Abstract
Background and Aims
Geographical variation in foliar and floral traits and their degree of coupling can provide relevant information on the relative importance of abiotic, biotic and even neutral factors acting at geographical scales as generators of evolutionary novelty. Geographical variation was studied in leaves and flowers of Embothrium coccineum, a species that grows along abrupt environmental gradients and exhibits contrasting pollinator assemblages in the southern Andes.
Methods
Five foliar and eight floral morphological characters were considered from 32 populations, and their patterns of variation and covariation were analysed within and among populations, together with their relationship with environmental variables, using both univariate and multivariate methods. The relationships between foliar and floral morphological variation and geographical distance between populations were compared with Mantel permutation tests.
Key Results
Leaf and flower traits were clearly uncoupled within populations and weakly associated among populations. Whereas geographical variation in foliar traits was mostly related to differences in precipitation associated with geographical longitude, variation in floral traits was not.
Conclusions
These patterns suggest that leaves and flowers responded to different evolutionary forces, environmental (i.e. rainfall) in the case of leaves, and biotic (i.e. pollinators) or genetic drift in the case of flowers. This study supports the view that character divergence at a geographical scale can be moulded by different factors acting in an independent fashion.
Key words: Embothrium coccineum, Proteaceae, geographical variation, foliar morphology, floral morphology, uncoupling, selective forces, environmental conditions, pollinators, south Andes
INTRODUCTION
Patterns of geographical variation in foliar and floral traits and their potential relationship with different selection agents can provide clues on adaptation and the evolutionary forces that result in genetic divergence and speciation (Endler, 1977; Grant, 1991; Herrera, 1996; Judd et al., 1999; Herrera et al., 2006). Therefore, the study of variation within widespread species at large spatial scales offers unique opportunities to observe evolution in progress, and is the first step to identify and determine the relative importance of different factors that promote phenotypic differentiation (e.g. Boyd, 2002; Herrera et al., 2002; Mascó et al., 2004; Herrera, 2005). There are two main evolutionary forces that promote geographical variation and morphological differentiation at intraspecific levels: (1) natural selection exerted by geographically patterned abiotic factors and/or biotic factors, and (2) random events such as genetic drift. On the contrary, gene flow has the opposite effect, linking populations through interbreeding and preventing differentiation and speciation in the long term (Slatkin, 1983; Grant, 1991).
Natural selection can act at different levels, or in different ways, resulting in independent trait evolution when different parts of a plant respond to distinctive selective mechanisms, or a given force affects differentially different organs or even different traits of the same organ. For example, flower size can be under disruptive selection exerted by different pollinators, independently of environmental conditions, whereas leaf size can be under selection pressure of environmental factors, such as increasing aridity. On the other hand, a given selective force affecting either flowers or leaves can result in correlated trait evolution because of either pleiotropism or gene linkage (e.g. Slatkin, 1983; Grant, 1991). After all, floral organs are leaves transformed into reproductive structures during the course of evolution, and if foliar and floral traits were tightly correlated genetically, then we might expect a high degree of phenotypic correlation between these two types of structures (coupling). On the contrary, if these two structures were genetically unlinked, we would expect them to respond independently to different evolutionary forces (uncoupling). For example, it has been hypothesized that floral morphology of plants specialized on one or few pollinators should be largely uncoupled from variation exhibited by vegetative traits such as leaf size, whereas at the same time selection through pollination should favour the integration of different floral traits (Berg, 1959, 1960; Armbruster et al., 1999; Herrera et al., 2002). Therefore, the assessment of the degree of coupling and/or uncoupling among foliar and floral traits in a plant species that grows along steep environmental gradients and also exhibits contrasting pollinator assemblages over its range, can provide relevant insights into the origins of morphological differentiation and into incipient speciation processes at a geographical scale (Thompson, 1994, 2005).
Although differential association of leaves and flowers with different factors that vary at a geographical scale can provide evidence of uncorrelated character evolution, evidence of morphological uncoupling should be better searched at the within-population level (Armbruster, 1991; and references therein). Particularly, an unexpectedly low correlation of foliar and floral traits among individuals growing under a common environment can be indicative of a lack of pleiotropism and more generally of a poor genetic correlation (Armbruster, 1991). Even more, two characters that appear unrelated within populations can be correlated among populations if they evolve independently in the same direction in response to a common selection factor. Therefore, the study of patterns of morphological covariation within and among levels can provide complementary evidence on the processes operating on character divergence at a geographical scale (Armbruster, 1991; Armbruster and Schwaegerle, 1996).
The orographic barrier of the Andes in southern South America stops the westerly, moisture-laden winds from the Pacific, creating a strong rainfall gradient that determines different transitional forest types. These include temperate rainforest at average lower altitudes on the western (Chilean) slope of the Andes, to xeric temperate forest at somewhat average higher altitudes on the eastern (Argentinean) slope. Embothrium coccineum is an endemic forest species from southern South America that grows all along this precipitation gradient, from sites with >4000 mm to sites with <700 mm. This species also has a broad latitudinal and altitudinal distribution in the southern Andes, from approx. 35°S to 55°S in Tierra del Fuego (Moore, 1983) and from sea level to 1200 m (Romero et al., 1987). Thus, E. coccineum is exposed to a high variety of environmental conditions. In addition, contrasting pollinator assemblages, which may exert divergent selection pressures on floral morphological characters, have been reported for populations from the western (Chilean) and eastern (Argentinean) side of the Andes (Smith-Ramírez and Armesto, 1998) and also among Argentinean populations (Devoto et al., 2006).
In this paper, the geographical pattern of morphological variation is analysed as well as the degree of coupling or uncoupling between foliar and floral traits within and among populations of E. coccineum from both sides of the austral Andes. Here the following questions are addressed: (a) What is the extent of morphological variation within and among populations of E. coccineum? (b) What is the degree of coupling/uncoupling between foliar and floral traits? (c) Which are the most probable evolutionary forces (i.e. genetic drift, or natural selection by environmental and/or biotic factors) explaining the pattern of variation in morphological traits at a geographical level? By answering these questions we seek to approach a more fundamental issue: to determine the relative importance of abiotic vs. biotic, or even neutral factors, acting at large spatial scales as generators of evolutionary novelty.
MATERIALS AND METHODS
Study species and sites
Embothrium coccineum J. R. Forster and G. Forst is a proteaceous shrub or small tree, which occupies numerous habitats within the temperate forest of southern Argentina and Chile (Sleumer, 1984). This biome extends as a narrow strip, 100–200 km wide, from 35°S to 55°S and from the Pacific Ocean to the eastern slopes of the Patagonian Andes, and is generally dominated by evergreen or deciduous Nothofagus species (Cabrera and Willink, 1980). Because of its bright red flowers, E. coccineum is known as ‘Chilean fire-bush’ and cultivated worldwide as an ornamental in temperate areas with mild climate (Wiersema and León, 1999). Flower colour and shape vary considerably in wild populations, and different varieties have been described from the shape and size of the leaves (Sleumer, 1984; Gardner, 1997). This shrub is ecologically important as it behaves as an early colonizer and regenerates vigorously in cleared areas, and its populations can increase under fragmentation (Mathiasen et al., 2007). It has a predominantly outcrossing mating system (Rovere et al., 2006), and flowers from October to January in the northern portion of its range (Brion et al., 1988; Smith-Ramírez and Armesto, 1998). Its seeds are winged and wind-dispersed, although they mostly fall within a few metres of the maternal tree (Rovere and Premoli, 2005).
The protandrous flowers of Embothrium coccineum are red, tubular, have four tepals fused with the anthers, and secrete abundant nectar of variable sugar composition among populations (Smith-Ramírez and Armesto, 1998; Chalcoff et al., 2006). The nectar is secreted by a gland that is found at the base of a stipe supporting the ovary. As in other members of the Proteaceae, the anthers dehisce previous to flower anthesis and the pollen is loaded on to a specialized part of the stigma called the ‘pollen presenter’ from where the pollen is transferred to a pollinator's body (i.e. there is a secondary pollen presentation) (Ladd, 1994). After the pollen is deposited on the presenter, the tepals open and curl downwards, leaving the style and stigma free. The stigma matures after the pollen is removed, and this dichogamous mechanism prevents autogamy.
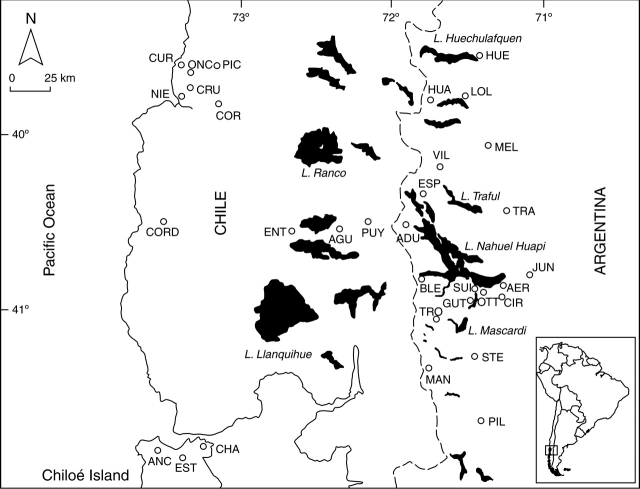
From October to January, a total of 32 populations of E. coccineum were surveyed over three consecutive flowering seasons (2003–2004, 2004–2005 and 2005–2006) in the northern part of the temperate forest of southern South America on both sides of the Andes (Appendix 1 and Fig. 1). Thirteen populations were located on the western side of the Andes (i.e. Chile), and 19 populations on the eastern side of the Andes (i.e. Argentina). Despite that populations were surveyed on a relatively reduced geographical area (approx. 60 000 km2), in this region, E. coccineum occurred in a highly heterogeneous landscape with elevations from sea level to 1100 m, and westerly winds that produce annual precipitations from >3000 mm on the west to 700 mm on the east of the Andes (Appendix 1).
Fig. 1.
Location of the 32 sampled populations of Embothrium coccineum (open circles) in the temperate forest of South America. Full location names are provided in Appendix 1.
Sampling procedures and traits measured
Approximately ten leaves and five flowers were collected at random from four or five mature individuals in each of the 32 populations (i.e. a total of 156 individuals). The number of plants per population and flowers or leaves per individual varied due to the different availability of reproductive individuals in the populations studied (Appendix 1). Only fully mature leaves, characterized by a tough texture and a dark green colour, and flowers in the early male stage, that roughly represents the midpoint in flower lifespan (approx. 4 d), were collected.
Thirteen morphological characters, five foliar and eight floral, were considered in the study (abbreviations in parentheses). The foliar characters measured were total leaf length including petiole (TLL), leaf lamina length (LLL), leaf width (LW), petiole length (PtL) and leaf lamina length/leaf width ratio (L/W) as an estimator of leaf shape, whereas the floral characters measured were corolla length (CL), corolla width (CW), pistil length (PL), stipe plus pistil total length (PPL), nectary length (NL), nectary width (NW), stigma length (SL), and stigma width (SW) (Fig. 2). The stipe plus pistil total length was considered an important trait because it represents the real distance between the source of nectar and the site of pollen presentation. These foliar and floral characters are among those usually measured to characterize leaf and flower size and overall shape, and have been proposed as likely selection targets of different abiotic and biotic factors (e.g. Ezcurra et al., 1997; Boyd, 2002; Herrera, 2005). Variation in these morphological traits also has functional consequences. For instance, leaf size and shape (i.e. L/W) determine at least in part evapotranspiration rates (e.g. Parkhurst and Loucks, 1972), and therefore are traits likely to be influenced by precipitation and temperature, whereas nectary size may relate to nectar production (Fahn, 1949), a likely selection target of pollinators. All morphometric measurements on leaves (n = 1559) and flowers (n = 776) were obtained from fresh material using a digital caliper, and measured by the same person. In addition, geographical co-ordinates were obtained for each population in situ (i.e. latitude, longitude and elevation), and environmental information on bioclimatic variables with a spatial resolution of 1 km2 from WorldClim Global Climate GIS data sets available online at www.WorldClim.org (Hijmans et al., 2005).
Fig. 2.
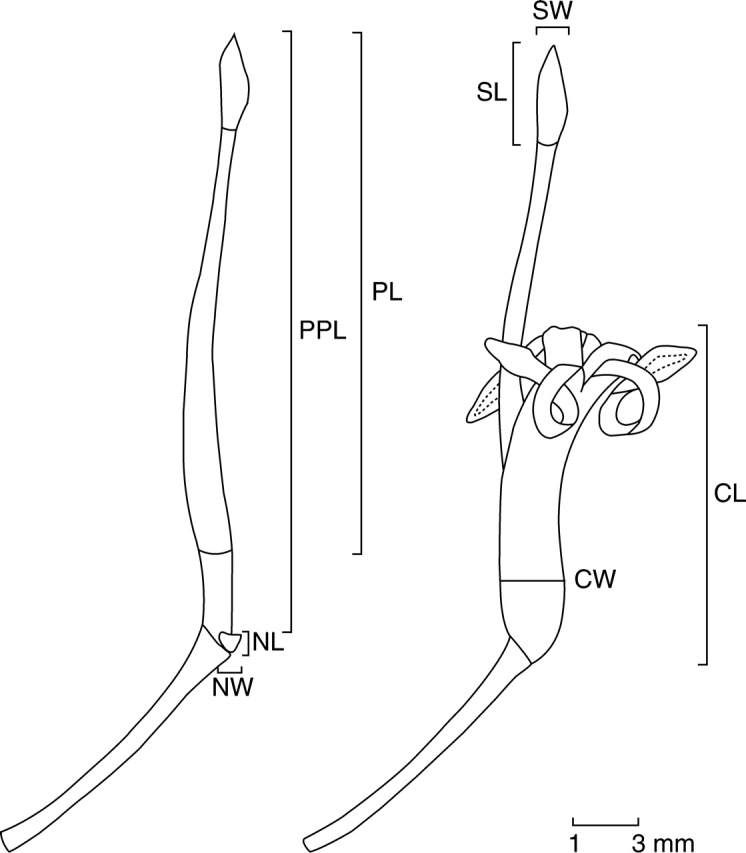
Schematic view of the flowers and morphometric characters measured (in mm) for Embothrium coccineum. The codes for the different morphological variables are provided in Materials and Methods.
Statistical analyses
Variation and covariation within and among populations in leaf and floral morphology were analysed using both univariate and multivariate methods. A nested ANOVA was used to partition the total variance of each of the 13 leaf and flower morphological variables into its hierarchical components. The levels considered for the variance hierarchical analysis were location in relation to the Andean continental divide (i.e. east or west), populations within east or west, individuals within populations, and leaves or flowers within individuals (this last level was used as the error term; Sokal and Rohlf, 1981). Despite that not all populations were sampled during the same year, repeated-measure ANOVAs based on the survey of six populations over 3 years showed high temporal consistency in the study of foliar and floral traits (results not shown). To satisfy assumptions of parametric statistical methods, all morphometric variables were log-transformed (x = log10 x; Sokal and Rohlf, 1981).
Nested analysis of covariance (nested ANCOVA) was also performed to partition the total covariance in hierarchical components to analyse the degree of coupling or uncoupling between foliar and floral traits or between each pair of morphological characters (Bell, 1989). As independent but complementary information is provided by patterns of character covariation within and among populations (see Introduction), covariation between all pairs of morphological variables was analysed at these two levels using the mean values of each foliar and floral trait studied for each individual tree. From this analysis, the intra-class (i.e. within population) and inter-class (i.e. among population) correlation were estimated [i.e. r′xy = COVxy/(Vx × Vy)1/2, where COVxy is the covariance component, and Vx and Vy are the variance component of traits x and y] for each pair of variables (n = 78 pairs). Ten of these r′xy corresponded to correlations between foliar traits (FOL vs. FOL), 28 corresponded to correlations between floral traits (FLO vs. FLO), and 40 corresponded to correlations between foliar vs. floral traits (FOL vs. FLO). Estimations of variance component correlations were carried using the PROC NESTED in SAS (9·1).
The mean variance component correlation at the within and among population levels, for each of these three types of comparisons, was compared with a distribution of randomized mean correlations. The expected mean correlation value and the corresponding 2·5 and 97·5 percentiles were obtained from random subgroups drawn without replacement from the 78 original variance component correlations (based on subsamples with n = 10 for comparisons among foliar characters, n = 28 for pairwise comparisons among floral characters, and n = 40 for comparisons among floral versus foliar characters). Randomizations were run with Resampling Stats (Simon, 1992).
To describe the multivariate pattern of morphological variation among populations we used principal components analysis (PCA). Two separate PCAs, one including foliar traits and another including floral traits only, were performed. Also the position of each population on the first axis of each of the two PCAs was related to its geographical longitude with regression analysis to visualize the influence of the strong west–east environmental gradient on leaf and flower morphology.
To analyse further the relationship between these two sets of morphological traits (vegetative and floral) and their association with environmental factors, multivariate analyses of redundancy (RDA) were performed using CANOCO (ter Braak and Šmilauer, 2002). This multivariate analysis was chosen because the morphological characters varied linearly along the gradient studied (see ter Braak and Šmilauer, 2002). Eight explanatory variables that were hypothesized to be important determinants of variation in the morphology of leaves and flowers were selected. These included geographical latitude and longitude, elevation, and from WorldClim, mean annual temperature, mean temperature of the warmest quarter (December–February), mean annual precipitation, and mean precipitation of the warmest quarter. As these environmental variables are measured in different units (e.g. m, mm, °C, etc.), these data were standardized according to: (data – mean)/SD (ter Braak and Šmilauer, 2002). Temperature and precipitation of the warmest quarter were selected because this period includes the flowering time of E. coccineum, the boom of pollinator activity, and also the months of highest vegetative growth. Therefore, water deficits or extreme temperatures should be particularly relevant during this period. The only qualitative explanatory variable included was position, east or west, with respect to the Andean continental divide. This qualitative variable, transformed into a binary dummy variable (east = 0, west = 1), can be considered as a surrogate for the different pollination modes reported for this species (see Discussion). A population × environmental synthetic matrix (each entry xij in this matrix represents the value of the environmental variable i for population j) was constructed that was compared with the population × morphology synthetic matrix used in the PCAs (each entry xij in this matrix represents the mean of each morphological variable i for population j). Appendix 1 shows the value for each of these eight variables for each population of E. coccineum. The significance of the variability explained by each environmental variable was analysed by forward stepwise selection using a Monte Carlo test with 999 permutations. In this procedure, the variable best fitting the data is selected first and then the next best fitting variable is added (ter Braak and Šmilauer, 2002).
Finally, geographical patterning in foliar and floral morphology was assessed by relating phenotypic distance matrixes of leaves and flowers and geographical distance between populations with a Mantel permutation test with 10 000 randomizations (Mantel, 1967; Bonnet and Van de Peer, 2002). Phenotypic dissimilarities were calculated as the Euclidean distances between each pair of populations based on morphological data, whereas linear geographical distances between each pair of populations were calculated using the Earth software (Byers, 1997). The confidence intervals delimited by the 2·5 and 97·5 percentiles were obtained by bootstrapping with Resampling Stats (Simon, 1992).
RESULTS
Morphological variation and covariation in foliar and floral traits
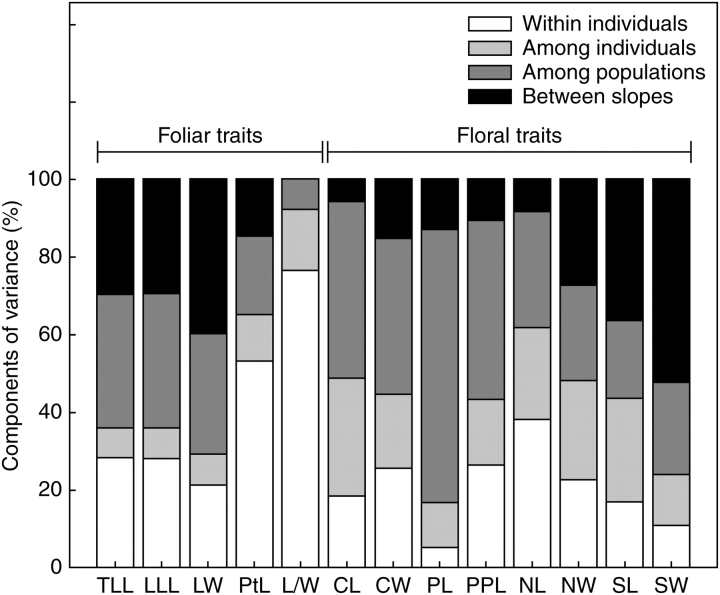
The morphological traits studied here showed coefficients of variation that ranged from 12·3 % to 53·3 % among populations (Appendix 2). In general terms, leaf traits exhibited higher coefficients of variation (29·0–53·3 %) than floral traits (12·3–28·6 %). Most morphological variables differed significantly between east and west of the Andes, among populations within each of the two regions, and among individuals. Only leaf length/leaf width, corolla length and pistil length did not exhibit significant variation between both Andean slopes (Appendix 2). Generally, variation between Andean slopes or among populations of each slope in foliar and floral traits was higher than among individuals within populations and among leaves and flowers from the same individual, and with few exceptions, variation among populations (including variability due to their west or east location) accounted for >50 % of all variation in leaf and flower morphology (Appendix 2 and Fig. 3). Only leaf length/leaf width, petiole length, and nectary length showed more variation within individuals, respectively, than at the population level (Appendix 2 and Fig. 3). In no case, variation among individuals within populations was the highest.
Fig. 3.
Variance components as estimated from the nested ANOVA model expressed as percentages of total variance. These components were between populations from the eastern (Argentinean) and western (Chilean) Andean slopes, among populations within each of these two regions, among individuals within populations, and within individuals. Significance levels for these components are provided in Appendix 2.
According to the nested ANCOVA, of all possible pairwise combinations between leaf and flower variables, approx. 24 % and 3 % exhibited relatively high correlation (>0·70) among and within populations, respectively (Table 1). Some examples of consistent high correlation at both levels (i.e. among and within populations) was the relationship between leaf lamina length (LLL) and total leaf length (TLL), or pistil length (PL) and stipe plus pistil total length (PPL), that represent classical examples of expected high associations due to correlations between a ‘part’ and the ‘whole’ (Sokal and Rohlf, 1981). The mean variance component correlation (r′xy) among populations, averaged over all pairs of foliar traits, was higher than the mean correlation between all pairs of floral traits and mixed pairs of foliar–floral traits, (0·59, 0·42 and 0·47, respectively). In no case, however, the average among-population correlation for any of these three categories was smaller or larger than the lower or upper limits of their respective intervals based on randomized values (Fig. 4A). In the case of the character covariation within populations, the average correlation between pairs of foliar traits was similar to the average correlation between pairs of floral traits and well within expectations based on the randomization procedure. However, these two mean correlations were higher than the mean correlation estimated from mixed pairs of foliar-floral traits, (0·41, 0·41 and 0·16, respectively). Figure 4B shows that, unlike the other two, this latter average within-population mean correlation was smaller than the lower limit of the range of expected values, denoting uncoupling between leaves and flowers.
Table 1.
Covariation component coefficents (r′xy) between pairs of leaf and flower traits among and within 32 populations of Embothrium coccineum from temperate forest of South America obtained from nested ANCOVA
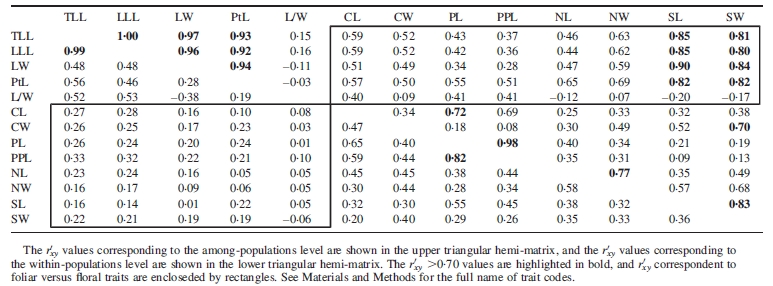 |
Fig. 4.
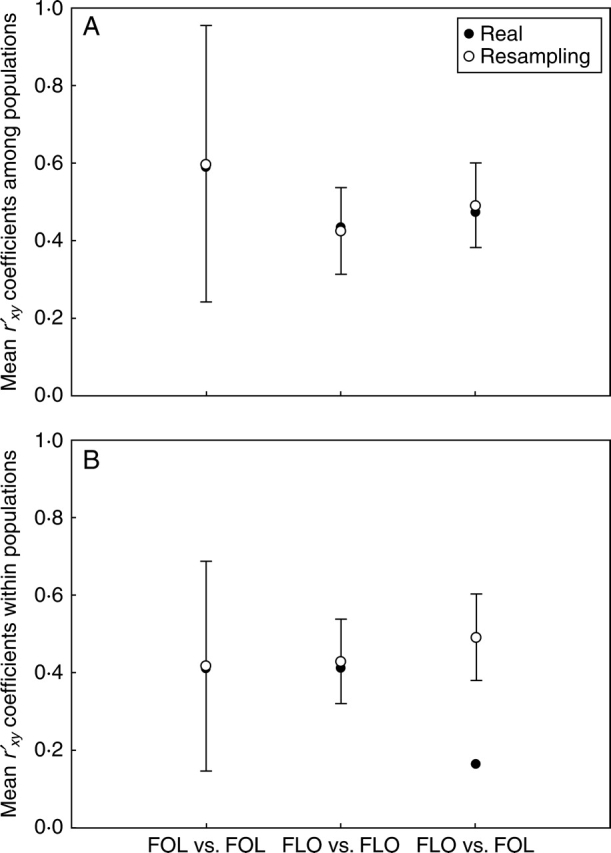
Mean observed component correlations (r′xy) (A) among and (B) within populations for the three types of comparisons of trait pairs: between pairs of foliar traits (FOL vs. FOL), between pairs of floral traits (FLO vs. FLO), and between foliar and floral traits (FOL vs. FLO). The expected mean value and the 2·5 and 97·5 percentiles were provided of the distribution of expected values obtained after 10 000 randomizations where, without replacement, an equal number of correlation coefficients, as observed for each type of comparison, were sampled (see Materials and Methods for a more detailed explanation).
Geographical patterns and contribution of environmental factors
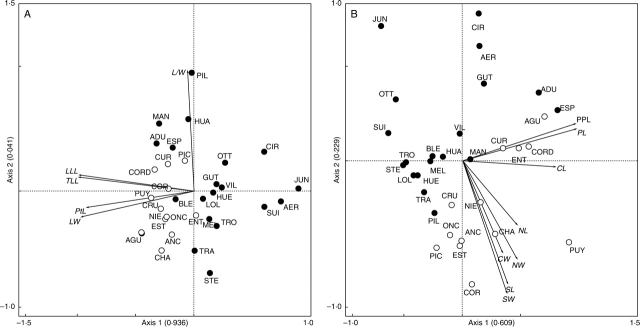
In the PCA analysis of foliar traits, the first two axes accounted for 97·7 % of variation across populations (Fig. 5A). In this ordination, there was a clear partition between populations located on the west and east sides of the Andes. The first axis generally separated western populations to the left according to leaf size (e.g. higher contributions of leaf lamina length and leaf width), and the second axis eastern populations to the top, mostly according to leaf shape (ratio leaf lamina length/leaf width). Thus, western populations were generally characterized by larger and rounder leaves and eastern populations by smaller and, in many cases, more elongated leaves.
Fig. 5.
Biplots of the first two axes of the PCA ordinations of 32 populations of Embothrium coccineum based on (A) five foliar morphological traits and (B) eight floral morphological traits. The eigenvalues associated with each axis are provided in parentheses. Open circles are the populations from the western slopes of the Andes (Chile), and closed circles, the populations from the eastern slopes of the Andes (Argentina). Full names for the acronyms for the different populations are provided in Appendix 1 and codes for the different morphological variables (in italics for clarity) are in Materials and Methods. Arrows show the contribution of each variable to the first two axes.
In the PCA analysis of floral traits, the first two axes accounted for 83·8 % of the variation among populations (Fig. 5B). In this figure, a partition similar to the one observed in the leaf ordination could be observed. The first axis generally separated western populations to the right according to floral length (i.e. corolla length, pistil length and stipe plus pistil total length), and the second axis, western populations to the bottom, mostly according to stigma length and width. Therefore, western populations were generally characterized by large flowers and large stigmas and eastern populations by short flowers and small stigmas.
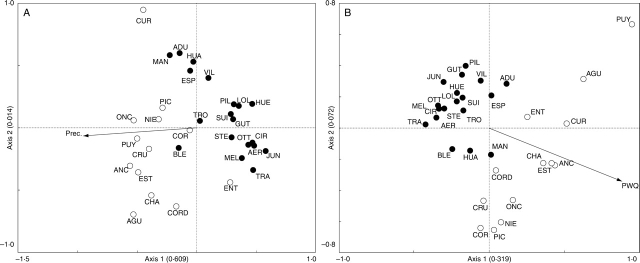
The RDA analysing the association between leaf traits and environmental variables showed that mean annual precipitation was the only climatic variable contributing significantly to the model (P < 0·05; Fig. 6A). The arrow representing the direction of maximum change for each explanatory variable showed that annual precipitation was correlated to the first axis, with populations from hyperhumid and wet sites (the Chilean and some Argentinean populations close to the international border) characterized by larger and rounder leaves on the left side of the figure. The RDA analysing the association between floral traits and environmental variables showed that precipitation during the warmest quarter contributed significantly to the model (P < 0·05; Fig. 6B). So in this case, the precipitation during the warmest quarter was associated with differences in floral traits between populations at eastern and western slopes of the Andes.
Fig. 6.
Biplots of the first two axes of the RDA ordinations of 32 populations of Embothrium coccineum; included are the explanatory variables that appeared to be significant (P < 0·05) determinants of variation in (A) leaf and (B) flower morphology. The explanatory variables are described in Materials and Methods and their values in Appendix 1. The eigenvalue associated with each axis is provided in parentheses. Open circles are the populations occurring on the western slope of the Andes (Chile) and closed circles the populations on the eastern slope of the Andes (Argentina). Full names for population acronyms are given in Appendix 1.
The comparison between the eigenvalues of the two first axes between the PCA and RDA, however, can provide a general assessment of whether the chosen explanatory variables can account for a large proportion of variation (ter Braak and Šmilauer, 2002). In this case, it seems that the environmental variables explain foliar variation better than floral variation, as the two multivariate analyses using foliar traits have more similar eigenvalues (Figs 5A vs. 6A and 5B vs. 6B). Thus, whereas the environment provides a satisfactory explanation for variation in leaf morphology, it does not do so for variation in floral morphology.
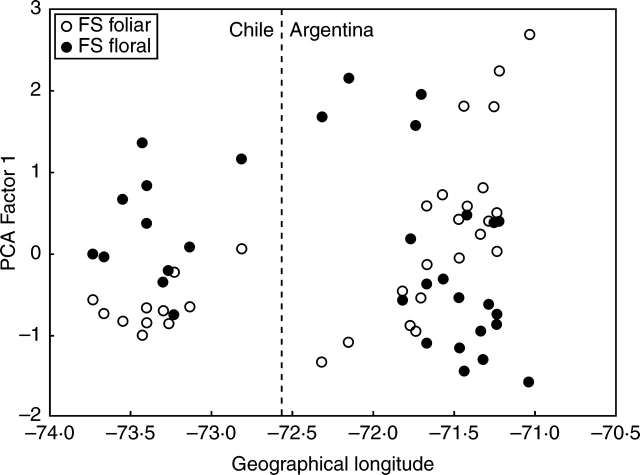
Figure 7 shows the position of each population on the first axes of the PCAs of foliar and floral traits, related to longitudinal geographical position (W long). Whereas variation in foliar morphology was associated with geographical longitude (R2 = 0·4, P < 0·001), variation in flower morphology was not (R2 = 0·1, P > 0·05). Mantel tests detected a weak positive association between geographical distance and foliar morphology (r = 0·12, P = 0·058, CI = 0·03–0·21), but a negative association between geographical distance and floral morphology among populations (r = –0·14, P = 0·005, CI = –0·22 to –0·05). Thus, populations that have similar leaves do not tend to share similar flowers, suggesting the existence of a differential geographical pattern between leaf and flower morphologies. On the other hand, leaf morphology between neighbouring populations tended to be more similar than randomly expected, whereas flower morphology showed the opposite pattern. Whereas geographical similarity in leaf morphology evidences the influence of the strong west–east rainfall gradient, flower characters seem to be more influenced by local-acting factors.
Fig. 7.
Relationship between each population score in the first axis of both PCAs of floral and leaf traits, and longitudinal geographical position (W long). Population characterization in terms of foliar morphology is indicated with closed circles, and in terms of floral morphology with open circles.
DISCUSSION
Associated with the high environmental heterogeneity of its native range, Embothrium coccineum exhibits large variation in both foliar and floral traits among populations, suggesting the operation of factors favouring trait-divergence even within a relatively small geographical region, as the study area. Nevertheless, despite a common ontogenetic origin, intraspecific variation in leaf morphology was mostly unrelated to variation in flower morphology, denoting strong decoupling between these traits within populations. Also, whereas geographical variation in leaf traits (particularly size and shape) was quite strongly associated with the abrupt west–east precipitation gradient that characterizes the northern part of the Patagonian Andes, variation in flower morphology was, at best, weakly related to this gradient. Therefore, in this region, foliar traits seem to vary according to a clinal longitudinal pattern, whereas floral traits appear to vary according to a fine-grained geographical mosaic. Thus, floral traits seem to be more influenced by local-acting factors, perhaps by idionsyncratic pollinator assemblages that can vary greatly over space, even at the scale of few kilometres (Smith-Ramírez and Armesto, 1998; Devoto et al., 2006; V. R. Chalcoff, pers. obs.). These two different patterns suggest the action of different factors affecting leaves and flower morphologies.
The present results suggest that populations of E. coccineum are under the effect of strong forces that foster intraspecific variation. This statement is supported by the fact that this species shows high morphological variation in leaves and flowers, and although this variation was observed at all levels studied, most of it could be attributed to morphological differences among populations, including their location east or west of the Andes. This is in contrast to other studies that found that most intraspecific variation in morphological traits occurred within, rather than among populations (e.g. Herrera et al., 2002). In the present case, closer populations did tend to share similar leaves, whereas they did not tend to exhibit more similar flowers. The results suggest that variation among populations of E. coccineum, at least in leaf morphology, seems to be shaped principally by natural selection or phenotypic plasticity mediated by environmental factors, rather than random drift. Nevertheless, plasticity appears not to be important, as different horticultural cultivars have been described that maintain their original characteristics after being grown in other regions of the world (Gardner, 1997). On the other hand, the results provide indirect evidence that gene flow is limited. If this flow were considerable, more floral similarity would be expected among nearby populations sharing a similar genetic template (Slatkin, 1983). Additionally, relatively high rates of gene flow in E. coccineum have only been found among populations growing in small forest fragments (Mathiasen et al., 2007).
Flowers are structures derived from modified leaves during the course of evolution, so leaf and floral traits can be phenotypically correlated through genetic pleiotropic effects or tight genetic linkage (Slatkin, 1983). However, ‘disarrangements’ between foliar and floral morphology can occur when these two types of organs are subject to disparate evolutionary forces if some degree of genetic uncoupling exists between these two types of structures (the ‘correlation pleiades’ hypothesis; Berg, 1959, 1960). Although genetic correlation is difficult to estimate in long-lived organisms, the sign and magnitude of covariation between a set of morphological traits within and among populations could provide a measure of the degree of morphological and functional organismic integration (Berg, 1959, 1960; Armbruster, 1991; Armbruster and Schwaegerle, 1996; Armbruster et al., 1999; Herrera et al., 2002).
Patterns of covariation between vegetative and reproductive traits within and among species have been evaluated previously, providing only partial support for Berg's hypothesis (e.g. Conner and Via, 1993; Conner and Sterling, 1996; Armbruster et al., 1999 and references therein; Herrera, 2005; Lambrecht and Dawson, 2006; Medrano et al., 2006; Hansen et al., 2007). In the present case, it was found that the mean leaf–floral covariation within populations was lower than randomly expected. Additionally, it was observed that foliar traits among populations of E. coccineum clearly vary according to a longitudinal clinal pattern, whereas floral traits seem to vary according to a more complex pattern, influenced, in part, by longitude and latitude and also by local-acting factors resulting in a geographical mosaic. Therefore, populations that have similar leaves do not necessarily have similar flowers. All these results denote uncoupling between foliar and floral structures, suggesting that floral morphology is able to evolve independently of variation in leaf morphology, if the low phenotypic correlations between foliar versus floral characters also reflect a low genetic covariation (see Herrera et al., 2002, and references therein).
Additionally in the present study, leaves and flowers showed differential responses to environmental factors. It was found that leaves of E. coccineum were apparently more influenced by climate, particularly water availability, than flowers. The relationship between the size and shape of leaves and environmental factors has been shown previously for many other species (e.g. Rico-Gray and Palacios-Rios, 1996; Ezcurra et al., 1997; Sapir et al., 2002). In addition, variation in leaf morphology of E. coccineum has also been reported to reflect adaptive differences associated with distinctive climatic characteristics across its whole distribution area (Souto et al., 2008). A common pattern reported for many species is a reduction in leaf size as environmental aridity increases, representing an adaptive strategy because smaller leaves exhibit lower evapotranspiration (Parkhurst and Loucks, 1972; Dudley, 1996). In this study, it was found that populations of E. coccineum showed a continuous reduction in leaf size according to their location along the west–east precipitation gradient rather than to their geographical proximity. For instance, Argentine populations near the continental divide (e.g. Blest), where precipitation can exceed 3000 mm, have leaves more similar to many relatively distant Chilean populations than to other nearby dryer Argentinean populations to the east. Thus, water availability seems to be the overriding factor shaping leaf morphology of the present study species in this region.
On the other hand, flower morphology can respond to selection exerted directly on flowers or indirectly on leaves or other plant organs by a series of environmental factors (Slatkin, 1983; Lambrecht and Dawson, 2006), or more traditionally to selection exerted by efficient pollinators (Faegri and van der Pijl, 1971). For example, clinal variation in flower size has been reported along precipitation gradients (Sapir et al., 2002; Silva-Montellano and Eguiarte, 2003; Herrera, 2005; Lambrecht and Dawson, 2006), as well as along gradients in pollinator body or bill size (Boyd, 2002; Herrera, 2005; Nattero and Cocucci, 2007). Finally, geographical variation in phenotypic traits can be to a large degree the product of genetic drift as proposed by Domínguez et al. (1998) for Rhizophora mangle and by Herrera et al. (2002) to explain flower divergence among Iberian populations of Helleborus foetidus. In the present study, floral morphology of E. coccineum does not seem to have a longitudinal clinal component as marked as in the case of leaves, responding in any event more directly to precipitation during the flowering season than to annual precipitation (Fig. 6A vs. B). Thus, whereas smaller corollas could represent an adaptation to increasing aridity (Lambrecht and Dawson, 2006), this sole factor does not provide a complete explanation of the geographical pattern of variation in flower morphology observed.
Since Darwin's time, it has been hypothesized that selection exerted by disparate pollinator groups is an important force promoting evolutionary divergence within plant lineages and convergence of floral-traits among plant lineages (i.e. pollinator syndromes; Fenster et al., 2004; and references therein). Therefore, species with generalized flowers or pollinated by different pollinator assemblages throughout their geographical ranges will exhibit a lower degree of floral integration than specialized flowers (Berg, 1959, 1960; Armbruster et al., 1999; Herrera et al., 2002; Fenster et al., 2004). In the case of E. coccineum, the pattern of variation of floral traits could coincide with the contrasting pollinator assemblages associated with different populations, with varying participation of hummingbirds, passerines and long-proboscis flies from the genus Tricophthalma (Nemestrinidae) (Devoto et al., 2006; V. R. Chalcoff, pers. obs.). As a general trend, pollinator assemblages associated with western populations are more diverse than those associated with eastern populations, but even nearby populations may show marked contrasts in assemblage composition. Despite that the passerine Elaenia albiceps has been reported to visit and pollinate E. coccineum flowers in Chilean populations only (Smith-Ramírez and Armesto, 1998), the relative abundance of this passerine, the hummingbird S. sephaniodes, and insects is quite variable among these western populations (V. R. Chalcoff, unpubl. res.). Thus, the geographical pattern of variation in the composition of this plant's pollinator assemblages is better described by a mosaic than a cline. This mosaic pattern could also describe the overall pattern of flower morphological variation in this proteaceous species, as environmental variables can only provide a partial account of the multivariate pattern in flower morphology (Fig. 6B).
Because of the obligate outcrossing system of E. coccineum, pollinators should exert strong selection on floral traits that maximize efficiency in pollen transfer (Harder and Barrett, 1993). Interestingly, pistil length, a trait that because of secondary pollen presentation could relate to both the location of pollen deposition onto a pollinator's body and its transfer to flowers' stigmas, was the measured morphological variable that exhibited the largest percent of variability among rather than within populations. Therefore, although genetic drift cannot be discarded as another contributing factor for increasing floral similarity between close populations (see Herrera et al., 2002), the present results are consistent with the view that a geographical mosaic in floral traits is modelled principally by pollinator assemblages that change over space in a non-clinal fashion (Thompson, 1994).
One key question that remains to be answered is how much genetic influence underlies the phenotypic variation patterns observed in leaf and flower morphology. For instance, the dramatic reduction in leaf size along the sampled precipitation gradient, although adaptive, could represent just a plastic response of plants to increasing drier conditions. However, plants of Embothrium coccineum developing from seeds of different populations growing under a common environment maintain striking differences in vegetative and reproductive traits (M. A. Aizen, pers. obs.), and different horticultural cultivars that reproduce by cuttings have been described (Gardner, 1997), suggesting a genetic basis in morphological variation. Also, in this species morphological divergence seems to be accompanied by processes of genetic differentiation (Souto and Premoli, 2007). It is not known how much genetic covariation exists between leaf and flower characters. However, the within-population and geographical morphological uncoupling between leaves and flowers observed is suggestive that vegetative and reproductive traits are responding independently to different forces, thus increasing the potential for evolutionary novelty.
ACKNOWLEDGEMENTS
We thank APN Argentina, CONAF Chile, CODEFF-Valdivia and ‘Senda Darwin’–Chiloé Biological Station for allowing us to work in their parks and reserves. We also wish to especially thank Cecilia Smith-Ramírez for her help during trips to Chile. Thanks are also due to Andrea Craig, Leonardo Galetto and Andrea Premoli for critically reading and commenting on an earlier draft of this manuscript, and to two anonymous reviewers and the editor Don Levin for their useful suggestions. This work was partially supported by an IAPT grant 2005 to the first author, and by funding to project B125 from Universidad Nacional del Comahue. V.R.C. holds a doctoral scholarship from the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (CONICET). C.E. and M.A.A. are scientific research members of the same institution.
APPENDIX 1
Geographical co-ordinates and environmental characteristics of the 32 populations of Embothrium coccineum studied. Information on number of individuals and total number of leaves and flowers sampled in each population is provided. Code = population code name; Name = population name; Geographical data: Lat. (S) = geographical latitude of populations, Long. (W) = geographical longitude populations, Elev. = elevation of populations (in metres above sea level); Environmental data (obtained from Worldclim Global Climate GIS data sets): Temp. = mean annual temperature, Prec. = mean annual precipitation, TWQ = mean temperature of warmest quarter, PWQ = mean precipitation of warmest quarter; Pos. = position in relation to the Andes, western (W) or eastern (E); N ind = number of individuals sampled per population; Leaves = number of leaves sampled per individual; Flowers = number of flowers sampled per individual.
| Geographical data |
Environmental data |
Samples |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Name | Lat. (S) | Long. (W) | Elev. | Temp. | Prec. | TWQ | PWQ | Pos. | N ind | Leaves | Flowers |
| ADU | Aduana | 40°40 | 71°44 | 893·0 | 8·3 | 1539 | 13·4 | 187 | E | 5 | 50 | 25 |
| AER | Aeropuerto Bariloche | 41°07 | 71°13 | 828·0 | 8·4 | 863 | 14 | 84 | E | 5 | 50 | 25 |
| AGU | Aguas Calientes | 40°44 | 72°19 | 440·0 | 8·9 | 2331 | 13·6 | 296 | W | 5 | 50 | 25 |
| ANC | Ancud | 41°52 | 73°44 | 12·1 | 10·6 | 2362 | 13·4 | 323 | W | 4 | 40 | 20 |
| BLE | Puerto Blest | 41°03 | 71°02 | 780·0 | 7 | 1533 | 12 | 190 | E | 5 | 50 | 25 |
| CHA | Chacao | 41°51 | 73°33 | 12·1 | 10·6 | 2152 | 13·5 | 298 | W | 5 | 50 | 25 |
| CIR | Cicunvalación | 41°09 | 71°15 | 888·0 | 8·1 | 858 | 13·7 | 84 | E | 5 | 50 | 25 |
| COR | Camino a Corral | 39°56 | 73°08 | 10·0 | 12·2 | 1933 | 16·2 | 172 | W | 5 | 50 | 25 |
| CORD | Cordillera de la Costa | 40°37 | 73°25 | 332·0 | 9·6 | 1750 | 12·7 | 199 | W | 5 | 50 | 25 |
| CRU | Río Cruses | 39°50 | 73°16 | 49·0 | 11·8 | 2228 | 15·8 | 213 | W | 5 | 50 | 25 |
| CUR | Curiñanco | 41° | 71°49 | 397·0 | 11·8 | 2250 | 15·6 | 220 | W | 5 | 49 | 25 |
| ENT | Entre Lagos | 40°37 | 72°48 | 129·0 | 11·4 | 1561 | 15·6 | 196 | W | 5 | 50 | 25 |
| ESP | Lago Espejo | 40°39 | 71°42 | 860·0 | 8·2 | 1464 | 13·4 | 176 | E | 4 | 40 | 20 |
| EST | Estación biológica | 41°53 | 73°40 | 12·1 | 10·6 | 2298 | 13·5 | 315 | W | 4 | 40 | 20 |
| GUT | Gutiérrez | 41°10 | 71°25 | 1058·0 | 7·3 | 978 | 12·8 | 107 | E | 5 | 50 | 25 |
| HUA | Hua Hum | 41°31 | 71°28 | 645·0 | 9·6 | 1491 | 14·7 | 156 | E | 4 | 40 | 20 |
| HUE | Lago Huechulafquen | 41°08 | 71°26 | 916·0 | 9 | 817 | 14·4 | 71 | E | 5 | 50 | 25 |
| JUN | Laguna Juncos | 40°27 | 71°34 | 995·0 | 7·9 | 702 | 13·7 | 64 | E | 5 | 50 | 25 |
| LOL | Lago Lolog | 40°23 | 71°17 | 917·0 | 8·7 | 927 | 14 | 86 | E | 5 | 50 | 24 |
| MAN | Manso | 41°34 | 71°46 | 460·0 | 9·5 | 1898 | 14·9 | 233 | E | 5 | 50 | 25 |
| MEL | Lago Meliquina | 40°37 | 71°29 | 980·0 | 7·5 | 863 | 12·9 | 85 | E | 5 | 50 | 25 |
| NIE | Niebla | 39°52 | 73°24 | 20·0 | 11·7 | 2204 | 15·4 | 222 | W | 5 | 50 | 25 |
| ONC | Parque Oncol | 39°48 | 73°18 | 236·0 | 10·5 | 2354 | 14·4 | 234 | W | 5 | 50 | 25 |
| OTT | C° Otto | 40°03 | 71°20 | 900·0 | 7·8 | 902 | 13·4 | 93 | E | 5 | 50 | 25 |
| PIC | Aeropuerto Pichoy | 39°48 | 73°14 | 20·4 | 11·9 | 2224 | 16 | 207 | W | 5 | 50 | 24 |
| PIL | C° Piltriquitron | 39°43 | 73°24 | 1100·0 | 7·3 | 962 | 12·8 | 103 | E | 5 | 50 | 25 |
| PUY | Puyehue | 40°40 | 72°09 | 460·0 | 10·2 | 2382 | 15·2 | 305 | W | 5 | 50 | 25 |
| STE | Lago Steffen | 41°16 | 71°40 | 928·0 | 7·4 | 1043 | 12·9 | 114 | E | 5 | 50 | 25 |
| SUI | Colonia Suiza | 40°42 | 71°14 | 878·0 | 8·2 | 1065 | 13·6 | 120 | E | 5 | 50 | 25 |
| TRA | Lago Traful | 41°53 | 73°40 | 847·0 | 7·7 | 876 | 13·4 | 89 | E | 5 | 50 | 24 |
| TRO | C°Tronador | 41°09 | 71°19 | 902·0 | 7·2 | 1343 | 12·5 | 161 | E | 5 | 50 | 25 |
| VIL | Lago Villarino | 39°47 | 71°14 | 1007·0 | 7·9 | 1219 | 13·2 | 133 | E | 5 | 50 | 24 |
APPENDIX 2
Mean values (s.d.) of the leaf and floral traits measured for the 32 populations of Embothrium coccineum studied. See Appendix 1 for the full names of population acronyms and Materials and methods for full names of trait codes. Mean and CV are the average values (s.d.) and coefficient of variation of populations means (CV = SD/mean × 100). VBS, VAP, VAI and VWI are the component of variance estimated from of the nested ANOVA between Andean slopes (i.e. east or west), among populations, among individuals, and within individuals respectively, expressed as a percentage of total variance (*P < 0·05, **P < 0·005, ***P < 0·0005).
| Pop. | TLL | LLL | LW | PtL | L/W | CL | CW | PL | PPL | NL | NW | SL | SW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADU | 80·1 (19·9) | 73·3 (18·7) | 20·9 (3·5) | 6·7 (2·6) | 3·6 (1·0) | 19·0 (2·0) | 3·1 (0·5) | 35·4 (2·6) | 42·4 (3·2) | 0·8 (0·2) | 1·6 (0·2) | 3·6 (0·3) | 1·0 (0·1) |
| AER | 29·4 (8·6) | 26·0 (8·0) | 8·8 (2·3) | 3·5 (1·0) | 3·1 (1·0) | 15·2 (0·9) | 3·3 (0·2) | 30·8 (1·5) | 37·8 (1·9) | 0·7 (0·1) | 1·6 (0·2) | 2·8 (0·3) | 0·9 (0·1) |
| AGU | 81·9 (17·5) | 75·2 (16·2) | 24·2 (3·8) | 6·7 (1·8) | 3·1 (0·7) | 19·2 (0·9) | 3·1 (0·3) | 35·3 (1·3) | 43·1 (1·5) | 0·8 (0·1) | 1·7 (0·2) | 3·9 (0·4) | 1·1 (0·1) |
| ANC | 66·6 (11·7) | 60·1 (10·8) | 21·4 (3·2) | 6·5 (1·6) | 2·8 (0·4) | 16·4 (1·0) | 3·3 (0·2) | 25·8 (1·5) | 31·7 (1·5) | 1·0 (0·2) | 2·1 (0·2) | 3·8 (0·3) | 1·3 (0·1) |
| BLE | 66·7 (19·2) | 60·9 (17·8) | 20·2 (3·8) | 5·8 (2·4) | 3·0 (0·8) | 16·3 (1·3) | 3·1 (0·3) | 25·1 (2·7) | 30·3 (3·1) | 0·6 (0·1) | 1·5 (0·1) | 3·6 (0·6) | 1·1 (0·1) |
| CHA | 71·5 (15·9) | 65·4 (15·1) | 24·8 (4·2) | 6·1 (1·5) | 2·7 (0·6) | 18·0 (2·0) | 3·2 (0·2) | 28·7 (2·7) | 34·8 (3·6) | 0·8 (0·2) | 2·0 (0·3) | 4·3 (0·9) | 1·3 (0·1) |
| CIR | 35·1 (10·7) | 31·8 (10·2) | 9·6 (1·6) | 3·3 (1·1) | 3·4 (1·2) | 17·3 (1·4) | 2·9 (0·3) | 30·3 (2·9) | 37·4 (3·2) | 0·7 (0·1) | 1·5 (0·2) | 2·8 (0·2) | 0·8 (0·1) |
| COR | 71·3 (28·1) | 65·5 (25·3) | 21·2 (5·6) | 5·8 (3·3) | 3·1 (1·0) | 18·0 (1·4) | 3·6 (0·4) | 25·2 (2·5) | 31·3 (7·2) | 0·8 (0·2) | 2·0 (0·3) | 4·3 (0·8) | 1·5 (0·1) |
| CORD | 79·9 (19·5) | 73·5 (18·2) | 22·7 (5·4) | 6·4 (1·9) | 3·3 (0·7) | 18·7 (1·6) | 3·3 (0·4) | 33·9 (1·6) | 40·3 (2·4) | 0·6 (0·1) | 1·6 (0·4) | 3·9 (0·5) | 1·3 (0·1) |
| CRU | 75·0 (26·9) | 69·5 (26·4) | 24·3 (7·5) | 5·5 (5·4) | 2·9 (0·8) | 16·5 (1·0) | 3·6 (0·2) | 25·5 (1·0) | 31·7 (1·4) | 0·7 (0·1) | 1·6 (0·3) | 3·7 (0·6) | 1·3 (0·2) |
| CUR | 72·8 (18·6) | 67·2 (17·4) | 20·7 (5·2) | 5·7 (1·8) | 3·3 (0·8) | 17·1 (1·6) | 3·4 (0·2) | 31·6 (2·1) | 37·6 (2·1) | 0·6 (0·1) | 1·6 (0·1) | 4·1 (0·4) | 1·1 (0·1) |
| ENT | 56·3 (15·4) | 50·8 (14·4) | 17·4 (3·7) | 5·5 (1·9) | 2·9 (0·6) | 17·3 (1·8) | 3·1 (0·4) | 32·4 (3·7) | 40·2 (5·2) | 0·8 (0·2) | 1·7 (0·2) | 3·8 (0·5) | 1·3 (0·2) |
| ESP | 71·0 (21·6) | 65·1 (20·0) | 18·9 (5·6) | 5·9 (2·0) | 3·5 (0·8) | 20·5 (1·8) | 3·5 (0·2) | 35·8 (2·2) | 44·8 (2·1) | 0·9 (0·1) | 1·8 (0·3) | 3·6 (0·3) | 1·0 (0·2) |
| EST | 71·3 (13·5) | 65·3 (12·6) | 22·9 (3·9) | 5·9 (2·7) | 2·9 (0·5) | 15·3 (0·7) | 2·8 (0·1) | 26·1 (1·6) | 31·6 (1·5) | 1·0 (0·1) | 2·1 (0·3) | 4·1 (0·4) | 1·3 (0·1) |
| GUT | 48·9 (10·9) | 43·7 (10·1) | 13·9 (2·5) | 5·2 (1·5) | 3·3 (1·2) | 17·3 (2·0) | 3·1 (0·4) | 30·0 (1·1) | 36·5 (1·7) | 0·8 (0·2) | 1·6 (0·2) | 3·3 (0·2) | 0·9 (0·1) |
| HUA | 64·9 (16·9) | 60·1 (15·8) | 16·6 (3·0) | 4·8 (1·6) | 3·6 (0·9) | 16·8 (1·0) | 3·2 (0·3) | 25·6 (1·0) | 31·1 (1·0) | 0·7 (0·1) | 1·4 (0·3) | 3·5 (0·4) | 1·2 (0·1) |
| HUE | 50·1 (9·3) | 45·1 (8·4) | 14·8 (2·6) | 4·9 (1·2) | 3·1 (0·8) | 16·8 (0·6) | 3·6 (0·2) | 23·1 (1·0) | 28·4 (1·3) | 0·7 (0·1) | 1·6 (0·2 | 3·5 (0·2) | 1·0 (0·1) |
| JUN | 26·1 (4·5) | 23·9 (4·1) | 8·3 (2·2) | 2·3 (0·7) | 3·1 (1·1) | 15·5 (1·0) | 2·6 (0·1) | 22·1 (0·9) | 28·6 (1·1) | 0·5 (0·1) | 1·2 (0·1) | 3·0 (0·3) | 0·7 (0·1) |
| LOL | 54·2 (10·4) | 49·4 (9·5) | 16·7 (2·6) | 4·8 (1·2) | 3·0 (0·8) | 16·0 (0·6) | 3·2 (0·2) | 22·9 (0·6) | 28·7 (1·1) | 0·7 (0·1) | 1·6 (0·2) | 3·5 (0·3) | 1·1 (0·2) |
| MAN | 80·6 (20·5) | 75·0 (19·1) | 21·1 (4·9) | 5·6 (2·1) | 3·6 (0·9) | 19·0 (0·9) | 3·3 (0·2 | 26·8 (1·0) | 33·6 (1·5) | 0·7 (0·1) | 1·7 (0·1) | 3·4 (0·3) | 1·2 (0·1) |
| MEL | 51·1 (10·1) | 46·5 (9·1) | 16·4 (2·0) | 4·6 (1·7) | 2·8 (0·5) | 17·0 (1·1) | 3·2 (0·2) | 23·8 (1·1) | 30·5 (1·7) | 0·7 (0·1) | 1·5 (0·1) | 3·6 (0·4) | 1·1 (0·1) |
| NIE | 74·3 (16·1) | 68·3 (15·5) | 23·3 (4·2) | 6·0 (1·5) | 3·0 (0·6) | 18·0 (1·9) | 3·4 (0·3) | 27·8 (2·9) | 34·2 (4·1) | 0·6 (0·1) | 1·7 (0·3) | 4·1 (0·7) | 1·3 (0·1) |
| ONC | 70·6 (15·0) | 64·6 (13·9) | 22·3 (4·0) | 6·1 (1·7) | 2·9 (0·5) | 16·0 (1·3) | 3·6 (0·3) | 25·5 (2·2) | 29·8 (6·6) | 0·7 (0·1) | 1·6 (0·2) | 4·2 (0·6) | 1·3 (0·2) |
| OTT | 47·1 (8·7) | 42.5 (8·3) | 13·2 (1·9) | 4·2 (0·8) | 3·3 (0·7) | 16·3 (0·8) | 2·6 (0·2) | 21·9 (1·0) | 28·5 (1·1) | 0·6 (0·1) | 1·4 (0·1) | 3·2 (0·2) | 0·9 (0·1) |
| PIC | 64·9 (16·2) | 60·5 (15·4) | 19·2 (5·0) | 4·4 (1·7) | 3·2 (0·8) | 17·1 (1·2) | 3·9 (0·4) | 23·1 (2·9) | 27·3 (8·9) | 0·8 (0·1) | 1·7 (0·2) | 3·9 (0·6) | 1·2 (0·2) |
| PIL | 65·0 (25·0) | 60·1 (23·5) | 14·8 (2·9) | 4·9 (2·1) | 4·2 (2·0) | 15·0 (1·2) | 3·4 (0·2) | 24·5 (1·7) | 30·2 (1·9) | 0·6 (0·1) | 1·9 (0·1) | 4·1 (0·3) | 1·1 (0·1) |
| PUY | 79·3 (14·1) | 71·3 (13·1) | 22·6 (4·4) | 8·0 (1·8) | 3·2 (0·6) | 21·5 (2·7) | 3·8 (0·3) | 35·8 (2·8) | 42·1 (4·2) | 0·8 (0·2) | 1·8 (0·2) | 4·7 (0·6) | 1·5 (0·1) |
| STE | 48·2 (10·7) | 43·0 (10·4) | 17·2 (3·6) | 5·2 (4·8) | 2·6 (0·7) | 14·2 (2·1) | 3·0 (0·3) | 23·1 (1·2) | 28·4 (2·3) | 0·7 (0·2) | 1·3 (0·2) | 4·0 (0·3) | 1·0 (0·1) |
| SUI | 33·4 (5·7) | 29·9 (5·2) | 10·5 (2·6) | 3·5 (0·9) | 3·0 (0·9) | 15·2 (0·8) | 3·2 (0·2) | 21·3 (1·1) | 27·8 (1·5) | 0·6 (0·1) | 1·5 (0·1) | 2·9 (0·4) | 1·1 (0·1) |
| TRA | 55·3 (12·6) | 49·7 (10·6) | 18·6 (2·9) | 5·6 (2·7) | 2·7 (0·6) | 16·0 (1·0) | 3·4 (0·1) | 23·8 (1·4) | 28·9 (1·7) | 0·7 (0·1) | 1·6 (0·2) | 3·6 (0·5) | 1·1 (0·1) |
| TRO | 48·2 (7·1) | 44·3 (6·7) | 16·5 (2·8) | 3·9 (0·9) | 2·8 (0·6) | 16·8 (1·2) | 3·1 (0·2) | 22·2 (0·9) | 28·1 (0·9) | 0·6 (0·1) | 1·4 (0·1) | 3·6 (0·3) | 1·1 (0·1) |
| VIL | 47·8 (9·8) | 44·0 (9·0) | 14·8 (1·8) | 3·8 (1·3) | 3·0 (0·7) | 17·6 (0·9) | 3·2 (0·2) | 27·3 (1·5) | 29·3 (9·3) | 0·7 (0·1) | 1·5 (0·1) | 3·8 (0·3) | 1·1 (0·1) |
| Mean | 60·4 (22·2) | 55·2 (20·8) | 18·2 (9·7) | 5·2 (2·5) | 3·1 (0·9) | 17·1 (2·1) | 3·2 (0·4) | 27·3 (4·9) | 33·3 (6·3) | 0·7 (0·2) | 1·6 (0·3) | 3·7 (0·6) | 1·1 (0·2) |
| CV | 36·8 | 37·7 | 53·3 | 48·1 | 29 | 12·3 | 12·5 | 17·9 | 18·9 | 28·6 | 18·8 | 16·2 | 18·2 |
| VBS | 29·7** | 29·3** | 39·8*** | 14·8** | 0·2 | 5·6 | 15·3* | 13 | 10·6* | 8·2* | 27·3** | 36·2*** | 52·4*** |
| VAP | 34·5*** | 34·7*** | 30·8*** | 19·8*** | 7·4*** | 45·3*** | 39·9*** | 70·3*** | 46·1*** | 29·7*** | 24·4*** | 20·0*** | 23·2*** |
| VAI | 7·7*** | 7·9*** | 8·1*** | 12·2*** | 15·6*** | 30·5*** | 19*** | 11·3*** | 16·8*** | 23·9*** | 25·5*** | 26·8*** | 13·4*** |
| VWI | 28·2 | 28·1 | 21·3 | 53·3 | 76·8 | 18·6 | 25·8 | 5·5 | 26·6 | 38·2 | 22·8 | 17 | 11 |
LITERATURE CITED
- Armbruster WS. Multilevel analysis of morphometric data from natural plant populations: insights into ontogenetic, genetic, and selective correlations in Dalechampia scandens. Evolution. 1991;45:1229–1244. doi: 10.1111/j.1558-5646.1991.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Schwaegerle KE. Causes of covariation of phenotypic traits among populations. Journal of Evolutionary Biology. 1996;9:261–276. [Google Scholar]
- Armbruster WS, Di Stilio V, Tuxill JD, Flores TC, Velazquez Punk JL. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg's correlation-pleiades concept. American Journal of Botany. 1999;86:39–55. [PubMed] [Google Scholar]
- Bell G. A comparative method. The American Naturalist. 1989;133:553–571. [Google Scholar]
- Berg RL. A general evolutionary principle underlying the origin of developmental homeostasis. The American Naturalist. 1959;93:103–105. [Google Scholar]
- Berg RL. The ecological significance of correlation pleiades. Evolution. 1960;14:171–180. [Google Scholar]
- Bonnet E, Van de Peer Y. zt: a software tool for simple and partial Mantel tests. Journal of Statistical Software. 2002;7:1–12. [Google Scholar]
- Boyd A. Morphological analysis of Sky Island populations of Macromeria viridiflora (Boraginaceae) Systematic Botany. 2002;27:116–126. [Google Scholar]
- ter Braak CJF, Šmilauer P. CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination (version 4·5) Ithaca NY: Microcomputer Power; 2002. [Google Scholar]
- Brion C, Puntieri J, Grigera D, Calvelo S. Flora de Puerto Blest y sus alrededores. San Carlos de Bariloche, Argentina: Universidad Nacional del Comahue; 1988. [Google Scholar]
- Byers JA. Surface distance between two points of latitude and longitude. 1997. Agricultural Research Service. http://www.chemical-ecology.net/java/lat-long.htm .
- Cabrera AL, Willink A. Biogeografía de América Latina. Washington DC: OEA; 1980. Biological Series Monograph No. 13. [Google Scholar]
- Chalcoff VR, Aizen MA, Galetto L. Nectar concentration and composition in 26 species from the temperate forest of South America. Annals of Botany. 2006;97:413–421. doi: 10.1093/aob/mcj043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JK, Sterling A. Selection for independence of floral and vegetative traits: evidence from correlation patterns in five species. Canadian Journal of Botany. 1996;74:642–644. [Google Scholar]
- Conner JK, Via S. Patterns of phenotypic and genetic correlations among morphological and life-history traits in wild radish. Evolution. 1993;47:704–711. doi: 10.1111/j.1558-5646.1993.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Devoto M, Montaldo NH, Medan D. Mixed hummingbird–long proboscid-fly pollination in ‘ornithophilous’ Embothrium coccineum (Proteaceae) along a rainfall gradient in Patagonia, Argentina. Austral Ecology. 2006;31:512–519. [Google Scholar]
- Domínguez CA, Eguiarte LE, Núñez-Farfan J, Dirzo R. Flower morphometry of Rhizophora mangle (Rhizophoraceae): geographical variation in Mexican populations. American Journal of Botany. 1998;85:637–643. [PubMed] [Google Scholar]
- Dudley SA. Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution. 1996;50:103–110. doi: 10.1111/j.1558-5646.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- Endler JA. Geographic variation, speciation and clines. Princeton, NJ: Princeton University Press; 1977. [PubMed] [Google Scholar]
- Ezcurra C, Ruggiero A, Crisci JV. Phylogeny of Chuquiraga Sect. Acanthophyllae (Asteraceae-Barnadesioideae), and the evolution of its leaf morphology in relation to climate. Systematic Botany. 1997;22:151–163. [Google Scholar]
- Faegri F, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1971. [Google Scholar]
- Fahn A. Studies in ecology of nectar secretion. Palestine Journal of Botany, Jerusalem Series. 1949;4:207–224. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–473. [Google Scholar]
- Gardner MF. The Chilean firebush, Embothrium coccineum. The New Plantsman. 1997;4:194. [Google Scholar]
- Grant V. The evolutionary process. New York, NY: Columbia University Press; 1991. [Google Scholar]
- Hansen TF, Pélabon C, Armbruster WS. Comparing variational properties of homologous floral and vegetative characters in Dalechampia scandens: testing the Berg hypothesis. Evolutionary Biology. 2007;34:86–98. [Google Scholar]
- Harder LD, Barrett SCH. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialization. Ecology. 1993;74:1059–1072. [Google Scholar]
- Herrera CM. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In: Lloyd DG, Barrett SCH, editors. Floral biology. New York, NY: Chapman and Hall; 1996. pp. 65–87. [Google Scholar]
- Herrera CM, Cerda X, Garcia MB, Guitan J, Medrano M, Rey PJ, et al. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. Journal of Evolutionary Ecology. 2002;15:108–121. [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York, NY: Oxford University Press; 2006. pp. 278–294. [Google Scholar]
- Herrera J. Flower size variation in Rosmarinus officinalis: individuals, populations and habitats. Annals of Botany. 2005;95:431–437. doi: 10.1093/aob/mci041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Judd WS, Campbell CS, Kellog EA, Stevens PF. Plant systematics, a phylogenetic approach. Sunderland, MA: Sinauer Associates; 1999. [Google Scholar]
- Ladd PG. Pollen presenters in the flowering plants: form and function. Botanical Journal of the Linnean Society. 1994;115:165–195. [Google Scholar]
- Lambrecht SC, Dawson TE. Correlated variation of floral and leaf traits along a moisture availability gradient. Oecologia. 2006;151:574–583. doi: 10.1007/s00442-006-0617-7. [DOI] [PubMed] [Google Scholar]
- Mantel NA. The detection of disease clustering and a generalized regression approach. Cancer Research. 1967;27:209–220. [PubMed] [Google Scholar]
- Mascó M, Noy-Meir I, Sérsic AN. Geographic variation in flower color patterns within Calceolaria uniflora in southern Patagonia. Plant Sytematics and Evolution. 2004;244:77–91. [Google Scholar]
- Mathiasen P, Rovere AE, Premoli AC. Genetic structure and early acting effects of inbreeding in fragmented temperate forests of a self-incompatible tree, Embothrium coccineum. Conservation Biology. 2007;21:232–240. doi: 10.1111/j.1523-1739.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- Medrano M, Castellanos MC, Herrera CM. Comparative floral and vegetative differentiation between two European Aquilegia taxa along a narrow contact zone. Plant Systematics and Evolution. 2006;262:209–224. [Google Scholar]
- Moore DM. Flora of Tierra del Fuego. St Louis, MO: Missouri Botanical Garden; 1983. [Google Scholar]
- Nattero J, Cocucci AA. Geographical variation in floral traits of the tree tobacco in relation to its hummingbird pollinator fauna. Biological Journal of the Linnean Society. 2007;90:657–667. [Google Scholar]
- Parkhurst DF, Loucks OI. Optimal leaf size in relation to environment. Journal of Ecology. 1972;60:505–537. [Google Scholar]
- Rico-Gray V, Palacios-Rios M. Leaf area variation in Rhizophora mangle L. (Rhizophoraceae) along a latitudinal gradient in Mexico. Global Ecology and Biogeography Letters. 1996;5:30–35. [Google Scholar]
- Romero MM, Riveros MC, Cox M, Alberdi M. Growth dynamics and phenology of Embothrium coccineum Forst. at different altitudes. Revista Brasileira de Botánica. 1987;10:139–135. [Google Scholar]
- Rovere AE, Premoli AC. Dispersión asimétrica de semillas de Embothrium coccineum (Proteaceae) en el bosque templado de Chiloé, Chile. Ecología Austral. 2005;15:1–7. [Google Scholar]
- Rovere AE, Smith-Ramírez C, Armesto JJ, Premoli AC. Breeding system of Embothrium coccineum (Proteaceae) in two populations on different slopes of the Andes. Revista Chilena de Historia Natural. 2006;79:225–232. [Google Scholar]
- Sapir Y, Shmida A, Fragman O, Comes HP. Morphological variation of the Oncocyclus irises (Iris: Iridaceae) in the southern Levant. Botanical Journal of the Linnean Society. 2002;139:369–382. [Google Scholar]
- Silva-Montellano A, Eguiarte LE. Geographic patterns in the reproductive ecology of Agave lechuguilla (Agavaceae) in the Chihuahuan desert. I. Floral characteristics, visitors, and fecundity. American Journal of Botany. 2003;90:377–387. doi: 10.3732/ajb.90.3.377. [DOI] [PubMed] [Google Scholar]
- Simon JL. Resampling Stats. 1992:13. IBM Version 3. [Google Scholar]
- Slatkin M. Genetic background. In: Futuyma DJ, Slatkin M, editors. Coevolution. Sunderland, MA: Sinauer Associates; 1983. pp. 14–32. [Google Scholar]
- Sleumer HO. Proteaceae. In: Correa MN, editor. Flora Patagonica 4a. INTA: Buenos Aires, Argentina; 1984. pp. 20–28. [Google Scholar]
- Smith-Ramírez C, Armesto JJ. Nectarivoría y polinización por aves en Embothrium coccineum (Proteaceae) en el bosque templado del sur de Chile. Revista Chilena de Historia Natural. 1998;71:53–65. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York, NY: Freeman; 1981. [Google Scholar]
- Souto CP, Premoli AC. Genetic variation in the widespread Embothrium coccineum (Proteaceae) endemic to Patagonia: effects of phylogeny and historical events. Australian Journal of Botany. 2007;55:809–817. [Google Scholar]
- Souto CP, Premoli AC, Reich PB. Leaf trait variation in Embothrium coccineum (Proteaceae) is shaped by complex Patagonian physiographic gradients. Revista Chilena de Historia Natural. 2008 (in press) [Google Scholar]
- Thompson JN. The coevolutionary process. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Thompson JN. Geographic mosaic of coevolution. Chicago, IL, USA: University of Chicago Press; 2005. [Google Scholar]
- Wiersema JH, León B. World economic plants, a standard reference. Boca Raton, FL: CRC Press; 1999. [Google Scholar]