Abstract
Background and Aims
Plants are naturally exposed to multiple, frequently interactive stress factors, most of which are becoming more severe due to global change. Established plants have been reported to facilitate the establishment of juvenile plants, but net effects of plant–plant interactions are difficult to assess due to complex interactions among environmental factors. An investigation was carried out in order to determine how two dominant evergreen shrubs (Quercus ilex and Arctostaphylos uva-ursi) co-occurring in continental, Mediterranean habitats respond to multiple abiotic stresses and whether the shaded understorey conditions ameliorate the negative effects of drought and winter frosts on the physiology of leaves.
Methods
Microclimate and ecophysiology of sun and shade plants were studied at a continental plateau in central Spain during 2004–2005, with 2005 being one of the driest and hottest years on record; several late-winter frosts also occurred in 2005.
Key Results
Daytime air temperature and vapour pressure deficit were lower in the shade than in the sun, but soil moisture was also lower in the shade during the spring and summer of 2005, and night-time temperatures were higher in the shade. Water potential, photochemical efficiency, light-saturated photosynthesis, stomatal conductance and leaf 13C composition differed between sun and shade individuals throughout the seasons, but differences were species specific. Shade was beneficial for leaf-level physiology in Q. ilex during winter, detrimental during spring for both species, and of little consequence in summer.
Conclusions
The results suggest that beneficial effects of shade can be eclipsed by reduced soil moisture during dry years, which are expected to be more frequent in the most likely climate change scenarios for the Mediterranean region.
Key words: Frost, climate change, shade, drought, plant–plant interactions, Quercus ilex, Arctostaphylos uva-ursi, soil moisture, facilitation
INTRODUCTION
Global climate change is bringing to Mediterranean regions not only a general increase of temperatures but also an increased aridity and a higher frequency of extreme climatic events such as heat waves and late-winter frosts (Diffenbaugh et al., 2005; Christensen et al., 2007). Responses of Mediterranean plants to summer stresses (i.e. drought, heat and high light) have been intensively studied (see Sánchez-Gómez et al., 2006; Allards et al., 2008, and references therein), but the impacts of low temperatures remain poorly understood (Egerton et al., 2000; Cavender-Bares et al., 2005; Pratt et al., 2005). The effects of freezing temperatures on Mediterranean-type vegetation deserve more attention, not only due to their highly deleterious effects (Ball, 1991; Larcher, 2000; Bertrand and Castonguay, 2003; Rochette et al., 2004; Pratt et al., 2005) but also because episodes of late-winter frost may be disproportionately important in plant evolution (Inouye, 2000; Agrawal et al., 2004). In general, plants handle one environmental stress and its typical correlates (e.g. drought and heat), but polytolerance (i.e. the capacity to cope with more than one distinct stress, such as shade and drought) seems to be very rare in the woody flora of the Northern Hemisphere due to functional trade-offs and constraints (Niinemets and Valladares, 2006). Continental, Mediterranean regions impose multiple, uncorrelated stresses, with climate change exacerbating them and representing, thus, a complex challenge for plants dwelling in these ecosystems. However, at present, our understanding of the impacts of climate change is limited by fragmentary knowledge of how plants cope with several interacting stresses (Aranda et al., 2005; Davis et al., 2005; Mittler, 2006; Valladares et al., 2007; Valladares, 2008).
Plants living in adverse habitats can benefit from facilitation by neighbours (Callaway, 1995). Shade and improved soil conditions under the canopy are mechanisms by which established plants facilitate other plants (Pugnaire et al., 2004; Gómez-Aparicio et al., 2005). However, the notion that facilitation among plants increases with stress (the stress-gradient hypothesis; Bertness and Callaway, 1994) has been challenged in arid environments, where the empirical evidence reveals a more complex picture (e.g. Hastwell and Facelli, 2003; Maestre et al., 2006; Brooker et al., 2008). Similarly, the notion that facilitation increases during adverse years has also been challenged (Tielborger and Kadmon, 2000; Maestre et al., 2004). Increasing severity of droughts in Mediterranean-type ecosystems has been predicted to lead to an increasing role for facilitation, but the current uncertainties surrounding the models upon which such predictions are based, and the complexities of the responses to multiple stresses, indicate that further research is clearly needed (Brooker, 2006; Valladares et al., 2007). The issue is particularly timely because plant–plant interactions are an important part of the response of plant communities to global change (Damgaard, 2005; Brooker, 2006).
The aim of the present study was to compare the ecophysiological performance of dominant, evergreen woody species growing in sun and shade microhabitats at a dry, continental Mediterranean site. The study was carried out during 2004 and 2005; 2005 was a particularly adverse year, being one of the driest and hottest years of the last 60 years. In addition, the winter of 2005 contained unusually late frost events. The experiments conducted here sought to establish the extent to which shade mitigated the impacts of drought, heat and freezing stresses. The species studied were Holm oak (Quercus ilex subsp. ballota), a dominant tree or shrub in the western Mediterranean Basin that is of Tertiary/tropical origin, and bearberry (Arctostaphylos uva-ursi), a widespread circumpolar dwarf shrub, restricted to mountain systems and continental sites in its southern range. Although both species normally spread vegetatively, seedlings can establish, particularly in shaded microhabitats to which they are typically dispersed (Stiles, 1980; Gómez et al., 2004). The species differ in shade tolerance, with Q. ilex being more tolerant of shade than A. uva-ursi (Niinemets and Valladares, 2006). The hypotheses here were: (a) the two species both exhibit maximal carbon gain during spring and minimal carbon gain during the summer drought and hea, as expected from long-term monitoring of carbon balance in these ecosystems (e.g. Allards et al. 2008); (b) shade is beneficial over winter by conferring protection against frosts and detrimental in summer due to its association with water depletion by canopy trees; and (c) species differ in their tolerance to the interactive effects of drought and extremes of temperature and irradiance, and thus in their performance in the two microhabitats. Shaded microhabitats are expected to enhance physiological performance, survival and growth by reducing the impact of abiotic stresses as suggested by a number of studies in arid ecosystems (see Flores and Jurado, 2003; Prider and Facelli, 2004, and references therein; Gómez-Aparicio et al., 2005). However, since shaded sites can be drier than sunny sites due to rainfall interception and extreme water depletion from the soil by canopy plants, particularly during adverse years (Tielborger and Kadmon, 2000; Valladares and Pearcy, 2002), it was hypothesized (d) that shade does not enhance physiological performance during adverse years, but, on the contrary, performance in the shade may be worse than in the sun when climatic conditions become exceptionally unfavourable.
MATERIALS AND METHODS
Study site
The study was carried out during 2004–2005 at Los Cerrillos Biological Station, Villar de Cobeta (40°48′N, 2°12′W), within the Alto Tajo Natural Park (Guadalajara, Spain). Climate is dry, continental Mediterranean, with contrasting temperatures both over the year and during the day, and a pronounced summer drought (Fig. 1). Climatic records from two meteorological stations (Molina de Aragón, Guadalajara, and Teruel, run by the Spanish Institute of Meteorology) revealed that 2005 was one of the five driest and hottest of the last 60 years, and exhibited unusually long frosts during the late winter–spring transition. While precipitation in the area typically averages 450 mm year−1, it was only 280 mm at the field site during the study period. The site is at a mean elevation of 950 m a.s.l., with steep slopes and cliffs covered with Mediterranean shrublands, and 2–5 m high open forests dominated by Holm oak, Q. ilex, L. subsp. ballota (Desf.) Samp. on south-facing slopes and Pinus nigra on north-facing slopes. The upper parts of the plateaus are dominated by Juniperus thurifera, Pinus sylvestris and Buxus sempervirens. Soils are basic, rocky and poorly developed over the limestone bedrock.
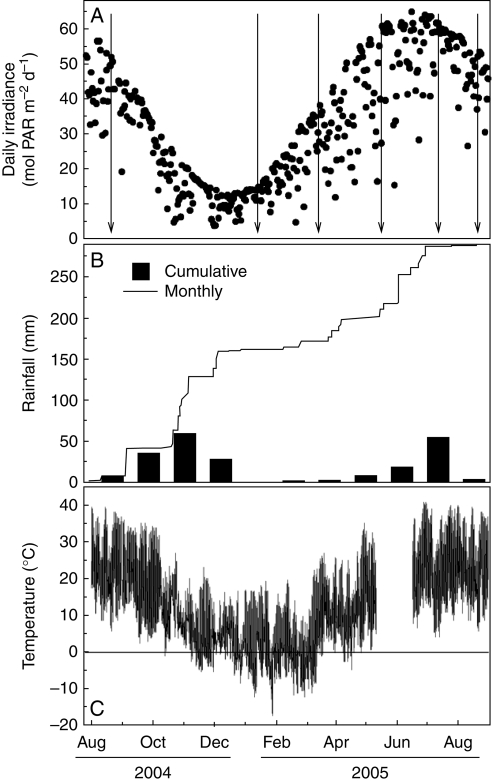
Fig. 1.
(A) Daily irradiance, (B) cumulative and monthly rainfall, and (C) air temperature over the study period (August 2004–September 2005) at an open location in the study site. The vertical arrows in the top panel indicate the six field campaigns.
Microclimatic measurements
One micro-meteorological station was installed in an open site (referred to as sun habitat hereafter) and another one was placed in the understorey of a Q. ilex forest (referred to as shade habitat hereafter). Each meteorological station was installed in June 2004 and included sensors for air temperature and relative humidity (Hobo H08-032-08, Onset, Pocasset, MA), soil moisture (ECH2O EC-20, Decagon Devices, Pullman, WA) and solar irradiance (Apogee quantum sensor QSO-SUN, Logan, UT), cross-calibrated with a Li-Cor SA Li-190 quantum sensor (Li-Cor, Lincoln, NE). Even though sensors were non-aspirated, biases in temperature and humidity were thought to be moderate due to the existence of a constant breeze even in the understorey. Readings of each sensor were recorded every 10 min with a Hobo H08-006-04 data-logger. Precipitation was recorded in the open with a Rain-o-matic small rain gauge (Pronamic Co. Ltd, Silkeborg, Denmark) attached to a Hobo H7 event data logger. Two additional data loggers were installed to record leaf temperature measured with sensors attached to the abaxial side of leaves of four plants in each light environment.
Twelve individual plants of both oak and bearberry target species [Q. ilex and A. uva-ursi (L.) Spreng.] were randomly selected and tagged in each habitat during the first field campaign. Selected individuals of Q. ilex were small shrubs 20–110 cm high, while those of A. uva-ursi were prostrate mats of 40–260 cm in diameter. All individuals were non-reproductive at the time of measurements, and were ≥10 years old; each individual was functionally independent and were not sprouts from adult trees. Hemispherical photographs were taken over each shade plant to determine the fraction of global irradiance (i.e. direct plus diffuse radiation) reaching the plants sampled in the understorey. Photographs were taken using a horizontally levelled digital camera (CoolPix 4500, Nikon, Tokyo, Japan), mounted on a tripod and aimed at the zenith, using a fish-eye lens of 180 ° field of view (FCE8, Nikon). Photographs were analysed for canopy openness using Hemiview canopy analysis software version 2.1 (1999, Delta-T Devices Ltd, Cambridge, UK). Daily irradiance in the shade was calculated by multiplying daily irradiance in the open by the global site factor obtained with Hemiview. Further details on the protocol for hemispherical photography are provided in Valladares and Guzmán (2006). The main microclimatic features of sun and shade habitats are shown in Fig. 2 and Table 1.
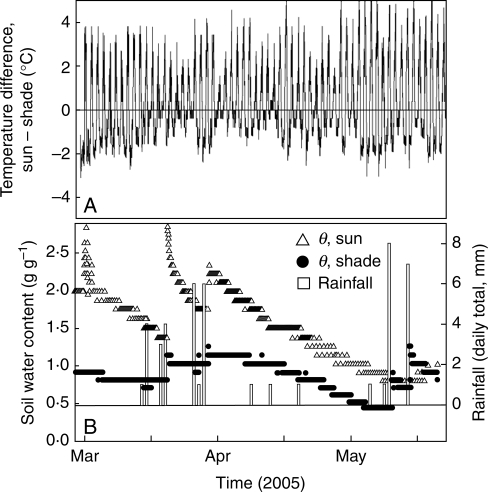
Fig. 2.
Microclimatic differences between sun and shade habitats. (A) Air temperature difference between sun and shade habitats. (B) Soil water content (θ) in the sun and in the shade, and rainfall events (daily totals). Only data for the winter–summer transition are shown for clarity. Each mark on the x-axis represents 2 weeks.
Table 1.
Solar irradiance [photosynthetically active radiation (PAR) integrated over the day], minimum relative humidity and maximum vapour pressure deficit (VPD) of the air in sun and shade habitats for the 2004–2005 study period
| Solar irradiance (mol PAR m−2 d−1) |
Minimum relative humidity of air (%) |
Maximum VPD (hPa) |
|||||
|---|---|---|---|---|---|---|---|
| Mean | s.e. | Mean | s.e. | Mean | s.e. | ||
| Summer | Sun | 45·1 | 2·7 | 14·7 | 2·5 | 38·6 | 1·9 |
| Shade | 13·5 | 0·8 | 21·7 | 2·9 | 30·0 | 1·6 | |
| Autumn | Sun | 28·6 | 1·7 | 33·0 | 2·5 | 16·6 | 1·4 |
| Shade | 8·6 | 0·5 | 42·8 | 2·7 | 13·0 | 1·1 | |
| Winter | Sun | 12·5 | 0·8 | 48·0 | 3·0 | 7·2 | 0·5 |
| Shade | 3·8 | 0·2 | 57·1 | 3·0 | 5·7 | 0·5 | |
| Spring | Sun | 37·2 | 2·2 | 17·0 | 1·5 | 25·3 | 1·3 |
| Shade | 11·2 | 0·7 | 26·8 | 1·9 | 18·8 | 1·1 | |
Values are daily totals or daily minima or maxima, and the mean and standard error for each season are provided; values of summer represent the mean for 2004 and 2005 summers.
Soil moisture (θ) in the understorey of each selected plant was determined at two points below the canopy: midway from the main stem and at the edge of the crown. Initially, θ was measured at two depths (20 and 50 cm) but, since these two data sets were highly correlated, with slightly higher values at the lower depth (data not shown), mean values at the upper depth were considered for these campaigns and θ was measured only at 20 cm depth in subsequent campaigns. This estimator of θ can be considered reliable because (a) the plants are juveniles, with relatively shallow roots; and (b) all water is coming from direct precipitation and the rocky soils on the slopes of the study site do not accumulate water for long periods of time so there is no chance for any plant to reach phreatic water. In addition to measuring θ below each plant, four sensors were also buried in the soil at 20 cm depth but avoiding the proximity or direct influence of woody vegetation and rocky patches; sensors integrated θ over 10 cm of the soil profile in the 15–25 cm depth range.
Ecophysiological measurements
A total of six field campaigns were carried out from August 2004 to August 2005 to record plant performance under different climatic conditions (Fig. 1). In each field campaign, soil moisture (θ) under each plant, water potential (Ψ), photochemical efficiency of photosystem II (PSII) (Fv/Fm) and leaf gas exchange were measured with a Theta-probe ML 2X (Delta-T Devices Ltd), a Scholander pressure chamber (self-made, using nitrogen gas), an FMS-2 fluorometer (Hansatech, King's Lynn, UK) and a Li-Cor 6400 photosynthesis system (Li-Cor), respectively. The youngest fully expanded leaves were sampled in August 2004 (leaves emerged in April 2004), and they were followed during winter 2004/2005 and spring 2005; new fully expanded, current year leaves were followed in summer 2005. Ψ was determined before dawn (‘pre-dawn’, 0630–0730 h solar time) in two small twigs (since leaves of the two species have very short petioles) with 2–3 leaves from each plant immediately after excision with a razor blade from the crown. Midday measurements were also taken on the first campaign but not thereafter because midday and pre-dawn values were highly correlated (data not shown). Fv/Fm was measured pre-dawn and at midday in leaves adapted to dark for 30 min using the leaf clips provided by the manufacturer. Net photosynthetic CO2 uptake (Pnet) and stomatal conductance (gs) were determined at different times over the day at prevailing ambient air temperature, and an atmospheric CO2 concentration of 400 µmol mol−1 (using the built-in Li-Cor 6400 CO2 controller). At both sites, Pnet was measured at two irradiances: the prevailing ambient irradiance and at saturating irradiance (800 µmol photons m−2 s−1) using the built-in Li-Cor 6400 blue–red light source. Pnet was measured during three consecutive days in each campaign. The flow rate was set to 500 µmol s−1 and ambient relative humidity [typically 35–55 %, involving vapour pressure deficits (VPDs) from 1·5 to 4 kPa depending on air temperature]. For each sampling period, four replicates were included (using the same plants but different leaves each time since leaves were collected in every sampling period for area and fresh/dry mass determinations) in the sun and shade sites. To account for changes in environmental variables during the course of a day, measurements of leaf gas exchange of individual replicates were alternated between the sun and shade sites over 1–2 h periods (one starting at 1000 h and the other at 1400 h). Sustained aridity and low VPDs over most of the year together with a late sunrise due to the topography of the site prevented plants from achieving maximum gas exchange values before 1000 h. Daily maximum values of Pnet and gs for each plant were used for statistical comparisons and to calculate intrinsic water use efficiency (WUE; Pnet/gs determined at saturating light) and instantaneous WUE (Pnet/transpiration determined at ambient light). Gas exchange data for the first campaign (August 2004) had fewer replicates per species and habitat and were thus discarded for subsequent statistical analyses.
At the end of the last campaign (August 2005), two current-year leaves from the upper and most exposed part of the crown of each selected plant were collected, dried and ground to analyse their carbon isotope composition (δ13C). δ13C is an index of intrinsic WUE integrated over the time of plant organ growth (Dawson et al., 2002). Determination of δ13C was performed on 0·5–1 mg sub-samples of dried and ground materials by combusting at 1020 °C in a Carlo Erba EA1500 NC elemental analyser on-line with a Finnigan Delta Plus XL mass spectrometer. Stable isotope abundance (13C/12C) was expressed in standard δ notation relative to V-PDB [Vienna international standard series supplied by the IAEA vs. the classical calcite standard from Pee Dee Belemnite (PDB)] according to
where R = (13C/12C), of the sample and standards, respectively. All samples for isotope composition were analysed twice and on different days, with two standards every ten samples. Based on numerous measurements of inorganic and organic international reference standards, the analytical precision of the system was about ±0·1 ‰ (1σ).
Statistical analyses
A three-factor analysis of variance (ANOVA; species, habitat and time of the year) was applied to all variables, except δ13C and gas exchange parameters, using the statistical software Statistica 6·1 (2004, StatSoft, Inc., Tulsa, OK). Repeated-measures ANOVA was applied to the data since the same individuals were followed over time. Significant differences between the levels of each factor (two for species and habitat, six for time of the year, except for gas exchange where only five campaigns were considered) were estimated by the Fisher least significant difference (l.s.d.) post hoc test; 95 % confidence intervals for the mean are provided in the graphs, and F- and P-values for main effects and interactions are given in Table 2. In all combinations of species–habitat–date, the number of individual plants (i.e. replicates) was six; the two values of soil water content taken for each plant were averaged and the mean used in the analyses. In the case of δ13C, only two factors, species and habitat, could be included in the analyses, while in the case of gas exchange parameters (Pnet and gs), the unequal distribution of reliable data over the field campaigns meant that separate two-factor ANOVAs had to be determined for each species, and interspecific comparisons were carried out only for time periods with enough data for both species (May, July and August 2005).
Table 2.
Results of the two- and three-factor ANOVA (F and P values) for seven study variables
| Variable | Species | Date | Habitat | Species × date | Species × habitat | Date × habitat | |
|---|---|---|---|---|---|---|---|
| Soil water content | F | 43·43 | 73·43 | 2·65 | 5·91 | 7·29 | 29·26 |
| P | <0·0001 | <0·0001 | 0·1102 | 0·0052 | 0·0078 | <0·0001 | |
| Leaf water potential | F | 17·58 | 39·76 | 0·34 | 1·90 | 5·05 | 16·15 |
| P | <0·0001 | <0·0001 | 0·5651 | 0·1759 | 0·0118 | 0·0002 | |
| Pre-dawn Fv/Fm | F | 7·97 | 37·76 | 32·51 | 13·04 | 29·26 | 4·17 |
| P | 0·0122 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | 0·0432 | |
| Midday Fv/Fm | F | 0·28 | 33·20 | 29·50 | 14·70 | 26·80 | 4·50 |
| P | 0·6044 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | 0·0412 | |
| Net photosynthetic rate | F | 5·66 | 19·20 | 6·95 | 14·23 | 0·63 | 10·56 |
| P | 0·0059 | <0·0001 | 0·0133 | 0·0003 | 0·4162 | 0·0031 | |
| Stomatal conductance | F | 4·12 | 12·99 | 0·14 | 13·38 | 14·68 | 6·44 |
| P | 0·0432 | <0·0001 | 0·6154 | 0·0021 | 0·0006 | 0·0098 | |
| δ13C | F | 4·66 | 2·65 | 5·91 | |||
| P | 0·0303 | 0·1102 | 0·0052 |
Only main effects (species, date and habitat, with 1, 5 and 1 d.f., respectively) and first order interactions are shown for clarity.
Significant effects are highlighted in bold (P<0·05).
RESULTS
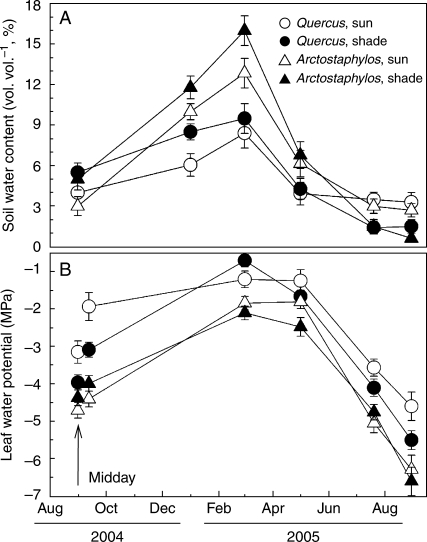
Most of the precipitation recorded during 2004–2005 fell during the autumn of 2004, with almost no precipitation from December 2004 until June 2005 (Fig. 1). Freezing below −10 °C occurred on several consecutive days during February and March. Many of these cold, dry days had clear skies with many hours of intense irradiance (>1900 µmol PAR m−2 s−1), giving daily irradiances <30 mol PAR m−2 d−1 after March 2005 (Fig. 1). Air temperature was higher and varied more at the sun than shade site; minimum temperatures in the sun were >2·5 °C lower and maximum temperatures were up to 4·5 °C higher than those in the shade (Fig. 2). Leaf temperatures were very close to that of the air in the shade, while they were up to 4 °C higher than that of the air in the sun during the central hours of clear spring and summer days (data not shown). Air humidity was higher in the shade than in the sun throughout the year, leading to VPDs significantly lower than those in the sun (Table 1). Daily irradiance was, on average, only one-third in the shade of that in the sun (Table 1). Away from plants, soil water content (θ) at 20 cm depth was greater and more affected by precipitation in the sun than in the shade (Fig. 2). However, a different and more complex seasonal pattern was found for the θ below the sampled plants. It was higher in the shade than in the sun from August 2004 until March 2005, but by the end of the dry spring and during the 2005 summer the pattern was reversed and soils were slightly but significantly more moist below plants in the sun (Fig. 3). Depending on the species, this seasonal pattern of θ paralleled that of the seasonal changes in Ψ of the target plants (Table 2, Fig. 3). θ of Q. ilex was less negative in the sun than in the shade in both summers but, in contrast, differences for A. uva-ursi were mostly insignificant (Fig. 3). Ψ was significantly lower at the end of the summer 2005 than in 2004, reaching values as low as –6·5 MPa in A. uva-ursi.
Fig. 3.
(A) Soil water content under the study plants. (B) Water potential (Ψ; pre-dawn measurements plus midday values in August 2004) of Quercus ilex and Arctostaphylos uva-ursi in sun and shade habitats. Each symbol is the mean of a minimum of six plants. Error bars indicate the 95 % confidence intervals for the mean; thus, non-overlapping bars reveal significant differences (ANOVA, Fisher l.s.d. post hoc test).
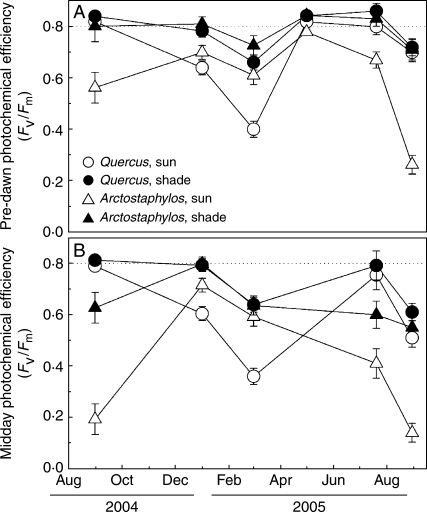
Photochemical efficiency of PSII (Fv/Fm) differed significantly among species, habitats and times of the year, with complex interactions between these three factors (Table 2, Fig. 4). Pre-dawn Fv/Fm tended to be lower in sun than in shade plants, and were particularly so for A. uva-ursi, at the end of both summers (Fig. 4A). In contrast, Q. ilex exhibited similar values in the shade and in the sun for most of the year, with the exception of March 2005, where the combination of freezing temperatures and high irradiance resulted in mean Fv/Fm values as low as 0·4 at the sun site (Fig. 4A). Values of pre-dawn Fv/Fm at the end of August 2005 were significantly lower than those of August 2004 for all combinations of species and habitats. Although midday Fv/Fm (measured after 30 min in darkness) was lower than pre-dawn Fv/Fm, the seasonal and species trends were similar to those observed pre-dawn, with very low values in the sun for A. uva-ursi in summer and for Q. ilex in early spring (Fig. 4B). However, midday Fv/Fm was also reduced in the shade at some times of the year, with mean values of 0·6 during the summer in A. uva-ursi and during the early spring in Q. ilex. As was the case for pre-dawn Fv/Fm, midday Fv/Fm was significantly lower in August 2005 than in August 2004.
Fig. 4.
Pre-dawn (A) and midday (B) photochemical efficiency (Fv/Fm) of Quercus ilex and Arctostaphylos uva-ursi in sun and shade habitats. Dashed lines indicate the threshold of 0·8 as a reference for optimum Fv/Fm. Each symbol is the mean of a minimum of six plants. Error bars indicate the 95 % confidence intervals for the mean; thus, non-overlapping bars reveal significant differences (ANOVA, Fisher l.s.d. post hoc test).
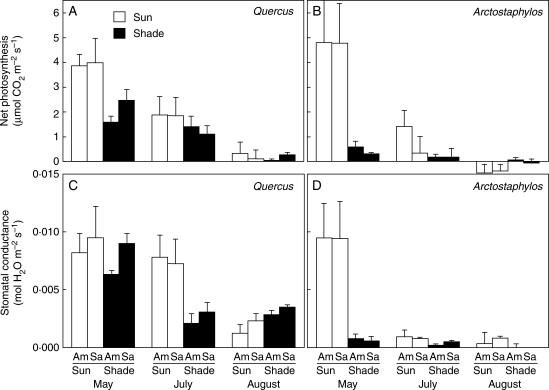
Maximum rates of light-saturated net photosynthesis (Psat; Fig. 5A, B) and stomatal conductances (gs; Fig. 5C, D) were low for both species during all seasons and in both habitats. However, when considering data from both species for May, July and August 2005 (i.e. the dates where full sets of gas exchange measurements were obtained for both species), significant interactions between habitat and date were found, and between species and date, with season being the main source of variation for these two gas exchange variables (Fig. 5; Table 2). While maximum rates of Psat were significantly higher in the sun than in the shade in both species during the spring (i.e. May), there were no differences between species and habitats in summer (July–August; Fig. 5). Sun and shade plants also exhibited similar rates of Psat in mid-winter (i.e. January 2005); for Q. ilex, average rates of Psat (in μmol CO2 m−2 s−1 ± s.e.) were 3·56 ± 0·32 (shade) and 3·33 ± 0·50 (sun). In contrast, rates of Q. ilex Psat in early spring (i.e. March 2005) were higher in the shade (1·77 ± 0·13) than in the sun (0·25 ± 0·24). At saturating irradiance, gs decreased significantly with the onset of drought during the summer in both species (Fig. 5). gs was significantly higher in the shade than in the sun in Q. ilex during the winter (data not shown) and early spring (Fig. 5), while gs of A. uva-ursi was always very low in the shade (Fig. 5).
Fig. 5.
(A, B) Maximum net photosynthetic rate (Psat) and (C, D) stomatal conductance (gs) under ambient (Am) and saturating (Sa) light at ambient temperature and CO2 concentrations of Quercus ilex (A, C) and Arctostaphylos uva-ursi (B, D) in sun and shade habitats. Data are given for the three campaigns (May, July and August 2005) with reliable data for the two species. Error bars indicate the 95 % confidence intervals for the mean; thus, non-overlapping bars reveal significant differences (ANOVA, Fisher l.s.d. post hoc test).
To gain an insight into actual rates of carbon assimilation in the shaded environment, Pnet of shade plants was also measured at the prevailing ambient irradiance of the understorey (i.e. typically 100–450 µmol photons m−2 s−1). For Q. ilex, Pnet at ambient irradiance (Pamb) was available for all months. However, for A. uva-ursi, Pamb rates were only available for May, July and August 2005; Fig. 5 shows values for both species from May to August 2005. For other months, Pamb values (in μmol CO2 m−2 s−1 ± s.e.) of Q. ilex shaded plants were: August 2004 = 1·07 ± 0·18; January 2005 = 3·56 ± 0·32; March 2005 = 1·01 ± 0·39. Comparison of these rates with those in Fig. 5 shows that in late winter (i.e. March) and spring (May), Pamb was lower than Psat in Q. ilex; however, following the onset of summer, no differences were found between Pamb and Psat. Thus, while low irradiances at the shade site limited Pnet in winter/spring, factors other than irradiance limited Pnet of shaded plants in the summer. For A. uva-ursi, little difference was found between Pamb and Psat of shaded plants through May–August, with Pamb declining with the onset of summer (Fig. 5).
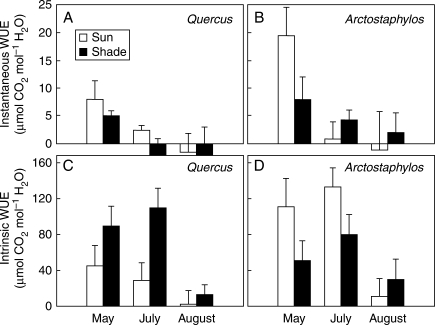
The very low gas exchange rates of both species in the two habitats over most of the year led to low WUEs (Fig. 6). However, significant differences were found among species and habitats, with A. uva-ursi exhibiting greater WUE than Q. ilex in the sun, particularly during the spring. Intrinsic WUE was greater in the shade than in the sun in Q. ilex, while both intrinsic and instantaneous WUEs were in general greater in the sun than in the shade in A. uva-ursi (Fig. 6).
Fig. 6.
Instantaneous and intrinsic water use efficiency [WUE, mean Pnet/mean transpiration under ambient light (A, B); and mean maximum net photosynthetic rate/mean maximum conductance (Psat/gs) under saturating light (C, D), respectively] of Quercus ilex (A, C) and Arctostaphylos uva-ursi (B, D) in sun and shade habitats. Data are given for the three campaigns (May, July and August 2005) with reliable data for the two species. Error bars indicate the 95 % confidence intervals for the mean; thus, non-overlapping bars reveal significant differences (ANOVA, Fisher l.s.d. post hoc test).
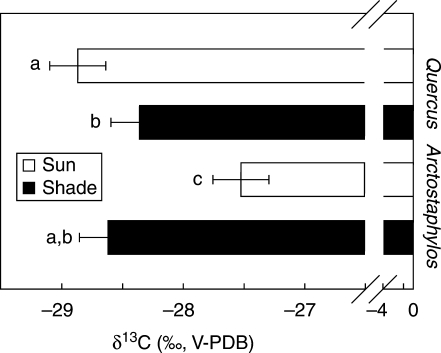
Carbon isotopic composition (δ13C) of the leaves collected at the end of the period of the study exhibited a significant species × habitat interaction (Table 2): δ13C was more negative in the sun than in the shade in Q. ilex, whereas the reverse was true in A. uva-ursi (Fig. 7). No differences were found between the two species in the shade, while the highest differences were found between sun individuals of the two species.
Fig. 7.
Carbon isotope composition (δ13C) of leaves of Quercus ilex and Arctostaphylos uva-ursi in sun and shade habitats collected at the end of the summer 2005; letters at the base of each bar indicate statistically homogenous groups (ANOVA, Fisher l.s.d. post hoc test). Each data point is the mean of a minimum of six plants. Error bars indicate the 95 % confidence intervals for the mean.
DISCUSSION
Transient and species-dependent responses
The year 2005 was particularly harsh, with plants experiencing adverse conditions through much of the year. Given this, it was not surprising that the ecophysiological performance of the two study species was very poor in 2005, with extremely low values of θ, Pnet, gs and Fv/Fm, particularly at the end of the summer. However, each species was affected differently by the adverse environmental conditions: for example, while photoinhibition in the more shade-tolerant Q. ilex was significantly more pronounced during late-winter frost than during the summer drought, the reverse was true for the less shade-tolerant A. uva-ursi. The two species also differed in their relative performance in the shade in terms of WUE estimated by both gas exchange and carbon isotopic composition of leaves, with A. uva-ursi having better WUEs in the sun than in the shade and Q. ilex exhibiting the reverse pattern. The relatively high intrinsic WUE of Q. ilex in the shade (higher photosynthesis/gs ratio under saturating light) is likely to enhance the performance of this species in an understorey that is characterized by numerous and intense sunflecks (Valladares and Guzman, 2006).
Importantly, the results also highlighted the extent to which the effects of shade (and drought) were transient, particularly when considering the physiological performance of the shade-tolerant Q. ilex. In winter, growth of Q. ilex in the shade protected leaves from the effects of cold-induced photoinhibition that severely reduced rates of Pamb in sun-exposed leaves. This contrasted with the situation in spring, where shade-grown Q. ilex plants exhibited lower rates of Pamb than their sun-grown counterparts, reflecting the fact that in spring, sun leaves had recovered from photoinhibition and CO2 uptake was not limited by low gs; Q. ilex Pamb was thus light-limited in the shade in spring. For shade-grown A. uva- ursi, there was no significant difference between photosynthesis measured at ambient and saturating irradiance in all months; this lack of light sensitivity probably reflects persistent limitations in gs, and explains why rates of Pamb and Psat in spring were so much lower in shaded A. uva-ursi than in their sun-grown counterparts. Similarly, in Q. ilex, low gs in summer would have played an important role in limiting rates of net carbon gain. In addition, high air temperatures would have increased the proportion of daily fixed carbon released by photorespiration and non-photorespiratory mitochondrial respiration (Zaragoza-Castells et al., 2008). Leaf respiration rates (Rdark) of A. uva-ursi (measured at the prevailing ambient temperatures) were consistently lower in shaded plants than in their sun-grown counterparts, being higher in early summer than in spring or late summer; for shaded plants, rates of Rdark consistently exceeded rates of Pamb. Rdark was also consistently lower in shaded plants in Q. ilex (data not shown, but for extensive data on Q. ilex see Zaragoza-Castells et al., 2008).
Occurrence and impact of combined shade, drought and extreme temperatures
To understand why the effects of shade varied through time and differed between the two species requires an understanding of how an overlying canopy alters the abiotic environment in the shaded understorey. Although, strictly speaking, shade only refers to low light conditions, functionally and ecologically shade involves a whole suite of effects on plants and environmental factors (Valladares, 2008). In addition to limiting the light reactions of photosynthesis, shade is often associated with cooler temperatures, greater humidity and greater θ (depending on canopy interception of rainfall), all of which can be beneficial to plants. However, shade in the understorey is also often associated with greater competition for below-ground resources because it occurs in high density vegetation, with the result that θ can be lower in shaded habitats than more open, sun-exposed sites (Valladares and Pearcy, 2002). At the field site in the present study, air temperature and VPD were lower in the shade than in the sun over all the seasons. However, θ was higher in the sun than in the shade after rainfall events and during many moments of the year, revealing complex seasonal and spatial patterns (Table 1, Fig. 2). Away from the direct influence of woody plants, θ was higher in the sun than in the shade during autumn and winter, becoming similar between the two habitats during the spring and summer. Below the study plants, θ exhibited a different pattern, being higher in the shade than in the sun during autumn and winter, similar during spring, and lower in the shade than in the sun during summer. However, irrespective of the season, θ was clearly often lower in the shaded habitat at the field site, as has been reported previously for other temperate and Mediterranean sites (Abrams and Mostoller, 1995; Valladares and Pearcy, 2002). The present results suggest that the lower θ values in the shade could act as a strong ecological filter for species such as A. uva-ursi, particularly during years of extreme drought such as occurred in 2005.
In contrast to A. uva-ursi, Q. ilex was sensitive to frost and more capable of dealing with drought and moderate shade. Moreover, in winter, shade-grown Q. ilex benefited from the protection conferred by a canopy, as discussed for the case of Eucalyptus pauciflora in Australia (Ball et al., 1991; Egerton et al., 2000). Previous studies have also highlighted the sensitivity of Q. ilex to low temperatures (Garcia-Plazaola et al., 2003; Martínez-Ferri et al., 2004; Corcuera et al., 2005). The latter authors found a higher sensitivity to winter than to summer stress at the upper altitudinal limit of the species in a milder location with fewer freezing events and warmer temperatures than the present study site. An important difference between the present results and those of Corcuera et al. (2005) is that while they found a negligible impact of the summer stress, in the present case summer stress was also important, emphasizing the very adverse climatic conditions of the continental plateaus of the centre of the Iberian Peninsula (Martinez-Ferri et al., 2000, 2004; Garcia-Plazaola et al., 2003; Valladares et al., 2005).
Although there is strong support for the hypothesis that the different physiological performances of Q. ilex and A. uva-ursis in late winter reflected contrasting susceptibilities to the combined stresses of high light and freezing, it is also possible that direct effects of freezing and/or drought were responsible. Evergreen leaves depend on a sustained water supply from the stem xylem, and thus are influenced by xylem anatomy, which in turn influences the overall vulnerability to freezing- and drought-induced embolism (Cavender-Bares and Holbrook, 2001; Davis et al., 2005). Quercus ilex has significantly larger vessels than A. uva-ursis, which may contribute to the higher vulnerability of the former to frost events. Moreover, the very low temperatures in late winter (down to –18 °C in February 2005) might have directly caused mesophyll cell death in the sun and thus may not necessarily represent interactions with light. However, several facts suggest that the interpretation of interactive effects of high light and low temperatures being the main determinant of reduced physiological performance in Q. ilex is correct: (a) leaves exposed to such low temperatures did not show visual symptoms of death afterwards; (b) photochemical efficiency (Fv/Fm) was not that low in the shade, where temperatures as low as −16 °C were experienced by the study leaves; and (c) lethal temperatures (LT50) determined in the laboratory for Q. ilex revealed a remarkable freezing tolerance (down to −15 °C; T. E. Gimeno and F. Valladares, unpubl. res.), which was highly dynamic, varying remarkably according to the previous thermal history and with significant differences throughout the day and the seasons and among individuals within a population, in agreement with previous studies (e.g. Boorse et al., 1998). It is suggested, therefore, that the different physiological performances of Q. ilex and A. uva-ursis in late winter reflected contrasting susceptibilities to the combined stresses of high light and freezing.
Water use efficiency: an integrated view on performance
The WUE of plants is a key aspect of their water economy and of their performance and survival in arid sites (Damesin et al., 1997; Nativ et al., 1999; Bacon, 2004). In oaks and many other species, δ13C, an estimator of WUE during the period of plant growth, has been related to a decline in performance of seedlings and saplings after drought (Bacon, 2004; Lloret et al., 2004). δ13C has been associated with pre-dawn θ, and values similar to those found here have been reported for oaks from other Mediterranean ecosystems (Damesin et al., 1998; Xu et al., 2000; Dawson et al., 2002). High WUE is hard to achieve in dry, unproductive Mediterranean areas, and its achievement is further complicated when plants are exposed to limitations in other important resources such as light. In a factorial experiment with cork oak seedlings, WUE estimated by δ13C increased with light regardless of the availability of water, although it was higher under drought (Aranda et al., 2007). In the current study, this pattern was found in only one species (A. uva-ursi), while the other (Q. ilex) exhibited the reverse, suggesting that WUE is influenced not only by the shade tolerance of each species but also by the co-occurrence of other stresses that differentially affect plant performance across light and moisture gradients.
Insights on facilitation by canopy trees
The present results strongly suggest that facilitation, taken here as the positive effect of shade by established plants on leaf physiological performance of understorey plants, is species specific. Previous studies with plants from Mediterranean and arid ecosystems have also found that shade tolerance of each species influences the extent of facilitation (Prider and Facelli, 2004). Moreover, shrub–seedling and nurse–protégée interactions were found to be species specific in Mediterranean ecosystems due to differential modification of both above-ground (light availability) and below-ground (soil compaction, water content and fertility) abiotic factors by different nurse plants (Pugnaire et al., 2004; Gómez-Aparicio et al., 2005). The present results also suggest that facilitation of leaf physiological performance is transient, since leaf-level performance in the shade was only improved in late winter for one of the two species (Q. ilex), and shade had negative or neutral effects at other times of the year. Further work is needed to assess the overall impact of such leaf-level facilitation on plant survival, growth and reproduction.
Although the notion of transient facilitation is not new, relatively few studies have provided empirical evidence of its importance under field conditions (Brooker et al., 2006). Most studies of plant ecophysiological performance in the field and of plant–plant interactions under natural conditions span relatively short periods of time and only rarely include extreme events or particularly adverse years (e.g. Hastwell and Facelli, 2003; Martínez-Ferri et al., 2004; Pugnaire et al., 2004). This is important because an unusually adverse year (such as in 2005) or climatic event can have a disproportionate influence on performance and survival, becoming a demographic bottleneck and severely affecting plant–plant interactions (Abrams and Mostoller, 1995; Tielborger and Kadmon, 2000; Peñuelas et al., 2001; Valladares and Pearcy, 2002). Thus, long-term data are needed to know whether increasing climatic severity in the Mediterranean will increase facilitation, as predicted by Brooker (2006), or not, as suggested by the present study.
CONCLUSIONS
The results on leaf-level physiological performance of open vs. understorey plants revealed that shade was beneficial for leaf-level physiology in Q. ilex during winter, detrimental during spring for both species, and of little consequence in summer. This in turn suggests that multiple co-occurring factors can make facilitation transient, with the impacts of shade/drought/high light on leaf performance differing between the two study species, which supports the notion that facilitation is species specific. Importantly, the results point to the beneficial effects of shade (e.g. avoidance of photoinhibition and extreme temperatures) being eclipsed by reduced θ, particularly in dry, adverse years, suggesting that the role of positive plant–plant interactions might not increase in the most likely global change scenarios.
ACKNOWLEDGEMENTS
Thanks are due to Rafael Ruiz López de la Coba and the staff of the Alto Tajo Natural Park and Delegación Provincial de Medio Ambiente (Junta de Castilla La Mancha); Adrian Escudero for help in finding the right field site and for insightful comments on earlier drafts of the manuscript; Fernando Maestre and Rob Brooker for comments on an earlier version of the manuscript; Ismael Aranda for providing fitting readings; members of GLOBIMED (www.globimed.net) for valuable insights; and Oscar Godoy, Virginia Sanz, Jose Alberto Ramírez and the many students who helped us in the field campaigns. Financial support was provided by the Spanish Ministry of Education and Science (ECOCLIM, CGL2007-66066-C04-02/BOS) and by the Programa de Actividades de I + D de la Comunidad de Madrid (Consejería de Educación) REMEDINAL-CM (S-0505/AMB/000335) to F.V., by a University of York Innovation & Research Primer Grant to O.K.A. and a University of York PhD studentship to J.Z.C.
LITERATURE CITED
- Abrams MD, Mostoller SA. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiology. 1995;15:361–370. doi: 10.1093/treephys/15.6.361. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Conner JK, Stinchcombe JR. Evolution of plant resistance and tolerance to frost damage. Ecology Letters. 2004;7:1199–1208. [Google Scholar]
- Allard V, Ourcival JM, Rambal S, Joffre R, Rocheteau A. Seasonal and annual variation of carbon exchange in an evergreen Mediterranean forest in southern France. Global Change Biology. 2008;14:714–725. [Google Scholar]
- Aranda I, Castro L, Pardos M, Gil L, Pardos JA. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. Forest Ecology and Management. 2005;210:117–129. [Google Scholar]
- Aranda I, Pardos M, Puértolas J, Jiménez M-D, Pardos JA. Water use efficiency in cork oak (Quercus suber L.) is modified by the interaction of water and light availabilities. Tree Physiology. 2007;27:671–677. doi: 10.1093/treephys/27.5.671. [DOI] [PubMed] [Google Scholar]
- Bacon MA. Water use efficiency in plant biology. Oxford: Blackwell Publishing Ltd/CRC Press; 2004. [Google Scholar]
- Ball MC, Hodges VS, Laughlin GP. Cold-induced photoinhibition limits regeneration of snow gum at tree-line. Functional Ecology. 1991;5:663–668. [Google Scholar]
- Bertness MD, Callaway RM. Positive interactions in communities. Trends in Ecology and Evolution. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Bertrand A, Castonguay Y. Plant adaptations to overwintering stresses and implications of climate change. Canadian Journal of Botany-Revue Canadienne De Botanique. 2003;81:1145–1152. [Google Scholar]
- Boorse GC, Ewers FW, Davis SD. Response of chaparral shrubs to below-freezing temperatures: acclimation, ecotypes, seedlings vs. adults. American Journal of Botany. 1998;85:1224–1230. [PubMed] [Google Scholar]
- Brooker RW. Plant–plant interactions and environmental change. New Phytologist. 2006;171:271–284. doi: 10.1111/j.1469-8137.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Brooker RW, Scott D, Palmer SCF, Swaine E. Transient facilitative effects of heather on Scots pine along a grazing disturbance gradient in Scottish moorland. Journal of Ecology. 2006;94:637–645. [Google Scholar]
- Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, et al. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology. 2008;96:18–34. [Google Scholar]
- Callaway RM. Positive interactions among plants. Botanical Review. 1995;61:306–349. [Google Scholar]
- Cavender-Bares J, Holbrook NM. Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with, contrasting habitats. Plant, Cell and Environment. 2001;24:1243–1256. [Google Scholar]
- Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A. Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of co-occurring Mediterranean oaks that differ in leaf lifespan. New Phytologist. 2005;168:597–611. doi: 10.1111/j.1469-8137.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- Corcuera L, Morales F, Abadia A, Gil-Pelegrin E. Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. Tree Physiology. 2005;25:599–608. doi: 10.1093/treephys/25.5.599. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, et al. Regional climate projections. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Solomon S, Qin D, Manning M, et al., editors. Climate change 2007: the physical science basis. Cambridge: Cambridge University Press; 2007. pp. 847–943. [Google Scholar]
- Damesin C, Rambal S, Joffre R. Between-tree variations in leaf delta13C of Quercus pubescens and Quercus ilex among Mediterranean habitats with different water availability. Oecologia. 1997;111:26. doi: 10.1007/s004420050204. [DOI] [PubMed] [Google Scholar]
- Damesin C, Rambal S, Joffre R. Seasonal and annual changes in leaf delta C-13 in two co-occurring Mediterranean oaks: relations to leaf growth and drought progression. Functional Ecology. 1998;12:778–785. [Google Scholar]
- Damgaard C. Evolutionary ecology of plant–plant interactions. Aarhus: Aarhus University Press; 2005. [Google Scholar]
- Davis SD, Ewers FW, Pratt RB, Brown PL, Bowen TJ. Interactive effects of freezing and drought on long distance transport: a case study for chaparral shrubs of California. In: Holbrook NM, Zwieniecki A, editors. Vascular transport in plants. Oxford: Elservier/AP co-imprint; 2005. pp. 564–584. [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics. 2002;33:507–559. [Google Scholar]
- Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F. Fine-scale processes regulate the response of extreme events to global climate change. Proceedings of the National Academy of Sciences; USA. 2005. pp. 15774–15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton JJG, Banks JCG, Gibson A, Cunningham RB, Ball MC. Facilitation of seedling establishment: reduction in irradiance enhances winter growth of Eucalyptus pauciflora. Ecology. 2000;81:1437–1449. [Google Scholar]
- Flores JLF, Jurado E. Are nurse–protégé interactions more common among plants from arid environments? Journal of Vegetation Science. 2003;14:911–916. [Google Scholar]
- Garcia-Plazaola JI, Olano JM, Hernandez A, Becerril JM. Photoprotection in evergreen Mediterranean plants during sudden periods of intense cold weather. Trees – Structure and Function. 2003;17:285–291. [Google Scholar]
- Gómez JM, Valladares F, Puerta-Piñero C. Differences between structural and functional heterogeneity caused by seed dispersal. Functional Ecology. 2004;18:787–792. [Google Scholar]
- Gómez-Aparicio L, Valladares F, Zamora R, Quero JL. Response of tree seedlings to the abiotic heterogeneity generated by nurse shrubs: an experimental approach at different scales. Ecography. 2005;28:757–768. [Google Scholar]
- Hastwell GT, Facelli JM. Differing effects of shade-induced facilitation on growth and survival during the establishment of a chenopod shrub. Journal of Ecology. 2003;91:941–950. [Google Scholar]
- Inouye DW. The ecological and evolutionary significance of frost in the context of climate change. Ecology Letters. 2000;3:457–463. [Google Scholar]
- Larcher W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosystems. 2000;134:279–295. [Google Scholar]
- Lloret F, Peñuelas J, Ogaya R. Establishment of co-existing Mediterranean tree species under a varying soil moisture regime. Journal of Vegetation Science. 2004;15:237–244. [Google Scholar]
- Maestre F, Cortina J, Bautista S. Mechanisms underlying the interaction between Pinus halepensis and the native late-successional shrub Pistacia lentiscus in a semi-arid plantation. Ecography. 2004;27:776–786. [Google Scholar]
- Maestre FT, Valladares F, Reynolds JF. The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: further insights from arid environments. Journal of Ecology. 2006;94:17–22. [Google Scholar]
- Martinez-Ferri E, Balaguer L, Valladares F, Chico JM, Manrique E. Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiology. 2000;20:131–138. doi: 10.1093/treephys/20.2.131. [DOI] [PubMed] [Google Scholar]
- Martínez-Ferri E, Manrique E, Valladares F, Balaguer L. Winter photoinhibition in the field involves different processes in four co-occurring Mediterranean tree species. Tree Physiology. 2004;24:981–990. doi: 10.1093/treephys/24.9.981. [DOI] [PubMed] [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Nativ R, Ephrath JE, Berliner PR, Saranga Y. Drought resistance and water use efficiency in Acacia saligna. Australian Journal of Botany. 1999;47:577–586. [Google Scholar]
- Niinemets U, Valladares F. Tolerance to shade, drought and waterlogging of temperate, Northern hemisphere trees and shrubs. Ecological Monographs. 2006;76:521–547. [Google Scholar]
- Peñuelas J, Lloret F, Montoya R. Severe drought effects on Mediterranean woody flora in Spain. Forest Science. 2001;47:214–218. [Google Scholar]
- Pratt RB, Ewers FW, Lawson MC, Jacobsen AL, Brediger MM, Davis SD. Mechanisms for tolerating freeze–thaw stress of two evergreen chaparral species: Rhus ovata and Malosma laurina (Anacardiaceae) American Journal of Botany. 2005;92:1102–1113. doi: 10.3732/ajb.92.7.1102. [DOI] [PubMed] [Google Scholar]
- Prider JN, Facelli JM. Interactive effects of drought and shade on three arid zone chenopod shrubs with contrasting distributions in relation to tree canopies. Functional Ecology. 2004;18:67–76. [Google Scholar]
- Pugnaire FI, Armas C, Valladares F. Soil as a mediator in plant–plant interactions in a semi-arid community. Journal of Vegetation Science. 2004;15:85–92. [Google Scholar]
- Rochette P, Belanger G, Castonguay Y, Bootsma A, Mongrain D. Climate change and winter damage to fruit trees in eastern Canada. Canadian Journal of Plant Science. 2004;84:1113–1125. [Google Scholar]
- Sánchez-Gómez D, Valladares F, Zavala MA. Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade-offs and evidence for niche differentiation. New Phytologist. 2006;170:795–806. doi: 10.1111/j.1469-8137.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- Stiles EW. Patterns of fruit presentation and seed dispersal in bird-disseminated woody plants in the Eastern deciduous forest. American Naturalist. 1980;116:670–688. [Google Scholar]
- Tielborger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Valladares F. A mechanistic view of the capacity of forests to cope with climate change. In: Bravo F, May VL, Jandl R, von Gadow K, editors. Managing forest ecosystems: the challenge of climate change. Berlin: Springer-Verlag; 2008. [Google Scholar]
- Valladares F, Guzmán B. Canopy structure and spatial heterogeneity of understory light in abandoned Holm oak woodlands. Annals of Forest Science. 2006;63:749–761. [Google Scholar]
- Valladares F, Pearcy RW. Drought can be more critical in the shade than in the sun: a field study of carbon gain and photoinhibition in a Californian shrub during a dry El Niño year. Plant, Cell and Environment. 2002;25:749–759. [Google Scholar]
- Valladares F, Arrieta S, Aranda I, Lorenzo D, Tena D, Sánchez-Gómez D, et al. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental-Mediterranean sites. Tree Physiology. 2005;25:1041–1052. doi: 10.1093/treephys/25.8.1041. [DOI] [PubMed] [Google Scholar]
- Valladares F, Gianoli E, Gómez JM. Ecological limits to plant phenotypic plasticity. Tansley review. New Phytologist. 2007;176:749–763. doi: 10.1111/j.1469-8137.2007.02275.x. [DOI] [PubMed] [Google Scholar]
- Xu ZH, Saffigna PG, Farquhar GD, Simpson JA, Haines RJ, Walker S, Osborne DO, Guinto D. Carbon isotope discrimination and oxygen isotope composition in clones of the F-1 hybrid between slash pine and Caribbean pine in relation to tree growth, water-use efficiency and foliar nutrient concentration. Tree Physiology. 2000;20:1209–1217. doi: 10.1093/treephys/20.18.1209. [DOI] [PubMed] [Google Scholar]
- Zaragoza-Castells J, Sánchez-Gómez D, Hartley IP, Matesanz S, Valladares F, Lloyd J, et al. Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of thermal acclimation in both high-light and shaded habitats. Functional Ecology. 2008;22:172–184. [Google Scholar]