Summary
Males should adjust their behavior and its neural substrates according to the quality of competition that they assess by eavesdropping on other males' courtship signals. In European starlings (Sturnus vulgaris), females base mate choice, in part, on aspects of male song associated with its length, which positively correlates with the males' reproductive success, immunocompetence, age and ability to repel competing males. To determine how variation in the quality of male courtship song affects the brain and behavior of incidental male receivers, we exposed adult male starlings to either long or short songs periodically over 7 days, followed by 1 day of no song. We found no difference between groups in the length (i.e. quality) of songs that subjects produced during the experiment. However, compared with males exposed to short songs, those exposed to long songs sang more songs, exhibited more non-singing activity and, by the end of the experiment, weighed less and had a 30% larger robust nucleus of the arcopallium (RA), a forebrain nucleus that translates pre-motor signals into the appropriate combination of respiratory and syringeal activity. The change in RA volume was not entirely due to variation in song output, suggesting, for the first time, the possibility of acoustically driven plasticity in this motor nucleus. We hypothesize that such neuroplasticity helps prepare the individual for future song output tailored to the prevailing competitive environment.
Keywords: bird song, European starling (Sturnus vulgaris), intra-specific competition, neuroplasticity, robust nucleus of the arcopallium (RA), song-control system
INTRODUCTION
In many animal species, females base mate-choice decisions, in part, on variation between males in their sexual advertisement signals (Andersson, 1994). One of the leading hypotheses explaining this phenomenon is that the signals convey information about the quality of the male (Zahavi and Zahavi, 1997). Because communication often involves incidental receivers, it stands to reason that a receiver other than a prospecting female may also glean information about the quality of the signaler (Naguib and Dietmar, 1997; McGregor and Peake, 2000; Ophir and Galef, 2003). Moreover, information about the quality of a signaling competitor may benefit an eavesdropping male if it enables him to modify his own behavior adaptively.
In songbirds, male song is often used in sexual advertisement (Catchpole and Slater, 1995) and is a learned behavior (Marler, 1997) controlled by an interconnected circuit of forebrain nuclei, including the HVC (initials used as a proper name), the robust nucleus of the arcopallium (RA), Area X, and the lateral portion of the magnocellular nucleus of the anterior nidopallium (lMAN) (Nottebohm et al., 1976). The HVC and RA are part of a motor pathway that is primarily involved in song production, whereas Area X and lMAN are part of an anterior forebrain pathway primarily involved in song learning and song plasticity (Brainard, 2004). The volumes and therefore functionality of these brain nuclei can be highly sensitive to stimuli from the physical environment, such as a changing photoperiod (Ball et al., 2006; Meitzen et al., 2007), and stimuli from the social environment, such as the presence of a female (Tramontin et al., 1999; Balthazart et al., 2008). However, the extent to which the volumes of the vocal-control nuclei are sensitive to subtle variations in advertisement signals from same-sex competitors is unknown.
In European starlings (Sturnus vulgaris L.), males sing from nesting cavities, thereby attracting not only females for mating purposes but also other males, which may compete with the singer for the cavity (Mountjoy and Lemon, 1991). Starling song length positively correlates with the singer's reproductive success (Mountjoy and Lemon, 1996), immunocompetence (Duffy and Ball, 2002) and age (Feare, 1984). Thus, it is not surprising that females prefer longer songs over shorter songs in a mate-choice context (Gentner and Hulse, 2000). More complex or longer songs attract fewer (or repel more) males from nest cavities than do simple or shorter songs, probably because song length reflects the quality of the singer and therefore his ability to defend the nesting cavity (Mountjoy and Lemon, 1991).
We conducted a laboratory experiment on European starlings to examine how song competition affects male singing behavior and the volumes of the vocal-control nuclei. Because song length reflects several measures of male quality and his ability to defend an important resource from other males, we reasoned that the quality of the song environment, reflected by the prevailing song length to which a male is chronically exposed, influences his perception of competition and thus his sexual advertisement behavior and the neural substrates that control it. We note that length is probably not the feature of the song that is most relevant to the male or female receiver. Instead, some aspect of the song that is correlated with its natural length appears to drive the behavioral responses of both male and female starlings to songs (Gentner and Hulse, 1998; Gentner and Hulse, 2000). Longer songs tend to have more unique and more repeated motifs per bout than shorter songs (Gentner and Hulse, 2000). Consequently, one correlate of song length to which male and female receivers attend in making behavioral decisions may be the stereotypy of motif transitions (Gentner and Hulse, 1998). Other correlates of a song's length also might influence male and female behavior. By using recordings of unmanipulated songs that differ naturally in length, as we did in this study (see Materials and methods), we should have captured such correlates that affect receiver behavior (see citations in previous paragraph) and that reflect the degree of competition.
MATERIALS AND METHODS
Animals and housing
European starlings were captured on 12 December 2005 in Pennsylvania, USA (41.75°N, 80.35°W) and transferred to large, outdoor flight cages at the University of North Carolina at Chapel Hill (USA), where we conducted the university-approved study (IACUC protocol 04-241.2). For the entire study, we provided the birds with food (Daily Maintenance, Roudybush; Woodland, CA, USA) and water ad libitum. We identified males based on the presence of a blue proximal region of the bill (Kessel, 1951) and later confirmed their sex (see `Experimental procedure'). On 10 May 2006, we paired 20 male subjects in 10 indoor cages on a photoperiod (16 h L:8 h D) that synchronizes the birds' reproductive cycles by driving the birds first through a reproductive-like state and then into a non-reproductive state (Nicholls et al., 1988). On 13 July, we changed the photoperiod to 8 h L:16 h D in order to begin the process of re-instating sensitivity to reproductive stimuli (Nicholls et al., 1988).
Experimental procedure
This study consisted of five sessions each involving four subjects. On 18 October, we transferred the four subjects of the first session (two of the pairs mentioned above) into each of four sound-attenuation chambers located together in one room on an 11 h L:13 h D photoperiod. Each foam-lined chamber had a cage with two perches, an air intake and fan-driven exhaust, a fluorescent light that maintained the 11 h L:13 h D photoperiod within the chamber, a speaker (Pioneer Corp. TS-G1040R; Tokyo, Japan) and an omni-directional microphone (Sennheiser ME 62; Old Lyme, CT, USA). We powered speakers by a daisy chain of four mono-block amplifiers interfaced with a computer.
The next day, we began the simultaneous broadcast of one of two acoustic treatments through each chamber's speaker (at approximately 70 dB 5 cm from the speaker), a set of long songs or a set of short songs (hereafter termed song environment treatment; see `Song recordings used for playbacks'). We balanced treatment levels between members of the original pairs described above and spatially interspersed among the chambers each replicate of the long song environment with each replicate of the short song environment. Using procedures described previously (Sockman and Salvante, 2008), we exposed the subjects to the song sets for 5.5 h per day for 7 days at partially randomized 30 min intervals during the photophase only and randomized the order of songs played within each 30 min period. Broadcasts began at the onset of the photophase each day. No more than two 30 min broadcasts occurred in a row (i.e. without at least one intervening 30 min silent period), and no broadcast occurred during the last 30 min of the photophase each day. This approach, along with the use of an 11 h L:13 h D photoperiod, was intended to mimic the natural sexual signaling environment of free-living starlings early in the breeding season. We constantly broadcast white-noise in the room to help mask sound between chambers. On the 8th day of the session (hereafter termed 1 day after playback), the subjects received no song playback.
During the photophase of each of the first 8 days, we collected audio recordings of the song produced by the subjects, using the microphone in each chamber, which we interfaced with a computer programmed (Sound Analysis Pro software, version 1.02; http://ofer.sci.ccny.cuny.edu:2001/html/sound_analysis.html) to store recordings only if 27 consecutive peaks in the recording's oscillogram exceeded an amplitude threshold of 80 dB on average (depending on the sound-attenuation chamber) for at least 3 s. Stored recordings began 20 ms prior to the peak train and ended when sound dropped below the amplitude threshold for 10 s. These settings enabled us to record all of the subjects' songs and to minimize the recording of other sounds. Nonetheless, we generated a large number of audio files, many of which contained the subjects' songs and many others of which contained only the cage noises produced when the subjects moved (see `Quantification and analysis').
Starting 4 h after the onset of the photophase on the 9th day of the session, we began exposing the subjects to an additional 30 min song treatment as part of another study. However, because this treatment had no effect on any of the measurements of interest in this paper and because the two levels of the treatment were perfectly balanced between levels of the song environment and among sessions, we do not discuss this further.
At 90 min following the onset of the second song treatment, we weighed the subjects, rapidly decapitated them, removed their brains, and confirmed that each had testes. Using previously described protocols (Sockman and Salvante, 2008), we fixed, stored and later sectioned one hemisphere (alternating left and right with each treatment group) at 40 μm in the sagittal plane. We repeated these procedures for the remaining four sessions of four subjects each, resulting in a total of 20 subjects divided among 10 replicates for each of the two treatment groups, counterbalanced among the chambers and sessions.
Song recordings used for playbacks
Details of the song recordings used for the song environment treatment have been described previously (Gentner and Hulse, 2000). Briefly, a library of complete song bouts was recorded from a single, laboratory-housed male directing song at a female. From these, 12 songs were selected, which, based on length, were divided into two sets of six: a long-song set and a short-song set with mean song lengths of 55.2 and 26.0 s, respectively. Importantly, neither total song duration nor total silence duration differed between the long-song and short-song treatment levels, and therefore we exposed all males to the exact same amount of song, regardless of group. By necessity, the two levels of the treatment also differed in the repetition rate of individual songs. However, a previous study reported that song length (or a correlate thereof) but not repetition rate affected expression of immediate, early genes in the auditory forebrain of female European starlings (Gentner et al., 2001). We assume that this sensitivity specifically to a correlate of song length over song repetition rate also applies to our dependent measures on males, but we do not know this with certainty.
Quantification and analysis
We defined a song as a series of at least two different motifs containing a combined total of at least five notes, wherein we defined a note as a continuous trace on a spectrogram and a motif as a group of notes arranged in a fixed order (Adret-Hausberger and Jenkins, 1988; Eens et al., 1989). The subjects produced almost no song on day 1, but there were too many songs produced on the other days to analyze them all. Therefore, we employed the following sampling technique. Using a random number generator, we selected approximately 45% of the song files recorded on each of days 4 and 7 of playback and 1 day after playback (day 8). (We distinguish song files from songs because individual files sometimes contained multiple songs, and individual songs sometimes spanned multiple files.) For each of these 3 days, the proportion of files selected from each individual reflected the proportion of all files that the individual produced. For example, if all 20 subjects produced a total of 1000 song files on day 4, we analyzed 450 of these files (45% of 1000 files=450 files). If 25% of the total number of song files on day 4 came from individual X, then we randomly selected and analyzed 113 of individual X's day 4 song files (113 files=25% of 450 song files analyzed from day 4). Within this random subset of song files, we then counted the number of songs and measured the duration (using Raven software, version 1.2.1, Cornell Lab of Ornithology) of their computer-drawn spectrograms. This was simply the difference in time between the end and beginning of a complete song, including the brief silent intervals between the individual motifs of a song, and is consistent with other studies measuring starling song length (Feare, 1984; Mountjoy and Lemon, 1996; Gentner and Hulse, 2000; Gentner et al., 2001; Duffy and Ball, 2002). We were able to distinguish between the playback and subject songs because the playback songs were of lower amplitude and produced unique spectrograms. We also used the count of cage noise files as an index of non-singing activity. Use of file sizes (as opposed to file counts) produced qualitatively identical results, which we do not present.
We mounted every third brain section onto glass slides, dehydrated the tissue, and stained the Nissl bodies with thionin. Using a microscope, imaging system and computer described previously (Sockman and Salvante, 2008), we collected digital brightfield images of Area X magnified 20 times, of the HVC and RA magnified 80 times, and of lMAN magnified 160 times. Blind to the experimental treatment of the subjects, we used the software ImageJ (version 1.34 s, National Institutes of Health) to measure the area contained within the Nissl-defined boundaries of the brain nuclei and calculated the volume of nuclei using a previously described protocol (Smith et al., 1997; Tramontin et al., 1998). For the RA, we also collected a rostral and a caudal image magnified 567 times from each of a medial, central and lateral section (six images per individual). For each of these images, we used ImageJ to count the number of cell profiles and to measure the area of five cell profiles, always selecting the closest cell to each of the image's four corners and its center point. We then calculated the mean spacing of cells (cell profiles/mm2) and the mean area of cell profiles (μm2) for each individual. Because of a procedural error, we lost and therefore did not analyze the tissue from all subjects in sessions 2 and 4.
Our data consisted of a combination of fixed and hierarchically structured random effects, each of which may differ from the others in its correlation structure. Therefore, we used a mixed, multi-level modeling framework (using Stata IC software, version 10.0, College Station, TX, USA), which is readily amenable to data sets such as this (Rabe-Hesketh and Skrondal, 2005) (Table 1). We primarily used the multi-level mixed-effects linear regression command but also used the multi-level mixed-effects Poisson regression command for the song count data. These models estimated parameters with restricted maximum likelihood and used z-tests to test the null hypothesis that a coefficient equaled 0. For models in which we had multiple observations per individual (analyses of song length, song count and non-singing activity), we nested observation within individual and individual within session, each as a random intercept. For models with just one observation per individual (analyses of body mass and brain measures), we nested individual within session as a random intercept. On a few occasions, we had to exclude the nested random effect in order to get the models to converge on a solution (see Table 1). Plots of some data (song length and song count) suggested a non-linear effect of the variable `day of observation'. For these analyses, we modeled the independent contrasts that made up the dummy variable set into which we expanded day of observation as a categorical variable (see Table 1). Otherwise, we treated day of observation as a continuous variable. For additional information on using this mixed, multi-level modeling approach, see Sockman et al. (Sockman et al., 2008).
Table 1.
Results from the statistical analyses of behavioral and brain measurements collected from adult male European starlings whose song environment was experimentally manipulated
| Analysis Predictor | Estimate | Standard error | z-score | P-value |
|---|---|---|---|---|
| 1. Song length (s) [52/20/5] | ||||
| Intercept | 33.578 | 2.832 | 11.86 | <0.001 |
| Song environment | 0.345 | 2.761 | 0.12 | >0.2 |
| Day 4:day 7 | –6.476 | 2.078 | –3.12 | 0.002 |
| Day 7:day 8 | –1.023 | 1.822 | –0.56 | >0.2 |
| Song environment × day 4:day 7 | 2.635 | 2.938 | 0.90 | >0.2 |
| Song environment × day 7:day 8 | –0.644 | 2.694 | –0.24 | >0.2 |
| 2. Song length (s) [39/13/4]* | ||||
| Intercept | 36.490 | 2.584 | 14.12 | <0.001 |
| Song environment | –0.890 | 2.844 | –0.31 | >0.2 |
| Day 4:day 7 | –7.114 | 2.017 | –3.53 | <0.001 |
| Day 7:day 8 | –1.550 | 2.017 | –0.77 | >0.2 |
| Song environment × day 4:day 7 | 3.587 | 2.969 | 1.21 | >0.2 |
| Song environment × day 7:day 8 | 1.457 | 2.969 | 0.49 | >0.2 |
| 3. Song count [60/20/5] | ||||
| Intercept | 3.658 | 0.352 | 10.39 | <0.001 |
| Song environment | –0.448 | 0.341 | –1.31 | 0.19 |
| Day 4:day 7 | –0.570 | 0.079 | –7.20 | <0.001 |
| Day 7:day 8 | –0.594 | 0.080 | –7.45 | <0.001 |
| Song environment × day 4:day 7 | 0.228 | 0.110 | 2.08 | 0.037 |
| Song environment × day 7:day 8 | 0.321 | 0.109 | 2.94 | 0.003 |
| 4. Song count on day 4 of playback [20/5] | ||||
| Intercept | 2.305 | 1.067 | 2.16 | 0.031 |
| Song environment | 0.172 | 0.086 | 2.01 | 0.045 |
| 5. Song count on day 7 of playback [20/5] | ||||
| Intercept | 3.675 | 0.221 | 16.63 | <0.001 |
| Song environment | –0.056 | 0.068 | –0.82 | >0.2 |
| 6. Song count 1 day after playback [20/5] | ||||
| Intercept | 3.023 | 0.279 | 10.83 | <0.001 |
| Song environment | 0.265 | 0.085 | 3.11 | 0.002 |
| 7. Non-singing activity [60/20/5] | ||||
| Intercept | 27.343 | 19.907 | 1.37 | 0.17 |
| Song environment | 63.486 | 23.878 | 2.66 | 0.008 |
| Day | 2.207 | 1.992 | 1.22 | >0.2 |
| Song environment × day | –3.369 | 2.818 | –1.20 | >0.2 |
| 8. Body mass (g) [20/5] | ||||
| Intercept | 89.840 | 2.216 | 40.55 | <0.001 |
| Song environment | –7.640 | 2.394 | –3.19 | 0.001 |
| 9. Body mass (g) [20/5] | ||||
| Intercept | 88.557 | 3.255 | 27.20 | <0.001 |
| Song environment | –8.681 | 2.834 | –3.06 | 0.002 |
| Song count | 0.005 | 0.021 | 0.25 | >0.2 |
| Non-singing activity | 0.002 | 0.004 | 0.65 | >0.2 |
| 10. HVC volume (mm3) [12/3] | ||||
| Intercept | 4.555 | 0.630 | 7.23 | <0.001 |
| Song environment | –0.303 | 0.415 | –0.73 | >0.2 |
| 11. IMAN volume (mm3) [12] | ||||
| Intercept | 0.270 | 0.029 | 9.19 | <0.001 |
| Song environment | –0.001 | 0.041 | –0.02 | >0.2 |
| 12. Area × volume (mm3) [12] | ||||
| Intercept | 35.784 | 2.653 | 13.49 | <0.001 |
| Song environment | –0.795 | 3.752 | –0.21 | >0.2 |
| 13. RA volume (mm3) [12/3] | ||||
| Intercept | 1.519 | 0.152 | 9.96 | <0.001 |
| Song environment | 0.468 | 0.154 | 3.04 | 0.002 |
| 14. RA volume (mm3) [12] | ||||
| Intercept | 1.321 | 0.145 | 9.09 | <0.001 |
| Song environment | 0.447 | 0.157 | 2.85 | 0.004 |
| Song count | 0.002 | 0.001 | 2.10 | 0.035 |
| 15. RA cell profiles per mm2 [12] | ||||
| Intercept | 79.750 | 2.431 | 32.80 | <0.001 |
| Song environment | 0.528 | 3.438 | 0.15 | >0.2 |
| 16. RA cell area (μm2) [12] | ||||
| Intercept | 8361.556 | 389.953 | 21.44 | <0.001 |
| Song environment | 8.111 | 551.477 | 0.01 | >0.2 |
The hierarchical nesting structure of each model is indicated in brackets. Models with no nested random effect have one number corresponding to the number of individuals. Two numbers indicate the number of individuals, followed by the number of sessions in which the individuals were nested as a random intercept. Three numbers indicate the number of observations, followed by the number of individuals in which observations were nested as a random intercept, followed by the number of sessions in which individuals were nested as a random intercept. Song environment was coded 0 for short song and 1 for long song. Day 4:day 7 is the contrast between day 4 (coded 1) and day 7 (coded 0) of playback. Day 7:day 8 is the contrast between day 7 (coded 0) of playback and 1 day after playback (coded 1). Non-singing activity is the count of non-singing audio files. HVC is a proper name. IMAN is the lateral portion of the magnocellular nucleus of the anterior nidopallium. RA is the robust nucleus of the arcopallium. P-values that are less than 0.05 and which correspond to effects of interest (i.e. not intercepts) are in bold type. *Analyzed with only the subset of individuals that sang on each of the 3 days of observation
RESULTS
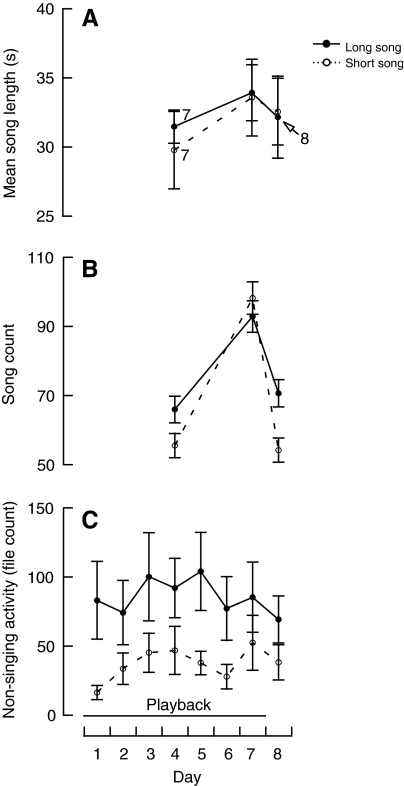
On day 4 of playback, seven of the 10 males in each song environment sang, and on day 7 of playback all males sang. One day after playback, all males in the short-song environment and eight in the long-song environment sang. Our first goal was to determine whether song environment affected the quality of a male's own song (i.e. mean daily song length; Table 1, analysis 1). We found that although song length increased within both song environments from day 4 to day 7 of playback (P=0.002), we observed no change from day 7 of playback to 1 day after playback (P>0.2) and no differences between song environments in either song length (P>0.2) or the change in song length with day (interactions between song environment and each day contrast: P>0.2; Fig. 1A). It is possible that the change from day 4 to day 7 was not due to the individuals' changing their song length but rather to the recruitment of three males on day 7 that were not singing on day 4. When we reanalyzed these data, this time using only those males that sang on each of the three days (Table 1, analysis 2), the results were qualitatively unchanged (change from day 4 to day 7 of playback: P<0.001; all other predictors: P>0.2), indicating that the change from day 4 to day 7 was due to a within-individual change in song length.
Fig. 1.
The effects of song environment on (A) mean song length, (B) song count and (C) non-singing activity (means±s.e.m.) of male European starlings. Male starlings (N=10 unless otherwise indicated on figure) were exposed to 7 days of either long songs or short songs, followed by a day with no song exposure.
Next, we assessed whether song environment affected singing effort (i.e. daily song count; Table 1, analysis 3). We found that, regardless of song environment, song count increased from day 4 to day 7 (P<0.001) and then decreased 1 day after playback (P<0.001; Fig. 1B). However, the degree to which song count changed over each of these intervals depended on song environment, as revealed by the interaction between song environment and the change from day 4 to day 7 of playback (P=0.037) and the interaction between song environment and the change from day 7 of playback to 1 day after playback (P=0.003). Specifically, the increase from day 4 to day 7 was less for the individuals in the long-song environment than for those in the short-song environment. This is because the individuals in the long-song environment were singing more on day 4 than those in the short-song environment (P=0.045), as indicated by a post-hoc analysis of day 4 only (Table 1, analysis 4). The same post-hoc analysis, but instead for day 7 only (Table 1, analysis 5), did not reveal a reliable difference between song environments (P>0.2). Additionally, the decrease from day 7 of playback to 1 day after playback was less for the individuals in the long-song environment than for those in the short-song environment because the former were singing more 1 day after playback than the latter were (P=0.002). This was supported by the same post-hoc analysis just described, but for 1 day after playback only (Table 1, analysis 6).
We observed considerable non-singing activity, which we then analyzed in relation to song environment (Table 1, analysis 7). Although we observed no general tendency for non-singing activity to change over the course of the observation period in either song environment (day: P>0.2; Fig. 1C), activity as a whole was greater in the long-song than in the short-song environment (P=0.008).
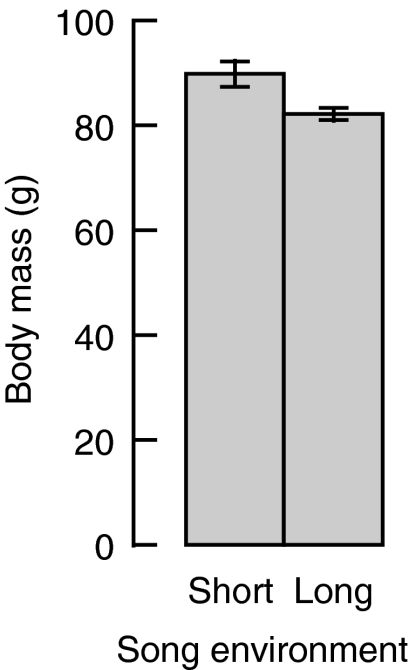
Given the difference in singing effort and non-singing activity between song environments, we wondered whether song environment also affected body mass at the end of the experiment (Table 1, analysis 8). Individuals in the short-song environment were approximately 10% heavier at the end of the experiment than were individuals in the long-song environment (P=0.001; Fig. 2). Moreover, the effect of song environment remained reliable (P=0.002), even when we added to the model total song count (P>0.2) together with total non-singing activity (P>0.2) as predictors (Table 1, analysis 9), indicating that these singing and non-singing activity differences cannot completely account for the effects of song environment on body mass.
Fig. 2.
The effect of song environment (see Fig. 1) on body mass (means±s.e.m.) of male European starlings. N=10 males in each group.
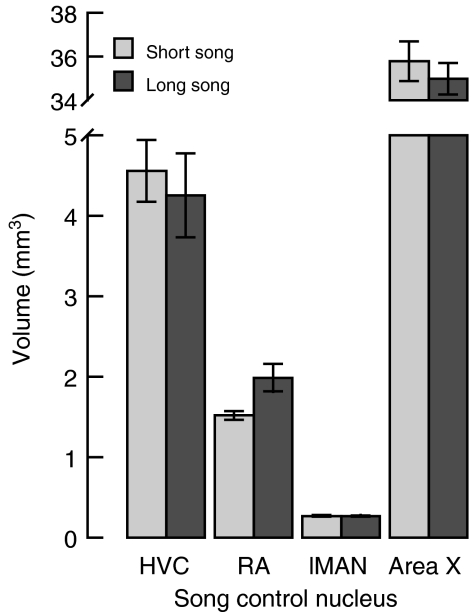
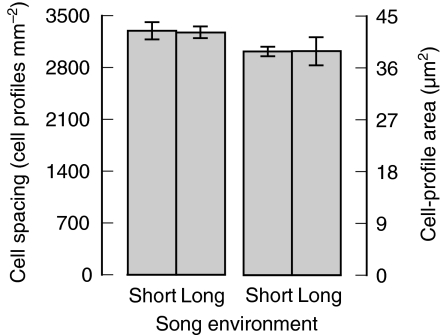
Finally, we examined the effects of song environment on the volumes of the four principal song-control nuclei of the songbird forebrain (Table 1, analyses 10–13). We observed no effect of song environment on the volumes of the HVC, lMAN or Area X (each nucleus: P>0.2; Fig. 3). However, the RA of individuals in the long-song environment was about 30% larger than it was for individuals in the short-song environment (P=0.002). The effects of song environment on singing effort described above cannot completely account for the effect of song environment on RA volume because when total song count is added to the model (Table 1, analysis 14) the effect of song environment remains reliable (P=0.004), despite there being a positive correlation between song count and RA volume (P=0.035). We did not detect a reliable difference between song environments either in cell spacing (P>0.2) or in the area of cell profiles (P>0.2) in the RA (Table 1, analyses 15 and 16; Fig. 4).
Fig. 3.
The effect of song environment (see Fig. 1) on the volume (means±s.e.m.) of forebrain vocal-control nuclei HVC (used as a proper name), robust nucleus of the arcopallium (RA), lateral portion of the magnocellular nucleus of the nidopallium (lMAN) and Area X. N=6 for each song environment.
Fig. 4.
The effects of song environment (see Fig. 1) on cell spacing (left) and on the area of individual cell profiles (right) (means±s.e.m.) in photomicrographs of the RA of male European starlings. N=6 males in each group.
DISCUSSION
We found that variation in the acoustic courtship signals of male European starlings affected the brain, behavior and body mass of male receivers. Compared with those exposed to short-bout songs, male starlings chronically exposed to periodic playback of long-bout songs sang more, engaged in more non-singing activity, weighed less and had an approximately 30% larger vocal-control nucleus RA. Although the adaptive significance of these responses is unclear, one possibility is that eavesdropping males adjust their behavior and its underlying neural substrates according to information about other males' competitive abilities, reflected in their sexual advertisement signals (Mountjoy and Lemon, 1991).
Over the period from day 4 to day 7 of song playback, male receivers increased song length by about 10% (Fig. 1A). However, males responded to variation in the length of songs to which they were exposed not by modulating their own song length but instead by modulating their singing effort. By 4 days into playback, males exposed to longer songs sang approximately 25% more songs per day than males exposed to shorter songs (Fig. 1B). This difference dissipated by the end of the 7 day playback period, possibly because of habituation to repetition of the same set of songs. Interestingly, cessation of playback rescued this response differential, suggesting the persistence of a memory trace of the song environment. How the restoration occurred is not clear, but slight changes in the context of a song cue – for example, the location from which it occurs – can restore otherwise habituated neural responses in the auditory forebrain of songbirds (Kruse et al., 2004). Evidently the new context of no song, at the time when individuals should be accustomed to the presence of song, was sufficient to restore behavioral sensitivity to the song memory.
Strong territorial or courtship responses to the loss of social stimuli are not uncommon in vertebrates (e.g. Sockman et al., 2005b) and, in at least some groups, may be under the control of immediate, early genes that are sensitive to a changing social context and which modulate synaptic properties of hypothalamic brain regions [e.g. teleost fish (Burmeister et al., 2005)]. In birds, one such region is the medial preoptic nucleus, which activates appetitive aspects of male sexual behavior, such as courtship (Balthazart and Ball, 2007), and, in male starlings, may regulate singing motivation and effort (Riters and Ball, 1999). In a breeding context, singing effort in starlings is correlated with the expression of the above-mentioned immediate, early genes in the medial preoptic nucleus (Heimovics and Riters, 2005), as well as in other areas of the basal forebrain and midbrain implicated in the regulation of social behavior (Goodson et al., 2005), including the medial bed nucleus of the stria terminalis, the anterior hypothalamus, the ventromedial nucleus of the hypothalamus, and the lateral septum (Heimovics and Riters, 2006; Heimovics and Riters, 2007). Clearly, future studies may benefit from a thorough examination of how the song environment, which affects singing effort, also affects these brain areas implicated in the neural control of singing effort.
Given the effects of song environment on singing and non-singing activity (Fig. 1B,C), it may not be surprising that males exposed to short songs were about 10% heavier by the end of the study than males exposed to long songs (Fig. 2). At first pass, one might think that this mass difference was due to an indirect effect of the acoustic environment on metabolic activity. For example, male túngara frogs (Physalemous pustulosus) consume more oxygen when exposed to a conspecific chorus than do males exposed to no chorus (Bucher et al., 1982), most likely because chorus exposure elevates locomotor (M. J. Ryan, personal communication) and presumably metabolic activity. However, our statistical analyses ruled out the possibility that singing and non-singing activity, as we measured them, could entirely explain the effect of song environment on body mass. It is possible that other forms of activity, such as those below our amplitude threshold (see `Experimental procedure'), caused the mass difference, but it would seem that the activities we did quantify would correlate with and therefore account for most other forms. Thus, as an alternative suggestion, perhaps song environment influences attention levels, which, in turn, influence the time an individual spends eating and therefore its body mass. An elevation in attention with competitiveness of the environment would seem adaptive, if attention levels enable individuals to better adjust to the environment and prepare for having to compete at a higher level; for example, by sustaining elevated singing effort. Thus, it seems unlikely that the decline in body mass under exposure to a more competitive song environment is itself adaptive. Rather, the decline in body mass may be a byproduct of an adaptive elevation in attention. We have no data in direct support of this idea, which, at the moment, remains merely a hypothesis. Nonetheless, the hypothesis, together with activity-independent effects of song environment on body mass (Fig. 2) and on RA volume (Fig. 3), does raise the question of how an animal might adjust to an unexpected elevation in competition. Below, we first discuss the role of the nucleus RA and effects of song environment on the size of this nucleus, before proposing a possible neural adjustment to an elevation in perceived competition.
The forebrain vocal-control nucleus RA is thought to translate pre-motor signals from the HVC and the anterior forebrain pathway into the appropriate combination of respiratory movements and movements of individual syringeal muscles that result in song (Vicario, 1991). The change in RA volume we observed (Fig. 3) is particularly intriguing not merely because volume changes in any brain area may have implications on the physiology, behavior and fitness of the individual and not merely because the volume of one area is likely to influence the volume of other areas within the rigid spatial confines of the adult vertebrate cranium. What also makes this response interesting is that the difference between the stimuli to which these birds were exposed seems to us quite subtle, even though the responses were not. That is, for a duration of only 1 week, we exposed these individuals to song sets that were largely identical, in that they contained identical amounts of song and silence and were recorded from the same single male. The song sets differed only in whether the songs within them were organized into more repetitions of short songs or fewer repetitions of long songs. That treatment difference, applied over 7 days followed by a 1 day no-treatment period, resulted in an approximately 30% difference in RA volume.
A change in the volume of a brain nucleus could arise because of a change in cell recruitment (i.e. the number of cells), cell spacing, cell size or any combination of these. Our failure to find a difference either in cell spacing or in the area of cell profiles (Fig. 4) suggests that the song environment affects RA volume specifically by affecting cellular recruitment. We do not know whether such recruitment would be of neurons or glia or whether recruitment differences would be due to differences in cell death or cell genesis. Further studies using cell-type markers and markers of cell division and apoptosis would be helpful in this regard.
Additionally, we do not know what might mediate this environmentally induced change in RA volume, but results from this and other studies rebut some possibilities, while supporting others. For instance, even though testosterone levels affect the volume of several vocal control nuclei, including the RA (Bernard et al., 1999; Brenowitz and Lent, 2002), it seems unlikely that the particular effect of song environment on RA volume was mediated by changing testosterone levels, because testosterone levels also affect the volume of the HVC, which did not appear to differ between song environments (Fig. 3). Of course, this is not conclusive evidence that variation in the circulating concentrations of testosterone or some other steroid, such as corticosterone, do not account for the variation in RA volume we observed. Moreover, variation in the concentrations of plasma steroids may have driven the body mass differences described above. Future studies would benefit from examining the effects of song environment on such steroids. Still, given the lack of effect of song environment on other brain nuclei, a role for elevated plasma steroids seems unlikely for this particular effect. We observed no difference between groups in the size of the testes (K.G.S., D.M.R., C.R.C. and K.W.S., unpublished), suggesting that there were no large differences in reproductive development, though more subtle group differences in reproductive development might have occurred.
In male starlings (Sartor and Ball, 2005) and male canaries (Alvarez-Borda and Nottebohm, 2002), the mere production of song influences the volume of the HVC (RA volume was not reported) in a manner that is independent of testosterone levels [but see Brenowitz et al. for results on white-crowned sparrows, Zonotrichia leucophrys (Brenowitz et al., 2007)]. Consistent with this, we found a positive correlation between song count and RA volume (see Results). So, one possibility is that a high-quality song environment elevates RA volume by elevating singing effort and motor-driven cell genesis in the RA. However, addition of song count to the statistical model did not entirely explain the effect of song environment on RA volume, and song environment had no discernible effect on evoked song length and presumably song complexity and motif stereotypy, because both of these measures correlate with song length (Gentner and Hulse, 2000). Thus, our findings cannot be explained entirely by motor-driven cell genesis. However, consistent with studies in zebra finches (Taeniopygia guttata) demonstrating strictly auditory responses in this motor nucleus (Dave et al., 1998; Shank and Margoliash, 2009), our findings raise, for the first time, the possibility of acoustically driven plasticity in the RA.
If acoustically driven plasticity in the RA did, in fact, occur, what might be the mechanism? Results from other studies implicate possible roles of the auditory telencephalon and the HVC. In particular, the quality of the song environment influences the responsiveness of the auditory telencephalon (caudomedial mesopallium and caudomedial nidopallium) in female European starlings to the length of novel songs (Sockman et al., 2002; Sockman et al., 2005a). This responsiveness was measured as expression of the immediate, early gene ZENK (egr-1) and may be influenced by song environment through the secretion of norepinephrine (noradrenaline) or other monoamines in at least one of these areas of the auditory telencephalon (Sockman, 2007; Sockman and Salvante, 2008). Recently, it was demonstrated that activity in the caudal mesopallium is necessary for auditory-evoked activity in the HVC and that the caudal mesopallium makes direct projections to the HVC (Bauer et al., 2008), which itself projects directly and indirectly (via the anterior forebrain pathway) to the RA (Nottebohm et al., 1976). Consequently, it is conceivable that the effect of song environment on RA volume is mediated by acoustic input via the caudal mesopallium and HVC.
Given the RA's important role in coordinating respiratory and syringeal activity for song production (Vicario, 1991), one possibility for the functional significance of this type of acoustically driven plasticity in the RA is that new cells are being incorporated in direct response to the perception of elevated song competition. In the zebra finch, the RA is myotopically organized, with specific regions controlling subsets of syringeal muscles important for vocal output (Vicario, 1991). We hypothesize that hypertrophy or hyperplasia in the syrinx is necessary for sustaining elevated song output under elevated competition, and that the control of the re-organized syringeal musculature requires new cells in the motor nucleus RA, which primarily controls the activity of these muscles. We emphasize that this is a hypothesis at this point, and we further emphasize the preliminary nature of our support that the plasticity in the RA that we demonstrated is acoustically driven.
Regardless of the relationship between song environment and RA volume or any of our other response variables, the interpretation of our results merits some caution, in that the way in which our treatment group was constructed limits the scope of our conclusions. Specifically, because both levels of our treatment were constructed from the recordings of a single male, we cannot extend our conclusions to the effects of song length in general (Kroodsma et al., 2001; Wiley, 2003). A previous study showed that females in a mate-choice context prefer this exact long-song set to this exact short-song set (Gentner and Hulse, 2000), and consequently our results are based on responses to stimuli that are known to differ in their attractiveness to females and, presumably, to differ in the content of information about the competitiveness of the signaler. Nonetheless, future studies would likely benefit from examining the effects of the songs of multiple males.
We found that variation in the song environment influenced the singing effort, non-singing activity, body mass and volume of a vocal control nucleus in male receivers. Although we do not know their mediating mechanisms, these responses, we hypothesize, reflect adaptive adjustments to the competitive environment by eavesdropping males. Future studies might examine how such responses affect the fitness of the individual and how social competition in general influences the brain and behavior.
We thank T. Gentner for the song recordings used in the playbacks, A. Troyer and family for capturing the birds used in this study, and K. Simmons, K. Suppler, B. Stout, S. Vora, S. Cavadel and A. Byerly for their help with bird care. This study was supported by NIH R01 NS055125 to K.W.S. Deposited in PMC for release after 12 months.
References
- Adret-Hausberger, M. and Jenkins, P. F. (1988). Complex organization of the warbling song in the European starling Sturnus vulgaris. Behaviour 107, 138-156. [Google Scholar]
- Alvarez-Borda, B. and Nottebohm, F. (2002). Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J. Neurosci. 22, 8684-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, M. (1994). Sexual Selection. Princeton, NJ: Princeton University Press.
- Ball, G. F., Sockman, K. W., Duffy, D. L. and Gentner, T. Q. (2006). A neuroethological approach to song behavior and perception in European starlings: interrelationships among testosterone, neuroanatomy, immediate early gene expression, and immune function. Adv. Study Behav. 36, 59-121. [Google Scholar]
- Balthazart, J. and Ball, G. F. (2007). Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocrinol. 28, 161-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart, J., Boseret, G., Konle, A. T. M., Hurley, L. L. and Ball, G. F. (2008). Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur. J. Neurosci. 27, 801-817. [DOI] [PubMed] [Google Scholar]
- Bauer, E. E., Coleman, M. J., Roberts, T. F., Roy, A., Prather, J. F. and Mooney, R. (2008). A synaptic basis for auditory-vocal integration in the songbird. J. Neurosci. 28, 1509-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, D. J., Bentley, G. E., Balthazart, J., Turek, F. W. and Ball, G. F. (1999). Androgen receptor, estrogen receptor α, and estrogen receptor β show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology 140, 4633-4643. [DOI] [PubMed] [Google Scholar]
- Brainard, M. S. (2004). Contributions of the anterior forebrain pathway to vocal plasticity. Ann. NY Acad. Sci. 1016, 377-394. [DOI] [PubMed] [Google Scholar]
- Brenowitz, E. A. and Lent, K. (2002). Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc. Natl. Acad. Sci. USA 99, 12421-12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz, E. A., Lent, K. and Rubel, E. W. (2007). Auditory feedback and song production do not regulate seasonal growth of song control circuits in adult White-crowned sparrows. J. Neurosci. 27, 6810-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, T. L., Ryan, M. J. and Bartholomew, G. A. (1982). Oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol. Zool. 55, 10-22. [Google Scholar]
- Burmeister, S. S., Jarvis, E. D. and Fernald, R. D. (2005). Rapid behavioral and genomic responses to social opportunity. PLOS Biol. 3, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole, C. K. and Slater, P. J. B. (1995). Bird Song. Cambridge: Cambridge University Press.
- Dave, A. S., Yu, A. C. and Margoliash, D. (1998). Behavioral state modulation of auditory activity in a vocal motor system. Science 282, 2250-2254. [DOI] [PubMed] [Google Scholar]
- Duffy, D. L. and Ball, G. F. (2002). Song predicts immunocompetence in male European starlings (Sturnus vulgaris). Proc. Biol. Sci. 269, 847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens, M., Pinxten, R. and Verheyen, R. F. (1989). Temporal and sequential organization of song bouts in the starling. Ardea 77, 75-85. [Google Scholar]
- Feare, C. J. (1984). The Starling. Oxford: Oxford University Press.
- Gentner, T. Q. and Hulse, S. H. (1998). Perceptual mechanisms for individual vocal recognition in European starlings, Sturnus vulgaris. Anim. Behav. 56, 579-594. [DOI] [PubMed] [Google Scholar]
- Gentner, T. Q. and Hulse, S. H. (2000). Female European starling preference and choice for variation in conspecific male song. Anim. Behav. 59, 443-458. [DOI] [PubMed] [Google Scholar]
- Gentner, T. Q., Hulse, S. H., Duffy, D. and Ball, G. F. (2001). Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J. Neurobiol. 46, 48-58. [DOI] [PubMed] [Google Scholar]
- Goodson, J. L., Evans, A. K., Lindberg, L. and Allen, C. D. (2005). Neuro-evolutionary patterning of sociality. Proc. Biol. Sci. 272, 227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics, S. A. and Riters, L. V. (2005). Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 65, 207-224. [DOI] [PubMed] [Google Scholar]
- Heimovics, S. A. and Riters, L. V. (2006). Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris). Horm. Behav. 50, 726-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics, S. A. and Riters, L. V. (2007). ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris). Behav. Brain Res. 176, 333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel, B. (1951). Criteria for sexing and aging European starlings (Sturnus vulgaris). Bird Banding 22, 16-23. [Google Scholar]
- Kroodsma, D. E., Byers, B. E., Goodale, E., Johnson, S. and Liu, W. C. (2001). Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61, 1029-1033. [Google Scholar]
- Kruse, A. A., Stripling, R. and Clayton, D. F. (2004). Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol. Learn. Mem. 82, 99-108. [DOI] [PubMed] [Google Scholar]
- Marler, P. (1997). Three models of song learning: evidence from behavior. J. Neurobiol. 33, 501-516. [PubMed] [Google Scholar]
- McGregor, P. K. and Peake, T. M. (2000). Communication networks: social environments for receiving and signalling behaviour. Acta Ethol. 2, 71-81. [Google Scholar]
- Meitzen, J., Perkel, D. J. and Brenowitz, E. A. (2007). Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. J. Comp. Physiol. A 193, 677-683. [DOI] [PubMed] [Google Scholar]
- Mountjoy, D. J. and Lemon, R. E. (1991). Song as an attractant for male and female European starlings, and the influence of song complexity on their response. Behav. Ecol. Sociobiol. 28, 97-100. [Google Scholar]
- Mountjoy, D. J. and Lemon, R. E. (1996). Female choice for complex song in the European starling: a field experiment. Behav. Ecol. Sociobiol. 38, 65-71. [Google Scholar]
- Naguib, M. and Dietmar, T. (1997). Effects of dyadic vocal interactions on other conspecific receivers in nightingales. Anim. Behav. 54, 1535-1543. [DOI] [PubMed] [Google Scholar]
- Nicholls, T. J., Goldsmith, A. R. and Dawson, A. (1988). Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 68, 133-176. [DOI] [PubMed] [Google Scholar]
- Nottebohm, F., Stokes, T. M. and Leonard, C. M. (1976). Central control of song in the canary, Serinus canaria. J. Comp. Neurol. 165, 457-486. [DOI] [PubMed] [Google Scholar]
- Ophir, A. G. and Galef, B. G., Jr (2003). Female Japanese quail that `eavesdrop' on fighting males prefer losers to winners. Anim. Behav. 66, 399-407. [Google Scholar]
- Rabe-Hesketh, S. and Skrondal, A. (2005). Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press.
- Riters, L. V. and Ball, G. F. (1999). Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris). Horm. Behav. 36, 276-286. [DOI] [PubMed] [Google Scholar]
- Sartor, J. J. and Ball, G. F. (2005). Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris). Behav. Neurosci. 119, 233-244. [DOI] [PubMed] [Google Scholar]
- Shank, S. S. and Margoliash, D. (2009). Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 458, 73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. T., Brenowitz, E. A. and Wingfield, J. C. (1997). Seasonal changes in the size of the avian song control nucleus HVC defined by multiple histological markers. J. Comp. Neurol. 381, 253-261. [DOI] [PubMed] [Google Scholar]
- Sockman, K. W. (2007). Neural orchestration of mate-choice plasticity in songbirds. J. Ornithol. 148, S225-S230. [Google Scholar]
- Sockman, K. W. and Salvante, K. G. (2008). The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev. Neurobiol. 68, 656-668. [DOI] [PubMed] [Google Scholar]
- Sockman, K. W., Gentner, T. Q. and Ball, G. F. (2002). Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc. Biol. Sci. 269, 2479-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman, K. W., Gentner, T. Q. and Ball, G. F. (2005a). Complementary neural systems for the experience-dependent integration of mate-choice cues in the European starling. J. Neurobiol. 62, 72-81. [DOI] [PubMed] [Google Scholar]
- Sockman, K. W., Sewall, K. B., Ball, G. F. and Hahn, T. P. (2005b). Economy of mate attraction in the Cassin's finch. Biol. Lett. 1, 34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman, K. W., Weiss, J., Webster, M. S., Talbott, V. and Schwabl, H. (2008). Sex-specific effects of yolk-androgens on growth of nestling American kestrels. Behav. Ecol. Sociobiol. 62, 617-625. [Google Scholar]
- Tramontin, A. D., Smith, G. T., Breuner, C. W. and Brenowitz, E. A. (1998). Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J. Comp. Neurol. 396, 186-192. [DOI] [PubMed] [Google Scholar]
- Tramontin, A. D., Wingfield, J. C. and Brenowitz, E. A. (1999). Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J. Neurosci. 19, 476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario, D. S. (1991). Organization of the zebra finch song control system: II. Functional organization of outputs from nucleus Robustus Archistriatalis. J. Comp. Neurol. 309, 486-494. [DOI] [PubMed] [Google Scholar]
- Wiley, R. H. (2003). Is there an ideal behavioural experiment? Anim. Behav. 66, 585-588. [Google Scholar]
- Zahavi, A. and Zahavi, A. (1997). The Handicap Principle: A Missing Piece of Darwin's Puzzle. New York: Oxford University Press.