Abstract
Background and Aims
Most neotropical Melastomataceae have bee-pollinated flowers with poricidal anthers. However, nectar rewards are known to be produced in about 80 species in eight genera from four different tribes. These nectar-producing species are pollinated by both vertebrates and invertebrates.
Methods
The floral morphology and anatomy of 14 species was studied in six genera of nectar-producing Melastomataceae (Blakea, Brachyotum, Charianthus, Huilaea, Meriania and Miconia). Anatomical methods included scanning electron microscopy, and serial sections of paraffin-embedded flowers.
Key Results
All vertebrate-pollinated melastome flowers have petals that do not open completely at anthesis, thus forming a pseudo-tubular corolla, while closely related species that are bee pollinated have rotate or reflexed corollas. In most species, nectar secretion is related to stomatal or epidermal nectaries and not filament slits as previously reported. Moreover, the nectar is probably supplied by large vascular bundles near the release area. Blakea and Huilaea have nectary stomata located upon the dorsal anther connective appendages. Brachyotum also has nectary stomata on the anther connectives, but these are distributed lengthwise along most of the connective. Meriania may release nectar through the anther connective, but has additional nectary stomata on the inner walls of the hypanthium. Miconia has nectary stomata on the ovary apex. Charianthus nectaries were not found, but there is circumstantial evidence that nectar release occurs through the epidermis at the apex of the ovary and the lower portions of the inner wall of the hypanthium.
Conclusions
Nectar release in Melastomataceae is apparently related to nectary stomata and not filament slits. The presence of nectary stomata on stamens and on ovary apices in different lineages suggests that the acquisition of nectaries is a derived condition. Nectary location also supports a derived condition, because location is strongly consistent within each genus, but differs between genera.
Key words: Blakea, Brachyotum, Charianthus, Huilaea, Meriania, Melastomataceae, Miconia, nectaries, nectary stomata, pollination
INTRODUCTION
Melastomataceae flowers are known for usually providing pollen rewards to their pollinators. Pollen of Melastomataceae can be of great ecological importance in the neotropics, where melastome flowers have been shown to be visited by up to 40 % of bee species in a given locality (Renner, 1989; Harter et al., 2002). Their poricidal anthers have been seen as a specialization to protect the pollen, ensuring that it is only collected by bees. However, nectariferous Melastomataceae, which mostly retain poricidal anthers, have evolved other strategies for attracting pollinators.
The first report on nectar release in the Melastomataceae was made by Ule (1896) on Tibouchina, a large genus with over 350 species, most of which are bee-pollinated (Renner, 1989). Since then, nectar production has been reported in a handful of other species of Tibouchina (Vogel, 1957; Renner, 1989; Stein and Tobe, 1989), and in this genus it has been linked to pollination by hummingbirds, bats and bees (Vogel, 1957; Renner, 1989). Nectar production associated with pollination by hummingbirds has also been reported for several species of Brachyotum (Lagerheim, 1899; Stiles et al., 1992), and it is believed that all 50 species of this genus are nectariferous (Wurdack, 1953). Likewise, all species of the small genera, Charianthus and Huilaea (six and eight species, respectively), produce nectar and are bird-pollinated (Snow and Snow, 1980, Mendoza-Cifuentes and Prieto-Cruz, 2003; Penneys and Judd, 2005). In Blakea, a genus with about 100 species, rodent pollination has been reported or suspected in four species (Lumer, 1980; Lumer and Schoer, 1986; Almeda, 2000), and hummingbird pollination is presumed in two, B. fuchsioides (Almeda, 1989; D. S. Penneys, unpubl. res.) and B. purpusii (C Lumer, Cochise County Herbarium). Bat and bird pollination, with the associated nectar production, has also been reported in four species of Meriania and closely related Centronia, a mostly Andean group with 60–70 species (Vogel, 1988, 1997; Muchhala and Jarrin-V, 2002). Additionally, some insect-pollinated Miconia produce nectar (Mori and Pipoly, 1984; Renner, 1989; Vogel, 1997; Goldenberg and Shepherd, 1998). Amongst neotropical Melastomataceae, nectar production has been reported either in the literature or upon collection labels of about 80 species out of a total of over 3000 species (Renner, 1989).
The shift from pollen to nectar rewards in Melastomataceae has been interpreted as a response to selective forces due to the greater availability of vertebrate pollinators at higher altitudes (Stein and Tobe, 1989; Cruden, 1972), where nectar-producing genera usually occur (Renner, 1989). Hummingbirds are a very speciose group in the Andes and appear to have tracked the creation of new montane habitats (Bleiweiss, 1998). On the other hand, bee pollination services decline along an altitudinal gradient (Arroyo et al., 1982). This is suggestive of the ecogeographic scenario proposed by Thomson and Wilson (2008) in which a melitophilous linage evolves into an ornithophilous one. This theory would not apply to nectariferous insect-pollinated species of Miconia, usually found at lower elevations. But, as for species in higher altitudes, the shift to nectar-flowers in Miconia can also be interpreted as a change fostered by pollinators' unpredictability. Nectariferous species of Miconia do not have poricidal anthers, presenting a much more generalist pollination system (Goldenberg et al., 2008).
In most angiosperms, nectar is exuded from nectaries via epidermal cells or trichomes, pores, by rupturing or permeable cuticules, or by nectariferous tissues with stomata (Fahn, 1979). Vogel (1997, 1998a, b, c) studied several species in different families known to produce nectar, but that lacked obvious nectariferous tissues, the ‘remarkable nectaries’. Among these remarkable nectaries were the genera Brachyotum, Medinilla and Meriania in the Melastomataceae (Vogel, 1997).
Thus far, all studies focusing on floral nectaries in Melastomataceae (Stein and Tobe, 1989; Tobe et al., 1989; Vogel, 1997) report the absence of nectariferous tissues, and failed to find a structure related to nectar release (except for Medinilla magnifica). In most genera like Brachyotum, Tibouchina (Stein and Tobe; 1989; Vogel; 1997), Chalybea (Stein and Tobe, 1989) and Meriania (Vogel, 1997), the release has been suggested to be staminal with nectar seeping out of parenchyma on the adaxial surface of the filament geniculum. However, filament slits also occur in nectarless species (Vogel, 1997; Renner, 1989, 1993; F. A. Michelangeli, unpubl. res.), and they can be easily spotted as darker tissue on the filament. These are the product of damage caused by visiting bees while they grasp the anthers, and the dark colour is due to necrosis of the tissue (Renner, 1989). Moreover, at least in the case of Meriania phlomoides, the slits appear only after the flowers have been visited by hummingbirds and may also be the result of the damage caused by the birds (R. Kriebel, New York Botanical Garden, USA, and F. A. Michelangeli, unpubl. res.). Other nectar production mechanisms have been postulated, like the stigmatic secretion of nectar in Miconia (Stein and Tobe, 1989). Additionally, in Medinilla magnifica, nectary stomata were found on petal tips (Tobe et al., 1989; Vogel, 1997), but are probably not related to pollination (Vogel, 1997). Therefore, the question remains open as to where and how nectar is released.
In this study, neotropical nectar-producing Melastomataceae were investigated in order to clarify (a) if structures related to nectar production are present, and (b) if closely related taxa use similar morphological and/or anatomical features to release nectar.
MATERIALS AND METHODS
Species studied
Fourteen species, in six genera and four tribes of neotropical Melastomataceae were studied. All of them produce nectar, as observed in the field by the authors or collaborators, who also collected the samples (Table 1). The flowers and buds were fixed in 50–70 % ethanol for later anatomical studies.
Table 1.
Pollination mode, flower size, nectary features (location and size), thickness ratio among vascular bundle/filament and nectar location reports for species of nectar-producing Melastomataceae in the neotropics
| Tribe | Species | Pollinators | Floral tube width and height (mm) | Location of nectaries | Nectary stomata (μm) | Ratio of vascular bundle : filament size | Site of nectar release | Collector and no. of collection |
|---|---|---|---|---|---|---|---|---|
| Blakeeae | Blakea chlorantha | Rodents | 5·2 × 8 | Dorsal appendage of the connective | 18 | 0·33 | Base of filaments*; abaxial surface of filaments† | Penneys 1512 |
| Blakea fuchsioides | Hummingbirds | 7 × 19 | Dorsal appendage of the connective | 19 | 0·30 | – | Penneys 1744 | |
| Huilaea ‘calyptrata’ Penneys & Morales-P | Hummingbirds | 17 × 16 | Dorsal appendage of the connective | 15 | 0·63 | – | Penneys 1892 | |
| Huilaea ecuadorensis | Hummingbirds | 16 × 25 | Dorsal appendage of the connective | 35 | 0·36 | – | Penneys 1589 | |
| Melastomeae | Brachyotum confertum | Hummingbirds | 4 × 17 | Dorsal surface of the connective | 35 | 0·27 | – | C. Ulloa |
| B. ledifolium | Hummingbirds | 7 × 17 | Dorsal surface of the connective | 19 | 0·41 | Adaxial surface of filaments† | C. Ulloa | |
| B. microdon | Hummingbirds | 4·5 × 16 | Dorsal surface of the connective | 29 | 0·41 | – | M. Alford 54-184 | |
| Merianieae | Meriania phlomoides | Hummingbirds and bats | 4 × 19 | Dorsal and ventral surface of the connective and hypanthium | 26 | 0·38 | Release on the abaxial surface of filaments‡/nectar accumulated on petals | FAM 947; Penneys 1776 |
| Meriania tomentosa | Hummingbirds | 8 × 27 | Dorsal and ventral surface of the connective | – | 0·54 | Abaxial surface of filaments‡ | Penneys 1621 | |
| Miconieae | Charianthus alpinus | Hummingbirds | 4 × 16 | Ovary apex? | – | 0·20 | – | Penneys 1314 |
| C. dominicensis | Hummingbirds | 3 × 13 | Ovary apex? | – | – | – | Penneys 1316 | |
| C. nodosus | Hummingbirds | 3·5 × 12 | Ovary apex? | – | 0·25 | – | Penneys 1275 | |
| Miconia hyemalis | Wasps, moths | 1·5 × 3 | Ovary apex | 17 | 0·20 | Nectar stored over the floral tube, stamens | Reginatto 702 and 703 | |
| M. melanotricha | Hummingbirds | 3 × 10 | Dorsal and ventral surface of the anther | 24 | 0·19 | Penneys 1730 |
(–), Not observed.
* Lumer, 1980; † Stein and Tobe, 1989; ‡ Vogel, 1997.
Blakeeae
Blakea chlorantha is a rodent-pollinated species that secretes copious, mucilaginous nectar at night (Lumer, 1980). The flowers are pendant, with green sepals and petals that form a bowl-shaped corolla (Fig. 1A). Blakea fuchsioides, as well as the two species of Huilaea mentioned below, is presumed to be hummingbird-pollinated (Almeda, 2000). The flowers are pendant, with showy, red floral bracts and pink petals forming a tubular corolla (Fig. 1B). Huilaea ‘calyptrata’ has flowers that are mostly erect, with inconspicuous sepals, and light-pink petals forming a tubular corolla (Penneys, 2007; Fig. 1C). Huilaea ecuadorensis has pendant flowers, with pink to red, tubular corollas (Wurdack, 1990).
Fig. 1.
Nectar-producing flowers in Melastomataceae. (A) Blakea chlorantha, (B) B. fuschisoides, (C) Huilaea ‘calyptrata’, (D) Brachyotum ledifolium, (E) Meriania phlomoides, (F) M. tomentosa, (G) Charianthus nodosus, (H) Miconia hyemalis, and (I) M. melanotricha.
Melastomeae
Brachyotum is an Andean genus of hummingbird-pollinated shrubs with tubular corollas (Wurdack, 1953). Brachyotum confertum has pendant, nigrescent-purple flowers. Brachyotum ledifolium has showy, pendant flowers with red sepals and yellow petals (Fig. 1D). Brachyotum microdon has pendant flowers, with dark purple petals and a red hypanthium.
Merianieae
Meriania phlomoides is hummingbird- and bat-pollinated (Vogel, 1997; R. Kriebel, New York Botanical Garden, USA, and F. A. Michelangeli, unpubl. res.). The flowers are erect, with green sepals, white petals and bowl-shaped corollas (Fig. 1E). Meriania tomentosa is hummingbird-pollinated, and has erect flowers with brown sepals, red petals, and bowl-shaped corollas (Fig. 1F).
Miconieae
Charianthus is a hummingbird-pollinated genus of Lesser Antillean shrubs and small trees (Penneys and Judd, 2005). Charianthus alpinus and C. dominicensis have erect flowers with red sepals, petals and filaments. The corollas are tubular with stamens exserted in the former, and barely so in the latter. Charianthus nodosus has pendant, tubular flowers, with red sepals, pink petals and red, exserted stamens (Fig. 1G). Miconia hyemalis is pollinated mainly by wasps (I. G. Varassin, unpubl. res.). The flowers are erect, with brown sepals, white petals and stamens (Fig. 1H). Miconia melanotricha is a hummingbird-pollinated species (Stein and Tobe, 1989) with pendant flowers that have pink sepals, pink petals and red stamens (Fig. 1I). The corolla is tubular with stamens exserted.
Morphological and anatomical studies
Measurements were taken from flowers preserved in 50 % ethanol. Flower size was estimated by measuring the tube width at the outside torus level, and the tube height from the torus level to the distal tips of petals. The relative size of the vascular supply in the connective was calculated as the ratio between radial thickness of the vascular bundle and the radial thickness of the filament as previously suggested by Stein and Tobe (1989). All measurements were made at the basal portion of anther on sections mounted on microscopy slides. Stomata were measured in SEM photographs as the diameter parallel to the guard cells.
For light microscopy the samples were dehydrated in an ethanol–toluene series and embedded in Paraplast Plus. Cross and longitudinal serial sections of 8–10 µm were stained alternatively with periodic acid–Schiff reagent for carbohydrates, or with 0·5 % AstraBlue and 0·2 % Safranin as a general stain. Ninhydrin–Schiff reaction was used for proteins and amino acids. Polarized light was used to search for starch grains. Permanent slides were mounted with Permount. All slides were observed on a Zeiss Axioplan compound scope, and photographed with a Nikon DXM1200c digital camera along with its Nikon ACT-1c software.
For scanning electron microscopy, the samples were dehydrated in an ethanol–acetone series, critical point-dried, and coated for 4·5 min with gold–palladium in a Hummer 6·2 Sputter Coater. The occurrence of nectaries was checked on petals, hypanthium and dorsal and ventral side of the anthers in each species in a JEOL JSM-5410 at 15 kV.
RESULTS
Flowers of nectar-producing melastomes are usually large, with a floral tube always over 10 mm long, and most commonly over 15 mm long. In most cases, nectar-producing flowers are larger (or equal) to closely related, nectarless species (Fig. 1). In the present sample, hummingbird-pollinated flowers have floral tubes that appear long because the petals do not spread open at anthesis, as in Blakea fuchsioides, Brachyotum spp., Charianthus spp., Meriania spp. and Miconia melanotricha. In the rodent-pollinated Blakea chlorantha, and bat-pollinated Meriania phlomoides, the petals open (but never spread out completely), so the floral tube appears wide (Fig. 1A and E). Huilea spp. have large petals (16 × 25 mm) that by opening at small amount at anthesis (<30°) give the floral tube a bell shape that is both wide and long (Fig. 1C). The only species possessing flowers with petals <5 mm long, and that has no real tube because the petals spread at anthesis, is the insect-pollinated Miconia hyemalis (Fig. 1H and Table 1).
Nectary tissue and secretory structures are found in different organs of the flowers of neotropical Melastomataceae, but within each genus the structures are all similar. Staminal nectaries are found in Blakea, Brachyotum, Huilaea, Meriania and Miconia melanotricha. Nectary stomata occur in abundance on the proximal portion of the connective, with the exception of Meriania, in which stomata are absent. Nectaries occur on the ovary apex of Meriania phlomoides and Miconia hyemalis, where nectary stomata were found. All observed nectary stomata are formed by two guard cells with subsidiary cells lacking. The surface of the guard cell is always smooth while the non-specialized epidermal cells are covered by a ridged cuticule.
Blakeeae
Blakea
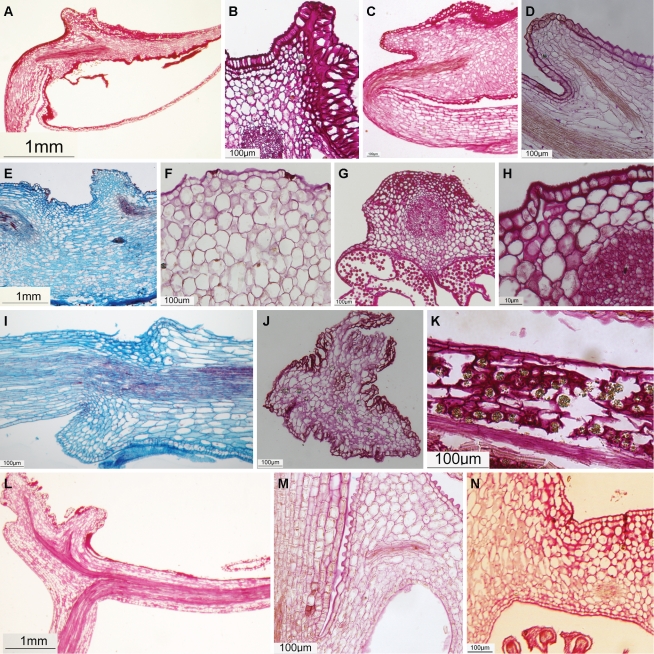
Blakea chlorantha and B. fuchsioides have staminal nectaries on the dorsal surface of the anther connective. The dorsal epidermis and parenchyma of the anther stained strongly for carbohydrates (Fig. 2A, B), even in areas where no nectary stomata are found. Nectary stomata distributed over the dorsal appendage of the connective (Fig. 3A–D) are supplied by a vascular bundle that diverges from the main bundle in the anther (Fig. 2C, D) and the parenchyma has copious, intercellular spaces. The appendage vascular bundle is only four to six cells distant from the epidermis and nectary stomata (Fig. 2C, D).
Fig. 2.
Aspects of nectary tissues in nectar-producing Melastomataceae. (A) B. chlorantha: dorsal epidermis and parenchyma of anther strongly stains for carbohydrates. (B) B. chlorantha: large and continuous intercellular space under the epidermis rich in carbohydrates. (C) B. fuschisoides: dorsal epidermis and parenchyma of anther strongly stains for carbohydrates. (D) B. fuschisoides: the dorsal appendage supplied by a vascular bundle that diverges from the main bundle in the anther. (E) Huilaea ‘calyptrata’: dorsal appendage supplied by a vascular bundle that diverges from the main bundle in the anther. (F) H. ecuadoriensis: intercellular spaces below stomata. (G) B. microdon: large vascular bundle in the connective close to the epidermis and stained for carbohydrates. (H) B. ledifolium: stomata and intercellular spaces – a path to vascular supply? (I) B. confertum: vascular bundle in the connective paralleling the epidermis. (J) M. phlomoides: connective strongly ornamented, with many channels between lobes. (K) M. phlomoides: hypanthium rich in carbohydrates. (L) M. tomentosa: connective vascular supply. (M) M. hyemalis: vascular supply in the apex of the ovary. (N) C. alpinus: weak reaction for carbohydrates in the apex of the ovary.
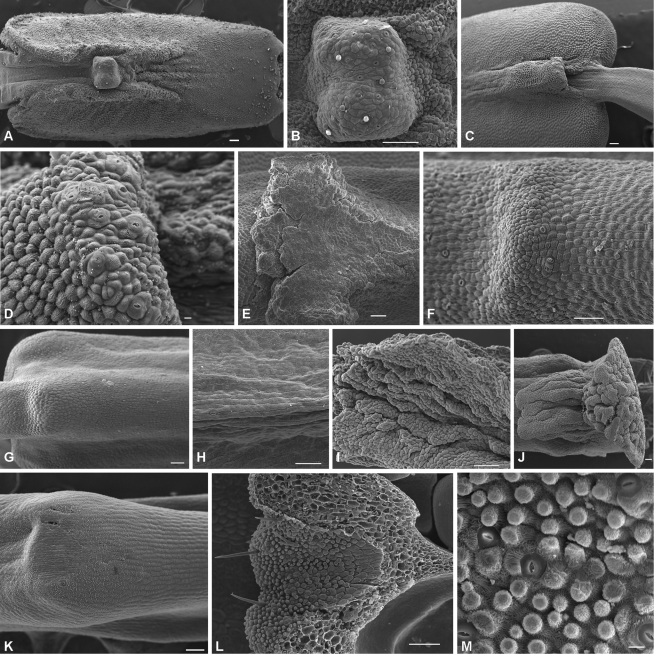
Fig. 3.
Stomata positioned upon the dorsal appendage of the anther: (A, B) Blakea chlorantha, (C, D) B. fuschisoides, (E) Huilaea ‘calyptrata’, (F) H. ecuadorensis. Stomata upon the anther connective: (G) Brachyotum ledifolium, (H) B. confertum. Lobed anther appendage: (I) Meriania phlomoides, (J) M. tomentosa. Stomata upon the anther connective: (K) Miconia melanotricha. Stomata upon the ovary apex: (L, M) M. hyemalis. Scale bars = 100 μm, except to (D) and (M) where scale bars = 10 μm.
Blakea chlorantha possesses large and continuous intercellular space beneath the epidermis of the distal portion of the anther, close to the dorsal appendage (Fig. 2B). This intercellular space is rich in carbohydrates (Fig. 2B).
Huilaea
Huilaea ‘calyptrata’ and H. ecuadorensis possess nectary stomata on the dorsal surface of the anther connective appendage (Fig. 3E, F). The dorsal epidermis and parenchyma of the anther strongly stains for carbohydrates (Fig. 2F) in areas where nectary stomata are found. The dorsal appendage is supplied by a vascular bundle that diverges from the main bundle in the anther (Fig. 2E). The appendage bundle is only three to six cell layers distant from the epidermis and nectary stomata (Fig. 2E). On the appendage, the parenchyma underlying the epidermis is rich in intercellular spaces (Fig. 2F). In H. ‘calyptrata’, the cells of this parenchyma possess thickened cell walls, and may store starch (Fig. 2E). The nectary stomata are small and very abundant over the appendage of connective and also occur on the first quarter of the connective close to the appendage (Fig. 3E). In H. ecuadorensis, nectary stomata occur over the concave depression close to the appendage (Fig. 3F).
Melastomeae
Brachyotum
Brachyotum confertum, B. ledifolium and B. microdon possess staminal nectaries on the dorsal surface of the anther connective. The dorsal epidermis and parenchyma of the anthers in Brachyotum spp. stains for carbohydrates (Fig. 2G–I), even in areas where no nectary stomata are found. Nectary stomata are distributed on the connective along the proximal third in B. microdon, two-thirds in B. confertum (Fig. 3H) or the entire connective in B. ledifolium (Fig. 3G); the stomata are absent from the dorsal crest of the connective. Nectary stomata are distributed in lines, tracking the position of vascular supply (Fig. 3G, H), and are positioned above large intercellular spaces (Fig. 2H) of the parenchyma. The vascular bundle in the connective is large, paralleling the epidermis by sometimes only three layers of parenchyma cells (Fig. 2G–I).
Merianieae
Meriania
Meriania phlomoides and M. tomentosa have strongly ornamented anther connective appendages with many channels between the lobes, and nectary stomata are lacking. The architecture of the appendage (Figs 2J, L and 3I, J) and the weak staining for carbohydrates along both faces of the ventral connective (Fig. 2J, L) suggests that the appendage may function as a nectary. In M. phlomoides, nectary stomata are found on the inner surface of the hypanthium, close to the ovary, where the parenchyma is rich in carbohydrates (Fig. 2K). Information about the hypanthium is currently lacking for M. tomentosa.
Miconieae
Charianthus
The source of nectar production in Charianthus remains enigmatic. No nectary stomata were observed on stamens, and only a single stomata was observed on the ovary apex of one specimen of C. nodosus. However, the parenchyma underlying the epidermis of the ovary apex is rich in carbohydrates (Fig. 2 N), and the vascular supply is abundant due to proximity with the ovary (Fig. 2 N).
Miconia
Miconia hyemalis and M. melanotricha are very distinct in their morphology and nectary stomata location. Miconia hyemalis has nectaries on the ovary apex possessing vascular bundles close to the epidermis and nectary stomata, and the parenchyma is rich in intercellular spaces (Fig. 2M). The nectary stomata are present on the inner face of the ovary apex, close to the style base (Fig. 3L). This area is rich in papillate trichomes (Fig. 3M). In M. melonotricha, nectary stomata are found on the ventral face of the anther and dorsal connective, where only two nectary stomata were observed (Fig. 3K), and are absent from the ovary apex. In these stamens, few nectary stomata were present. The vascular bundle is closer to the ventral face of the anther, which is probably associated with the richest area of nectary stomata.
DISCUSSION
Nectaries in Melastomataceae are composed of modified stomata with little differentiation in their adjacent parenchyma. Nectar secretion through stomata has been reported for many species (Davis and Gunning, 1992; Nepi et al., 1996; Gaffal et al., 1998; Razem and Davis, 1999; Fahn and Shimony, 2001; Wist and Davis, 2006; Paiva and Machado, 2008), but this is the first report on nectary stomata related to pollination in Melastomataceae. This kind of nectary seems to be the commonest method of nectar release in non-monocotyledoneous angiosperms (Endress, 1994; Bernardello, 2007). These modified stomata are all very similar, formed by two guard cells, with no subsidiary cells (Gaffal et al., 1998; Wist and Davis, 2006). A common feature in nectary stomata is the smoothness of guard-cells (Wist and Davis, 2006). Such nectaries are usually differentiated as a disc around the ovary in eudicots (Endress, 1994), but there are reports of nectar exudation on the stamens (Davis and Gunning, 1992) or in a staminal column (Razem and Davis, 1999).
Modified stomata associated with nectar secretion in Melastomataceae were first seen by Vogel (1997) who described this structure on petal tips of Medinilla magnifica. Although in M. magnifica the ecological function of the stomata on the petals is associated with protection by ants rather than with pollination, these stomata do also secrete sugars.
Nectary stomata may be homologous to leaf hydathodes (Vogel, 1997), which are almost twice as large as the aerial stomata in Medinilla magnifica. The nectary stomata on the species reported here (see Table 1) are from slightly bigger than the aerial stomata in M. magnifica, 13 µm (Vogel, 1997) to larger than its hydathodes, 21–25 µm (Vogel, 1997) as in Huilaea ecuadorensis, Brachyotum confertum and B. microdon.
Since it is difficult to distinguish nectariferous tissue from the surrounding tissues, Melastomataceae nectaries were originally described as non-structural (Stein and Tobe, 1989; Vogel, 1997) as defined by Zimmerman (1932). However, some differentiation may be found, such as the presence of intercellular spaces next to stomata or below the epidermis, the presence of thickened cell walls, and vascular bundles close to the nectary stomata.
The presence of intercellular spaces below the epidermis is a characteristic feature of nectaries with stomata (Fahn, 1979; Gaffal et al., 1998; Wist and Davis, 2006), where nectar may be discharged internally, from where it reaches the exterior via modified stomata (Durkee, 1983; Gaffal et al., 1998). It remains unknown how nectar reaches the intercellular spaces, but both apoplastic and symplastic pathways have been invoked to explain nectar release (Gaffal et al., 1998; Stpiczynska et al., 2005; Wist and Davis, 2006). The presence of sucrose-rich nectar in the species studied here would support the hypothesis of a phloem origin of nectar (Vogel, 1997). However, even considering the homology to hydathodes, there is some support for an active role of parenchyma cells in water exudation (Fahn, 1979) and nectar is, in many cases, hypertonic to phloem sap (Durkee, 1983).
The presence of thickened cell walls on the adjacent epidermis parenchyma cells, as in Huilaea ‘calyptrata’, was reported in some other species where nectary parenchyma cell walls are thick and heavily cutinized (Fahn, 1979; Durkee, 1983; Stpiczynska et al., 2005).
As reported here for many species, in vascularized nectaries the vascular bundle generally ends a few cells away from the secretory tissue (Durkee, 1983) and may be constituted only by phloem ends (Gaffal et al., 1998, Wist and Davis, 2006), or by both xylem and phloem (Stpiczynska et al., 2005). Supporting the idea that vascular supply is directly involved with nectar secretion, nectariferous species of Melastomataceae usually possess stamens with large vascular bundles whose diameter is approximately one-third that of the filament (Stein and Tobe, 1989). This pattern is true for all species investigated in this study that have staminal nectaries with the exception of Miconia melanotricha. Species with nectaries on the ovary apex, M. hyemalis, and perhaps Charianthus spp., all have a low thickness ratio (from 0·20 to 0·25). However, it should be noted that Miconia and Charianthus are the only genera studied here that belong to the tribe Miconieae, and this pattern might have a phylogenetic origin, rather than be a reflection of the position of the nectaries.
The staminal nectaries on the dorsal surface of the anther connective, the parenchyma rich in intercellular spaces, and the distribution of vascular supply on the connective are morphological similarities observed in Blakea and Huilaea. They support the recent hypothesis that Huilaea belongs in the tribe Blakeeae (Penneys et al., 2004; Penneys, 2007; M. E. Morales, Universidad Tecnologíca y Pedagógica de Colombia, Colombia, and D. S. Penneys, unpubl. res.), rather than in the Miconieae as previously suggested (Wurdack, 1957; Judd, 1989; Renner, 1993). In Blakea there has probably been a single shift from insect to vertebrate (hummingbirds and rodents) pollination (Penneys, 2007), where nectar production is expected.
All three species of Brachyotum studied also have staminal nectaries, but this genus is placed in the Melastomeae. The staminal nectaries found in the Blakeeae and Melastomeae could potentially reflect a phylogenetic affinity, as in some cladistic analyses (D. S. Penneys, unpubl. res.) these two lineages appear to be sister, though increased generic and tribal-level sampling is needed for confirmation. Staminal nectaries of Brachyotum differ from those of Blakea and Huilaea in that no stomata occur upon the dorsal appendage.
In Meriania, tribe Merianieae, nectar is released through the epidermis of the inner wall of the hypanthium, either by rupture of the cuticle, or by cuticle permeability as has been proposed for other species (Fahn, 1979). It is possible that in M. phlomoides nectar is released both in the stamens and the hypanthium. As nectary stomata have been described occurring in deep depressions (Nepi, 2007), it is also possible that in M. phlomoides and M. tomentosa they are enclosed and obscured by the connective lobes.
Miconia hyemalis and M. melanotricha have nectary stomata either on the ovary apex or on the ventral anther connective surface. This makes Miconia the only genus included in this study that shows different nectary locations. However, Miconia has over 1050 species, is widely distributed throughout the neotropics, and is paraphyletic (Michelangeli et al., 2004; Goldenberg et al., 2008). Miconia melanotricha is not closely related to M. hyemalis (Goldenberg et al., 2008). Miconia melanotricha is a hummingbird-pollinated species belonging to a mostly Caribbean clade, while M. hyemalis is an insect-pollinated species, from an eastern Brazilian clade (Goldenberg et al., 2008). Miconia hyemalis shares nectar production, a disagreeable flower scent, and fly pollination (I. G. Varassin, unpubl. res.) with other species from the same eastern Brazilian clade such as M. pepericarpa (Goldenberg and Shepherd, 1998) and M. angellana (Santos, 2008). It is predicted that nectary stomata are also found on the ovary apex of the other nectariferous species in this clade.
Charianthus, which also belongs to the Miconieae, obviously produces nectar and is hummingbird-pollinated (Penneys and Judd, 2005; Penneys, 2007). However, this study failed to reveal the source of the nectar. Because the stamens are exserted beyond the corolla tube, and because hummingbirds place their beak deep into the corolla tube, it is not surprising that nectar production does not seem to be associated with the anthers as in other species discussed above. The level of vascularization and the amount of carbohydrates at the base of the hypanthium suggest that nectar is produced in this region. Alternatively, the vascularization might be associated with vasculature that supplies the ovary rather than with nectar production.
A striking feature of nectar-producing melastomes is that the stamen appendages and basally prolonged connectives (termed pedoconnectives by Jacques-Felix, 1953) that are widespread in the family are both small or absent in these species (with the exception of the appendages of Meriania). In part, this is related to the fact that most nectar-producing species belong to clades that have reduced appendages or no pedoconnectives regardless of their pollination mode (e.g. Miconieae and Blakeeae), but it is even true for Brachyotum which belongs to the Melastomeae, a tribe with both pedoconnectives and large anther appendages. This may be due to the fact that these structures are associated with the buzz-pollination syndrome (Renner, 1989), and might have been lost in the hummingbird-pollinated lineages. Detailed phylogenetic analyses of the Melastomeae and reproductive biology studies for the same taxa are necessary in order to test this hypothesis.
The unusual presence of nectaries in the Melastomataceae species described here is also associated with a flower morphology shift, from rotate, to falsely tubular, pseudocampanulate flowers. It is important to clarify that these corollas are ‘tubular’ only from a functional point of view, because like in all other Melastomataceae, the petals are not connate and the tubular aspect is produced by corollas that do not spread open during anthesis (Fig. 1A–G). Nectar-producing flowers tend to be rather large, except for Miconia hyemalis, the only insect-pollinated one. According to Faegri and van der Pijl (1979), large flowers are expected in vertebrate-pollinated species.
Hummingbird flowers are usually associated with long-billed Phaethornithinae hummingbirds (Walther and Brieschke, 2001; Kaehler et al., 2005), and the flowers are mostly tubular (Buzato et al., 2000), as in Blakea fuchsioides, Brachyotum confertum, B. ledifolium, B. microdon, Charianthus alpinus, C. dominicensis, C. nodosus and Miconia melanotricha. There are also hummingbird-pollinated species with large corolla apertures (Buzato et al., 2000), such as Huilaea and Meriania.
Flower colours of Blakea fuchsioides, Brachyotum confertum, B. ledifolium, B. microdon, Charianthus alpinus, C. dominicensis, C. nodosus, Huilaea ‘calyptrata’, H. ecuadorensis, Meriania tomentosa and Miconia melanotricha are usually associated with hummingbird pollination. All of these species have showy flowers, with sepals, and/or petals usually pink to red, which are not exclusive to hummingbird-pollinated flowers, but are very common among them (Faegri and van der Pijl, 1979).
Wide floral tubes and bell- or brush-shaped flowers are common in bat-pollinated plants, as are dull colours (Proctor et al., 1996); these characteristics are present in Meriania phlomoides, and in another bat-pollinated species, M. pichichensis (Muchhala and Jarrin-V, 2002). However, M. phlomoides seems to be bat- and hummingbird-pollinated (Vogel, 1997; R. Kriebel, New York Botanical Garden, USA, and F. A. Michelangeli, unpubl. res.). Species that are pollinated by both of these vertebrate groups have been reported (Sazima et al., 1994), and M. phlomoiodes, which has diurnal and nocturnal anthesis along with large, whitish, pseudocampanulate corollas, may be another.
Flowers pollinated by non-flying mammals are usually robust, not vivid coloured, and secrete copious nectar (Proctor et al., 1996); characteristics found in Blakea chlorantha (and three closely related species). The structure of the nectary in B. chlorantha, with a large intercellular space rich in carbohydrates, may be a feature associated with voluminous nectar release as reported by Lumer (1980).
One of the major conclusions of this study is that nectar release in Melastomataceae is related to nectary stomata and not filament slits. Thus, the hypothesis that nectar production is primitive in Melastomataceae and lost in most modern members (Renner, 1989) is probably incorrect because it was proposed under the assumption that filament slits are widespread in the family, and in some lineages the slits were co-opted into nectaries (Renner, 1989). The presence of nectary stomata on stamens and ovaries in different lineages suggests that the acquisition of nectaries is a derived condition in the family, as proposed by Stein and Tobe (1989), and Vogel (1997), and subsequently subject to convergence. Within the Melastomataceae, it appears that pathways and locations exploitable for nectary stomata are conserved within closely related lineages. This is illustrated by the highly similar staminal nectaries of the Blakeeae (Blakea and Huilaea) and Melastomeae (Brachyotum), lineages that appear to be sister (D. S. Penneys, unpubl. res.), and also in the Merianieae (Meriania phlomoides) and Miconieae (Miconia hyemalis), although tribal-level phylogenetic affinities need to be confirmed.
This research on nectar-producing Melastomataceae is just beginning and this study should help guide future research. For example, we still have a very poor understanding about the nectar production and release in Charianthus. Detailed chemical analyses of Melastomataceae nectar are also necessary. Considering the diversity of vertebrate pollinators, and the occurrence of insect pollination within nectar-producing species in the family, it would be interesting to study whether some aspects of nectar secretion and composition are related to pollination syndromes. Since Melastomataceae usually have poricidal anthers that are buzz-pollinated, research on the morphological character changes of the anthers as related to pollen release in nectariferous species is also necessary.
ACKNOWLEDGMENTS
We thank Dennis Stevenson for helpful comments and support, Renato Goldenberg for suggestions and comments, C. Ulloa, M. Alford and M. Reginato for plant collections. This research was partially supported by the National Science Foundation (grant DEB-0515665 to F.A.M. and DEB-0508582 to D.S.P.).
LITERATURE CITED
- Almeda F. Five new berry-fruited species of tropical American Melastomataceae. Proceedings of the California Academy of Sciences. 1989;46:137–150. [Google Scholar]
- Almeda F. A synopsis of the genus Blakea (Melastomataceae) in Mexico and Central America. Novon. 2000;10:299–319. [Google Scholar]
- Arroyo MTK, Primack R, Armesto JJ. Community studies in pollination ecology in the high temperate Andes of central Chile. I. American Journal of Botany. 1982;69:82–97. [Google Scholar]
- Bernardello G. A systematic survey of floral nectaries. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 19–128. [Google Scholar]
- Bleiweiss R. Origin of hummingbird faunas. Biological Journal of the Linnaean Society. 1998;65:77–97. [Google Scholar]
- Buzato S, Sazima M, Sazima I. Hummingbird-pollinated floras at three Atlantic forest sites. Biotropica. 2000;32:824–841. [Google Scholar]
- Cruden RW. Pollinators in high-elevation ecosystems: relative effectiveness of birds and bees. Science. 1972;176:1439–1440. doi: 10.1126/science.176.4042.1439. [DOI] [PubMed] [Google Scholar]
- Davis AR, Gunning BES. The modified stomata of the floral nectary of Vicia faba L. 1. Development, anatomy and ultrastructure. Protoplasma. 1992;166:134–152. [Google Scholar]
- Durkee LT. The ultrastructure of floral and extrafloral nectaries. In: Bentley B, Elias T, editors. The biology of nectaries. New York, NY: Columbia University Press; 1983. pp. 1–29. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fahn A. Ultrastructure of nectaries in relation to nectar secretion. American Journal of Botany. 1979;66:977–985. [Google Scholar]
- Fahn A, Shimony C. Nectary structure and ultrastructure of unisexual flowers of Ecballium elaterium (L.) A. Rich. (Cucurbitaceae) and their presumptive pollinators. Annals of Botany. 2001;87:27–33. [Google Scholar]
- Gaffal KP, Heimler W, El-Gammal S. The floral nectary of Digitalis purpurea L.: structure and nectar secretion. Annals of Botany. 1998;81:251–262. [Google Scholar]
- Goldenberg R, Shepherd GJ. Studies on the reproductive biology of Melastomataceae in cerrado vegetation. Plant Systematics and Evolution. 1998;211:13–29. [Google Scholar]
- Goldenberg R, Penneys DS, Almeda F, Judd WS, Michelangeli FA. Phylogeny of Miconia (Melastomataceae): patterns of stamen diversification in a megadiverse neotropical genus. International Journal of Plant Sciences. 2008;169:963–979. [Google Scholar]
- Harter B, Leistikow C, Wilms W, Truylio B, Engels W. Bees collecting pollen from flowers with poricidal anthers in a south Brazilian Araucaria forest: a community study. Journal of Apicultural Research. 2002;40:9–16. [Google Scholar]
- Jacques-Felix H. Sur quelques Mélastomatacées d'Afrique. Bulletin de l'Institut Francais d'Afrique Noire. 1953;15:972–1001. [Google Scholar]
- Judd WS. Taxonomic studies in the Miconieae (Melastomataceae). III. Cladistic analysis of axillary flowered taxa. Annals of the Missouri Botanical Garden. 1989;76:476–495. [Google Scholar]
- Kaehler M, Varassin IG, Goldenberg R. Polinização em uma comunidade de bromélias em Floresta Atlântica Alto Montana no Estado do Paraná, Brasil. Revista Brasileira de Botânica. 2005;28:219–228. [Google Scholar]
- Lagerheim NG. Über die Bestäubungs- und Aussäungseinrichtungen von Brachyotum ledifolium (Desr.) Cogn. Botaniska Notiser. 1899;1899:105–122. [Google Scholar]
- Lumer C. Rodent pollination of Blakea (Melastomataceae) in a Costa Rican cloud forest. Brittonia. 1980;32:512–517. [Google Scholar]
- Lumer C, Schoer RD. Pollination of Blakea austin-smithii and B. penduliflora (Melastomataceae) by small rodents in Costa Rica. Biotropica. 1986;18:363–364. [Google Scholar]
- Mendoza-Cifuentes H, Prieto-Cruz A. Una especie nueva de Huilaea Wurdack (Melastomataceae) de Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales. 2003;27:39–43. [Google Scholar]
- Michelangeli FA, Penneys DS, Giza J, Soltis DE, Hils MH, Skean DJ., Jr A preliminary phylogeny of the tribe Miconieae (Melastomataceae) based on nrITS sequence data and its implications on inflorescence position. Taxon. 2004;53:279–290. [Google Scholar]
- Mori SA, Pipoly JS. Observations on the big bang flowering of Miconia minutiflora. Brittonia. 1984;36:337–341. [Google Scholar]
- Muchhala N, Jarrin-V P. Flower visitation by bats in cloud forests of western Ecuador. Biotropica. 2002;34:387–395. [Google Scholar]
- Nepi M. Nectary structure and ultrastructure. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 129–166. [Google Scholar]
- Nepi M, Ciampolini F, Pacini E. Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Annals of Botany. 1996;78:95–104. [Google Scholar]
- Paiva EAS, Machado SR. The floral nectary of Hymenaea stigonocarpa (Fabaceae, Caesalpinioideae): structural aspects during floral development. Annals of Botany. 2008;101:125–133. doi: 10.1093/aob/mcm268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penneys DS. Phylogeny and character evolution in the Blakeeae (Melastomataceae). USA: University of Florida; 2007. PhD Thesis. [Google Scholar]
- Penneys DS, Judd WS. A systematic revision and cladistic analysis of Charianthus (Melastomataceae) using morphological and molecular characters. Systematic Botany. 2005;30:559–584. [Google Scholar]
- Penneys DS, Whitten WM, Williams NH, Judd WS. Botany. Snowbird, UT: 2004. Huilaea and the Blakeeae (Melastomataceae): phylogenetic relationships reconsidered. 2004, 31 July–5 August. [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. London: Harper Collins Publishers; 1996. [Google Scholar]
- Razem FA, Davis AR. Anatomical and ultrastructural changes of the floral nectary of Pisum sativum L. during flower development. Protoplasma. 1999;206:57–72. [Google Scholar]
- Renner SS. A survey of reproductive biology in Neotropical Melastomataceae and Memecylaceae. Annals of the Missouri Botanical Garden. 1989;76:496–518. [Google Scholar]
- Renner SS. Phylogeny and classification of the Melastomataceae and Memecylaceae. Nordic Journal of Botany. 1993;13:519–540. [Google Scholar]
- Santos APM. Biologia reprodutiva de espécies endêmicas de Melastomataceae na Serra da Canastra. Brazil: Universidade Federal de Uberlândia; 2008. MSc Thesis. [Google Scholar]
- Sazima M, Sazima I, Buzato S. Nectar by day and night: Siphocampylus sulfurous (Lobeliaceae) pollinated by hummingbirds and bats. Plant Systematics and Evolution. 1994;191:237–246. [Google Scholar]
- Snow DW, Snow BK. Relationships between hummingbirds and flowers in the Andes of Colombia. Bulletin of the British Museum of Natural History (Zoology) 1980;38:105–139. [Google Scholar]
- Stein BA, Tobe H. Floral nectaries in Melastomataceae and their systematic and evolutionary implications. Annals of the Missouri Botanical Garden. 1989;76:519–531. [Google Scholar]
- Stiles FG, Ayala AV, Giron M. Pollination of the flowers of Brachyotum (Melastomataceae) by two species of Diglossa (Emberizidae) Caldasia. 1992;17:47–54. [Google Scholar]
- Stpiczynska M, Davies KL, Gregg A. Comparative account of nectary structure in Hexisea imbricata (Lindl.) Rchb.f. (Orchidaceae) Annals of Botany. 2005;95:749–756. doi: 10.1093/aob/mci081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JD, Wilson P. Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. International Journal of Plant Sciences. 2008;169:23–38. [Google Scholar]
- Tobe H, Hakki MI, Langhammer L. Floral nectary in Medinilla magnifica, an Old World Melastomataceae. Botanische Jahrbücher für Systematik. 1989;111:57–62. [Google Scholar]
- Ule E. Weiteres zur Bluetheneinrichtung von Purpurella cleistopetala und Verwandten. Berichte der Deutschen Botanischen Gesellschaft. 1896;14:169–178. [Google Scholar]
- Vogel S. Fledermausblumen in Südamerika. Oesterreichische botanische Zeitschrift. 1957;104:491–530. [Google Scholar]
- Vogel S. Neu er kannte bzw neu documentierte Fledermausblumen aus drei Kontinenten. Tagungsberichte der Deutschen Botanischen Gesellschaft, Giessen. 1988;1988:188. [Google Scholar]
- Vogel S. Remarkable nectaries: structure, ecology, organophyletic perspectives. I. Substitutive nectaries. Flora. 1997;192:305–333. [Google Scholar]
- Vogel S. Remarkable nectaries: structure, ecology, organophyletic perspectives. II. Nectarioles. Flora. 1998;a 193:1–29. [Google Scholar]
- Vogel S. Remarkable nectaries: structure, ecology, organophyletic perspectives. III. Nectar ducts. Flora. 1998;b 193:113–131. [Google Scholar]
- Vogel S. Remarkable nectaries: structure, ecology, organophyletic perspectives. IV. Miscellaneous cases. Flora. 1998;c 193:225–248. [Google Scholar]
- Walther BA, Brieschke H. Hummingbird flower relationships in a mid-elevation rain forest near Mimdo, northwest Ecuador. International Journal of Ornithology. 2001;4:115–135. [Google Scholar]
- Wist TJ, Davis AR. Floral nectar production and nectary anatomy and ultrastructure of Echinacea purpurea (Asteraceae) Annals of Botany. 2006;97:177–193. doi: 10.1093/aob/mcj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdack JJ. A revision of the genus Brachyotum (Tibouchineae–Melastomataceae) Memoirs of the New York Botanical Garden. 1953;8:343–407. [Google Scholar]
- Wurdack JJ. Certamen Melastomataceis IV. Brittonia. 1957;9:101–109. [Google Scholar]
- Wurdack JJ. Certamen Melastomataceis XXXIX. Phytologia. 1990;69:316–327. [Google Scholar]
- Zimmerman J. Über die extrafloralen Nektarien der Angiospermen. Beihefte zum Botanischen Centralblatt Abt I. 1932;49:99–196. [Google Scholar]