Abstract
Background and Aims
In neotropical forests, very small-seeded pioneer species (<0·1 mg seed mass) recruit preferentially in small tree fall gaps and at gap edges, but large-seeded pioneers do not. Since water availability is related to gap size, these differences in microsite preference may reflect in part species-specific differences in germination at reduced water potentials.
Methods
For 14 neotropical pioneer species, the hypothesis is tested that small-seeded species, with shallow initial rooting depths, reduce the risks associated with desiccation by germinating more slowly and at higher water potentials than large-seeded species.
Key Results
Germination occurred both more quickly and at lower water potentials with increasing seed mass. For example, Ochroma pyramidale (seed mass 5·5 mg) had a time to 50 % germination (T50) of 2·8 d and a median base potential for germination (ψb50) of −1·8 MPa while Clidemia quinquenervia (seed mass 0·017 mg) had a T50 of 17·6 d and ψb50 of −1·1 MPa.
Conclusions
These data suggest that small-seeded species germinate only in comparatively moist microsites, such as small canopy gaps, which may reduce the risk of drought-induced mortality. Conversely, large-seeded species are able to germinate in the drier environment of large gaps, where they benefit by enhanced seedling growth in a high irradiance environment. The positive association of seed size and canopy gap size for optimal seedling establishment is maintained by differential germination responses to soil water availability coupled with the scaling of radicle growth rate and seed size, which collectively confer greater drought tolerance on large-seeded species.
Key words: Germination, seed size, Panamá, neotropical, pioneer, water potential
INTRODUCTION
Seed size is a key variable for predicting patterns of plant regeneration. For example, in temperate herbaceous plant communities, seed persistence in the soil is associated with small seed size (Thompson, 1987; Thompson et al., 1993), whereas in neotropical forests long-term persistence of pioneer species is associated with relatively large-seeded species (Dalling and Burslem, 2005; Dalling and Brown, unpubl. res.). Across a range of ecosystems small-seeded species are also more likely to require light as a cue to germinate in canopy openings or gaps in leaf litter (Milberg et al., 2000; Pearson et al., 2002; Jankowska-Blaszczuk and Daws, 2007). However, the importance of seed mass for other aspects of germination, such as the response to water potential, has received little attention and yet is a likely determinant of successful establishment.
Among tropical shrubs and trees, a functional group labelled ‘pioneers’ has been identified that consists of fast-growing early successional species that need canopy gaps for establishment (Swaine and Whitmore, 1988). In central Panamá, pioneers have seed sizes from approx. 0·02 mg to 100 mg (Daws et al., 2007a) and occur in canopy gaps of differing size. Along the understorey–gap continuum, the quantity of irradiance, and hence the amplitude of soil temperature fluctuations and the rate of soil surface drying, increase with increasing gap size (Denslow et al.,1998; Daws et al., 2002, 2007a; Pearson et al., 2002). For example, in large canopy gaps at the present study site in central Panamá (Barro Colorado Island), irregular rainfall during the wet-season can result in the soil matric potential at 10 mm depth falling to less than −1·5 MPa (the permanent wilting point) after just 6 d of drying (Engelbrecht et al., 2006; Daws et al., 2007a; Marthews et al., 2008). In some small gaps, water extraction by the roots of the surrounding trees results in surface soil drying up to but not beyond the wilting point, while litter-free soil in the centre of large gaps continues to dry beyond the wilting point (Marthews et al., 2008). This potential for rapid drying beyond the wilting point implies that the risk of mortality from desiccation for germinating seeds/establishing seedlings increases with gap size (Engelbrecht et al., 2006; Daws et al., 2007a, b).
Large-seeded neotropical pioneer species generally have a distribution that is biased towards the ‘risky’ environment of larger sizes of canopy gap (Brokaw, 1987; Pearson et al., 2003a), although these studies did not invoke seed size to account for these differential distribution patterns (but see Dalling et al., 2004). In addition, germination requirements of large-seeded pioneers typically appear to be ‘cued’ to large gap environments, i.e. they require either a high ratio of red:far red light (R:FR) or a large amplitude of diurnal temperature fluctuations for germination (Vázquez-Yanes and Orozco-Segovia, 1982; Daws et al., 2002; Pearson et al., 2002).
The risk of desiccation-induced mortality may be lower for seedlings from large seeds since such seedlings can emerge from greater soil depths (Bond et al.,1999; Pearson et al., 2002) and have more rapid radicle growth rates (Daws et al., 2007a) than seedlings from small seeds. Consequently, it is hypothesized that large-seeded species may be better able to ‘cope’ with the risk associated with large canopy gaps. The high light environment of large gaps is likely to be advantageous for seedlings that can establish successfully, by facilitating rapid post-establishment growth, as long as this occurs in advance of the frequent dry spells in the early wet season. For these potentially ‘risk-taking’ species, the risk of rapid emergence may be tempered by a rapid radicle growth and hence access to soil water at depth (Daws et al., 2007a). However, for small-seeded species that can germinate only at shallow depths, where soil drying occurs rapidly, it is predicted that germination will occur more slowly. This response will limit germination either to large gaps in prolonged wet spells or to the less strongly drying environment of small gaps.
Soil water potential modulates seed germination, and species differ in the minimum water potential at which germination can occur (Kaufmann, 1969; Evans and Etherington, 1990; Choinski and Tuohy, 1991; Allen et al., 2000; Daws et al., 2002). For neotropical pioneer shrubs and trees, only four species from one genus have been characterized for their germination response to water availability (Daws et al., 2002). These congeners had a limited range of seed mass values and showed only modest differences in the base water potential for germination (Daws et al., 2002). Since large-seeded pioneers occur more frequently in large gap environments, it was predicted that they would germinate at lower matric potentials than small-seeded species. Such a response would potentially complement rapid germination in enabling rapid establishment in the unpredictable large gap environment.
Consequently, in this paper, the predictions that for pioneer species (a) large-seeded species germinate more rapidly than small-seeded species and (b) that large-seeded species are able to germinate under lower water potentials (drier conditions) than small-seeded species are tested. The results are discussed in the context of risk-spreading in environments of unpredictable rainfall.
MATERIALS AND METHODS
Study site and species
Ripe fruits of the ten study species (Table 1) were collected between February and July 1999 from semi-deciduous forest on Barro Colorado Island (BCI; 9°09'N, 79°51'W), Republic of Panamá. Mean annual rainfall at BCI is approx. 2700 mm with about 93 % of the total occurring in the wet season between May and December (Croat, 1978). Fruits were collected from at least five individual plants per species and seeds extracted by mashing the fruits in water and decanting off the fruit pulp. Subsequently, seeds were air-dried in the dark, transported to Aberdeen, UK, and stored at room temperature prior to the start of germination experiments. In addition, seed mass and germination data for a further four pioneer species from BCI (Piper dilatatum, P. hispidum, P. marginatum and P. peltatum) were obtained from Daws et al. (2002).
Table 1.
Summary information of seeds used in this study
| Species | Family* | Habitat information | Seed mass (mg) | T50 (d) |
|---|---|---|---|---|
| Apeiba tiborbou | Malvaceae–Tiliaceae | Abandoned farms, road edges, canopy gaps† | 5·6 | 7·1 |
| Cecropia insignis | Moraceae | Canopy gaps† | 0·68 | 5·6 |
| Cecropia longipes | Moraceae | Canopy gaps‡, open areas§ | 1·2 | 6·3 |
| Cecropia obtusifolia | Moraceae | Tree fall areas§ | 0·58 | 5·9 |
| Cecropia peltata | Moraceae | Tree fall areas§ | 0·59 | 14·0 |
| Clidemia octona | Melastomataceae | Forest edge§ | 0·015 | 21·4 |
| Clidemia quinquenervia | Melastomataceae | Edge of clearings§ | 0·017 | 17·6 |
| Luehea seemannii | Malvaceae–Tiliaceae | Along roads, canopy gaps† | 1·7 | 4·1 |
| Miconia argentea | Melastomataceae | Along roads, canopy gaps† | 0·076 | 11·3 |
| Ochroma pyramidale | Malvaceae–Bombacaceae | Large clearings† | 5·5 | 2·8 |
| Disturbed areas§ |
Seed germination
Seeds were subjected to water potentials of approx. 0, −0·25, −0·5 and −1·0 MPa by placing three replicates of 25 seeds each on the surface of a wick connected to a reservoir of polyethylene glycol 8000 (PEG), at a concentration appropriate to the intended treatment (Michel and Kaufmann, 1973), following the method outlined by Daws et al. (2002). The PEG solution flowed over the wick, maintaining the seeds at a constant water potential. Sub-samples of the wicks were removed after the experiment had been running for 20 d, and their actual water potential determined using an SC-10A Richards thermocouple psychrometer (Decagon Devices, Pullman, WA, USA). Measured rather than nominal values of water potential were used in all analyses; measured values were all within 20 % of the target values and had standard deviations <15 % of the mean value.
Seeds of Ochroma pyramidale and Apeiba tibourbou require a dormancy-breaking treatment. Consequently, to ensure germination in the water potential treatments, seeds of A. tibourbou and O. pyramidale were treated with hot water at 80 °C and 90 °C, respectively, for 2 min (Acuña and Garwood, 1987; Daws et al., 2006).
The germination system was housed in transparent polyethylene boxes and placed in a growth chamber at 26 °C, with a 12-h photoperiod. Although for some species base potential has been shown to be dependent on temperature (Dahal et al., 1993; Kebreab and Murdoch, 1999), a single temperature (26 °C) was used. Even though soil surface temperatures in large gaps are elevated close to mid-day, time-courses of temperature at 1-cm depth suggest that values for both small and large gaps are within 1 °C of 26 °C for a mean of 87·5 % and 67·4 %, respectively, each day (Daws et al., 2002). Irradiance inside the transparent boxes averaged 5 µmol m−2 s−1, the R : FR was approx. 2·0. Seeds were scored daily for germination for up to 50 d with germination defined as visible radicle emergence.
Data analysis
Time to 50 % of maximum germination (T50) at 0 MPa (water) was determined for each species from plots of germination against time.
The effect of water potential on time to germination has been described by the hydrotime model of germination (Bradford, 1990). In this model seeds germinate only when they have accumulated sufficient hydrotime (θH). At and below a base water potential (ψb) seeds do not start to germinate. Above this potential, seeds accumulate hydrotime. Within seed lots a normal distribution of base potentials is typically observed (Bradford, 1990; Dahal and Bradford, 1990; Gummerson, 1996) while the hydrotime for germination is constant for all individual seeds (Bradford, 1990; Dahal and Bradford, 1990). Thus, the fastest seeds to germinate in a population have the lowest base potentials and are able to accumulate hydrotime at lower potentials than slower-germinating seeds. Thus germination can be described as:
| 1 |
where θHg is the hydrotime (in MPa h−1) required for germination of proportion g of the seed lot, ψw is the actual water potential (MPa), ψbg is the base potential of proportion g of the seed lot and tg is the length of time since the start of imbibition.
By rearranging eqn (2) in terms of base potential:
| 2 |
it is possible to determine ψb50 and the standard deviation of base potential (σψb) by plotting experimentally determined germination progress curves, on a probit scale (for the experimentally studied range of water potentials), against base potential [ψw – (θH/tg)]. Thus, the unknown values of θH can be estimated iteratively (Bradford, 1990). If the value of θH is repeatedly changed until the best fit (i.e. the value with minimal residual variation) is obtained when a single least squares regression line is fitted to the data for the range of water potentials, then ψb50 corresponds to the base potential when germination is 50 % of the maximum observed germination level and the reciprocal of the slope of the regression line is the standard deviation of the base potential (σψb).
RESULTS
Effect of water potential on germination
For all ten species, germination percentage and rate of germination decreased as water potential became more negative. Thus, germination was most rapid and reached the highest percentage at 0 MPa (water) and was lowest at 1·0 MPa. In addition, the negative effect of −0·25 and −0·5 MPa was relatively minor for most species, with a greater impact of −1·0 MPa (data not shown).
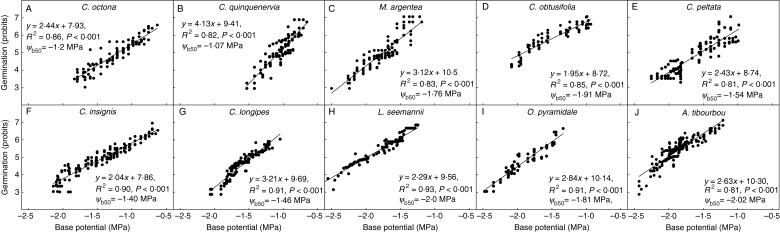
Plotting germination (in probits) against base potential indicated that the median base water potential (the base potential when germination in probits is 5; ψb50) ranged from −1·07 to −2·06 MPa (Clidemia quinquenervia and Apeiba tibourbou, respectively; Fig. 1). On Fig. 1 each point represents an observation from the four germination progress curves with values of base potential determined after iteration of the hydrotime constant, θH, in the expression [ψw –(θH/tg)] to maximize the fit of the linear regression. Therefore species that take longer to germinate have more observations per germination progress curve, which is reflected in a greater number of points on the corresponding graphs.
Fig. 1.
Plots of germination, on a probit scale (a probit value of 5 corresponds to 50 % germination), against the median base water potential for germination (ψb50) for the ten study species. Species (A–J) are ranked in order of increasing seed mass.
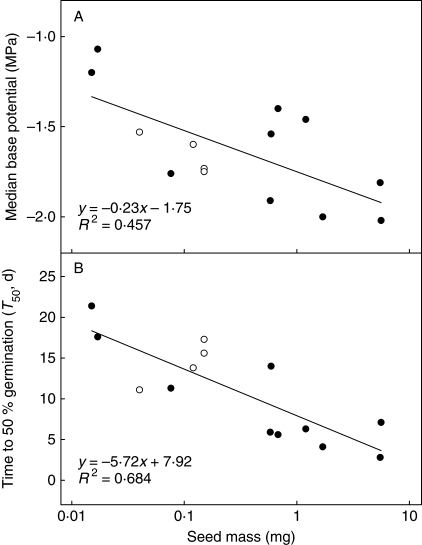
For the ten study species and four additional species from Daws et al. (2002), there was a significant negative relationship between the median base water potential and seed mass (linear regression, R2 = 0·457, d.f. = 12, P = 0·008): small-seeded species were less able to germinate at low water potentials (Fig. 2A).
Fig. 2.
The relationship between seed mass and (A) the median base water potential for germination (ψb50) and (B) time to 50 % germination (T50) at 0 MPa (water) for 14 pioneer species. Closed symbols refer to data from this current study, open symbols to data for Piper dilatatum, P. hispidum, P. marginatum and P. peltatum from Daws et al. (2002).
Time to 50 % germination and seed mass
Time to 50 % germination (T50) in the 0-MPa (water) treatment for the 14 species was significantly negatively related to seed mass (linear regression, R2 = 0·684, d.f. = 12, P < 0·001; Fig. 2B). For example, Ochroma pyramidale (seed mass = 5·5 mg) reached 50 % of maximum germination in 2·8 d while Clidemia octona (seed mass = 0·015 mg) took 21·4 d. In addition, T50 and ψb50 were significantly correlated, i.e. the faster-germinating species could germinate at more negative water potentials (Pearson's correlation coefficient, r = 0·573, d.f. = 12, P < 0·05).
DISCUSSION
There were substantial differences in the germination responses of BCI pioneer species in both T50 and ψb50 that were related to seed size. The low base potential and rapid germination (low T50) of large-seeded species suggests a ‘risky’ germination strategy that has the potential to enable germination in large gaps and result in microsite pre-emption in advance of slower-germinating species. However, smaller-seeded species are more ‘conservative’, germinating slowly and at less-negative water potentials, which may reduce the likelihood of seedling mortality by ensuring germination occurs primarily in either small gaps or large gaps during prolonged wet periods. For the 14 species examined here and by Daws et al. (2002) seed size differences are confounded by phylogenetic contrasts at the family level, and there were no relationships between T50 or ψb50 and seed size within any of the four families (Fig. 2). Consequently, phylogenetic effects independent of seed size may also be important: data from a larger sample of species and families would help to clarify this issue.
Base water potential and germination microsite
The 14 species exhibited differences of >1 MPa in ψb50. Species with more negative values of ψb50 (e.g. Ochroma pyramidale, −1·8 MPa) can progress towards germination under drier conditions, which may facilitate germination in large (drier) gaps. In contrast, a higher ψb50 will limit germination to small gaps and potentially also to large gaps during extended wet periods. A wide range of ψb50 values has also been reported for other species. For example, Allen et al. (2000) report values for 24 desert species ranging from −5·92 to −0·07 MPa, although most values were clustered in the range −1·8 to −0·07 MPa. Similarly, Evans and Etherington (1990) found that among 15 British plant species there was a wide range of responses to water potential with differences between species partly corresponding to habitat type. Thus, the three species with germination most sensitive to decreasing water potentials were all wet-land species (e.g. Juncus articulatus). However, among the remaining species there was little clear pattern in sensitivity to water potential in relation to habitat type.
Role of seed size in germination
Brokaw (1987) inferred gap partitioning among the pioneer species Miconia argentea, Cecropia insignis and Trema micrantha, based on the observation that plants of these species grow to maturity in gaps larger than 102, 215 and 376 m2, respectively, on BCI. While these differences have been related to a trade-off between growth-rate in high light and survival in the shade (Brokaw, 1987), seed size also increases in the same order in these three species: Miconia argentea < Cecropia insignis < Trema micrantha. Similarly, large-seeded species such as Ochroma pyramidale and Cecropia obtusifolia also occur preferentially in larger gap sizes (Croat, 1978; Pearson et al., 2003a). On the other hand, the small-seeded Clidemia octona and Clidemia quinquenervia occur preferentially in small gaps or the edges of large gaps (Croat, 1978). A role for seed mass in contributing to observed gap size preferences is supported by the correlation between ψb50 and seed mass: small-seeded species germinate less well at low water potentials and hence are less able to germinate in the drier environment of large gaps.
Successful establishment in large gaps may necessitate rapid germination to enable emergence in advance of potentially rapid soil drying (at least close to the soil surface), which is supported by the rapid germination of the larger-seeded species (Fig. 1B). Large seed size may also facilitate germination and establishment in large gaps by enabling emergence from greater soil depths (Bond et al., 1999; Pearson et al., 2002) where soil drying will be less rapid. However, even for shallowly buried large seeds, the risk of seedling mortality may be lower than for small-seeded species since the rate of radicle extension is allometrically related to seed mass: radicle growth of seedlings from large seeds may progress faster than the soil drying front in the event of a post-germination dry spell (Daws et al., 2007a). Indeed, several studies have reported that seedlings from small seeds have higher mortality under water stress than those from large seeds (Buckley, 1982; Leishman and Westoby, 1994). Thus, for these pioneer species it is proposed that for small-seeded species, a combination of rapid germination and germination at low water potentials is unlikely since it would result in extremely high seedling mortality in the event of a post-germination dry spell. Similarly, Doussi and Thanos (2002) suggested that slow germination of Mediterranean species was an adaptation to unpredictable water availability since this strategy ensures that germination only occurs with the onset of reliable winter rainfall (i.e. it prevents germination occurring following sporadic early autumn/winter rainfall).
However, the mechanism(s) resulting in slow germination in the small-seeded species is unclear. Small seeds have a faster water absorption capacity than large seeds resulting from a larger surface area to mass ratio (Kikuzawa and Koyama, 1999), suggesting that the opposite pattern might be expected. Alternatively, the mechanical restraint provided by the seed coat might contribute to delaying germination. However, Daws et al. (2007a) found no relationship between seed mass and the proportion of seed mass that constituted seed coat, which suggests that this may not contribute to the differences in T50. Consequently, further studies are needed to understand the physiological basis of these differences in germination timing.
The present data for neo-tropical pioneers suggest a continuum between large-seeded ‘risk-takers’, with rapid germination and a low ψb50, and small-seeded ‘risk-averse’ species with slow germination and a high ψb50. This trend is further supported by the pattern of response in germination observed in relation to seed mass and R : FR for pioneer taxa on BCI. Thus, Pearson et al. (2003b) reported that among species with photoblastic germination, large-seeded species require a higher R : FR to trigger germination than small seeds. In this scenario, large-seeded species only germinate in the higher risk environment of large gaps, while the small-seeded species can germinate in all gap sizes. However, these kinds of patterns are not evident in the data of Allen et al. (2000) for 24 desert species where no relationship between ψb50 and germination speed was observed. One explanation for this discrepancy is that the analysis of Allen et al. (2000) was potentially confounded by the inclusion of species belonging to various functional groupings (halophytes, psammophytes and generalists) while the present study is based on species from one functional group collected from the same location. Clearly, further studies are needed to test the generality of seed mass, germination rate and ψb50 relationships using co-occurring species from additional habitat types that span a range of seed masses and that belong to the same functional group.
ACKNOWLEDGEMENTS
We thank Charles Hutchinson for technical assistance. Prof. H. W. Pritchard (Royal Botanic Gardens, Kew) commented on an earlier draft of this manuscript. This work was funded by the Natural Environment Research Council (grant to D.F.R.P.B.).
LITERATURE CITED
- Acuña PI, Garwood NC. Effect of light and scarification on the germination of five species of tropical secondary trees. Revista de Biologia Tropicale. 1987;35:203–207. [Google Scholar]
- Allen PS, Meyer SE, Khan MA. Hydrothermal time as a tool in comparative germination studies. In: Black M, Bradford KJ, Vazquez-Ramos J, editors. Seed biology: advances and applications. Wallingford: CAB International; 2000. pp. 401–410. [Google Scholar]
- APGII. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnaean Society. 2003;141:399–436. [Google Scholar]
- Bond WJ, Honig M, Maze KE. Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia. 1999;120:132–136. doi: 10.1007/s004420050841. [DOI] [PubMed] [Google Scholar]
- Bradford KJ. A water relations analysis of seed germination rates. Plant Physiology. 1990;94:840–849. doi: 10.1104/pp.94.2.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw NVL. Gap-phase regeneration of three pioneer tree species in a tropical forest. Journal of Ecology. 1987;75:9–19. [Google Scholar]
- Buckley RC. Seed size and seedling establishment in tropical arid dunecrest plants. Biotropica. 1982;14:314–315. [Google Scholar]
- Choinski JS, Tuohy JM. Effect of water potential and temperature on the germination of four species of African savanna trees. Annals of Botany. 1991;68:227–233. [Google Scholar]
- Croat TB. Flora of Barro Colorado Island. Stanford, CA: Stanford University Press; 1978. [Google Scholar]
- CTFS. Trees of the Panama Canal area. 2004. http://ctfs.si.edu/webatlas/maintreeatlas.html .
- Dahal P, Bradford KJ. Effects of priming and endosperm integrity on seed germination rates of tomato genotypes. II. Germination at reduced water potential. Journal of Experimental Botany. 1990;41:1441–1453. [Google Scholar]
- Dahal P, Bradford KJ, Haigh AM. The concept of hydrothermal time in seed germination and priming. In: Côme D, Corbineau F., editors. Basic and applied aspects of seed biology. Angers: Proceedings of the Fourth International Workshop on Seeds; 1993. pp. 1009–1014. [Google Scholar]
- Dalling JW, Winter K, Hubbell SP. Variation in growth responses of neotropical pioneers to simulated forest gaps. Functional Ecology. 2004;18:725–736. [Google Scholar]
- Daws MI, Burslem DFRP, Crabtree LM, Kirkman P, Mullins CE, Dalling JW. Differences in seed germination responses may promote coexistence of four sympatric Piper species. Functional Ecology. 2002;16:258–267. [Google Scholar]
- Daws MI, Orr D, Burslem DFRP, Mullins CE. Effect of high temperature on chalazal plug removal and germination in Apeiba tibourbou Aubl. Seed Science and Technology. 2006;34:221–225. [Google Scholar]
- Daws MI, Ballard C, Mullins CE, Garwood NC, Murray B, Pearson TRH, et al. Allometric relationships between seed mass and seedling characteristics reveal trade-offs for neotropical gap-dependent species. Oecologia. 2007;a 154:445–454. doi: 10.1007/s00442-007-0848-2. [DOI] [PubMed] [Google Scholar]
- Daws MI, Bolton S, Burslem DFRP, Garwood NC, Mullins CE. Loss of desiccation tolerance during germination in neo-tropical pioneer seeds: implications for seed mortality and germination characteristics. Seed Science Research. 2007;b 17:273–281. [Google Scholar]
- Denslow JS, Ellison A, Sanford RE. Treefall gap size effects on above- and below-ground processes in a tropical wet forest. Journal of Ecology. 1998;86:597–609. [Google Scholar]
- Doussi MA, Thanos CA. Ecophysiology of seed germination in Mediterranean geophytes. 1. Muscari spp. Seed Science Research. 2002;12:193–201. [Google Scholar]
- Engelbrecht BMJ, Dalling JW, Pearson TRH, Wolf RL, Galvez DA, Koehler T, et al. Short dry spells in the wet season increase mortality of tropical pioneer seedlings. Oecologia. 2006;148:258–269. doi: 10.1007/s00442-006-0368-5. [DOI] [PubMed] [Google Scholar]
- Evans CE, Etherington JR. The effect of soil water potential on seed germination of some British plants. New Phytologist. 1990;115:539–548. doi: 10.1111/j.1469-8137.1990.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Gummerson RJ. The effect of constant temperatures and osmotic potentials on the germination of sugar beet. Journal of Experimental Botany. 1986;37:729–741. [Google Scholar]
- Jankowska-Blaszczuk M, Daws MI. Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Functional Ecology. 2007;21:1055–1062. [Google Scholar]
- Kaufmann MR. Effects of water potential on germination of lettuce, sunflower, and citrus seeds. Canadian Journal of Botany. 1969;47:1761–1764. [Google Scholar]
- Kebreab E, Murdoch AJ. Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiacea seeds. Journal of Experimental Botany. 1999;50:655–664. [Google Scholar]
- Kikuzawa K, Koyama H. Scaling of soil water absorption by seeds: an experiment using seed analogues. Seed Science Research. 1999;9:171–178. [Google Scholar]
- Leishman MR, Westoby M. The role of seed mass in seedling establishment in dry soil conditions – experimental evidence from semi-arid species. Journal of Ecology. 1994;82:249–258. [Google Scholar]
- Marthews TR, Burslem DFRP, Paton SR, Yangüez F, Mullins CE. Soil drying in a tropical forest: three distinct environments controlled by gap size. Ecological Modelling. 2008;216:369–384. [Google Scholar]
- Michel BE, Kauffmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiology. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg P, Andersson L, Thompson K. Large seeded species are less dependent on light for germination than small seeded ones. Seed Science Research. 2000;10:99–104. [Google Scholar]
- Pearson TRH, Burslem DFRP, Dalling JW, Mullins CE. Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size. Ecology. 2002;83:2798–2807. [Google Scholar]
- Pearson TRH, Burslem DFRP, Goeriz RE, Dalling JW. Regeneration niche partitioning in neotropical pioneers: effects of gap size, seasonal drought and herbivory on growth and survival. Oecologia. 2003;a 137:456–465. doi: 10.1007/s00442-003-1361-x. [DOI] [PubMed] [Google Scholar]
- Pearson TRH, Burslem DFRP, Mullins CE, Dalling JW. Functional significance of photoblastic germination in neotropical pioneer trees: a seed's eye view. Functional Ecology. 2003;b 17:394–402. [Google Scholar]
- Swaine MD, Whitmore TC. On the definition of ecological species groups in tropical rain forests. Vegetatio. 1988;75:81–86. [Google Scholar]
- Thompson K. Seeds and seed banks. New Phytologist. 1987;106(Suppl. S):23–34. [Google Scholar]
- Thompson K, Band SR, Hodgson JG. Seed size and shape predict persistence in soil. Functional Ecology. 1993;7:236–241. [Google Scholar]
- Tompsett PB, Pritchard HW. The effect of chilling and moisture stress on the germination, desiccation tolerance and longevity of Aesculus hippocastanum L. seeds. Annals of Botany. 1998;82:249–261. [Google Scholar]
- Vázquez-Yanes C, Orozco-Segovia A. Seed germination of a tropical rain forest pioneer tree (Heliocarpus donnell-smithii) in response to diurnal fluctuations in temperature. Physiologia Plantarum. 1982;56:295–298. [Google Scholar]
- Würth MKR, Winter K, Körner C. Leaf carbohydrate responses to CO2 enrichment at the top of a tropical forest. Oecologia. 1998;116:18–25. doi: 10.1007/PL00013821. [DOI] [PubMed] [Google Scholar]