Abstract
NY-ESO-1 elicits frequent antibody responses in cancer patients, accompanied by strong CD8+ T cell responses against HLA-A2-restricted epitopes. To broaden the range of cancer patients who can be assessed for immunity to NY-ESO-1, a general method was devised to detect T cell reactivity independent of prior characterization of epitopes. A recombinant adenoviral vector encoding the full cDNA sequence of NY-ESO-1 was used to transduce CD8-depleted peripheral blood lymphocytes as antigen-presenting cells. These modified antigen-presenting cells were then used to restimulate memory effector cells against NY-ESO-1 from the peripheral blood of cancer patients. Specific CD8+ T cells thus sensitized were assayed on autologous B cell targets infected with a recombinant vaccinia virus encoding NY-ESO-1. Strong polyclonal responses were observed against NY-ESO-1 in antibody-positive patients, regardless of their HLA profile. Because the vectors do not cross-react immunologically, only responses to NY-ESO-1 were detected. The approach described here allows monitoring of CD8+ T cell responses to NY-ESO-1 in the context of various HLA alleles and has led to the definition of NY-ESO-1 peptides presented by HLA-Cw3 and HLA-Cw6 molecules.
NY-ESO-1 is a member of the cancer/testis family of genes (1). Expression of NY-ESO-1 in normal tissues is limited to germ cells, but in cancer, NY-ESO-1 is expressed in a broad range of different tumor types. NY-ESO-1 was discovered by serological analysis of a recombinant cDNA expression library obtained from an esophageal cancer (1). NY-ESO-1 is one of the most immunogenic tumor antigens defined to date, eliciting a humoral response in nearly half of patients with advanced cancers expressing NY-ESO-1 (2) along with a strong CD8+ T cell (3–6) and CD4+ T cell (7) response.
Using optimized techniques for analyzing antibodies and newer methodologies for CD8+ T cell responses, such as HLA-A2/peptide tetramer complexes and enzyme-linked immunospot (Elispot) assays, we recently obtained a comprehensive picture of the spontaneous immune response against NY-ESO-1 in a cohort of patients with advanced tumors (4). The study showed that humoral and cellular responses occurred simultaneously against NY-ESO-1. Antibodies appeared to be good indicators of CD8+ T cell responses in this system.
Studies of T cell reactivity against NY-ESO-1, however, remain limited because of the few epitopes described thus far. With regard to HLA-A2-restricted epitopes, three overlapping NY-ESO-1 peptides are known (3). Wang et al. (8) defined epitopes presented by the HLA-A31 molecule, encoded by both the normal and an alternative reading frame of NY-ESO-1.
In the present study, we devised an assay to monitor CD8+ T lymphocyte responses to NY-ESO-1 in cancer patients that does not require knowledge of predefined peptide epitopes or particular HLA profiles. Recombinant adenovirus and vaccinia vectors were constructed to express the full-length NY-ESO-1 gene. The first vector was used to transduce antigen-presenting cells (APCs) with NY-ESO-1 and stimulate memory effector T cells from cancer patients, and the second was used to induce antigen expression in B cells, the targets for measuring T cell responses. Using this set of non-cross-reactive recombinant viruses, we recalled and characterized CD8+ T cell responses against naturally processed NY-ESO-1 epitopes.
Materials and Methods
Tumor Typing for NY-ESO-1 mRNA.
Expression of NY-ESO-1 mRNA in tumor specimens was assessed by reverse transcription–PCR, using previously described primers (1).
Assays for NY-ESO-1 Antibody.
NY-ESO-1 serum antibodies were assayed by ELISA and Western blots, using NY-ESO-1 recombinant protein purified from Escherichia coli (2).
Generation of Viral Vectors.
Adenoviral constructs Ad2/EV (empty vector) and Ad2/EGFP (encoding green fluorescent protein) have been described previously (9). Adenovirus recombinant for NY-ESO-1 (Ad2/ESO) was generated with a cloning method described previously (10). Briefly, pBK-CMV NY-ESO-1 (1) was digested with EcoRI and XbaI, and an 0.8-kb NY-ESO-1 fragment was isolated and cloned into EcoRI and XbaI sites of pSV2-ICEU-1 pAd Quick shuttle vector. The shuttle plasmid pSV2-ICEU-1 NY-ESO-1 was digested with ICEU-1, and the expression cassette comprising cytomegalovirus promoter/enhancer, NY-ESO-1 cDNA, and BGH poly(A) sequence was isolated and cloned into the unique ICEU-1 site of pAd Quick plasmid. Finally, the recombinant adenovirus was generated by transfecting 293 cells with SnaBI-digested pAd Quick NY-ESO-1 plasmid.
The vaccinia/NY-ESO-1 recombinant virus contains the full-length NY-ESO-1 cDNA (1) under the control of the vaccinia virus 40K promoter (11) and the E. coli lacZ gene under the control of the fowlpox virus C1 promoter (12). The foreign sequences are inserted into the thymidine kinase gene, located in the HindIII J region of the genome of the vaccinia virus Wyeth strain, using techniques as described (13).
Cell Lines.
The following lymphoblastoid B cells immortalized by Epstein–Barr virus (EBV) (EBV-B cells) were generated from healthy donor peripheral blood lymphocytes (PBLs) and typed for HLA class I: 9-EBV (A*0101,*0301; B*1501,*4402; Cw*0304,*0704), 10-EBV (A*3001,*3301; B*4501,*5301; Cw*0602), 19-EBV (A*0201,*2402; B*2705,*3701; Cw*0202,*0602), 20-EBV (A*0301,*2301; B*0702,*4403; Cw*0401,*0702), 21-EBV (A*2402,*3101; B*1501,*2705; Cw*0202,*0303), 26-EBV (A*0101,*0201; B*0801,*5701; Cw*0602,*0701), and 32-EBV (A*0101,*0201; B*0801,*1501; Cw*0303,*0701). Established human melanoma cell lines were obtained from the cell bank maintained at the New York Branch of the Ludwig Institute. All melanoma cell lines studied expressed NY-ESO-1 mRNA, except SK-MEL-23, and all expressed the HLA-A2 molecule, except SK-MEL-19. Stable transfectants of HLA class-I-negative 721.221 cells with plasmids encoding HLA-Cw*0304 or HLA-Cw*0401 genes were kindly provided by Peter Parham (Stanford University, Stanford, CA).
Peptides.
Long peptides spanning the entire sequence of NY-ESO-1 and consisting of 18 amino acids with overlapping sequences have been described (7). Shorter peptides from NY-ESO-1, including previously described HLA-A2-restricted peptide p157–165 (SLLMWITQC) (3), and two peptides described in this study, HLA-Cw3-restricted p92–100 (LAMPFATPM) and HLA-Cw6-restricted p80–88 (ARGPESRLL), were synthesized by Multiple Peptide Systems (San Diego), with a purity of >95%, as determined by reverse-phase HPLC.
In Vitro Sensitization with Peptides and Adenoviral Constructs.
PBLs were collected from cancer patients in sample sizes as low as 10 million cells. CD8+ T lymphocytes were separated from PBLs by antibody-coated magnetic beads (Minimacs; Miltenyi Biotec, Auburn, CA) and seeded into round-bottomed 96-well plates (Corning) at a concentration of 5 × 105 cells per well in RPMI medium 1640 supplemented with 10% human AB serum (NABI, Boca Raton, FL), l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), and 1% nonessential amino acids. As APCs, PBLs depleted of CD8+ T cells were either pulsed with 10 μM peptide or infected with adenoviral constructs at 1000 infectious units per cell, overnight at 37°C in 300 μl serum-free medium (X-VIVO-15; BioWhittaker). Pulsed or infected APCs were then washed, irradiated, and added to the plates containing CD8+ T cells, at a concentration of 1 × 106 APCs per well. After 8 h, IL-2 (10 units/ml; Roche Molecular Biochemicals) and IL-7 (20 ng/ml; R & D Systems) were added to culture wells, and this step was repeated every 3 days, until the cells were harvested for testing (see text).
Tetramer Synthesis.
HLA-A2 tetrameric complexes were synthesized as described (4, 14). Tetramers were assembled with NY-ESO-1-derived peptide p157–165.
Tetramer Assay and Cell Sorting.
Presensitized CD8+ T cells were stained in 50 μl PBS containing 1% FCS with phycoerythrin-labeled tetramer for 15 min at 37°C before the addition of Tricolor-CD8 mAb (Caltag, South San Francisco, CA) for 15 min on ice. After washing, results were analyzed by flow cytometry (FACSCalibur; Becton Dickinson). Cell sorting was performed after tetramer staining by flow cytometry (MoFlo; Cytomation, Fort Collins, CO). Subsequent culture of tetramer-positive and tetramer-negative populations was performed by stimulating sorted cells with allogeneic feeder PBL in the presence of 0.1 μg/ml phytohemagglutinin (0.1 μg/ml; Murex Diagnostics, Dartford, U.K.), IL-2 (10 units/ml), and IL-7 (20 ng/ml).
Target Cells.
EBV-B cells (5 × 105) were either pulsed with 10 μM peptide or infected at 30 plaque-forming units per cell with vaccinia virus wild-type or recombinant for NY-ESO-1, in 300 μl serum-free medium overnight (X-VIVO-15).
Elispot Assay.
Flat-bottomed, 96-well nitrocellulose plates (Millititer; Millipore) were coated with IFN-γ mAb (2 μg/ml, 1-D1K; MABTECH, Stockholm) and incubated overnight at 4°C. After the plates were washed with PBS, they were blocked with 10% human AB serum for 1 h at 37°C. Presensitized CD8+ T cells (1 × 103 to 5 × 104) and 5 × 104 target cells (peptide-pulsed or vaccinia-infected EBV-B or tumor cells) were added to each well and incubated for 20 h in RPMI medium 1640 lacking IL-2 and human serum. Plates were then washed thoroughly with PBS to remove cells, and IFN-γ mAb (0.2 μg/ml, 7-B6-1-biotin; MABTECH) was added to each well. After incubation for 2 h at 37°C, plates were washed and developed with streptavidin-alkaline phosphatase (1 μg/ml; MABTECH) for 1 h at room temperature. After washing, substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma) was added and incubated for 5 min. After final washes, plate membranes displayed dark-violet spots that were counted under the microscope.
Intracellular Staining of Cytokines (Cytospot).
The protocol was adapted from Jung et al. (15). Presensitized CD8+ T cells were incubated with peptide-pulsed or vaccinia-infected EBV-B targets at a 1:2 ratio in 300 μl serum-free medium (X-VIVO-15) for 30 min at 37°C. Brefeldin-A (Sigma) was added to the samples at 10 μg/ml for an additional 5 h. Cells were fixed, permeabilized (Becton Dickinson), and stained with Tricolor-labeled CD8 mAb (Caltag, CA), FITC-labeled IFN-γ mAb, and phycoerythrin-labeled tumor necrosis factor-α mAb (PharMingen) at room temperature for 15 min. Results were analyzed by flow cytometry by gating on CD8+ lymphocytes.
Cytotoxicity Assay.
NW29-EBV cells were labeled with Na 51CrO4 (DuPont) for 1 h and pulsed with NY-ESO-1 peptide p92–100 in dilutions ranging from 10 μM to 0.1 nM. After washing, labeled cells were incubated with CD8+ T cells at a 10:1 effector-to-target cell ratio for 4 h at 37°C in round-bottomed 96-well plates. Radioactivity in the supernatant was measured as described (4).
Results
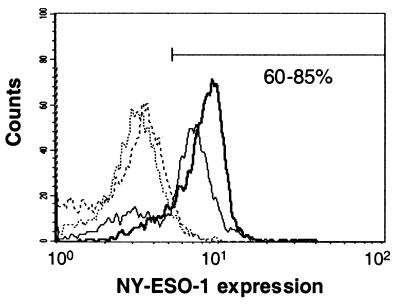
A range of variables were tested for the optimal in vitro sensitization of T cells by APCs infected with NY-ESO-1 recombinant adenovirus. Positively selected CD8+ T cells appeared to be more potent effectors than did whole PBL populations. As stimulators, PBLs depleted of CD8+ cells were equivalent to dendritic cells differentiated from adherent peripheral cells. CD8-depleted PBLs were thus used as APCs for infection with adenoviral constructs at a high multiplicity of infection. Immunostaining with anti-NY-ESO-1 mAb showed that up to 85% of infected PBLs expressed the recombinant protein (Fig. 1).
Figure 1.
NY-ESO-1 expression in PBLs infected with recombinant adenovirus. PBLs from a healthy donor (bold lines) and from a cancer patient (thin lines) were infected overnight with 1,000 infectious units per cell of adenovirus recombinant for NY-ESO-1 (solid lines) or with adenoviral empty vector (dashed lines). After permeabilization, cells were stained with 1 μg/ml mAb ES121 specific for NY-ESO-1 and analyzed by flow cytometry.
Sensitization with Recombinant Adenovirus Recalls Specific Responses to Known NY-ESO-1 T Cell Epitopes.
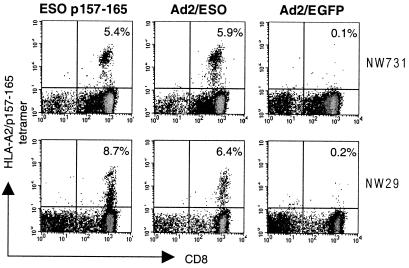
We first confirmed that stimulation with NY-ESO-1 recombinant adenovirus (Ad2/ESO)-infected APC was comparable to that with NY-ESO-1 peptide. Fig. 2 shows tetramer staining of sensitized cells from two melanoma patients, NW29 and NW731 (see Table 1 for patient characteristics), previously known to have a spontaneous T cell response against HLA-A2-restricted NY-ESO-1 peptide 157–165 (4). Frequencies of tetramer-positive populations were equivalent after 8 days of stimulation with NY-ESO-1 peptide or NY-ESO-1 recombinant adenovirus. The response was specific for the NY-ESO-1 sequence, as stimulation with APC infected with adenovirus encoding green fluorescent protein gave negative tetramer staining results (Fig. 2).
Figure 2.
Staining of presensitized CD8+ T cells with HLA-A2/NY-ESO-1 p157–165 tetrameric complexes. CD8+ T cells from patient NW731 (Upper) and patient NW29 (Lower) were stimulated for 8 days with autologous CD8-depleted PBLs either pulsed with NY-ESO-1 peptide 157–165 (Left) or infected with recombinant adenovirus encoding NY-ESO-1 (Center) or with adenovirus encoding green fluorescence protein (Right). Values indicate the percentage of tetramer-positive CD8+ T cells.
Table 1.
Patient characteristics
| Patient code | Cancer type | HLA class I typing | NY-ESO-1
|
|
|---|---|---|---|---|
| mRNA† | Ab status‡ | |||
| NW29 | Melanoma | A*0206,*2301; B*2705,*4403; Cw*0303,*0401 | + | + |
| NW731 | Melanoma | A*0201,*0301; B*0702,*1501; Cw*0304,*0702 | + | + |
| NW903 | Melanoma | A*0201,*1101; B*1501; Cw*0303 | + | + |
| NW634 | Melanoma | A*0101,*2402; B*0801,*3701; Cw*0602,*0701 | + | + |
| NW31 | Melanoma | A*2501,*2601; B*0801,*1801; Cw*0701,*1203 | + | − |
| NW46 | Melanoma | A*0201,*0301; B*0702,*2705; Cw*0202,*0702 | + | − |
†mRNA expression in tumor cells.
Antibodies against NY-ESO-1 in the serum.
Sensitized Effectors Recognize Naturally Processed NY-ESO-1.
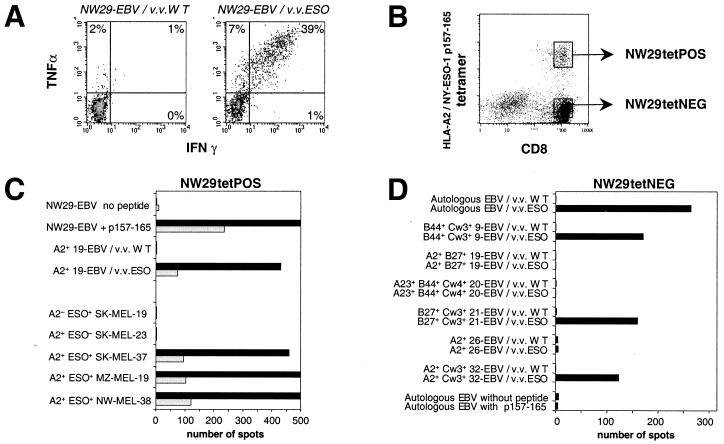
Effectors from patient NW29 stimulated with Ad2/ESO were able to produce high amounts of IFN-γ and tumor necrosis factor-α in response to autologous EBV-B cells infected with vaccinia virus recombinant for NY-ESO-1, but not in response to EBV-B cells infected with wild-type vaccinia (Fig. 3A). There was no cross-reaction between the adenoviral vector used for sensitization and the vaccinia vector used for read-out. Sensitized effectors had typical characteristics of activated memory T cells, with high expression of CD45RO and low CD62L, and could be maintained for over a month in culture without restimulation. Tetramer-positive CD8+ T cells from NW29 Ad2/ESO sensitization were sorted by flow cytometry (Fig. 3B). Subsequent culture of monospecific polyclonal line NW29tetPOS showed that it specifically recognized NY-ESO-1 p157–165 (Fig. 3C). Analysis of line NW29tetPOS also demonstrated that stimulation with NY-ESO-1 recombinant adenovirus could recall effector cells specifically recognizing HLA-A2+ tumor cells expressing NY-ESO-1 (Fig. 3C).
Figure 3.
Characterization of CD8+ T cells from patient NW29 after stimulation with autologous CD8-depleted PBLs infected with Ad2/ESO. (A) Cytospot analysis of IFN-γ and tumor necrosis factor-α production by NW29 effectors tested at day 27 against autologous EBV-B cells infected with wild-type vaccinia (Left) or with NY-ESO-1 vaccinia (Right). The cells were gated on CD8+ lymphocytes, and values indicate the percentage of cytokine-producing CD8+ T cells. (B) Tetramer staining of NW29 effectors presensitized for 13 days (see Fig. 2). Two polyclonal lines were derived after sorting of cell populations indicated by gates. (C) Elispot analysis of IFN-γ release by 1000 cells (solid bars) or 200 cells (open bars) from line NW29tetPOS in response to peptide-pulsed autologous EBV-B targets or vaccinia-infected HLA-A2+ histocompatible EBV-B targets, and to five melanoma cell lines (see Materials and Methods). (D) Elispot analysis of IFN-γ release by 5000 cells from line NW29tetNEG in response to a range of EBV-B cells infected with wild-type or NY-ESO-1 vaccinia virus. Only HLA alleles shared between NW29tetNEG effectors and EBV-B targets are indicated.
Spontaneous Responses to NY-ESO-1 T Cell Epitopes.
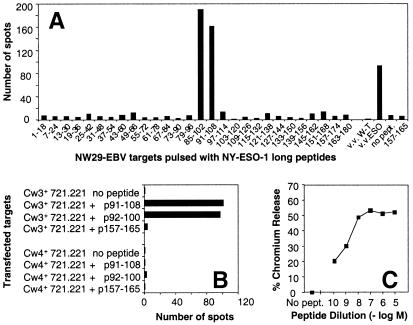
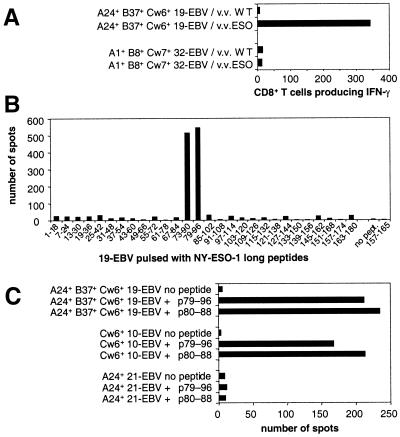
Tetramer-negative CD8+ T cells from NW29 Ad2/ESO sensitization were sorted by flow cytometry, and line NW29tetNEG was obtained (Fig. 3B). Line NW29tetNEG was also capable of recognizing autologous EBV-B cells infected with vaccinia virus recombinant for NY-ESO-1 (Fig. 3D). Thus, there was an additional NY-ESO-1 epitope recognized by CD8+ T cells from patient NW29 distinct from HLA-A2-restricted p157–165. To determine the restriction element of this response, several partially histocompatible EBV-B cells were assayed after infection with NY-ESO-1-recombinant or WT vaccinia viruses. Only HLA-Cw3+ EBV-B cells were capable of presenting NY-ESO-1 to NW29tetNEG cells (Fig. 3D). To define the epitope recognized by these effectors, long overlapping peptides spanning the entire sequence of NY-ESO-1 were pulsed on autologous EBV-B cells and assayed by Elispot (Fig. 4A). Only peptides 85–102 and 91–108 were recognized by NW29tetNEG. Knowledge of anchor motifs for HLA-Cw3 molecules helped designate peptide p92–100 as the minimal epitope (16). This peptide was synthesized and was very well presented by 721.221 cells transfected with HLA-Cw3, but not by 721.221 cells transfected with HLA-Cw4 (Fig. 4B). Serial dilutions of p92–100 showed that it was still recognized at concentrations of less than 1 nM (Fig. 4C). Both HLA-Cw*0303 and HLA-Cw*0304 subtypes could present p92–100.
Figure 4.
Characterization of line NW29tetNEG. (A) Elispot analysis of IFN-γ release by 2,500 cells from line NW29tetNEG in response to autologous EBV-B cells pulsed with long overlapping NY-ESO-1 peptides or infected with vaccinia constructs. (B) Elispot analysis of IFN-γ release by 1,000 cells from line NW29tetNEG in response to 721.221 cells transfected with HLA-Cw3 or HLA-Cw4 and pulsed with NY-ESO-1 peptides. (C) Cytotoxicity assay with line NW29tetNEG against autologous EBV-B targets pulsed with NY-ESO-1 peptide 92–100 ranging from 10 μM to 0.1 nM. The effector-to-target cell ratio was 10:1.
Spontaneous T Cell Responses to NY-ESO-1 in a Non-HLA-A2 Individual.
Patient NW634 was selected for her seropositivity to NY-ESO-1 (Table 1). Effector cells were stimulated in vitro by Ad2/ESO-infected CD8-depleted PBLs. After 9 days of culture, nearly 40% of sensitized CD8+ T cells were capable of specifically producing IFN-γ in response to NY-ESO-1 expressed by vaccinia-infected histocompatible EBV-B cells (Fig. 5A). Long NY-ESO-1 peptides p73–90 and p79–96 were the only ones recognized by NW634 presensitized effectors (Fig. 5B). With the approach described above with partially matching targets, HLA-Cw6 was identified as a restriction element for this response (Fig. 5C). Based on anchor motifs for HLA-Cw6 (16), peptide p80–88 was deduced as a candidate nonamer epitope within the region 73–96. This peptide was synthesized, and its recognition by NW634 effectors in a HLA-Cw*0602-restricted fashion was confirmed (Fig. 5C). It should be noted that activity of p80–88 decreased after storage at 4°C.
Figure 5.
Characterization of CD8+ T cells from patient NW634 after stimulation with autologous CD8-depleted PBLs infected with Ad2/ESO. (A) Cytospot analysis of IFN-γ production by NW634 CD8+ T cell effectors tested at day 11 against partially histocompatible EBV-B cells infected with wild-type or NY-ESO-1 vaccinia virus. Values represent the number of IFN-γ-producing cells per 1,000 CD8+ T cells. HLA alleles shared by effectors and targets are indicated. (B) Elispot analysis of IFN-γ release by 2,500 presensitized NW634 effector cells tested at day 14 in response to histocompatible EBV-B cells pulsed with long overlapping NY-ESO-1 peptides. (C) Elispot analysis of IFN-γ release by 2,000 presensitized NW634 effector cells tested at day 31 in response to different EBV-B target cells pulsed with NY-ESO-1 peptides. HLA alleles shared by effectors and targets are indicated.
Correlation of NY-ESO-1 CD8+ T Cell Responses and Antibody Status.
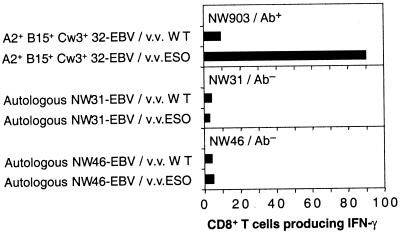
In our previous study of HLA-A2 patients (4), we showed that CD8+ T cell reactivity to NY-ESO-1 was found only in patients with antibodies to NY-ESO-1. To see whether this situation also holds for the adenovirus/vaccinia cross-sensitization procedure, three additional patients with NY-ESO-1-positive tumors were studied, one seropositive and two seronegative (see Table 1). CD8+ T cells from the seropositive patient, NW903, were stimulated with CD8-depleted PBLs infected with Ad2/ESO. NY-ESO-1-specific responses were observed against histocompatible EBV-B cells expressing NY-ESO-1 recombinant vaccinia (Fig. 6). Like patient NW29, both HLA-A2-restricted peptide p157–163 and HLA-Cw3-restricted peptide p92–100 were the targets of this response, although at lower frequencies. Results for the two seronegative patients, NW31 and NW46, are shown in Fig. 6. After Ad2/ESO sensitization of their CD8+ T cells, no responses were observed against autologous EBV-B cells expressing the NY-ESO-1 vaccinia recombinant.
Figure 6.
Cytospot analysis of IFN-γ production by CD8+ T cells from three melanoma patients after stimulation with Ad2/ESO. (Top) Effectors from NY-ESO-1 antibody-positive patient NW903 were tested at day 11 against partially histocompatible EBV-B cells infected with wild-type or NY-ESO-1 vaccinia virus. HLA alleles shared by effectors and targets are indicated. (Middle) Effectors from NY-ESO-1 antibody-negative patient NW31 were tested at day 8 against autologous EBV-B cells infected with wild-type or NY-ESO-1 vaccinia virus. Similar results were obtained at day 15. (Bottom) Effectors from NY-ESO-1 antibody-negative patient NW46 were tested at day 8 against autologous EBV-B cells infected with wild-type or NY-ESO-1 vaccinia virus. Similar results were obtained at day 15. Values represent the number of IFN-γ-producing cells per 2,000 CD8+ T cell effectors.
Discussion
Over the past 10 years, the definition of peptide epitopes recognized by T lymphocytes has provided the basis for detailed studies of T cell immunity to human tumor antigens (17). Peptides recognized in the context of HLA-A2 have received the most attention, and a substantial number of HLA-A2-restricted peptides encoded by melanocyte differentiation antigens (18–21), mutational antigens (22), overexpressed antigens (23, 24), and cancer/testis antigens, such as MAGE-3 (25) and NY-ESO-1 (3), have been defined. Although HLA-A2 is a common allele in populations from Caucasian and African descent, it accounts for less than half of patients who might benefit from monitoring and immunointervention. Thus, there is a need to broaden our knowledge of non-HLA-A2-restricted tumor peptides. In addition, a large number of identified tumor gene products have been defined by serological analysis of a recombinant cDNA expression library, and the profiles of HLA-A2 and non-HLA-A2-restricted peptides from these so-defined products await analysis (26).
Several approaches have been used to identify HLA-restricted peptides derived from human tumor antigens. Most of our knowledge comes from a general strategy developed by Boon et al. (27) to identify the peptide targets of CD8+ T cells with reactivity for autologous melanoma cells. This approach depends on transferring antigen expression to nonexpressing cells by transfecting target cells with cosmid (28) or cDNA vectors (29) coding for antigen, and, where required, with HLA class Irestriction elements (30). Once the coding sequence for the T cell-recognized tumor antigen has been defined, information about the binding motifs of known HLA-restricted peptides (31) greatly facilitates the identification of peptide targets for the tumor-reactive CD8+ T cell lines or clones. Another approach to defining T cell-recognized peptide epitopes was developed by Hunt et al. (32) and involves the elution of peptides from HLA molecules expressed by tumor cells, fractionation of peptides by HPLC, and structural identification of the T cell-recognized peptides. This method has been successfully used to identify gp100 (32), tyrosinase (33), and Melan-A/MART-1 peptides (34), but the technical challenges involved in this approach have precluded its more widespread application. With regard to the definition of peptide targets of known tumor antigens, infecting target cells with viral vectors coding for antigen has also proved of value, both in inducing a de novo specific response by naïve T cells (35, 36) and in stimulating and expanding in vivo sensitized T cells (37–40). These T cells can then be used to identify the naturally processed tumor peptides eliciting a T cell response.
We had two objectives in the present study: (i) to develop a general method to monitor CD8+ T cell responses to NY-ESO-1 in patients of any HLA class I type, even if no HLA-restricted NY-ESO-1 peptides are known, and (ii) to expand the list of NY-ESO-1 peptides recognized by CD8+ T cells. NY-ESO-1 is a particularly favorable antigen to study in this regard, because of its strong inherent immunogenicity in a high proportion of patients and because serum antibodies to NY-ESO-1 predict strong CD8+ T cell responses in HLA-A2 patients (4), and so can be used to select non-HLA-A2 patients for analysis. The approach we took involved stimulating CD8+ T lymphocytes from seropositive melanoma patients with CD8-depleted PBLs expressing NY-ESO-1 after infection with recombinant adenovirus. This nonreplicative adenoviral vector has high general infectability and low cellular toxicity. Sensitized effectors were assayed against autologous or histocompatible EBV-B target cells infected with vaccinia recombinants for NY-ESO-1. A strong polyclonal CD8+ T cell response to NY-ESO-1 was elicited by Ad2/ESO in antibody-positive patients. No response was detected by adenovirus/vaccinia cross-sensitization in individuals without antibody to NY-ESO-1. Thus, the relationship between antibody status and T cell reactivity to NY-ESO-1, previously described in HLA-A2 patients (4), also appears to be relevant in patients with other HLA class I alleles. Finally, using this approach, we defined two spontaneously processed peptides from NY-ESO-1, one presented by HLA-Cw3 and the other by HLA-Cw6, both of which are immunogenic in vivo.
The two HLA-C-restricted NY-ESO-1 peptides can be added to the list of HLA-C-restricted epitopes described for other cancer/testis antigens, like MAGE-1 (35, 41), MAGE-12 (42), BAGE (43), and GAGE-1 (44). Some HLA-C alleles have been shown to be the ligands of inhibitory receptors expressed by natural killer cells and a subset of T cells (45, 46). We are now investigating the possibility that peptides from NY-ESO-1 may alter the interaction with inhibitory molecules and possibly trigger a response from the innate arm of the immune system, adding a further dimension to the integrated immune response to NY-ESO-1.
Expanding the list of NY-ESO-1 peptides recognized by CD8+ T cell opens the way to addressing some critical questions related to NY-ESO-1 immunity. As the T cell response to NY-ESO-1 is polyclonal and can be simultaneously directed against two or more epitopes in a single patient, issues such as epitope immunodominance, epitope eclipse, epitope tolerance, and linkage of NY-ESO-1 responses to HLA class I and II alleles can be analyzed. In addition, the relation between immune response or lack of response to specific NY-ESO-1 epitopes and the emergence of escape variants of NY-ESO-1-positive tumor cells can be examined.
In light of clinical trials involving vaccination with NY-ESO-1 protein and viral recombinants, the approach described here should allow the monitoring of CD8+ T cell responses in all immunized patients, without requiring selection based on HLA alleles. More generally, it should prove useful for defining spontaneous T cell responses against the growing list of tumor antigens defined by serological analysis of a recombinant cDNA expression library (26) and other methods.
Acknowledgments
We are grateful to Hisashi Wada for establishing immortalized B cell lines from healthy donor PBLs. We thank Julia Karbach for PBL separation from patient blood. We further thank Thomas Delohery and Patrick Anderson for help with cell sorting. V.C. and P.R.D. received support from the Cancer Research Institute and the Cancer Research Campaign. B.D. was supported by National Institutes of Health Grants CA08748 and CA23766.
Abbreviations
- PBL
peripheral blood lymphocyte
- APC
antigen-presenting cell
- Elispot
enzyme-linked immunospot
- Cytospot
intracellular staining of cytokines by flow cytometry
- Ad2/ESO
NY-ESO-1 recombinant adenovirus
- Ad2/EGFP
green fluorescence protein recombinant adenovirus
- EBV
Epstein–Barr virus
- EBV-B
lymphoblastoid B cell line immortalized by EBV
References
- 1.Chen Y-T, Scanlan M J, Sahin U, Türeci Ö, Güre A O, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old L J. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockert E, Jäger E, Chen Y-T, Scanlan M J, Gout I, Karbach J, Arand M, Knuth A, Old L J. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jäger E, Chen Y-T, Drijfhout J W, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, et al. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar P R, Lee S Y, Jungbluth A, Jäger D, et al. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J-L, Dunbar P R, Gileadi U, Jäger E, Gnjatic S, Nagata Y, Stockert E, Panicali D L, Chen Y-T, Knuth A, et al. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 6.Valmori, D., Dutoit, V., Liénard, D., Rimoldi, D., Pittet, M. J., Champagne, P., Ellefsen, K., Sahin, U., Lejeune, F., Cerottini, J.-C., et al. (2000) Cancer Res., in press. [PubMed]
- 7.Jäger E, Jäger D, Karbach J, Chen Y-T, Ritter G, Nagata Y, Gnjatic S, Stockert E, Arand M, Old L J, et al. J Exp Med. 2000;191:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R F, Johnston S L, Zeng G, Topalian S L, Schwartzentruber D J, Rosenberg S A. J Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]
- 9.Kaplan J M, Yu Q, Piraino S T, Pennington S E, Shankara S, Woodworth L A, Roberts B L. J Immunol. 1999;163:699–707. [PubMed] [Google Scholar]
- 10.Souza D W, Armentano D. BioTechniques. 1999;26:502–508. doi: 10.2144/99263rr01. [DOI] [PubMed] [Google Scholar]
- 11.Gritz L, Destree A, Cormier N, Day E, Stallard V, Caiazzo T, Mazzara G, Panicali D. J Virol. 1990;64:5948–5957. doi: 10.1128/jvi.64.12.5948-5957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins S, Gritz L, Fedor C H, O'Neill E M, Cohen L K, Panicali D L. AIDS Res Hum Retroviruses. 1991;7:991–998. doi: 10.1089/aid.1991.7.991. [DOI] [PubMed] [Google Scholar]
- 13.Mazzara G P, Destree A, Mahr A. Methods Enzymol. 1993;217:557–581. doi: 10.1016/0076-6879(93)17089-n. [DOI] [PubMed] [Google Scholar]
- 14.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Falk K, Rötzschke O, Grahovac B, Schendel D, Stevanovic S, Gnau V, Jung G, Strominger J L, Rammensee H G. Proc Natl Acad Sci USA. 1993;90:12005–12009. doi: 10.1073/pnas.90.24.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boon T, Old L J. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 18.Brichard V, Van Pel A, Wölfel T, Wölfel C, De Plaen E, Lethé B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulie P G, Brichard V, Van Pel A, Wölfel T, Schneider J, Traversari C, Mattei S, De Plaen E, Lurquin C, Szikora J P. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins P F, Rivoltini L, Yannelli J R, Appella E, Rosenberg S A. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins P F, Sette A, Appella E, Rosenberg S A. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 22.Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Buschenfelde K H, Beach D. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 23.Cheever M A, Disis M L, Bernhard H, Gralow J R, Hand S L, Huseby E S, Qin H L, Takahashi M, Chen W. Immunol Rev. 1995;145:33–59. doi: 10.1111/j.1600-065x.1995.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 24.Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet J-G, Choppin J. J Immunol. 1998;160:328–333. [PubMed] [Google Scholar]
- 25.van der Bruggen P, Bastin J, Gajewski T, Coulie P G, Boël P, De Smet C, Traversari C, Townsend A, Boon T. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y-T, Scanlan M J, Obata Y, Old L J. In: Biologic Therapy of Cancer. de Vita V T, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 2000. pp. 557–570. [Google Scholar]
- 27.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Bruggen P, Traverseri C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1650. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Sakaguchi K, Appella E, Yannelli J R, Adema G J, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Plaen E, Lurquin C, Lethé B, van der Bruggen P, Brichard V, Renauld J C, Coulie P, Van Pel A, Boon T. Methods. 1997;12:125–142. doi: 10.1006/meth.1997.0462. [DOI] [PubMed] [Google Scholar]
- 31.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee H-G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 32.Cox A L, Skipper J, Chen Y, Henderson R A, Darrow T L, Shabanowitz J, Engelhard V H, Hunt D F, Slingluff C L., Jr Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 33.Skipper J C, Hendrickson R C, Gulden P H, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wölfel T, Slingluff C L, Jr, Boon T, et al. J Exp Med. 1996;183:527–234. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castelli C, Storkus W J, Maeurer M J, Martin D M, Huang E C, Pramanik B N, Nagabhushan T L, Parmiani G, Lotze M T. J Exp Med. 1995;181:363–368. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, Russo V, Schultz E, Cornelis G R, Boon T, et al. J Immunol. 1999;163:2928–2936. [PubMed] [Google Scholar]
- 36.Butterfield L H, Jilani S M, Chakraborty N G, Bui L A, Ribas A, Dissette V B, Lau R, Gamradt S C, Glaspy J A, McBride W H, et al. J Immunol. 1998;161:5607–5613. [PubMed] [Google Scholar]
- 37.Toso J F, Oei C, Oshidari F, Tartaglia J, Paoletti E, Lyerly H K, Talib S, Weinhold K J. Cancer Res. 1996;56:16–20. [PubMed] [Google Scholar]
- 38.Yee C, Gilbert M J, Riddell S R, Brichard V G, Fefer A, Thompson J A, Boon T, Greenberg P D. J Immunol. 1996;157:4079–4086. [PubMed] [Google Scholar]
- 39.Kim C J, Prevette T, Cormier J, Overwijk W, Roden M, Restifo N P, Rosenberg S A, Marincola F M. J Immunother. 1997;20:276–286. doi: 10.1097/00002371-199707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, et al. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 41.van der Bruggen P, Szikora J P, Boel P, Wildmann C, Somville M, Sensi M, Boon T. Eur J Immunol. 1994;24:2134–2140. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- 42.Panelli M C, Bettinotti M P, Lally K, Ohnmacht G A, Li Y, Robbins P, Riker A, Rosenberg S A, Marincola F M. J Immunol. 2000;164:4382–4392. doi: 10.4049/jimmunol.164.8.4382. [DOI] [PubMed] [Google Scholar]
- 43.Boël P, Wildmann C, Sensi M L, Brasseur R, Renauld J C, Coulie P, Boon T, van der Bruggen P. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 44.Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanier L L. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 46.Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, Mingari M C, Moretta L. Immunol Rev. 1997;155:105–117. doi: 10.1111/j.1600-065x.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]