Abstract
Background and Aims
The period during which seeds develop on the parent plant has been found to affect many seed characteristics, including dormancy, through interactions with the environment. Goodenia fascicularis (Goodeniaceae) seeds were used to investigate whether seeds of an Australian native forb, harvested from different environments and produced at different stages of the reproductive period, differ in dormancy status.
Methods
During the reproductive phase, plants were grown ex situ in warm (39/21 °C) or cool (26/13 °C) conditions, with adequate or limited water availability. The physiological dormancy of resulting seeds was measured in terms of the germination response to warm stratification (34/20 °C, 100 % RH, darkness).
Key Results
Plants in the cool environment were tall and had high above-ground biomass, yet yielded fewer seeds over a shorter, later harvest period when compared with plants in the warm environment. Seeds from the cool environment also had higher viability and greater mass, despite a significant proportion (7 % from the cool-wet environment) containing no obvious embryo. In the warm environment, the reproductive phase was accelerated and plants produced more seeds despite being shorter and having lower above-ground biomass than those in the cool environment. Ten weeks of warm stratification alleviated physiological dormancy in seeds from all treatments resulting in 80–100 % germination. Seeds that developed at warm temperatures were less dormant (i.e. germination percentages were higher) than seeds from the cool environment. Water availability had less effect on plant and seed traits than air temperature, although plants with reduced soil moisture were shorter, had lower biomass and produced fewer, less dormant seeds than plants watered regularly.
Conclusions
Goodenia fascicularis seeds are likely to exhibit physiological dormancy regardless of the maternal environment. However, seeds collected from warm, dry environments are likely to be more responsive to warm stratification than seeds from cooler, wetter environments.
Key words: Goodenia fascicularis, Goodeniaceae, Australia, physiological dormancy, seeds, temperature, soil moisture, maternal influence, climate
INTRODUCTION
The period during which seeds develop on the parent plant has been found to affect many seed characteristics, including dormancy, through interactions with the environment (Fenner, 1991; Wulff, 1995). As a result, seeds from a single species can vary greatly in dormancy status, depending on when and where they were collected. How the environment influences plant and seed traits will depend on the level of ‘stress’ experienced by the plant. Stress in this instance is defined as abiotic factors that reduce the rate of physiological processes (e.g. growth and reproduction), below the maximum rate that the plant could otherwise sustain. The causes, levels and timing of stress are all relative to a plant's natural environment; however, a typical response to stress will occur within seconds to days of stressful exposure and will result in a decline in plant performance (Lambers et al., 1998).
In the field, individual plants may experience different environmental conditions because they have different phenological schedules or they grow in different geographical regions. Influential environmental factors include air temperature and soil moisture (for reviews, see Fenner, 1991; Wulff, 1995; Gutterman, 2000), and the type of dormancy exhibited by a seed usually dictates the effect of an environmental parameter on seed germinability (Baskin and Baskin, 2001). Many studies carried out on crop and exotic species have found physiological dormancy (PD), the most common expression of seed dormancy (Baskin and Baskin, 2001), to be negatively correlated with air temperature during seed development. Thus, warmer temperatures during seed maturation usually result in decreased dormancy, while cooler temperatures have the opposite effect. For example, studies upon invasive weeds within Australia found that high maternal day/night temperatures (30/25 °C) during seed production reduced dormancy of Lolium rigidum (Poaceae) (Steadman et al., 2004), while higher germination percentages of Onopordum acanthium (Asteraceae) seeds were achieved after parent plants were grown in warm environments (28 or 30 °C) compared with cool (20 or 22 °C) (Qaderi et al., 2006). Only seeds exhibiting physical dormancy have been found to be conversely affected by maternal temperature (Fenner, 1991; Baskin and Baskin, 2001).
Dormancy status has also been found to vary with soil water stress/drought during seed development, depending on the species and the dormancy mechanism(s) involved (Fenner, 1991). Maternal drought reduces dormancy of many PD seeds, including the herbaceous weed Sinapis arvensis (Brassicaceae) (Luzuriaga et al., 2005) and Avena fatua (Poaceae) (Peters, 1982; Sawhney and Naylor, 1982).
Understanding the environmental determinants of variation in dormancy status can enable us to make predictions about dormancy loss, select for reduced dormancy in the field and use and conserve seeds more successfully. Whether Australian native seeds conform to the general trends in response to maternal air temperature and soil moisture is unknown. Goodenia fascicularis (Goodeniaceae) is a native Australian perennial forb that flowers throughout the year but particularly in spring (September to November). Seeds of this species dispersed in late spring in south-west Queensland (Qld) exhibit a deep level of PD that enables seedlings to avoid potentially fatal summer temperatures by postponing germination until the following autumn (Hoyle et al., 2008b). Widespread across all mainland states, in a large number of habitats and soil types (George, 1992), G. fascicularis has great potential for inclusion in land revegetation projects, particularly if there is an enhanced understanding of dormancy regulation in this species. Therefore G. fascicularis seeds collected in south-west Qld were used to investigate whether seeds of a native forb, harvested from different environments or produced at different stages of the reproductive period, differ in dormancy status. Experiments were designed to determine the effect of maternal air temperature and soil water availability during flowering, gamete production, fertilization, fruiting and seed maturation, on plant growth and development, and the viability and dormancy status of F1 seeds.
MATERIALS AND METHODS
Seed collection and storage
Seeds of Goodenia fascicularis F.Muell. & Tate. were collected in south-west Qld (28°03′39′′S, 145°49′7′′E), on 24 October 2004. At least 10 000 seeds were collected from >50 individual plants within one population. Only mature seeds were collected, i.e. seed capsules were dehiscing and seeds were brown in colour and dispersing naturally. In the laboratory, seeds were processed (non-seed material was removed by hand and with an aspirator), and stored at 15 °C and 15–20 % relative humidity (RH) until experimentation began in April 2006.
Seed germination
Goodenia fascicularis seeds require warm stratification to alleviate dormancy prior to being placed in germination test conditions (Hoyle et al., 2008a). To obtain seedlings for subsequent glasshouse experiments, multiple replicates of 20 seeds were sown into 9-cm-diameter plastic Petri dishes containing 1 % water–agar medium. Dishes were wrapped in aluminium foil to exclude light and stored at 34/20 °C (12/12 h thermoperiod) for 5 weeks. Seeds were then transferred to fresh agar, sealed in transparent plastic bags to prevent agar desiccation and placed into germination incubators at 20 °C, 12/12 h photoperiod. Light was provided by white fluorescent tubes (approx. 50 µmol m–2 s–1). Ten days after seed germination, which was defined as visible radicle emergence by at least 1 mm, seedlings were transplanted into a potting mix and transferred to a glasshouse (see below).
In a separate experiment, a light requirement for germination was investigated. Three replicates of 20 seeds received warm stratification for 0, 2 and 5 weeks before being transferred to fresh agar and sown in germination tests at 20 °C with a 12/12 h photoperiod or constant darkness. Germination was counted after 5 weeks and any non-germinated seeds were cut-tested and examined for viability. A firm, fresh endosperm and white embryo were considered viable and empty or necrotic seeds were excluded when calculating percentage germination.
Plant growth
Ten-day-old seedlings were transplanted into multiple-celled trays containing University of California mix (UC mix B) which is 1 : 1 (v/v) river sand and peat with 4 kg of stock fertilizer per 0·50 m–3 of mix; 1 kg of stock fertilizer contained blood and bone (185 g), potassium nitrate (30 g), potassium sulfate (15 g), superphosphate (185 g), dolomite (310 g), hydrate lime (185 g) and gypsum (90 g). Trays were placed on benches in ambient glasshouse conditions [12–31 (mean 18) °C and 21–94 (mean 68) % RH], and seedlings were watered every day. Fifty days later plants were transplanted into 15-cm-diameter pots containing 1·7 kg UC mix B and foliage was tied up with cotton around three stakes. Additional fertilizer was applied to pots at this stage and after flowering had begun, at a rate of 1 g L–1 of Aquasol™ (Yates Ltd) water-soluble fertilizer dissolved in distilled water, with 50 mL of the solution added per pot. Plants were watered every 2–4 d.
Temperature and soil moisture treatments
When individual plants had two or three open flowers they were randomly assigned to ‘wet’ or ‘dry’ soil moisture treatments (described below) and moved to either a ‘warm’ (minimum 21 °C, maximum 39 °C, mean 25·8 ± 0·1 °C) or ‘cool’ (minimum 13 °C, maximum 26 °C, mean 16·8 ± 0·1 °C) temperature controlled glasshouse. In the warm glasshouse, air RH was between 30 and 94 (mean 69·5 ± 0·2) %, while in the cool glasshouse it was between 47 and 93 (mean 79·6 ± 0·2) % RH. As it was not possible to replicate glasshouses, the experimental design consisted of two soil treatments nested within two temperature environments (four treatments altogether). All plants were moved into one of the four treatments within a 28-d period and distributed evenly between three blocks (later replicates) per glasshouse, each containing between 15 and 18 plants per treatment. At this stage, mean plant height was 31·0 ± 0·5 cm, and plants had an average of 4·5 ± 0·2 budding branches and 2·4 ± 0·1 open flowers.
A soil moisture retention curve for the UC potting mix was determined previously using the pressure plate method (O'Donnell and Adkins, 2001). The percentage available water content of the UC mix B was established using mean fresh and oven-dry soil weight (eqn 1). Three soil samples were taken at the beginning, middle and end of each potting-up session and closed tins were weighed (fresh soil weight). Lids were then removed and tins placed at 105 °C for at least 24 h before lids were replaced and tins re-weighed (oven-dry weight).
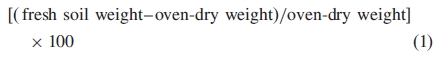 |
1 |
A soil moisture deficit treatment was applied once plants had been moved to the temperature-controlled glasshouses and an open watering system was adopted. ‘Wet’ plants were maintained at an average of 80 % field capacity or 24·7 % soil moisture content (–0·01 MPa) and ‘dry’ plants were allowed to dry down to 8·5 % soil moisture content (–1 MPa) before being watered back up to 80 % field capacity. This equated to ‘wet’ plants being watered daily (warm) or every 2 d (cool) and the ‘dry’ plants being watered every 4 d (warm) or every 7 d (cool). ‘Dry’ plant pots stood in trays for a few minutes after watering, to allow dry soil to take up water.
Pollination
Flowers of the Goodeniaceae are distinguished by having five stamens and a style bearing an indusium. Pollen is deposited into the indusium before flowers open, and growth of the stigma from within the indusium slowly forces pollen from the mouth of the indusium for presentation to the pollinating vector, usually an insect (Howell et al., 1993). The three successive events described for secondary pollen presentation in the Asterales–Campanulales complex (Leins and Erbar, 1990) were confirmed for G. fascicularis and pollination was achieved in two ways. First, fly screens were secured over glasshouse air vents and a bee hive installed into each glasshouse. Each hive contained approx. 10 000 native bees (Trigona carbonaria), up to 1000 of which were expected to forage at temperatures above 18 °C. Secondly, pollen was transferred by hand, via a small paintbrush, from the indusium of younger flowers to the stigma of older flowers, between different plants, at random. On average, each plant, including every open flower, was hand pollinated (pollen both taken and added) 3·0 ± 0·1 times over the flowering period.
Plant data collected
Plants were regularly examined for size, foliage colour and rate of development. Each plant's height (measured from soil level to the apical bud of the tallest flowering stem) and number of open flowers was recorded monthly. Above-ground plant dry biomass was calculated at the end of the experiment on 21 December 2006; the above-ground material of each plant was placed in a paper bag and dried in an oven at 65 °C for 48 h, before the contents were weighed.
Seed harvest and storage
As seeds reached maturity, capsules changed from green to brown and then dried and cracked open around the circumference. Seeds from each plant were harvested immediately after capsules began to open and were placed in separate paper envelopes, per plant, per day. The seed harvest period ranged from a total of 8–11 weeks in duration depending on the seed's maturation environment. Upon harvest, seeds were placed in drying conditions (15 °C, 15–20 % RH) for a total of 4 weeks. Within the first 2 weeks of drying, seeds were processed; chaff and seed capsules were removed by hand. At the end of 4 weeks, seeds were put into paper envelopes, then vacuum-sealed in aluminium envelopes and placed at –20 °C. All seeds were stored at –20 °C for the same length of time, 3 months. The process of drying seeds to between 15 and 20 % RH and storing at –20 °C are together referred to as ‘banking’, methods commonly employed by seed banks for germplasm conservation (Linington, 2003).
Assessing seed dormancy status
Three months post-banking, all aluminium foil bags containing seeds were removed from storage at –20 °C and allowed to thaw for 24 h at room temperature (approx. 20 °C) before being opened. Ten replicates of 50 randomly selected seeds, per block, per treatment, were weighed and both the total number of seeds harvested per plant and the mean seed mass, per treatment, estimated.
Approximately 80 % of all seeds collected from each treatment per week were bulked together and the seed dormancy status after various durations of warm stratification investigated. Multiple replicates of 50 seeds from each block were sown onto 1 % water–agar medium in 9-cm-diameter plastic Petri dishes, wrapped in aluminium foil and placed at 20/34 °C for 0, 1, 2, 3, 4, 6 or 10 weeks. One replicate per block (i.e. three replicates in total per treatment) was then transferred to fresh agar and sown in germination tests at either constant 20 °C or alternating 25/15 °C (12/12 h photoperiod). Final germination was counted after 5 weeks and any non-germinated seeds cut-tested for viability. Empty or necrotic seeds were excluded when calculating percentage germination.
The remaining 20 % of seeds from each treatment per week were used to investigate the effect of treatment duration on dormancy status of seeds produced. Multiple replicates of 20 seeds produced at the beginning and the end of the harvest period, per treatment, were sown onto 1 % water–agar medium and placed in the warm stratification treatment for 1, 2, 3, 4 or 6 weeks before three replicates per treatment were transferred to fresh agar and sown in germination tests at 20 °C 12/12 h photoperiod. Final germination was counted after 5 weeks and any non-germinated seeds cut-tested for viability. Due to variation in seed numbers, the seeds compared were as follows: week 1 vs. 9 (warm-wet treatment), weeks 1 and 2 vs. 10 and 11 (warm-dry treatment), weeks 1, 2, 3 and 4 vs. 8 and 9 (cool-wet treatment) and weeks 2, 3 and 4 vs. 7 and 8 (cool-dry treatment).
Assessing seed viability and germinability
Tests were also carried out to assess the effect of banking on seed viability and germinability. Twenty seeds per block were harvested from each treatment at the beginning, middle and end of the harvest period and, prior to drying and freezing, were sown in germination tests conditions (25/15 °C, 12/12 h photoperiod) with and without receiving a pre-germination test warm stratification treatment (4 weeks at 20/34 °C, 100 % RH, in darkness). Post-banking, these tests were repeated using seeds from the same harvest times and replicates. Final germination was counted after 5 weeks and any remaining non-germinated seeds were cut-tested for viability.
Seeds that did not receive warm stratification did not germinate and were therefore tested for viability using the tetrazolium chloride (TZ) staining technique (International Seed Testing Association, 2003). The gelatinous wing and at least one half of each seed coat were removed to expose the spatulate embryo beneath the opaque endosperm. Seeds were placed in glass Petri dishes, submerged in a 1 % aqueous TZ solution, wrapped in aluminium foil and placed at 30 °C for 24 h. Each seed was then dissected and the embryo examined for staining. Embryos uniformly stained dark pink/red were considered viable and those that were pale pink, unstained or irregularly stained were considered non-viable.
Statistical analysis
All statistical analysis was carried out in Minitab 15 (Minitab Inc., 2007). The effects of soil moisture and block (replicate) were analysed nested within temperature environment and data were transformed where necessary in order to meet the assumptions of ANOVA. Plant height data was log transformed and a nested MANOVA carried out in which Wilks' test was used to determine overall effect of temperature, soil moisture and block. Open flower data were square-root transformed and a nested ANOVA (GLM) compared day 40 data only. A nested ANOVA (GLM) also analysed plant biomass, seeds per plant (square-root transformed), mean seed weight, seeds without embryos (arcsine transformed), seed viability (arcsine transformed) and seed germinability (arcsine transformed) data. Sigmoidal Gompertz three-parameter curves were generated with SigmaPlot (V7, SPSS Inc.) and fitted to non-transformed germination data (one curve per replicate). A nested ANOVA (GLM) compared curve parameters a (maximum % germination), b (slope) and x0 (tG50, time to reach 50 % germination, in weeks). A one-way ANOVA compared curve parameters between the two germination temperature regimes. In addition, a nested MANOVA was performed on arcsine-transformed germination data. Lastly, a split-plot ANOVA (GLM) analysed the effect of treatment duration upon response to dormancy alleviation.
RESULTS
Parental seed germination
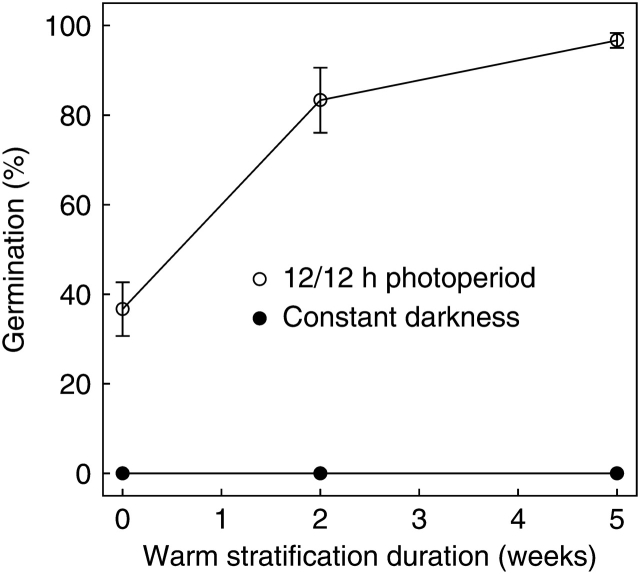
Parental G. fascicularis seeds (i.e. those collected from the field) were found to have an obligate light requirement for germination regardless of dormancy status (Fig. 1). Warm stratification for increasing durations prior to germination testing gradually alleviated PD of seeds until the majority germinated at 20 °C after 5 weeks stratification and developed into healthy experimental plants (data not shown).
Fig. 1.
Percentage germination (mean ± s.e.) of G. fascicularis seeds collected from the field, at 20 °C, 12/12 h photoperiod and constant darkness, after receiving increasing durations of warm stratification.
Glasshouse plants and their seeds
Within 40 d of growing in the glasshouse treatment conditions, G. fascicularis plants subjected to a cool (mean 17 °C) temperature regime during the reproductive phase were significantly taller (day 40, ANOVA: F = 8·18, d.f. = 1, P = 0·005) and exhibited twice as many flowers (day 40, ANOVA: F = 72·94, d.f. = 1, P ≤ 0·001) when compared with those in the warm (mean 26 °C) environment. Plants in the cool environment were taller, measuring an average of 52 ± 1 cm in height after 113 d, compared with 46 ± 1 cm for plants in the warm environment (MANOVA, Wilks': F = 8·8, d.f. = 5, P ≤ 0·001; Fig. 2A). After 113 d, plants in the warm environment no longer exhibited flowers, whereas those in the cool environment continued to exhibit flowers (whether they were new or original flowers is not known; Fig 2B). In the warm environment, the time between flowering and seed maturity was 42 d, compared with 66 d in the cool (Fig. 2C). Plants at warm temperatures yielded a total of approx. 8900 seeds compared with approx. 6000 seeds from plants in the cool; however, there was no difference in seed yield between wet and dry treatments (Fig. 2C).
Fig. 2.
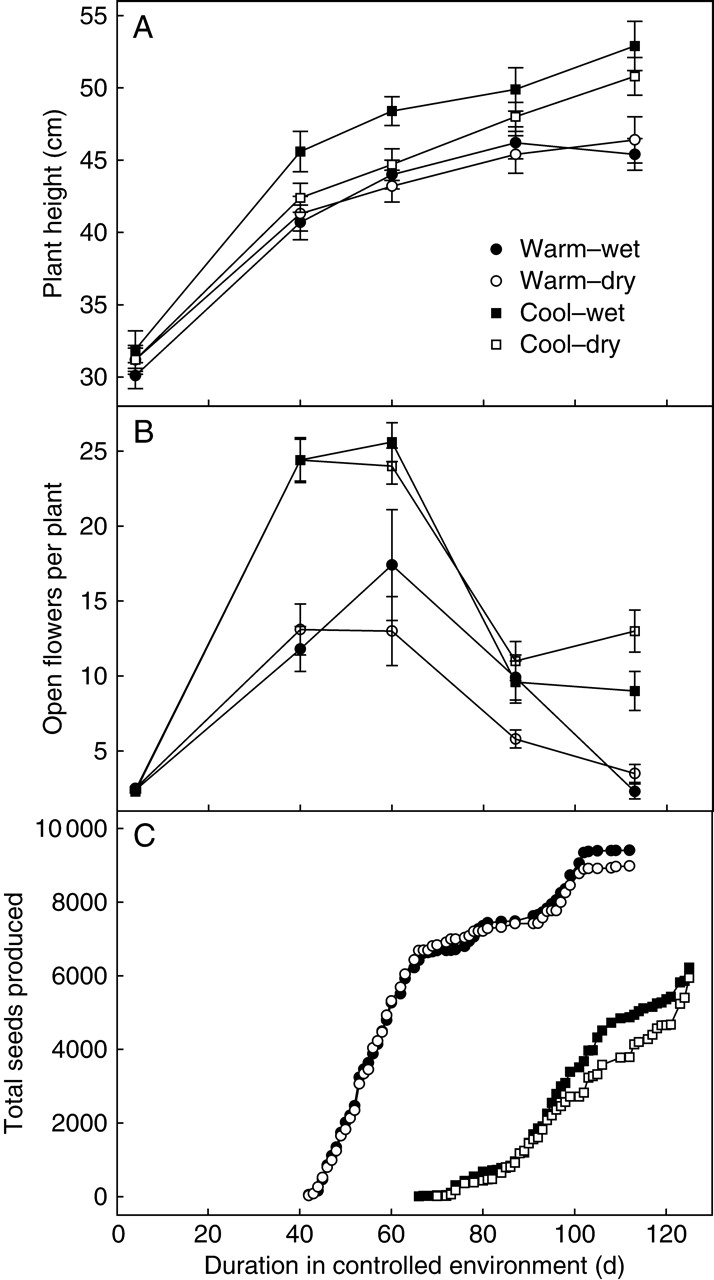
The effect of a warm-wet, warm-dry, cool-wet and cool-dry environments during the reproductive phase on G. fascicularis plant height (A), number of open flowers per plant (B) and cumulative total number of seeds harvested (C) (mean ± s.e.).
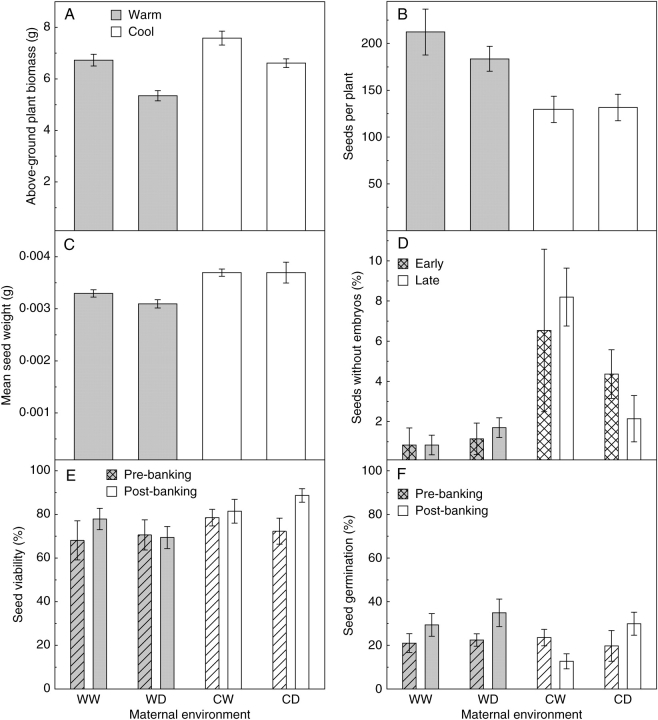
At the end of the experiment, plants from the cool environment had a greater above-ground biomass (mean 7·1 ± 0·2 g plant–1) than those from the warm environment (mean 6·0 ± 0·2 g plant–1; GLM, F = 21·6, d.f. = 1, P ≤ 0·001). Plants that received limited water were lower in biomass (mean 6·0 ± 0·2 g plant–1) than well-watered plants (mean 7·2 ± 0·2 g plant–1) (GLM, F = 5·24, d.f. = 6, P ≤ 0·001; Fig. 3A). Plants from the cool environment yielded approx. 70 fewer seeds per plant than those in the warm environment (GLM, F = 20·75, d.f. = 1, P ≤ 0·001; Fig. 3B). Of seeds from the cool environment, seed coats appeared to be paler/red (compared with brown) in colour (data not shown) and were approx. 0·5 mg greater in mass than seeds from the warm environment (GLM, F = 13·11, d.f. = 1, P = 0·001; Fig. 3C), despite a significant proportion containing no filled, spatulate embryo but rather an empty space (GLM, F = 12·54, d.f. = 1, P = 0·008), particularly those from the cool-wet treatment (approx. 7 %; Fig. 3D). This seed defect was not related to seeds being produced early or late during plant growth (GLM, F = 0·73, d.f. = 4, P = 0·597; Fig. 3D). Seeds from the cooler environment were more viable (mean 83·0 ± 1·7 %) than those from the warm (mean 71·6 ± 2·2 %; GLM, F = 17·02, d.f. = 1, P = 0·001), according to TZ staining patterns, and the seed-banking process had no effect on seed viability data (GLM, F = 4·23, d.f. = 4, P = 0·096; Fig. 3E) or seed germinability data (GLM, F = 0·58, d.f. = 4, P = 0·696; Fig. 3F).
Fig. 3.
The effect of a warm-wet (WW), warm-dry (WD), cool-wet (CW) and cool-dry (CD) environment during the reproductive phase of G. fascicularis on above-ground plant biomass (A), number of seeds produced per plant (B), mean seed weight (C), percentage of seeds containing no embryo, early and late harvest (D), seed percentage viability as determined by TZ staining pre- and post-banking (E) and seed percentage germination at 25/15 °C, 12/12 h photoperiod after 4 weeks of warm stratification, pre- and post-banking (F) (mean ± s.e.).
Seed dormancy status
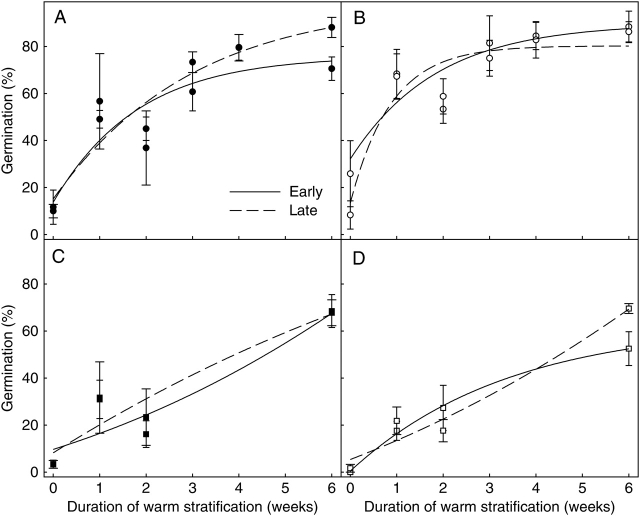
Increasing durations of warm stratification (20/34 °C, 100 % RH, darkness) resulted in dormancy alleviation of seeds from all treatments, evidenced by the increasing percentage of seed germination in germination test conditions (Fig. 4). Seeds responded differently to the two germination test temperature regimes. Times to 50 % germination were faster (one-way ANOVA, F = 19·54, d.f. = 1, P ≤ 0·001) and germination curve slopes steeper (one-way ANOVA, F = 18·58, d.f. = 1, P ≤ 0·001) at 20 °C when compared with 25/15 °C, although between 80 % and 100 % of seeds could germinate at either temperature regime after 10 weeks of warm stratification (Fig. 4). The effect of treatment on dormancy status was analysed in two ways. Analysis of Sigmoidal Gompertz curve parameters did not reveal any significant differences in maximum germination, tG50 or the slope of curves between treatments. A MANOVA revealed a significant overall effect of maternal air temperature on dormancy status of seeds germinated at constant 20 °C (MANOVA Wilks': F = 95·6, d.f. = 7, P = 0·010); cool maternal temperatures yielded seeds with greater dormancy (i.e. lower germination after warm stratification) when compared with those from warm maternal temperatures (Fig. 4B). The difference in dormancy status was particularly evident after 1, 3 and 4 weeks of warm stratification (P < 0·05). At 25/15 °C, the overall difference in germination response between treatments was less obvious than at 20 °C (MANOVA Wilks': F = 5·7. d.f. = 7, P = 0·158). However, seeds from the cooler temperatures were again significantly more dormant than those from the warm temperatures after 2, 4 and 10 weeks warm stratification (P < 0·05). There was no effect of maternal soil moisture upon dormancy status, regardless of stratification treatment duration, germination test temperature or data analysis. However, seeds matured in the warm-dry treatment tended to exhibit the least PD and those matured in the cool-dry treatment, the most PD (Fig. 4). This trend was also evident when examining only those seeds that developed early and late in the reproductive phase (Fig. 5). However, seeds germinated in response to warm stratification regardless of when plants committed to their production [GLM: P = 0·487 (warm-wet), 0·436 (warm-dry), 0·900 (cool-wet), 0·278 (cool-dry); Fig. 5).
Fig. 4.
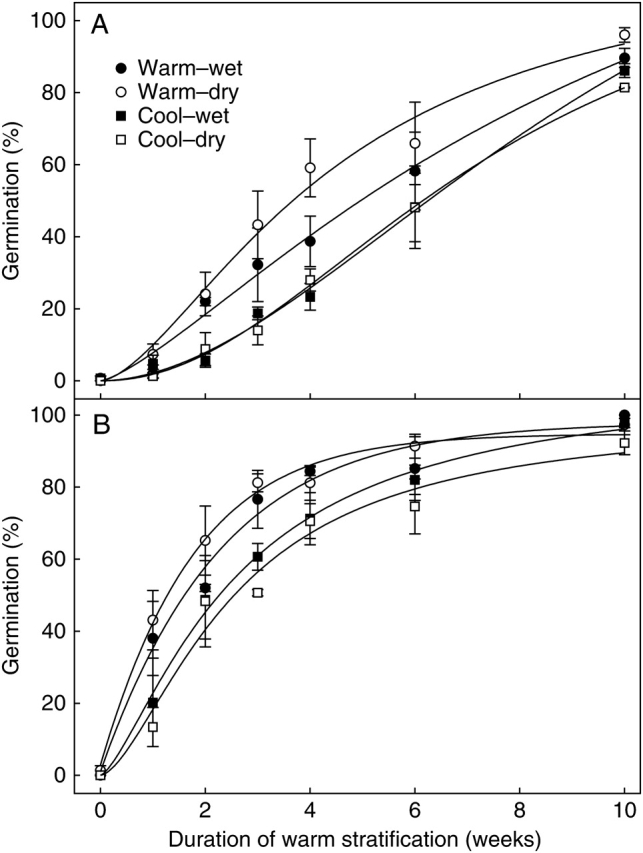
The effect of a warm-wet, warm-dry, cool-wet and cool-dry environments during the reproductive phase on final percentage germination (mean ± s.e.) of G. fascicularis at 25/15 °C (A) and constant 20 °C (B), 12/12 h photoperiod, after increasing durations of warm stratification.
Fig. 5.
The effect of early and late seed development on final percentage germination (mean ± s.e.) at 20 °C, 12/12 h photoperiod, of G. fascicularis seeds from a warm-wet (A), warm-dry (B), cool-wet (C) and cool-dry (D) environment after increasing durations of warm stratification.
DISCUSSION
Mature seeds of G. fascicularis exhibit a PD that is alleviated by the application of warm stratification (20/34 °C, 100 % RH, in darkness), a relatively new approach to alleviating seed dormancy ex situ (Hoyle et al., 2008a, b). The present study confirmed, with both parental and F1 seeds, that increasing durations of stratification can alleviate PD of G. fascicularis seeds until, after 10 weeks of warm stratification, 80–100 % of seeds germinate at temperatures between 15 and 25 °C. Germination was greater at constant 20 °C when compared with alternating 25/15 °C, and white light was necessary for germination, suggesting that in situ, seeds persist and germinate on or close to the soil surface. Goodenia fascicularis is commonly found in moisture-retentive soil, particularly adjacent to depressions which become flood-ways during periods of rain (Williams, 1988). Consequently, it is concluded that PD of G. fascicularis seeds dispersed in south-west Qld in spring will be gradually alleviated by the effects of a warm, wet stratification during summer rains, and germination will be stimulated by light and the onset of cooler autumn temperatures. Thus, seeds postpone germination until after the hot summer months.
The process of banking seeds (drying to 15–20 % RH and storing at –20 °C), commonly adopted in seed conservation and revegetation projects, did not significantly reduce the seed viability or germination at conditions tested. Results suggest that G. fascicularis seeds can be stored in seed banks for periods of at least 3 months while retaining high viability for subsequent use, as expected for orthodox seeds. However, in such storage conditions PD will persist, supporting the observation that dry storage does not alleviate PD of G. fascicularis (Hoyle et al., 2008a, b).
During the reproductive phase, air temperature was influential in determining, to some degree, dormancy loss in the resulting seeds. A warmer environment resulted in seeds being less dormant (i.e. requiring less warm stratification before germinating), when compared with seeds from a cooler environment. This was particularly evident after 2–4 weeks of warm stratification when seeds exhibited early and rapid dormancy loss. A relationship between maternal temperature and seed PD loss has not been previously reported in an Australian native plant. However, the present results are similar to those observed in PD crop and exotic species. In addition to examples already mentioned (Steadman et al., 2004; Qaderi et al., 2006), five genotypes of the wheat species Triticum aestivum (Poaceae) produced less dormant seed when parent plants experienced unusually warm conditions (>30 °C for >12 d) 30–50 d post-anthesis (Biddulph et al., 2007).
Seed production was earlier and greater, and seed viability reduced, in the warm environment when compared with the cool. Low production temperatures also reduced yield of Lactuca sativa seeds (Drew and Brocklehurst, 1990) and unusually cold temperatures in the Austrian Alps delayed the initiation of seed development in Gentianella germanica (Gentianaceae) (Wagner and Mitterhofer, 1998). Accelerated physiological and biochemical processes in the warm environment may have acted to circumvent stages in the development of dormancy mechanisms, such as accumulation of germination inhibitors, enzymes or hormones, resulting in seeds requiring less warm stratification prior to germination.
Increased dormancy in G. fascicularis seeds from the cool environment corresponded with increased seed mass and paler, red (compared with brown) seed coats of the less dormant seeds. Similarly, Lactuca sativa (Asteraceae) which also disperses PD seeds, produced heavier seeds at low temperatures, the thicker pericarps of which increased mechanical resistance to embryo growth (Drew and Brocklehurst, 1990). Although it is not known what tissues were affected, a role for the seed coat in the PD of G. fascicularis is supported by the fact that seeds responded to scarification as a means of alleviating dormancy (Hoyle et al., 2008b).
The effect of water availability was less pronounced; however, seeds from reduced soil moisture treatments tended to have reduced dormancy, particularly those from the warm environment. The present results match those found for many members of the Poaceae including Avena fatua (Peters, 1982; Sawhney and Naylor, 1982), Sorghum halepense (Benech Arnold et al., 1992) and Bromus tectorum (Meyer and Allen, 1999), and others such as Sinapis arvensis (Cruciferae) (Luzuriaga et al., 2005). Similarly, maternal temperature had a greater effect upon dormancy status of Alopecurus myosuroides (Poaceae) than soil moisture, but seeds produced under warm, dry conditions exhibited least dormancy (Swain et al., 2006).
The overall plant morphology of G. fascicularis was dependent on both temperature and soil moisture during the reproductive phase. Warmer maternal temperatures resulted in reduced plant height and biomass and plants that experienced reduced soil moisture were significantly smaller in biomass than those watered frequently. Reduced plant size in response to high temperatures and drought may help to reduce water loss in an attempt to withstand increased stress. Indeed temperatures in the cooler-treatment environment more closely matched those common to south-west Qld during autumn and winter (Bureau of Meteorology, 2007), when G. fascicularis is expected to grow and reproduce in situ. Results suggest that G. fascicularis traded-off growth and photosynthesis for reproduction in response to increased stress in the warmer environment.
While dormancy levels in G. fascicularis were affected by the maternal environment, these effects were comparatively minor. Nor was PD status affected by the time at which plants produced seeds during the treatments. Consequently, the present results suggest that these plants are highly adapted to, and tolerant of, variable temperatures and rainfall events. The high resistance to warm temperatures and reduced soil moisture parallels results found for species native to Mediterranean semi-arid regions (Llorens et al., 2003; Gonzalez-Rodriguez et al., 2005; Aragon et al., 2008). In the event of Qld's predicted climate change (increased temperatures, fewer/less predictable rain events), a large response to environmental stress may be a selective disadvantage, leading to less dormant seeds with lower viability, that are less discerning about when and where germination occurs. However, the present results suggest that G. fascicularis seeds are likely to exhibit PD regardless of maternal environment, and that maturation air temperature may only subtly regulate dormancy. Seeds of this forb collected from a warmer, drier location are likely to be more responsive to warm stratification as a means of alleviating PD. Since flowering is known to occur throughout the year (George, 1992), seeds that mature over winter and disperse in spring are likely to exhibit greater PD in preparation for postponing immediate germination. In contrast, seeds that mature later (during summer), and are dispersed in autumn when conditions favour subsequent plant growth, can ‘afford’ to be less particular about when and where they germinate.
AKNOWLEDGEMENTS
We are very grateful to Winston Bean, Lee Hickey, Ella Hoyle and Sarah Henrich for assistance in the glasshouses, Dr Tim Heard for the loan of his bees and Dr Olena Kravchuk for help with statistical analysis. Financial support from the Australian Mining Industry and the Millennium Seed Bank Project, The Royal Botanic Gardens, Kew, UK is gratefully acknowledged.
LITERATURE CITED
- Aragon CF, Escudero A, Valladares F. Stress-induced dynamic adjustments of reproduction differentially affect fitness components of a semi-arid plant. Journal of Ecology. 2008;96:222–229. [Google Scholar]
- Baskin C, Baskin J. Seeds, ecology, biogeography and evolution of dormancy and germination. London: Academic Press; 2001. [Google Scholar]
- Benech Arnold RL, Fenner M, Edwards PJ. Changes in dormancy level in Sorghum halepense seeds induced by water stress during seed development. Functional Ecology. 1992;6:596–605. [Google Scholar]
- Biddulph TB, Plummer JA, Setter TL, Mares DJ. Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.) Field Crops Research. 2007;103:139–153. [Google Scholar]
- Bureau of Meteorology. Climate statistics for Australian locations. 2007. [Accessed May 2007]. http://www.bom.gov.au/climate/averages/tables/cw_044026.shtml .
- Drew RLK, Brocklehurst PA. Effects of temperature of mother-plant environment on yield and germination of seeds of lettuce (Lactuca sativa) Annals of Botany. 1990;66:63–71. [Google Scholar]
- Fenner M. The effects of the parent environment on seed germination. Seed Science Research. 1991;1:75–84. [Google Scholar]
- George AS. Flora of Australia, Brunoniaceae, Goodeniaceae. Vol. 35. Canberra: Australian Government Publishing Service; 1992. Goodenia fascicularis F. Muell. & Tate; pp. 233–234. [Google Scholar]
- Gonzalez-Rodriguez AM, Martin-Olivera A, Morales D, Jimenez MS. Physiological responses of tagasaste to a progressive drought in its native environment on the Canary Islands. Environmental and Experimental Botany. 2005;53:195–204. [Google Scholar]
- Gutterman Y. Maternal effects on seeds during development. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CAB International; 2000. pp. 59–84. [Google Scholar]
- Howell GJ, Slater AT, Knox RB. Secondary pollen presentation in angiosperms and its biological significance. Australian Journal of Botany. 1993;41:417–438. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. Mimicking a semi-arid tropical environment achieves dormancy alleviation for seeds of Australian native Goodeniaceae and Asteraceae. Annals of Botany. 2008;a 101:701–708. doi: 10.1093/aob/mcn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GL, Steadman KJ, Daws MI, Adkins SW. Physiological dormancy in forbs native to south-west Queensland: diagnosis and classification. South African Journal of Botany. 2008;b 74:208–213. [Google Scholar]
- International Seed Testing Association. ISTA working sheets on tetrazolium testing. Vols I and II. Bassersdorf, Switzerland: ISTA; 2003. [Google Scholar]
- Lambers HF, Chapin S, III, Pons TL. Plant physiological ecology. New York, NY: Springer; 1998. Time scale of plant response to environment; pp. 4–5. [Google Scholar]
- Leins P, Erbar C. On the mechanisms of secondary pollen presentation in the Campanualales-Asterales-complex. Botanica Acta. 1990;103:87–92. [Google Scholar]
- Linington SH. The design of seed banks. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. [Google Scholar]
- Llorens L, Penuelas J, Estiarte M. Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiologia Plantarum. 2003;119:231–243. doi: 10.1034/j.1399-3054.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Luzuriaga AL, Escudero A, Perez-Garcia F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cuciferae) Weed Research. 2005;46:163–174. [Google Scholar]
- Meyer SE, Allen PS. Ecological genetics of seed germination regulation in Bromus tectorum L. II. Reaction norms in response to a water stress gradient imposed during seed maturation. Oecologia. 1999;120:35–43. doi: 10.1007/s004420050830. [DOI] [PubMed] [Google Scholar]
- O'Donnell CC, Adkins SW. Wild oat and climate change: the effect of CO2 concentration, temperature, and water deficit on the growth and development of wild oat in monoculture. Weed Science. 2001;49:694–702. [Google Scholar]
- Peters N. The dormancy of wild oat seed (Avena fatua L.) from plants grown under various temperature and soil moisture conditions. Weed Research. 1982;22:205–212. [Google Scholar]
- Qaderi MM, Cavers PB, Hamill AS, Downs MP, Bernards MA. Maturation temperature regulates germinability and chemical constitutes of Scotch thistle (Onopordum acanthium) cypselas. Canadian Journal of Botany. 2006;84:28–38. [Google Scholar]
- Sawhney R, Naylor JM. Dormancy studies in seed of Avena fatua. 13 Influence of drought stress during seed development on duration of seed dormancy. Canadian Journal of Botany. 1982;60:1016–1020. [Google Scholar]
- Steadman KJ, Ellery AJ, Chapman R, Moore A, Turner NC. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum) Australian Journal of Agricultural Research. 2004;55:1047–1057. [Google Scholar]
- Swain AJ, Huges ZS, Cook SK, Moss SR. Quantifying the dormancy of Alopecurus myosuroides seeds produced by plants exposed to different soil moisture and temperature regimes. Weed Research. 2006;46:470–479. [Google Scholar]
- Wagner J, Mitterhofer E. Phenology, seed development and reproductive success of an Alpine population of Gentianella germanica in climatically varying years. Botanica Acta. 1998;111:159–166. [Google Scholar]
- Williams KAW. Goodenia fascicularis. Native Plants Queensland. 1988;2:144. [Google Scholar]
- Wulff RD. Environmental maternal effects on seed quality and germination. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY: Marcel Dekker; 1995. pp. 491–505. [Google Scholar]