Summary
Survival requires the selection of appropriate behavioural responses in the face of danger. With respect to the threat of predation, both the decision to escape and the underlying neuronal mechanisms have been extensively studied, but processes that trigger evasion of abiotic stressors, which are potentially hazardous to survival, are less well understood. Here, we document the interplay between rhythmic locomotory and `C-start' escape swimming in Xenopus frog larvae when exposed to hyperthermic conditions. As temperature rises, swim cycle frequency increases while swim bout duration decreases, until swimming can no longer be initiated by sensory stimuli. Above a critical higher temperature, more intense sequences of spontaneous high amplitude C-start escape activity occur. Each C-start is followed by a few cycles of fast rhythmic swimming in which activity alternates between the two sides. The initial, high amplitude ventral root burst of an escape sequence propagates rostrocaudally approximately threefold faster than subsequent cycles. The high conduction velocity of this initial burst is consistent with the activation of a Mauthner neuron, one of a pair of giant reticulospinal neurons in fish and amphibians. In support of the involvement of a Mauthner neuron, unilateral lesions of the caudal hindbrain eliminated escape activity on the operated side, but activity remained on the un-operated side. Behaviourally, tadpoles responded to temperature ramps with a sequence of C-start responses in which the body arced through ∼130° in 22 ms, followed by high frequency swimming. These results suggest that high temperature activates the Mauthner neurons to trigger C-start escape behaviour.
Keywords: locomotion, escape behaviour, hyperthermia, tadpole
INTRODUCTION
There are strong selection pressures for the evolution of effective escape manoeuvres when organisms are faced with potentially threatening stimuli. To ensure success, escape behaviours must generally be both rapid and powerful. As a result of these requirements, certain design features of the central neuronal circuitry that are beneficial to these needs have been conserved across a diverse range of animal groups, thus representing the end points of convergent evolutionary processes. These design features include the involvement of relatively large neurons that conduct neural information rapidly, but that have a relatively high threshold for activation. In addition, there are normally relatively few synaptic connections in the pathway from sensory input to motor output and these connections, often involving an electrical component, have a high fidelity to ensure fail safe transmission. In addition, the muscles used for escape need to be activated fully and synchronously to generate the maximum force possible, and thus fast and efficient propulsion away from danger.
Although much is known about the activation of escape circuitry by the primary afferent inputs that signal approach of predators, such as acoustic, acousticolateralis, visual and mechanosensory inputs, far less is known about how the same circuits are engaged by abiotic stressors such as high or low temperature, or anoxia. The neural circuits controlling both rhythmic locomotion and escape have been shown to be susceptible to abiotic stressors and at extremes will cease to operate, thereby endangering the survival of the organism (Robertson, 2004a; Robertson, 2004b). This is particularly evident in poikilotherms, which, unlike homeotherms, cannot readily alter internal temperature and consequently are more vulnerable to thermal shock. In locusts, for example, the flight circuitry can operate at extremely high temperatures of over 40°C, but above this temperature the central pattern generator for flight fails, only to recover when temperatures return towards normal (Robertson et al., 1996). This adaptive shut down of circuit operation clearly serves a protective function and, moreover, the circuit displays long term (acclimation) and short term (heat shock) adaptive changes in the temperature at which the circuit fails in response to subsequent hyperthermia (Robertson, 2004b). Adaptive modifications induced by prior heat shock are also evident in the properties of a locust descending visual interneuron (Money et al., 2005) that is crucially important in relaying information about looming objects and triggering escape behaviours.
In the present study we have investigated how the neural circuitry controlling swimming locomotion in Xenopus laevis frog tadpoles responds to rising temperature. This system, near the time of hatching at developmental stage 37/8 (Nieuwkoop and Faber, 1967), has been extensively studied as a simple vertebrate model of locomotor rhythm generation and its development (for reviews, see McLean et al., 2000; Roberts et al., 1998) and a wealth of detailed information exists regarding the interneuronal circuits of the spinal cord and caudal hindbrain that generate rhythmic swimming (e.g. Li et al., 2002; Li et al., 2004; Li et al., 2006). In addition, there is an increasing knowledge about how the relatively simple network of the hatchling changes its output during larval development (Sillar et al., 1991; Sillar et al., 1992) (reviewed by McLean et al., 2000) and during metamorphosis (Combes et al., 2004) (reviewed by Sillar et al., 2007). Much less is known about the pathways that induce escape swimming. Anatomical evidence indicates that the tadpole brainstem is endowed with a pair of large reticulospinal Mauthner cells which, even before the time of hatching, are significantly larger than other neurons in the CNS (van Mier and ten Donkelaar, 1986). Homologous neurons in fish are command neurons for escape, and underlie the production of C-start escape behaviour, in which the near synchronous activation of the fast twitch myotomal muscles on the contralateral side to stimulation causes the body to adopt a C-shape and orientates the body away from the threat before swimming away (Faber and Korn, 1978; Fetcho, 1991; Korn and Faber, 2005). The main sources of sensory input to the Mauthner cells are from auditory afferents of the VIIIth cranial nerve, but also from lateral line fibres arising from hair cells that detect water vibrations. Other inputs from visual and vestibular systems have been described, although these may constitute signals that modify the final trajectory of the C-start (Korn and Faber, 2005). The acute effects of temperature change on Mauthner cell-mediated C-start behaviour have been investigated previously in fish (Preuss and Faber, 2003), but in this study responses to cooling from 18°C to 8°C were described. Longer term effects of temperature acclimation on neuronal properties of the Mauthner system have also been described (Szabo et al., 2008), but the acute effects of hyperthermia have not been documented previously.
We find that in immobilized stage 42 Xenopus tadpoles, fictive swimming activity evoked by electrical stimulation of cutaneous afferents in the skin persists during temperature ramps from room temperature (∼20°C) up to approximately 30°C. As the bath temperature increases, swim episode durations, cycle periods and ventral root burst amplitudes decrease, until eventually swimming in response to cutaneous stimulation fails completely. Fictive swimming triggered by cutaneous stimulation recovers on return towards room temperature at around 28°C. At temperatures above the failure of the cutaneously evoked swimming, a more vigorous and apparently spontaneous pattern of activity occurs with all the characteristics of escape C-start activity. Thus, the activity consists of an initial, unilateral high amplitude, high intensity ventral root burst that is conducted from head to tail at relatively high velocity (∼1 m s–1), usually followed by up to 10 cycles of fast swimming. The initial burst occurs either on the left or the right side of the body and alternates between several bouts on one side followed by several bouts on the opposite side. Unilateral lesions of the hindbrain confirmed that the escape responses were triggered by descending inputs from one or other side of the brain, suggesting the involvement of fast-conducting reticulospinal neurons, most probably the Mauthner neurons. High speed video analysis confirmed that hyperthermia triggered sequences of rapid movements in which the body arced into a C-shape. In summary, hyperthermia initially shuts down normal, sensory-evoked swimming to be replaced at higher, presumably damaging temperatures by an adaptive activation of Mauthner cell-mediated escape. Some aspects of this work have been published previously in abstract form (Sillar and Robertson, 2008).
MATERIALS AND METHODS
All experiments were performed on stage 42 Xenopus laevis Daudin larvae (Fig. 1A), staged according to the Nieuwkoop and Faber normal tables (Nieuwkoop and Faber, 1967). This work was performed in accordance with the UK 1986 Animals (Scientific Procedures) Act. Experimental animals were obtained following injection of pairs of adults, selected from an in-house laboratory colony, with human chorionic gonadotropin (HCG; 1000 i.u. ml–1; Sigma, St Louis, Mo, USA). Eggs were collected and reared in trays at temperatures between 17 and 23°C until they had reached the desired stage of development, after 3 to 5 days. Experiments began at room temperature (about 20°C).
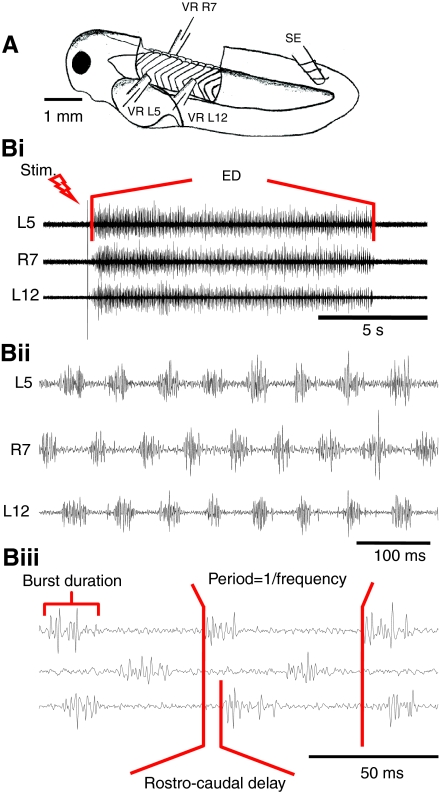
Fig. 1.
The preparation and fictive swimming. (A) Schematic representation of a stage 42 Xenopus larva dissected for recording from three ventral roots VR L5, L12 (on the left) and VR R7 (on the right). Swimming was evoked by electrical stimulation of the caudal skin on the left side using a glass suction electrode (SE). (B) Characteristics of stimulus evoked swimming. (i) Single 1 ms electrical pulse (Stim) evoked an episode of swimming lasting ∼12 s. (ii) Expanded excerpt showing eight cycles of swimming with ventral root activity alternating between left (L5) and right (L7) sides, and propagating from head to tail with a brief delay (L5,L12). (iii) In each cycle of activity, burst durations of approximately 20 ms occurred at cycle periods of ∼50–100 ms. Frequency was calculated as 1000/cycle period in milliseconds.
Before making extracellular recordings of ventral root activity, animals were immobilized in 12.5 μmol l–1 α-bungarotoxin (Sigma), as described previously (Sillar et al., 1991), and then transferred to a recording bath with circulating Hepes saline [composition (in mmol l–1): NaCl, 115; KCl, 2.5; NaHCO3, 2.5; Hepes, 10; MgCl2, 1; CaCl2, 4; pH 7.4 with NaOH]. Saline was gravity-fed from a 100 ml reservoir into a Perspex chamber (about 5 ml). The chamber housed a rotatable platform coated with a layer of Sylgard (Dow-Corning, Midland, MI, USA) on which the immobilized animals were secured using fine-etched tungsten pins. Animals were pinned through the notocord at the level of the otic capsule rostrally and approximately at the 13th to 15th post-otic myotomal segment. The flank skin on the left and right sides of the tadpoles was carefully pierced with fine-etched tungsten pins and gently removed using fine forceps to reveal the underlying myotomes (Fig. 1A). Glass suction electrodes (about 50 μm tip opening) were positioned over the intermyotomal clefts wherein the axons of motorneurons lie. To allow the unambiguous identification of fictive motor patterns underlying both swimming and struggling behaviours (Soffe, 1991), three such recording electrodes were routinely positioned at rostral and caudal locations on the left side and rostrally on the right side (Fig. 1A). The recordings on the left side were most usually made from the 4th or 5th and 11th or 12th post-otic intermyotomal clefts, a separation of approximately 1.5 mm (Fig. 1B). This made it possible to estimate the propagation rate of information along the spinal cord during motor activity. Contralateral activity was usually recorded with an electrode positioned at the 5th to the 8th ventral root cleft. The activity was usually initiated via a glass stimulating suction electrode placed on the tail skin (Fig. 1A; SE), which delivered a 1 ms current pulse from a DS2A isolated stimulator (Digitimer, Welwyn Garden City, UK), and the threshold voltage for initiating fictive swimming was determined for each episode. In some cases, swimming was initiated by transient dimming of the illumination. Signals were amplified using a differential AC amplifier (A-M Systems Model 1700, Carlsborg, WA, USA), digitized using an Axon Instruments Digidata 1322A data acquisition system and viewed on a PC computer using Axoscope software (version 10; Axon Instruments, Union City, CA, USA). In some experiments (N=5) one of the extracellular electrodes was positioned over the head skin to monitor the skin impulse (c.f. Alpert et al., 2007). The skin impulse initiated by stimulating the tail skin propagated throughout the epithelium and was recorded in the head region with a delay of approximately 100 ms.
Electrophysiological data (e.g. Fig. 1Bi–iii) were analysed using Dataview software (version 4.7c, courtesy of Dr W. J. Heitler). Statistical analysis of episode duration, cycle period, burst duration and rostrocaudal delay was performed using Sigmastat and Sigmaplot software. Statistical comparisons of changes in the amplitude, duration and frequency of motor busts during activity recorded at different temperatures were made using ANOVA, parametric t-tests or non-parametric Mann–Whitney U-tests, and differences were deemed significant at P<0.05. Increases in variance associated with temperature-induced changes in swimming were considered significant at P<0.05. The saline was locally heated just before it flowed into the preparation chamber and the temperature was recorded using a digital thermometer with a 0.5 mm diameter probe located adjacent to the tail of the preparation. Motor patterns were recorded while preparations were exposed to repeatable temperature ramps from room temperature (∼20°C) to ∼38°C over a 6 min period. Turning the heater off allowed a gradual return to room temperature in around 10 min.
The behavioural responses of tadpoles to increasing temperature were filmed either at 500 frames s–1 (2 ms frame intervals; N=32 tadpoles) or 600 frames s–1 (every 1.667 ms; N=18) using a PCO 1200HS camera (Optikon Corporation, Kitchener, Ontario, Canada). Tadpoles were filmed in a watchglass (5 cm diameter) containing approximately 3 ml of dechlorinated tap water, at room temperature. The water was changed between trials. The interior of the watchglass was lined with paper towel to reduce the shadow cast by the tadpole. The high intensity light illuminating the tadpoles (enabling short exposure times) was sufficient to raise the temperature by 5°C per minute (similar to the rates of temperature change in the electrophysiological experiments) and no other source of heat was required. Temperature was monitored with a BAT12 thermometer with a copper/constantan thermocouple in the watchglass. In five animals the responses to mechanical stimulation were recorded at a temperature of 27°C and the heating effects of the light source were mitigated using a fan to enhance evaporation from the surface of the water in the experimental chamber.
RESULTS
Effects of temperature change on swimming
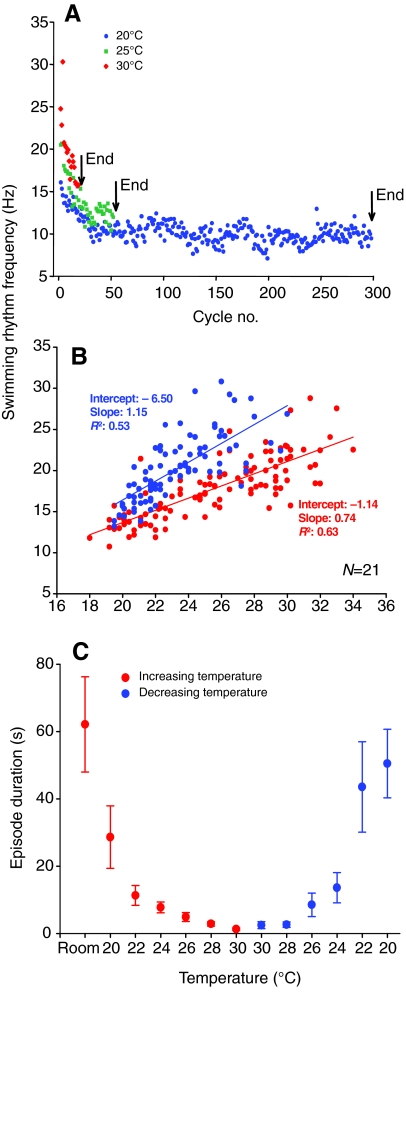
The bath temperature was gradually raised from room temperature (∼20°C) to around 38°C before being returned to room temperature again, a ramp that lasted approximately 10–12 min in total and was roughly a half sine wave in shape. During each temperature ramp several characteristic changes in the fictive swimming pattern evoked by skin stimulation were observed (Fig. 2A). Firstly, the frequencies attained during fictive swimming increased dramatically as the temperature rose (Fig. 2B,C, Fig. 3A). The relationship between temperature and rhythm frequency, which was approximately linear, was such that for a 10°C temperature change, swim frequencies increased on average from ∼14 Hz to 20 Hz at 30°C, representing a Q10 of around 1.5. The peak frequencies attained at room temperature were usually around 18–20 Hz, but at the higher temperature swim frequencies reached over 35 Hz, albeit transiently. This effect on frequency occurred throughout each episode; frequencies normally start high and then decline as the swim episode progresses but both the initial and the final frequencies increased at higher temperature (Fig. 3A). Secondly, the durations of swim episodes showed a corresponding decrease such that episodes that could approach a minute or more in duration at room temperature fell to only a few seconds or even less at 30°C (Fig. 2A, Fig. 3C). Thirdly, at temperatures above 30–32°C, there was a complete failure of fictive swimming following electrical stimulation of the skin (Fig. 2A, red arrows; Fig. 2B), even when the stimulus intensity was increased significantly above the previous threshold that had triggered swimming at lower temperatures (not illustrated). At 34°C the heater was turned off and, after peaking at 36–38°C, the bath temperature gradually returned towards room temperature. On recovery from hyperthermia, swimming could again be reliably evoked, but only once the bath temperature had fallen to ∼28°C or below (Fig. 2A; green up arrow). Thus, the recovery temperature was significantly (P<0.001) lower than the failure temperature (failure at 31.34±0.47°C, N=23; recovery at 26.5±0.42°C, N=20). Interestingly, the slope of the relationship between swim frequency and temperature was steeper during the recovery (Fig. 3B).
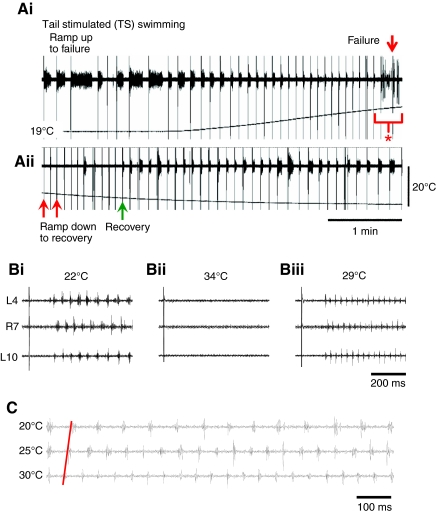
Fig. 2.
Effects of high temperature on fictive swim frequency and episode duration. (Ai) On the rising phase of a temperature ramp from 19°C, the duration of tail-skin stimulated (TS) swimming episodes gradually decreased until eventual failure (red down arrow indicates stimulus artefact). At even higher temperature `escape' activity occurred (bracketed at red asterisk; see also Fig. 4 and associated text). (Aii) On the falling phase of a temperature ramp swimming continued to fail (e.g. red up arrows), but eventually recovered (recovery; green up arrow). (B) Expanded excerpts from a different experiment showing complete failure of swimming at 34°C (Bii). The same stimulus reliably triggered swimming at room temperature (Bi, 22°C) and after recovery at 29°C (Biii). Note the faster swimming in Biii than in Bi. (C) The frequency of swimming increased with increasing temperature such that cycle periods from near the onset of a swim episode almost halved with an increase from 20 to 30°C (red line).
Fig. 3.
Quantification of temperature effects on swimming. (A) Graph of swimming frequency versus cycle in swim episode at 20, 25 and 30°C. Note decreasing episode durations at high temperature accompanied by higher frequencies throughout the episode. (B) Linear relationship between swim frequency and temperature during an increasing (red) and a decreasing ramp (blue). (C) Graphical representation of temperature ramps (N=23) showing decrease in episode duration with increasing (red) and decreasing (blue) phases of the ramp.
Hyperthermia induces escape swimming
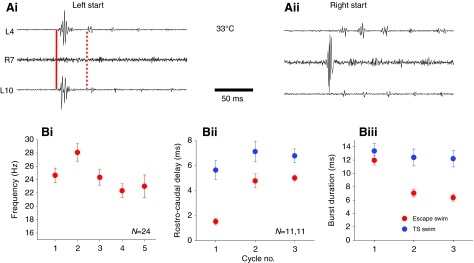
At, or more usually just above the cut off temperature at which swimming, evoked by skin stimulation, failed, a different, more vigorous and apparently spontaneous motor rhythm reliably appeared (asterisked in Fig. 2A). In the example shown in Fig. 4A, no skin stimuli were delivered during the ramp. A spontaneous swim episode occurred shortly after the onset of the ramp, which lasted approximately 1.5 min, but then no further spontaneous activity occurred until the temperature reached approximately 31.5°C. This rhythm, which we have termed escape swimming activity, had a similar coordination to the normal sensory-evoked swimming in that it was rhythmic, displayed left–right alternation and a rostrocaudal delay, but it differed in several important respects. First, the amplitude of the initial burst in the first cycle was significantly larger than the contralateral burst that followed it and in subsequent cycles on either side (Fig. 4B, Fig. 5A). Second, the frequency of the spontaneous rhythm was high, reaching up to 50 Hz in some preparations. Peak frequency was normally attained in the second cycle of escape swimming (Fig. 5B), probably due to the fact that the initial burst duration in an escape sequence was significantly longer than in the following cycles (Fig. 5Biii), but not any longer than the burst durations attained during normal swimming. Third, the rostrocaudal delay, although present was barely detectable, but on average was around 1–2 ms (Fig. 5Bii). With the distance between the two ipsilateral recording electrodes approximately 1.5 mm, this computes to a conduction velocity along the spinal cord during escapes of approximately 1 m s–1. This is rapid compared with the equivalent delays reported during fictive swimming in Xenopus embryos and larvae (Tunstall and Sillar, 1993) (see Discussion). Fourth, in contrast to normal swimming, the activity consisted of short bouts of three to ten cycles of activity, which began either on the right, or the left sides and indeed alternated between the two, such that a series of left-sided bouts would be followed by a series of right sided bouts and vice versa (Fig. 4B).
Fig. 4.
Characteristics of escape activity activated at high temperature. (A) Example of a temperature ramp in which a spontaneous episode of swimming began early in the ramp at ∼22°C before eventually petering out to be replaced by high amplitude and intensity spontaneous `escape' activity. (B) Excerpt from spontaneous escape activity at approximately 32°C showing an escape sequence with three bouts of activity starting on the left side (L), followed by five on the right side (R). Note the propensity for the first ventral root burst in each sequence to be of significantly larger amplitude than subsequent bursts.
Fig. 5.
Characteristics of escape swimming activity. (A) Expanded examples of left (i) and right (ii) sided escape sequences. Note in Ai a high amplitude initial burst occurs nearly synchronously (solid red line) at rostral (top trace) and caudal (bottom trace) recording sites on left side, whereas the next cycle has clear rostrocaudal delay (dotted red line). Initial burst is followed by a few cycles of left–right alternating swimming activity. (B) Graphical representations of C-start sequences. (Bi) Cycle frequency versus cycle in sequence averaged over 24 experiments. Note that the first cycle is at lower frequency than the second cycle because of the large initial burst duration (see text). (Bii) Rostrocaudal delays of the first three cycles of normal stimulus evoked swimming at high temperature (blue; before failure) compared with the first three cycles of escape activity (red). Note that the first cycle in escape is significantly shorter that either the second cycle of escape or any cycle of normal swimming. (Biii) Burst durations of initial burst in the escape sequence are significantly longer than subsequent cycles (red), but not longer than bursts at the onset of control swim episodes at lower temperature (blue).
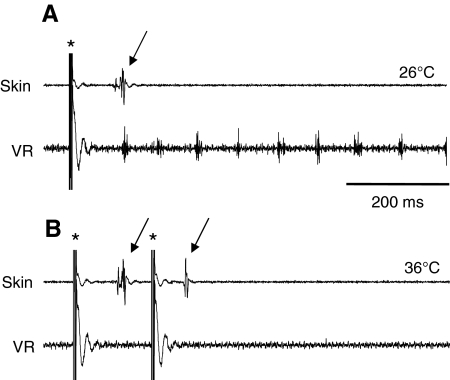
As already mentioned, activity at extremes of temperature could not be induced by sensory stimuli applied to the tail, suggesting that cutaneous pathways of the trunk and tail are somehow inhibited at high temperature. Two possible routes for the activation of a motor response following skin stimulation are: (1) the Rohon–Beard pathway, involving activation of primary afferents with free nerve endings in the skin (Clarke et al., 1984); and (2) the skin impulse pathway involving the electrically excitable epithelium (Roberts, 1998; Alpert et al., 2007); the skin impulse propagates through the epithelium via gap junctions between neighbouring skin cells and has access to the CNS via a branch of the trigeminal ganglion. We monitored the skin impulse with an extracellular suction electrode positioned rostrally on the head during escape swimming to test whether high temperatures triggered spontaneous skin impulses, which in turn triggered escape swimming (Fig. 6). However, we found no evidence of spontaneous skin impulses occurring coincident with escape swimming (not illustrated), despite the fact that the skin impulse could be recorded in response to electrical stimulation of the skin at the temperatures attained during expression of the escape pattern. Indeed the skin impulse was highly resistant to hyperthermia and was present even at the peak temperatures during a ramp (Fig. 6B), up to a maximum observed at 38°C. The possibility that high temperatures trigger spontaneous impulses in Rohon–Beard neurons was not investigated further but seems unlikely because this would be expected to trigger swimming (single Rohon–Beard impulses) or struggling [multiple Rohon–Beard impulses (Soffe, 1991)]. This suggests that the inability of skin stimulation to trigger either normal or escape swimming at high temperatures is because of a central rather than a peripheral inhibition of cutaneous pathways. In summary, the activity triggered at high temperatures had some unique characteristics that distinguished it from normal swimming. For these reasons we term this spontaneous activity `escape swimming'.
Fig. 6.
The skin impulse is resistant to hyperthermia. (A) At 26°C stimulation of the skin (at asterisk) elicited a skin impulse monitored extracellularly (top trace, arrow) with a delay of approximately 100 ms (cf. Alpert et al., 2007) and fictive swimming (bottom trace). (B) At a temperature of 36°C, which is above the failure temperature for fictive swimming (bottom trace), the skin impulse faithfully followed each of two successive skin stimuli.
Effects of hindbrain hemisections
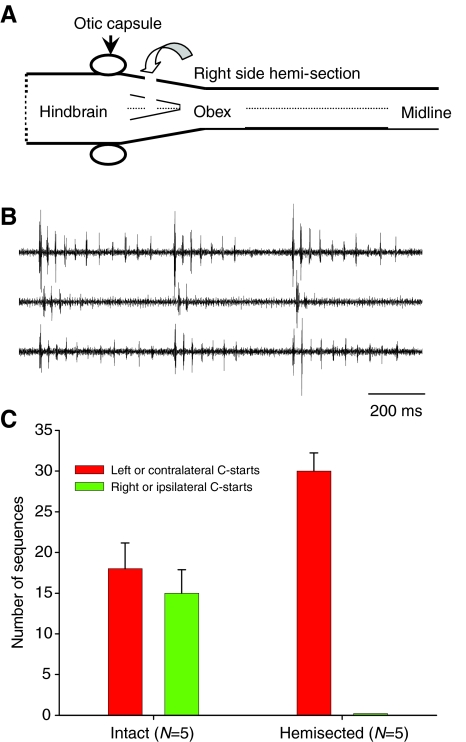
The brief, but detectable rostrocaudal delay in the initial, high amplitude ventral root burst suggested that the activation of escape swimming involved pathways that descend from the brainstem. Therefore we embarked on a series of lesion experiments in which the hindbrain was transected at the level of the otic capsule to block the transmission of information down one or other side of the central nervous system (Fig. 7A). Following unilateral spinalization, hyperthermic escape bursts were recorded only on the side contralateral to the lesion (Fig. 7B). In intact animals escape bursts were initiated in approximately equal numbers on the right and left sides (Fig. 7C, intact), whereas after hemisection 100% of escape bursts were initiated contralateral to the lesion (Fig. 7C, hemisected). Interestingly the total numbers of escape bursts were not significantly different (intact –33±3.2; hemisected –30±2.2; t-test, P<0.05). Bilateral spinalization did not affect tail skin-evoked swimming, but eradicated escape bursts during hyperthermia (not illustrated), providing further evidence that descending signals from the brain trigger escape swimming activity in the spinal cord.
Fig. 7.
Effects of hindbrain hemisections on escape swimming. (A) Schematic diagram of hindbrain and spinal cord in dorsal view showing the approximate location of the hindbrain hemisection on the left, rostral to the obex and caudal to the otic capsule. (B,C) C-start activity in response to hyperthermia was not abolished by surgery (B) but, compared with control preparations (C) in which activity occurred either on the right (green) or left (red) sides, activity only began on the side contralateral to the hemisection (B,C – right histogram). Note that the total number of escape sequences in each case (N=5 preparations analysed for control hemisectioned) was similar.
High speed video analysis of C-start behaviour
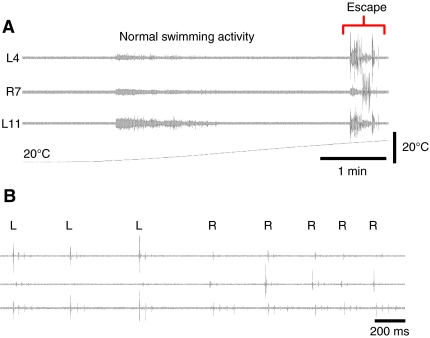
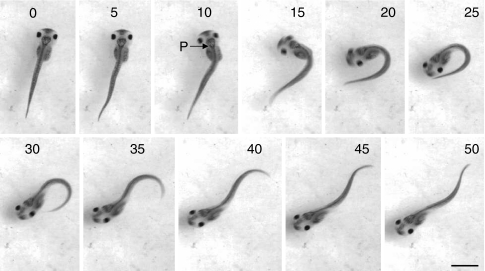
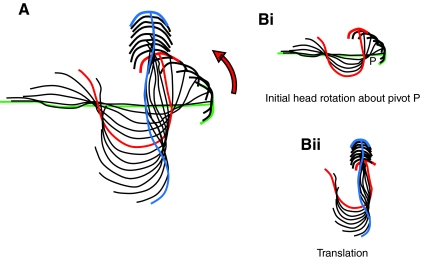
At developmental stage 42, Xenopus tadpoles are pre-feeding and have yet to become free swimming. They are normally motionless either suspended from an object in the dish or the meniscus of the water surface by the mucus secreted by the cement gland or lying on the bottom of their chamber. In keeping with the preceding findings using electrophysiology in immobilized tadpoles, high temperatures triggered vigorous escape-like behavioural responses in which the body adopted a characteristic C-shape (Figs 8 and 9). The temperature at which these C-starts occurred was 32.6±0.3°C (N=50), similar to, though slightly higher than, that which triggered the fictive C-start activity described above. During escape behaviour the head initially rotated through an angle of 131±6 degrees (N=47) in 22.2±0.5 ms (N=47), representing an angular head rotation rate of ∼6000 degrees per second. This initial C-start was non-propulsive but usually was followed by a short sequence of propulsive fast swimming lasting a few cycles (25±0.5 Hz; N=42). Each C-start thus consisted of two components (Fig. 9A,B): an initial rapid head rotation that was essentially non-propulsive (Fig. 9Bi); followed by a translation into a propulsive component, which moved the animal away from its starting point at high velocity (Fig. 9Bii). In some cases when the initial starting position of the tadpole was lying flat on the floor of the chamber, the force of the initial upward C-start trajectory was sufficient to cause the tadpole to breach the water surface (not illustrated).
Fig. 8.
C-start escape response of stage 42 tadpole. Single frames at 5 ms intervals of C-start escape sequence of a stage 42 tadpole triggered by hyperthermia and filmed at 500 frames s–1. From the first detectable movement (5 ms) to completion of the C-bend took 20 ms. The tadpole turned ∼160 degrees about a pivot point (P, at 10 ms) located at the position of the obex and then swam in approximately the opposite direction. The tadpole was 7 mm in length.
Fig. 9.
Trajectories of C-start escape responses of stage 42 tadpole. (A) Stick drawings showing head (arcs) and tail (lines) positions from consecutive frames during a C-start sequence of a stage 42 tadpole, triggered by hyperthermia. (B) The head undergoes an initial rapid rotation about a pivot point (P; i), located at the position of the obex. This phase of the escape sequence is non-propulsive but is followed by a translation phase which propels the tadpole forwards (ii). Drawings are from frames at 5 ms intervals.
DISCUSSION
When the saline temperature reaches a critical level of 28°C to 32°C, immobilized stage 42 Xenopus larvae respond to these hyperthermic conditions by initiating a series of fictive escape responses, initiated on one or other side with a large amplitude ventral root burst. The activity propagates at relatively high velocity (∼1 m s–1) along the body and is often followed by several cycles of fast fictive swimming in which ventral root activity alternates between the left and right sides. Within a sequence of escapes, which could last up to 30 s, left and right side escapes alternated. At the high temperatures that activated this escape response, normal swimming activity could not be evoked by skin stimulation suggesting that cutaneous activation pathways are inhibited and motor behaviour is selected centrally in response to this abiotic hyperthermic stress.
Normal and escape swimming activity differed in two ways. Firstly, the amplitude of the initial ventral root burst during escape was significantly larger than either during subsequent cycles of swimming in an escape sequence or in the first cycle of skin-stimulated swimming. This suggests that a complete or near complete activation of the myotomal motor pool innervating a given muscle segment only occurs during escape behaviour. Secondly, the initial burst of an escape sequence propagated along the trunk significantly faster than either during subsequent cycles of swimming in an escape sequence or in the first cycle of skin-stimulated swimming. These distinctions between fictive escape and normal swimming activity were also reflected in the behavioural responses to hyperthermia which involved a rapid initial, non-propulsive C-start followed by propulsive high speed swimming.
Probable involvement of Mauthner neurons
The brief, but detectable rostrocaudal delay during escape swimming activity suggests rostral centres are the probable source of the drive for the initiation of escape behaviour. This was confirmed by hemisections of the caudal hindbrain at the level of the otic capsule that resulted in unilateral escape bouts on the side opposite the transaction, and by complete transections that eliminated all escape swimming, but not stimulated swimming. The near instantaneous activation of the motorneurons along the activated side of the body also suggests that the pre-motor axons involved are relatively large and fast conducting, with estimated conduction velocities of around 1 m s–1. Although this would not be considered a high conduction velocity for adult myelinated vertebrate axons, it is approaching the maximum that might be expected for a small developing vertebrate in which myelination has not yet occurred (Yoshida, 1997). The initial burst of an escape sequence propagates significantly faster than during normal swimming activity at the same cycle frequency. Thus, in subsequent cycles of the escape bout the delay was approximately threefold longer than on the first cycle, despite the fact that the cycle period of the first cycle was lower than the second. The conduction velocity is also much higher than previous estimates in the Xenopus tadpole. For example, the conduction velocities of the peripheral and central processes of primary sensory Rohon–Beard neurons at stage 37 and 38 have been estimated at 0.05–0.2 and 0.15–0.4 m s–1, respectively (Clarke et al., 1984). The modal conduction velocity for Rohon–Beard neurons of 0.3 m s–1 is also similar to estimates of average conduction velocities along the spinal cord based upon the rostrocaudal delays during fictive swimming. A similar estimate of 0.12 m s–1 for spinal excitatory interneurons was calculated by Dale and Roberts (Dale and Roberts, 1985). Thus, the neurons that trigger escape swimming have significantly faster conduction velocities, around threefold higher than other neurons thus far studied in this preparation, and presumably therefore have larger diameter axons.
The largest and most conspicuous reticulospinal neurons in the amphibian brainstem are the bilaterally paired Mauthner cells (van Mier and ten Donkelaar, 1984), which are also renowned for being responsible for C-start escape behaviour in teleost fish (Korn and Faber, 2005; Zottoli, 1978). These neurons are distinguishable from others in the brainstem by their large size. Even as early in development as stage 30/31, only about 1.5 days after fertilization [when reared at 23°C (Nieuwkoop and Faber, 1967)] the soma is approximately 50 μm in diameter (van Mier and ten Donkelaar, 1984), and their axons have already invaded the spinal cord. By stage 35/36 the soma has increased to 80 μm in diameter and the decussating axons are distinctly larger than other descending projections. Given these statistics, the most probable explanation for the C-start escape behaviour recorded in the present study is that hyperthermia somehow triggers action potentials in the Mauthner neurons. It is interesting, in light of the fact that the Mauthner neurons are known to be coupled by reciprocal inhibition, that the hyperthermia-induced escape sequences consisted of several episodes beginning on one side followed by several episodes beginning on the opposite side. Although activation of the Mauthner neurons is the most probable causal explanation for the observed escape responses, there is no direct evidence for this and we cannot rule out the possibility that other reticulospinal neurons, such as Mauthner cell homologues, are additionally activated. It is also notable that despite three decades of research on the neural control of swimming in hatchling tadpoles, there have been no previous reports, to our knowledge, of the Mauthner neurons being activated by cutaneous stimulation of the skin, or indeed by other sensory stimuli, as a prelude to swimming activity, so this is the first report ascribing a possible function to them at these early stages of tadpole development.
Inhibition of sensory pathways for motor action
It has recently been shown that cellular and synaptic properties of the Mauthner system are affected when fish are acclimated to different environmental temperatures (Szabo et al., 2008). The mechanisms that activate the Mauthner neurons acutely in response to high temperature are not known. It seems unlikely that high temperature selectively activates them by causing direct depolarization because these are large neurons, most probably with a high activation threshold. It might be expected that if neurons are directly excited by high temperature, smaller neurons will be activated first leading to constant motor activity before failure. Another possibility that requires consideration is that high temperatures activate a sensory input pathway to the Mauthner neurons. Although this cannot be ruled out, it is notable that two skin sensory pathways that activate swimming do not appear to be activated by hyperthermia. For example, the electrically excitable skin cells, which are known to activate swimming (Roberts and Smyth, 1974; Roberts and Stirling, 1971), did not discharge spontaneously at high temperature, although skin impulses could still be evoked at high temperature (Fig. 6). This is presumably also true of the mechanosensory Rohon–Beard neurons which would be expected to trigger swimming if they discharged in response to hyperthermia; in fact, skin stimulation fails to trigger any motor activity at the temperatures that trigger spontaneous C-start activity. This suggests that sensory input pathways are inhibited rather than activated. It is conceivable that another sensory pathway (such as from the lateral line afferents) is selectively excited by hyperthermia to directly activate the Mauthner neurons, although the precise identity of the temperature sensor remains to be elucidated.
Possible behavioural relevance of hyperthermic escape
Despite the apparent inhibition of swimming at high temperatures, the activation of the C-start escape circuitry reveals that the tadpole has retained a residual, latent capacity to repeatedly generate short bouts of fast escape swimming. This in turn suggests that the inhibition of the swimming by cutaneous inputs serves a protective function, ensuring that once temperatures reach potentially damaging levels the tadpole becomes inactive, perhaps allowing it to sink to cooler climes. However, the organism is still able to respond with an abrupt C-start to orient it away from the heat while retaining the capacity to swim away in the opposite direction should these damaging higher temperatures be reached. But could this arrangement have any adaptive value in the wild? Xenopus tadpoles live in environments that are likely to experience a wide diurnal variation in temperatures and rapid changes of 15°C are not uncommon (Montgomery and MacDonald, 1990), such that at the extremes, thermal stress may compromise survival. One possible scenario in which thermal activation of Mauthner neurons could be engaged is when high daytime temperatures lead to significant evaporation in small ponds. In the lowered water levels it is possible that tadpoles could become isolated and temperatures could rise above that required to activate C-starts so that the organism escapes to cooler, deeper regions. A related possibility is that if rising temperatures cause the water level to drop to the extent that the tadpole becomes beached, the powerful flipping that would ensue could increase its chances of returning to the pond and surviving. It is important that the organism has a means of avoiding potentially damaging environments and the activation of C-start escape behaviour may be an appropriate method of evading hyperthermic conditions.
The decision to escape is undoubtedly complex, often relying on the convergence of inputs from multiple pathways, such as visual signals from approaching predators or conspecifics, which can influence escape threshold. As we show here environmental temperature may by an additional factor influencing the decision to escape. Thus both recent experience and evolutionary history can modify the utilization of escape circuitry. It remains to be determined whether the hyperthermic activation of the escape system described here represents a conserved mechanism for evading such abiotic stress.
Work in the authors' laboratories is supported by the BBSRC, the Wellcome Trust (K.T.S.) and the Natural Sciences and Engineering Research Council of Canada (R.M.R.). We thank Stuart McGregor for help in collecting the high speed imaging data, Rudi Winklbauer of the University of Toronto for providing the supply of tadpoles used in the high speed video analysis and Bill Heitler and HongYan Zhang for commenting on a draft of the manuscript. Deposited in PMC for release after 6 months.
References
- Alpert, M. H., Zhang, H., Molinari, M., Heitler, W. J. and Sillar, K. T. (2007). Nitric oxide modulation of the electrically excitable skin of Xenopus laevis frog tadpoles. J. Exp. Biol. 210, 3910-3918. [DOI] [PubMed] [Google Scholar]
- Clarke, J. D. W., Hayes, B. P., Hunt, S. P. and Roberts, A. (1984). Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J. Physiol. 348, 511-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes, D., Merrywest, S. D., Simmers, J. and Sillar, K. T. (2004). Developmental segregation of spinal networks driving axial and hindlimb locomotor behavior during amphibian metamorphosis. J. Physiol. 559, 17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, N. and Roberts, A. (1985). Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J. Physiol. 363, 35-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, D. S. and Korn, H. (1978). Neurobiology of the Mauthner Neuron. New York: Raven Press.
- Fetcho, J. (1991). The spinal network of the Mauthner cell. Brain Behav. Evol. 37, 298-316. [DOI] [PubMed] [Google Scholar]
- Korn, H. and Faber, D. S. (2005). The Mauthner cell half a century later: a neurobiological model for decision-making. Neuron 47, 13-28. [DOI] [PubMed] [Google Scholar]
- Li, W. C., Soffe, S. R. and Roberts, A. (2002). Spinal inhibitory neurons that modulate cutaneous sensory pathways during locomotion in a simple vertebrate. J. Neurosci. 22, 10924-10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. C., Soffe, S. R. and Roberts, A. (2004). Glutamate and acetylcholine corelease at developing synapses. Proc. Natl. Acad. Sci. USA 101, 15488-15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. C., Soffe, S. R., Wolf, E. and Roberts, A. (2006). Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J. Neurosci. 26, 4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, D. L., Merrywest, S. D. and Sillar, K. T. (2000). The development of neuromodulatory systems and the maturation of motor patterns in amphibian tadpoles. Brain Res. Bull. 53, 595-603. [DOI] [PubMed] [Google Scholar]
- Money, T. G. A., Anstey, M. L. and Robertson, R. M. (2005). Heat stress-mediated plasticity in a locust looming-sensitive visual interneuron. J. Neurophysiol. 93, 1908-1919. [DOI] [PubMed] [Google Scholar]
- Montgomery, J. C. and Macdonald, J. A. (1990). Effects of temperature on nervous system: implications for behavioural performance. Am. J. Physiol. 259, R191-R196. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Faber, J. (1967). Normal Table of Xenopus laevis (Daudin), 2nd edn. Amsterdam: North Holland Publishing.
- Preuss, T. and Faber, D. S. (2003). Central cellular mechanisms underlying temperature-dependent changes in the goldfish startle-escape behaviour. J. Neurosci. 23, 5617-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A. (1998). Skin sensory systems of amphibian embryos and young larvae. In Amphibian Biology, Volume 3: Sensory Perception (ed. H. Heatwole), pp. 923-935. Chipping Norton, Australia: Surrey Beaty.
- Roberts, A. and Smyth, D. (1974). The development of a dual touch sensory system in embryos of the amphibian Xenopus laevis. J. Comp. Physiol. 88, 31-42. [Google Scholar]
- Roberts, A. and Stirling, C. A. (1971). The properties and propagation of a cardiac-like impulse in the skin of young tadpoles. Z. Vgl. Physiol. 71, 295-310. [Google Scholar]
- Roberts, A., Soffe, S. R., Wolf, E. S., Yoshida, M. and Zhao, F. Y. (1998). Central circuits controlling locomotion in young frog tadpoles. Ann. NY Acad. Sci. 860, 18-34. [DOI] [PubMed] [Google Scholar]
- Robertson, R. M. (2004a). Modulation of neural circuit operation by prior environmental stress. Integr. Comp. Biol. 44, 21-27. [DOI] [PubMed] [Google Scholar]
- Robertson, R. M. (2004b). Thermal stress and neural function: adaptive mechanisms in insect model systems. J. Therm. Biol. 29, 351-358. [Google Scholar]
- Robertson, R. M., Xu, H., Shoemaker, K. L. and Dawson-Scully, K. (1996). Exposure to heat shock affects thermosensitivity of the locust flight system. J. Neurobiol. 29, 367-383. [DOI] [PubMed] [Google Scholar]
- Sillar, K. T. and Robertson, R. M. (2008). Thermal activation of escape swimming circuitry in Xenopus laevis tadpoles. Program No. 575.4. 2008 Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience.
- Sillar, K. T., Wedderburn, J. F. S. and Simmers, A. J. (1991). The postembryonic development of locomotor rhythmicity in Xenopus laevis tadpoles. Proc. Biol Sci. 246, 147-153. [DOI] [PubMed] [Google Scholar]
- Sillar, K. T., Wedderburn, J. F. S. and Simmers, A. J. (1992). The post-embryonic development of cell properties and synaptic drive underlying locomotor rhythm generation in Xenopus larvae. Proc. Biol. Sci. 249, 65-70. [DOI] [PubMed] [Google Scholar]
- Sillar, K. T., Combes, D., Ramanathan, S., Molinari. M and Simmers, A. J. (2007). Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res. Rev. 57, 94-102. [DOI] [PubMed] [Google Scholar]
- Soffe, S. R. (1991). Triggering and gating of motor responses by sensory stimulation: behavioural selection in Xenopus embryos. Proc. Biol. Sci. 246, 197-203. [DOI] [PubMed] [Google Scholar]
- Szabo, T. M., Brookings, T., Preuss, T. and Faber, D. S. (2008). Effects of temperature acclimation on a central neural circuit and its behavioural output. J. Neurophysiol. 100, 2997-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall, M. J. and Sillar, K. T. (1993). Physiological and developmental aspects of intersegmental coordination in Xenopus embryos and tadpoles. Semin. Neurosci. 5, 29-40. [Google Scholar]
- van Mier, P. A. and ten Donkelaar, H. J. (1984). Early development of descending pathways from the brain stem to the spinal cord in Xenopus laevis as revealed by anterograde labelling. Anat. Embryol. 170, 295-306. [DOI] [PubMed] [Google Scholar]
- Yoshida, A. (1997). Oligodendreocyte maturation in Xenopus laevis. J. Neurosci. Res. 50, 169-176. [DOI] [PubMed] [Google Scholar]
- Zottoli, S. J. (1978). Comparative morphology of the Mauthner cell in fish and amphibians. In Neurobiology of the Mauthner Cell (ed. D. S. Faber and H. Korn), pp. 13-46. New York: Raven Press.