Abstract
Background and Aims
While within-species competition for light is generally found to be asymmetric – larger plants absorbing more than proportional amounts of light – between-species competition tends to be more symmetric. Here, the light capture was analysed in a 5-year-old competition experiment that started with ten genotypes of the clonal plant Potentilla reptans. The following hypotheses were tested: (a) if different genotypes would do better in different layers of the canopy, thereby promoting coexistence, and (b) if leaves and genotypes with higher total mass captured more than proportional amounts of light, possibly explaining the observed dominance of the abundant genotypes.
Methods
In eight plots, 100 leaves were harvested at various depths in the canopy and their genotype determined to test for differences in leaf biomass allocation, leaf characteristics and the resulting light capture, calculated through a canopy model using the actual vertical light and leaf area profiles. Light capture was related to biomass to determine whether light competition between genotypes was asymmetric.
Key Results
All genotypes could reach the top of the canopy. The genotypes differed in morphology, but did not differ significantly in light capture per unit mass (Φmass) for leaves with the laminae placed at the same light levels. Light capture did increase disproportionately with leaf mass for all genotypes. However, the more abundant genotypes did not capture disproportionately more light relative to their mass than less-abundant genotypes.
Conclusions
Vertical niche differentiation in light acquisition does not appear to be a factor that could promote coexistence between these genotypes. Contrary to what is generally assumed, light competition among genetic individuals of the same species was size-symmetric, even if taller individual leaves did capture disproportionately more light. The observed shifts in genotype frequency cannot therefore be explained by asymmetric competition for light.
Key words: Potentilla reptans, light, competition, symmetric, clonal, genotype, investment, petiole, canopy, allocation
INTRODUCTION
Plants in nutrient-rich environments are thought to compete predominantly for light (Goldberg and Miller, 1990; Tilman and Pacala, 2006). Because light is a unidirectional resource, successful competitors are usually described as having ‘traits leading to overtopping of the neighbours’ (Aerts, 1999). Taller individuals can increase their fitness directly by increasing their light capture, and indirectly by making the resource unavailable to competitors (Falster and Westoby, 2003). As a result taller plants may catch a disproportional share of incident light, i.e. they can catch more light per unit biomass than smaller individuals, a phenomenon that is also called asymmetric competition (Weiner, 1990; Anten and Hirose, 1998; Schwinning and Weiner, 1998; Berntson and Wayne, 2000). At high densities, this may lead to high mortality of subordinate plants (Weiner and Solbrig, 1984; Weiner and Thomas, 1986; Nagashima et al., 1995).
Increased height growth, however, occurs at a cost. To maintain mechanical stability, tall plants invest disproportionately more in stems and relatively less in leaves (Ballaré et al., 1987). Therefore the leaf mass ratio (LMR, g invested in leaves g−1 total biomass) generally decreases with plant height (Givnish, 1982, 1995; Anten and Hirose, 1998). Plants also increase the leaf area per unit leaf mass invested in leaves (specific leaf area, SLA, m2 g−1 leaf biomass) in response to shade (Corré, 1983a, b). Consequently, tall plants with leaves exposed to higher light availability have a relatively low leaf area per unit plant mass (LAR; LAR = SLA × LMR; Hirose and Werger, 1995). To analyse the benefits (light capture) and costs (above-ground biomass) of different plants within a dense canopy, Hirose and Werger (1995) developed an approach in which they calculated the light captured per unit biomass (Φmass), with Φmass being the product of the light interception per unit of leaf area (Φarea) and the LAR of a plant. They showed that within a multi-species grassland, tall dominant species captured more light per unit of leaf area than subordinate ones. Subordinate species on the other hand had considerably higher LMR, SLA and thus LAR values, which compensated for their lower Φarea, resulting in similar or even higher Φmass (Φmass = LAR × Φarea ). Thus in spite of the strong gradient in light availability in the canopy, taller and shorter species captured light in proportion to their size expressed in terms of mass, i.e. light competition was size symmetric (sensu Weiner, 1990). Size-symmetric competition for light has been demonstrated to result in size-symmetric growth (growth being proportional to size) and the maintenance of a relatively constant size distribution among plants of different species in crowded populations (van Kuijk et al., 2008). The large difference in LAR between species probably largely resulted from contrasting intrinsic architectures. This may contribute to coexistence of differently sized species in dense grasslands (Hirose and Werger, 1995; Anten and Hirose, 1999; Werger et al., 2002).
Within species, however, the variation in SLA and LMR depends on mean values and plasticity that modifies a common architectural design and it might not be large enough to allow subordinate individuals to persist in the lower layers of the vegetation (Anten and Hirose, 1998). Indeed several studies found that in dense monospecific stands taller dominant individuals had higher Φmass values than subordinate individuals (Anten and Hirose, 1998; Hikosaka et al., 1999, 2003). It could hence be argued that, within species, selection should favour genotypes that have a strong height growth, enabling them to capture a disproportional amount of light relative to their size.
However, selection within a monospecific stand of a stoloniferous species that can only increase its height through petiole elongation could still favour different height growth strategies. In general, meristems are placed in the axils of leaves (Bell, 1991). The activity of these meristems are suppressed by low light and low R : FR ratio (Schmitt and Wulff, 1993; Bonser and Aarssen, 2003). Light levels and R : FR ratio increase with height in the vegetation (Ballaré et al., 1990; Schmitt et al., 2003). An erect-growing plant can place its meristems at higher levels through stem elongation, which could reduce the apical dominance (Ongaro and Leyser, 2008), allowing the plant to branch, and consequently to increase its growth by placing more leaves at more favorable light conditions. This will increase the benefits of increased height growth. Since stoloniferous plants that form basal rosettes can only increase in height through the elongation of their petioles (Dong, 1995; Huber, 1996; Huber et al., 1998), each leaf has to be supported separately. As this is less efficient in terms of biomass use than producing a single stem (Liu et al., 2007), they have to invest relatively more in height than erect plants in order to place every leaf at the top of the canopy. This in turn might allow subordinate stoloniferous plants with smaller investment in petioles to capture similar amounts of light per unit mass (Φmass) values as their taller competitors.
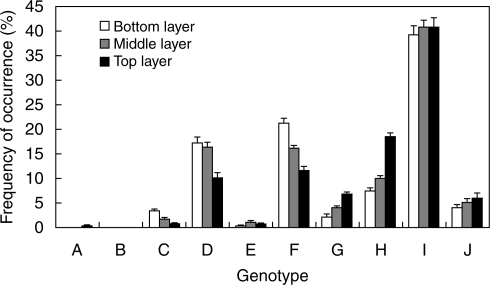
In 1998 an experiment was started with ten genotypes of the clonal stoloniferous plant Potentilla reptans, all growing together in competition starting at equal frequencies. Analysis of the relative frequency of these genotypes after 5 years using ISSR markers revealed that one genotype had become the most abundant genotype (±40 % of all leaves), while several others were still present in approximately the same frequency as at the start of the experiment, an indication that the increase in abundance of the dominant genotypes had not reduced the frequency of these genotypes (Fig. 1; data from J. F. Stuefer et al., detailed results to be published at a later date). Still other genotypes, however, had declined in frequency, which suggests that selection had occurred.
Fig. 1.
Average frequency per plot (% of total leaves + 1 s.e.) of all genotypes in the three layers harvested: bottom, middle and top layer. Data are from J. F. Stuefer et al. (unpubl. res.).
Data collected from this experiment were used to compare leaf biomass allocation, leaf characteristics and the resulting light capture between genotypes. Different genotypes were tested to see if they were able to capture light efficiently at different layers in the canopy and, if so, whether this resulted in differences in light acquisition between genotypes being proportional to their size. This would support the idea that the genotypes may coexist due to vertical niche space differentiation. Alternatively, if this did not occur, it was expected that the few dominant genotypes would capture disproportionately more light relative to their mass, i.e. that competition for light would be asymmetric, which would in part explain the abundance of a few dominant genotypes.
MATERIALS AND METHODS
Plant material
Potentilla reptans is a stoloniferous herb found in moderately disturbed, productive pastures and mown grasslands, and on lake and river shores, road margins and other man-made habitats (Van der Meijden, 1996). This species has been found to form dense mono-specific stands (P. J.Vermeulen, pers. obs.). The plant produces sympodially growing stolons with rooted ramets on its nodes. In the absence of physical disturbance, the ramets remain interconnected throughout one growing season (Stuefer et al., 2002). Because internodes between rosette leaves do not elongate, height growth is exclusively achieved by petiole elongation (Huber, 1995). Each leaf consists of five to seven palmately arranged leaflets borne on a vertically orientated petiole attached to the ground rosette. Petiole elongation stops when the lamina reaches the top of the canopy (see Vermeulen et al., 2008).
Ten genotypes of P. reptans were collected in a wide range of habitats in The Netherlands. The sites included river shores, mown pastures, car parks and relatively undisturbed grasslands. Differences between genotypes thus represent within-species variation. The genotypes were propagated in the botanical gardens of Utrecht University. In shading experiments these genotypes differed in several traits, such as SLA, LMR and petiole length (Liu et al., 2007; Vermeulen, 2008).
Experimental set-up
In the botanical gardens of Utrecht University 16 plots of 2 × 2 m were established in the spring of 1998 as part of a larger experiment (J. F. Stuefer et al., unpubl. res.). In this paper, only the eight plots of the undisturbed control treatment were used. In these plots, 100 planting points were positioned on a regular grid. For each plot ten similar-sized juvenile ramets per genotype were taken from the stock population and randomized over these planting points. Every genotype thus started with an initial frequency of 10 %. At the beginning of July 2003, 5 years after the start of the experiment, 100 leaves in each plot were harvested at randomly chosen grid points in the vegetation (the frequency harvest). The vegetation of the plot was visually divided into three layers and the layer from which the leaf had to be sampled was drawn randomly. The leaf closest to the grid point compared with the other leaves with the lamina placed in the same layer was sampled. Only leaves with fully developed laminas were taken into account. Since the plots were part of an ongoing experiment, only leaves (i.e. petioles and leaf laminas, which in this species are the units of vertical growth) were sampled, leaving stolons and roots intact.
Leaf measurements
For each leaf the height of the lamina above the ground and the height of the vegetation at the position of the sampled leaf were measured. From these two measurements, the depth of the vegetation at the height of the lamina was calculated.
The lamina was separated from the petiole after which the lamina was cut in two halves. Then the lamina area (LA) of both halves was measured using a Licor LI-3100 leaf-area meter. One half was used to determine the identity of the genotype using ISSR (J. F. Stuefer et al., unpubl. res.). The other half was used to measure dry weight. This lamina half and the petiole were dried for at least 3 days at 65 °C.
Dry weight was then determined (accuracy 0·1 mg), after which the specific lamina area (SLamA, m2 g−1) of the dried lamina part was calculated. The total lamina weight was then calculated using this SLA and the total leaf area of both halves together.
The other parameters were then calculated:
Light capture
Every plot was divided into four subplots, in each of which a light profile was measured under an overcast sky. Starting at the top of the vegetation two measurements were made at 5-cm intervals using a ceptometer (Delta-T Devices, Cambridge, UK). Photosynthetic photon flux density (PPFD) above the canopy (PPFDo) was measured simultaneously using a Licor Li 185A photometer. The relative PPFD (rPPFD) was calculated for each point of measurement and the two values obtained per point were averaged. The rPPFD within the interval between two measurement heights was estimated by means of interpolation. Because only the differences in relative light capture were of interest in the present study, an accurate measurement of the daily PPFD above the canopy was not necessary. Therefore, the daily light availability for each height was calculated from the rPPFD, assuming an average day of 12 h and an average light availability above the vegetation of 1000 µmol m−2 s−1, which by summation gives a reasonable estimate of the total daily PPFD on a clear summer day at the study site.
Daily light availability at the height of the lamina (PPFDh, mol m−2 d−1) was taken from the light profile of the subplot in which the leaf was collected using the depth of the vegetation at lamina height. Differences in leaf angle between genotypes were not taken into account since this variation was small. To determine the light extinction coefficient (K) and the leaf area per layer, for every plot a stratified clipping in a 30 × 30 cm subplot was done. Every 5 cm the relative light intensity was measured in the same way as in the subplots. Leaf area was determined by taking a subsample from the laminas that were cut from the 5-cm layer, and by calculating the SLA of the subsample. Total leaf area of the layer was then calculated using this SLA and the total lamina weight. The extinction coefficient (K) was then calculated following Anten and Hirose (2001):
| 1 |
with PPFDb the light at the bottom of the canopy, PPFD0 the light above the canopy and Lc the cumulative LAI, which ranged between 3·8 and 6·1. K was found to be on average 0·83 ± 0·009 (1 s.e.), a normal value for a dicotyledonous species (Monsi and Saeki, 1953).
Daily light capture per lamina (Φd, mol d−1) was calculated using the PPFDh, the lamina area (LA, m2) and the leaf absorbance (α) and the K:
| 2 |
Leaf absorbance was taken to be 0·8 (Goudriaan, 1977).
Light capture per unit biomass (Φmass, mol g−1 d−1) was calculated adjusting the formula from Hirose and Werger (1995), using the total leaf weight (TLW):
| 3 |
Note that leaf area, biomass and light acquisition are defined at the level of individual leaves and not of whole plants.
Next, the plot Φmass (Φmass,p, mol g−1 d−1) of each genotype, i.e. the total light capture per genotype in a plot divided by its total mass in that plot, was calculated as a measure of the overall light capture efficiency of the genotypes within each plot. Note that in order to do so, two points have to be considered. First, the three layers in which the leaves were sampled differed in the amount of leaves and the total lamina area, which has to be taken into account when calculating plot Φmass. Secondly, the frequency harvest is a subsample that does not supply information on the total amount of leaves (or total lamina area) in the vegetation. For this the total number of leaves of each genotype within each layer was estimated using the LAI data from the stratified clipping.
The total number of leaves per square metre of each layer (LNL) for all genotypes combined was estimated using the LAI of that layer in the stratified clipping (LAIL) and the average lamina area per leaf of all leaves harvested (all genotypes pooled) within that layer in the frequency harvest (LAT,A):
| 4 |
The total number of leaves per layer per genotype (LNLG) can then be calculated through the proportion of lamina area from the harvested leaves in the frequency harvest that belonged to the genotype:
| 5 |
with LALG the summarized lamina area of all leaves of that genotype within the layer and LAL the total lamina area of all leaves measured in that layer.
Next, using the data from the frequency harvest, the average layer Φ per leaf of a given genotype (ΦL) and the average leaf weight (TLWL) were calculated by dividing the summarized total light capture (Φd) and the summarized TLW of all leaves of a genotype harvested in that layer by the number of leaves that was harvested in that layer.
Then the weighted plot total light capture (Φp) and plot total leaf weight (TLWp) for each genotype was calculated by summarizing the light capture and total leaf weight:
| 6 |
and
| 7 |
Plot Φmass (Φmass,p) can be calculated by replacing Φd and TLW in eqn (3) by Φp and TLWp:
| 8 |
Statistics
Genotype A was left out of the analyses because only one leaf was found in all eight plots together.
Two-way covariance analyses (ANCOVA) with plot as a block factor, genotype as a random factor and rPPFD as the covariate were used to test for differences between genotypes for SLamA, LamMR, LamAR and Φmass. All data were log transformed to meet demands of normality and homoscedasticity.
To see whether light capture increased disproportionately with total leaf weight (within genotypes), a linear regression line was fitted following Anten and Hirose (1998), with light capture (log-transformed) as dependent variable and total leaf weight (log transformed) as predictor:
| 9 |
This was done for every plot, since plot was a significant factor in the covariance analysis (see Table 1). Since the genotype was not a significant factor, all leaves within a plot were pooled. If the coefficient β was larger than one, light capture increased exponentially and thus disproportionately with total leaf weight. A t-test using the coefficient βs from the eight plots was performed to test if the coefficient was significantly larger than one.
Table 1.
Results of two-way analysis of covariance (ANCOVA)
| Dependent | Covariate | Factor | Among slopes | Among intercepts |
|---|---|---|---|---|
| Specific lamina area (log) | Relative PPFD (log)*** | Genotype | 0·458 | 0·012* |
| Plot | 0·205 | 0·167 | ||
| G × P | 0·794 | 0·795 | ||
| Lamina mass ratio (log) | Relative PPFD (log)*** | Genotype | 0·196 | 0·035* |
| Plot | 0·932 | 0·293 | ||
| G × P | 0·423 | 0·878 | ||
| Lamina area ratio (log) | Relative PPFD (log)*** | Genotype | 0·500 | 0·022* |
| Plot | 0·226 | 0·499 | ||
| G × P | 0·916 | 0·909 | ||
| Total leaf weight (log) | Relative PPFD (log)*** | Genotype | 0·435 | 0·000* |
| Plot | 0·106 | 0·019* | ||
| G × P | 0·752 | 0·099 | ||
| Φmass (log) | Relative PPFD (log)*** | Genotype | 0·486 | 0·099 |
| Plot | 0·690 | 0·401 | ||
| G × P | 0·660 | 0·787 | ||
| Φ (log) | Tdw (log)*** | Genotype | 0·431 | 0·070 |
| Plot | 0·103 | 0·003* | ||
| G × P | 0·291 | 0·915 |
All values are P values. All data have been log transformed.
*, *** Significant effects: P < 0·05, P < 0·001, respectively.
To see whether plot light capture increased disproportionately with plot total leaf weight (between genotypes), eqn (9) was again used, replacing replacing Φd and TLW by Φp and TLWp. Note that now each point represents one genotype.
Genotypic differences in Φmass,p were tested in an ANOVA with plot as a block factor and genotype as a random factor. For all analyses, SPSS version 12·1 was used.
RESULTS
Frequency of genotypes
Figure 1 (data from J. F. Stuefer et al., unpubl. res.) shows the frequency of the ten genotypes in the vegetation after 5 years of competition. One genotype (genotype I) was most abundant in all three layers of the vegetation. No leaves were found of genotype B. Of the other genotypes some had a frequency close to or higher than their initial abundance of 10 % (genotypes D, F and H), while the other genotypes had decreased in frequency after 5 years. All remaining genotypes except genotype A were present in all three layers, but they differed in their frequency of leaves in the different layers, with some having relatively more leaves in the lower layer (genotypes C, D and F) while others had more leaves in the top layer (genotypes G, H and J). Genotypes E and F had a more or less even distribution of leaves over the three layers (see Fig. 1).
Leaf architecture
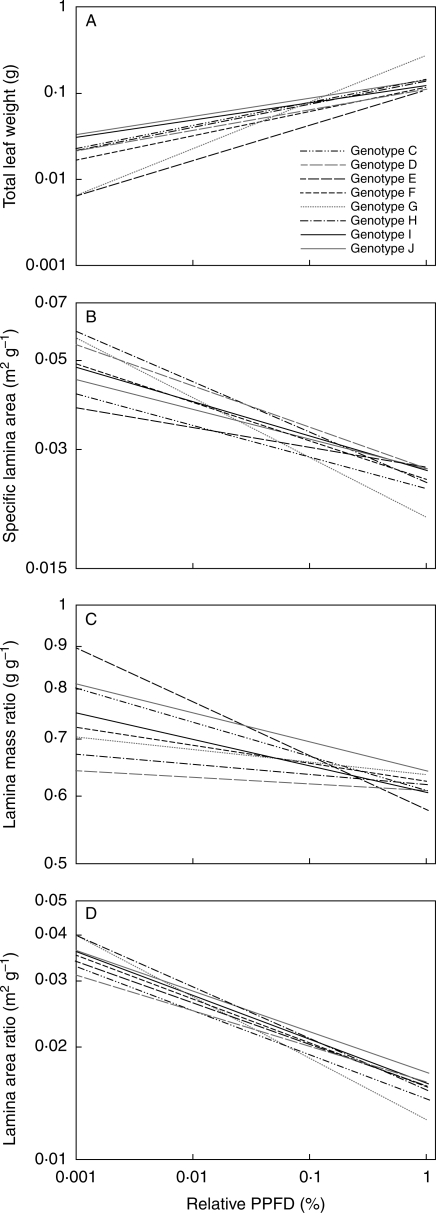
Average lamina height of the top leaves, a proxy for the height of the vegetation, was 28·6 cm ± 0·54 (1 s.e., data not shown). Light availability increased with height within the plots. All morphological characteristics changed with increasing available PPFD. Total leaf weight increased while SLamA, LamMR and LamAR all decreased with increasing light availability (Fig. 2). No interaction was found between the relative PPFD and the morphological traits of the genotypes (Table 1, Among slopes). The genotypes, however, did differ in all leaf characteristics (Table 1, Among intercepts). Although genotypes differed in their LamAR, the variation in this trait between genotypes appeared to be smaller than that in SLamA and LamMR. The three genotypes with the highest SLamA had the lowest LamMR, while for the genotypes with the lowest SLamA the reverse was true. The most abundant genotype in general had average values for leaf characteristics.
Fig. 2.
Allometric relationships between leaf characters of eight genotypes of Potentilla reptans and relative PPFD at the position of each leaf lamina: (A) total leaf weight (g); (B) specific lamina area (m2 g−1); (C) lamina mass ratio (g g−1); (D) lamina area ratio (m2 g−1). Lines represent linear regression lines of the genotypes as indicated, based on log-transformed data of all plots pooled together. Covariance analysis is given in Table 1.
Light capture efficiency
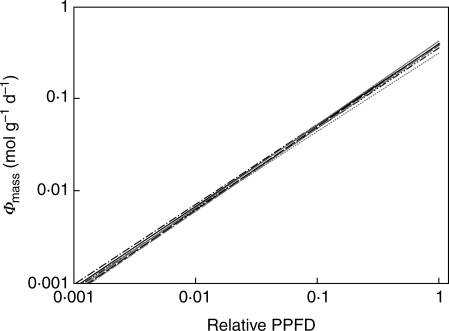
For all genotypes light capture per unit leaf mass (Φmass) increased with increasing PPFD (Fig. 3). As the PPFD decreases with increasing depth, leaves in the top layers thus had higher Φmass than lower-placed leaves. Light capture of a leaf (Φd) increased disproportionately with increasing leaf weight (Fig. 4A). No significant differences in the light capture efficiency (Φmass) of leaves positioned at the same light availability were found between genotypes, indicating that this relationship did not differ among genotypes (Table 1, among intercepts). Also no interaction was found between the genotypes and increasing light availability (among slopes).
Fig. 3.
Relationship between light capture efficiency (Φmass, mol g−1 d−1) and relative PPFD. Lines represent linear regression; for explanation of genotypes, see key in Fig. 2A. Note log scales. For statistics see Table 1.
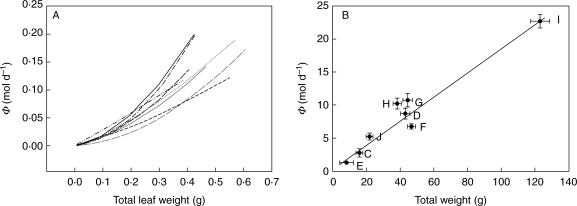
Fig. 4.
Power relationship between light capture (mol d−1) and mass (g). Data are represented on a normal scale for clarity. (A) Power relationship between light capture of individual leaves (mol d−1) and total leaf weight (TLW, g). β is the regression coefficient in the linear expression: log Φd = log α + β logTLW, with β > 1 indicating a disproportional increase of Φ with total leaf weight. Different lines indicate the regressions for different plots. Average β = 1·56, which was significantly larger than 1 (t = 5·42, P = 0·001). (B) Power relationship between average total light capture of the genotypes (mol d−1) and total weight of the genotypes (g). The line represents the regression of all measurement of all eight plots. Average β for all eight plots = 1·03, which was not significantly different from 1 (t = 0·034, P = 0·974).
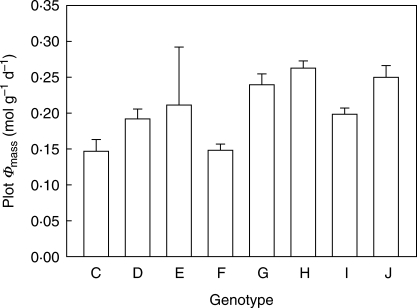
Genotypes with larger amounts of total leaf mass within a plot (TLWp) did not capture disproportionately more light in that plot (Φp) than those with less total leaf mass within a plot (Fig. 4B). Genotypes with relatively more leaves in the upper layer of the vegetation (genotypes G, H and J; Fig. 1) had higher Φmass,p values (Fig. 5). However, the most abundant genotype (I) did not have the highest Φmass,p values, nor did all genotypes that had declined in frequency (such as G and J) have lower Φmass,p values.
Fig. 5.
Plot Φmass (mol g−1 d−1) for the eight genotypes (+ 1 s.e.) as calculated from all leaves within a plot. Results of ANOVA analysis: genotype, F = 3·49, P = 0·005; plot, F = 3·09, P = 0·01.
DISCUSSION
Fitness of a modular organism has been argued to be a product of the response of individual plant parts to the individual growth conditions they experience (De Kroon et al., 2005). Therefore it was expected that the long-term performance of the genotypes would depend on the positioning of the leaves and the efficiency with which these leaves captured light per unit of biomass (Φmass). In contrast to expectation, however, the light capture efficiency was not related to the observed shifts in frequency.
The genotypes did differ in all leaf characteristics for leaves that were placed at the same light availability, including SLamA and LamMR. This, however, did not result in differences in Φmass. In general SLA is considered to be an important factor in determining differences in the relative growth rate between species, which in turn is linked with plant performance; the LMR usually is unrelated (Poorter and Remkes, 1990; Westoby et al., 2002; Reich et al., 2003; Shipley, 2006). In the present study the most abundant genotype did not have the highest SLamA. More remarkably, the genotypes with the highest SLamA had the lowest LamMR. At this point, no definite explanation for this apparent trade-off can be offered. As a consequence the variation in LamAR between the genotypes was small. Because the genotypes did not differ in leaf angle, this in turn means that the genotypes differed little in Φmass for leaves placed at the same height. Also, all genotypes were present in all layers of the canopy. Thus the idea of vertical niche space differentiation whereby each genotype uses a different canopy layer most efficient for light acquisition, as has been found for species in multi-species stands (Hirose and Werger, 1995; Anten and Hirose, 1999), did not apply here for genetically different individuals of the same species.
Since leaves placed at the same light availability did not differ in Φmass and Φmass increased with increasing PPFD, differences between genotypes in light capture efficiency per plot (Φmass,p) depended on the amount of leaves placed in the upper layers of the vegetation. Within genotypes, total daily light capture (Φd) increased disproportionately with total leaf weight, which was reflected in taller leaves having higher Φmass values than shorter ones. These results were consistent with other findings for monospecies stands (Anten and Hirose, 1998; Hikosaka et al., 2003). Taller, heavier leaves thus capture disproportionately more light per unit invested biomass than leaves placed lower in the canopy. This indicates that it is most efficient to place leaves at the top of the canopy as there light conditions are better and it results in shading of other, lower placed leaves. Therefore genotypes that had relatively more leaves placed in the top layer were on average more efficient in light capture and thus captured more light per unit biomass.
This may seem to support the general notion that in dense vegetation stands within-species competition is asymmetric (Hikosaka et al., 1999; Anten and Hirose, 1998; Aan et al., 2006). However, all genotypes could reach the top layers and therefore the most dominant genotypes were not larger in terms of absolute height, and the most dominant genotype did not have relatively more leaves at the top of the canopy. Therefore neither the final height of a genotype, nor the relative number of leaves at the top was correlated to its total mass. As a consequence, total light interception per genotype was linearly related to their total biomass, indicating that the more abundant genotypes that constituted most of the biomass did not capture more than proportionate amounts of light (i.e. they did not have higher Φmass,p values). This in turn indicates that competition for light between different genotypes was size-symmetric, in the sense that in terms of biomass larger individuals did not obtain a disproportionate share of the resource (sensu Weiner, 1990).
The reason why competition between species may be symmetric, while within species competition is asymmetric might stem from the relatively large variation in LMR, SLA and thus LAR between species, which results partly from intrinsic differences in shoot architecture (Anten and Hirose, 1999; Anten, 2005). Such differences obviously did not exist between the different genotypes in this study, and therefore cannot account for the symmetric competition for light that was found among genotypes in this experiment. It is possible that the symmetry in light competition found here is associated with the intrinsic architecture of many stoloniferous plants. It has been pointed out that the degree of asymmetry may be more related to the speed of height increment of plants in a stand and not so much to the height ultimately achieved (Schwinning, 1996; van Kuijk et al., 2008). In stands of stoloniferous plants such P. reptans each new leaf has to start from the bottom of the vegetation, which causes height increment of the vegetation to be slower than in stands of stem plants where leaves are formed at the top of the canopy. In an experiment with five of the genotypes that were used in the present study, it was found that when the increase in the light gradient was slow, all five genotypes used could reach the top, despite initial differences in height and in plasticity therein (Vermeulen et al., 2008). The height increase in that experiment was comparable to the height growth measured in the present competition experiment. This vegetation may thus be an example where the plasticity of the genotypes and the low height growth rate of the vegetation allows them all to reach the top of the canopy, thereby preventing the occurrence of asymmetric competition for light (Aphalo et al., 1999; Ballaré, 1999).
The reason why no asymmetric competition for light was found, while genotypes differed in the relative number of leaves at the top of the canopy, may be due to the dynamics of the system. Surveys in 2005 showed that leaf turnover is very high in this canopy (six to eight leaves were formed and shed between early April and the end of June; Vermeulen, 2008). Genotypes of Potentilla reptans place their leaves at or near the top of a light gradient (Vermeulen et al., 2008). These leaves will be shaded by new leaves that are placed above the older ones. A possible explanation why the dominant genotype still had a lot of leaves at the lower part of the canopy, and therefore lower Φmass,p values, is that it retained its old leaves longer. Other genotypes with relatively more leaves at the top may have shed their lower-placed leaves, and thus part of the invested mass has already been lost. Possibly, there are two opposing mechanisms at work each of which may promote coexistence: a high leaf turnover that allows for investing the carbon and nutrients in new leaves at the top of the canopy (Oikawa et al., 2006; Boonman et al., 2006; Selaya, 2007), or a longer leaf longevity, which could lead to a higher life-time carbon gain of a leaf (see also Poorter, 1994; Westoby et al., 2000). Yet measured at one point in time, the light capture per unit mass will be higher for the former strategy.
Asymmetric competition implies that differences between plants in relative growth rate will increase and thus that size inequality increases (Weiner and Thomas, 1986; Weiner, 1990; Hara, 1992). Our finding that competition is not asymmetric suggests that the differences in frequency between the dominant genotypes and the others will not increase in this way, and thus that the dominant genotype is not able to exclude all other genotypes from the vegetation through differences in light capture (see also De Kroon et al., 1992). Other, so far unknown factors may be better able to explain the shifts in frequencies that have occurred over 5 years. Competition for below-ground resources, for example, has been shown to be asymmetric in some cases (see Weiner and Thomas, 1986; Hikosaka and Hirose, 2001). In addition asymmetry in growth results not only from asymmetry in resource acquisition but can also be due to differences in resource-use efficiency and herbivory levels.
In conclusion, the present data show that spatial niche differentiation in light acquisition is not a factor that could explain the possible coexistence between genotypes in our study. Contrary to what is generally assumed, however, the present data show that in these vegetation stands of stoloniferous plants, competition for light between individuals of the same species can be symmetric, even if taller leaves capture disproportionately more light per unit mass. The shifts in genotype frequency that occurred in the present 5-year study can therefore not be explained by differences in light-capture efficiency.
ACKNOWLEDGEMENTS
We thank Annemiek Smit-Tiekstra, Henri Noordman, Sander van Hal, Betty Verduyn and Sonja Huggers for technical assistance and Prof. M. J. A. Werger and Prof. T. Hirose for comments on the manuscript.
LITERATURE CITED
- Aan A, Hallik L, Kull O. Photon flux partitioning among species along a productivity gradient of an herbaceous plant community. Journal of Ecology. 2006;94:1143–1155. [Google Scholar]
- Aerts R. Interspecific competition in natural plant communities: mechanisms, trade-offs and plant-soil feedbacks. Journal of Experimental Botany. 1999;50:29–37. [Google Scholar]
- Anten NPR. Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Annals of Botany. 2005;95:495–506. doi: 10.1093/aob/mci048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Hirose T. Biomass allocation and light partitioning among dominant and subordinate individuals in Xanthium canadense stands. Annals of Botany. 1998;82:665–673. [Google Scholar]
- Anten NPR, Hirose T. Limitations on photosynthesis of competing individuals in stands and the consequences for canopy structure. Oecologia. 2001;129:186–196. doi: 10.1007/s004420100718. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Hirose T. Interspecific differences in above-ground growth patterns result in spatial and temporal partitioning of light among species in a tall-grass meadow. Journal of Ecology. 1999;87:583–597. [Google Scholar]
- Aphalo PJ, Ballaré CL, Scopel AL. Plant–plant signalling, the shade-avoidance response, and competition. Journal of Experimental Botany. 1999;50:1629–1634. [Google Scholar]
- Ballaré CL. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends in Plant Science. 1999;4:201. doi: 10.1016/s1360-1385(99)01408-9. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant, Cell & Environment. 1987;10:551–557. [Google Scholar]
- Ballaré CL, Scopel AL, Sanchez RA. Far-red radiation reflected from adjacent leaves – an early signal of competition in plant canopies. Science. 1990;247:329–332. doi: 10.1126/science.247.4940.329. [DOI] [PubMed] [Google Scholar]
- Bell AD. Plant form. Oxford: Oxford Univerisity Press; 1991. [Google Scholar]
- Berntson GM, Wayne PM. Characterizing the size dependence of resource acquisition within crowded plant populations. Ecology. 2000;81:1072–1085. [Google Scholar]
- Bonser SP, Aarssen LW. Allometry and development in herbaceous plants: functional responses of meristem allocation to light and nutrient availability. American Journal of Botany. 2003;90:404–412. doi: 10.3732/ajb.90.3.404. [DOI] [PubMed] [Google Scholar]
- Boonman A, Anten NPR, Dueck TA, Jordi WJRM, Van der Werf A, Voesenek LACJ, et al. Functional significance of shade-induced leaf senescence in dense canopies: an experimental test using transgenic tobacco. American Naturalist. 2006;168:597–607. doi: 10.1086/508633. [DOI] [PubMed] [Google Scholar]
- Corré WJ. Growth and morphogenesis of sun and shade plants. I. The influence of light intensity. Acta Botanica Neerlandica. 1983;a 32:49–62. [Google Scholar]
- Corré WJ. Growth and morphogenesis of sun and shade plants. II. The influence of light quality. Acta Botanica Neerlandica. 1983;b 32:185–202. [Google Scholar]
- De Kroon H, Hara T, Kwant R. Size hierarchies of shoots and clones in clonal herb monocultures – do clonal and nonclonal plants compete differently. Oikos. 1992;63:410–419. [Google Scholar]
- De Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytologist. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Dong M. Morphological responses to local light conditions in clonal herbs from contrasting habitats, and their modification due to physiological integration. Oecologia. 1995;101:282–288. doi: 10.1007/BF00328813. [DOI] [PubMed] [Google Scholar]
- Falster DS, Westoby M. Plant height and evolutionary games. Trends in Ecology & Evolution. 2003;18:337–343. [Google Scholar]
- Givnish TJ. On the adaptive significance of leaf height in forest herbs. American Naturalist. 1982;120:353–381. [Google Scholar]
- Givnish TJ. Plant stems: biomechanical adaptation for energy capture and influence on species distribution. In: Gartner BL, editor. Plant stems: physiology and functional morphology. San Diego, CA: Academic Press; 1995. pp. 3–49. [Google Scholar]
- Goldberg DE, Miller TE. Effects of different resource additions on species-diversity in an annual plant community. Ecology. 1990;71:213–225. [Google Scholar]
- Goudriaan J. Crop micrometeorology: a simulation study. Wageningen: Pudoc; 1977. [Google Scholar]
- Hara T. Effects of the mode of competition on stationary size distribution in plant populations. Annals of Botany. 1992;69:509–513. [Google Scholar]
- Hikosaka K, Hirose T. Nitrogen uptake and use by competing individuals in a Xanthium canadense stand. Oecologia. 2001;126:174–181. doi: 10.1007/s004420000517. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Sudoh S, Hirose T. Light acquisition and use by individuals competing in a dense stand of an annual herb. Xanthium canadense. Oecologia. 1999;118:388–396. doi: 10.1007/s004420050740. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Yamano T, Nagashima H, Hirose T. Light-acquisition and use of individuals as influenced by elevated CO2 in even-aged monospecific stands of Chenopodium album. Functional Ecology. 2003;17:786–795. [Google Scholar]
- Hirose T, Werger MJA. Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology. 1995;76:466–474. [Google Scholar]
- Huber H. Growth form and plasticity of the hybrid Potentilla anglica and its two parent species. Abstracta Botanica. 1995;19:61–73. [Google Scholar]
- Huber H. Plasticity of internodes and petioles in prostrate and erect Potentilla species. Functional Ecology. 1996;10:401–409. [Google Scholar]
- Huber H, Fijan A, During HJ. A comparative study of spacer plasticity in erect and stoloniferous herbs. Oikos. 1998;81:576–586. [Google Scholar]
- van Kuijk M, Anten NPR, Oomen RJ, Van Bentum DW, Werger MJA. The limited importance of size-asymmetric light competition and growth of pioneer species in early secondary forest succession in Vietnam. Oecologia. 2008;157:1–12. doi: 10.1007/s00442-008-1048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schieving F, Stuefer JF, Anten NPR. The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Annals of Botany. 2007;99:121–130. doi: 10.1093/aob/mcl230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsi M, Saeki T. Über den lichtfaktor in den pflanzengesellschaften und seine bedeutung für die stoffproduktion. Japanese Journal of Botany. 1953;15:60–82. [Google Scholar]
- Nagashima H, Terashima I, Katoh S. Effects of plant-density on frequency-distributions of plant height in Chenopodium album stands – analysis based on continuous monitoring of height growth of individual plants. Annals of Botany. 1995;75:173–180. [Google Scholar]
- Oikawa S, Hikosaka K, Hirose T. Leaf lifespan and lifetime carbon balance of individual leaves in a stand of an annual herb. Xanthium canadense. New Phytologist. 2006;172:104–116. doi: 10.1111/j.1469-8137.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- Ongaro V, Leyser O. Hormonal control of shoot branching. Journal of Experimental Botany. 2008;59:67–74. doi: 10.1093/jxb/erm134. [DOI] [PubMed] [Google Scholar]
- Poorter H. Construction cost and payback time of biomass: a whole plant perspective. In: Roy E, Garnier E, editors. A whole plant perspective on carbon–nitrogen interactions. The Hague: SPB Academic; 1994. pp. 111–127. [Google Scholar]
- Poorter H, Remkes C. Leaf-area ratio and net assimilation rate of 24 wild-species differing in relative growth-rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, et al. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences. 2003;164:S143–S164. [Google Scholar]
- Schmitt J, Wulff RD. Light spectral quality, phytochrome and plant competition. Trends in Ecology and Evolution. 1993;8:47–51. doi: 10.1016/0169-5347(93)90157-K. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Stinchcombe JR, Heschel MS, Huber H. The adaptive evolution of plasticity: phytochrome-mediated shade avoidance responses. Integrative and Comparative Biology. 2003;43:459–469. doi: 10.1093/icb/43.3.459. [DOI] [PubMed] [Google Scholar]
- Schwinning S. Decomposition analysis of competitive symmetry and size structure dynamics. Annals of Botany. 1996;77:47–57. [Google Scholar]
- Schwinning S, Weiner J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia. 1998;113:447–455. doi: 10.1007/s004420050397. [DOI] [PubMed] [Google Scholar]
- Selaya NG. Sprinting, climbing and persisting; light interception and carbon gain in a secondary tropical forest succession. The Netherlands: Utrecht University; 2007. PhD thesis. [Google Scholar]
- Shipley B. Net assimilation rate, specific leaf area and leaf mass ratio: which is most closely correlated with relative growth rate? A meta-analysis. Functional Ecology. 2006;20:565–574. [Google Scholar]
- Stuefer JF, Van Hulzen JB, During HJ. A genotypic trade-off between the number and size of clonal offspring in the stoloniferous herb Potentilla reptans. Journal of Evolutionary Biology. 2002;15:880–884. [Google Scholar]
- Tilman D, Pacala S. The maintenance of species richness in plant communities. In: Ricklefs RE, Schluter D, editors. Species diversity in ecological communities: historical and geographical perspectives. Chicago, IL: University of Chicago Press; 2006. pp. 13–25. [Google Scholar]
- Van der Meijden R. Heukels' Flora van Nederland. Groningen: Wolters-Noordhoff; 1996. [Google Scholar]
- Vermeulen PJ. Plastic responses in the competition for light among genotypes of a stoloniferous plant. The Netherlands: Utrecht University; 2008. PhD Thesis. [Google Scholar]
- Vermeulen PJ, Anten NPR, Schieving F, Werger MJA, During HJ. Height convergence in response to neighbour growth: genotypic differences in the stoloniferous plant Potentilla reptans. New Phytologist. 2008;177:688–697. doi: 10.1111/j.1469-8137.2007.02301.x. [DOI] [PubMed] [Google Scholar]
- Weiner J. Asymmetric competition in plant-populations. Trends in Ecology and Evolution. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- Weiner J, Solbrig OT. The meaning and measurement of size hierarchies in plant populations. Oecologia. 1984;61:334–336. doi: 10.1007/BF00379630. [DOI] [PubMed] [Google Scholar]
- Weiner J, Thomas SC. Size variability and competition in plant monocultures. Oikos. 1986;47:211–222. [Google Scholar]
- Werger MJA, Hirose T, During HJ, Heil GW, Hikosaka K, Ito T, et al. Light partitioning among species and species replacement in early successional grasslands. Journal of Vegetation Science. 2002;13:615–626. [Google Scholar]
- Westoby M, Warton D, Reich PB. The time value of leaf area. American Naturalist. 2000;155:649–656. doi: 10.1086/303346. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]