Abstract
Background and Aims
Flowering phenology is a critical life-history trait that influences reproductive success. It has been shown that genetic, climatic and other factors such as plant size affect the timing of flowering and its duration. The spatial and temporal variation in the reproductive phenology of the columnar cactus Stenocereus thurberi and its association with plant size and environmental cues was studied.
Methods
Flowering was monitored during 3 years in three populations of S. thurberi along a latitudinal gradient. Plant size was related to phenological parameters. The actual and past weather were used for each site and year to investigate the environmental correlates of flowering.
Key Results
There was significant variation in the timing of flowering within and among populations. Flowering lasted 4 months in the southern population and only 2 months in the northern population. A single flowering peak was evident in each population, but ocurred at different times. Large plants produced more flowers, and bloomed earlier and for a longer period than small plants. Population synchrony increased as the mean duration of flowering per individual decreased. The onset of flowering is primarily related to the variance in winter minimum temperatures and the duration to the autumn–winter mean maximum temperature, whereas spring mean maximum temperature is best correlated with synchrony.
Conclusions
Plant size affects individual plant fecundity as well as flowering time. Thus the population structure strongly affects flowering phenology. Indications of clinal variation in the timing of flowering and reproductive effort suggest selection pressures related to the arrival of migrating pollinators, climate and resource economy in a desert environment. These pressures are likely to be relaxed in populations where individual plants can attain large sizes.
Key words: Flowering phenology, optimal timing, plant size, Sonoran Desert, Stenocereus thurberi, temperature
INTRODUCTION
Plant phenology involves the timing, duration and abundance of recurrent biological phenomena, including reproductive events such as flowering, fruiting, seed dispersal and germination. Flowering phenology is a critical life-history trait that strongly influences reproductive success (Rathcke and Lacey, 1985). Many species show gradual changes in flowering time over geographical and environmental gradients (Jackson, 1966; Harris, 1970; Hodgkinson and Quinn, 1978; Blionis et al., 2001). Among the multiple explanations given for the evolution of flowering time (Janzen, 1967; Bawa, 1983; Bawa et al., 2003), it has been hypothesized that competition for pollinators has shaped, at least in part, the temporal segregation of flowering by members of the same pollinator guild (Pleasants, 1980; Gleeson, 1981; Armbruster, 1986; Ashton et al., 1988; Murali and Sukumar, 1994; Lobo et al., 2003). Also, the timing of flowering, as well as its duration and intensity, may be affected by other biotic factors such as competition for seed dispersers and herbivory (Rathcke and Lacey, 1985; Wheelwright, 1985; Marquis, 1988; van Schaik et al., 1993; Bronstein, 1995; Brody, 1997; Pilson, 2000; Lobo et al., 2003). Some studies also indicate that different species could increase their fitness by flowering in synchrony. Pollination is then facilitated by combining efforts that increase resource density and local pollinator attraction (Schemske, 1981; Thompson, 1982).
Several climatic variables such as temperature (Ashton et al., 1988; Pfeifer et al., 2006), photoperiod (van Schaik et al., 1993; Rivera et al., 2002), precipitation (Opler et al., 1976; Stiles, 1977; Domínguez and Dirzo, 1995; Tyler, 2001; Inouye et al., 2003; Borchert et al., 2004; Pfeifer et al., 2006), irradiance (Wright and van Schaik, 1994; Hamann, 2004) and other variables (e.g. soil nutrient concentration, Dahlgren et al., 2007) can trigger flowering. In tropical dry forests and deserts, seasonal variation in rainfall and soil water availability have been proposed as the primary abiotic factors affecting phenological patterns (Reich and Borchert, 1984; Borchert, 1994; Bowers and Dimmitt, 1994; Borchert et al., 2004). For columnar cacti in the Sonoran Desert it has been hypothesized that variation in rainfall and spring temperatures affects the timing of flowering (Fleming et al., 2001). However, direct effects have not been documented. In some columnar cacti of South America, rainfall before the reproductive season can trigger flowering, but it stops or is uncorrelated with flowering in other species (Ruíz et al., 2000; Petit, 2001). The variation in phenological events caused by temperature or photoperiod has never been examined for columnar cacti.
Divergence in flowering times may also be due to biotic factors such as plant size (Schmitt, 1983; O'Neil, 1997; Bishop and Schemske, 1998; Ollerton and Lack, 1998; Petit, 2001; McIntosh, 2002; Bowers, 2006), competition for pollinators (Mosquin, 1971; Stiles, 1977) and the intensity of seed predation (Ollerton and Lack, 1992), which may act together to modulate fecundity. Finally, phylogenetic constraints influence many traits that affect flowering phenology such as flower number per functional module, or the timing of flower development (Kochmer and Handel, 1986; Dorn and Mitchell-Olds, 1991; Harvey and Pagel, 1991; Morales, 2000).
Spatial and temporal variation in flowering time is crucial to plants pollinated by animals with specific emergence times or subject to seasonal migration. For example, Waser (1979) showed concordance between the timing of flowering of Fouquieria splendens and hummingbird spring migration in the Sonoran Desert. With few exceptions (see Scott, 2004), plants pollinated by migrating bats also show adaptative latitudinal variation in their flowering times. Phenological data for columnar cacti and paniculate agaves suggest that both groups form a nectar corridor along the bat migration route in western Mexico (Gentry, 1982; Arita, 1991; Fleming et al., 1993). However, few studies have examined the spatio-temporal variability in flowering phenology and its effects on the reproductive success of columnar cacti (see Fleming et al., 2001).
Although some authors have studied the geographic differentiation in flowering duration between species of columnar cacti (e.g. Valiente et al., 1996; Fleming et al., 2001), most studies are restricted to a single or a few dates during the blooming season. Furthermore, timing, duration and intensity of flowering have been examined in isolation from one another. However, the interactions of these parameters are likely to act synergisticaly in shaping reproductive success, particularly when coupled with pollinator and resource availability. The interactions among timing, duration and intensity have not been fully explored, but it can be hypothesized that a selective component is associated with individual and populational variation in flowering phenology.
Stenocereus thurberi is one of the most common columnar cacti of the Sonoran Desert. Its extensive distribution and large population numbers allow the exploration of the timing, duration and intensity of flowering at the individual and population levels, and the relationship between flowering phenology and fruit production in a resource-limited (low water–high temperature variance) environment. Three populations were choosen at the northern, central and southern range of the species to study their phenology. The aims of this study were: (a) to describe the geographic variation in flowering phenology; (b) to compare the timing and duration of flowering at the individual and population level; (c) to examine how plant size affects flowering phenology; and (d) to evaluate the effect of past weather on the onset, synchrony and duration of flowering.
MATERIALS AND METHODS
The plant
Stenocereus thurberi (Engelm.) Buxb. is a columnar cactus 3–10+ m tall distributed on the Pacific slope of northwestern Mexico, from northern Sinaloa and western Chihuahua to southwestern Arizona in the USA (Fig. 1). It is also common in Mexico throughout Baja California and some of the Gulf of California islands (Turner et al., 1995). It has many ribbed and spiny stems arising close to the ground or from a short trunk. Flowering starts in mid May, and lasts for about 15 weeks (Turner et al., 1995; Fleming, 2000). Flowers open for only one night and the morning after. They show features associated with bat pollination, although hummingbirds have been reported as the main pollinators in some sites (Fleming et al., 1996, 2001). Fruit ripens during the summer, and bats, humans and many other mammals consume them and are good dispersal agents (Fleming and Sosa, 1994; Yetman and Búrquez, 1996). Birds also consume the fruits, but some are seed predators (Godínez-Alvarez et al., 2002; Wolf et al., 2002), while others, such as woodpeckers and orioles, are probably efficient dispersal agents (E. Bustamante, pers. obs.).
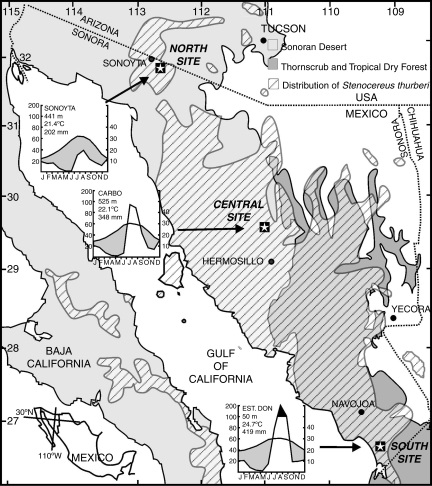
Fig. 1.
Geographic distribution of S. thurberi (modified from Turner et al., 1995) showing the location of the three study sites (stars). The climograms illustrate the change in mean monthly precipitation and temperature for each site.
Study area
Three populations along a transect spanning about 800 km and 5° of latitude were studied in Sonora, Mexico. These will be referred to hereafter as North, Central and South sites (Fig. 1). The North site (31°48′17″N, 112°51′56″W, 550 m altitude) is located in northwestern Sonora, about 6 km southeast of the town of Sonoyta. It lies at the western edge of the crassicaulescent desert of the Arizona Upland subdivision of the Sonoran Desert (Shreve, 1951). The Sonoyta meteorological station, located 7 km from the site, reports a mean annual precipitation of 202 mm, a mean annual temperature of 21·4 °C (mean annual thermal fluctuation = 21·7 °C; n = 60 years). The Central site (29°34′06″N, 111°05′29″W, 525 m altitude) is located near the town of Carbó, in central Sonora. The vegetation is an arbosuffrutescent community typical of the Sonoran Desert subdivision Plains of Sonora. The climate (Carbó meteorological station, approx. 18 km north-east of the site; n = 45 years) is dominated by summer rains, with 348 mm mean annual precipitation and 22·1 °C mean annual temperature (mean thermal fluctuation = 15·3 °C). The South site (26°38′12″N, 109°18′39″W, 60 m altitude) is near Coteco, a Mayo village in south-western Sonora. The vegetation is a coastal thornscrub (Búrquez et al., 1999) with a denser and taller vegetation than any of the Sonoran Desert sites. About 35 km south-east of the site, and in the same extensive plain, the meteorological station of Estación Don records a mean annual precipitation of 419 mm and a mean annual temperature of 24·7 °C (mean thermal fluctuation = 15·4 °C; n = 20 years, Fig. 1). Although the sites were selected by the high ecological prominence of S. thurberi, large differences in density were apparent, the South site being the densest (325 ± 29 s.e. individuals ha−1), the Central site the sparsest (13 ± 1), and the North site intermediate in density (136 ± 10).
Phenological parameters
In early 2003, reproductive plants of all size classes in the North (n = 110), Central (n = 67) and South sites (n = 86) were randomly selected, located with a GPS and numbered with aluminium tags. Flowering and fruiting were then monitored every 2–4 weeks in 2003 and every 2 weeks during 2004. Additional data for the southern population were gathered every 2 weeks during 2001. A few individuals did not flower every year, and for that reason the number of reproductive plants varied slightly between seasons in each site.
Using 1 April as day 1, six flowering phenology variables were estimated for each plant in each site and year: (1) number of open flowers; (2) onset (date when the first open flower was recorded); (3) end date (date when the last open flower was recorded); (4) flowering duration (number of days of flower production, e.g. difference between onset and end date); (5) mean flowering date [mean of census dates weighted by the number of open flowers produced on each date (after Bishop and Schemske, 1998)]; and (6) flowering synchrony (see below).
Flowering synchrony was quantified at both the individual and the population levels. Individual flowering synchrony (Xi) is the relative overlap between the blooming period of a given individual and all other plants in the population. It was calculated according to Augspurger's method (1983), modified from Primack (1980) as follows:
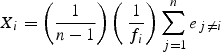 |
where, ei is the number of census dates at which plants i and j are both in flower, fi is the number of census dates at which individual i is flowering and n is the number of individuals in the population. Xi varies from 1 (plant flowering overlaps completely with all other individuals) to 0 (no overlap). The overall synchrony of the population (Z) is the average syncrhrony of individual plants.
To test the hypothesis that at least some multimodal flowering phenologies could result from random errors caused by the sampling of low numbers of individuals, a comparison was established between the actual distribution (based on a sample of 86 individuals of the South site) and simulated phenologies of 20 randomly selected individuals each time from the total sampled population.
Plant size
For all plants, three components of plant size (number of stems, height and plant canopy cover) were measured to assess the relationship between size and phenology. In all populations, plant size was measured before the 2003 flowering season. Height was measured from the base to the tip of the highest stem. Plant canopy cover was defined as the ellipse drawn by the vertical projection of the exterior arms to the ground surface measured along two perpendicular axes (Mueller-Dombois and Ellenberg, 1974).
Effects of climatic variables on phenology
To investigate the environmental flowering triggers on phenology, the relationship between several climatic indices and the onset and duration of flowering at the study sites in different years was tested. It was expected that site and year differences in climatic conditions would be related to flowering phenology. Based on the literature the following hypotheses were proposed: (a) the onset of flowering should be delayed when minimum temperatures during winter and/or spring are low or have a large variation (Fleming et al., 2001; Bowers, 2007); (b) synchrony will be higher in more stable environments in terms of temperature or precipitation (i.e. having less variance on these weather variables); and (c) duration should be related to the general water status of the plant, a feature known to be dependent on the previous summer or winter precipitation in several species of the Sonoran Desert (Bowers and Dimmitt, 1994).
Three phenological response variables were used at the population level: (a) the onset of flowering, defined as the time when 5 % of the reproductive individuals had at least one open flower; (b) the total duration of flowering, determined as the time interval between the opening of the first flower and the last flower in the population during a reproductive season; and (c) the time interval when more than half of the individuals were flowering (>50 % duration).
Records of daily rainfall and maximum and minimum temperatures up to 1 year before flowering were obtained from the meteorological station closest to each site (for the purpose of this study, the climatic year began in May and ended in April). The year was divided into five seasons following Dimmitt (1991): foresummer or dry summer (the hottest and driest time of year, from 1 May to 30 June = 10–12 months before flowering); summer monsoon or warm rainy season (1 July to 15 September = 7·5–10 months before flowering), autumn (similar to the dry summer, but less severe, from 16 September to 30 November = 5–7·5 months before flowering); winter (some years with abundant cold season rainfall, from 1 December to 15 February = 2·5–5 months before flowering); and spring drought (16 February to 30 April = 0–2·5 months before flowering). The average and variance of daily precipitation and temperature (mean, minimum and maximum) were calculated for each interval and for the following combinations: dry summer–summer monsoon, summer monsoon–autumn, autumn–winter and winter–spring. These indices were correlated with the phenological parameters to assess the relationship of past climatic events with phenology. As causality was difficult to ascribe to a given climatic factor (because of collinearity between indices), the phenological variables were correlated with only those environmental variables for which a sensible relationship of causation could be formulated.
Statistical analysis
One-way analyses of variance (ANOVAs) after logarithmic transformation of the 2004 data were used to analyse the differences among populations in plant size and phenological parameters. Post hoc comparisons among means were made using a Student–Newman–Keuls test. Pearson product–moment correlation coefficients tested the association between parameters. Principal component analysis (PCA) was used to reduce size parameters into fewer components. Linear regressions on log-transformed data were performed to assess the allometric coefficients of size and phenological factors (as they were used only for comparative purpose, there was no need for reduced major axis regression; see Warton et al., 2006), and analyses of covariance (ANCOVAs) assessed differences in the coefficients among populations. Using data from 2001, 2003 and 2004, the effect of climatic variables on flowering was examined with stepwise multiple regression analysis; the analysis was useful to detect which of the climatic variables had the strongest impact on onset, duration and >50 % duration. All statistical analyses were performed using SPSS 13·0 (SPSS Inc. 2004).
RESULTS
Flowering patterns
The southern and central populations started to flower between mid and late April, while the northern population started flowering much later, between mid May and early June (Fig. 2). The southern population flowered for 19–21 weeks, setting the last flowers by late September. The central population had a shorter flowering duration (16–17 weeks), finishing by mid August, and the northern population had a brief blooming period of only 10–14 weeks. There was considerable variation in the duration of flowering within and among populations, suggesting a latitudinal trend in the flowering duration. The North site had the shortest and the South site the longest flowering duration (Table 1).
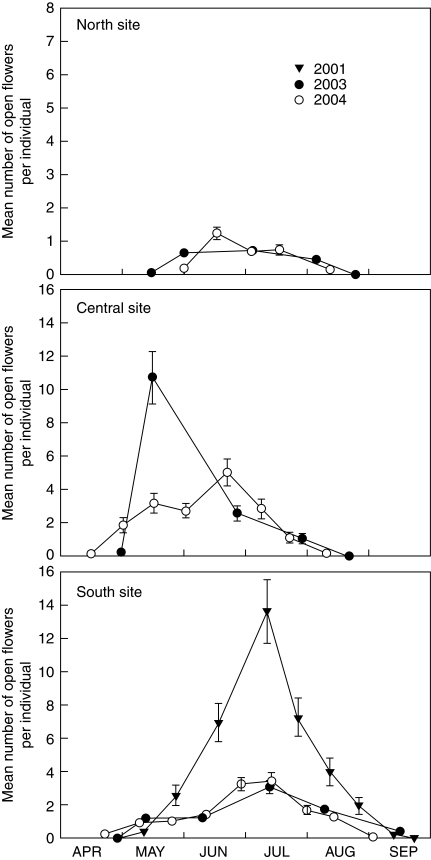
Fig. 2.
Flowering phenology of three populations of S. thurberi during 2001, 2003 and 2004 as indicated. The symbols show the mean number of open flowers per individual ± s.e.m. Note that the y-axis for the North site has a different scale.
Table 1.
Comparison of means ± s.e. of phenological and morphological data among three populations of Stenocereus thurberi in Sonora, Mexico
| North site (n = 86) | Central site (n = 66) | South site (n = 76) | F | W | |
|---|---|---|---|---|---|
| Height (m) | 2·98 ± 0·08a | 4·50 ± 0·12b | 4·57 ± 0·16b | 55·24 | 70·97 |
| Number of stems | 19·87 ± 1·21a | 68·53 ± 8·02b | 14·70 ± 1·47a | 46·34 | 23·24 |
| Canopy cover (m2) | 2·60 ± 0·22a | 10·93 ± 1·29b | 4·91 ± 0·58c | 31·65 | 25·42 |
| Flowers per plant per day | 0·63 ± 0·06a | 2·19 ± 0·27b | 1·43 ± 0·14c | 22·81 | 26·94 |
| Mean flowering date* | 91·08 ± 1·60a | 71·77 ± 1·98b | 92·90 ± 1·49a | 44·77 | 40·30 |
| Synchrony | 0·49 ± 0·01a | 0·57 ± 0·01b | 0·65 ± 0·01c | 46·02 | 50·29 |
| Duration (d) | 28·65 ± 1·50a | 57·00 ± 3·30b | 69·45 ± 3·45c | 61·21 | 77·29 |
| Total fruits per plant | 56·90 ± 5·67a | 413·45 ± 45·39b | 120·99 ± 11·58a | 59·06 | 40·50 |
As some samples did not exhibit homogeneity of variances (Levene test), an additional robust test for equality of means is presented (Welch test). In all cases, there are significant differences (P < 0·0001) for the F and W statistics. Letters next to each value indicate sites significantly different using a Student–Newman–Keuls post hoc test. Values with the same letter are not significantly different.
* Days since 1 April, 2004.
Flowering intensity (i.e. the number of open flowers present at a given time) was high in the Central and South sites, and much lower in the North site where individuals usually had less than one flower open per census (Fig. 2). The timing and magnitude of the peaks differed among populations. In the northern population, a barely discernible peak occurred during mid June. In the Central site, the peak varied between mid May and late June, and in the southern population the peak was always in mid July. A single flowering peak was evident in each population, but there was considerable variation in the peak number of open flowers within populations among years. For example, in the southern population it varied >4-fold: from an average of 14 flowers per individual per day during 2001 – the highest value observed for any population and year – to only three in 2003 and 2004 (Fig. 2).
The comparisons of flowering schedules between the data set and the simulated phenologies of six randomly chosen sets of 20 individuals showed a general agreement between both data sets. However, in some cases large discrepancies were observed, highlighting the risk of making a type I error by drawing conclusions from small samples where spurious secondary peaks appear (see Fig. S1 in Supplementary Information, available online). Also, flowering peak intensity could vary from two to six flowers per individual, but flowering duration was the same for all simulations.
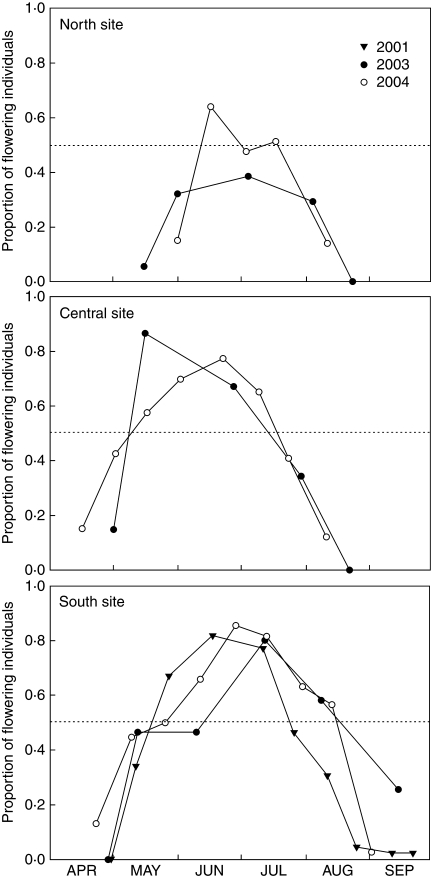
In all sites, most reproductive plants flowered every year (≥93 %). The proportion of individuals that flowered on a given day differed within and among populations and years, closely following the pattern of flowering intensity (Fig. 3). The proportion of flowering individuals peaked at around 0·80 in the Central and South sites each year, whereas the North site had lower peaks of 0·38 and 0·64. Therefore, in the central and southern populations, the synchrony among individuals was higher than in the northern population. Each year, the central and southern populations had a period of at least 2 months when >50 % of the individuals were flowering. In the northern population, it lasted only 1 month during 2004, and never reached this level of flowering during 2003. The overall flowering synchrony (Z) was rather constant within populations. It did not vary between 2003 and 2004 in the northern population (0·49), and it was much higher in the central (0·65 in 2003 and 0·57 in 2004) and southern population (0·65 in both years).
Fig. 3.
Proportion of individuals flowering at the same time in three populations of S. thurberi [North (n = 110), Central (n = 68) and South (n = 86)] during 2001, 2003 and 2004 as indicated. The dotted line shows the point when half of the individuals flower at the same time.
There was a N–S trend in synchrony and flowering duration, but the central population produced many more fruits; as much as 8- and 3·5-fold more than the northern and southern populations, respectively. The Central site also had a significantly earlier mean flowering date than any of the other populations (Table 1).
Relationship among phenological parameters
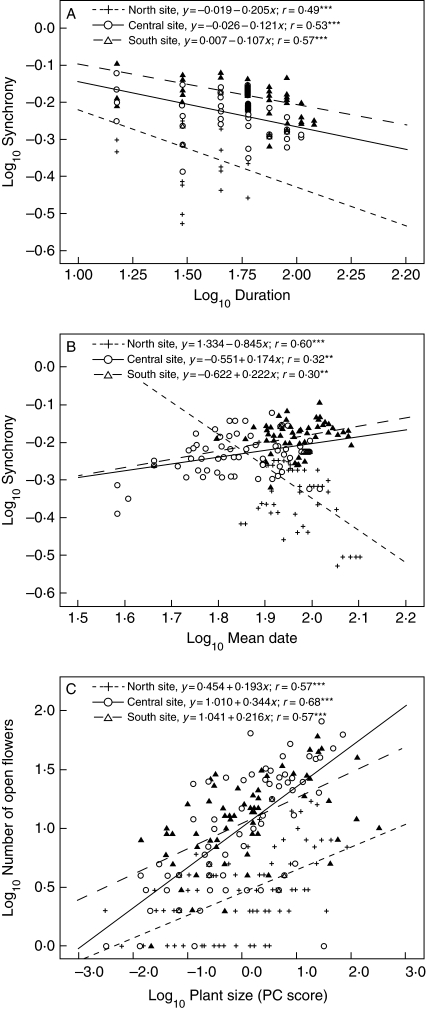
The number of open flowers was highly correlated with the number of fruits (Table 2). Flowering duration and the number of open flowers, as well as the number of fruits, were positively correlated in all populations (Table 2). Flowering duration explained 62–74 % of the variance in the number of open flowers, and the slopes of the log–log regression were significantly different among sites (ANCOVA F(2,211) = 0·146; P = 0·003). The number of open flowers increased faster than the flowering duration, and that effect was more marked in the Central site. Individual flowering synchrony was inversely correlated with flowering duration – plants that flowered for a longer period had a smaller overall synchrony (Table 2). The regression slopes were significantly different among sites (ANCOVA F(2,211) = 3·09; P = 0·003; Fig. 4A), with the highest rate of change in the North and the lowest in the South site. Mean flowering date was only correlated with synchrony: inversely in the North and directly in the Central and South sites (Table 2). In the northern population, the least synchronous individuals started reproducing later in the season, while the more synchronous reproduced earlier, opposite to the trend found in the other populations (ANCOVA F(2,211) = 38·237; P < 0·001; Fig. 4B). Mean flowering date explained much more of the variance in the synchrony of the North site (36 %) than in the other sites (10 %). Flowering synchrony was negatively correlated with the number of fruits produced per individual in the central and southern populations, but not in the northern population (Table 2). In the central and southern populations, the more synchronous individuals produced fewer fruits (ANCOVA F(2,211) = 3·787; P = 0·024).
Table 2.
Pearson correlation matrix for the morphological and phenological traits (during 2004) in three populations of Stenocereus thurberi
| Plant size (PC score) | Flowers per plant per day | Mean flowering date† | Synchrony | Duration (d) | |
|---|---|---|---|---|---|
| North site (n = 83) | |||||
| Plant size (PC score) | 1 | ||||
| Flowers per plant per day | 0·570** | 1 | |||
| Mean flowering date† | 0·025 | 0·049 | 1 | ||
| Synchrony | −0·251* | −0·373** | −0·603** | 1 | |
| Duration (d) | 0·486** | 0·859** | 0·157 | −0·493** | 1 |
| Total fruits per plant | 0·274* | 0·677** | 0·199 | −0·135 | 0·567** |
| Central site (n = 64) | |||||
| Plant size (PC score) | 1 | ||||
| Flowers per plant per day | 0·676** | 1 | |||
| Mean flowering date† | 0·070 | 0·010 | 1 | ||
| Synchrony | −0·486** | −0·494** | 0·317* | 1 | |
| Duration (d) | 0·636** | 0·886** | −0·010 | −0·533** | 1 |
| Total fruits per plant | 0·756** | 0·853** | −0·038 | −0·419** | 0·776** |
| South site (n = 70) | |||||
| Plant size (PC score) | 1 | ||||
| Flowers per plant per day | 0·574** | 1 | |||
| Mean flowering date† | 0·100 | −0·095 | 1 | ||
| Synchrony | −0·119 | −0·395** | 0·301* | 1 | |
| Duration (d) | 0·329** | 0·790** | −0·192 | −0·575** | 1 |
| Total fruits per plant | 0·406** | 0·722** | −0·045 | −0·353** | 0·670** |
Two-tailed significance ** P <0·01; * P <0·05.
† Days since 1 April.
Fig. 4.
Variation among sites in the allometry of duration and synchrony (A), mean flowering date and synchrony (B), and plant size and number of open flowers (C) in three populations of S. thurberi. At the Noth, Central and South sites as indicated. Regression equations and their significance are shown: *** P <0·001; ** P <0·05.
Effect of plant size on phenology
Reproductive plants in the Central site were significantly larger than plants in any of the other sites. The mean canopy cover of the plants in the Central site was four times higher than in the North site, and twice higher than those in the South site. Plants in the Central site also had about four times more stems than plants in any other site, and were much taller than in the North site (Table 1).
Height, canopy cover and number of stems were highly correlated in all sites (r = 0·47–0·69). A PCA was used to reduce log-transformed plant size measures. PCA extracted only one component (eigenvalue >1), which accounted for 71·5 % of the cumulative variance in the North site, 75·3 % in the Central site and 63·4 % in the South site, and was positively related to each one of the size measures (all size variables in the PCA had component loadings in the range of 0·67–0·94). The resulting component score of size for each plant was used to examine its relationships with the log10-transformed phenological parameters: number of open flowers, mean flowering date, duration, synchrony and number of fruits.
Plant size was significantly correlated with the number of open flowers and with the number of fruits produced by each plant in all studied populations (Table 2). Within populations, plant size explained 33–46 % of the variance in the number of open flowers (Fig. 4C), and 8–46 % in the number of fruits. The results indicate that even after adjusting for plant size (using it as a covariate among populations in the ANOVA), the scores for the number of flowers were still different among populations (ANCOVA F(2,211) = 4·329; P = 0·014), as well as the number of fruits (ANCOVA F(2,211) = 5·661; P = 0·004). At the individual level, flowering duration was also well correlated with plant size in all populations. Size explained 11 % of the variance in the South site, 24 % in the North site and 40 % in the Central site, but, despite the large variance in plant size, the regression slopes were marginally different among sites (ANCOVA F(2,211) = 2·995; P = 0·052). Flowering synchrony was not correlated with plant size in the South site, was poorly correlated in the North site, but in the Central site 24 % of the variance on the flowering synchrony was explained by size. However, there were no significant differences among populations (ANCOVA F(2,211) = 2·250; P = 0·108). Mean flowering date did not show any significant correlation with plant size (Table 2). In summary, small individuals had smaller flowering intensity, shorter duration and were more synchronized than large individuals. A graphic comparison of individual flowering phenologies between populations is available online as Supplementary Information (Fig. S2).
Effect of climatic variables on phenology
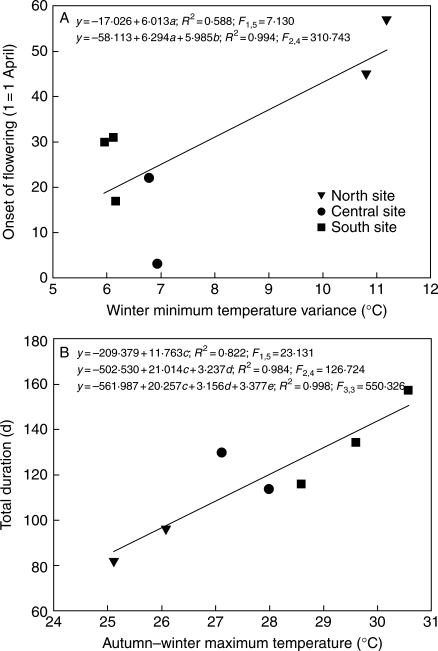
Although many of the bivariate combinations between the three phenological and the several climatic variables were highly significant; of all climatic variables tested using a stepwise multiple regression, winter minimum temperature variance explained 59 % of the variation in the onset of flowering (Fig. 5A). When combined with summer monsoon–autumn minimum temperature variance, it explained >99 % of the variation. The autumn–winter mean maximum temperature in combination with dry summer–summer monsoon maximum temperature variance explained 98 % of the variation in total duration of flowering (Fig. 5B). The time interval when more than half of the population is flowering (>50 % duration) was only positively correlated with the spring mean maximum temperature, which explained 75 % of its variation.
Fig. 5.
Effect of winter minimum temperature variance on the onset of flowering (A) and of the autumn–winter maximum temperature on total duration (B). Stepwise multiple regression equations are shown: a = winter minimum temperature variance, b = summer monsoon minimum temperature variance, c = autumn–winter mean maximum temperature, d = dry summer–monsoon summer maximum temperature variance, e = summer monsoon mean minimum temperature.
DISCUSSION
Regardless of the geographic location, S. thurberi shows a unimodal flowering phenology with a peak at the end of the dry season. This pattern is common to many other columnar cacti with lengthy flowering periods (2–4 months; Fleming et al., 2001; Pavón and Briones, 2001; Petit, 2001; Esparza-Olguín and Valverde, 2003; Otero-Arnaiz et al., 2003; Ibarra-Cerdeña et al., 2005). This mode of flowering, associated with the opening of only a few flowers per night on each plant, may favour the transference of pollen by forcing pollinators to visit more individuals (Ruiz et al., 2000). Flowering during the dry season allows the dispersal of seeds and the recruitment of seedlings during the ensuing rainy season. The timing of dispersal is particularly important for long-lived desert species with episodic recruitment, because the seedlings establish and survive only in years that are particularly benign, especially in terms of the quantity and distribution of rainfall (Steenbergh and Lowe, 1977; Jordan and Nobel, 1982; Godínez-Alvárez et al., 2003). Bimodal phenological patterns have been reported in some columnar cacti with widely extended flowering seasons. These include S. griseus in the arid inter-Andean valleys of Colombia (Ruiz et al., 2000) and S. queretaroensis in the aridlands of Guanajuato, Mexico (Castillo Landero, 2003). However, in other dry regions, these species exhibit unimodal flowering phenologies (Petit, 2001; Ibarra-Cerdeña et al., 2005). Also, Pachycereus schottii (Fleming et al., 2001) and Cereus hexagonus (Ruiz et al., 2000) have been reported as multimodal species. It is possible that some of the reported multimodal phenologies result from random errors caused by the sampling of low numbers of individuals as has been shown in this study (see Supplementary Information, available online).
The striking differences in flowering duration among populations follow a geographical trend, the northern population having the shortest duration, while the central and southern populations have the longest. For two populations at the northern and western edge of distribution, Fleming et al. (2001) described a pattern which corresponded closely with that of the northern population studied here, and was dissimilar in duration and flowering intensity to the central and southern populations. This trend suggests that peripheral populations in a more limited environment (i.e. less precipitation, extreme temperatures) have a shorter flowering season, while the central populations, with larger individuals, extend their blooming. The biological advantages of extended blooming include according to Bawa (1983): (a) a reduced risk of reproductive failure; (b) an increased chance of mating with more individuals; and (c) a better control of the relative investment in flowers and fruits. All three seem to be acting in the columnar cacti populations studied here. Selection for extended blooming is more likely to occur in self-incompatible species such as S. thurberi, as shown by de Jong et al. (1992). Extended blooming, however, is probably limited by resource availability, a pattern consistent with the N–S trend (and also E–W; see Búrquez et al., 1999) of rainfall, the incidence of damaging low temperatures, which restricts the distribution of columnar cacti and many others Sonoran Desert plants (Niering et al., 1963; Kalisz and Wardle, 1994; Turner et al., 1995; Weiss and Overpeck, 2005), and the larger variation in the number of pollinators in the northern edge of distribution (Rojas-Martínez et al., 1999).
As expected, the negative correlation between synchrony and duration holds for all three populations (Augspurger, 1983; de Jong et al., 1992). However, the northern population had the shortest mean flowering duration, and also its individuals were less synchronized and produced fewer flowers than the other populations; a surprising result that can only be explained by the brief duration of reproduction in many individuals in that population. Under this scenario, long duration and high synchrony increase the chances for interbreeding among the individuals. As a result, the southern and central populations may have a higher chance of interbreeding with more individuals (i.e. towards more panmixia) than in the northern population, where usually less than half of the population is blooming simultaneously. This is particularly important if the timing of flowering initiation of each individual is consistent year to year, as has been recorded for some populations of organ pipe cactus (Fleming, 2006).
The size of the reproductive individuals was highly variable: individuals in the northern population not only were considerably smaller, but started reproduction at a smaller size than in any other population. Mean growth rates of reproductive stems showed significant differences between populations (6·28, 8·76 and 8·33 cm year−1 in the North, Central and South sites, respectively; ANOVA F(1,2) = 8·407, P < 0·001; average of 3 years; E. Bustamante and A. Búrquez, unpubl. res.). Thus, reproductive individuals of a given size in the northern population are probably much older than similarly sized individuals in the central and southern populations. If the trigger for reproduction in this species is size related, it is likely that this difference results from genetic differentiation for this particular feature. Variable plant size and age relationships are known to reflect adaptation to uncertain environments (Wilbur and Rudolf, 2006). The N–S trend in phenological traits seems to be mediated through plant size, and also possibly through changes in the root:shoot ratio of each population (larger in the North site, see Deng et al., 2006). It is possible that the same trend is present across the Sonoran Desert along the W–E precipitation gradient from the coast to the mountains (see Búrquez et al., 1999). Large plants produced more flowers and fruits, both within and among populations, they flowered for longer periods and also showed less synchrony. Because the population size structure varies in time and space by the episodic nature of recruitment, size distributions could strongly influence phenology (Bishop and Schemske, 1998). Populations with many young individuals will have phenological patterns different from those of old populations. Plant size is affected by the genetic make-up of each population (i.e. population differentiation), and by factors related to the severity of the physical environment (i.e. precipitation, extreme temperatures, etc.). In spite of not knowing the relative contribution of each of these factors, it is relevant to consider variation in plant size when studying the effect of flowering time on reproductive success.
Rainfall has been suggested as the primary cause of variation in the onset and duration of flowering in communities with a marked dry season (Petit, 2001; Borchert et al., 2004). However, the present data demonstrated that mean temperatures and its variances are also important environmental cues that affect different phenological processes. For S. thurberi, these are processes that occur well before blooming: the variance in winter minimum temperature for the onset, the maximum temperatures during autumn–winter of the previous year for flowering duration, and the maximum temperatures of spring drought just before flowering for >50 % duration. When the winter minimum temperature is highly variable, the onset of flowering shifts to a later date in the year, suggesting that decelerating response functions (Ruel and Ayres, 1999), such as the occurrence of uneven temperatures, affect the internal clock of the plant. The variance in temperature probably influences the activity of molecular and physiological functions related to flowering (e.g. Valverde et al., 2004; Tookel et al., 2005). In the same sort of response, when the variance in autumn–winter maximum temperature is larger, flowering duration increases. As predicted, the variance in the winter minimum temperature is strongly correlated with the onset of flowering, especially in the northern population where freezing temperatures are frequent.
The lack of correlation (and direct dependence) of phenological parameters with precipitation is not unexpected for cacti: a group particularly adapted to water limitation. Cacti are well buffered against drought because of their massive water storage. Temperatures, on the other hand, could affect factors such as the future pollinator activity (affecting their migration), or the pace at which physiological processes occur. Dependence on the past temperature regime for phenological patterns has been documented for dipterocarp forests (Ashton, 1988), a species of orchid in Germany (Pfeifer et al., 2006) and desert shrubs (Bowers, 2007). Here, it is reported for the first time that past temperature variance (several months before flowering), instead of the mean values, is closely associated with the onset of flowering. Also, past temperatures seem to be more important than past precipitation for synchrony in the studied populations of S. thurberi. If the maximum temperature during spring is high, the plants could bloom in a more synchronous manner and for a longer period, increasing the time interval during which a larger fraction of the population is flowering.
Several studies have shown significant variation in the date of onset of flowering as a result of climate change (Fitter and Fitter, 2002; Bowers, 2007); for this reason long-term studies are necessary for a better undertanding of the evolution of flowering time of S. thurberi, that also could modify its interactions with migratory pollinators. As Wiess and Overpeck (2005) point out, the significant increase of the minimum temperatures, the decreasing frequency of freezing temperatures and the increasing freeze-free season length in the Sonoran Desert region could drive the distribution of species into new areas. More subtle consequences in the short term point to a trend for earlier flowering times and changes in the migration patterns of pollinators of columnar cacti.
SUPPLEMENTARY INFORMATION
Supplementary information is available online at www.aob.oxfordjournals.org/. Figure S1 shows the actual phenology based on a sample of 86 individuals and six phenological curves resulting from the random sampling of 20 individuals from the total population in the South site. Figure S2 is a graphical summary of the variation within and among populations of the individual phenologies of 68 randomly drawn individuals from each of the three populations studied of S. thurberi during the 2004 season.
ACKNOWLEDGEMENTS
We thank Rita Dávila, Rosalio León, Consuelo Orozco and Vicente Tajia for their field assistance. The Mayo community of Masiaca, Club de Caza El Venadito and Patronato del Centro de Investigaciones Pecuarias del Estado de Sonora kindly gave permission for work on their land. David Yetman and Vicente Tajia provided ideas, encouragement and support. Alejandro Casas, Angelina Martínez-Yrízar, Carlos Montaña, Peter Scott and two anonymous reviewers read and commented on the manuscript. E.B. wishes to thank the Posgrado en Ciencias Biológicas and Dirección General de Estudios de Posgrado of the Universidad Nacional Autónoma de México, and Consejo Nacional de Ciencia y Tecnología for logistical and financial support. A.B. also gratefully acknowledges funding from Secretaría del Medio Ambiente y Recursos Naturales-Consejo Nacional de Ciencia y Tecnología (C01-0580).
LITERATURE CITED
- Arita HT. Spatial segregation in long-nosed bats Leptonycteris nivalis and Leptonycteris curasoae, in Mexico. Journal of Mammalogy. 1991;72:706–714. [Google Scholar]
- Armbruster WS. Reproductive interactions between sympatric Dalechampia species: are natural assemblages ‘random’ or organized? Ecology. 1986;67:522–533. [Google Scholar]
- Ashton PS, Givnish TJ, Appanah S. Staggered flowering in the Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. American Naturalist. 1988;132:44–66. [Google Scholar]
- Augspurger CK. Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica. 1983;15:257–267. [Google Scholar]
- Bawa KS. Patterns of flowering in tropical plants. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold; 1983. pp. 394–410. [Google Scholar]
- Bawa KS, Kang H, Grayum MLH. Relationships among time, frequency, and duration of flowering in tropical rain forest trees. American Journal of Botany. 2003;90:877–887. doi: 10.3732/ajb.90.6.877. [DOI] [PubMed] [Google Scholar]
- Bishop JG, Schemske DW. Variation in flowering phenology and its consequences for lupines colonizing Mount St. Helens. Ecology. 1998;79:534–546. [Google Scholar]
- Blionis GJ, Halley JM, Vokou D. Flowering phenology of Campanula on Mt Olympos, Greece. Ecography. 2001;24:696–706. [Google Scholar]
- Borchert R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology. 1994;75:1437–1449. [Google Scholar]
- Borchert R, Meyer SA, Felger RS, Porter-Bolland L. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forest. Global Ecology and Biogeography. 2004;13:409–425. [Google Scholar]
- Bowers JE. Branch length mediates flower production and inflorescence architecture of Fouquieria splendens (ocotillo) Plant Ecology. 2006;186:87–95. [Google Scholar]
- Bowers JE. Has climatic warming altered spring flowering date of Sonoran Desert shrubs? Southwestern Naturalist. 2007;52:347–355. [Google Scholar]
- Bowers JE, Dimmitt MA. Flowering phenology of six woody plants in the northern Sonoran Desert. Bulletin of the Torrey Botanical Club. 1994;121:215–229. [Google Scholar]
- Brody AK. Effects of pollinators, and seed predators on flowering phenology. Ecology. 1997;78:1624–1631. [Google Scholar]
- Bronstein JL. The plant-pollinator landscape. In: Hansson L, Fahrig L, Merriam G, editors. Mosaic landscape and ecological processes. London: Chapman & Hall; 1995. pp. 256–288. [Google Scholar]
- Búrquez A, Martínez-Yrizar A, Felger RS, Yetman D. Vegetation and habitat diversity at the southern edge of the Sonoran Desert. In: Robichaux RH, editor. Ecology of Sonoran Desert plants and plants communities. Tucson, AZ: University of Arizona Press; 1999. pp. 36–67. [Google Scholar]
- Castillo-Landero JP. Biología de la polinización de Stenocereus queretaroensis (Weber.) Buxbaum., una cactácea con floración biestacional. México, D.F: Universidad Nacional Autónoma de México; 2003. Bachelor thesis. [Google Scholar]
- Dahlgren JP, von Zeipel H, Ehrlén J. Variation in vegetative and flowering phenology in a forest herb caused by environmental heterogeneity. American Journal of Botany. 2007;94:1570–1576. doi: 10.3732/ajb.94.9.1570. [DOI] [PubMed] [Google Scholar]
- Deng J, Wang G, Morris EC, Wei X, Li D, Chen B, Zhao C, Liu J, Wang Y. Plant mass–density relationship along a moisture gradient in north-west China. Journal of Ecology. 2006;94:953–958. [Google Scholar]
- Dimmitt M. Arizona Upland: the land of five seasons. Sonorensis. 1991;12:2–4. [Google Scholar]
- Domínguez CA, Dirzo R. Rainfall and flowering synchrony in a tropical shrub: variable selection on the flowering time of Erythroxylon havanense. Evolutionary Ecology. 1995;9:204–216. [Google Scholar]
- Dorn LA, Mitchell-Olds T. Genetics of Brassica campestris. 1. Genetic constrains on evolution of life-history characters. Evolution. 1991;45:371–379. doi: 10.1111/j.1558-5646.1991.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Esparza-Olguín L, Valverde T. Estudio comparativo de la fenología de tres especies de Neobuxbaumia que difieren en su nivel de rareza. Cactáceas y Suculentas Mexicanas. 2003;48:68–83. [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Fleming TH. Pollination of cacti in the Sonoran desert. American Scientist. 2000;88:432–439. [Google Scholar]
- Fleming TH. Reproductive consequence of early flowering in organ pipe cactus. Stenocereus thurberi. International Journal of Plant Sciences. 2006;167:473–481. [Google Scholar]
- Fleming TH, Sosa V. Effects of nectarivorous and frugivorous mammals on reproductive success of plants. Journal of Mammalogy. 1994;75:845–851. [Google Scholar]
- Fleming TH, Nuñez RA, Sternberg LSL. Seasonal changes in the diets of migrant and non-migrant nectarivorous bats as revealed by carbon stable isotope analysis. Oecologia. 1993;94:72–75. doi: 10.1007/BF00317304. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Tuttle MD, Horner MA. Pollination biology and the relative importance of nocturnal and diurnal pollinators in three species of Sonoran Desert columnar cacti. Southwestern Naturalist. 1996;41:257–269. [Google Scholar]
- Fleming TH, Sahley CT, Holland JN, Nason JD, Hamrick JL. Sonoran desert columnar cacti and the evolution of generalized pollination systems. Ecological Monographs. 2001;71:511–530. [Google Scholar]
- Gentry HS. Agaves of continental North America. Tucson, AZ: University of Arizona Press; 1982. [Google Scholar]
- Gleeson SK. Character displacement in flowering phenologies. Oecologia. 1981;51:294–295. doi: 10.1007/BF00540618. [DOI] [PubMed] [Google Scholar]
- Godínez-Alvarez H, Valiente-Banuet A, Rojas-Martínez A. The role of seed disperser in the population dynamics of the columnar cactus Neobuxbaumia tetetzo. Ecology. 2002;83:2617–2629. [Google Scholar]
- Godínez-Alvarez H, Valverde T, Ortega-Baes P. Demographic trends in the Cactaceae. Botanical Review. 2003;69:173–203. [Google Scholar]
- Hamann A. Flowering and fruiting phenology of a Philippine submontane rain forest: climatic factors as proximate and ultimate causes. Journal of Ecology. 2004;92:24–31. [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. New York: Oxford University Press; 1991. [Google Scholar]
- Harris W. Genecological aspects of flowering and vegetative reproduction in Rumex acetosella L. New Zealand Journal of Botany. 1970;8:99–113. [Google Scholar]
- Hodgkinson KC, Quinn JA. Environmental and genetic control of reproduction in Danthonia caespitosa populations. Australian Journal of Botany. 1978;26:351–364. [Google Scholar]
- Ibarra-Cerdeña CN, Iñiguez-Dávalos LI, Sánchez-Cordero V. Pollination ecology of Stenocereus queretaroensis (Cactaceae), a quiropterophilous columnar cactus, in a tropcal dry forest of Mexico. American Journal of Botany. 2005;92:503–509. doi: 10.3732/ajb.92.3.503. [DOI] [PubMed] [Google Scholar]
- Inouye DW, Saavedra F, Lee-Yang W. Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae) American Journal of Botany. 2003;90:905–910. doi: 10.3732/ajb.90.6.905. [DOI] [PubMed] [Google Scholar]
- Jackson MT. Effects of microclimate on spring flowering phenology. Ecology. 1966;47:407–415. [Google Scholar]
- Janzen DH. Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution. 1967;21:620–637. doi: 10.1111/j.1558-5646.1967.tb03416.x. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Klinkhamer PGL, Van Staalduinen MJ. The consequences of pollination biology for selection of mass or extended blooming. Functional Ecology. 1992;6:606–615. [Google Scholar]
- Jordan PW, Nobel PS. Height distributions of two species of cacti in relation to rainfall, seedling establishment and growth. Botanical Gazette. 1982;143:511–517. [Google Scholar]
- Kalisz S, Wardle GM. Life history variation in Campanula americana (Campanulaceae): population differentiation. American Journal of Botany. 1994;81:521–527. [Google Scholar]
- Kochmer JP, Handel SN. Constraints and competition in the evolution of flowering phenology. Ecological Monographs. 1986;56:303–325. [Google Scholar]
- Lobo JA, Quesada M, Stoner KE, Fuchs EJ, Herrerías-Diego Y, Rojas J, Saborío G. Factors affecting phenological patterns of bombacaceous trees in seasonal forest in Costa Rica and Mexico. American Journal of Botany. 2003;90:1054–1063. doi: 10.3732/ajb.90.7.1054. [DOI] [PubMed] [Google Scholar]
- Marquis RJ. Phenological variation in the neotropical understory shrub Piper arielanum: causes and consequences. Ecology. 1988;69:1552–1565. [Google Scholar]
- McIntosh ME. Flowering phenology and reproductive output in two sister species of Ferocactus (Cactaceae) Plant Ecology. 2002;159:1–13. [Google Scholar]
- Morales E. Estimating phylogenetic inertia in Tithonia (Asteraceae): a comparative approach. Evolution. 2000;54:475–484. doi: 10.1111/j.0014-3820.2000.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Mosquin T. Competition for pollinators as a stimulus for the evolution of flowering time. Oikos. 1971;22:398–402. [Google Scholar]
- Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. New York: John Wiley and Sons; 1974. [Google Scholar]
- Murali KS, Sukumar R. Reproductive phenology of a tropical dry forest in Mudumalai, southern India. Journal of Ecology. 1994;82:759–767. [Google Scholar]
- Niering WA, Whittaker RH, Lowe CH. The saguaro: a population in relation to environment. Science. 1963;142:15–23. doi: 10.1126/science.142.3588.15. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Lack AJ. Flowering phenology: an example of relaxation of natural selection? Trends in Ecology and Evolution. 1992;7:274–276. doi: 10.1016/0169-5347(92)90175-B. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Lack AJ. Relationships between flowering phenology, plant size and reproductive success in Lotus corniculatus (Fabaceae) Plant Ecology. 1998;139:35–47. [Google Scholar]
- O'Neil P. Natural selection on genetically correlated phenological characters in Lythrum salicaria L. (Lythraceae) Evolution. 1997;51:267–274. doi: 10.1111/j.1558-5646.1997.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Opler PA, Frankie GW, Baker HG. Rainfall as a factor in the release, timing, and synchronization of anthesis by tropical trees and shrubs. Journal of Biogeography. 1976;3:231–236. [Google Scholar]
- Otero-Arnaiz A, Casas A, Bartola C, Pérez-Negrón E, Valiente-Banuet A. Evolution of Polaskia chichipe (Cactaceae) under domestication in the Tehuacán Valley, Central Mexico: reproductive biology. American Journal of Botany. 2003;90:593–602. doi: 10.3732/ajb.90.4.593. [DOI] [PubMed] [Google Scholar]
- Pavón NP, Briones O. Phenological patterns of nine perennial plants in an intertropical semi-arid Mexican scrub. Journal of Arid Environments. 2001;49:265–277. [Google Scholar]
- Petit S. The reproductive phenology of three sympatric species of columnar cacti on Curaçao. Journal of Arid Environments. 2001;49:521–531. [Google Scholar]
- Pfeifer M, Heinrich W, Jetschke G. Climate, size and flowering history determine flowering pattern of an orchid. Botanical Journal of the Linnean Society. 2006;151:511–526. [Google Scholar]
- Pilson D. Herbivory and natural selection on flowering phenology in wild sunflower. Helianthus annuus. Oecologia. 2000;122:72–82. doi: 10.1007/PL00008838. [DOI] [PubMed] [Google Scholar]
- Pleasants JM. Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology. 1980;61:1446–1459. [Google Scholar]
- Primack RB. Variation in the phenology of natural populations of montane shrubs in New Zealand. Journal of Ecology. 1980;68:849–862. [Google Scholar]
- Rathcke BJ, Lacey EP. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics. 1985;16:179–214. [Google Scholar]
- Reich PB, Borchert R. Water stress and tree phenology in atropical dry forest in the lowlands of Costa Rica. Journal of Ecology. 1984;72:61–74. [Google Scholar]
- Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VTR, Borchert R. Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees. 2002;16:445–456. [Google Scholar]
- Rojas-Martínez A, Valiente-Banuet A, Arizmendi A, Alcántara-Egúren A, Arita H. Seasonal distribution of the long-nosed bat (Leptonycteris curasoae) in North America: does a generalized migration pattern really exist? Journal of Biogeography. 1999;26:1065–1077. [Google Scholar]
- Ruel JL, Ayres MP. Jensen's inequality predicts effects of environmental variation. Trends in Ecology and Evolution. 1999;14:361–366. doi: 10.1016/s0169-5347(99)01664-x. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Santos M, Cavelier J, Soriano PJ. Estudio fenológico de cactáceas en el enclave seco de la Tatacoa, Colombia. Biotropica. 2000;32:397–407. [Google Scholar]
- van Schaik CPV, Terborgh JW, Wright SJ. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics. 1993;24:353–377. [Google Scholar]
- Schemske DW. Floral convergence and pollinator sharing in two bee pollinated tropical herbs. Ecology. 1981;62:946–954. [Google Scholar]
- Schmitt J. Individual flowering phenology, plant size, and reproductive success in Linanthus androsaceus, a California annual. Oecologia. 1983;59:135–140. doi: 10.1007/BF00388084. [DOI] [PubMed] [Google Scholar]
- Scott PE. Timing of Agave palmeri flowering and nectar-feeding bat visitation in the Peloncillos and Chiricahua Mountains. Southwestern Naturalist. 2004;49:425–434. [Google Scholar]
- Shreve F. Vegetation of the Sonoran Desert. 1951 Carnegie Institution of Washington, Publication 591. [Google Scholar]
- Steenbergh WF, Lowe CH. Ecology of the saguaro II: reproduction, germination, establishment, growth and survival of the young plant. Washington, DC: Goverment Printing Office; 1977. National Park Service Scientific Monograph Series No. 8. [Google Scholar]
- Stiles FG. Coadapted competitors: the flowering seasons of hummingbird-pollinated plants in a tropical forest. Science. 1977;198:1170–1178. doi: 10.1126/science.198.4322.1177. [DOI] [PubMed] [Google Scholar]
- Thompson JD. Patterns of visitation by animal pollinators. Oikos. 1982;39:241–250. [Google Scholar]
- Tookel F, Ordidge M, Chiurugwi T, Battey N. Mechanisms and function of flower and inflorescence reversion. Journal of Experimental Botany. 2005;56:2587–2599. doi: 10.1093/jxb/eri254. [DOI] [PubMed] [Google Scholar]
- Turner RM, Bowers JE, Burgess TL. Sonoran Desert plants: an ecological atlas. Tucson, AZ: University of Arizona Press; 1995. [Google Scholar]
- Tyler G. Relationships between climate and flowering of eight herbs in a Swedish deciduous forest. Annals of Botany. 2001;87:623–630. [Google Scholar]
- Valiente-Banuet A, Aizmendi MC, Rojas-Martínez A, Domínguez-Canseco L. Ecological relationships between columnar cacti and nectar-feeding bats in Mexico. Journal of Tropical Ecology. 1996;12:103–119. [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of constans protein and the mechanism of photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biological Reviews. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Waser NM. Pollinator availability as a determinant of flowering time in ocotillo (Fouquieria splendens) Oecologia. 1979;39:107–121. doi: 10.1007/BF00346001. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Overpeck JT. Is the Sonoran Desert losing its cool? Global Change Biology. 2005;11:2065–2077. doi: 10.1111/j.1365-2486.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- Wheelwright NT. Competition for dispersers, and the timing of flowering and fruiting in a guild of tropical trees. Oikos. 1985;44:465–477. [Google Scholar]
- Wilbur HM, Rudolf VHW. Life-history evolution in uncertain environments: bet hedging in time. American Naturalist. 2006;168:398–411. doi: 10.1086/506258. [DOI] [PubMed] [Google Scholar]
- Wolf BO, Martínez del Río C, Babson J. Stable isotopes reveal that saguaro fruit provides different resources to two desert dove species. Ecology. 2002;83:1286–1293. [Google Scholar]
- Wright SJ, van Schaik CP. Light and the phenology of tropical trees. American Naturalist. 1994;143:192–199. [Google Scholar]
- Yetman DA, Búrquez A. A tale of two species: speculation on the introduction of Pachycereus pringlei in the Sierra Libre, Sonora, Mexico by Homo sapiens. Desert Plants. 1996;12:23–32. [Google Scholar]