but no embryo growth appears to occur prior to germination (personal observation).
The embryo started to grow from the moment that the seeds of S. bifolia and N. pseudonarcissus were dispersed in spring, and continued during summer (Fig. 2). Embryo growth in seeds of N. pseudonarcissus and S. bifolia was completed in autumn and winter, respectively. In seeds of Anemone nemorosa, embryo growth also starts from the moment the seeds are dispersed in spring and it continues until they germinate in autumn (Ali et al., 2007). Continuous embryo growth over an extended period of time is a common feature in species occurring in the temperate woodland habitat. This can be understood by the fact that seeds have to be imbibed for embryo development to occur and that woodland soils are less susceptible to drying, especially in summer (Fenner and Thompson, 2005). The present experiments in controlled conditions showed that embryo growth was initiated during a 16 week period at 23 °C, but a transfer of the seeds to 5 °C was required to complete embryo growth. Even in seeds of H. non-scripta, the embryos of which grew very little, a sequence of high temperatures followed by low temperatures was necessary to induce germination. This sequence, to a certain extent, simulates the summer–autumn–winter temperatures in natural conditions. Similar patterns were obtained for other woodland species such as Erythronium japonicum (Kondo et al., 2002) and Fraxinus excelsior (Finch-Savage and Leubner-Metzger, 2006).
Almost all seeds of N. pseudonarcissus and H. non-scripta, sown in spring, germinated in a short time interval in the following autumn, when temperatures decreased. Caldwell and Wallace (1955) observed that up to 50 % of N. pseudonarcissus seeds sown in Devon, UK, germinated in November and December. Hyacinthoides non-scripta seeds, collected in Warwickshire, UK, apparently germinated from the beginning of October until the end of December (Knight, 1964). It seems, however, very plausible that these authors equated germination to seedling emergence, instead of radicle protrusion. In the present experiment, seeds of S. bifolia did not start to germinate until mid-winter, when the soil temperature dropped below 5 °C.
It has been suggested that seeds of H. non-scripta have a cold stratification requirement for germination (Blackman and Rutter, 1954; Slade and Causton, 1979). Seeds of H. non-scripta were also included in the large-scale screening study of germination requirements of plants in the Sheffield, UK, region (Grime et al., 1981). All H. non-scripta seeds in this study germinated during 2 months at 5 °C, following a 3 month moist incubation period at 15 °C. However, Thompson and Cox (1978), who examined germination of H. non-scripta in sequences of decreasing temperatures, found 11 °C to be the optimal temperature for germination, agreeing with the present results. Moreover, like these authors, it was observed here that extending the period of pre-treatment at higher temperatures improved subsequent germination (Fig. 4). A similar germination pattern was found for seeds of N. pseudonarcissus in the present experiments, although final germination percentages were lower than those of H. non-scripta. Seeds of S. bifolia, on the contrary, germinated best at 5 °C, indicating a lower temperature optimum for germination than H. non-scripta and N. pseudonarcissus. This discrepancy in temperature optimum reflects the period of germination in the field. While seeds of N. pseudonarcissus and H. non-scripta germinate best at 10 °C, matching autumn temperature conditions, seeds of S. bifolia germinate at lower temperature conditions during winter.
Once seeds have germinated in natural conditions, it takes a couple more weeks before seedlings actually emerge. Seedlings of S. bifolia take about 7 weeks to emerge after the onset of germination, and up to approx. 14 and 18 weeks were required for emergence of H. non-scripta and N. pseudonarcissus, respectively. The pattern whereby a considerable time lag exists between germination and seedling emergence has frequently been referred to as epicotyl dormancy (Barton, 1933; Baskin and Baskin, 1998). However, when looking at the morphology and temperature requirements for seedling development of the studied species, one can easily imagine that the term ‘epicotyl dormancy’ is not appropriate in this case. First of all, an epicotyl is not formed in seedlings of H. non-scripta, N. pseudonarcissus and S. bifolia. Moreover, it is absent in all monocotyledon species (Muller, 1978). Nevertheless, the term ‘epicotyl dormancy’ has been applied to monocotyledons as well (Barton, 1936, 1944; Kondo et al., 2002, 2004). Secondly, although low temperature conditions are required for further seedling development of the studied species, a dormant stage is never observed. Once the radicle protrudes through the seed coat, seedlings of S. bifolia grow continuously until the cotyledon emerges above soil level. Narcissus pseudonarcissus and H. non-scripta did develop normal seedlings when incubated at 5 or 10 °C, but seedling development was arrested when incubated at 20/10 and 23 °C (Table 2). The time lag between seed germination and seedling emergence of N. pseudonarcissus and H. non-scripta can be attributed to the fact that low temperature conditions (<10 °C) are required for development of normal seedlings. Similarly, a time lag between germination and emergence of Viburnum tinus has also been attributed to a retarded seedling development at low winter temperatures (Karlsson et al., 2005).
Frost damage and competition for light are two factors that can be particularly hazardous for seedling establishment of temperate deciduous woodland plants. It has been shown that emerging early in the growing season enhances plant fitness and survival rates (Jones et al., 1997; Donohue, 2002; Verdu and Traveset, 2005). When winters are mild, emerging in autumn can provide a selective advantage, resulting in plants that flower earlier and grow taller than spring germinators (Arthur et al., 1973). Severe winter conditions can, however, over-ride this advantage through increased seedling mortality. In the present study, the order of seedling emergence was the same in the outdoor and move-along experiments: seedlings of H. non-scripta emerged first, followed by N. pseudonarcissus and S. bifolia (Figs 2 and 7). Seeds buried in 2005 in general emerged earlier as compared with seeds buried in 2007. This was the case for all three species studied, although more pronounced for S. bifolia. Seedlings of H. non-scripta in Belgium started to emerge in January, but in England seedlings can be observed even as early as October and November (Knight, 1964). These differences are very probably caused by spatial and temporal climatic differences. Similarly, differences in the amount of seedlings emerged between the 2005 and 2007 batches could be attributed to climatic effects prior to or during the period of emergence. Seedlings of the 2007 N. pseudonarcissus batch, for example, were severely hampered by intermittent frost periods during emergence.
Thompson and Cox (1978) argued that the distribution range of H. non-scripta in Europe is determined by climatic factors and is restricted to places where the mean daily minimum temperature remains above freezing point. The same reasoning can be followed for N. pseudonarcissus, also showing an Atlantic distribution pattern and a germination and seedling emergence pattern very similar to that of H. non-scripta. Scilla bifolia, on the other hand, occurs in Central Europe and mountainous regions of Southern Europe. In these regions, winter conditions are harsher, due to a reduced tempering impact of the Gulf Stream and differing prevailing winds (Seager et al., 2002). The increased probability of frost damage in continental Europe might therefore explain the adaptation of S. bifolia seedlings to emerge later than both Atlantic species. Note that seeds of both H. non-scripta and S. bifolia were sampled from populations at the edge of the distribution range. A study of several populations occurring throughout the distribution range might show finer adaptations to local conditions.
Once seeds of S. bifolia, N. pseudonarcissus and H. non-scripta are dispersed, the underdeveloped embryo grows continuously until seeds germinate in autumn or winter. The seedlings in their turn grow continuously but slowly until they emerge above the soil surface. The different stages of development, from embryo growth to leaf development, occur continuously in response to different temperatures during the year after dispersal. This prolonged germination process leads to an emergence timing well suited to the climates where the species occur, without dormancy periods for the seed or seedling. Differences in timing of emergence between the three species studied are caused by a subtle difference in temperature effects on germination and seedling development. It can be concluded that although H. non-scripta is phylogeneticaly more related to S. bifolia, its germination and seedling emergence pattern resembles more that of N. pseudonarcissus which has a similar European Atlantic distribution pattern.
LITERATURE CITED
- Ali N, Probert R, Hay F, Davies H, Stuppy W. Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research. 2007;17:155–163. [Google Scholar]
- Arthur AE, Gale JS, Lawrence MJ. Variation in wild populations of Papaver dubium. VII. Germination time. Heredity. 1973;30:189–197. [Google Scholar]
- Barkham JP. Population dynamics of the wild daffodil (Narcissus pseudonarcissus). I. Clonal growth, seed reproduction, mortality and the effects of density. Journal of Ecology. 1980;68:607–633. [Google Scholar]
- Barkham JP. Population dynamics of the wild daffodil (Narcissus pseudonarcissus). IV. Clumps and gaps. Journal of Ecology. 1992;80:797–808. [Google Scholar]
- Barton LV. Seedling production of tree peony. Contributions from Boyce Thompson Institute. 1933;5:451–460. [Google Scholar]
- Barton LV. Germination and seedling production in Lilium sp. Contributions from Boyce Thompson Institute. 1936;8:297–309. [Google Scholar]
- Barton LV. Some seeds showing special dormancy. Contributions from Boyce Thompson Institute. 1944;13:259–271. [Google Scholar]
- Baskin CC, Baskin JM. Seeds. Ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin CC, Baskin JM. When breaking seed dormancy is a problem try a move-along experiment. Native Plants. 2003;4:17–21. [Google Scholar]
- Baskin JM, Baskin CC. Epicotyl dormancy in seeds of Cimicifuga racemosa and Hepatica acutiloba. Bulletin of the Torrey Botanical Club. 1985;112:253–257. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Blackman GE, Rutter AJ. Biological flora of the British Isles: Endymion non-scriptus (L.) Garcke. Journal of Ecology. 1954;42:629–638. [Google Scholar]
- Caldwell J, Wallace TJ. Biological flora of the British Isles: Narcissus pseudonarcissus L. Journal of Ecology. 1955;43:331–341. [Google Scholar]
- Chase MW, Soltis DE, Soltis PS, Rudall PJ, Fay MF, Hahn WH, et al. Higher-level systematics of the monocotyledons: an assessment of the current knowledge and a new classification. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne: CSIRO; 2000. pp. 3–16. [Google Scholar]
- Corner EJH. The seeds of dicotyledons. Cambridge: Cambridge University Press; 1976. 2 Vols. [Google Scholar]
- Donohue K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology. 2002;83:1006–1016. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Grime JP, Mason G, Curtis AV, Rodman J, Band SR, Mowforth MAG, et al. A comparative study of germination characteristics in a local flora. Journal of Ecology. 1981;69:1017–1059. [Google Scholar]
- Grushvitzky IV. After-ripening of seeds of primitive tribes of angiosperms, conditions and peculiarities. In: Borris H, editor. Physiologie, ökologie und biochemie der keimung. Greifswald, Germany: Ernst-Moritz-Arndt Universität; 1967. pp. 329–336. [Google Scholar]
- Harper JL. Population biology of plants. London, UK: Academic Press; 1977. [Google Scholar]
- Hegi G. Illustrierte flora von Mittel-Europa. Monocotyledons. München, Germany: J. F. Lehmanns; 1975. [Google Scholar]
- Jones RH, Allen BP, Sharitz RR. Why do early-emerging tree seedlings have survival advantages? A test using Acer rubrum (Aceraceae) American Journal of Botany. 1997;84:1714–1718. [PubMed] [Google Scholar]
- Karlsson LM, Hidayati SN, Walck JL, Milberg P. Complex combination of seed dormancy and seedling development determine emergence of Viburnum tinus (Caprifoliaceae) Annals of Botany. 2005;95:323–330. doi: 10.1093/aob/mci029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Scilla bifolia L. in the second forest district of the canton Aargau (Jura mountains, Switzerland) – on the sociological behaviour of a non-frequent spring geophyte. Botanica Helvetica. 2004;114:15–34. [Google Scholar]
- Knight GH. Some factors affecting the distribution of Endymion non-scriptus (L.) Garcke in Warwickshire woods. Journal of Ecology. 1964;52:405–421. [Google Scholar]
- Kondo T, Okubo N, Miura T, Honda K, Ishiwaki Y. Ecophysiology of seed germination in Erythronium japonicum (Liliaceae) with underdeveloped embryos. American Journal of Botany. 2002;89:1779–1784. doi: 10.3732/ajb.89.11.1779. [DOI] [PubMed] [Google Scholar]
- Kondo T, Miura T, Okubo N, Shimada M, Baskin CC, Baskin JM. Ecophysiology of deep simple epicotyl morphophysiological dormancy in seeds of Gagea lutea (Liliaceae) Seed Science Research. 2004;14:371–378. [Google Scholar]
- Kondo T, Sato C, Baskin JM, Baskin CC. Post-dispersal embryo development, germination phenology, and seed dormancy in Cardiocrinum cordatum var. glehnii (Liliaceae s. str.), a perennial herb of the broadleaved deciduous forest in Japan. American Journal of Botany. 2006;93:849–859. doi: 10.3732/ajb.93.6.849. [DOI] [PubMed] [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. American Midland Naturalist. 1946;36:513–660. [Google Scholar]
- Muller FM. Seedlings of the North-western European lowland. A flora of seedlings. The Hague, The Netherlands: Dr W. Junk B.V. Publishers; 1978. [Google Scholar]
- Nikolaeva MG. Factors controlling the seed dormancy pattern. In: Khan AA, editor. The physiology and biochemistry of seed dormancy and germination. Amsterdam: North-Holland; 1977. pp. 51–74. [Google Scholar]
- Nikolaeva MG. Patterns of seed dormancy and germination as related to plant phylogeny and ecological and geographical conditions of their habitats. Russian Journal of Plant Physiology. 1999;46:369–373. [Google Scholar]
- Pfosser M, Speta F. Phylogenetics of Hyacinthaceae based on plastid DNA sequences. Annals of the Missouri Botanical Garden. 1999;86:852–875. [Google Scholar]
- Seager R, Battisti DS, Yin J, Gordon N, Naik N, Clement AC, Cane MA. Is the Gulf Stream responsible for Europe's mild winters? Quarterly Journal of the Royal Meteorological Society. 2002;128:2563–2586. [Google Scholar]
- Skordilis A, Thanos CA. Seed stratification and germination strategy in the Mediterranean pines Pinus brutia and P. halepensis. Seed Science Research. 1995;5:151–160. [Google Scholar]
- Slade EA, Causton DR. Germination of some woodland herbaceous species under laboratory conditions: a multifactorial study. New Phytologist. 1979;83:549–557. [Google Scholar]
- Tamura MN, Yamashita J, Fuse S, Haraguchi M. Molecular phylogeny of monocotyledons inferred from combined analysis of plastid matK and rbcL gene sequences. Journal of Plant Research. 2004;117:109–120. doi: 10.1007/s10265-003-0133-3. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Cox SA. Germination of Bluebell (Hyacinthoides non-scripta (L.) Chouard) in relation to its distribution and habitat. Annals of Botany. 1978;42:51–62. [Google Scholar]
- Verdu M, Traveset A. Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology. 2005;86:1385–1394. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology. 1995;83:1031–1037. [Google Scholar]