Abstract
Background
Tension wood evolved in woody angiosperms to allow stems with secondary thickening to bend and thus maintain an optimal orientation. Stem bending is the result of longitudinal tensile stress that develops in tension wood tissues. In many species, a specialized secondary cell wall layer, the so-called gelatinous (G)-layer, develops, containing longitudinally orientated crystalline cellulose fibrils; these have been recently shown to generate the tensile stress by an unknown mechanism. The cellulose fibrils cannot, however, work in isolation. Both coherence between the fibrils and adherence of the G-layer to the adjacent cell wall layers are required to transfer the tensile stresses of the cellulose fibrils to the tissue. Previous work had not identified hemicelluloses within the G-layer.
Recent Progress
Sugar composition and polysaccharide linkage analyses of pure G-layers isolated by sonication have recently identified xyloglucan as the main non-cellulosic component of the G-layer. Xyloglucan has been detected by immunolabelling with the CCRC-M1 monoclonal antibody and by in-situ activity assays using XXXG–sulforhodamine substrate in the developing G-layers but not in the mature ones. However, xyloglucan endotransglucosylase/hydrolase (XTH) proteins persist in the G-layer for several years and the corresponding xyloglucan endotransglucosylase (XET) activity (EC 2·4·1·207) occurs in the adjacent layers. Correspondingly, several XTH-encoding transcripts were found to be up-regulated in developing tension wood compared with normal wood.
Scope
We propose that, during cellulose crystallization, a part of the xyloglucan is trapped inside the crystal, inducing longitudinal tensile stress within it; another part of it is accessible and present between the G-layer and the outer wall layers. XET activity that occurs persistently in the G-fibres maintains coherence between the G-layer and the adjacent secondary wall layers. It is postulated that these activities are essential for generation of tensile stress during fibre maturation in tension wood.
Key words: Tension wood, gelatinous layer, Populus tremula x tremuloides, growth stress, reaction wood, XET, aspen, cellulose microfibril, G-layer, xyloglucan, Xyloglucan endotransglucosylase
INTRODUCTION
What is tension wood?
Tension wood is a specialized tissue that evolved in angiosperms to allow tree trunks and other stems with secondary thickening to bend, so that they are able to maintain plant architecture and an appropriate orientation in the gravitational field (Jourez, 1997a, b). Tension wood is produced in the trunk when its orientation is shifted from the vertical, for example by landslides, wind-throw or snow damage. Tension wood is also formed when mechanical reinforcement is needed. For example, in branches it is permanently present in the upper side of the stem, where it counteracts the increasing branch mass and regulates branch angle. Tension wood tissue is, therefore, essential for shaping the architecture of dicotyledonous woody species and, in so doing, it optimizes leaf exposure to sunlight.
The bending of stems with secondary thickening is explained by the growth stress hypothesis. Growth stresses are mechanical stresses arising in tissues during growth and differentiation. They have been most extensively studied in growing tree trunks. Growth stress increases during wood cell maturation, leading to the generation of longitudinal tensile stress at the stem surface (Hejnowicz, 1997; Plomion et al., 2001). When tensile stresses are uneven around the stem circumference, a bending moment is created and the trunk bends. Tension wood can thus be defined as wood that develops a high tensile stress compared with normal wood or opposite wood (Almeras et al., 2005; Clair et al., 2006a).
Presence of the G-layer in tension wood fibres
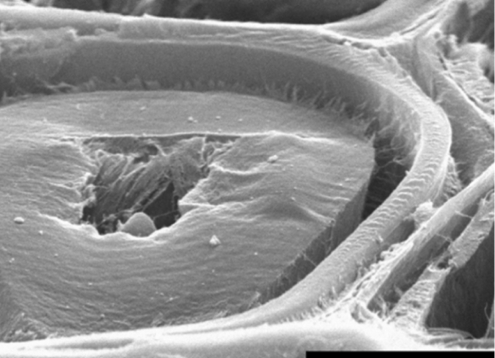
Tension wood can be detected macroscopically by an eccentric growth ring pattern: the cambial growth is increased on the tension wood side of the stem compared with the opposite side (Jourez, 1997a). At a microscopic level, tension wood is characterized by fewer vessels (and correspondingly more fibres), and by differences in the cell wall architecture of the fibres. Tension wood fibres of many species, including several poplar (Populus) species, form a thick specialized cell wall layer called the gelatinous layer (G-layer) inside the secondary cell wall layers. In poplar, the G-layer partially replaces the second layer of secondary walls (S2 layer) (reviewed by Mellerowicz et al., 2001) and is often seen in transverse sections as being detached from other cell wall layers (Fig. 1). This, however, is an artefact of sectioning and is seen only close to the section surface (Clair et al., 2005).
Fig. 1.
The G-layer in poplar tension wood is under tensile stress; this has been demonstrated by Clair and Thibault (2001), who observed its excessive shrinkage compared with other layers after transverse cutting of fibres in water (to prevent dehydration, which causes even greater G-layer shrinkage). The detachment of the G-layer from the adjacent S2 layer shows that the boundary between layers is a weak area, which does not contain many reinforcing cellulose fibrils. Therefore the connection must be maintained by compounds other than cellulose. Scale bar = 5 µm. Photograph from Clair and Thibault (2001), reprinted with permission.
Early investigations into the carbohydrate composition of the G-layer in poplar had suggested that the layer contains almost pure cellulose (Norberg and Meier, 1966). The 1,4-β-glucan chains of cellulose in the G-layer form crystals which, according to X-ray diffraction analysis, have a cross-sectional area approximately four times larger than the crystals in the adjacent S2 layer (Müller et al., 2006). This suggests that the tendency to form larger crystal aggregates of microfibrils, so called macrofibrils, is higher in the G-layer than in the S2 layer owing to the low lignin and hemicellulose content. [We use the term ‘microfibril’ for the cellulose fibril coming out of a single rosette and having a diameter of approx. 3 nm, the term ‘macrofibril’ for microfibril aggregates, which in the G-layers were reported to be approx. 6·5 nm in diameter (Müller et al., 2006), and the general term ‘fibril’ for any type of cellulose crystallite in cell wall.] A similar aggregate formation is known to occur during wood pulp preparation, when lignin and hemicelluloses are removed (Hult et al., 2001). It is still not clear, however, whether the macrofibrils observed in the G-layer are formed in situ or whether their formation is triggered by sample preparation. It is well established that the orientation of cellulose fibrils in the G-layer is almost parallel to the cell axis, whereas it is it at an angle in the adjacent secondary (S) layers (Chaffey, 2000; Müller et al., 2006). The G-layer is also known to be almost devoid of lignin, whereas the secondary cell walls in the adjacent layers have a normal or higher lignin content than the analogous layers in normal wood (Pilate et al., 2004). These differences in chemical composition and cell wall architecture between the G-layer and adjacent layers must be the reason for their differential shrinkage, which can be observed upon stress release by transverse cutting of fibres (Fig. 1).
Current ideas about the mechanism of action of the G-layer
Although there is much debate on the origin of tensile growth stress in the wood (reviewed by Pilate et al., 2004), it is clear from the G-layer behaviour (Fig. 1) that this layer can be responsible for tensile stress generation in wood. Recent data on the crystal structure of cellulose fibrils, obtained using a synchrotron X-ray beam, have revealed that the cellulose crystals have a greater lattice spacing under tensile stress in situ than observed following tensile stress release (Clair et al., 2006b). This has demonstrated, for the first time, that tensile stress develops in cellulose during maturation. The mechanism responsible for this build-up of tensile stress is not yet clear but one possibility is that it is mediated by lateral crystallization of parallel microfibrils, as indicated by measurements of crystal fibre diameter (Müller et al, 2006). Comparing the macrofibrils from a few different sources, Clair et al. (2006b) did, indeed, observe a positive correlation between lattice spacing and macrofibril diameter.
The emerging picture of G-layer architecture and the proposed mechanism for its action raises several questions. If lateral microfibril crystallization occurs, why would it lead to the development of tensile stress? How is the coherence of cellulose in the G-layer maintained? And how can the G-layer transfer its tensile stresses to the tissue when it is only loosely attached to the adjacent layers?
A NEW HYPOTHESIS OF G-LAYER FUNCTION
Carbohydrate analysis in the G-layer reveals the presence of xyloglucan and arabinogalactan–proteins
The analysis of the polysaccharide sugar composition in pure G-layers, isolated from poplar (P. tremula) tension wood by sonication, indicated that the G-layer contains less cellulose and more other carbohydrates than previously believed based on analyses by Norberg and Meier (1966). The following molar percentages of neutral sugars were found: rhamnose, 0·6 %; fucose, 1·3 %; arabinose, 0·7 %; xylose, 5·6 %; mannose, 2·1 %; galactose, 1·1 %; and glucose, 88·6 % (Nishikubo et al., 2007). Similar findings were reported by Furuya et al. (1970), who additionally observed pectin (homogalacturonan) in the G-layers of P. euroamericana. Linkage analysis (Nishikubo et al., 2007) suggests the presence of xyloglucan – a hemicellulose known to occur in primary cell walls. This polymer has a repetitive cellotetraose backbone of which three contiguous glucose residues are substituted by (1 → 6)-α-xylopyranose, which can be further decorated with galactose and fucose (Hayashi and Takeda, 1994). The presence of 4,6-linked glucose supported the demonstration of the presence of xyloglucan. Based on the neutral sugar composition and linkage analyses, it can be deduced that the xyloglucan content is probably between 10 % and 15 %, and, thus, it is the most significant non-cellulosic component of the G-layer (Nishikubo et al., 2007).
Another significant component appears to be type II arabinogalactan. This polymer forms the glycan part of arabinogalactan–proteins (AGPs) and has a backbone of (1 → 3)-linked β-galactopyranose units that are substituted at O-6 by side-chains of 1,6-β-galactopyranose units (Gaspar et al., 2001). The presence of 3,6-linked galactose in G-layers is, therefore, indicative of the presence of AGPs. The presence of AGPs in the G-layers was demonstrated by use of monoclonal antibodies (Lafarguette et al., 2004; Bowling and Vaughn, 2008). These observations are consistent with analyses of gene expression (Lafarguette et al., 2004; Andersson-Gunnerås et al., 2006), which showed a spectacular up-regulation of one class of AGPs similar to Arabidopsis FLA12 and containing the fasciclin domain in developing tension wood compared with normal or opposite wood. Neutral sugar and linkage analyses (Nishikubo et al., 2007) suggest that there is about 2 % arabinogalactan, although this estimate may be too low because AGPs are readily dissolved in water, so can be lost during G-layer isolation. Besides xyloglucan and arabinogalactan, approx. 2 % of the sugars appears to belong to glucomannan. Recently, pectin (rhamnogalacturonan I) epitopes have also been detected in the G-layers of sweetgum and hackberry by immunolocalization (Bowling and Vaughn, 2008). Pectin content might have been underestimated in previous studies owing to losses during G-layer isolation. Thus, the composition of the G-layer is quite different from that of either primary or other secondary cell wall layers.
Immunolocalization of xyloglucan with the monoclonal antibody CCRC-M1, which recognizes the fucose residue of xyloglucan (Puhlmann et al., 1994), has revealed the presence of xyloglucan in the developing G-layer and in primary wall layers. In contrast, mature G-layers appeared devoid of the label apart from a faint trace on the inner surface (Nishikubo et al., 2007; K. Baba, T. Hayashi et al., unpubl. res.). We presume that this partially reflects some masking of the CCRC-M1 epitope associated with the maturation process. Similarly, immunolocalization of AGPs by use of the JIM14 monoclonal antibody did not detect a signal in the mature part of the G-layer; only the innermost (developing) part was labelled (Lafarguette et al., 2004).
Xyloglucan endotransglucosylase (XET) activity shifts from the G-layer to outside, where it persists in the dead fibres
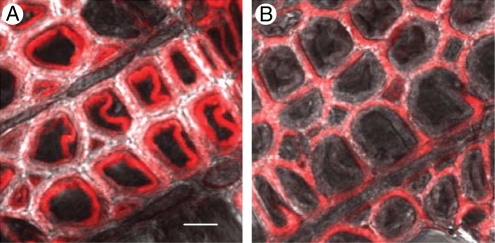
Considering the presence of xyloglucan in the G-layers, we wondered whether XET activity was also present there. By use of an antibody against aspen PttXET16A (Bourquin et al., 2002), the presence of xyloglucan endotransglucosylase/hydrolase (XTH) protein in the G-layer can be readily detected (Nishikubo et al., 2007). The protein was observed in cell walls during fibre differentiation. In the mature wood, XTH protein was present in the tension wood fibres, where it was specifically localized in mature G-layers, but was absent from secondary wall layers. Unexpectedly, the protein persisted in the G-layers of dead fibres for several years. Transglycosylation reactions do not require energy or reducing power and, theoretically, can occur outside the living cell (Fry, 2004). Is it possible that XET acts in the G-layers post mortem? XET activity can be monitored in situ by observation of the incorporation of a fluorescently labelled acceptor substrate into the cell wall (Vissenberg et al., 2000). In normal wood fibres, the activity is observed during the early stages of secondary wall synthesis, when the xyloglucan is still being deposited in the compound middle lamella via the developing secondary wall layers (Bourquin et al., 2002). This activity, however, does not continue during the later stages of secondary wall biosynthesis. In contrast, in developing tension wood fibres, XET activity is visualized by use of XXXG–sulforhodamine (Nishikubo et al., 2007) or xyloglucan–FITC (fluorescein isothiocyanate) (Takeda et al., 2002; K. Baba, T. Hayashi et al., unpubl. res.) at the inner surfaces during the early stages of secondary wall development, and it can also be seen clearly with these probes during the later stages, when the G-layer is being synthesized. Soon after the synthesis is completed, XET activity can no longer be detected in the layer by any of the probes. Instead, with XXXG–sulforhodamine, XET activity can be seen immediately outside the G-layer (Fig. 2), where it is detectable for several years after fibre formation (Nishikubo et al., 2007). Thus, during G-layer maturation, some, as yet unknown, process makes the G-layers impermeable to the incorporation of the fluorescent substrates. It is probably the same process that masks CCRC-M1 epitopes in mature G-layers.
Fig. 2.
XET activity in tension wood fibres, visualized by the incorporation of XXXG–sulforhodamine. Recently formed fibres show XET activity in the developing G-layers (A). At a later developmental stage, the activity is seen adjacent to the G-layer, where it persists for several years (B). Scale bar = 10 µm. Figure based on Nishikubo et al. (2007).
Several XTH genes are up-regulated during G-layer biosynthesis
XET activities (or XETs) are encoded by the XTH gene family (41 genes in poplar; Geisler-Lee et al., 2006). To determine which genes might be responsible for the observed XET activity in the G-fibres during the primary and secondary wall development stages, gene-specific macroarrays comprising 16 poplar XTH genes found to be expressed in mature stem tissues were used (Nishikubo et al., 2007). During primary wall development, there was less of the PttXET16-35 transcript in the tension wood than in the normal wood, while some other XTH genes were up-regulated. As a result, the overall abundance of XTH transcripts was similar to that found in normal wood. In contrast, during secondary wall development, there was a 2-fold increase in XTH transcripts in tension wood, with at least four different genes (XTH14, -21, -27 and -36) being induced. None of these up-regulated genes shares the conserved residues found in xyloglucan endohydrolases (Baumann et al., 2007), the other enzymic activity known within the XTH family. These genes are, therefore, probably responsible for the observed XET activity in tension wood fibres (Fig. 2).
New ideas about the role of XET activity in the G-layers
Based on the above findings, we postulate that xyloglucan and XET play essential roles in the mechanism of the generation of maturation stress and stem bending by tension wood. The maturation stress is proposed to develop first within the cellulose macrofibril, and then to be transmitted to the G-layer, to the G-fibre and to the tissue, as presented below and illustrated in Fig. 3.
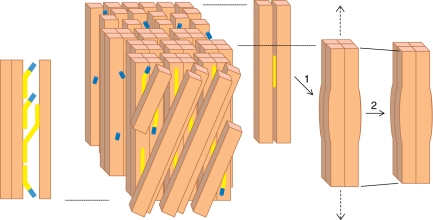
Fig. 3.
Model of the G-layer and its attachment to the S2 layer, depicting the proposed roles of xyloglucan (yellow), XTH with XET activity (blue) and cellulose fibrils (beige) in the development of tensile stress during maturation. The G-layer is shown from outside through two layers of S2 cellulose microfibrils orientated at an angle. The G-layer has several layers of cellulose microfibrils orientated axially, which aggregate during maturation to form a lattice-like structure (not pictured here) locally forming groups of four microfibrils that could trap short xyloglucan chains inside, as shown in 1. This induces the longitudinal tensile stress that leads to macrofibril shortening, as shown in 2. Xyloglucan makes cross-links between G and S2 layers as shown on the left. The XTH enzyme saturates the cut ends of xyloglucan ready to reconnect them to a suitable acceptor. Mechanical rupture of a cross-link would thus be rapidly repaired by transglucosylation. Xyloglucan-free XTH enzyme is stored in the G-layer. For more details see the text.
Development of tensile stress in cellulose macrofibrils
The microfibrils of the G-layers readily crystallize into macrofibrils because of the relatively low hemicellulose content. This process involving, for example, four adjacent microfibrils (Müller et al., 2006) would entrap xyloglucan inside the cellulose macrofibril and make it inaccessible to antibodies or XET activity. Such xyloglucan would correspond to the most tightly bound hemicellulose fraction, which is only released upon cellulose swelling (Hayashi, 1989). Crystallization around the xyloglucan would lead to the development of longitudinal tensile stress in the cellulose (Fig. 3, process 1) that upon release would shorten the macrofibril to accommodate the presence of xyloglucan in the pocket (Fig. 3, process 2), with a conformational change of the lattice as observed experimentally (Clair et al., 2006b). Other hemicelluloses and pectins that directly interact with cellulose microfibrils could play a similar role, but it appears from the chemical analysis that xyloglucan is the most important polysaccharide (Nishikubo et al., 2007). Interestingly, the presence of an intra-fibrillar substance in the G-layers was proposed long ago by Sachsse (1964) based on a honeycomb-like appearance of the cellulose network not seen in the secondary wall layers. The honeycomb structure probably corresponds to xyloglucan pockets within cellulose macrofibrils. In the S layers, the more abundant hemicelluloses would immediately interact with newly synthesized cellulose microfibrils preventing the formation of macrofibrils as reflected in cellulose fibril diameter data (Müller et al., 2006).
Development of tensile stress in the G-layer
Cellulose microfibrils in cell walls could crystallize with different partners along their length to form macrofibrils, as suggested by the atomic force microscopy images of Ding and Himmel (2006). This process would create a united cellulose network that would allow the macrofibrils to shrink uniformly as a layer, when the tensile stress is relieved, which was observed under experimental conditions (Fig. 1). Since the cellulose macrofibrils of the G-layer are axially orientated, the relative shrinkage of each macrofibril (Sm), expressed as a percentage of initial macrofibril length, would be equal to the relative shrinkage of the G-layer (Sg):
However, in the secondary wall layers, the same degree of shrinkage of cellulose macrofibrils Sm would result in:
shrinkage of the S layer, where α is the S layer microfibril angle, which is typically between 10° and 30°. Thus, the larger the angle (the more transversely orientated microfibrils) the less longitudinal wall layer shrinkage would be observed.
Development of the tensile stress in the wood
The tensile stress in the G-layers is transferred to entire G-fibres because all cell wall layers are thought to be connected to each other by hemicelluloses, as explained below, and further to the wood because all wood cells are connected within the tissue by pectins and lignin. Since cellulose fibrils are axially orientated in the G-layers, and the G-fibres are also axially orientated, the same degree of the shrinkage will be observed at the stem surface of the tension wood as in the G-fibres and in the individual macrofibrils of the G-layer, as indeed was observed by Clair et al. (2006b).
Xyloglucan is thought to ‘glue’ the tension-stressed G-layers to the adjacent outer wall layers. Xyloglucan deposition in this area is very intense during the early stage of G-layer deposition, and has been recorded in the form of high XET activity and CCRC-M1 labelling (Nishikubo et al., 2007). We hypothesize that the xyloglucan that is seen in the G-layers is mainly en route to the outer wall layers, as is observed in normal wood fibres (Bourquin et al., 2002). The xyloglucan migration may be assisted by molecular chaperones, such as AGPs (Braam, 1999), which would prevent xyloglucan interacting with already crystallized macrofibrils. A strong connection between cell wall layers is essential for efficient tensile stress transfer: any slippage would reduce the efficacy of the macrofibril pulling action. According to our model, XTH is present as a xyloglucan partner between the layers, where it probably saturates all available ends of the xyloglucan; any rupture of a xyloglucan cross-link could be rapidly repaired by transglucosylation. When xyloglucan is secreted on to the inner surface during macrofibril layering, XTH could bind the mid-chain region of the secreted xyloglucan, cleave it, and transfer this newly generated reducing end to the non-reducing end of wall-bound xyloglucan at the interface between the G- and S2 layers. Thus, the xyloglucans in the walls would form a larger network during endotransglucosylation, and XET activity would not work to loosen but to tighten the connection between the layers. We presume that the high hydrophobicity of the adjacent layer, due to lignification, excludes the enzyme from this layer, while the hydrophilic environment, produced by the abundant AGPs (Braam, 1999), attracts the enzyme to the G-layer. This way, the cutting of xyloglucan cross-links by XET activity, which would weaken the connection, is minimized.
It seems that Nature has evolved a clever mechanism not only to create these connections but also to maintain them for as long as possible. G-fibres accumulate large supplies of XTH protein in their G-layers. This reserve is enough to last for several years after cell death and provide XET activity as required, as long as there is sufficient moisture around the enzyme. It is likely that the abundant AGPs create an appropriate moisture environment in the cell wall and, by so doing, promote XET activity (Takeda and Fry, 2004).
The role of xyloglucan (and also other hemicelluloses) and XET activity (and possibly other transglycosylase activities as recently identified within the XTH family; Ait Mohand and Farkaš, 2006; Hrmová et al., 2007; Fry et al., 2008) in tensile stress development during maturation, as described above, may be more generally applicable. Such a growth stress also develops in upright trees, albeit to a lesser extent than in tension wood. In support of this, XET activity has been reported during secondary wall deposition in normal wood fibres (Bourquin et al., 2002). In normal wood fibres, the S2 layer could play a role similar to that of the G-layer in tension wood fibres, and it could drive development of tensile stress because of its cellulose microfibril orientation, which is relatively axial, and the low lignin content compared with that of the outer wall layers (reviewed by Mellerowicz and Sundberg, 2008) creating a more hydrophilic environment. However, unlike in the G-fibres, so far no transglycosylating protein has been reported in the S2 wall layers.
FUTURE PERSPECTIVES
Although the evidence presented here strongly supports the role of XET and xyloglucan in tension wood-mediated stem bending, direct experimental confirmation is still required. Xyloglucan-deficient poplars were recently obtained in the laboratory of one of us (Park et al., 2004) and these lines can be used directly to test our hypotheses. The xyloglucan-modified poplars should be tested for stem bending, as well as for maturation stress generation. Recently a number of mechanical tests were developed for biological materials; in combination with mutant analyses, these should allow us to understand the mechanical construction of the plant cell wall (Burgert, 2006).
Most hypotheses regarding the roles proposed above relating to AGPs in maturation stress generation need to be verified experimentally. In this context, the characterization of the phenotypes of AGP-modified trees is a promising route. It is also necessary to measure the AGP content, composition and interactions with other cell wall components in the secondary wall layers.
Information on the kinds of transglycosylation reactions that may occur in the secondary walls is lacking. Although enzyme-dependent incorporation of the XXXG–sulforhodamine (acceptor substrate) into different secondary cell wall layers has been observed, it is not known which donor became attached to the XXXG–sulforhodamine. It was recently demonstrated that XTH family proteins might use other donors such a mixed-linkage glucan in addition to xyloglucan (Ait Mohand and Farkaš, 2006; Hrmová et al., 2007; Fry et al., 2008) and perhaps such donors play a role in maturation stress management in secondary walls. Cell wall layers adjacent to the G-layer must have evolved together to permit transglucosylations between these layers. Analysis of the composition of these different cell wall layers would help to resolve these questions.
Although we, and others, proposed microfibril crystallization as being a driving force in the generation of maturation stress, this hypothesis needs to be verified experimentally. The lattice architecture and macrofibril ultrastructure with entrapped hemicellulose still need to be demonstrated experimentally and the crystal structure, with and without the xyloglucan pockets, determined. How the crystallization process is controlled in the cell wall is largely unknown, but recently evidence has been obtained that cellulose crystallinity in cell walls is regulated by KORRIGAN1 (a membrane-bound cellulase of family GH9 implicated in cellulose biosynthesis), possibly by splitting cellulose macrofibrils (J. Takahashi, E. J. Mellorowicz et al., unpubl. res.). In this case, it should be possible to use KORRIGAN1-modified plants to test the effects on maturation tensile stress. In summary, new tools are already available to test experimentally many of the hypotheses described above; this should help to resolve the long-standing debate on the mechanism of tension wood action.
ACKNOWLEDGEMENTS
We thank Dr Tatyana Gorshkova, Dr Ingo Burgert and two anonymous reviewers for the comments on the manuscript and discussions. Swedish Research Council, FORMAS, SSF, Wallenberg Foundation, JSPS KAKENHI (No. 19208016) and the JSPS Global COE Program (E-04): In Search of Sustainable Humanosphere in Asia and Africa are thanked for financial support.
LITERATURE CITED
- Ait Mohand F, Farkaš V. Screening for hetero-transglycosylating activities in extracts from nasturtium (Tropaeolum majus) Carbohydrate Research. 2006;341:577–581. doi: 10.1016/j.carres.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Almeras T, Thibault A, Gril J. Effect of circumferential heterogeneity of wood maturation strain, modulus of elasticity and radial growth on the regulation of stem orientation in trees. Trees – Structure and Function. 2005;19:457–467. [Google Scholar]
- Andersson-Gunnerås S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, et al. Making cellulose enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. The Plant Journal. 2006;45:144–165. doi: 10.1111/j.1365-313X.2005.02584.x. [DOI] [PubMed] [Google Scholar]
- Baumann MJ, Eklof JM, Michel G, Kallas AM, Teeri TT, Czjzek M, et al. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. The Plant Cell. 2007;19:1947–1963. doi: 10.1105/tpc.107.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin V, Nishikubo N, Abe H, Brumer H, Denman S, Eklund M, et al. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. The Plant Cell. 2002;14:3073–3088. doi: 10.1105/tpc.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling AJ, Vaughn KC. Immunocytochemical characterization of tension wood: gelatinous fibers contain more than just cellulose. American Journal of Botany. 2008;95:655–663. doi: 10.3732/ajb.2007368. [DOI] [PubMed] [Google Scholar]
- Braam J. If walls could talk. Current Opinion in Plant Biology. 1999;2:521–524. doi: 10.1016/s1369-5266(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Burgert I. Exploring the micromechanical design of plant cell walls. American Journal of Botany. 2006;93:1391–1401. doi: 10.3732/ajb.93.10.1391. [DOI] [PubMed] [Google Scholar]
- Chaffey I. Microfibril orientation in wood cells: new angles on an old topic. Trends in Plant Science. 2000;5:360–362. doi: 10.1016/s1360-1385(00)01695-2. [DOI] [PubMed] [Google Scholar]
- Clair B, Thibault B. Shrinkage of the gelatinous layer of poplar and beech tension wood. International Association of Wood Anatomists Journal. 2001;22:121–131. [Google Scholar]
- Clair B, Thibault B, Sugiyama J. On the detachment of the gelatinous layer in tension wood fiber. Journal of Wood Science. 2005;51:218–221. [Google Scholar]
- Clair B, Almeras T, Sugiyama J. Compression stress in opposite wood of angiosperms: observations in chestnut, mani and poplar. Annals of Forest Science. 2006;a 63:507–510. [Google Scholar]
- Clair B, Almeras T, Yamamoto H, Okuyama T, Sugiyama J. Mechanical behavior of cellulose microfibrils in tension wood, in relation with maturation stress generation. Biophysical Journal. 2006;b 91:1128–1135. doi: 10.1529/biophysj.105.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SY, Himmel ME. The maize primary cell wall microfibril: a new model derived from direct visualization. Journal of Agriculture and Food Chemistry. 2006;54:597–606. doi: 10.1021/jf051851z. [DOI] [PubMed] [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BHWA, Franková L. Mixed-linkage β-glucan^:^xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. The Plant Journal. 2008;55:240–252. doi: 10.1111/j.1365-313X.2008.03504.x. [DOI] [PubMed] [Google Scholar]
- Furuya N, Takahashi S, Miyazaki M. The chemical composition of the gelatinous layer from the tension wood of Populus euroamericana. Mokuzai Gakkaishi. 1970;16:26–30. [Google Scholar]
- Gaspar YM, Johnson KL, McKenna JA, Bacic A, Schultz CJ. The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Molecular Biology. 2001;47:161–176. [PubMed] [Google Scholar]
- Geisler-Lee J, Geisler M, Coutinho PM, Segerman B, Nishikubo N. Poplar carbohydrate-active enzymes (CAZymes): gene identification and expression analyses. Plant Physiology. 2006;140:1–17. doi: 10.1104/pp.105.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T. Xyloglucans in the primary cell wall. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:139–168. [Google Scholar]
- Hayashi T, Takeda T. Compositional analysis of the oligosaccharide units of xyloglucan from suspension-cultured poplar cells. Japan Society for Bioscience, Biotechnology and Agrochemistry. 1994;58:1707–1708. doi: 10.1271/bbb.58.1707. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z. Graviresponses in herbs and trees: a major role for the redistribution of tissue and growth stresses. Planta. 1997;203:S136–S146. doi: 10.1007/pl00008102. [DOI] [PubMed] [Google Scholar]
- Hrmová M, Farkaš V, Lahnstein J, Fincher GB. A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-β-d-glucans. Journal of Biological Chemistry. 2007;282:12951–12962. doi: 10.1074/jbc.M611487200. [DOI] [PubMed] [Google Scholar]
- Hult EL, Larsson PT, Iversen T. Cellulose fibril aggregation – an inherent property of kraft pulps. Polymer. 2001;42:3309–3314. [Google Scholar]
- Jourez B. Tension wood. 1. Definition and distribution in the tree. BASE: Biotechnologie, Agronomie, Société et Environnement. 1997;a 1:100–112. [Google Scholar]
- Jourez B. Tension wood. 2. Quantitative evaluation, formation and role in the tree. BASE: Biotechnologie, Agronomie, Société et Environnement. 1997;b 1:167–177. [Google Scholar]
- Lafarguette F, Leple JC, Dejardin A, Laurans F, Costa G, Lesage-Descauses MC, et al. Poplar genes encoding fasciclin-like arabinogalactan proteins are highly expressed in tension wood. New Phytologist. 2004;164:107–121. doi: 10.1111/j.1469-8137.2004.01175.x. [DOI] [PubMed] [Google Scholar]
- Mellerowicz EJ, Sundberg B. Wood cell walls: biosynthesis, developmental dynamics and their implications for wood properties. Current Opinion in Plant Biology. 2008;11:293–300. doi: 10.1016/j.pbi.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W. Unraveling cell wall formation in the woody dicot stem. Plant Molecular Biology. 2001;47:239–274. [PubMed] [Google Scholar]
- Müller M, Burghammer M, Sugiyama J. Direct investigation of the structural properties of tension wood cellulose microfibrils using microbeam X-ray fibre diffraction. Holzforschung. 2006;60:474–479. [Google Scholar]
- Nishikubo N, Awano T, Banasiak A, Bourquin V, Ibatullin F, Funada R, et al. Xyloglucan endo-transglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar—a glimpse into the mechanism of the balancing act of trees. Plant Cell Physiology. 2007;48:843–855. doi: 10.1093/pcp/pcm055. [DOI] [PubMed] [Google Scholar]
- Norberg PH, Meier H. Physical and chemical properties of the gelatinous layer in tension wood fiber of aspen (Populus tremula L.) Holzforschung. 1966;20:174–178. [Google Scholar]
- Park YW, Baba K, Furuta Y, Iida I, Sameshima K, Arai M, et al. Enhancement of growth and cellulose accumulation by overexpression of xyloglucanase in poplar. FEBS Letters. 2004;564:183–187. doi: 10.1016/S0014-5793(04)00346-1. [DOI] [PubMed] [Google Scholar]
- Pilate G, Chabbert B, Cathala B, Yoshinaga A, Leplé J-C, Laurans F, et al. Lignification and tension wood. Comptes Rendus Biologies. 2004;327:889–901. doi: 10.1016/j.crvi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Physiology. 2001;127:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain M J, Dunning N, Albersheim P, Darvill AG, et al. Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. characterization of a monoclonal antibody to a terminal α-(1 → 2)-linked fucosyl-containing epitope. Plant Physiology. 1994;104:699–710. doi: 10.1104/pp.104.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsse H. The submicroscopic structure of the fibre cell wall in poplar tensionwood. Holz Roh- und Werkstoff. 1964;22:169–174. [Google Scholar]
- Takeda T, Fry SC. Control of xyloglucan endotransglucosylase activity by salts and anionic polymers. Planta. 2004;219:722–732. doi: 10.1007/s00425-004-1267-9. [DOI] [PubMed] [Google Scholar]
- Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T. Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proceedings of the National Academy of Sciences of the USA. 2002;99:9055–9060. doi: 10.1073/pnas.132080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Martinez-Vilchez IM, Verbelen JP, Miller JG, Fry SC. In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of arabidopsis roots. The Plant Cell. 2000;12:1229–1237. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]